Abstract
Asthma is one of the most common chronic immunological diseases in humans, affecting people from childhood to old age. Progress in treating asthma has been relatively slow and treatment guidelines have mostly recommended empirical approaches on the basis of clinical measures of disease severity rather than on the basis of the underlying mechanisms of pathogenesis. An important molecular mechanism of asthma is type 2 inflammation, which occurs in many but not all patients. In this Opinion article, I explore the role of type 2 inflammation in asthma, including lessons learnt from clinical trials of inhibitors of type 2 inflammation. I consider how dichotomizing asthma according to levels of type 2 inflammation — into ‘T helper 2 (TH2)-high’ and ‘TH2-low’ subtypes (endotypes) — has shaped our thinking about the pathobiology of asthma and has generated new interest in understanding the mechanisms of disease that are independent of type 2 inflammation.
The type 1 and type 2 immune response paradigm describes distinct immune responses that are mainly regulated by subpopulations of CD4+ T cells known as T helper 1 (TH1) and TH2 cells, respectively. TH1 cells secrete interleukin-2 (IL-2), interferon-γ (IFNγ) and lymphotoxin-α, and stimulate type 1 immunity, which is characterized by prominent phagocytic activity1. By contrast, TH2 cells mainly secrete the prototypical cytokines IL-4, IL-5 and IL-13, and stimulate type 2 immunity, which is characterized by high antibody titres and eosinophilia2. Type 2 immune responses are induced by parasitic helminths and are associated with atopic diseases, such as allergy and asthma. Airway type 2 immune responses are mainly mediated by eosinophils, mast cells, basophils, TH2 cells, group 2 innate lymphoid cells (ILC2s) and IgE-producing B cells. Type 2 immune responses are characteristic of allergic rhinitis in the upper airways and asthma in the lower airways, and the details of these responses are understood in great detail3 (FIG. 1). Despite some gaps in our knowledge — such as the relative roles of TH2 cells versus ILC2s as sources of type 2 cytokines (such as IL-4, IL-5 and IL-13) — it is generally accepted that upstream events in the airway epithelium (involving master regulators such as thymic stromal lymphopoietin (TSLP), IL-25 or IL-33) result in increased production of type 2 cytokines that drive a cascade of downstream events. These include IgE-triggered hypersensitivity to aeroallergens, activation of airway epithelial cells, chemoattraction of effector cells (mast cells, eosinophils and basophils), and remodelling of the epithelium and subepithelial matrix.
Figure 1. Type 2 immune responses in asthma.
Release of epithelial cell cytokines, particularly interleukin-33 (IL-33) and thymic stromal lymphopoeitin (TSLP), induces the expression of OX40 ligand (OX40L; also known as TNFSF4) on dendritic cells (DCs) to promote their mobilization to local draining lymph nodes where they activate naive CD4+ T cells to an IL-4-competent state. These IL-4-competent T cells in the lymph nodes migrate to B cell zones where they differentiate into T follicular helper (TFH) cells and move into the circulation to complete maturation as T helper 2 (TH2) cells. IL-4-secreting TFH cells in parafollicular B cell areas mediate IgE class-switching in B cells, whereas TH2 cells that migrate to the airway epithelium and to the subepithelial mucosa secrete IL-5 and IL-13 to mediate inflammatory and remodelling changes in the airway mucosa that predispose an individual to asthma and to asthma exacerbations. ILC2, group 2 innate lymphoid cell; TSLPR, TSLP receptor.
There has been much recent discussion of the role of the microbiome and early-life exposure to bacterial antigens in the origins of asthma4. I do not attempt to cover these important issues in this Opinion article and instead focus on how type 2 inflammation in asthma is initiated at the molecular level. Type 2 inflammatory responses in the lungs often start in childhood, when environmental stimuli — such as viral respiratory tract infections or exposures to oxidants, such as cigarette smoke or other airborne pollutants — can activate airway epithelial cells to produce IL-25, IL-33 or TSLP. This initiates a pathogenic cascade, which leads to the development of asthma in children who are susceptible because they have pre-existing atopy, specific genetic risk factors in regulators of type 2 inflammation or other less well-understood vulnerabilities. The reasons why type 2 immune responses that are initiated during childhood become persistent are not well understood. It may be that aberrant immune programmes become fixed because they are established during crucial time windows in early life when the immune system is particularly plastic. During this time period, either innate or adaptive immune cells may be susceptible to epigenetic changes that lead to persistent changes in cell behaviour5.
Type 2 inflammation is suppressed by glucocorticoids, which have long been the mainstay controller medication for asthma. As steroids have multiple local side effects (including dysphonia and candidiasis) and systemic side effects (including cataracts, osteoporosis and adrenal suppression), particularly when used in high doses over prolonged periods of time, the advent of more specific inhibitors of type 2 inflammation in the past 10–15 years has raised hope that these drugs will provide similar benefits to patients with asthma without the same side effect profile. However, as detailed below, compared with glucocorticoids, specific inhibitors of type 2 inflammation have a more limited range of effects on airway function and asthma control, and their emerging role is as adjunctive treatments to steroids rather than as replacement therapies. In this Opinion article, I outline type 2 inflammation in asthma, summarize the lessons that have been learnt from clinical trials of inhibitors of type 2 inflammation and consider how these lessons are informing concepts of asthma heterogeneity, asthma biology and the future of personalized therapies for asthma.
Phenotypic heterogeneity of asthma
Approximately 300 million people in the world have asthma — with at least 250,000 deaths attributed to the disease each year — which makes it the most common chronic lung disease6. It is defined as a disease that is characterized by recurring symptoms of reversible airflow obstruction, bronchial hyperresponsiveness (BHR) and airway inflammation7 (FIG. 2), and its clinical manifestations can range from mild to severe (BOX 1). Multiple pathological changes occur in the airway epithelium and submucosa in asthma and these changes are collectively referred to as airway remodelling (BOX 2).
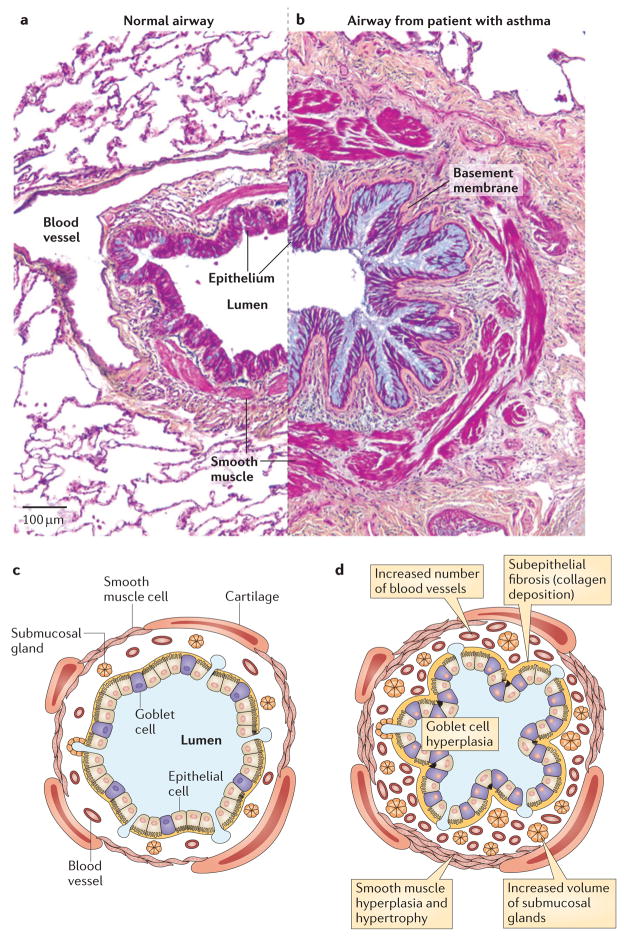
Figure 2. Airway pathology in asthma.
Airway structures in medium-sized healthy airways (part a; a schematic representation is depicted in part c) and in a patient with asthma (part b; a schematic representation is depicted in part d). The airways in asthma show considerable structural remodelling, including goblet cell hyperplasia, subepithelial fibrosis and increases in smooth muscle volume. Figure parts a and b republished with permission of Dove Medical Press, from the Journal of Asthma and Allergy, Clinical update on the use of biomarkers of airway inflammation in the management of asthma, Wadsworth, S., Sin, D. and Dorscheid, D., 4, 2011; permission conveyed through Copyright Clearance Center, Inc.
Box 1. Clinical features of asthma.
Asthma is characterized by episodes of shortness of breath (dyspnoea) that are relieved by treatment with β2-adrenergic receptor agonists. Many patients with asthma have intermittent symptoms that can be controlled as needed with β2-adrenergic receptor agonists and low doses of inhaled corticosteroids or a leukotriene receptor antagonist. A considerable subgroup of individuals with asthma suffer from more severe disease that is characterized by more frequent symptoms and exacerbations. Asthma exacerbations are a complex clinical phenomenon involving a loss of asthma control that leads to symptoms, including shortness of breath, wheeze, cough and sputum production. Airway narrowing during asthma exacerbations results not only from concentric smooth muscle contraction but also from mucosal oedema and the formation of pathological intraluminal mucus. Exacerbations can range from mild to severe and can result in near-fatal or fatal episodes of respiratory failure. The most common cause of asthma exacerbation is viral upper respiratory tract infections37. Common causative viruses include respiratory syncytial virus in children and rhinoviruses in adults64.
Box 2. Airway remodelling in asthma.
Asthma is pathologically characterized by abnormal structural changes in the airway epithelium and submucosa. Changes in the epithelium include goblet cell metaplasia, hyperplasia and increases in epithelial mucin stores. Changes in the submucosa include subepithelial fibrosis (increased deposition of collagen I, collagen III and collagen V, as well as fibronectin and tenascin C, in the lamina reticularis of the basement membrane zone)65–67 and changes in submucosal gland cells (to increase gland volume), smooth muscle cells (to cause hypertrophy and hyperplasia)68,69 and blood vessel cells (to cause increases in the number of blood vessels)70,71. Pathological changes in the airway are thought to predispose individuals with asthma to exacerbations by narrowing baseline airway calibre and by altering structural elements in a manner that predisposes to exaggerated responses to inhaled exacerbants64.
It has long been evident that asthma severity varies greatly between patients but this phenotypic heterogeneity has recently been investigated in a more systematic way. Specifically, cluster analyses of large numbers of highly characterized individuals with asthma have shown that they can be grouped into 4–5 phenotypic clusters according to age, gender, atopy, lung function, health care utilization and body mass index (BMI)8,9. These cluster-based studies highlight the clinical heterogeneity of asthma; for example, the largest clusters are characterized by mild airflow obstruction and relative resistance to exacerbations, but other clusters with considerably sized subgroups of patients are characterized by more severe airflow obstruction and are more prone to exacerbations. The clusters of patients with more severe asthma typically include those who are older and those who are obese9.
Increased awareness of the phenotypic heterogeneity of asthma has spurred interest in whether this heterogeneity can be explained by specific underlying cellular and molecular mechanisms. Linking phenotypes to mechanisms requires concomitant analysis of biospecimens for markers of inflammation, and this approach is now being undertaken by the U-BIOPRED research group in Europe and the SARP research group in the United States. The association of obesity and increasing age with more severe asthma provides some clues about the potential mechanisms of disease. As an example of one among many potential mechanisms, it is possible that the systemic inflammation that is associated with obesity, which includes increased oxidative stress and increased production of IL-1, IL-6 or tumour necrosis factor (TNF) by white adipose tissue and inflammatory macrophages10, may have effects in the airways to worsen asthma. However, phenotypic heterogeneity is limited in how much it can inform our understanding of disease mechanisms because phenotypic traits such as airway narrowing can be caused by multiple disease mechanisms. Therefore, a physiological test showing a reduction in FEV1(forced expired volume in 1 second) in any one patient does not reveal a specific mechanism or point to a specific anti-inflammatory treatment. For this reason, it has been suggested that disease endotypes represent a better conceptual framework than disease phenotypes11 to accelerate progress towards personalized treatments, and endotypes have now been adopted as a useful framework for asthma research12,13.
Asthma endotypes — TH2-high asthma
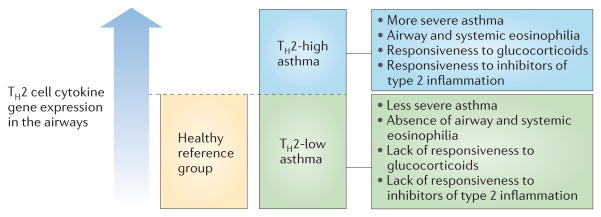
The term endotype was proposed in 2008 as a conceptual framework to guide new thinking about the molecular heterogeneity of asthma14. Specifically, it was proposed that an endotype is a disease subtype that is defined by a distinct functional or pathobiological mechanism. It is distinct from a disease phenotype, which is any observable characteristic or trait of a disease without any implication for mechanism. The advantage of classifying asthma as groups of endotypes would be that each endotype could logically be targeted by treatments that are specific for the causative molecular mechanism. So far, there is only one proposed endotype of asthma — ‘TH2-high’ asthma — which is characterized by increased levels of type 2 inflammation in the airways compared with the normal reference range of healthy controls (FIG. 3).
Figure 3. Asthma can be divided into TH2-low and TH2-high subgroups.
Levels of gene expression for T helper 2 (TH2) cell cytokines76 or for the activation of epithelial cells by TH2 cell cytokines77 show a continuum in the airways of patients with asthma (rather than a bimodal distribution). Individuals with asthma who have expression levels higher than the range found in healthy controls have specific clinical, pathological and treatment-response characteristics. This suggests a threshold effect of TH2 cell cytokines in the airways above which type 2 inflammation influences the clinical features of asthma and the responsiveness to specific treatments. Biomarkers of type 2 inflammation in blood and exhaled air can identify individuals with asthma who are above and below this threshold, and are showing promise as predictors of responsiveness to a growing list of type 2 cytokine inhibitors that are in late-phase clinical trials for asthma.
It has been known for some time that some individuals with asthma have airway eosinophilia whereas others do not and that eosinophilia predicts a favourable response to glucocorticoid treatment14–16. The stability of these eosinophilic subtypes was not clear until recently, but it is now apparent that large subgroups of patients with asthma are persistently lacking airway eosinophilia17. The transition from a concept of eosinophilic asthma to TH2-high asthma came from array-based gene expression studies of airway epithelial brushings from patients with mild to moderate asthma, which showed that approximately half of the patients had a gene expression profile that was consistent with the activation of epithelial cells by the type 2 cytokine IL-13 and half did not18,19. This approximately even division of patients with asthma into those with and without an activation signature for IL-13 is consistent with the proportion of individuals with asthma who are found to have airway eosinophilia16,17,20–22. Patients with TH2-high asthma have eosinophilia and other signs of airway type 2 inflammation, including increased numbers of airway mast cells23. The gene expression signature for IL-13-mediated activation of airway epithelial cells is characterized by marked upregulation of the gene encoding periostin, which has an unusual localization to the subepithelial region of the airway18; this can be explained by the rapid basal secretion of periostin by IL-13-activated epithelial cells24. This observation raised the possibility that periostin might enter subepithelial blood vessels and be detectable as a blood-based biomarker of IL-13-activated airway epithelial cells — a possibility that has now been supported by biomarker validation studies25 and by clinical trials showing that blood periostin levels predict responsiveness to IL-13-directed treatment in patients with asthma26. However, the widespread use of periostin as a biomarker in the general population of individuals with asthma requires further investigation (see later).
An important characteristic of TH2-high asthma is that it is responsive to treatment with inhaled corticosteroids, whereas TH2-low asthma (classified as having levels of type 2 inflammation in the airways that are comparable to the normal reference range of healthy controls) is not19. This finding advances our understanding of mechanisms of steroid resistance in asthma (BOX 3), and it raises questions about the treatment approaches that should be used for TH2-low asthma and about how best to address unmet needs for treatment approaches beyond corticosteroids for patients with TH2-high.asthma.
Box 3. Pharmacological treatment of asthma.
Inhaled β2-adrenergic receptor agonists were approved for the treatment of asthma in 1969 and inhaled glucocorticoids were approved in 1974. These two drug classes continue to be the main treatment for individuals with asthma, although leukotriene receptor antagonists and IgE-directed therapies are now available as additional treatment options. The subgroup of patients with severe disease needs a combination of therapies to achieve asthma control and to prevent asthma exacerbations. Typical treatment regimens include some combination of a long-acting β2-adrenergic receptor agonist, higher dose inhaled corticosteroid, oral corticosteroid, leukotriene receptor antagonist, long-acting anticholinergic medication and IgE-directed therapies. Current treatment guidelines recommend increasing treatment as disease becomes more severe with specific recommendations to increase the dose of corticosteroid and to add adjunctive treatments, such as IgE-directed therapies, as disease severity worsens72. As corticosteroids and IgE-directed therapies target type 2 inflammation, the guidelines assume a relationship between the severity of asthma and the strength of type 2 inflammation that is only partly supported by research. Although outcomes of type 2 inflammation such as eosinophil numbers are higher in the airways of patients with severe asthma than in those with mild asthma73, large subgroups of individuals with asthma, including many with severe disease, do not have airway eosinophilia17. Lack of corticosteroid response in asthma has been referred to as ‘steroid resistance’, with the implication being that steroid-resistant individuals with asthma have a defect in corticosteroid responsiveness that renders them resistant to an otherwise effective drug class74. One strategy to overcome this type of steroid resistance is to increase the dose of steroids and this is one rationale for guideline recommendations to treat patients who have poorly controlled asthma with higher doses of corticosteroids. However, true steroid resistance is relatively uncommon and a more probable explanation is that these individuals with asthma do not have a disease endotype that is sensitive to steroid treatment.
The lack of an effective controller medication for the TH2-low asthma subgroup is a considerable clinical problem and one without an obvious solution at present. The mechanisms of asthma other than type 2 inflammation are not well understood. Patients with TH2-low asthma probably constitute a mix of multiple disease endotypes that each affect relatively small subgroups of patients. It is important to note that patients who lack evidence of type 2 inflammation do indeed have asthma, as evidenced by a lack of smoking history and the presence of bronchial hyperreactivity and bronchodilator responsiveness17,19. Possible mechanisms of asthma in these patients include intrinsic abnormalities in airway smooth muscle or effects of oxidative stress, IL-17 or neutrophil products on structural elements of the airway that result in airway hyperresponsiveness and airway obstruction. Although there are no proven pathways to target at this point, this situation should improve as biomarkers are used to identify TH2-high asthma, so that TH2-low patients can be analysed separately in unbiased biospecimen analyses using ‘omics’ and other technologies that have broad potential to uncover mechanisms.
Although TH2-high asthma is generally a corticosteroid-responsive endotype16,17,19; a notable subgroup of patients with this endotype have persistent symptoms and uncontrolled asthma despite corticosteroid treatment27–30. This failure to fully respond to corticosteroids may reflect high levels of type 2 inflammation that cannot be controlled with steroids or a subgroup of patients with true steroid resistance. TH2-high asthma constitutes 50% of mild to moderate asthma19 and probably a larger proportion of patients with more severe asthma, so that the subgroups of patients with the TH2-high endotype who are not optimally controlled on low or medium doses of steroids are large enough to warrant additional treatment strategies. In addition, there are subgroups of patients with TH2-high asthma who require high doses of steroids to maintain asthma control and these patients need a steroid-sparing strategy to reduce their risk of dose-dependent side effects of corticosteroids31. Together, these reasons constitute the rationale for pursuing novel treatments for type 2 inflammation in asthma. The use of these type 2-targeted treatments in recent years has improved our understanding of how type 2 inflammation drives the physiological and pathological abnormalities of asthma.
Effects of inhibiting type 2 inflammation
The discovery of IgE in the 1970s and the progressive increase in our knowledge of type 2 inflammation in the 1990s and early 2000s provided multiple potential therapeutic targets for asthma, including IgE, IL-5, IL-13 and TSLP. It is now possible to assess the lessons that have been learnt from the initial clinical studies of these type 2-directed therapies. So far, clinical trial data of treatments in Phase II clinical trials or beyond are available for inhibitors of IgE, IL-5 and IL-13 (TABLE 1).
Table 1.
Summary of available data from trials of treatments directed at type 2 inflammation in asthma*
| Therapeutic antibody | Isotype | Targeted epitope | Relative affinity | Main effects in human asthma trials |
|---|---|---|---|---|
| Omalizumab(Genentech/Roche and Novartis) | Humanized IgG1 | IgE (CH2 and CH3 domains) | 0.06 nM78 | Decrease in asthma exacerbation rates and reductions in maintenance doses of oral corticosteroids36,37. Small effects on FEV1 and asthma symptoms. |
| Mepolizumab(GlaxoSmithKline) | Humanized IgG1 | IL-5 | NA | Decrease in asthma exacerbation rates when used to treat patients with asthma who have persistent eosinophilia despite corticosteroid treatment27–29. |
| Benralizumab (MedImmune/ AstraZeneca) | Humanized IgG1 | IL-5Rα | NA | Decrease in asthma exacerbation rates when used to treat patients with asthma who have persistent eosinophilia despite corticosteroid treatment45. |
| Reslizumab(Teva Pharmaceutical Industries) | Humanized IgG4 | IL-5 | 20 pM | Improvements in airway function and a trend towards greater asthma control when used to treat patients with asthma who have persistent eosinophilia despite corticosteroid treatment46. |
| Lebrikizumab(Genentech/Roche) | Human IgG4 | IL-13 (IL-4Rα-binding epitope) | <10 pM79 | No effect on FEV1 in steroid-naive individuals with asthma47. Improvements in FEV1 and asthma exacerbations in steroid-treated patients with moderate and severe asthma48. Greatest effects in patients with high serum periostin levels. |
| GSK679586(GlaxoSmithKline) | Human IgG1 | IL-13Rα1 and IL-13Rα2 | 300–400 pM80 | No improvement in FEV1 or exacerbations in patients with moderate to severe asthma. |
| Tralokinumab(MedImmune/AstraZeneca) | Human IgG4 | IL-13Rα1 and IL-13Rα2 | 165 pM81 | Limited effects on FEV1 but effective in reducing asthma exacerbations. Greatest effects in patients with high serum periostin levels. |
| Dupilumab(Regeneron Pharmaceuticals) | Human IgG4 | IL-4Rα | NA | Maintenance of asthma control and FEV1 when corticosteroid dose is tapered in patients with moderate to severe asthma30. Effects are greatest in patients with high blood eosinophil levels. |
The table is restricted to data from Phase II trials or beyond. FEV1, forced expired volume in 1 second; IL, interleukin; IL-5Rα, α-chain of the IL-5 receptor;
NA, not applicable.
Inhibiting IgE
Omalizumab (Xolair; Genentech/Roche and Novartis) is a non-anaphylactogenic monoclonal antibody that is specific for IgE and was approved for the treatment of asthma in 2002. Its dosing is determined on an individual basis from body weight and serum IgE level. Omalizumab depletes IgE, ‘disarms’ mast cells and basophils, and blocks the effects of IgE on dendritic cells. Early-phase clinical studies showed that omalizumab inhibits airway responses to inhaled allergens32,33, decreases airway eosinophilia34 and improves asthma control35. Phase III registration studies showed that omalizumab decreases asthma exacerbation rates and allows for reductions in maintenance doses of oral corticosteroids36,37. These effects are apparent in both children and adults, and have been confirmed in multiple post-registration studies38. In addition, omalizumab is well tolerated and apart from injection site reactions and a small risk of anaphylaxis, it is generally considered safe. Concerns about increased morbidity from geohelminth infections and about increased risk of malignancy have not been realized39,40.
Inhibiting IL-5
Three antibody therapeutics that target IL-5 or its receptor have advanced to Phase II clinical trials or beyond in asthma, and these drugs are also being targeted to eosinophilic disorders such as hypereosinophilic syndrome, eosinophilic oesophagitis and eosinophilic granulomatosis with polyangiitis. Mepolizumab (proposed trade name Bosatria; GlaxoSmithKline) is a humanized monoclonal antibody that binds IL-5 to prevent its interaction with the α-chain of the IL-5 receptor (IL-5Rα). Benralizumab (MedImmune/AstraZeneca) is a humanized recombinant IgG1 antibody that binds to IL-5Rα. Reslizumab (Cinquil; Teva Pharmaceutical Industries) is a humanized monoclonal antibody comprising the complementarity-determining regions of a murine antibody specific for human IL-5 that has been grafted onto human framework regions41.
An initial large multicentre clinical trial of the efficacy of mepolizumab in individuals with asthma who have persistent symptoms despite inhaled corticosteroid therapy showed that it had no significant effect on multiple outcomes of asthma control, including exacerbation rates42. However, this trial did not specifically target individuals with asthma who might be expected to benefit most — namely, the subgroup with persistent eosinophilia (eosinophilia that is not suppressed by corticosteroids), which is indicative of ongoing type 2 inflammation. The most recent studies of mepolizumab have focused on this asthma subgroup and these targeted trials have consistently shown that mepolizumab significantly decreases asthma exacerbation rates and is oral corticosteroid sparing27–29,43,44. Recently presented data from Phase II clinical trials of benralizumab and reslizumab show that they too are effective in improving asthma control or in reducing asthma exacerbations when used to treat patients with asthma who have persistent eosinophilia despite corticosteroid treatment45,46.
Inhibiting IL-13
IL-13 binds to two receptors. The first is the heterodimeric combination of the α1 chain of the IL-13 receptor (IL-13Rα1) and the α-chain of the IL-4 receptor (IL-4Rα), and the second is the monomeric IL-13Rα2. Of these, the heterodimeric receptor is also bound by IL-4. Four antibody therapeutics that prevent binding of IL-13 to its receptors have now been tested in Phase II clinical studies in asthma. Lebrikizumab (Genentech/Roche) interferes with IL-13 binding to IL-4Rα to prevent formation of IL-13–IL-13Rα1–IL-4Rα signalling complexes, and GSK679586 (GlaxoSmithKline) and tralokinumab (MedImmune/AstraZeneca) block binding of IL-13 to IL-13Rα1 and to IL-13Rα2. By contrast, dupilumab (Regeneron Pharmaceuticals) binds to IL-4Rα and so blocks signalling of both the IL-4 and IL-13 pathways. Although these antibodies have subtly different effects according to the epitopes that they bind and their effect on receptor internalization, the clinical trial data for IL-13-directed treatments can be summarized as follows: inhibiting IL-13 in steroid-naive individuals with asthma does not improve FEV1 (REF. 47), which indicates that IL-13 is not a mediator of airway smooth muscle dysfunction in asthma; and inhibiting IL-13 in individuals with asthma who are insufficiently controlled on inhaled corticosteroids improves lung function, asthma control and asthma exacerbation rates, particularly in individuals who have evidence of type 2 inflammation (systemic eosinophilia or increased serum periostin levels)47,48. One of the IL-13 inhibitors (GSK679586) has not proven to be as effective as the others49 for reasons that may be related to the relatively low epitope affinity of the antibody (TABLE 1) or because the clinical trial design did not specifically target individuals with asthma who have evidence of persistent type 2 inflammation despite treatment with corticosteroids.
Lessons from targeting type 2 inflammation
The safety data for IgE-directed therapy are reassuring50 and safety data for inhibitors of type 2 cytokines are currently being collected in multiple clinical trials. From the efficacy data collected so far in clinical trials of these drugs, it is clear that although study results are highly dependent on the patient population included in the trial, five important general lessons can be learnt.
IgE has an important role in mechanisms of asthma exacerbations
Omalizumab is effective in preventing exacerbations in many individuals with asthma, particularly in those with evidence of type 2 inflammation51. These data reveal an important role for IgE in the pathogenesis of asthma exacerbations. Although the clinical trials do not explain the mechanism, of which there are several possibilities, I believe that this might relate to the effects of IgE on IFNα secretion by plasmacytoid dendritic cells (pDCs). Specifically, IgE crosslinking impairs IFNα production and hence antiviral responses by pDCs52, so that omalizumab may reduce virus-induced asthma exacerbations (which are the most common type of exacerbations53) by restoring the antiviral activity of pDCs.
IL-5 and IL-13 are important mediators of asthma exacerbations
The clinical trial data for IL-5-directed and IL-13-directed therapies show a robust effect of decreasing asthma exacerbations in patients with type 2 inflammation, which implicates IL-5 and IL-13 as mediators of airway susceptibility to asthma exacerbation (most probably virus-induced asthma exacerbation) in these patients. Such susceptibility may result from the effects of these cytokines to promote airway eosinophilia or airway remodelling. Regarding eosinophils, respiratory viruses may activate eosinophils either directly or indirectly54 and products of activated eosinophils, such as eosinophil cationic protein or eosinophil myeloperoxidase, may then mediate exaggerated cellular responses to virus in epithelial cells, mucus cells, smooth muscle cells and endothelial cells.
Biomarkers have the potential to guide asthma treatment
Only when clinical trials of mepolizumab used persistent eosinophilia as a biomarker was it possible to show the efficacy of targeting IL-5 in decreasing asthma exacerbation rates. Similarly, the clinical efficacy of IL-13-directed therapies has been most apparent in the subgroups of individuals with asthma who have type 2 inflammation, as indicated by eosinophilia or increased serum periostin levels. Indeed, these clinical trials have highlighted the potential for blood-based biomarkers to guide treatment for individuals with asthma. Specifically, it is now clear that the benefit of treatments targeting type 2 cytokines is restricted to patients who have biomarkers of type 2 inflammation. This is the first evidence that asthma management could progress to a phase in which treatment guidelines can move from empirical recommendations on the basis of asthma severity (BOX 3) towards biomarker-based recommendations that promise to increase treatment precision.
Additional research will be required to identify the best and most practical biomarkers to guide asthma treatment. The number of eosinophils in peripheral blood has emerged as a useful biomarker of treatment response in recent studies of dupilumab and mepolizumab30,43,44, but questions remain about the standardization of blood eosinophil counts in clinical service laboratories and whether this test can be reliably used in clinical practice. Similarly, serum periostin level has shown promise as a biomarker of treatment response in clinical trials of lebrikizumab, but the specificity of this marker for type 2 responses in the general population of individuals with asthma requires further investigation and the use of this test in less well-controlled clinical scenarios is unknown.
Type 2 inflammation is a disease modifier
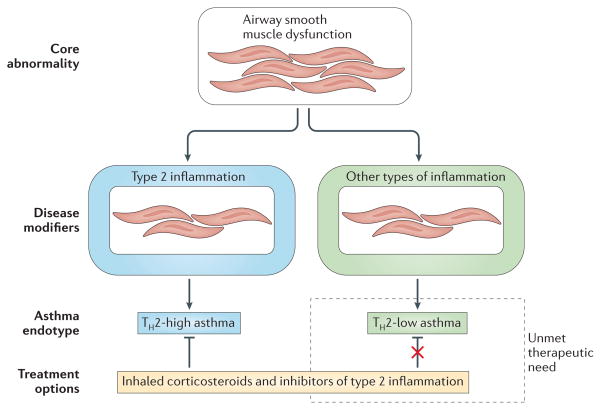
Inhibiting triggers and mediators of type 2 inflammation — such as IgE, IL-5 and IL-13 — has very limited effects on baseline asthma indices such as measures of airflow limitation or BHR. Indeed, in steroid-naive patients, IL-13-directed therapy has little effect on FEV1, even in individuals with asthma who have high serum periostin levels47. Similarly, a recent meta-analysis of seven clinical trials of mepolizumab showed that it has no significant effect on lung function55. This lack of effect of lebrikizumab and mepolizumab on FEV1 and BHR is in contrast to the robust effects of these drugs on asthma exacerbation rates and asthma control, as measured by the asthma control questionnaire. This should be compared with the effects of corticosteroids on both baseline measures of asthma and asthma exacerbations. The data showing that many individuals with asthma have no evidence of active type 2 inflammation in their airways, despite evidence of active smooth muscle dysfunction, indicate that the core physiological abnormalities of excessive smooth muscle tone and BHR in asthma are not driven by type 2 inflammation. Rather, the effect of type 2 inflammation might be to modify asthma to worsen asthma control and increase susceptibility to exacerbation (FIG. 4).
Figure 4. Asthma as a core disease of smooth muscle that is modified by inflammation.
Lessons learnt from clinical trials of inhibitors of type 2 inflammation suggest a conceptualization of asthma as a disease with a core abnormality in airway smooth muscle function that can be modified by inflammation to worsen disease severity and to promote susceptibility to asthma exacerbations. Although type 2 inflammation has been shown to be an important disease modifier by the effects of its inhibition in clinical trials, these same clinical trials also highlight that additional types of inflammation must contribute to certain forms of asthma. These non-type 2 inflammatory pathways are not well understood but may include those associated with obesity, infection or neutrophilia. TH, T helper.
Non-type 2 disease mechanisms must be present in subgroups of patients with asthma
Clinical trials that have selected patients with asthma on the basis of persistent eosinophilia despite treatment with inhaled corticosteroids have identified many patients who do not have evidence of type 2 inflammation but who have uncontrolled asthma. In addition, treatment of individuals with asthma who do have evidence of type 2 inflammation with IgE-directed, IL-5-directed or IL-13-directed therapies improves asthma but does not eliminate exacerbations or completely suppress other outcomes of poor asthma control. Therefore, non-type 2 inflammatory mechanisms must drive asthma in some subgroups of patients and must account for residual asthma in patients who have been successfully treated with inhibitors of type 2 inflammation.
Inhibiting regulators of type 2 cytokines
IL-25, IL-33 and TSLP are thought to be master regulators of type 2 inflammation in diseases such as asthma56,57, nasal polyposis58 and eosinophilic oesophagitis56. They are produced by airway epithelial cells, which gives them a suitable cellular location for a role in regulating responses to inhaled aeroallergens. The genes encoding TSLP and IL-33 (as well as the IL-33 receptor) consistently feature in lists of genes that are associated with asthma and atopy in genome-wide association studies59,60. IL-25, IL-33 and TSLP can all activate innate and adaptive immune cells to secrete IL-5 and IL-13, and the effects of IL-33 and TSLP are highly synergistic for this function61. There has consequently been considerable interest in targeting IL-25, IL-33 and TSLP as a treatment strategy for asthma. No inhibitors of IL-25 or IL-33 have reached the clinic yet, but inhibitors of TSLP are now completing early-phase studies; for example, AMG 157 (Amgen) is a fully human TSLP-specific monoclonal IgG2λ antibody that blocks the interaction of TSLP with its receptor complex (which consists of a unique TSLP receptor chain and the IL-7 receptor α-chain). AMG 157 has recently been tested for its effects on airway responses to aeroallergens in patients with mild asthma as a proof-of-concept study. AMG 157 attenuated both the early- and the late-phase responses to allergens62, a result that predicts success in subsequent clinical trials focused on asthma control outcomes. AMG 157 also attenuated allergen-induced increases in airway eosinophilia. As early-phase bronchoconstriction in response to allergens is considered a type I hypersensitivity response that is mediated by mast cell or basophil degranulation, the ability of AMG 157 to attenuate the early phase also suggests that inhibition of TSLP decreases the number of airway mast cells (or basophils). This might occur because mast cells or basophils rely on TSLP as a trophic signal for their accumulation in the airways63. It could also mean that TSLP has some role in mechanisms of IgE-mediated ‘arming’ of mast cells or basophils. Indeed, the effects of TSLP inhibition on airway responses closely mirror those of omalizumab32. Taken together, these data indicate that TSLP — perhaps working together with IL-33 — is an important and non-redundant upstream regulator of type 2 inflammation in the airways of patients with asthma.
Conclusion and future directions
Type 2 inflammation is an important disease mechanism in a large subgroup of individuals with asthma who have the TH2-high asthma endotype. Blood-based biomarkers, such as blood eosinophil counts and serum periostin levels, show promise for their ability to identify those patients with a TH2-high endotype so that personalized medicine targeting the type 2 inflammatory pathway should soon become a reality for these patients. The advent of specific inhibitors of type 2 inflammation has provided new insights into the role of type 2 inflammation in asthma and the importance of other disease pathologies. Specifically, type 2 inflammation seems to have a limited role in the mechanisms of airway smooth muscle dysfunction but it seems to have a major role in driving susceptibility to asthma exacerbations. In addition, many patients with asthma do not have type 2 inflammation and do not benefit from treatment with inhibitors of type 2 inflammation. Therefore, although the emphasis on dissecting mechanisms of type 2 inflammation in asthma is now benefitting many patients with TH2-high asthma, there remains a large subgroup of patients who do not have this endotype and so have unmet therapeutic needs. Meeting these needs will require a new research emphasis to better understand non-type 2 mechanisms of asthma.
Comprehensive and integrated efforts to improve disease understanding and to identify disease endotypes are in their infancy. Recent efforts include multicentre research programmes in severe asthma in Europe (U-BIOPRED) and in the United States (SARP) that aim to collect high quality biospecimens from highly characterized individuals with asthma with a view to using omics, and systems-based and hypothesis-driven experimental approaches to uncover novel mechanisms of disease. These efforts signal a trend towards a more disease biology-based approach, in which all the pieces of the asthma puzzle (clinical, cellular and molecular) are examined together to better identify currently hidden patterns or links between clinical traits and specific disease mechanisms. Understanding disease biology will require higher quality interactions between different investigator groups who have historically tended to work in silos based on research interest. Broader considerations of mechanisms combined with more interdisciplinary research are necessary so that asthma — a common disease with a high public health impact — can advance beyond knowledge of a single disease endotype (TH2-high asthma) to knowledge of multiple endotypes, which would provide a rational basis for new and personalized treatments.
Acknowledgments
Acknowledgements
The author would like to thank the following colleagues and collaborators who have contributed to his understanding of type 2 inflammation in asthma: P. Woodruff, M. Peters, E. Gordon, N. Bhakta, S. Christenson, H. Boushey, S. Lazarus, R. Locksley and M. Ansel (all at the University of California, San Francisco, USA), and M. Seibold (National Jewish Health, Denver, Colorado, USA) and J. Arron (Genentech Inc., South San Francisco, California, USA).
Glossary
- Asthma control questionnaire
A tool for measuring quality of life and symptoms in patients with asthma. It assesses whether an individual wakes in the mornings with symptoms, the limitations of daily activities, shortness of breath and wheeze
- Atopy
A tendency to develop IgE-mediated allergic disease, such as asthma, eczema (atopic dermatitis), allergic rhinitis (hay fever) or allergic conjunctivitis
- Bronchial hyperresponsiveness
(BHR). A state in which the airways are hyperreactive to various bronchoconstrictor stimuli, including methacholine, histamine, hypertonic saline, distilled water, exercise or eucapnic hyperventilation. Hyperresponsiveness in this context means a bronchoconstrictor response at ‘doses’ that normally have no bronchoconstrictor effect
- FEV1
(Forced expired volume in 1 second). A measure of airflow. Decreases in FEV1 are characteristic of asthma and are one metric of asthma severity
- Glucocorticoids
Steroid hormones that are produced in the adrenal glands. Synthetic analogues are available as anti-inflammatory drugs that are particularly effective in suppressing type 2 immune responses
- Group 2 innate lymphoid cells
(ILC2s). ILC2s are a subtype of innate lymphoid cells, which are a novel family of haematopoietic effector cells with heterogeneous location, cytokine production and effector functions. ILC2s specifically produce type 2 cytokines and depend on GATA-binding protein 3 and retinoic acid receptor-related orphan receptor-α for their development and function
- SARP
(Severe Asthma Research Program). A network of seven asthma research centres and one data coordination centre in the United States. SARP aims to improve understanding of the heterogeneity of asthma, including asthma endotypes, with the ultimate goal of better treatments. It is funded by the US National Heart, Lung and Blood Institute
- U-BIOPRED
(Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes). A European-wide project that aims to uncover biomarkers of asthma and to identify mechanisms of disease subtypes to improve the treatment of asthma. It is funded by the Innovative Medicine Initiative (IMI) and the European Federation of Pharmaceutical Industries and Associations (EFPIA)
Footnotes
Competing interests statement
The author declares competing interests: see Web version for details.
References
- 1.Spellberg B, Edwards JE., Jr Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 2.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigelman A, Weinstock GM, Bacharier LB. The relationships between environmental bacterial exposure, airway bacterial colonization, and asthma. Curr Opin Allergy Clin Immunol. 2014;14:137–142. doi: 10.1097/ACI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begin P, Nadeau KC. Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin Immunol. 2014;10:27. doi: 10.1186/1710-1492-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol. 2014;795:17–29. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 7.National Asthma Education and Prevention Program, National Heart, Lung and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis & Management of Asthma. 2007 http://www.ncbi.nlm.nih.gov/books/NBK7232/
- 8.Haldar P, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol. 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 12.Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discov Med. 2013;15:243–249. [PubMed] [Google Scholar]
- 13.Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13:249–256. doi: 10.1097/ACI.0b013e32836093dd. [DOI] [PubMed] [Google Scholar]
- 14.Green RH, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel SE, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 16.Berry M, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath KW, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodruff PG, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodruff PG, et al. T helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Silva L, et al. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can Respir J. 2011;18:144–148. doi: 10.1155/2011/430317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw DE, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132:1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
- 22.van Veen IH, et al. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol. 2009;124:615–617.e2. doi: 10.1016/j.jaci.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Dougherty RH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in TH2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053.e8. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidhu SS, et al. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia G, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–654.e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corren J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 27.Nair P, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 28.Haldar P, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavord ID, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel S, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 31.Randhawa I, Klaustermeyer WB. Oral corticosteroid-dependent asthma: a 30-year review. Ann Allergy Asthma Immunol. 2007;99:291–302. doi: 10.1016/S1081-1206(10)60543-1. [DOI] [PubMed] [Google Scholar]
- 32.Fahy JV, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 33.Boulet LP, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–1840. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 34.Djukanovic R, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 35.Milgrom H, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 study group. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 36.Soler M, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 37.Busse W, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 38.Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–340.e1. doi: 10.1016/j.jaip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Cooper PJ, et al. Geohelminth infections: a review of the role of IgE and assessment of potential risks of anti-IgE treatment. Allergy. 2008;63:409–417. doi: 10.1111/j.1398-9995.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 40.Long A, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134:560–567.e4. doi: 10.1016/j.jaci.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Egan RW, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 1999;49:779–790. doi: 10.1055/s-0031-1300502. [DOI] [PubMed] [Google Scholar]
- 42.Flood-Page P, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 43.Bel EH, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 44.Ortega HG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 45.Castro M, et al. Benralizumab reduces exacerbations and improves lung function in adults with uncontrolled eosinophilic asthma. Am Thorac Soc Abstr. 2014;B101:abstr. A3699. [Google Scholar]
- 46.Castro M, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 47.Noonan M, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J Allergy Clin Immunol. 2013;132:567–574.e12. doi: 10.1016/j.jaci.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 48.Hanania NA, et al. Efficacy and safety of lebrikizumab in severe uncontrolled asthma: results from the Lute and Verse Phase II randomized, double-blind, placebo-controlled trials. J Allergy Clin Immunol. 2014;133:abstr. AB402. [Google Scholar]
- 49.De Boever EH, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol. 2014;133:989–996. doi: 10.1016/j.jaci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Corren J, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788–797. doi: 10.1111/j.1365-2222.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 51.Sorkness CA, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol Pract. 2013;1:163–171. doi: 10.1016/j.jaip.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill MA, et al. Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davoine F, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77.e2. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Zhang S, Li DW, Jiang SJ. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PLoS ONE. 2013;8:e59872. doi: 10.1371/journal.pone.0059872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of TH2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 57.Beale J, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagarkar DR, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffatt MF, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira MA, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133:1564–1571. doi: 10.1016/j.jaci.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 62.Gauvreau GM, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 63.Siracusa MC, et al. Thymic stromal lymphopoietin-mediated extramedullary hematopoiesis promotes allergic inflammation. Immunity. 2013;39:1158–1170. doi: 10.1016/j.immuni.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karjalainen EM, et al. Airway inflammation and basement membrane tenascin in newly diagnosed atopic and nonatopic asthma. Respir Med. 2003;97:1045–1051. doi: 10.1016/s0954-6111(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 66.Beasley R, Roche W, Holgate ST. Inflammatory processes in bronchial asthma. Drugs. 1989;37 (Suppl 1):117–122. doi: 10.2165/00003495-198900371-00021. [DOI] [PubMed] [Google Scholar]
- 67.Brewster CE, et al. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 68.Woodruff PG, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 69.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. 2010;36:1174–1184. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 70.Wadsworth S, Sin D, Dorscheid D. Clinical update on the use of biomarkers of airway inflammation in the management of asthma. J Asthma Allergy. 2011;4:77–86. doi: 10.2147/JAA.S15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siddiqui S, et al. Vascular remodeling is a feature of asthma and nonasthmatic eosinophilic bronchitis. J Allergy Clin Immunol. 2007;120:813–819. doi: 10.1016/j.jaci.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 72.Reddy AP, Gupta MR. Management of asthma: the current US and European guidelines. Adv Exp Med Biol. 2014;795:81–103. doi: 10.1007/978-1-4614-8603-9_6. [DOI] [PubMed] [Google Scholar]
- 73.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6:256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 74.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 75.Woodruff PG, Fahy JV. Airway remodeling in asthma. Semin Respir Crit Care Med. 2002;23:361–367. doi: 10.1055/s-2002-34331. [DOI] [PubMed] [Google Scholar]
- 76.Peters MC, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133:388–394. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhakta NR, et al. A qPCR-based metric of TH2 airway inflammation in asthma. Clin Transl Allergy. 2013;3:24. doi: 10.1186/2045-7022-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng YG, Singh N, Wong WL. Binding of cynomolgus monkey IgE to a humanized anti-human IgE antibody and human high affinity IgE receptor. Mol Immunol. 1996;33:635–642. doi: 10.1016/0161-5890(96)00024-7. [DOI] [PubMed] [Google Scholar]
- 79.Ultsch M, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425:1330–1339. doi: 10.1016/j.jmb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 80.Hodsman P, et al. A Phase I, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics. Br J Clin Pharmacol. 2013;75:118–128. doi: 10.1111/j.1365-2125.2012.04334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.May RD, et al. Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol. 2012;166:177–193. doi: 10.1111/j.1476-5381.2011.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]