Abstract
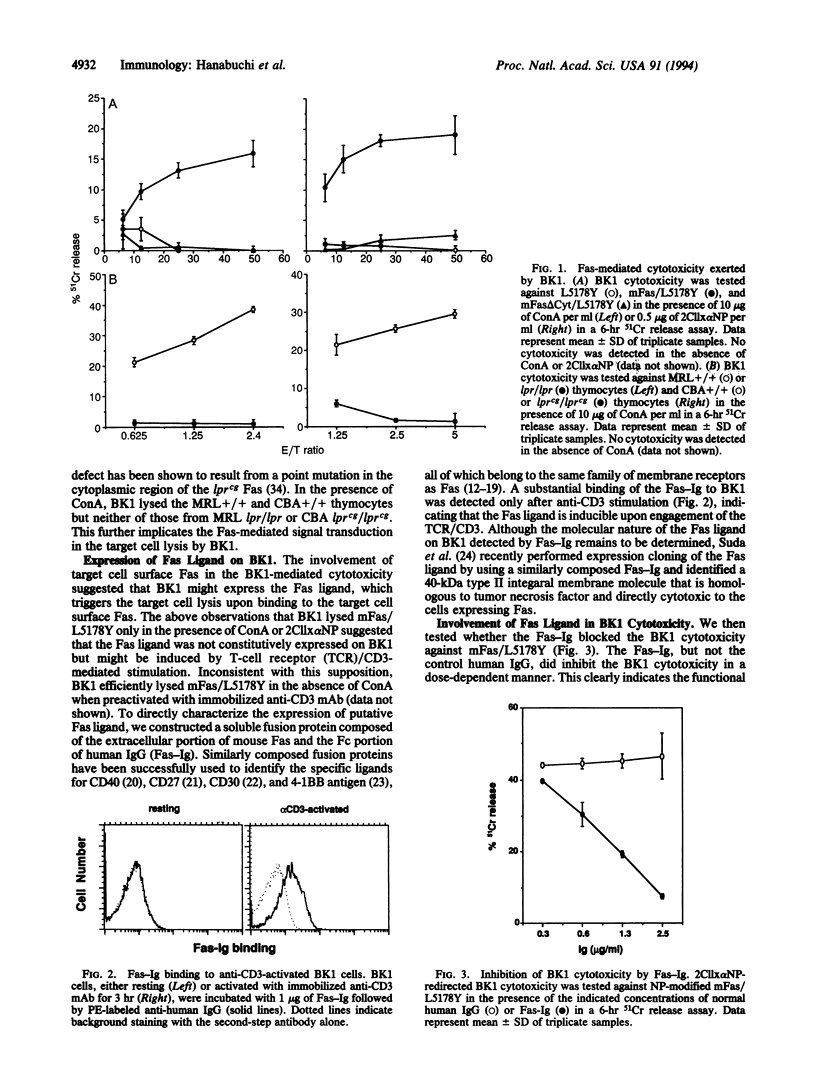
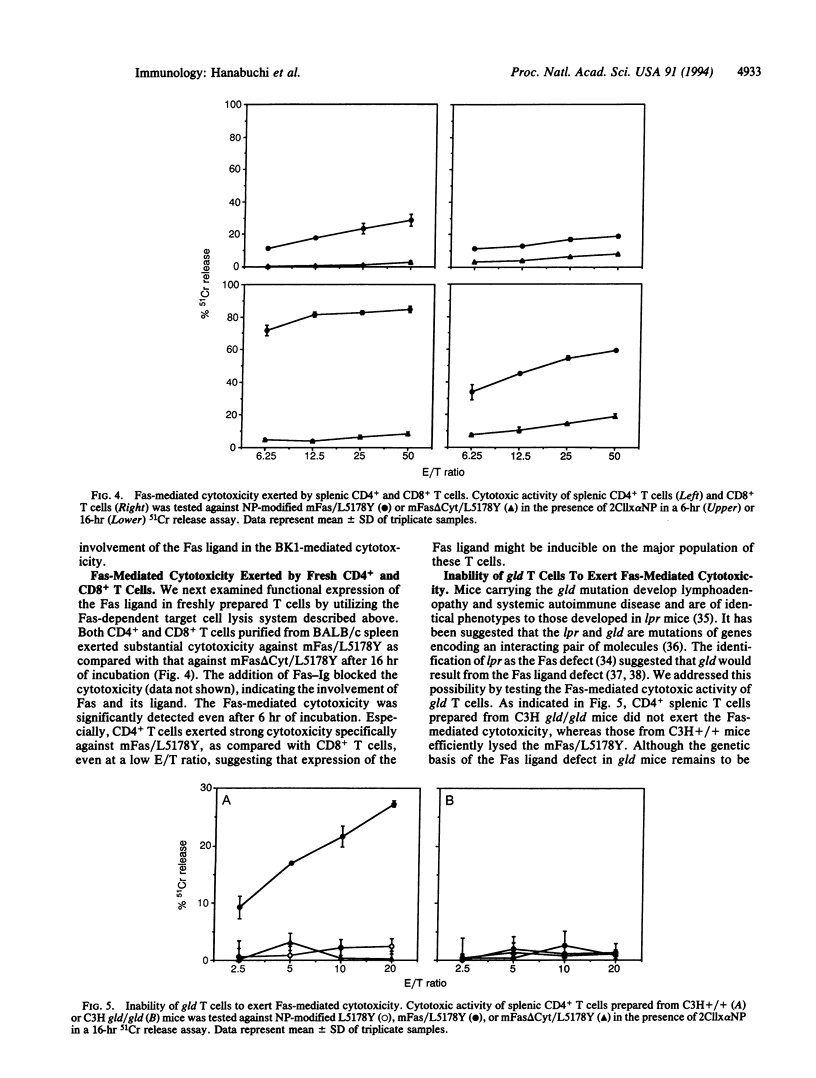
To investigate the mechanisms of T-cell-mediated cytotoxicity, we estimated the involvement of apoptosis-inducing Fas molecule on the target cells and its ligand on the effector cells. When redirected by ConA or anti-CD3 monoclonal antibody, a CD4+ T-cell clone, BK1, could lyse the target cells expressing wild-type Fas molecule but not those expressing death signaling-deficient mutants. This indicates the involvement of Fas-mediated signal transduction in the target cell lysis by BK1. Anti-CD3-activated but not resting BK1 expressed Fas ligand as detected by binding of a soluble Fas-Ig fusion protein, and the BK1-mediated cytotoxicity was blocked by the addition of Fas-Ig, implicating the inducible Fas ligand in the BK1 cytotoxicity. Ability to exert the Fas-mediated cytotoxicity was not confined to BK1, but splenic CD4+ T cells and, to a lesser extent, CD8+ T cells could also exert the Fas-dependent target cell lysis. This indicates that the Fas-mediated target cell lytic pathway can be generally involved in the T-cell-mediated cytotoxicity. Interestingly, CD4+ T cells prepared from gld/gld mice did not mediate the Fas-mediated cytotoxicity, indicating defective expression of functional Fas ligand in gld mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Alderson M. R., Armitage R. J., Maraskovsky E., Tough T. W., Roux E., Schooley K., Ramsdell F., Lynch D. H. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993 Dec 1;178(6):2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., Marshall J. D., Roths J. B., Sidman C. L. Differences defined by bone marrow transplantation suggest that lpr and gld are mutations of genes encoding an interacting pair of molecules. J Exp Med. 1990 Nov 1;172(5):1367–1375. doi: 10.1084/jem.172.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Camerini D., Walz G., Loenen W. A., Borst J., Seed B. The T cell activation antigen CD27 is a member of the nerve growth factor/tumor necrosis factor receptor gene family. J Immunol. 1991 Nov 1;147(9):3165–3169. [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. The lpr and gld genes in systemic autoimmunity: life and death in the Fas lane. Immunol Today. 1992 Nov;13(11):427–428. doi: 10.1016/0167-5699(92)90066-G. [DOI] [PubMed] [Google Scholar]
- Dürkop H., Latza U., Hummel M., Eitelbach F., Seed B., Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992 Feb 7;68(3):421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Alderson M. R., Smith C. A., Armitage R. J., VandenBos T., Jerzy R., Tough T. W., Schoenborn M. A., Davis-Smith T., Hennen K. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993 May 7;73(3):447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Din W. S., Davis-Smith T., Anderson D. M., Gimpel S. D., Sato T. A., Maliszewski C. R., Brannan C. I., Copeland N. G., Jenkins N. A. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993 Oct;23(10):2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Two distinct functional high affinity receptors for mouse interleukin-3 (IL-3). EMBO J. 1992 May;11(5):1875–1884. doi: 10.1002/j.1460-2075.1992.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kumar V., Kono D. H., Urban J. L., Hood L. The T-cell receptor repertoire and autoimmune diseases. Annu Rev Immunol. 1989;7:657–682. doi: 10.1146/annurev.iy.07.040189.003301. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancki D. W., Hsieh C. S., Fitch F. W. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991 May 1;146(9):3242–3249. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Mapara M. Y., Bargou R., Zugck C., Döhner H., Ustaoglu F., Jonker R. R., Krammer P. H., Dörken B. APO-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993 Mar;23(3):702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- Masson D., Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987 Jun 5;49(5):679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- Nakata M., Kawasaki A., Azuma M., Tsuji K., Matsuda H., Shinkai Y., Yagita H., Okumura K. Expression of perforin and cytolytic potential of human peripheral blood lymphocyte subpopulations. Int Immunol. 1992 Sep;4(9):1049–1054. doi: 10.1093/intimm/4.9.1049. [DOI] [PubMed] [Google Scholar]
- Nitta T., Yagita H., Azuma T., Sato K., Okumura K. Bispecific F (ab')2 monomer prepared with anti-CD3 and anti-tumor monoclonal antibodies is most potent in induction of cytolysis of human T cells. Eur J Immunol. 1989 Aug;19(8):1437–1441. doi: 10.1002/eji.1830190814. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Rush B. J., Abrams S. I., Wang R. Sensitivity of T cells to anti-CD3-stimulated suicide is independent of functional phenotype. Eur J Immunol. 1992 Jun;22(6):1655–1658. doi: 10.1002/eji.1830220648. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Shiver J. W., Su L., Henkart P. A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992 Oct 16;71(2):315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Gruss H. J., Davis T., Anderson D., Farrah T., Baker E., Sutherland G. R., Brannan C. I., Copeland N. G., Jenkins N. A. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993 Jul 2;73(7):1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- Sobel E. S., Kakkanaiah V. N., Cohen P. L., Eisenberg R. A. Correction of gld autoimmunity by co-infusion of normal bone marrow suggests that gld is a mutation of the Fas ligand gene. Int Immunol. 1993 Oct;5(10):1275–1278. doi: 10.1093/intimm/5.10.1275. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E. A., Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989 May;8(5):1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takayama H., Shinohara N., Kawasaki A., Someya Y., Hanaoka S., Kojima H., Yagita H., Okumura K., Shinkai Y. Antigen-specific directional target cell lysis by perforin-negative T lymphocyte clones. Int Immunol. 1991 Nov;3(11):1149–1156. doi: 10.1093/intimm/3.11.1149. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Yagita H., Nakata M., Kawasaki A., Shinkai Y., Okumura K. Role of perforin in lymphocyte-mediated cytolysis. Adv Immunol. 1992;51:215–242. doi: 10.1016/s0065-2776(08)60488-5. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Liu C. C., Persechini P. M., Cohn Z. A. Perforin-dependent and -independent pathways of cytotoxicity mediated by lymphocytes. Immunol Rev. 1988 Mar;103:161–202. doi: 10.1111/j.1600-065x.1988.tb00755.x. [DOI] [PubMed] [Google Scholar]


