Abstract
Long-term catheterization inevitably leads to a catheter-associated bacteriuria caused by multispecies bacterial biofilms growing on and in the catheters. The overall goal of the presented study was (1) to unravel bacterial community structure and function of such a uropathogenic biofilm and (2) to elucidate the interplay between bacterial virulence and the human immune system within the urine. To this end, a metaproteomics approach combined with in vitro proteomics analyses was employed to investigate both, the pro- and eukaryotic protein inventory. Our proteome analyses demonstrated that the biofilm of the investigated catheter is dominated by three bacterial species, that is, Pseudomonas aeruginosa, Morganella morganii, and Bacteroides sp., and identified iron limitation as one of the major challenges in the bladder environment. In vitro proteome analysis of P. aeruginosa and M. morganii isolated from the biofilm revealed that these opportunistic pathogens are able to overcome iron restriction via the production of siderophores and high expression of corresponding receptors. Notably, a comparison of in vivo and in vitro protein profiles of P. aeruginosa and M. morganii also indicated that the bacteria employ different strategies to adapt to the urinary tract. Although P. aeruginosa seems to express secreted and surface-exposed proteases to escape the human innate immune system and metabolizes amino acids, M. morganii is able to take up sugars and to degrade urea. Most interestingly, a comparison of urine protein profiles of three long-term catheterized patients and three healthy control persons demonstrated the elevated level of proteins associated with neutrophils, macrophages, and the complement system in the patient's urine, which might point to a specific activation of the innate immune system in response to biofilm-associated urinary tract infections. We thus hypothesize that the often asymptomatic nature of catheter-associated urinary tract infections might be based on a fine-tuned balance between the expression of bacterial virulence factors and the human immune system.
Catheter-associated urinary tract infections (CAUTIs)1 account for up to 40% of all nosocomial infections and are thus the most prevalent source of hospital-acquired infectious diseases (1, 2). CAUTIs are mostly “asymptomatic” and characterized by less than 105 colony-forming units per milliliter urine, which do not cause any signs of infection or symptoms. A symptomatic CAUTI, usually correlated to a number of colony-forming units (CFUs) exceeding the above mentioned threshold, is diagnosed when symptoms commonly associated with urinary tract infections (e.g. fever, dysuria, urgency, flank pain, or leukocytosis) occur (3). The risk that CAUTIs become symptomatic increases dramatically during catheterization because of the formation of bacterial biofilms on catheter surfaces (4). This explains why the urinary tract of long-term hospitalized patients represents the part of the human body with the highest risk for acquiring sepsis caused by Gram-negative bacteria (5, 6). Long-term catheterization is commonly applied to elderly or disabled persons often for many years (3). Considering the actual demographic development in industrialized nations, problems caused by long-term urinary tract catheterization will certainly increase.
Biofilm formation of bacteria on medical devices, including implants, central venous catheters, and urinary tract catheters has become a worldwide and severe problem (7–9). Surface-associated bacteria, which are embedded in a complex matrix of extracellular polymeric substances (EPS), are highly resistant to antibiotics as well as to the human immune system and therefore hard to eradicate (10–12). Biofilms growing on urinary tract catheters have been demonstrated to often consist of multiple (two to six) species (5). Most frequently Pseudomonas aeruginosa, Proteus mirabilis, Providencia stuartii, Morganella morganii, Enterococcus faecalis, and Klebsiella pneumoniae have been identified in biofilms of long-term catheterized patients (13, 14).
Until now, the global adaptation mechanisms of uropathogens to their respective habitats, including P. mirabilis, E. faecalis, and Escherichia coli, have been preferentially analyzed by ex vivo cultivation in human urine (15–17), by employing murine models (18, 19) or in the human urinary tract (20). The mentioned studies identified the lack of freely available trace metals, especially iron, as a major limiting factor in human (and murine) urine during urinary tract infections (UTIs). For uropathogenic P. aeruginosa a strong iron limitation response was observed when cultured as colony biofilm for 6 days on artificial urine medium (AUM) agar (21). Moreover, fimbrial genes, like mrp and fim, were found highly expressed in uropathogenic P. mirabilis and E. coli (18), highlighting the importance of bacterial adherence during CAUTIs. Notably, mechanisms required for immune evasion including changes in surface structures (19) and secreted proteases cleaving proteins of the host immune system (20) have been identified in uropathogenic E. coli strains. The facultative anaerobic Gram-negative M. morganii is a typical secondary invader during multispecies infections. It can be isolated from infected wounds, septicemia, and CAUTIs (22–24). This natural commensal of the human intestinal tract is often regarded as a harmless opportunistic pathogen (25). However, some strains are associated with large nosocomial outbreaks (26). During infection, urease (27) and beta-lactamase (28) are considered to be involved in the maintenance of bacterial fitness in M. morganii. Until today, transcriptome or proteome studies targeting global gene expression of M. morganii are lacking and its adaptation strategies to the urinary tract environment remain widely unexplored.
Host response to UTIs is achieved by innate and adaptive immunity (reviewed in (29, 30)). Initiated by pathogen recognition via Toll-like receptors, a complex mixture of cytokines, antimicrobial peptides, and proteins is released by infiltrating neutrophils and the urothelium (31–33). Intensely studied protein-effectors are for example, defensins (34), cathelicidin (35), lactoferrin (36), and the tamm-horsfall protein (37). Moreover, the complement system is known to play an important role during urinary tract innate immune response (38) by recognizing bacterial surface structures, followed by an activation of complement cascades. During this process, the central complement compound C3 is activated by C3 convertases, resulting in activated C3a, a chemoattractant for neutrophils. Furthermore, activated C3b covers the pathogen, which subsequently becomes phagocytosed. Finally, the membrane attack complex (C5–C9) forms a pore in the bacterial membrane resulting in cell-lysis (38, 39). The role of the adaptive immunity during UTIs has been discussed controversially. However, it is well-accepted that the humoral (40) and the cellular (41) immunity are involved in the defense against uropathogens. In contrast to the multitude of studies dealing with the human immune response during planktonic UTIs, the impact of catheter-associated UTIs on the innate and adaptive human immunity has not yet been investigated in detail.
The presented study therefore focuses on (1) the comprehensive analysis of structure and functionality of a multispecies catheter biofilm dominated by P. aeruginosa and M. morganii and (2) the elucidation of the molecular basis of the complex interplay between bacterial virulence of P. aeruginosa, M. morganii, and the human immune system in the urinary tract environment. For this purpose, a semiquantitative gel-free metaproteomics approach was employed to obtain a global view on the corresponding in vivo protein profiles. Additionally, in vitro proteome analyses of P. aeruginosa and M. morganii grown in artificial urine medium were performed. To our knowledge, here we present the first comprehensive metaproteomics investigation of host–pathogen interactions induced by catheter-associated microbial biofilms. The knowledge gained in our study strongly contributes to a better understanding of P. aeruginosa and M. morganii urinary tract niche adaptation, bacteria–bacteria and bacteria–host interactions and the corresponding human immune response and will thus also promote the development of novel strategies to fight CAUTIs.
EXPERIMENTAL PROCEDURES
Sample Collection
Suprapubic urinary catheters and urinary drainage bags were collected by the attending urologist after 28 days of use from long-term patients with asymptomatic UTI living in a German nursing home. All samples were completely anonymized. Sampling and further processing have been approved by the ethical review committee of the University Medicine in Greifswald (Internal Registration Number BB 035/13). Immediately after collection, catheters and urine were cooled on dry ice and stored for up to two hours at 4 °C until sample preparation. Catheter tips were cut with a sterile scalpel in 1 cm pieces, which were further processed (see below).
Protein Extraction from Catheter Biofilm
Small pieces of the catheter tip (two times 1 cm) were transferred to 1 ml of urea-containing buffer (11 m urea, 3 m thiourea, 70 mm dithiothreitol (DTT), 4% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)). The sample was incubated on ice for 5 min, mixed vigorously for 30 s and then incubated on ice for 10 min; those steps were repeated twice. Sonication was performed for 30 s and repeated five times to dissolve and lyse the cells using a Sonicator (Type UW 2070, Bandelin Electronics, Berlin, Germany). Subsequently, the catheter pieces were removed and the remaining cell lysate was mixed with ice-cold acetone (1:7, v/v). After centrifugation (14,000 × g, 12 °C, 40 min) the resulting protein pellet was dried and solubilized in urea-containing buffer supplemented with 1% SDS. Total protein concentration was determined according to Bradford (42) employing the Coomassie PlusTM Protein Assay (Thermo Fisher Scientific Inc.). The absorbance was measured at 595 nm. The protein concentration was calculated using a bovine serum albumin (BSA) standard (Thermo Fisher Scientific Inc.). Metaproteomics analysis was performed in three technical replicates, that is, the protein extract from the catheter biofilm was divided into three subsamples.
Protein Extraction from Urine
Urine samples (200 ml) from three urinary drainage bags from catheterized patients or collected from three healthy control persons were centrifuged for 20 min at 4 °C at 6000 × g and the resulting supernatants were mixed with 20% (w/v) trichloroacetic acid and kept overnight at 4 °C. Subsequently, samples were centrifuged for 40 min at 14,000 × g at 4 °C, the resulting protein pellets were washed twice with 70% EtOH and thereafter solubilized in a urea-containing buffer with 1% SDS. Protein concentrations were determined as described above. Metaproteomics analyses were performed in three technical replicates, that is, the protein extracts from urine were divided into three subsamples.
Protein Extraction from P. aeruginosa and M. morganii Planktonic Cultures and In Vitro Biofilms
All in vitro proteomics analyses were performed with protein extracts of three biological replicates. Planktonic cultures of the P. aeruginosa and M. morganii isolates (see below) were grown in artificial urine medium (AUM) based on the recipe of Brooks and Keevil (43) with slight modifications (uric acid 0.333 g/l, Na2SO4 1.4 g/l). To this end, 100 ml media were inoculated to an OD600 nm of 0.01 with freshly grown AUM overnight cultures and subsequently incubated at 180 rpm at 37 °C. Cells were harvested in the early stationary growth phase by centrifugation at 6,000 × g for 10 min at 4 °C. Proteins were extracted from the resulting cell pellet as described above. The corresponding supernatants were filtered through a 0.2 μm pore filter and concentrated by a vivaspin 20 concentrator (3 kDa MWCO, GE Healthcare) followed by protein precipitation with ice-cold acetone (1:7, v/v) for the investigation of the secretome.
Biofilms were grown on silicone catheters, which were placed into planktonic AUM cultures, inoculated to an OD600 nm of 0.01 and cultivated for 24 h at 50 rpm at 37 °C. Before harvesting the biofilm, the catheter was washed three times with ¼ ringer solution. Protein extraction from biofilm-grown P. aeruginosa and M. morganii was performed as described above.
One-Dimensional SDS-PAGE and Tryptic Digestion
The extracted proteins (30–40 μg of protein per biological or technical replicate) were separated on a 12% SDS-polyacrylamide gel (44) and stained overnight with Colloidal Coomassie Brilliant Blue G-250 as described previously (45). Gel blocks were excised from the gel and proteins were digested with trypsin as follows: the excised gel pieces were destained using 50% (v/v) methanol in 100 mm NH4HCO3. Proteins were reduced in 50 mm NH4HCO3 by using 10 mm DTT for 30 min at 60 °C and carbamidomethylated/alkylated in 50 mm NH4HCO3 containing 50 mm iodoacetamide for 60 min in the dark at RT. Subsequently, gel pieces were dehydrated using 100% ACN and allowed to dry. Modified trypsin (sequencing grade, Promega, Fitchburg, WI) was added to a final ratio of 1:10 (trypsin/sample) in 50 mm Tris/HCl, pH 7.5, and the sample incubated at 37 °C overnight. Peptides were iteratively extracted from the gel by a six-step procedure, using ACN, 1% (v/v) formic acid in H2O, acetonitrile, 10% (v/v) formic acid and two times acetonitrile. Peptide-containing supernatants were pooled and completely dried using a Speedvac concentrator (Eppendorf AG, Hamburg, Germany). Samples were subsequently resolved in buffer A (5% (v/v) ACN, 0.1% (v/v) formic acid) and desalted using ZipTips (C18, Merck Millipore, Billerica, MA). Finally, peptides were again vacuum-dried and stored at −20 °C.
Mass Spectrometric Analyses
The tryptic digest was applied to a reversed phase (RP) chromatographic system (Easy-nLC II operated in “one column setup” (Thermo Fisher Scientific, Waltham, MA)) equipped with a self-packed RP C18 separation column (100-μm i.d. × 200 mm length) as published by Teeling et al.(46). Peptides were loaded and desalted on the column followed by elution from the column by a binary gradient of buffers A (0.1% (v/v) acetic acid) and B (99.9% (v/v) ACN, 0.1% (v/v) acetic acid) over a time of 100 min at 300 nl/min. For the analysis of peptide mixtures derived from gel blocks of one gel lane (corresponding to one sample) injection volumes were kept constant. The chromatographic system was coupled on-line to an LTQ-Orbitrap-Velos mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a nanoelectrospray ion source. Samples were measured in data-dependent manner with repeated cycles of overview scans in the Orbitrap (r = 30,000) with the lock-mass option enabled, followed by MS/MS acquisition of the 20 most intensive precursor ions in the linear ion trap. Dynamic exclusion was enabled.
Database Assembly and Data Analysis
Phylogenetic information obtained by 16S rRNA gene sequencing was used for the construction of a sample-specific virtual metagenome database designated as “catheterDB” as described by Okuda et al. (47). This database contained 342.642 nonredundant protein entries, that is, all available proteins from Homo sapiens, P. aeruginosa, M. morganii, and Bacteroides sp. extracted from the NCBInr protein database (version 18.01.13). A second database used for protein assignments from urine samples was designated as “urineDB” and contained 46,415 entries, that is, reference proteomes of H. sapiens, P. aeruginosa, M. morganii, E. faecalis, E. coli, P. mirabilis, and Bacteroides fragilis (Uniprot, version 17.10.14). Because we were in particular interested in human proteins identified in the urine, the additional microbial entries (compared with “catheterDB”) were thought to minimize false positive assignments to human proteins.
Proteome discovererTM software (version 1.4, Thermo Fisher Scientific Inc., Waltham, MA) was used to validate MS/MS-based peptide and protein identifications. Sequest HT database searches were performed with raw files as MudPit experiments. The following search parameters were used: enzyme type, trypsin (KR); peptide tolerance, 10 ppm; tolerance for fragment ions, 0.6 Da; b- and y-ion series; variable modification, methionine (15.99 Da) and carbamidomethylation (57 Da); a maximum of three modifications per peptide was allowed. The percolator node was used to filter peptide identifications based on a fixed false discovery rate (FDR) of 1% depending on a target-decoy approach. Peptide identifications were accepted when they could be established on Sequest: deltaCn scores of greater than 0.05 and XCorr scores of greater 2.2, 3.3, and 3.75 (XCorr filter) for doubly, triply, and quadruply charged peptides. Protein identifications were based on at least two identified distinct peptides per protein. Moreover, only proteins were taken into account that were at least identified in two out of three technical or biological replicates (replicate filter). After application of the filters (XCorr and replicate filter) the initial FDR of 1% was reduced to 0.0001%. Proteins that contained identical peptides and could not be differentiated based on MS/MS analysis alone were grouped together.
Quantification was based on integration of the area under the curve (AUC) from MS1 spectrum peaks performed by the “Precursor Ions Area Detector” node integrated in the proteome discoverer software. Proteome discoverer identification and quantification results (*.msf) were imported into Scaffold (version 4.4, Proteome Software Inc., Portland, OR) and protein quantities were determined as the average of the three highest peptide intensity values for a protein (48). If only two peptides could be quantified, the average of the two highest peptide intensity values for a protein was calculated. Identical distinct peptides identified and quantified from different gel blocks or retention times within a sample were considered. Normalization of these data was achieved by using the following equation:
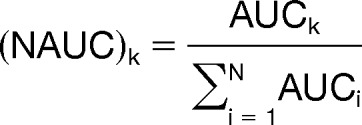 |
NAUC is the normalized intensity value (AUCk) for a protein divided by the sum of all intensity values per sample (AUCi). AUCk is the average of the three highest peptide intensity values for a protein.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (49) via the PRIDE partner repository with the data set identifier PXD000164. All identified proteins and their respective NAUCs are listed in the supplementary Files S2, S4, S5, and S6. A quantitative comparison of the in vivo and the in vitro proteome data of P. aeruginosa and M. morganii proteins was achieved by the separate analysis of proteins identified under both (in vivo and in vitro) conditions. Quantitative protein data were normalized as described above and used as the basis for fold-change calculation. Proteins detected under in vivo conditions were only considered as “highly expressed” if their fold-change in abundance compared with in vitro conditions was at least twofold or if they were uniquely identified in vivo. Identification of statistical differences in relative protein amounts was performed using t test (p < 0.05) including adjusted Bonferroni correction and all possible permutations.
Data Processing and Visualization
For metaproteomics data analyses we used the Prophane bioinformatics pipeline (www.prophane.de, (50)). Briefly, corresponding search results were merged using the Scaffold software version 3.6.1 (Proteome Software Inc., Portland, OR). The exported protein reports were submitted to Prophane to screen peptide-sharing proteins for taxonomic or functional similarities. Protein matches pointing to reverse sequences or contaminations were excluded from analyses. Peptide-sharing proteins missing unique peptide identifications were grouped together. The common taxonomic origin of those groups was evaluated considering different taxonomic levels (superkingdom, order, class, family, genus, and species). Groups without common taxonomic origin of all protein members were called “heterogeneous” regarding their taxonomy. Protein functions were transferred by RPSBLAST (51) and HMMER3 (52) alignments, respectively. Using the RPSBLAST algorithm prokaryotic (eukaryotic) protein sequences were aligned versus the COG (KOG) database collection (release 22.03.2003) (53). Functional annotation of the first hit (e-value ≤ 1E-20) was considered for each protein. Using the HMMER3 algorithm protein sequences were aligned versus the TIGRFAMs 15.0 (54) and PFAMs 27.0 (55) collections. Functional annotation of all hits (e-value ≤ 1E-5) was considered for each protein. Functional annotation was assigned to each group if respective protein members show the same functional description (proteins without prediction were not considered). If functional prediction varies between protein members group function was named “heterogeneous.” In case of multiple TIGRFAMs or PFAMs hits shared by protein group members the hit with the lowest e-value over all proteins were selected. Details regarding the taxonomic and functional assignment of proteins are provided in Suppl. File 1. After computational assignments of protein functions, assignments were carefully checked, completed, and manually curated.
Protein abundances in in vivo versus in vitro biofilms were illustrated using treemaps (56). Genes/proteins were functionally classified according TIGRFAMs and visualized in multiple level hierarchically organized regions (e.g. energy metabolism) subdivided into subregions (e.g. TCA).
DNA Extraction for High-Throughput Sequencing
Total DNA was extracted from the catheter biofilm using the FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA) (57). Cell disruption was performed employing the Fast Prep® instrument for 40 s with an intensity of 5.5. DNA quality and quantity were determined using a NanoDrop 200 spectrophotometer (Thermo Scientific, Waltham, MA). The V1–2 region of the 16S rRNA gene was amplified using primers based on the 27F and 338R primers as previously described (58) and paired end sequenced on a GAIIx Genome Analyzer (Illumina, Inc., San Diego, CA). Image analysis and base calling were accomplished using the Illumina Pipeline (version 1.7). Sequences were quality filtered, trimmed, collapsed into representative reads and clustered as previously described (58). Phylogenetic assignments were performed manually based on the Ribosomal Database Project (RDP) (http://rdp.cme.msu.edu/) database.
Isolation of Biofilm-Associated Uropathogens
For the isolation of biofilm-inhabiting uropathogens, 0.5 cm of the catheter tips were transferred in 1 ml of ¼ ringer solution, mixed, and incubated for 5 min at RT. After repeating this procedure twice, the resulting bacterial suspension was mixed with 50% (v/v) glycerol (98%) and stored at −80 °C. Dilutions of the glycerol-suspensions were plated on cystine-lactose-electrolyte-deficient (CLED)-agar (Roth, Hamburg, Germany). Single colonies were picked and streaked on the same agar plates. After repeating this procedure four times, cell morphology of the pure cultures was inspected by microscopy and isolates were finally identified by colony PCR as described below. The isolated strains were stored at −80 °C as glycerol stocks for further analysis.
Identification of Isolates by 16S rRNA Gene Sequencing
Genomic DNA of the isolates was extracted using the FastDNA Spin Kit for soil (MP Biomedicals, Santa Ana, CA) following the manufacturer's instructions. 16S rRNA genes were amplified by PCR using the primers Com1f and 1492R (Com1f: CAG CCG CGG TAA TAC and 1492R: AGA AAG GAG GTG ATC CAG CC) (59), which were synthesized by Metabion, Martinsried, Germany. Reaction mixtures contained 1x ThermoPol buffer, deoxynucleoside triphosphates (20 μm of each dNTP), 0.5 μm of each primer, 2.5 U of DNA polymerase (ThermoTaq Polymerase, NEB) and 100 ng genomic DNA. Cycle conditions for the reactions were: initial denaturation at 95 °C (2 min), 28 cycles of 95 °C (20 s), 52 °C (2 min), and 72 °C (2 min) with a final extension at 72 °C (6 min). PCR products were purified by the QIAquick PCR Purification Kit (Qiagen). The DNA sequence of the PCR product was determined using the BigDye® Terminator v1.1 Cycle Sequencing Kit and the 310 genetic analyzer (Applied Biosystems, Waltham, MA) according to the manufacturer's instructions. For sequencing primers 27f and 1492r were used. Identification of the isolates was performed by a BLAST search of the determined 16S rRNA gene fragment sequence against the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (60).
Phenotypic Characterization of Isolates
Biofilm forming capacity of the isolates was tested by a microtiter plate assay according to O'Toole and Kolter (61). Briefly, the bacterial cells were grown in artificial urine medium (AUM) in 96-well microtiter plates (Nunclon, PS) for 24 h at 37 °C. Bacterial growth was monitored by measuring the optical density of the planktonic cultures at 550 nm (Synergy Mx, BioTek, Bad Friedrichshall, Germany). After removing planktonic cells, the remaining biofilms were washed three times with ddH2O, incubated for 30 min with 0.1% crystal violet and washed again with ddH2O. Dried biofilms were dissolved in 120 μl DMSO and incubated for 20 min at RT. Finally, the absorbance at 570 nm was measured and the biofilm-index was calculated (62).
Antibiotic susceptibility of the isolates was tested by a standardized single disk approach (63) with the following antibiotics (μg/disk): nitrofurantoin 100 μg, gentamycin 10 μg, cefalexin 30 μg, sulfamethoxazole/trimethoprim 23.75 μg/1.25 μg, penicillin G 10 μg, trimethoprim 5 μg, tetracycline 30 μg, ciprofloxacin 5 μg, levofloxacin 5 μg, amoxicillin 25 μg, and ampicillin 10 μg (Oxoid Limited, Hampshire, United Kingdom).
Urease production of the isolates was tested in 5 ml urea-containing liquid media (0.1 g/l yeast extract; 9.1 g/l potassium phosphate, monobasic; 9.1 g/l potassium phosphate, dibasic; 20 g/l urea; 0.01 g phenol red), which were inoculated with the test strains (1% (v/v) overnight cultures in LB broth) and incubated at 37 °C for 24 and 48 h at 180 rpm. P. mirabilis HI432 was used as positive control (64). After 24 (48) h, the pH-dependent color change (light yellow to dark red) was visually determined as an indicator for urease activity.
Protease activity in cell-free supernatants of the isolates was determined on azocasein (65). Briefly, overnight cultures were centrifuged and the supernatants filtered under sterile conditions. One hundred microliters supernatant and 250 μl substrate solution (2% azocasein, 50 mm Tris/HCl, pH 7.5) were incubated for 3 h at 40 °C. Undigested azocasein was precipitated with 10% trichloroacetic acid and protease activity was quantified by measuring the absorption of the remaining dye at 410 nm. As a positive control P. aeruginosa PAO1 (66) was used.
RESULTS
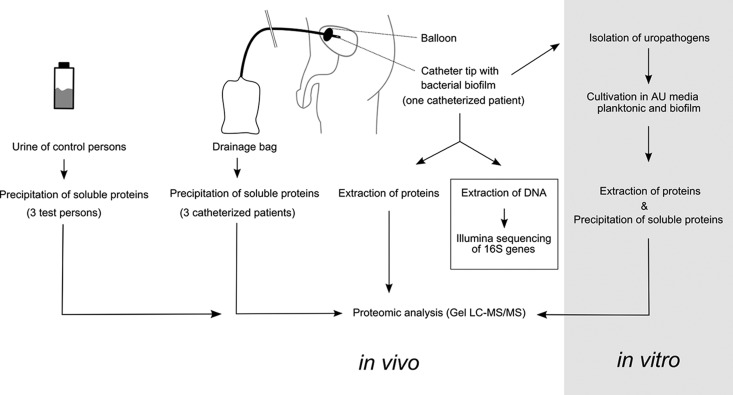
CAUTIs can cause serious problems in long-term patients because of the polymicrobial nature of catheter-associated biofilms and the high bacterial load of up to 5 × 109 cells/cm biofilm. Metaproteomics has been increasingly recognized as a powerful tool to investigate the physiology of complex microbial communities in their natural environment (50, 67–70). Therefore, we have decided to employ such a state-of-the-art approach to elucidate the molecular mechanisms underlying pathogen host adaptation and host immune response to bacterial invaders during a multispecies CAUTI of one long-term catheterized patient. An overview on the experimental setup is shown in Fig. 1. A specific patient was selected as his catheter biofilm was found to consist of two major components, P. aeruginosa and M. morganii, the latter of which can still be considered as poorly characterized; moreover, nothing is known on the interactions between these two opportunistic pathogens during CAUTI. For a further detailed view of bacterial response to the urinary tract the two major bacterial constituents of the biofilm were cultivated in artificial urine medium and their protein profiles were analyzed by in vitro proteome analyses (Fig. 1, right panel). Finally, to determine to what extent the biofilm versus immune system interaction is reflected by the urine composition of CAUTI patients, the urinary metaproteome of three healthy individuals was compared with that of three CAUTI patients (Fig. 1, left panel).
Fig. 1.
Schematic overview of the experimental setup. A representative multispecies biofilm derived from a long-term catheterized patient was processed together with the corresponding urine for culture-independent metaproteomics analyses. Illumina 16S rDNA sequencing was employed to confirm the phylogenetic composition of the biofilm. Moreover, abundant uropathogens were isolated from the biofilms. The isolates, identified as P. aeruginosa and M. morganii, were further analyzed by in vitro proteome analyses of the extracellular proteins and biofilm-associated cells grown in artificial urine medium (AUM).
Metaproteomics Analyses of a Multispecies Catheter-Biofilm and Associated Urine
Spectra recorded for the trypsin-digested proteins extracted from the multispecies biofilm and urine samples were assigned to a total of 1064 and 896 proteins, respectively (Fig. 2, supplemental File S2). Although in the biofilm the number of bacterial proteins (612) clearly exceeded the number of human proteins (452), much fewer microbial proteins could be detected in the urine (509 human proteins versus 387 bacterial proteins). However, relative protein quantification indicates a much higher abundance of human proteins on the catheter biofilm (92%) and in the urine (99%) compared with bacterial proteins. The dominance of human proteins is also reflected by a threefold higher median of the NAUC values from human proteins compared with bacterial proteins (supplemental File S3, supplemental Fig. S1).
Fig. 2.
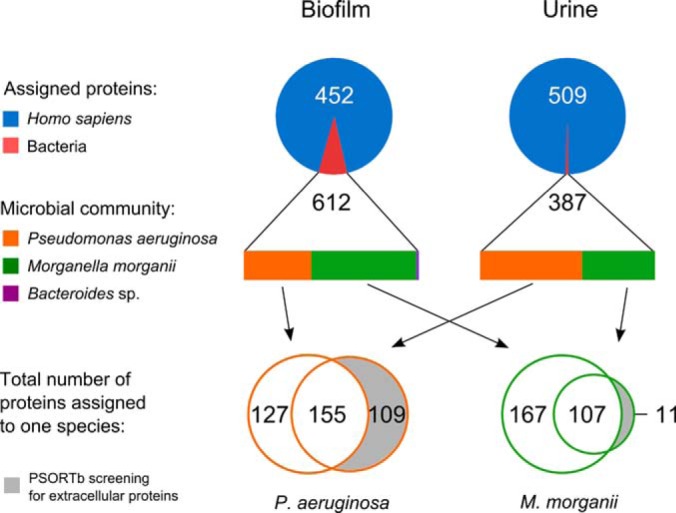
Taxonomic distribution of assigned proteins resulting from the metaproteomic analysis of the catheter-associated biofilm and urine of a long-term catheterized patient. Numbers indicate the assigned proteins per sample (average of three technical replicates), the chart area displays the quantitative distribution (NAUC values) of the assigned proteins to the kingdom or the species level.
A Polymicrobial Community Forms the Biofilm
The quantification of the metaproteomics data based on NAUC values revealed that two opportunistic pathogens, that is, M. morganii (57%) and P. aeruginosa (40%), dominated the biofilm, whereas the obligate anaerobe Bacteroides sp. represented only a minor fraction (2.5%) of the biofilm colonizing community (Fig. 2). The observed coefficient of variation (CV) between replicates with regard to the bacterial composition in the biofilm or urine was about 0.1. Relative protein abundance between replicates exhibited a CV of ∼0.25. Moreover, 75% of all proteins identified by two distinct peptides were identified in two out of three replicates. The taxonomic composition of the catheter biofilm was confirmed by a 16S rDNA sequencing approach using DNA directly extracted from the catheter biofilm (Fig. 1). Clearly, M. morganii and P. aeruginosa were confirmed as the predominant biofilm bacterial inhabitants (supplemental File S3, supplemental Tables S1 and S2). The proteins from the urinary sample were assigned to the same taxa found in the biofilm, but the NAUC-based quantification resulted in a predominant occurrence of P. aeruginosa proteins (57%), followed by M. morganii (41,5%) proteins. Remarkably, only one protein expressed by Bacteroides sp. was identified in the urinary proteome (Fig. 2). Notably, a culture-dependent approach gave rise to the isolation and identification of the two dominant biofilm inhabitants P. aeruginosa and M. morganii (supplemental File S3, supplemental Table S3), which were used for further investigations (Fig. 1, right panel).
Adaptation of P. aeruginosa to the Urinary Tract and the Host Defense
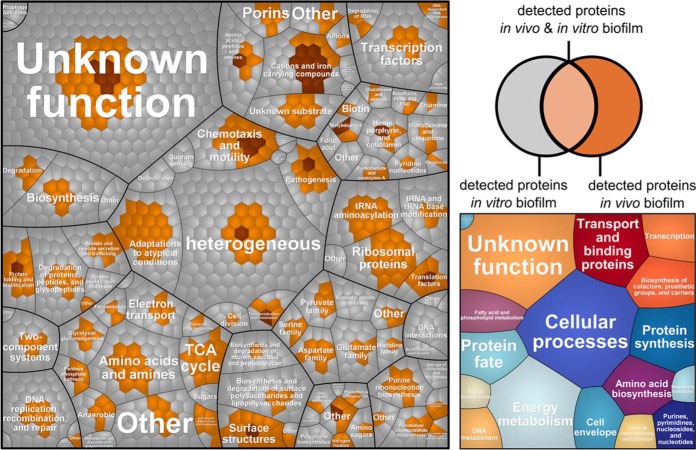
To elucidate the molecular adaptation strategies of P. aeruginosa to the infected bladder environment, its catheter-derived proteome profile (282 identified proteins) was compared with the in vitro biofilm proteome profile (1332 identified proteins) obtained for the isolated strain grown as biofilm in AUM on a urinary tract silicone catheter (Figs. 1 and 3, supplemental File S4). Interestingly, 28 proteins of P. aeruginosa were exclusively detected under in vivo conditions on the catheter biofilm (Table I). Moreover, based on our fold-change calculation (described in detail in the experimental procedures), 27 proteins were found to be more abundant in the in vivo biofilms compared with the in vitro biofilm cultures (Table I, Fig. 3). In the following paragraphs the 55 proteins, which were highly abundant in vivo, are described.
Fig. 3.
Voronoi treemaps (109) comparing protein expression profiles of P. aeruginosa grown in catheter biofilms (in vivo) or in a catheter model system in AUM (in vitro). Gene functions are based on TIGRFAMS, major TIGRFAMS roles are displayed in the right map and the minor TIGRFAMS roles the left map. Gray proteins were exclusively identified in vitro, light brown proteins were identified in vivo and in vitro and dark brown proteins were exclusively identified in vivo.
Table I. Proteins of P. aeruginosa exclusively identified or highly expressed in the catheter-biofilm compared to the in vitro biofilm. Proteomic data were compared to published transcriptome analyses (22) that have identified genes, expression of which was induced by either iron-limitation (iron), or surface-associated growth (biofilm). Genes induced under various stress conditions were designated as “core”.
| Representative GI accession nr.a | Protein description | Protein name | PAO1 homolog | ON/FCb | Corec | Ironc | Biofilmsc |
|---|---|---|---|---|---|---|---|
| Iron uptake | |||||||
| 15595869 | Heme oxygenase | HemO | PA0672 | ON | + | + | |
| 313105815 | Ferric enterobactin receptor | PirA | PA0931 | ON | + | ||
| 152989712 | l-ornithine N5-oxygenase | PvdA | PA2386 | ON | + | ||
| 254235400 | Pyoverdine synthetase | PvdF | PA2396 | ON | + | ||
| 152989260 | 2,4-diaminobutyrate 4-transaminase | PvdH | PA2413 | ON | + | ||
| 107102212 | Ferric enterobactin receptor | PfeA | PA2688 | ON | + | ||
| 116051427 | Heme uptake outer membrane receptor | HasR | PA3408 | ON | + | ||
| 313109326 | Fe(III) dicitrate transport protein | FecA | PA3901 | 706,7 | + | ||
| 424938959 | Second ferric pyoverdine receptor | FpvB | PA4168 | ON | + | ||
| 107103807 | Heme-transport protein | PhuT | PA4708 | ON | + | ||
| 254244389 | Putative heme degradation protein | PhuS | PA4709 | ON | + | ||
| 386068373 | Dihydroaeruginoic acid synthetase | PchE | - | 15,1 | |||
| 170282658 | Ferripyoverdine receptor | FpvA | - | ON | |||
| Biofilm/Chemotaxis | |||||||
| 418593466 | Twitching motility protein | PilU | PA0396 | 2,9 | |||
| 392981824 | Twitching motility protein | PilJ | PA0411 | 4,5 | + | ||
| 107103325 | Type 4 fimbrial biogenesis protein | PilF | PA3805 | ON | |||
| 319918960 | Type 4 fimbrial biogenesis protein | PilX | PA4553 | ON | |||
| 313107199 | Type 4 fimbrial biogenesis protein | PilY1 | PA4554 | ON | |||
| 421156565 | Type 4 fimbrial biogenesis protein | PilQ | PA5040 | 4,7 | + | ||
| 152985916 | Type 4 fimbrial biogenesis protein | PilN | PA5043 | 9,0 | + | ||
| Defense mechanisms/Pathogenicity | |||||||
| 254239129 | Serine protease | MucD | PA0766 | 2,3 | |||
| 15596159 | Dna-binding stress protein | Dps | PA0962 | 2,7 | + | ||
| 15596166 | TolQ protein | TolQ | PA0969 | 7,7 | |||
| 15598322 | Heat-shock protein | IbpA | PA3126 | 3,7 | |||
| 313109552 | Beta-lactamase | AmpC | PA4110 | 7,5 | + | ||
| 421170117 | Insulin-cleaving metalloproteinase | IcmP | PA4370 | 4,5 | + | ||
| General or unknown functions | |||||||
| 15595620 | Hypothetical protein | PasP | PA0423 | 12,5 | |||
| 313111914 | Putative ClpA/B protease ATP binding subunit | - | PA0459 | 2,7 | |||
| 218892989 | Pyridoxamine 5′-phosphate oxidase | PdxH | PA1049 | 2,8 | |||
| 190613598 | Hypothetical protein | - | PA1195 | ON | + | ||
| 15596990 | Peptidyl-prolyl cis-trans isomerase B | PpiB | PA1793 | 2,2 | + | ||
| 107101462 | Hypothetical protein | - | PA2033 | ON | + | ||
| 15597519 | Glyceraldehyde-3-phosphate dehydrogenase | - | PA2323 | 68,4 | |||
| 296388958 | Hypothetical protein | - | PA2575 | 9,5 | |||
| 15598049 | Outer membrane lipoprotein | OprI | PA2853 | ON | |||
| 15598843 | Hypothetical protein | - | PA3647 | 2,7 | |||
| 308198348 | Peptide chain release factor 1 | PrfB | PA3701 | ON | |||
| 116051948 | Hypothetical protein | - | PA3911 | ON | |||
| 15599110 | Molybdopterin biosynthetic protein B1 | MoaB1 | PA3915 | ON | |||
| 218889787 | Hypothetical protein | - | PA3931 | 3,1 | |||
| 254242900 | Probable ATP-binding component of ABC transporter | - | PA4222 | ON | + | ||
| 107100081 | Hypothetical protein | - | PA4336 | 11,0 | |||
| 15599565 | Hypothetical protein | - | PA4369 | ON | |||
| 313107055 | Fumarate hydratase | FumC1 | PA4470 | 15,3 | + | ||
| 15599949 | Transcription elongation factor | GreA | PA4755 | 2,8 | |||
| 15599987 | Hypothetical protein | - | PA4793 | 2,8 | |||
| 15600040 | Biotin carboxyl carrier protein | AccB | PA4847 | ON | |||
| 15600128 | 30S ribosomal protein S6 | RpsF | PA4935 | 3,2 | |||
| 116053356 | Hypothetical protein | - | PA5208 | ON | |||
| 392983995 | Serine hydroxymethyltransferase | GlyA1 | PA5415 | ON | + | ||
| 15600668 | Hypothetical protein | - | PA5475 | ON | + | ||
| 15600747 | ATP synthase F0F1 subunit beta | AtpD | PA5554 | 2,0 | |||
| 15600750 | ATP synthase F0F1 subunit delta | AtpH | PA5557 | 2,9 | + | ||
| 30141367 | Aminoglycoside acetyltransferase | - | - | 3,9 | |||
| 313107144 | Hypothetical protein | - | ON |
a Representative GI accession number of P. aeruginosa protein groups.
b The change (n-fold) was calculated by comparing relative protein amounts of P. aeruginosa grown in vivo as a biofilm to P. aeruginosa grown in vitro as a biofilm. Only proteins identified as in vivo highly expressed that demonstrated a change of equal to or greater than threefold and exhibiting a statistically significant change (p ≤ 0.05) are reported. Proteins exclusively detected in vivo are indicated by “ON”.
c Induced expression of the corresponding transcripts during iron-limitation (iron), surface-associated growth (biofilm), or various physiological conditions (core) are indicated (+).
Thirteen of these proteins are involved in iron acquisition. Their expression is mostly dependent on the iron-binding Fur regulator (71). Levels of proteins of the pyochelin and the pyoverdine siderophore systems were elevated under in vivo conditions, pyoverdine synthase PvdAFH and the corresponding receptors FpvAB were exclusively identified in the catheter biofilms, additionally the pyochelin synthase (PchE) was found to be more abundant in vivo. Furthermore, proteins involved in (1) heme/hemin degradation and uptake (HemO, HasR, PhuST), (2) transport of Fe (III) citrate, and (3) uptake of heterologous siderophores (PirA and PfeA receptors) were found to be more abundant under in vivo conditions (Table I). Moreover, proteins involved in various functions such as motility and biofilm formation were strongly expressed in the catheter biofilms (Table I): (1) proteins important for biofilm formation/development (i.e. the fimbrial proteins PilF, J, N, Q, U, X, and Y1) (68), (2) proteins related to antibiotic resistance and the general stress response (i.e. AmpC, Dps, IdpA), and (3) an insulin-cleaving membrane proteinase (IcmP), which has been shown to degrade plasminogen activator, complement convertase and kallikrein (72) and a protease involved in pathogenicity (i.e. MucD).
Phenotypic assays were performed to validate biofilm-forming capacity of the catheter isolates. Interestingly, P. aeruginosa exhibited a significantly higher (about twofold) biofilm formation than M. morganii in AUM (Table II). Both isolated strains were found resistant to all tested antibiotics. Especially, ciprofloxacin and levofloxacin inhibiting the growth of P. aeruginosa PAO1 and P. mirabilis HI432 were found inactive with the CAUTI isolates (Table II).
Table II. Phenotypic characterization of isolated P. aeruginosa and M. morganii strains. P. mirabilis HI432 and P. aeruginosa PAO1 were used as control strains. (–) no signal, (+) weak signal, (+++) strong signal, (n.a.) not analyzed, (+) resistant or (-) sensitive to the respective antibiotic.
| P. aeruginosa Isolate | M. morganii Isolate | P. mirablis HI432 | P. aeruginosa PAO1 | |
|---|---|---|---|---|
| Urease | – | + | +++ | n.a. |
| Protease | 1.33 ± 0.05 | 0 | n.a. | 1.39 ± 0.05 |
| Biofilm Index | 282 ± 58 | 141 ± 8 | n.a. | 107 ± 16 |
| Ciprofloxacin | + | + | - | - |
| Levofloxacin | + | + | - | - |
| Tetracycline | + | + | + | + |
| Amoxillin | + | + | + | + |
| Ampicillin | + | + | + | + |
| Penicillin | + | + | + | + |
| Cephalexin | + | + | + | + |
| Sulfamethoxazol | + | + | + | + |
| Nitrofurantion | + | + | + | + |
| Trimethoprim | + | + | + | + |
| Gentamicin | + | + | + | + |
P. aeruginosa proteins found in the urinary proteome but not on the catheter biofilms might be secreted virulence factors, which are involved in host-pathogen interactions (Fig. 2). Only those proteins were considered, which harbored a signal peptide and were also identified in the in vitro secretome (supplemental Table S3). This protein group included (Table III): the PvdS regulated protease PrpL, elastase LasB and alkaline protease AprA, the hemolysin-coregulated protein Hcp, the lipase LipA, the chitin binding protein CbpD, a putative aminopeptidase, and a colicin M-like bacteriocin. These findings were supported by an in vitro protease assay, by which a high protease activity was found in cell-free supernatants of the recovered P. aeruginosa strain (Table II).
Table III. Potentially secreted P. aeruginosa proteins exclusively identified in the catheter-associated urine when compared to the catheter biofilm proteome. Protein sequences were analyzed by pSORTb and only extracellular and proteins of unknown localization were listed. An identification of the respective protein in the secretome and/or whole cell proteome of the in vitro AUM cultures is indicated by “+”.
| GI Accession Nr.a | Protein Annotation | Protein name | pSORTb | Protein function | Reference | USCb |
in vitro proteomics |
|
|---|---|---|---|---|---|---|---|---|
| Secretome | Whole-cell proteome | |||||||
| 313111732 | Phage tail tube protein FII | Unknown | Phage tail | 8.3 | + | + | ||
| 116052217 | Pvds-regulated endoprotease | PrpL | Extracellular | Protease (casein, lactoferrin, transferrin, elastin, decorin) | (90) | 10.0 | + | - |
| 313105877 | Colicin M-Like Bacteriocin | PaeM | Unknown | Bacteriocin (inhibits cell wall peptidoglycan biosynthesis) | (93) | 4.7 | - | + |
| 15598135 | Putative aminopeptidase | Extracellular | Peptidase | 9.3 | + | + | ||
| 313111732 | Phage tail sheath protein FI | Unknown | Phage tail | 7.3 | + | + | ||
| 421182152 | Chitin-binding protein | CbpD | Extracellular | Pathogenicity | (110) | 5.3 | + | + |
| 15599752 | Type 4 fimbrial biogenesis protein | PilE | Extracellular | Chemotaxis | 2.0 | - | + | |
| 107104369 | Hemolysin-coregulated protein | Hcp | Extracellular | Pathogenicity | (111) | 2.0 | + | - |
| 386066386 | Enterochelin esterase | Unknown | Hydrolysis of ferric enterochelin | (112) | 8.3 | + | - | |
| 107103721 | Lactonizing lipase | LipA | Extracellular | Lipase activity | (113) | 2.0 | - | + |
| 107100698 | Alkaline metalloproteinase | AprA | Extracellular | Protease (transferrin, complement, cytokines) | (101–104) | 2.3 | + | - |
| 107103238 | Elastase LasB | LasB | Extracellular | Protease (lactoferrin, transferrin, elastin, immunglobulin, collagen, complement) | (101, 103, 105, 106, 114, 115) | 2.7 | + | - |
| 421169436 | Flagellar hook-associated protein | FlgK | Extracellular | Cell motility | 1.7 | + | + | |
a Representative GI accession number of P. aeruginosa protein groups.
b USC (Unique spectral counts), average of three replicates.
Adaptation of M. morganii to the Urinary Tract and the Host Defense
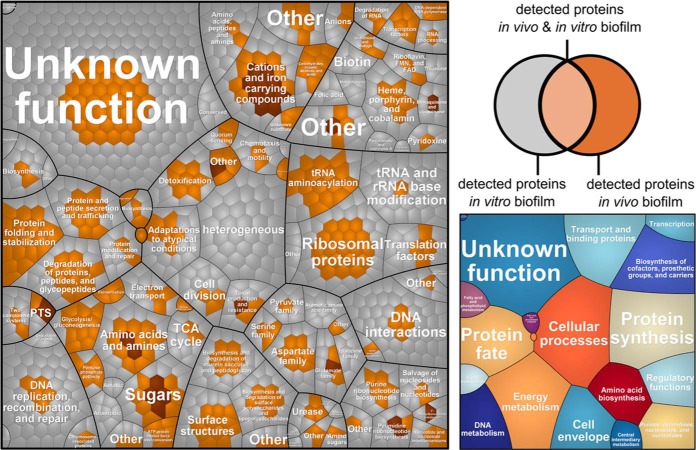
As described above for P. aeruginosa, protein expression profiles of M. morganii grown either in mixed catheter-associated biofilms (274 proteins) or monospecies in vitro cultured biofilms (1165 proteins) were compared (Figs. 1 and 4, supplemental File S5). Interestingly, 48 proteins were either uniquely detected or highly abundant in the in vivo proteome based on the abundance ranking (Table IV, Fig. 4). A major part of the proteins found to be highly expressed in vivo is involved in iron and manganese uptake, that is, a ferrous iron transport system (EfeB, EfeO), a manganese uptake system (SitA, SitB), and different siderophore receptors (i.e. two TonB-dependent receptors, ferric iron ABC transporter, iron ABC transporter substrate-binding protein, and periplasmic protein p19 involved in high-affinity Fe (II) transport). Moreover, proteins participating in carbohydrate uptake and metabolism appeared to be strongly expressed in the catheter-associated biofilms when compared with the in vitro biofilms. Among these proteins were components of phosphotransferase systems (PTS) (i.e. N-acetylgalactosamine-specific IIB component, mannose-specific IID component) and various enzymes involved in glycolysis, pentose phosphate pathway, and sugar conversion (i.e. d-mannonate oxidoreductase, mannonate dehydratase, 2-dehydro-3-deoxygluconate kinase, enolase, uronate isomerase, 6-phosphofructokinase, NAD-dependent glyceraldehyde-3-phosphate dehydrogenase, galactosamine-6-phosphate isomerase, and gluconokinase). Additionally a β-lactamase was found to be abundant in vivo, expression of which was confirmed by our antibiotic susceptibility test revealing that the isolate is multidrug-resistant (Table II). Moreover, proteins involved in general and oxidative stress response were found to be unique or more abundant in vivo, that is, a molecular chaperone, a tellurium resistance protein, an iron–sulfur cluster assembly protein, and proteins involved in transport and assembly of lipopolysaccharides.
Fig. 4.
Voronoi treemaps (109) comparing protein expression profiles of M. morganii grown in catheter biofilms (in vivo) or in a catheter model system in AUM (in vitro). Gene functions are based on TIGRFAMS, major TIGRFAMS roles are displayed in the right map and the minor TIGRFAMS roles the left map. Gray proteins were exclusively identified in vitro, light brown proteins were identified in vivo and in vitro and dark brown proteins were exclusively identified in vivo.
Table IV. Proteins of M. morganii exclusively identified or highly expressed in the catheter-biofilm compared to the in vitro biofilm.
| Representative GI accession nr.a | Protein description | Protein name | MU9 homolog | ON/FCb |
|---|---|---|---|---|
| Iron and cation uptake | ||||
| 410084858 | Ferric iron ABC transporter, iron-binding protein | - | MU9_1225 | 2,2 |
| 421493824 | TonB-dependent receptor | - | MU9_2104 | ON |
| 421492020 | Manganese ABC transporter, periplasmic-binding protein | SitA | MU9_2451 | 41,7 |
| 410086754 | Manganese ABC transporter, ATP-binding protein | SitB | MU9_2452 | 26,1 |
| 421494274 | Iron ABC transporter substrate-binding protein | - | MU9_2748 | 7,5 |
| 410086951 | Ferrous iron transport peroxidase | EfeB | MU9_2852 | ON |
| 421494564 | Ferrous iron transport periplasmic protein | EfeO | MU9_2853 | ON |
| 410086947 | Periplasmic protein p19 involved in high-affinity Fe2+ transport | - | MU9_2856 | 6,6 |
| 421494894 | TonB-dependent receptor | - | MU9_2961 | ON |
| Carbohydrate uptake and metabolism | ||||
| 410086198 | 2-dehydro-3-deoxygluconate kinase | - | MU9_376 | ON |
| 421494175 | Uronate isomerase | UxaC | MU9_377 | 34,2 |
| 410086200 | d-mannonate oxidoreductase | - | MU9_378 | ON |
| 410086201 | Mannonate dehydratase | UxuA | MU9_379 | ON |
| 410086204 | TRAP-type C4-dicarboxylate transport system, periplasmic component | - | MU9_382 | 15,3 |
| 410087600 | 6-phosphofructokinase [Morganella morganii SC01] | PfkA | MU9_571 | 2,5 |
| 410084920 | Enolase | Eno | MU9_1162 | 4,0 |
| 410087699 | PTS system, mannose-specific IID component | - | MU9_1934 | 9,4 |
| 410085950 | NAD-dependent glyceraldehyde-3-phosphate dehydrogenase | - | MU9_2337 | 9,8 |
| 410088110 | PTS system, N-acetylgalactosamine-specific IIB component | - | MU9_3143 | ON |
| 410088115 | Galactosamine-6-phosphate isomerase | - | MU9_3148 | ON |
| Defense mechanisms/Pathogenicity | ||||
| 421493572 | Molecular chaperone | Skp | MU9_943 | 7,8 |
| 410084895 | Tellurium resistance protein | TerD | MU9_1186 | 2,6 |
| 410087933 | LPS-assembly lipoprotein | IptE | MU9_1348 | 2,2 |
| 410085873 | Iron-sulfur cluster assembly protein | SufD | MU9_2411 | ON |
| 40781704 | Beta-lactamase, class C | - | MU9_3299 | 19,0 |
| 410087140 | LPS transport protein | LptA | MU9_3355 | 2,2 |
| Amino acids and amines | ||||
| 410085074 | Methylaspartate mutase, E subunit | - | MU9_1022 | ON |
| 410085059 | Ethanolamine utilization protein | EutQ | MU9_1035 | 3,2 |
| 410085048 | Ethanolamine ammonia-lyase heavy chain | - | MU9_1045 | 4,1 |
| 421494057 | N-acetylneuraminate lyase | - | MU9_1230 | ON |
| General or unknown functions | ||||
| 410086339 | LSU ribosomal protein L9p | RplI | MU9_205 | 2,5 |
| 410086267 | Protein yifE | YifE | MU9_269 | 3,1 |
| 410088675 | LSU ribosomal protein L3p | RplC | MU9_310 | 2,7 |
| 410086197 | 4-Hydroxy-2-oxoglutarate aldolase | - | MU9_375 | 13,7 |
| 410086264 | Glutathione S-transferase | - | MU9_450 | 15,0 |
| 410085838 | ATP synthase beta chain | AtpD | MU9_474 | 2,4 |
| 410088376 | Translation elongation factor Ts | Tsf | MU9_935 | 2,2 |
| 410085053 | Acetaldehyde dehydrogenase, ethanolamine utilization cluster | - | MU9_1040 | 8,4 |
| 410087956 | Ribonucleotide reductase of class Ib (aerobic), beta subunit | - | MU9_1325 | ON |
| 410085589 | Outer membrane receptor protein | - | MU9_1681 | ON |
| 410087848 | SAM-dependent methyltransferase | - | MU9_2098 | ON |
| 421493822 | Hypothetical protein | - | MU9_2102 | ON |
| 410087871 | NAD(P) transhydrogenase alpha subunit | - | MU9_2119 | 19,0 |
| 410087905 | N-ethylmaleimide reductase | - | MU9_2153 | ON |
| 421494395 | Alcohol dehydrogenase | - | MU9_2315 | 43,3 |
| 421493969 | Pseudouridine-5′-phosphate glycosidase | PsuG | MU9_3155 | ON |
| 410088124 | Pseudouridine kinase | - | MU9_3156 | ON |
| 410088158 | Putative ATP-binding protein | YbbA | MU9_3188 | 3,3 |
a Representative GI accession number of M. morganii protein groups.
b The change (n-fold) was calculated by comparing relative protein amounts of M. morganii grown in vivo as a biofilm to M. morganii grown in vitro as a biofilm. Only proteins identified as in vivo highly expressed that demonstrated a change of equal to or greater than threefold and exhibiting a statistically significant change (p ≤ 0.05) are reported. Proteins exclusively detected in vivo are indicated by “ON”.
Notably, urease (UreG, α- and β-unit), an important fitness factor in the urinary tract degrading urea to CO2 and ammonia (73, 74), was detected in the in vivo and in vitro proteome of M. morganii but not in P. aeruginosa. Accordingly, urease activity was only observed for M. morganii (Table II).
Only 11 proteins of M. morganii were solely present in the urine and not in the catheter biofilm (Fig. 2), of which only one protein, protease III, was considered as a potential secreted virulence factor. However, M. morganii protease III does not contain any signal peptide, no protease was found in the in vitro secretome (data not shown) and no protease activity was detected in the supernatant of the M. morganii isolate (Table II). These observations indicate that the investigated M. morganii strain was not capable to secrete proteases into the extracellular matrix of the biofilm or into the urine.
Human Proteins of the Innate Immune System are Abundant on the Bacterial Biofilm
In order to elucidate the human immune response on the basis of the identified proteins to the catheter-associated biofilm (Fig. 1), 452 human proteins were assigned to different functional categories (Fig. 2).
More than 30 of the detected human proteins are directly involved in the human innate immunity including antimicrobial proteins, proteins of the complement system and peptides secreted by epithelial cells or proteins associated to neutrophils or macrophages (Table V, supplemental File S2). Many of these proteins are used by neutrophils to kill bacteria in the phagolysosome in either a reactive oxygen species-(ROS) dependent or ROS-independent manner. Proteins involved in ROS-dependent killing mechanisms are cytochrome b245, neutrophil NADPH oxidase factor 4 and the myeloperoxidase. Interestingly, almost all ROS-independent effector molecules, known to be stored in azurophilic (i.e. myeloperoxidase, cathepsin G, proteinase 3, azurocidin, bacterial permeability increasing protein and Hnp-3 defensins) and specific granules (lactoferrin, cathelicidin, lysozyme, cytochrome b245, and neutrophil gelatinase-associated lipocalin) were identified. Besides neutrophil-associated proteins, eosinophil-specific proteins were found, namely eosinophil peroxidase and eosinophil cationic protein (Table V).
Table V. Human proteins identified from the catheter-biofilm and assigned to the innate immune system (complement proteins are not shown). References indicate cells/tissues in which expression of corresponding proteins has been demonstrated.
| GI accession nr. | Protein description | Function | Azurophil granules of neutrophils | Specific granules of neutrophils | Expression in neutrophils | Expression in epithelial cells | Expression in kidney | Spectral counts (biofilm) |
|---|---|---|---|---|---|---|---|---|
| 34719 | Myeloperoxidase | Oxidative burst | (116) | (117) | 48 | |||
| 20664221 | Cathepsin G | Protease | (116) | (118) (119, 120) | 13 | |||
| 1633225 | Proteinase 3 (Myeloblastin) | Protease | (116) | (121) (122) | (123) | 4 | ||
| 227250 | Azurocidin | Protease | (116) | (124) | 10 | |||
| 157830420 | Bacterial permeability increasing protein | Antimicrobial peptides | (116) | (125) | 8 | |||
| 109156990 | Alpha-Defensin-4 | Antimicrobial peptides | (116) | (126–128) | 3 | |||
| 229858 | Defensin HP-3 | Antimicrobial peptides | (116) | 6 | ||||
| 6996021 | Cytochrome b245 | Oxidative burst | (116) | (129) | 6 | |||
| 54607120 | Lactoferrin | Iron chelating | (116) | (130) | (131) | 75 | ||
| 348041314 | Cathelicidin | Antimicrobial peptides | (116) | (132) | (133) | (35) | 7 | |
| 157832581 | Lysozyme | Cell wall lysis | (116) | (117) | 17 | |||
| 119587431 | Neutrophil collagenase | Protease | (116) | (134) | 10 | |||
| 300181 | Neutrophil gelatinase-associated lipocalin | Binding of bacterial siderophores | (116) | (135) | (136) | 13 | ||
| 62898209 | Neutrophil NADPH oxidase factor 4 | Oxidative burst | 6 | |||||
| 31183 | Eosinophil peroxidase | Oxidative burst | (137) | 21 | ||||
| 13400006 | Eosinophil Cationic Protein (RNase 3) | Cell wall lysis | (137, 138) | 6 |
Moreover, a significant number of proteins of the complement system, one of the most important components of the innate immune system, were found to be highly abundant in the catheter biofilm, that is,core proteins of the complement system C1–C9, complement inhibitors (H, I, AFD), and activators (D, B).
Human Proteins of the Innate Immune System were also identified in Cell-free Urine
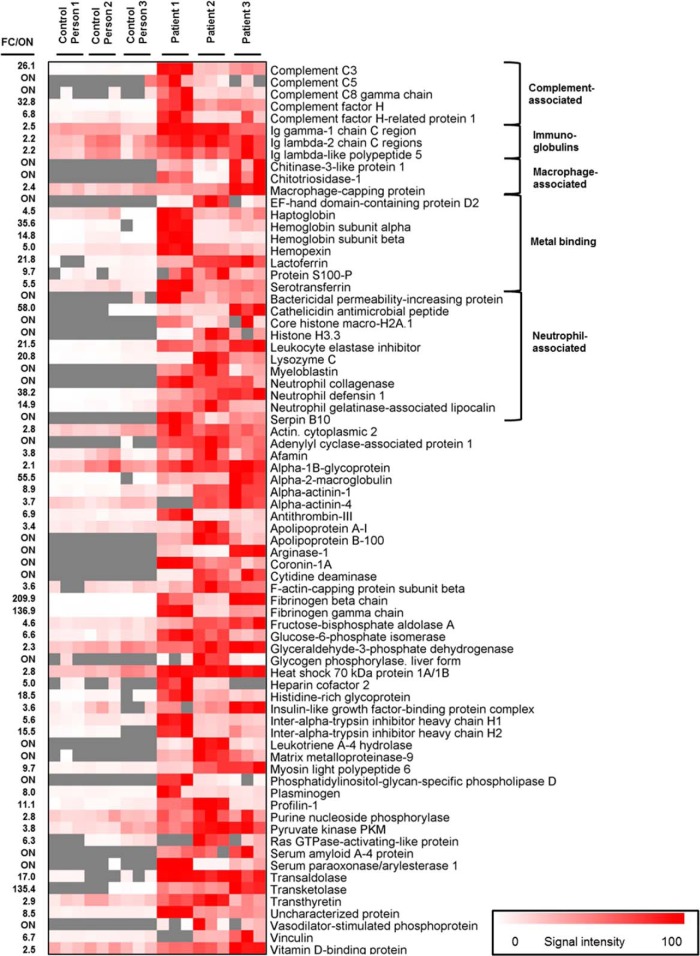
In order to prove whether the high abundance of proteins involved in innate immunity is caused by the presence of the catheter-associated biofilm, the protein profiles of urine of three long-term catheterized patients were compared with the ones of three healthy control persons (Fig. 1, Fig. 5, supplemental File S6). Forty-seven proteins were found to be significantly more abundant or uniquely identified in the patient urine, 30 of which are functionally involved in innate and adaptive immunity, among them: (1) key compounds of the complement system (i.e. C3, C5, C8, factor H, and factor H related protein 1), (2) antimicrobial peptides and proteins commonly associated with neutrophils, for example, neutrophil collagenase, myeloblastin, bacterial permeability increasing protein, neutrophil defensin 1, cathelicidin, lysozyme C, leukocyte elastase inhibitor, neutrophil gelatinase-associated lipocalin, and histones H3.3 and H2A.1, and 3) iron and calcium binding proteins, that is, lactoferrin, serotransferrin, haptoglobin, hemopexin, hemoglobin, S100-P, EF-hand domain-containing protein D2, and (IV) macrophage-associated proteins that is, chitotriosidase-1, macrophage capping protein, and chitinase-3-like protein, respectively. Interestingly, numerous proteins considered as “acute-phase” proteins expressed in response to microbial infections and other diseases were found to be more abundant in patient urine, that is, fibrinogen, haptoglobin, and serum amyloid A-4 protein (75). Moreover, also proteins considered as “negative acute-phase” proteins were strongly expressed in patient urine, that is, transthyretin and transferrin (75). Lastly, immunoglobulins were found to be more abundant in patient urine.
Fig. 5.
Heat map of host-derived proteins involved in innate and adaptive immunity identified in cell-free urine of three catheterized patients and three healthy control persons. The heat map indicates the relative abundance of the given proteins which were at least twofold higher abundant (p < 0.05) or uniquely identified in the patients urine (corresponding proteins not detected in the control persons urine are gray).
DISCUSSION
Experimental Design
Until recently, most of the global studies investigating host-pathogen interactions have been employed a murine pathogenicity model system infected with well-characterized laboratory strains (e.g. (76–78). For transferring the derived results to human it has to be considered that (1) the murine immune systems differs substantially from the human immune system (79) and (2) pathogenicity and adaptation strategies might vary between laboratory strains and clinical strains isolated from the infection of interest (80). Aiming at a comprehensive and realistic insight into bacterial adaptation mechanisms to the human urinary tract environment and the response of the human host to the long-term colonization of urinary tract catheters, we employed a semiquantitative state-of-the-art metaproteomics approach to investigate a bacterial biofilm directly derived from a catheterized patient. In contrast to metagenome analyses, metatranscriptomics and metaproteomics allow a direct linkage of structure and functionality in microbial communities (68). However, with a metatranscriptomics approach we would probably not detect human mRNA coding for granulocyte-associated proteins because these immune cells originate from the bone marrow and are already differentiated when they reach the site of infection (81, 82). Thus, we would miss mRNA coding for proteins secreted by the urothel as we had only access to catheter biofilms and urine.
Metaproteome coverage (spectra to peptide matching) can be significantly improved if the MS and MS/MS data can be searched against a sample-specific metagenome-based database. However, a recent study demonstrated that virtual metagenome databases based on 16S rDNA sequence information can replace a matching metagenome-based database in case the organisms present in the sample have been already sequenced (47, 83). We thus constructed an appropriate database based on the phylogenetic information obtained by 16S rDNA sequencing, which contained all protein sequences of the identified species available in the NCBI database. Nevertheless, as we did not sequence the genomes of the biofilm isolates, we might have missed to identify proteins specific for the strains present in our samples. In contrast to the majority of other metaproteome studies, all identified proteins were exclusively assigned to one of the species present in the biofilm. This is probably because of the specifically designed database, the stringent filter settings, and the limited diversity of the biofilm compared with other environments such as the human intestinal tract or soil.
Even though recent advances in mass spectrometry have turned metaproteomics into a powerful tool for the investigation of complex biological systems, quantification of proteins derived from clinical samples is often still not very accurate and mostly based on NSAFs. Unfortunately, the same applies to our study, in which, albeit we applied more precise NAUC quantification, an accurate comparison of the expression rates of proteins identified from the in vivo and in vitro samples was hampered by the strongly differing complexity of the two sample types. The catheter metaproteome is certainly much more complex than the proteomes of pure bacterial cultures. The catheter biofilm is composed of at least three bacterial species and contains an enormous amount of human proteins (min. 30,000 proteins), whereas the pure P. aeruginosa or M. morganii cultures contain a maximum of about 5600 and 3500 proteins, respectively. In addition, bacterial proteins are much less abundant compared with human proteins in the in vivo sample (Fig. 2, supplemental File S3, and supplemental Fig. S1). As proteins exclusively identified or highly expressed under in vitro conditions, but missing in the in vivo data set might simply have escaped mass spectrometric analyses because of the comparably high complexity of the metaproteomics sample, only those proteins were reported that appeared to be exclusively or highly expressed under in vivo conditions.
Iron was Identified as one of the Major Limiting Factors in the Bladder Environment
Proteins involved in iron-acquisition were among the most abundant proteins in the metaproteomics data set (Tables I and IV; Figs. 3 and 4), which indicates that iron is highly limited in the urinary tract environment and thus strongly affects protein expression of the uropathogens. Our findings are in good agreement with earlier studies demonstrating an increase of iron-uptake mechanisms on the transcriptome level when opportunistic pathogens were cultivated in urine (E. coli, P. mirabilis), in the urinary tract (E. coli) or on AUM agar plates (P. aeruginosa) (18–21). In an infected bladder environment the availability of iron is even more restricted by competition for iron in multispecies biofilms (84) and the presence of siderophore- and iron-binding proteins such as NGAL and lactoferrin in the urine representing the nutritional immunity (Fig. 5, supplemental File S6) (85).
The expression of P. aeruginosa outer membrane receptors for heterologous siderophores (PirA and PfeA) produced by cocolonizers, supports the hypothesis that biofilm inhabitants in the bladder environment strongly compete for iron. These receptors have been described to be involved in ferric enterobactin/enterochelin uptake (86, 87). Our findings suggest that P. aeruginosa is able to utilize siderophores produced by M. morganii and/or Bacteroides sp., which are structurally related to enterobactin (Fig. 6).
Fig. 6.
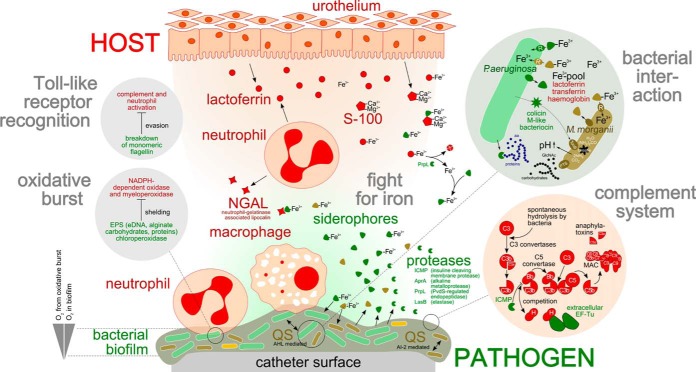
Proposed host-pathogen interactions during the catheter-associated urinary tract infection. P. aeruginosa (green), M. morganii (brown), and Bacteroides sp. (yellow) form a multispecies biofilm on the catheter surface. Cell-to-cell communication, that is, N-acylhomoserine lactone- and autoinducer 2-mediated quorum sensing, might contribute to the expression of virulence factors and the overall biofilm physiology. Various secreted or surface-exposed virulence factors of P. aeruginosa could be involved in immune evasion, that is, elastase (LasB), alkaline metalloprotease (AprA), insulin cleaving membrane proteinase (ICMP), elongation factor Tu (EF-Tu), as well as in iron acquisition, that is, PvdS-regulated endoprotease (PrpL) and siderophores. Host-factors possibly involved in innate immune response have been identified, that is, various compounds of the complement system (orange circle), and proteins related to neutrophils, macrophages and the urothelium. Lactoferrin, neutrophil-gelatinase associated lipocalin (NGAL) and S100 are secreted host factors, which are known to bind siderophores and calcium. The left gray circles summarize the proposed bacterial strategies to evade immune recognition and the oxidative burst because of neutrophil activation. Suggested interspecies interactions and differential adaptation strategies of P. aeruginosa and M. morganii in the catheter biofilm are depicted in the right greenish circle. Both bacteria produce siderophores (green and brown triangles) and express specific siderophore-receptors (R) to compensate iron limitation in the bladder environment. P. aeruginosa expresses also receptors for heterologous siderophores (PirA, PfeA) probably enabling the uptake of siderophores produced by M. morganii. Moreover, P. aeruginosa secretes a colicin M-like bacteriocin capable of inhibiting peptidoglycan biosynthesis in M. morganii. The bacteriocin might be taken up by M. morganii via TonB-dependent transport systems (blue oval), which are known to be involved in iron acquisition. The predominant carbohydrates taken up by P. aeruginosa are most probably peptides and amino acids (AS) whereas M. morganii might utilize carbohydrates (N-acetylglucosamine) and urea.
As mentioned above, the concentration of freely available iron in the urinary tract is further decreased by lactoferrin, which is secreted by the urothelium and expressed in neutrophils. Lactoferrin has been identified as a biofilm-associated protein and was also found to be highly abundant in the urine of catheterized patients compared with the control persons (supplemental Files S1 and S6). It has been shown that lactoferrin reduces the extracellular free iron concentration to ∼10−18 m (88) and inhibits P. aeruginosa biofilm formation (89). In the urine the siderophore pyoverdine and the high-abundant PvdS-regulated lactoferrin degrading protease PrpL were identified, which compensate iron limitation induced by the nutritional immunity of the host (90) (Fig. 6). Moreover, NGAL was found to be expressed in neutrophils that is able to bind ferric-siderophores and thereby interferes with the bacterial siderophore uptake (91). It has been demonstrated that P. aeruginosa pyoverdine is able to evade NGAL recognition (92), suggesting that pyoverdine-mediated iron acquisition of P. aeruginosa is not hampered by NGAL. Notably, a P. aeruginosa colicin M-like bacteriocin was identified in the urine, which has been demonstrated to inhibit peptidoglycan biosynthesis (93). Colicin M-like bacteriocins produced by Pectobacterium carotovorum and E. coli have been shown to enter the periplasm of potential competitors via TonB-dependent iron-uptake systems (94, 95). These observations suggest, that iron limitation promotes colicin uptake of cocolonizing bacterial species.
The Catheter Biofilm is Characterized by an Oxygen Gradient
It is well known that bacterial biofilms are characterized by a decreasing oxygen gradient from the biofilm-surface to the bottom. The identification of proteins involved in the adaptation to microaerobic conditions indicates that also cells in the catheter-associated biofilm are exposed to oxygen-limiting conditions. These results are supported by several other findings: First, the obligate anaerobe (96) Bacteroides sp. was identified in the biofilm (Fig. 2). Second, the P. aeruginosa proteins NirS, NosZ, and NarG, which are involved in denitrification and used for energy production under oxygen limitation, were found. They are part of the anaerobic regulon induced by Anr, Dnr, and NarXL (97, 98). Third, proteins of the innate immune system involved in ROS production, that is, myeloperoxidase, NADH peroxidase, and eosinophil peroxidase, were identified from the biofilm (99) (Fig. 5, Table V). Notably, a significant number of proteins associated with neutrophils were found in the biofilm metaproteome. This supports the finding of Jesaitis and colleagues, who demonstrated that neutrophils fusing with P. aeruginosa biofilms contribute to oxygen limitation by the consumption of oxygen during ROS generation (100) (Fig. 6).
Functional Assignment of Proteins Suggests Different Adaptation Strategies of the Major Biofilm Formers
Although both dominating biofilm inhabitants employ similar strategies to overcome the major limitation in the bladder environment, that is, restriction of iron, the differential expression of carbohydrate-transporters, urease and protease(s), suggests different nutrient acquisition strategies of the two opportunistic pathogens (Fig. 6).
The finding that protein components of a PTS uptake system (N-acetylglucosamine-specific) together with proteins involved in carbohydrate metabolism were found to be strongly expressed in M. morganii catheter biofilms suggests that this organism gains energy from cell wall compounds of lysed bacteria taken up from the infected bladder environment. In contrast, P. aeruginosa is able to generate energy from the fermentation of amino acids (91), which might be released by its various secreted proteases (Fig. 6) and taken up by transporters such as an amino acid ABC transporter, which appeared to be highly abundant in the catheter biofilm (Table I). This hypothesis is supported by the finding that P. aeruginosa PAO1 is able to grow in AUM with peptone as the sole carbon source (21).
Moreover, our data indicate that M. morganii expresses urease in vivo and is able to metabolize urea in vitro, whereas no urease was assigned to P. aeruginosa and no urease activity was detected in vitro. Our results are in good agreement with the findings of Tielen et al. who showed that P. aeruginosa PAO1 is not able to grow on urea as a sole carbon source in AUM (21).
P. aeruginosa Evades Recognition by the Immune System in the Urinary Tract
In the urine various P. aeruginosa virulence factors involved in immune system evasion were identified, many of which are known to exhibit proteolytic activity (Table III, Fig. 6). (1) Alkaline protease A (AprA) degrades the complement compounds C2 (101) and C3 (102) as well as IFN-gamma (103). Moreover, AprA is able to degrade monomeric but not polymeric flagella-forming flagellin thereby preventing the recognition of monomeric flagellin by TLR-5 (104). (2) Elastase (LasB) degrades pulmonary surfactant protein D (105), different surface receptors (106), and cytokines (103). (3) PvdS-regulated endoprotease (PrpL) cleaves lactoferrin and transferrin (90) and (IV) insulin-cleaving membrane proteinase (ICMP) degrades plasminogen activator, complement convertase and kallikrein (72). However, neither a protease secretion nor a proteolytic activity of the M. morganii culture-supernatant was observed. Therefore, we suggest that only P. aeruginosa is involved in the protease-dependent immune evasion and in the degradation of host proteins/tissue.
Notably, complement negative regulator factor H was found to be highly abundant on the catheter biofilm and in the corresponding urine (Fig. 5). Factor H can be bound by different human pathogens to mask themselves (107). P. aeruginosa is able to bind inhibitory factor H by surface-located elongation factor EF-Tu (108), too, which was found to be highly expressed in the biofilm (Fig. 6).
The Multispecies Biofilm Seems to Induce an Innate Immune Response
Proteins associated with the complement system, one of the central effectors of the innate immune system, are highly abundant in our metaproteomics data set and suggest an important role of the complement system in the defense of catheter-associated urinary tract infections. The innate immune system works clinically silently (30), which might explain why CAUTIs are mostly asymptomatic. The comparison of urine protein profiles of three patients and three control persons revealed that the expression of most of these proteins is at least associated with the presence of a catheter biofilm (Fig. 5). Neutrophils, known to be the first defense line of the immune system have been proven to be active during kidney infections and urinary tract infections (29, 30, 38). The high abundance of neutrophil-associated proteins suggests that neutrophils are also active during catheter-associated asymptomatic urinary infections.
Our finding that “acute-phase” and “negative acute-phase” proteins were significantly more abundant in patient urine compared with urine from healthy control persons might suggest a so far uncharacterized pronounced immune response to the catheter biofilm. However, because of the limited number of analyzed samples from CAUTI patients and healthy control persons, these results can only be considered as first evidence for a specific function of the innate immune system during CAUTIs. Moreover, additional controls, that is, urine from noncatheterized patients suffering from UTI or patients prior to catheterization, should be analyzed to verify that the observed immune response is specific for catheter-associated infections.
CONCLUSIONS
Combining metaproteomics analyses of a selected multispecies urinary tract catheter biofilm and the associated urine together with in vitro proteome analyses of P. aeruginosa and M. morganii isolated from the biofilm enabled us to unravel different molecular adaptation strategies of these important uropathogens to the urinary tract and co-infecting biofilm inhabitants as well as the response of the human innate immune system to the asymptomatic infection process. Our proteomics analysis strongly supports earlier studies that have demonstrated that iron restriction is one of the major limiting factors in the bladder environment. P. aeruginosa overcomes iron restriction by the host and cocolonizing competitors by the expression of proteins involved in siderophore-production, lactoferrin-degradation, and heme- and siderophore uptake. Moreover, the identification of complement-degrading proteases in vivo indicates that P. aeruginosa is able to evade the human immune response. Interestingly, M. morganii is not involved in protease secretion and might therefore often be considered as a harmless opportunistic pathogen. Our study indicates differential adaptation strategies of P. aeruginosa and M. morganii in nutrient acquisition in the urinary tract. Although P. aeruginosa seems to express secreted and surface-exposed proteases to metabolize amino acids, M. morganii is able to take up sugars and to degrade urea. Highly abundant proteins associated with neutrophils, eosinophils, and the complement system found in the biofilm and urine might suggest an activation of the innate immune system in response to the catheter biofilm. Even though extended clinical proteomics studies are urgently needed to consolidate our hypothesis, our data provides first evidence for a sophisticated interplay between the different members of the mixed biofilm and the host and will serve as a good basis for further studies aiming at a deeper insight into detailed molecular mechanisms of synergistic or competitive interactions observed in the urinary tract.
Supplementary Material
Acknowledgments
We thank the BMBF for financial support (UroGenOmics, grant 0315833B) and Holger Kock, Alexander Graf and Stefanie Dietz for critical reading of the manuscript.
Footnotes
Author contributions: C.L. and K.R. designed research; C.L., M.B., and D.C. performed research; C.L., M.B., D.C., and R.J. analyzed data; C.L. and K.R. wrote the paper; A.O. and C.H. mass spectrometry; S.F. provided bioinformatic tools; J.B. provided visualization tool; R.N. provided samples; D.B. provided mass spectrometry knowhow; D.H.P., M.J., and D.J. contributed to writing.
 This article contains supplemental Files S1 to S6.
This article contains supplemental Files S1 to S6.
1 The abbreviations used are:
- AUM
- artificial urine medium
- BLAST
- basic local alignment search tool
- UTI
- urinary tract infection
- CAUTI
- catheter-associated urinary tract infection
- CLED
- cystine lactose electrolyte deficient-agar
- CV
- coefficient of variation
- COG
- clusters of orthologous groups
- dNTP
- deoxynucleoside triphosphates
- FDR
- false discovery rate
- INF
- interferon
- NGAL
- neutrophil gelatinase associated lipocalin
- NSAF
- normalized spectral abundance factor
- NAUC
- normalized area under the curve
- RPSBLAST
- reversed position specific BLAST
- PTS
- phosphotransferase systems
- PRIDE
- Proteomics Identifications database
- RDP
- ribosomal database project
- ROS
- reactive oxygen species
- RT
- room temperature
- TLR
- toll-like receptor.
REFERENCES
- 1. Stamm W. E. (1991) Catheter-associated urinary tract infections: Epidemiology, pathogenesis, and prevention. Am. J. Med. 91, 65S–71S [DOI] [PubMed] [Google Scholar]
- 2. Warren J. W. (1991) The catheter and urinary tract infection. Med. Clin. North. Am. 75, 481–493 [DOI] [PubMed] [Google Scholar]
- 3. Tambyah P. A., Maki D. G. (2000) Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1497 catheterized patients. Arch. Intern. Med. 160, 678–682 [DOI] [PubMed] [Google Scholar]
- 4. Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. (1982) A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146, 719–723 [DOI] [PubMed] [Google Scholar]
- 5. Warren J. W. (2001) Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17, 299–303 [DOI] [PubMed] [Google Scholar]
- 6. Warren J. W., Damron D., Tenney J. H., Hoopes J. M., Deforge B., Muncie H. L., Jr. (1987) Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J. Infect. Dis. 155, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 7. Stickler D. J. (2008) Bacterial biofilms in patients with indwelling urinary catheters. Nat. Clin. Pract. Urol. 5, 598–608 [DOI] [PubMed] [Google Scholar]
- 8. Donlan R. M. (2001) Biofilms and device-associated infections. Emerg. Infect. Dis. 7, 277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall-Stoodley L., Costerton J. W., Stoodley P. (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 [DOI] [PubMed] [Google Scholar]
- 10. Donlan R. M. (2002) Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart P. S., Costerton J. W. (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138 [DOI] [PubMed] [Google Scholar]
- 12. Lewis K. (2001) Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45, 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonkat G., Widmer A. F., Rieken M., van der Merwe A., Braissant O., Müller G., Wyler S., Frei R., Gasser T. C., Bachmann A. (2012) Microbial biofilm formation and catheter-associated bacteriuria in patients with suprapubic catheterisation. World J. Urol. 31, 565–557 [DOI] [PubMed] [Google Scholar]
- 14. Macleod S. M., Stickler D. J. (2007) Species interactions in mixed-community crystalline biofilms on urinary catheters. J. Med. Microbiol. 56, 1549–1557 [DOI] [PubMed] [Google Scholar]
- 15. Vebø H. C., Solheim M., Snipen L., Nes I. F., Brede D. A. (2010) Comparative genomic analysis of pathogenic and probiotic Enterococcus faecalis isolates, and their transcriptional responses to growth in human urine. PloS One 5, e12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alteri C. J., Mobley H. L. (2007) Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect. Immun. 75, 2679–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roos V., Klemm P. (2006) Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 74, 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearson M. M., Yep A., Smith S. N., Mobley H. L. (2011) Transcriptome of Proteus mirabilis in the murine urinary tract: virulence and nitrogen assimilation gene expression. Infect. Immun. 79, 2619–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snyder J. A., Haugen B. J., Buckles E. L., Lockatell C. V., Johnson D. E., Donnenberg M. S., Welch R. A., Mobley H. L. (2004) Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72, 6373–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagan E. C., Lloyd A. L., Rasko D. A., Faerber G. J., Mobley H. L. (2010) Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6, e1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tielen P., Rosin N., Meyer A. K., Dohnt K., Haddad I., Jänsch L., Klein J., Narten M., Pommerenke C., Scheer M., Schobert M., Schomburg D., Thielen B., Jahn D. (2013) Regulatory and metabolic networks for the adaptation of Pseudomonas aeruginosa biofilms to urinary tract-like conditions. PloS One 8, e71845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Hara C. M., Brenner F. W., Miller J. M. (2000) Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 13, 534–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams E. W., Hawkey P. M., Penner J. L., Senior B. W., Barton L. J. (1983) Serious nosocomial infection caused by Morganella morganii and Proteus mirabilis in a cardiac surgery unit. J. Clin. Microbiol. 18, 5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falagas M. E., Kavvadia P. K., Mantadakis E., Kofteridis D. P., Bliziotis I. A., Saloustros E., Maraki S., Samonis G. (2006) Morganella morganii infections in a general tertiary hospital. Infection 34, 315–321 [DOI] [PubMed] [Google Scholar]
- 25. Khatri I., Dureja C., Raychaudhuri S., Subramanian S. (2013) Draft genome sequence of the opportunistic human pathogen Morganella morganii SC01. Genome Announc. 1, e00051–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tucci V., Isenberg H. D. (1981) Hospital cluster epidemic with Morganella morganii. J. Clin. Microbiol. 14, 563–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young G. M., Amid D., Miller V. L. (1996) A bifunctional urease enhances survival of pathogenic Yersinia enterocolitica and Morganella morganii at low pH. J. Bacteriol. 178, 6487–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poirel L., Guibert M., Girlich D., Naas T., Nordmann P. (1999) Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weichhart T., Haidinger M., Hörl W. H., Säemann M. D. (2008) Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Invest. 38, 29–38 [DOI] [PubMed] [Google Scholar]
- 30. Zasloff M. (2007) Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 18, 2810–2816 [DOI] [PubMed] [Google Scholar]
- 31. Agace W. W., Hedges S. R., Ceska M., Svanborg C. (1993) Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Invest. 92, 780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haraoka M., Hang L., Frendéus B., Godaly G., Burdick M., Strieter R., Svanborg C. (1999) Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180, 1220–1229 [DOI] [PubMed] [Google Scholar]
- 33. Hedges S., Anderson P., Lidin-Janson G., de Man P., Svanborg C. (1991) Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect. Immun. 59, 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Selsted M. E., Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 [DOI] [PubMed] [Google Scholar]
- 35. Chromek M., Slamova Z., Bergman P., Kovacs L., Podracka L., Ehren I., Hökfelt T., Gudmundsson G. H., Gallo R. L., Agerberth B., Brauner A. (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12, 636–641 [DOI] [PubMed] [Google Scholar]
- 36. Håversen L. A., Engberg I., Baltzer L., Dolphin G., Hanson L. A., Mattsby-Baltzer I. (2000) Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect. Immun. 68, 5816–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bates J. M., Raffi H. M., Prasadan K., Mascarenhas R., Laszik Z., Maeda N., Hultgren S. J., Kumar S. (2004) Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 [DOI] [PubMed] [Google Scholar]
- 38. Chowdhury P., Sacks S. H., Sheerin N. S. (2004) Minireview: functions of the renal tract epithelium in coordinating the innate immune response to infection. Kidney Int. 66, 1334–1344 [DOI] [PubMed] [Google Scholar]
- 39. Walport M. J. (2001) Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 40. Hopkins W. J., Uehling D. T., Balish E. (1987) Local and systemic antibody responses accompany spontaneous resolution of experimental cystitis in cynomolgus monkeys. Infect. Immun. 55, 1951–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jones-Carson J., Balish E., Uehling D. T. (1999) Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J. Urol. 161, 338–341 [PubMed] [Google Scholar]
- 42. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 43. Brooks T., Keevil C. W. (1997) A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 24, 203–206 [DOI] [PubMed] [Google Scholar]
- 44. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 45. Neuhoff V., Arold N., Taube D., Ehrhardt W. (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9, 255–262 [DOI] [PubMed] [Google Scholar]
- 46. Teeling H., Fuchs B. M., Becher D., Klockow C., Gardebrecht A., Bennke C. M., Kassabgy M., Huang S., Mann A. J., Waldmann J., Weber M., Klindworth A., Otto A., Lange J., Bernhardt J., Reinsch C., Hecker M., Peplies J., Bockelmann F. D., Callies U., Gerdts G., Wichels A., Wiltshire K. H., Glöckner F. O., Schweder T., Amann R. (2012) Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336, 608–611 [DOI] [PubMed] [Google Scholar]
- 47. Okuda S., Tsuchiya Y., Kiriyama C., Itoh M., Morisaki H. (2012) Virtual metagenome reconstruction from 16S rRNA gene sequences. Nat. Commun. 3, 1203. [DOI] [PubMed] [Google Scholar]
- 48. Silva J. C., Gorenstein M. V., Li G. Z., Vissers J. P., Geromanos S. J. (2006) Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol. Cell. Proteomics 5, 144–156 [DOI] [PubMed] [Google Scholar]
- 49. Vizcaino J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Rios D., Dianes J. A., Sun Z., Farrah T., Bandeira N., Binz P. A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R. J., Kraus H. J., Albar J. P., Martinez-Bartolomé S., Apweiler R., Omenn G. S., Martens L., Jones A. R., Hermjakob H. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider T., Schmid E., de Castro J. V., Jr., Cardinale M., Eberl L., Grube M., Berg G., Riedel K. (2011) Structure and function of the symbiosis partners of the lung lichen (Lobaria pulmonaria L. Hoffm.) analyzed by metaproteomics. Proteomics 11, 2752–2756 [DOI] [PubMed] [Google Scholar]
- 51. Schäffer A. A., Wolf Y. I., Ponting C. P., Koonin E. V., Aravind L., Altschul S. F. (1999) IMPALA: matching a protein sequence against a collection of PSI-BLAST-constructed position-specific score matrices. Bioinformatics 15, 1000–1011 [DOI] [PubMed] [Google Scholar]
- 52. Eddy S. R. (2011) Accelerated Profile HMM Searches. PLoS Comput. Biol. 7, e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tatusov R. L., Fedorova N. D., Jackson J. D., Jacobs A. R., Kiryutin B., Koonin E. V., Krylov D. M., Mazumder R., Mekhedov S. L., Nikolskaya A. N., Rao B. S., Smirnov S., Sverdlov A. V., Vasudevan S., Wolf Y. I., Yin J. J., Natale D. A. (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haft D. H., Selengut J. D., Richter R. A., Harkins D., Basu M. K., Beck E. (2013) TIGRFAMs and Genome Properties in 2013. Nucleic Acids Res. 41, D387–D395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finn R. D., Bateman A., Clements J., Coggill P., Eberhardt R. Y., Eddy S. R., Heger A., Hetherington K., Holm L., Mistry J., Sonnhammer E. L., Tate J., Punta M. (2014) Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bernhardt J., Michalik S., Wollscheid B., Völker U., Schmidt F. (2013) Proteomics approaches for the analysis of enriched microbial subpopulations and visualization of complex functional information. Curr. Opin. Biotechnol. 24, 112–119 [DOI] [PubMed] [Google Scholar]
- 57. Camarinha-Silva A., Wos-Oxley M. L., Jáuregui R., Becker K., Pieper D. H. (2012) Validating T-RFLP as a sensitive and high-throughput approach to assess bacterial diversity patterns in human anterior nares. FEMS Microbiol. Ecol. 79, 98–108 [DOI] [PubMed] [Google Scholar]
- 58. Camarinha-Silva A., Jáuregui R., Chaves-Moreno D., Oxley A. P., Schaumburg F., Becker K., Wos-Oxley M. L., Pieper D. H. (2014) Comparing the anterior nare bacterial community of two discrete human populations using Illumina amplicon sequencing. Environ. Microbiol. 16, 2939–2952 [DOI] [PubMed] [Google Scholar]
- 59. Lane D. J. (1991) 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., eds. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley and Sons; 1991. pp. 115–175 [Google Scholar]
- 60. Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410 [DOI] [PubMed] [Google Scholar]
- 61. O'Toole G. A., Kolter R. (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28, 449–461 [DOI] [PubMed] [Google Scholar]
- 62. Savoia D., Zucca M. (2007) Clinical and environmental Burkholderia strains: biofilm production and intracellular survival. Curr. Microbiol. 54, 440–444 [DOI] [PubMed] [Google Scholar]
- 63. Bauer A. W., Kirby W. M., Sherris J. C., Turck M. (1966) Antibiotic susceptibility testing by a standardized single disk method. Am. J Clin. Pathol. 45, 493–496 [PubMed] [Google Scholar]
- 64. Mobley H. L., Warren J. W. (1987) Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25, 2216–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hanlon G. W., Hodges N. A. (1981) Bacitracin and protease production in relation to sporulation during exponential growth of Bacillus licheniformis on poorly utilized carbon and nitrogen sources. J. Bacteriol. 147, 427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holloway B. W. (1955) Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13, 572–581 [DOI] [PubMed] [Google Scholar]
- 67. Schneider T., Keiblinger K. M., Schmid E., Sterflinger-Gleixner K., Ellersdorfer G., Roschitzki B., Richter A., Eberl L., Zechmeister-Boltenstern S., Riedel K. (2012) Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 6, 1749–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneider T., Riedel K. (2010) Environmental proteomics: analysis of structure and function of microbial communities. Proteomics 10, 785–798 [DOI] [PubMed] [Google Scholar]
- 69. Lichtman J. S., Marcobal A., Sonnenburg J. L., Elias J. E. (2013) Host-centric proteomics of stool: a novel strategy focused on intestinal responses to the gut microbiota. Mol. Cell. Proteomics 12, 3310–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Verberkmoes N. C., Russell A. L., Shah M., Godzik A., Rosenquist M., Halfvarson J., Lefsrud M. G., Apajalahti J., Tysk C., Hettich R. L., Jansson J. K. (2009) Shotgun metaproteomics of the human distal gut microbiota. ISME J. 3, 179–189 [DOI] [PubMed] [Google Scholar]
- 71. Ochsner U. A., Vasil A. I., Vasil M. L. (1995) Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J. Bacteriol. 177, 7194–7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fricke B., Parchmann O., Kruse K., Rücknagel P., Schierhorn A., Menge S. (1999) Characterization and purification of an outer membrane metalloproteinase from Pseudomonas aeruginosa with fibrinogenolytic activity. Biochim. Biophys. Acta 1454, 236–250 [DOI] [PubMed] [Google Scholar]
- 73. Jacobsen S. M., Stickler D. J., Mobley H. L., Shirtliff M. E. (2008) Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21, 26–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Griffith D. P., Musher D. M., Itin C. (1976) Urease. The primary cause of infection-induced urinary stones. Invest. Urol. 13, 346–350 [PubMed] [Google Scholar]
- 75. Gruys E., Toussaint M. J., Niewold T. A., Koopmans S. J. (2005) Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B. 6, 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Becker D., Selbach M., Rollenhagen C., Ballmaier M., Meyer T. F., Mann M., Bumann D. (2006) Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature 440, 303–307 [DOI] [PubMed] [Google Scholar]
- 77. Twine S. M., Mykytczuk N. C., Petit M. D., Shen H., Sjöstedt A., Wayne Conlan J., Kelly J. F. (2006) In vivo proteomic analysis of the intracellular bacterial pathogen, Francisella tularensis, isolated from mouse spleen. Biochem. Biophys. Res. Commun. 345, 1621–1633 [DOI] [PubMed] [Google Scholar]
- 78. Kruh N. A., Troudt J., Izzo A., Prenni J., Dobos K. M. (2010) Portrait of a pathogen: the Mycobacterium tuberculosis proteome in vivo. PloS One 5, e13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mestas J., Hughes C. C. (2004) Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 [DOI] [PubMed] [Google Scholar]
- 80. Fux C. A., Shirtliff M., Stoodley P., Costerton J. W. (2005) Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 13, 58–63 [DOI] [PubMed] [Google Scholar]
- 81. Bainton D. F., Ullyot J. L., Farquhar M. G. (1971) The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134, 907–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bjerregaard M. D., Jurlander J., Klausen P., Borregaard N., Cowland J. B. (2003) The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood 101, 4322–4332 [DOI] [PubMed] [Google Scholar]
- 83. Tanca A., Palomba A., Deligios M., Cubeddu T., Fraumene C., Biosa G., Pagnozzi D., Addis M. F., Uzzau S. (2013) Evaluating the impact of different sequence databases on metaproteome analysis: insights from a lab-assembled microbial mixture. PloS One 8, e82981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Purschke F. G., Hiller E., Trick I., Rupp S. (2012) Flexible survival strategies of Pseudomonas aeruginosa in biofilms result in increased fitness compared with Candida albicans. Mol. Cell. Proteomics 11, 1652–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schaible U. E., Kaufmann S. H. (2004) Iron and microbial infection. Nat. Rev. Microbiol. 2, 946–953 [DOI] [PubMed] [Google Scholar]
- 86. Ghysels B., Ochsner U., Möllman U., Heinisch L., Vasil M., Cornelis P., Matthijs S. (2005) The Pseudomonas aeruginosa pirA gene encodes a second receptor for ferrienterobactin and synthetic catecholate analogs. FEMS Microbiol. Lett. 246, 167–174 [DOI] [PubMed] [Google Scholar]
- 87. Dean C. R., Poole K. (1993) Cloning and characterization of the ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J. Bacteriol. 175, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cherayil B. J. (2011) The role of iron in the immune response to bacterial infection. Immunol. Res. 50, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh P. K., Parsek M. R., Greenberg E. P., Welsh M. J. (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555 [DOI] [PubMed] [Google Scholar]
- 90. Wilderman P. J., Vasil A. I., Johnson Z., Wilson M. J., Cunliffe H. E., Lamont I. L., Vasil M. L. (2001) Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect Immun. 69, 5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Borregaard N., Cowland J. B. (2006) Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals 19, 211–215 [DOI] [PubMed] [Google Scholar]
- 92. Peek M. E., Bhatnagar A., McCarty N. A., Zughaier S. M. (2012) Pyoverdine, the Major Siderophore in Pseudomonas aeruginosa, Evades NGAL Recognition. Interdiscip. Perspect. Infect. Dis. 10.1155/2012/843509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barreteau H., Tiouajni M., Graille M., Josseaume N., Bouhss A., Patin D., Blanot D., Fourgeaud M., Mainardi J. L., Arthur M., van Tilbeurgh H., Mengin-Lecreulx D., Touzé T. (2012) Functional and structural characterization of PaeM, a colicin M-like bacteriocin produced by Pseudomonas aeruginosa. J. Biol. Chem. 287, 37395–37405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cascales E., Buchanan S. K., Duché D., Kleanthous C., Lloubes R., Postle K., Riley M., Slatin S., Cavard D. (2007) Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Grinter R., Milner J., Walker D. (2012) Ferredoxin containing bacteriocins suggest a novel mechanism of iron uptake in Pectobacterium spp. PloS One 7, e33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baughn A. D., Malamy M. H. (2004) The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427, 441–444 [DOI] [PubMed] [Google Scholar]
- 97. Schobert M., Jahn D. (2010) Anaerobic physiology of Pseudomonas aeruginosa in the cystic fibrosis lung. Int. J. Med. Microbiol. 300, 549–556 [DOI] [PubMed] [Google Scholar]
- 98. Trunk K., Benkert B., Quäck N., Münch R., Scheer M., Garbe J., Jänsch L., Trost M., Wehland J., Buer J., Jahn M., Schobert M., Jahn D. (2010) Anaerobic adaptation in Pseudomonas aeruginosa: definition of the Anr and Dnr regulons. Environ. Microbiol. 12, 1719–1733 [DOI] [PubMed] [Google Scholar]
- 99. Dahlgren C., Karlsson A. (1999) Respiratory burst in human neutrophils. J. Immunol. Methods. 232, 3–14 [DOI] [PubMed] [Google Scholar]
- 100. Jesaitis A. J., Franklin M. J., Berglund D., Sasaki M., Lord C. I., Bleazard J. B., Duffy J. E., Beyenal H., Lewandowski Z. (2003) Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171, 4329–4339 [DOI] [PubMed] [Google Scholar]
- 101. Hong Y. Q., Ghebrehiwet B. (1992) Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol. 62, 133–138 [DOI] [PubMed] [Google Scholar]
- 102. Laarman A. J., Bardoel B. W., Ruyken M., Fernie J., Milder F. J., van Strijp J. A., Rooijakkers S. H. (2012) Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J. Immunol. 188, 386–393 [DOI] [PubMed] [Google Scholar]
- 103. Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. (1990) Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect. Immun. 58, 3009–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bardoel B. W., van Kessel K. P., van Strijp J. A., Milder F. J. (2012) Inhibition of Pseudomonas aeruginosa virulence: characterization of the AprA-AprI interface and species selectivity. J. Mol. Biol. 415, 573–583 [DOI] [PubMed] [Google Scholar]
- 105. Alcorn J. F., Wright J. R. (2004) Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J. Biol. Chem. 279, 30871–30879 [DOI] [PubMed] [Google Scholar]
- 106. Leduc D., Beaufort N., de Bentzmann S., Rousselle J. C., Namane A., Chignard M., Pidard D. (2007) The Pseudomonas aeruginosa LasB metalloproteinase regulates the human urokinase-type plasminogen activator receptor through domain- specific endoproteolysis. Infect. Immun. 75, 3848–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zipfel P. F., Skerka C. (2009) Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740 [DOI] [PubMed] [Google Scholar]
- 108. Kunert A., Losse J., Gruszin C., Hühn M., Kaendler K., Mikkat S., Volke D., Hoffmann R., Jokiranta T. S., Seeberger H., Moellmann U., Hellwage J., Zipfel P. F. (2007) Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J. Immunol. 179, 2979–2988 [DOI] [PubMed] [Google Scholar]
- 109. Bernhardt J. F., S., Hecker M., Siebourg J. (2009) Sixth international symposium on Voronoi diagrams in science and engineering. Computer Society Digital Library CSDL, 233–241 [Google Scholar]
- 110. Folders J., Tommassen J., van Loon L. C., Bitter W. (2000) Identification of a chitin-binding protein secreted by Pseudomonas aeruginosa. J. Bacteriol. 182, 1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mougous J. D., Cuff M. E., Raunser S., Shen A., Zhou M., Gifford C. A., Goodman A. L., Joachimiak G., Ordonez C. L., Lory S., Walz T., Joachimiak A., Mekalanos J. J. (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312, 1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Porra R. J., Langman L., Young I. G., Gibson F. (1972) The role of ferric enterochelin esterase in enterochelin-mediated iron transport and ferrochelatase activity in Escherichia coli. Arch. Biochem. Biophys. 153, 74–78 [DOI] [PubMed] [Google Scholar]
- 113. Wohlfarth S., Hoesche C., Strunk C., Winkler U. K. (1992) Molecular genetics of the extracellular lipase of Pseudomonas aeruginosa PAO1. J. Gen. Microbiol. 138, 1325–1335 [DOI] [PubMed] [Google Scholar]
- 114. Obernesser H. J., Doring G., Botzenhart K. (1981) Extracellular toxins of Pseudomonas aeruginosa. I. Purification and characterization of two exoproteases. Zentralbl. Bakteriol. A 249, 76–88 [PubMed] [Google Scholar]
- 115. Heck L. W., Alarcon P. G., Kulhavy R. M., Morihara K., Russell M. W., Mestecky J. F. (1990) Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J. Immunol. 144, 2253–2257 [PubMed] [Google Scholar]
- 116. Pham C. T. (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 6, 541–550 [DOI] [PubMed] [Google Scholar]
- 117. Baggiolini M., Hirsch J. G., De Duve C. (1969) Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J. Cell Biol. 40, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Odeberg H., Olsson I. (1976) Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect. Immun. 14, 1276–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ohlsson K., Olsson I. (1977) Neutral proteases of human granulocytes. IV. Interaction between human granulocyte collagenase and plasma protease inhibitors. J. Lab. Clin. Med. 89, 269–277 [PubMed] [Google Scholar]
- 120. Olsson I., Venge P. (1974) Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood 44, 235–246 [PubMed] [Google Scholar]
- 121. Baggiolini M., Bretz U., Dewald B., Feigenson M. E. (1978) The polymorphonuclear leukocyte. Agents Actions 8, 3–10 [DOI] [PubMed] [Google Scholar]
- 122. Campanelli D., Melchior M., Fu Y., Nakata M., Shuman H., Nathan C., Gabay J. E. (1990) Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J. Exp. Med. 172, 1709–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schwarting A., Hagen D., Odenthal M., Brockmann H., Dienes H. P., Wandel E., Rumpelt H. J., Zum Buschenfelde K. H., Galle P. R., Mayet W. (2000) Proteinase-3 mRNA expressed by glomerular epithelial cells correlates with crescent formation in Wegener's granulomatosis. Kidney Int. 57, 2412–2422 [DOI] [PubMed] [Google Scholar]
- 124. Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. (1990) Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J. Clin. Invest. 85, 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Weiss J., Elsbach P. (1977) The use of a phospholipase A-less Escherichia coli mutant to establish the action of granulocyte phospholipase A on bacterial phospholipids during killing by a highly purified granulocyte fraction. Biochim. Biophys. Acta 466, 23–33 [DOI] [PubMed] [Google Scholar]
- 126. Zeya H. I., Spitznagel J. K. (1963) Antibacterial and enzymic basic proteins from leukocyte lysosomes: separation and identification. Science 142, 1085–1087 [DOI] [PubMed] [Google Scholar]
- 127. Zeya H. I., Spitznagel J. K. (1966) Antimicrobial specificity of leukocyte lysosomal cationic proteins. Science 154, 1049–1051 [DOI] [PubMed] [Google Scholar]
- 128. Zeya H. I., Spitznagel J. K., Schwab J. H. (1966) Antibacterial action of PMN lysosomal cationic proteins resolved by density gradient electrophoresis. Proc. Soc. Exp. Biol. Med. 121, 250–253 [DOI] [PubMed] [Google Scholar]
- 129. Segal A. W., Jones O. T. (1979) Identification of a previously undescribed cytochrome b in human neutrophils and its relationship to phagocytosis-induced oxidase activity. Biochem. Soc. Trans. 7, 187–188 [DOI] [PubMed] [Google Scholar]
- 130. Masson P. L., Heremans J. F., Schonne E. (1969) Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 130, 643–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Abrink M., Larsson E., Gobl A., Hellman L. (2000) Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int. 57, 2004–2010 [DOI] [PubMed] [Google Scholar]
- 132. Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jörnvall H., Wigzell H., Gudmundsson G. H. (2000) The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 [PubMed] [Google Scholar]
- 133. Bals R., Goldman M. J., Wilson J. M. (1998) Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 66, 1225–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. (1990) Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J. Biol. Chem. 265, 11421–11424 [PubMed] [Google Scholar]
- 135. Bundgaard J. R., Sengelov H., Borregaard N., Kjeldsen L. (1994) Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem. Biophys. Res. Commun. 202, 1468–1475 [DOI] [PubMed] [Google Scholar]
- 136. Cowland J. B., Sorensen O. E., Sehested M., Borregaard N. (2003) Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J. Immunol. 171, 6630–6639 [DOI] [PubMed] [Google Scholar]
- 137. Egesten A., Alumets J., von Mecklenburg C., Palmegren M., Olsson I. (1986) Localization of eosinophil cationic protein, major basic protein, and eosinophil peroxidase in human eosinophils by immunoelectron microscopic technique. J. Histochem. Cytochem. 34, 1399–1403 [DOI] [PubMed] [Google Scholar]
- 138. Peters M. S., Rodriguez M., Gleich G. J. (1986) Localization of human eosinophil granule major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin by immunoelectron microscopy. Lab. Invest. 54, 656–662 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.