Abstract
Alzheimer's disease is the 3rd most costly disease and is estimated to be the 6th leading cause of death. Alzheimer's disease (AD) is fatal and affected individuals can sometimes linger many years. Current treatments are palliative and transient, not disease modifying. This article reviews progress in the search to identify the primary AD-causing toxins. We summarize the shift from an initial focus on amyloid plaques to the contemporary concept that AD memory failure is caused by small soluble oligomers of the Aβ peptide, toxins that target and disrupt particular synapses. Evidence is presented that links Aβ oligomers to pathogenesis in animal models and humans, with reference to seminal discoveries from cell biology and new ideas concerning pathogenic mechanisms. These findings have established the oligomer hypothesis as a new molecular basis for the cause, diagnosis, and treatment of AD.
Keywords: Alzheimer's disease, oligomer, neurodegeneration
At an annual cost estimated to exceed $180 billion in the US alone, Alzheimer’s is the 3rd most costly disease, afflicting 1 person in 8 over 65, and almost 1 in 2 over 85 (Alzheimer's Association, 2010; Hebert et al., 2003). It is the leading cause of dementia in the elderly. Progressively incapacitating, Alzheimer’s disease (AD) can linger many years. The average is 8, but it can be as long as 20 (Alzheimer's Association, 2011). Ultimately, AD is fatal and is estimated to be the 6th leading cause of death.
Current treatments are palliative and transient, not disease modifying. This is not surprising, as the disease is complex with multiple clinical manifestations. Although AD is classically defined as dementia with plaques and tangles, its inherent complexity and variability have led some to suggest that AD might be a spectrum of diseases, not just one. Its complexity increases even further with the occurrence of age-related co-morbidities. The broad clinical phenotype and underlying neuropathology of AD are summarized in Table 1.
TABLE 1.
Complex clinical and pathological characteristics of Alzheimer's Disease
| Clinical | Neuropathology |
|---|---|
Mild Cognitive Impairment
|
|
Early AD
| |
Moderate AD
| |
Severe AD
|
(Left) Clinical characteristics of AD by disease stage (Alzheimer's Association, 2010; Apostolova and Cummings, 2007). (Right) Neuropathology markers of the disease (Apostolova and Cummings, 2007; Berti et al., 2010; Braak et al., 1998; Hampel et al., 2009; Hampel et al., 2010; Hoozemans et al., 2006; Lacor, 2007; Masters et al., 2011; Morris et al., 1989; Mrak, 2009; Nelson et al, 2009; Perl, 2010). Organized by Kirsten L. Viola.
This article reviews progress in the search for primary AD-causing toxins. Despite the complexity of AD, the symptom that first brings patients to a physician is a troubling inability to form new memories, and early AD is largely regarded as a disease of memory formation. If we could regard AD as having a single disease-initiating cytotoxin (albeit one likely elicited by multiple factors), its action minimally must provide a basis for memory dysfunction. If the toxin were to serve as an optimal target for therapeutics, its actions should also account for the major facets of AD neuropathology. We summarize here why attention has shifted away from amyloid plaques as the toxic agents of AD to the contemporary concept that memory failure is caused by small soluble oligomers of the Aβ peptide, toxins that target and disrupt particular synapses. Evidence is presented that links Aβ oligomers to pathogenesis in animal models and humans, with reference to seminal discoveries from cell biology and new ideas concerning pathogenic mechanisms. This evidence has established the oligomer hypothesis as an appealing molecular basis for the cause, diagnosis, and treatment of AD.
The original amyloid cascade hypothesis
Neurodegeneration in AD can be linked to multiple cellular abnormalities. At one time or another, a number of these have been considered upstream events that initiate neurological damage (e.g., anomalies in tau and the cytoskeleton; mitochondrial dysfunction and oxidative damage; inflammation and gliosis (Butterfield et al., 2001; Finch et al., 2001; Mandelkow and Mandelkow, 1998)). However, at least until recently, the dominant theory for AD has attributed disease onset to the toxicity of amyloid plaques. The amyloid plaque hypothesis, or “amyloid cascade,” emerged in the late 1980s (Allsop et al., 1988; Selkoe, 1989) and was formalized in 1992 in a review by Hardy and Higgins (Hardy and Higgins, 1992). The hypothesis attributed dementia to nerve cell death caused by the toxicity of large insoluble amyloid fibrils. The straightforward therapeutic consequences were appealing: clear away fibrils, block nerve cell death, eliminate dementia.
The hypothesis was in harmony with precedents from a wide range of degenerative diseases involving various amyloids. Amyloid is a generic term that refers to fibrillar protein assemblies that bind dyes such as Congo Red or Thioflavin T and show birefringence under polarized light. While the “amyloid” in Alzheimer’s-affected brain comprises fibrils of a peptide called amyloid beta (Aβ), a number of proteins and peptides other than Aβ also produce amyloids associated with more than two dozen different disease states. These include Parkinson’s disease, Creutzfeld-Jacob and related prionoses, and type 2 diabetes (Ferreira et al., 2007). A link between amyloid deposits and AD pathogenesis is thus intuitively appealing.
Support for the original amyloid cascade hypothesis was substantial. Opening the door were two seminal discoveries – first, that amyloid fibrils in AD are polymers made from a ∼4.5 kDa peptide (amyloid beta; Aβ; (Glenner and Wong, 1984)) , and second, that Aβ comes from a precursor membrane protein of ∼ 110 kDa (amyloid precursor protein; APP; (Koo et al., 1990)). These molecular-level discoveries were followed by a third major breakthrough -- the discovery that a simple mutation in APP can cause familial AD (FAD;(Goate et al., 1991)). Observations from human genetics earlier determined that some cases of familial AD show linkage to chromosome 21 (Tanzi et al., 1988), which was found to be the locus for the APP gene. An additional indication that APP might play a critical role in AD were the findings that it is overexpressed in Down syndrome (Podlisny et al., 1987), which is caused by an extra chromosome 21, and that individuals with Down syndrome invariably develop AD (Giaccone et al., 1989; Lemere et al., 1996).
Although APP is not overexpressed in AD (Podlisny et al., 1987), its metabolism is altered significantly by mutations that cause FAD. Physiologically, APP is broken down by alternative proteolytic pathways, some of which generate the Aβ peptide (Cole and Vassar, 2008; Gralle and Ferreira, 2007). Despite being the subunit of amyloid fibrils, Aβ is in fact a normal metabolite. However, its production is increased by mutations either in APP or in the γ-secretase protease complex that helps generate Aβ from APP (Bertram et al., 2010; Citron et al., 1997; De Strooper et al., 2010; Goate et al., 1991; Scheuner et al., 1996; Selkoe, 2000; Sherrington et al., 1995). The increase in Aβ characteristically is for a sequence with 42 amino acids. There also is a more abundant species comprising 40 amino acids, which is less hydrophobic, not as prone to self-association, and whose concentration correlates less well with AD. Although the FAD pool represents only a small fraction of all AD patients, less than 10%, Aβ also is elevated in sporadic, late-onset disease. What promotes Aβ accumulation in sporadic AD remains largely a mystery (as discussed below).
Besides the evidence from molecular pathology and human genetics, discoveries from cell biology provided further support for the amyloid cascade by linking assemblies of Aβ to neurotoxicity (Kowall et al., 1991; Lorenzo and Yankner, 1994; Pike et al., 1991a; Pike et al., 1991b). Monomeric Aβ is innocuous to cultured neurons, but it becomes neurotoxic upon self-association. This gain of function initially was found to be accompanied by a robust accumulation of Aβ fibrils (Lorenzo and Yankner, 1994). Nonetheless, despite strong evidence indicating that deposition of toxic amyloid fibrils causes AD, some neuropathologists rejected the hypothesis, emphasizing that a poor correlation exists between plaque load and cognitive function (Terry et al., 1994).
In fact, while toxicity does depend on Aβ self-association, fibrils are not the only toxin formed by Aβ, and probably not the most significant ones. As discussed below, it now appears the most pathogenically relevant Aβ-derived toxins are small soluble oligomers, not readily detected. Moreover, the disease-initiating pathology does not appear to be nerve cell death but rather an oligomer-induced disruption of the mechanisms of synaptic plasticity.
Amyloid and nerve cell death are not the primary disease-causing pathologies
As early as 1975, Scheibel and colleagues introduced an interesting concept presaging the modern view of dementia mechanisms (Scheibel et al., 1975). Investigating neuronal morphology in the aging human brain, Scheibel found that neurons in demented individuals showed drastic deterioration in their dendritic arbors. Scheibel proposed that senility is a function of damaged neuropil, arguing it to be at least equal in importance to nerve cell death. A classic study by Terry, Masliah and colleagues subsequently confirmed that synapse loss is the best pathological correlate of Alzheimer’s dementia (Terry et al., 1991).
Synapse deterioration manifests in transgenic mouse AD models as well as in human AD brain. Significantly, in comparing several mouse lines carrying APP transgenes, Mucke and colleague found that synapse loss does not require the presence of amyloid deposits (Mucke et al., 2000). This disconnect between amyloid and disease state extends to behavior. In two startling observations, Dodart, Paul and colleagues found that memory loss was reversed in APP tg-mice injected with antibodies against Aβ and that this reversal, moreover, did not require removal of amyloid plaques (Dodart et al., 2002). Similar findings with a different memory task, antibody, and animal model were obtained by Ashe’s group (Kotilinek et al., 2002). Thus, while Aβ is essential for memory loss, the fibrillar Aβ in amyloid deposits is not the agent. Amyloid in fact is a poor correlate of disease state in AD models as in humans (Terry et al., 1991),
An earlier clue strongly indicating a disconnect between amyloid and Aβ-dependent pathogenesis was reported by Oda, Finch and colleagues (Oda et al., 1994; Oda et al., 1995). In toxicology experiments with the PC12 pheochromocytoma cell line, they looked at the effect of exposing Aβ to clusterin (apoJ), a protein that is elevated in AD and that occurs in amyloid deposits. Surprisingly, rather than promoting amyloidogenesis, clusterin was found to prevent it. Even more surprisingly, clusterin-Aβ solutions appeared to be more cytotoxic than amyloid-containing controls.
Memory loss is a disruption of synaptic plasticity: the oligomer hypothesis
Investigation into the action of clusterin by Lambert, Klein and colleagues established that, concomitant with inhibition of amyloid formation, clusterin promotes accumulation of soluble Aβ oligomers (Lambert et al., 1998). Small oligomers were evident by gel electrophoresis in the presence of SDS, with larger SDS-unstable species also present in aqueous buffer. Imaging the oligomers by atomic force microscopy showed globular proteins a few nanometers in diameter, about the dimensions of average-sized globular proteins. Oligomers were not in complexes with clusterin, and they also formed in the absence of clusterin when solutions contained very low levels of Aβ. The oligomers were relatively long-lived, not converting into amyloid over periods of several days.
Most importantly, these amyloid-free Aβ oligomers were potent CNS neurotoxins. In a discovery providing the first molecular-level insight into AD memory loss, oligomers were found to inhibit functional synaptic plasticity (Lambert et al., 1998). Long-term potentiation (LTP) was inhibited within minutes. This rapid loss of LTP, measured in rat brain slices and in situ in mice, occurred without impact on baseline excitability, indicating impairment of signaling rather than degeneration. With chronic exposure, neurons ultimately were killed. Death was selective for subpopulations of vulnerable neurons and was prevented by knockout of Fyn, a protein tyrosine kinase linked to NMDA receptor signaling. Consistent with dependence on signal transduction, toxicity required maturation of the hippocampus and association of oligomers with protease-sensitive cell surface toxin receptors.
These findings led to a new hypothesis for the role of Aβ in Alzheimer’s disease, the “oligomer hypothesis.” Memory loss, beginning early in the disease, was attributed to oligomer-induced disruption of synaptic plasticity, with later stages of dementia attributed to oligomer-induced cellular degeneration and death. Based on a central role for impaired signaling, the oligomer hypothesis predicted that early memory loss should be reversible. This prediction was confirmed in the transgenic mouse experiments mentioned above (Dodart et al., 2002; Kotilinek et al., 2002). The impact of oligomers is in harmony with recent findings that clusterin, which prevents amyloid formation and promotes oligomer formation, is an AD risk factor (Harold et al., 2009; Lambert et al., 2009; Thambisetty et al., 2010).The conclusion that Aβ oligomers can be neurologically significant toxins, possibly the most important ones for AD, is now supported by more than a decade of further investigation, with over 1,000 papers addressing the oligomer hypothesis.
Clinical relevance: build up of toxic oligomers in AD and AD animal models
The oligomer hypothesis evolved from experiments with synthetic preparations applied to experimental models. Its clinical relevance has been established by evidence that equivalent oligomers accumulate in AD-affected human brain and animal AD models.
Although oligomers assemble from soluble Aβ monomers, which are abundant in normal brain tissue, their detection proved feasible through development of sensitive, conformation-dependent antibodies. These antibodies target oligomers without binding Aβ monomers (Kayed et al., 2003; Lambert et al., 2007; Lambert et al., 2001). Dot immunoblots of soluble extracts from human brain show major increases in oligomers in AD-affected tissue (Gong et al., 2003; Kayed et al., 2003; Lambert et al., 2001). Immunohistochemistry confirms that oligomers associate with neurons, accumulating very early in disease progression (Lacor et al., 2004), in loci distinct from amyloid deposits (Kayed et al., 2003). Recent studies have shown that oligomers also manifest in the Aβ-related muscle disease inclusion body myositis (Nogalska et al., 2010). Furthermore, oligomers accumulate with age in diseased brain of essentially all tg AD models examined so far, including mouse, rat, and C. elegans (Chang et al., 2003; Kotilinek et al., 2002; Leon et al., 2010; Wu et al., 2006) for reviews of tg models used in AD research, including their limitations, see (Wisniewski and Sigurdsson, 2010) and (Ashe and Zahs, 2010)).
Synthetic and brain-derived oligomers appear structurally equivalent, consistent with the ability of conformation sensitive antibodies generated against oligomers formed in vitro to bind oligomers formed in situ. Analyzed by two-dimensional gel electrophoresis, synthetic and brain-derived oligomer show biochemical identity (Gong et al., 2003). Although synthetic oligomers have a tendency to clump into large aggregates, this is fostered by high peptide concentrations required by relatively insensitive analytical assays (Hepler et al., 2006). The toxicology of synthetic and brain-derived oligomers also appears parallel, and effects of brain-derived oligomers are prevented by conformation-sensitive antibodies (De Felice et al., 2008). The ability of synthetic oligomers to mimic the structure and activity of brain-derived species validates their use for experimentation.
One caveat is that oligomers of different apparent sizes have been reported. At present it is uncertain whether one class is more germane to pathogenesis than another. Some groups suggest dimers through tetramers are most critical (Jin et al., 2011; Klyubin et al., 2008; Shankar et al., 2007; Shankar et al., 2008; Townsend et al., 2006; Walsh et al., 2005), while others suggest higher order oligomers are most relevant, including12mers (Gong et al., 2003; Lesne et al., 2006) and 24 mers (Peng et al., 2009). Part of the uncertainty derives from inadequate means to measure size, which is typically determined by SDS-PAGE and Western blots. These assays measure oligomers that are SDS-resistant. The spectrum of SDS-resistant species is not equivalent to the spectrum of oligomers that are stable only in aqueous buffers (Chromy et al., 2003; Lambert et al., 1998). Other factors that affect detection of particular species in gels include choice of antibody, whether samples are heat denatured, and the presence or absence of detergent during protein transfer. Innovative crosslinking approaches (Bernstein et al., 2009; Bitan et al., 2003) have elucidated the stages of in vitro oligomerization, lending support to a prominent 12mer product. Formation of SDS-stable12mers is promoted in vitro by factors likely to be disease-relevant, such as prostaglandins (Boutaud et al., 2006), metal ions (Bush and Tanzi, 2008), and insulin receptor dysfunction (Zhao et al., 2009). The discovery that memory failure in tg mice coincides with the appearance of SDS-stable 12mers is compelling in situ evidence that this species is pathogenic (Lesne et al., 2006). However, extensive evidence also has been marshaled in support of smaller species (Klyubin et al., 2008; Shankar et al., 2008). While the issue of size awaits resolution, it is not unreasonable that oligomers of different sizes could be neurologically relevant, consistent with the generation of multiple pathologies, as considered below. If so, targeting different species may be an important factor in developing treatment strategies.
Typical of novel phenomena, a confusing nomenclature has developed to describe toxic oligomers, e.g., globulomer, Abeta*56, Abeta-o, amylospheroid, protofibril, etc. The term first applied was ADDLs, for Abeta-Derived Diffusible Ligands with dementing action. It was introduced to distinguish toxic oligomers, which are non-fibrillar, from the well-known fibrillar amyloid. By definition, ADDLs and toxic oligomers refer to the same set of pathogenic molecules. Which term is most suitable remains to be settled.
It should be noted that oligomers actually had been found in AD brain extracts several years before the discovery of their toxicity (Frackowiak et al., 1994). At first, however, they were considered irrelevant to pathogenesis, regarded only as indicators of ongoing formation of amyloid, which was considered the actual pathogenic culprit. And in fact, not all oligomers are toxic, even those oligomers of a given size. Subtle conformation changes affect toxicity and immunoreactivity (Chromy et al., 2003; Pitt et al., 2009). However, those oligomers that are toxic appear germane to the disease process, likely at its earliest stages.
It is now known that a large variety of amyloidogenic peptides and proteins that are disease-associated make soluble cytotoxic oligomers. Prominent examples include prion protein in Creutzfeld-Jacob diseases (Martins et al., 2006), islet amyloid polypeptide in diabetes, and α-synuclein in Parkinson’s (Ferreira et al., 2007; Klein, 2006). Cytotoxicity of oligomers thus has emerged as a new basis for understanding pathogenic mechanisms in an array of degenerative diseases. Aβ oligomers simply were the first to be discovered in this new structural class of toxin.
Why do toxic oligomers accumulate?
In FAD, metabolic effects of mutations promote increased levels of total Aβ, or increased Aβ42 to Aβ40 ratio (Borchelt et al., 1996; Citron et al., 1992), conditions that favor oligomerization. Relative levels of Aβ42 to Aβ40 appear to influence the mechanism of oligomerization, with higher levels of Aβ40 in normal individuals likely impeding formation of stable toxic oligomers (Giuffrida et al., 2009).
It is not known why toxic oligomers accumulate in sporadic AD. Most likely, accumulation is a consequence of various factors. Those affecting Aβ clearance may be especially germane, as the Aβ monomer is a metabolite with rapid turnover, determined by extracellular microdialysis in animal models and human subjects (Cirrito et al., 2005; Kang et al., 2007; Kang et al., 2009); Recent findings by Bateman and colleagues show that clearance of Aβ in AD patients is reduced by 30% (Mawuenyega et al., 2010). The mechanisms underlying reduced clearance are not known, but expression of the ApoE4 allele, a major risk factor for sporadic AD (Corder et al., 1993; Schmechel et al., 1993; Strittmatter et al., 1993), may be involved. Individuals homozygous for ApoE4 show an 8-fold elevated risk for AD compared to those with the more common ApoE3 allele. Biochemically, ApoE3 helps clear extracellular Aβ, whereas ApoE4 does not (Yang et al., 1999), but whether this underlies elevated risk has not been determined. Comorbidity factors also are important. For example, individuals with type2 diabetes have an elevated AD risk (Luchsinger, 2008). At the cellular level, deficient insulin signaling results in impaired removal of Aβ (Plum et al., 2005) and Aβ oligomers from the extracellular milieu (Zhao et al., 2009). Somewhat surprisingly, acutely elevated oligomer levels have been noted after exposure to general anesthetics (Eckenhoff et al., 2004). Traumatic brain injury also is associated with acutely elevated levels in CSF, with a positive correlation between oligomer levels and severity of sequelae (David Brody, MP Lambert, and WL Klein, unpublished). This is in harmony with rapid plaque formation seen in about 30% of brain injury patients (Johnson et al., 2009). Association with injury is consistent with APP, a trauma-induced protein that is upregulated in the optic nerve in shaken baby syndrome (Gleckman et al., 2000), being the link between mechanical trauma and AD. Suggestions also have been made that AD could be transmissible, like a prion disease (Soto et al., 2006), and it is possible that regulation of APP translation may be stimulated by oligomers themselves, producing a pathogenic spiral (discussed below).
Neurological consequences: Oligomers impair synapse plasticity and learning
The conclusion that oligomers are neurologically active is firmly established for synthetic and metabolically derived oligomers (i.e., obtained from AD brain extracts or from cells transfected with human APP). In hippocampal slices, synthetic oligomers cause rapid LTP inhibition (Barghorn et al., 2005; Fa et al., 2010; Jurgensen et al., 2011; Lambert et al., 1998; Nimmrich et al., 2008; Rammes et al., 2011; Wang et al., 2002). Inhibition also has been observed in a number of experiments using uncharacterized Aβ preparations (Cullen et al., 1997; Freir et al., 2001). In the latter cases, the presence of oligomers can be inferred, as Aβ42 rapidly oligomerizes at 10 nM and presumably lower concentrations (Chang et al., 2003). Remarkably, at sub-nanomolar doses, the effect on LTP is reversed, and Aβ markedly enhances LTP. Discovered by Arancio and colleagues in a detailed analysis (Puzzo et al., 2008), enhancement occurs over a narrow range of concentrations and is associated with presynaptic activation of alpha7 nicotinic receptors. A role for nicotinic receptors in mediating presynaptic Aβ responses had been suggested earlier by experiments with synaptosomes (Dougherty et al., 2003). Functional effects observed at extremely low doses suggest a physiological role for synaptic Aβ. This possibility is consistent with the axonal trafficking of APP to the presynaptic terminal (Koo et al., 1990). Additional electrophysiological effects of soluble Aβ in various states of assembly have now been reported (Varghese et al., 2010). APP also is translated within dendritic terminals (Westmark and Malter, 2007), where the impact of toxic oligomers appears focused ((Lacor et al., 2004; Lacor et al., 2007); discussed below).
Disruption of functional plasticity includes LTD, which is promoted, not inhibited (Shankar et al., 2008; Wang et al., 2002). The overall impact of oligomers thus is depressed synaptic output. Because synaptic depression is linked in development to synapse pruning, it was predicted that oligomers would initiate synapse loss (Wang et al., 2002). This prediction has indeed been confirmed (Lacor et al., 2007), as have the related predictions that oligomers cause dysfunctional trafficking of ionotropic glutamate receptors and metabotropic glutamate receptors (Gong et al., 2003; Lacor et al., 2004).
Plasticity failure has amply been substantiated in situ. Compelling evidence was first provided in cerebral microinjection experiments by Walsh, Rowan, Selkoe and colleagues, who used oligomers from the conditioned medium of APP-transfected cell cultures (Walsh et al., 2002). Their soluble preparations were fibril free, and Aβ monomers were eliminated by insulin degrading enzyme, which proteolyzes monomers but not oligomers. Those oligomers were found to be particularly potent, possibly as a result of oligomerization occurring at low concentrations in culture media. High concentrations of Aβ foster assembly of seeds that catalyze formation of amyloid fibrils, and fibrils have minimal impact on plasticity. Protofibrils derived from Aβ dimers, however, have been shown to cause plasticity dysfunction (O'Nuallain et al., 2010). Small oligomers obtained from AD-affected human brain also are active in plasticity assays (Barry et al., 2011; Klyubin et al., 2008; Li et al., 2011).
Tg-mouse AD models manifest a spontaneous age-onset impairment of LTP (Gong et al., 2004) that presumably is linked to impaired performance in memory tests (Arendash et al., 2001). Functional recovery provided by oligomer-directed antibodies indicates the deficits are induced by toxic oligomers. In an important developmental analysis, Lesne, Ashe and colleagues showed memory failure coincides with extracellular accumulation of SDS-stable12mers (Lesne et al., 2006). The size of these oligomers is identical to prominent species observed in AD-affected human brain (Gong et al., 2003). Involvement of the 12mer was validated by direct tests in which immuno-isolated 12mers injected into brains of wild-type rats caused impairment of spatial memory. Another paradigm, an operant task called the alternating level cyclic ratio (ALCR) test, has been used to confirm oligomer-induced impairment of memory function, although results point to oligomeric species comprising trimers and dimers rather than 12mers (Cleary et al., 2005). Recently, spontaneous impairment of memory has been observed in Aβ transgenic Drosophila, opening powerful new opportunities for genetic studies of toxic mechanisms (Chiang et al., 2010; Iijima et al., 2008).
The oligomer hypothesis of AD memory failure, initially proposed based on the finding that synthetic oligomers inhibit LTP in hippocampal slices, is now supported by strong in situ evidence. Synapse plasticity and memory formation are impaired by intracerebral injections of oligomers, whether made in vitro or in vivo, and transgenic models that are cognitively impaired show significant benefits from immuno-depletion of endogenous oligomers. Oligomers thus arguably can account for the primary aspect of dementia in early AD. As presented next, oligomers can also be linked to the major facets of AD neuropathology.
Synapse-specific and other pathological cellular consequences of ADDL binding to neurons
Once bound to synapses, Aβ oligomers instigate a variety of pathological processes, some of which affect synapses specifically (e.g., inhibition of LTP and interference with mechanisms of plasticity) and some of which appear to cause more general neuronal dysfunction (e.g., tau hyperphosphorylation, impairment of fast axonal transport of organelles). Many of these pathologies appear directly correlated to changes in brain function in AD. A comprehensive review of all cellular functions and pathways affected by Aβ oligomers would be beyond the scope of the present review. Such a task would be further complicated by the fact that published studies have made use of different oligomer preparations (which differ substantially in terms of the composition of oligomer species), in the concentrations and incubations times employed, and in other experimental conditions that conceivably may affect the outcome of the studies, such as brain region and maturation stage of neuronal cultures in vitro.
Some of the most notable effects of Aβ oligomers in terms of their connection to AD-specific neuropathologies are highlighted in Tables 2 and 3. Synapse-specific effects of Aβ oligomers (Table 2) include alterations in neurotransmitter (glutamate/D-serine) levels, down-regulation (due to abnormal trafficking or degradation) of plasticity-related receptors at the plasma membrane, altered plasticity (inhibition of long term potentiation and/or facilitation of long term depression), cytoskeletal abnormalities and synapse loss. More generalized neuronal impacts of Aβ oligomers (Table 3) include Ca2+ dyshomeostasis, oxidative stress and mitochondrial damage, proteasome inhibition, tau accumulation and hyperphosphorylation, impairment of fast axonal transport, ER stress, inhibition of the proteasome, cell cycle re-entry and, ultimately, cell death.
TABLE 2.
Synaptic impact of ADDLs
| Synaptic defects | Refs | |
|---|---|---|
| Alterations in neurotransmitter release/uptake | ||
| Changes in receptor levels or localization | Internalization of NMDARs | |
| Internalization of AMPARs | ||
| Internalization/degr adation of EphB receptors | ||
| Internalization of insulin receptors | ||
| Impairment of plasticity | Inhibition of LTP | |
| Facilitation /potentiation of LTD | ||
| Cytoskeletal abnormalities |
|
|
| Synapse deterioration /elimination | ||
TABLE 3.
Broader cellular impact of ADDLs
| Broader cellular defects | Refs |
|---|---|
| Dysregulation of Ca2+ Homeostasis | |
| Oxidative stress, mitochondrial damage, energy depletion | |
| Tau accumulation and hyperphosphorylation | |
| Inhibition of axonal transport of vesicles and mitochondria | |
| ER stress | |
| Proteasome inhibition | |
| Cell cycle re-entry | |
| Impact (recruitment/activation) on astrocytes and microglia | |
| Neuronal death |
In conclusion, besides directly affecting memory-related processes, the various impacts of Aβ oligomers on neurons have the potential to account for major facets of AD neuropathology (e.g., tau hyperphosphorylation, oxidative stress, synapse loss), supporting the concept that Aβ oligomers provide a unifying mechanism for initiation of AD pathogenesis.
Neuronal insulin resistance induced by Aβ oligomers: AD as a novel form of brain-specific diabetes
In a recent and surprising twist in the Alzheimer field, clinical studies have revealed that type 2 diabetes patients are at higher risk of developing AD (for reviews, see (Carlsson, 2010; Kopf and Frolich, 2009; Riederer et al., 2011)). For several years, however, the basis for this link between diabetes and AD remained elusive. Recent evidence suggests that insulin resistance, a hallmark of diabetes, develops in Alzheimer’s brains (, 2000; Craft et al., 1999; de la Monte, 2009; Moroz et al., 2008; Pedersen and Flynn, 2004; Razay and Wilcock, 1994; Watson and Craft, 2003). This led to the intriguing notion that a form of brain-specific diabetes, also referred to as type 3 diabetes, develops in AD (de la Monte and Wands, 2005; Steen et al., 2005). Supporting this idea, AD-like changes have been observed in experimental models of insulin resistance, including animals fed a high fat diet or receiving intracerebral or IP injections of streptozotocin (Cao et al., 2007; Labak et al., 2010; Lester-Coll et al., 2006; Ma et al., 2009; Pratchayasakul et al., 2011; Tong et al., 2009; Zhang et al., 2009). Further support was provided by studies in AD transgenic mice, in which hyperinsulinemia develops in parallel to accumulation of Aβ deposits and cognitive impairment (Pedersen and Flynn, 2004). Experimentally-induced diabetes moreover causes accelerated cognitive failure in AD mouse models (Cao et al., 2007; Ho et al., 2004; Plaschke et al., 2010; Wang et al., 2010).
Brain insulin signaling declines with age (Frolich et al., 1998; , 1999; Hoyer, 1998; Rivera et al., 2005), the primary risk factor for AD. As noted, an elevated risk for AD also exists in type 2 diabetes patients, who manifest deficient CNS insulin signaling (Matsuzaki et al., 2010; Ott et al., 1999; Toro et al., 2009; Watson and Craft, 2003), likely as a consequence of decreased insulin uptake into the brain following sustained peripheral hyperinsulinemia (Gerozissis et al., 1993; Kaiyala et al., 2000; Schwartz et al., 1990). Significantly, levels of brain insulin and insulin receptors (IRs) are lower in AD (Frolich et al., 1998; Rivera et al., 2005). As a result, brain insulin signaling, which plays important roles in learning and memory (see below), is thought to be impaired in AD.
The molecular mechanisms by which neurons become insulin-resistant in AD are becoming apparent from recent studies using hippocampal cell cultures. Aβ oligomers induce a striking redistribution of IRs, causing their internalization from dendritic membranes and accumulation in the cell body. This renders neurons insulin resistant (De Felice et al., 2009; De Felice et al., 2008; Zhao et al., 2008). Internalization occurs by a mechanism mediated by casein kinase 2 (CK2) and Ca2+/calmodulin-dependent kinase 2 (CaMK II), which also are involved in ADDL-induced internalization of NMDA receptors (De Felice et al., 2009). Removal of IRs from the surface of neurons was subsequently verified in AD brains (Moloney et al., 2010), establishing the pathophysiological significance of the initial cell-based findings.
Recent results show that Aβ oligomers further impact insulin signaling by inducing serine phosphorylation of the insulin receptor substrate (IRS-1) (Bomfim, De Felice and collaborators, submitted). Physiologically, IRS-1 is phosphorylated at tyrosine residues by the tyrosine kinase activity of the IR, which activates downstream signaling in the insulin pathway (Araki et al., 1994; White, 2003). Serine phosphorylation precludes tyrosine phosphorylation of IRS-1, blocking downstream insulin signaling. Insulin signaling in ADDL-attacked neurons thus is effectively blocked by the combined effect of internalization of IRs and aberrant serine phosphorylation of IRS-1.
Although the brain once was considered insulin-insensitive, it is now clear that CNS insulin signaling is important for many aspects of neuronal survival and function (Broughton and Partridge, 2009; de la Monte and Wands, 2005; Reagan, 2007; Zhao and Alkon, 2001; Zhao et al., 2004). IRs are present in the brain, where they are abundantly distributed in synaptic membranes of the cerebral cortex and hippocampus (Abbott et al., 1999; Havrankova et al., 1981; Marks et al., 1990; Unger and Betz, 1998). Neuronal IRs are involved in diverse brain functions including synapse plasticity, learning and memory (Chiu et al., 2008; Craft et al., 1996; Kern et al., 2001; Lee et al., 2005; Park et al., 2000). Therefore, impairment in insulin signaling instigated by Aβ oligomers may be a central mechanism by which memory deficits develop in the earliest stages of AD.
Interestingly, and somewhat surprisingly, insulin itself blocks the pathological binding of Aβ oligomers and the resulting redistribution of IRs and synapse loss (De Felice et al., 2009). The mechanism of protection does not involve direct competition between Aβ oligomers and insulin for a common binding site on the neuronal surface. Rather, it consists of an IR signaling-dependent down-regulation of neuronal ADDL binding sites. The insulin-sensitizing drug rosiglitazone, a PPAR-γ agonist, potentiates the ability of insulin to protect synapses against Aβ oligomers, while a cell-permeant inhibitor of the tyrosine kinase activity of the IR abrogates the protection by insulin. Thus, physiological insulin signaling may play an important role in defending neurons against AD, suggesting the loss of CNS insulin signaling in aging and diabetes is a potential risk factor for AD. Consistent with expectations based on this idea, human subjects have been found to respond to CNS insulin signaling stimulation with enhanced verbal memory performance (Reger et al., 2008a). When given to subjects with early AD, intranasal insulin also improves performance (Reger et al., 2008b) but only at higher doses, consistent with expected insulin resistance and suggesting possible involvement of additional mechanisms other than stimulation of plasticity. The beneficial effect of insulin in AD may derive, at least in part, from an acute decrease in ADDL synaptotoxicity. In harmony with this possibility, it was recently shown that insulin ameliorates Aβ oligomer-induced inhibition of LTP (Lee et al., 2009). Long-term stimulation of CNS insulin signaling potentially could be of further benefit to AD patients by reducing ADDL-induced neuronal deterioration. Besides decreasing ADDL binding, ADDL-induced spine degeneration, oxidative stress and insulin receptor loss (De Felice et al., 2009), stimulation of insulin signaling also protects against accumulation of hyperphosphorylated tau (Escribano et al., 2010), a pathological hallmark of AD that is induced by Aβ oligomers (De Felice et al., 2008).
A possible caveat of the insulin signaling approach in AD therapeutics is that, as noted above, Aβ oligomers induce a marked removal of IRs from synapses, decreasing the responsiveness of nerve cells to insulin. As a consequence, use of insulin or IR agonists might not be the best way to combat AD. To overcome this hurdle, alternative approaches are currently being developed to bypass IRs and activate pathways common to insulin signaling by targeting other receptors, e.g., glucagon-like peptide 1 receptors (Bomfim, Klein, Ferreira De Felice and colleagues, unpublished).
Initiating mechanisms – the targets are synapses
A premise for investigating the effects of exogenous oligomers on memory and neuropathology is that oligomers presumably act from the extracellular milieu. Although consistent with evidence presented below, this premise is not uniformly accepted, largely because of findings that all mouse, rat, and invertebrate transgenic AD models show oligomers that are abundantly intracellular (Oakley et al., 2006; Oddo et al., 2003). A factor that likely contributes to this intracellular abundance in the models is the excessively high level of Aβ fostered by the transgenes.
It is noteworthy that one mouse model clearly mimics the human disease state. In the E693Delta APP mutation discovered in a Japanese kindred (Nishitsuji et al., 2009), the presence of intracellular oligomers is essentially identical in patients and in the transgenic mouse model (Tomiyama et al., 2010). What is especially compelling for the oligomer hypothesis is that this AD phenotype and its mouse model manifest only oligomers, no amyloid plaques.
For the various animal models, it is unknown if oligomers maintain their intracellular localization and attack cells only from within. Evidence indicates, in fact, that a dynamic equilibrium exists between intracellular and extracellular oligomers (Oddo et al., 2003). This possibility is in harmony with the ability of antibodies to reverse intracellular tau and synapse pathology (Chauhan et al., 2008; Oddo et al., 2006) and to provide cognitive benefits (Hillen et al., 2010; Takamura et al., 2011). Extracellular occurrence of oligomers is consistent with the rapid outpouring of Aβ, and perhaps small oligomers, into brain interstitial fluid, measured by microdialysis in mouse models and humans (Cirrito et al., 2005; Kang et al., 2007; Kang et al., 2009). Direct evidence that oligomers do occur extracellularly has come from nanotechnology-based immunoassays. These assays, which can be many orders of magnitude more sensitive than ELISAs, have established an AD-dependent accumulation of oligomers in human CSF (Fukumoto et al., 2010; Gao et al., 2010; Georganopoulou et al., 2005; Haes et al., 2005). The clear difference in CSF levels between AD and normal individuals has not yet been exploited as a biomarker, however, as measuring extremely low oligomer levels is inherently challenging.
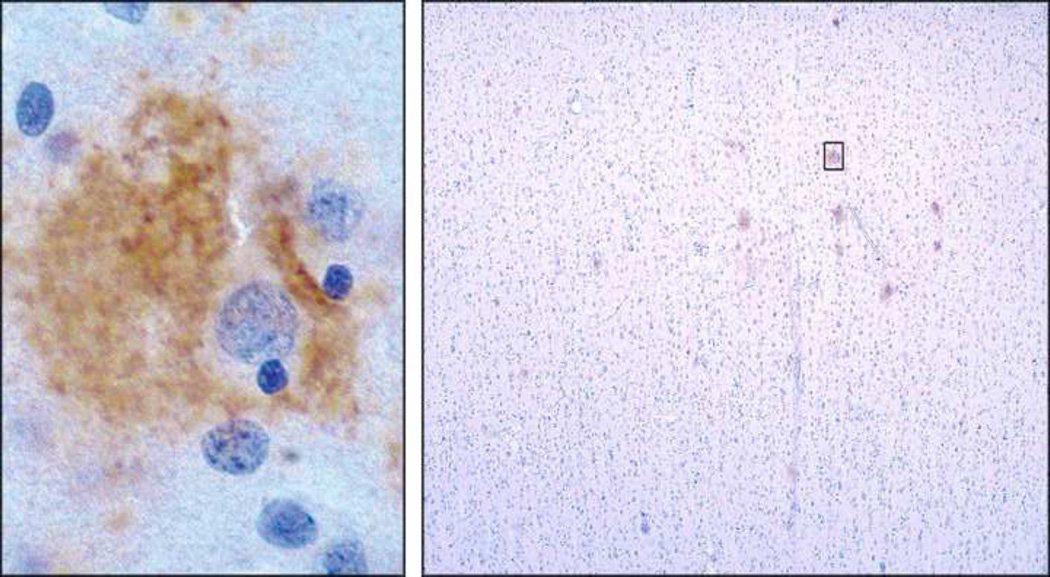
The possible source of extracellular oligomers and how they interact with neurons in human brain are suggested by their peri-somatic localization, observed in early-stage disease (Figure 1, left; (Lacor et al., 2004)). These oligomer-targeted neurons are distributed sparsely, indicating oligomers might originate within the cells they surround (Figure 1, right). While immunohistochemistry cannot determine precise distribution, and some indications from electron microscopy suggest an intracellular distribution (Takahashi et al., 2002), it is clear that oligomers are not abundant in the soma. This is in marked contrast to the pattern in tg-mouse brain. High levels of somatic expression found in transgenic mice may mask any peri-somatic distribution. Importantly, the peri-somatic distribution in human brain is evident in the complete absence of amyloid plaques, indicating oligomer pathology occurs early in the disease process.
Figure 1. Dendritic oligomer pathology occurs early in disease progression.
(Left) Perineuronal localization of oligomers detected using oligomer-specific antibody, enlarged from field at right (box). Stain is associated with dendritic arbor, not somatic cytoplasm. (Right) Low magnification of human brain section stained with anti-oligomer antibody. Pattern indicates association with only scattered individual neurons early in disease, prior to amyloid plaques. Taken from Lacor et al, 2004 (Lacor et al., 2004).
Cell biology experiments indicate the pattern in human brain might be attributable to autocrine-like ligand interactions with sites in the dendritic arbor. Cultured hippocampal neurons incubated with brain-derived oligomers show patterns that recapitulate the in situ distribution (Gong et al., 2003). Dendrites of particular neurons richly manifest bound oligomers. Significantly, some neurons are targeted while others are not, establishing clear-cut specificity. Patterns observed in culture reveal details not evident in tissue sections. Attachment of brain-derived oligomers shows a highly punctate nature. The same binding specificity and punctate pattern is observed for synthetic oligomers, particularly those greater than 50 kDa (Lacor et al., 2004).
Significantly, the sites at which oligomers accumulate in culture comprise synapses, consistent with the rapid and specific effects seen on LTP and LTD. Oligomers show > 90% co-localization with the post-synaptic marker PSD-95 (Lacor et al., 2004). High resolution confocal imaging confirms accumulation of oligomers at dendritic spines (Lacor et al., 2007). Double-labeling with synaptophysin or FM463 establishes this localization to be at functional synapses (Renner et al., 2010). As expected, the pattern of binding depends on cell maturation. Clustering of oligomers at synapses, moreover, shows a cause-and-effect relationship with characteristic Alzheimer neuropathologies. AD type tau hyperphosphorylation (De Felice et al., 2008), redistribution of insulin receptors (De Felice et al., 2009; Zhao et al., 2008), spine loss (Lacor et al., 2007) and oxidative stress (De Felice et al., 2007; Decker et al., 2010a) are manifest exclusively in neurons showing synapses targeted by oligomers. It can be concluded that oligomers are gain-of-function pathogenic ligands that target and disrupt signaling at particular synapses, a specificity that may explain why early AD is a disease of memory.
How spines are targeted by Aβ oligomers – receptors, co-receptors, scaffolds and toxic clusters
A major challenge in AD research has been to determine whether the deleterious impact of Aβ oligomers on neurons is mediated by one or more specific receptors on the neuronal surface and, if so, to identify such receptors. Despite evidence that oligomers bind specifically to dendritic spines, some studies have suggested that, due to their hydrophobic nature, Aβ and oligomers may insert into the neuronal plasma membrane, perturbing normal membrane structure and dynamics and creating large conductance pores that disrupt ion homeostasis (Arispe, 2004; Arispe et al., 1993a; Arispe et al., 1993b; Bhatia et al., 2000; Demuro et al., 2005; Lashuel et al., 2002; Lin et al., 2001). However, mounting and convincing evidence indicates that interaction of Aβ oligomers with synaptic membranes is mediated by cell surface proteins that act as toxin receptors. Supporting this contention, ADDL binding to synapses displays saturable kinetics (Renner et al., 2010) and early studies showed that binding is lost upon controlled trypsin treatment of neurons (Lambert et al., 1998).
A number of candidate oligomer-binding proteins have been proposed in the past few years. A controversial but highly interesting recent study from Lauren, Strittmatter and colleagues showed that Aβ oligomers bind with nanomolar affinity to cellular prion protein (PrPC) and that anti-PrP antibodies reduce oligomer binding to neurons and rescue synaptic plasticity (Lauren et al., 2009). Similarly, oligomer binding is significantly reduced in PrPC knock-out cells (Lauren et al., 2009). These findings suggest that PrPC is involved in synaptic binding and synaptotoxicity of Aβ oligomers (Gimbel et al., 2010). On the other hand, other studies have shown that PrP-expressing and PrP-knockout mice are equally susceptible to long-term memory impairment induced by Aβ oligomers (Balducci et al., 2010; Calella et al., 2010), supporting the notion that, at least in part, the deleterious effects of Aβ oligomers on plasticity are independent of PrPC (Kessels et al., 2010). Although the involvement of PrPC in the synaptic impact of Aβ oligomers is still a matter of debate, it is noteworthy that Aβ oligomers were recently shown to cause a rapid increase in surface exposure and clustering of PrPC in hippocampal neurons and cell lines (Caetano et al., 2011). This suggests that initial ADDL binding recruits more PrPC molecules (presumably by exocytosis of vesicles located proximal to the plasma membrane; (Caetano et al., 2011)) to form a higher order complex that may initiate toxic signaling at the neuronal membrane.
The glutamate receptor family of proteins appears to be centrally involved in neuronal targeting by Aβ oligomers. Co-immunoprecipitation and photoactivated amino acid cross-linking studies indicated that Aβ oligomers interact with complexes containing the GluR2 subunit of AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptors, a conclusion that was corroborated by the observation that pharmacological inhibition or removal of surface AMPA receptors reduced oligomer binding to neurons (Zhao et al., 2010). Moreover, single particle tracking of quantum dot-labeled Aβ oligomers has demonstrated the participation of metabotropic glutamate receptors (mGluR5) in oligomer binding and clustering at synapses (Renner et al., 2010). Additionally, NMDARs co-immunoprecipitate with Aβ oligomers from detergent-extracted oligomer-treated rat synaptosomal membranes, and an N-terminal anti-NR1 antibody markedly reduced oligomer binding to dendrites (De Felice et al., 2007). Consistent with those findings, oligomer binding is virtually abolished in dendrites of NMDAR knock-down neurons (Decker et al., 2010a). As predicted, NMDAR knock-down also abrogates neuronal oxidative stress induced by Aβ oligomers, which is mediated by aberrant activation of NMDARs (De Felice et al., 2007). NMDARs thus are specifically required for pathogenic oligomer binding to dendrites.
Interestingly enough, however NMDARs do not appear to be directly responsible for ADDL binding. In hippocampal cultures, between 50% and 85% of the neurons bind Aβ oligomers, while the others are spared. If NMDARs were directly responsible for ADDL binding, the amount of bound Aβ oligomers should correlate with surface levels of NMDARs. However, the ADDL-attacked and ADDL-spared neurons exhibit similar levels of surface NMDARs (Decker et al., 2010a). Additionally, NMDARs are not down-regulated in insulin-treated neurons, despite decreases in ADDL binding sites induced by insulin (Decker et al., 2010a). These observations indicate that NMDARs are necessary but not sufficient for ADDL binding, and suggest that ADDL binding to synapses may involve the assembly of a multi-protein receptor whose assembly is promoted by NMDARs and likely involves other participants, including PrPC.
Other putative receptors/co-receptors involved in neuronal binding of Aβ oligomers include the p75 neurotrophin receptor (p75NTR) (Knowles et al., 2009), the receptor for advanced glycation end products (RAGE) (Sturchler et al., 2008), nicotinic acetylcholine receptors (Magdesian et al., 2005) and Frizzled (Magdesian et al., 2008). The latter appears particularly noteworthy as Frizzled-mediated Wnt/β-catenin signaling has been shown to play an important role in synaptic plasticity (Chen et al., 2006; Varela-Nallar et al., 2010; Varela-Nallar et al., 2009). In a very interesting recent report, Mucke and colleagues (Cisse et al., 2011) showed that Aβ oligomers bind to and trigger subsequent proteasomal degradation of the receptor tyrosine kinase EphB2. EphB2 receptors interact functionally and physically with NMDARs at synapses, and thus are greatly appealing as candidate components of an ADDL receptor complex. A previous study established that EphB2 receptor levels are reduced in parallel with NMDAR levels in ADDL-treated hippocampal neurons (Lacor et al., 2007).
Novel insight into the dynamics of ADDL interactions with synapses has come from high resolution particle tracking experiments. In these, the movements of individual quantum dot-labeled ADDL molecules were followed after their binding to membranes of living neurons (Renner et al., 2010). Initially, Aβ oligomers diffused as if bound to an untethered protein. Within minutes, however, movement slowed and oligomers became immobilized, typically at active synapses labeled with FM4-64. The synaptic trapping of oligomers was accompanied by co-clustering and immobilization of mGluR5 metabotropic glutamate receptors.
What emerges from current evidence is that Aβ oligomers first bind to the neuronal plasma membrane at freely moving receptors, but lateral diffusion leads to interactions between Aβ oligomers and membrane proteins such as mGluR5 that ultimately target Aβ oligomers to synaptic sites. At synapses, the relatively diffusible complex between Aβ oligomers and mGluR5 (or other component) becomes trapped in a larger, diffusion-restricted complex whose assembly is organized by key synaptic proteins. These “organizers” likely include NMDARs and PrPC, explaining the finding that knocking-down these proteins or adding antibodies against their extracellular domains blocks the accumulation of ADDL clusters (“hotspots”) at synapses (De Felice et al., 2007; Decker et al., 2010a; Lauren et al., 2009). In this regard, it is interesting to note that PrPC has been proposed to act as a cell-surface platform that orchestrates several physiological signaling pathways at the neuronal plasma membrane (for reviews, see (Linden et al., 2008; Martins et al., 2010)). Interaction with Aβ oligomers could potentially dysregulate such signaling pathways, resulting in neurotoxicity. On the other hand, NMDARs are known to < interact physically with a number of proteins in the post-synaptic density. Of particular relevance may be the fact that NMDARs are anchored by PSD-95, which acts as a scaffold to organize multiple membrane-associated proteins at synapses (Kennedy, 2000; Kim and Sheng, 2004; Siekevitz, 1985; Ziff, 1997). Both PrPC and NMDARs are thus poised to act as platforms that drive the assembly of a multi-protein complex responsible for the accumulation of synaptotoxic ADDL clusters.
Oligomer-induced Ca++ elevation: Consequences and a novel triggering mechanism
The mechanism of oligomer synapto-toxicity appears to be tied closely to accumulation of excessive levels of Ca++ (Marx, 2007), consistent with involvement of NMDA and mGluR5 receptors. One rapid consequence is an aberrant expression of Arc (Activity-Regulated Cytoskeletal associated protein; (Lacor et al., 2004)). Arc is an immediate early gene product that is translated from mRNA in dendritic spines (Bramham, 2008). Synaptically controlled transient expression of Arc is essential for LTP and memory formation (Guzowski et al., 2000; Kelly and Deadwyler, 2003). Within minutes of exposure to oligomers, neurons show a localized over-expression of Arc, which later spreads ectopically throughout the dendrite. Because Arc is an actin binding protein, its aberrant expression was predicted to disrupt receptor trafficking (Lacor et al., 2004), which could consequently disrupt plasticity (Gong et al., 2003). This prediction was first confirmed for NMDA-Rs (Lacor et al., 2004; Lacor et al., 2007; Snyder et al., 2005). NMDA-R down-regulation appears to explain why glutamate stimulation of the CREB pathway is impaired by oligomers (Tong et al., 2001; Vitolo et al., 2002). Receptor downregulation has been extended to other plasticity-associated receptors, including EphB2 receptors (Lacor et al., 2007), AMPA-Rs (Jurgensen et al., 2011; Zhao et al., 2010), and insulin Rs (Zhao et al., 2008). Although receptors are lost from the surface, total levels are at first unchanged, indicating dysfunctional trafficking, and pharmacological experiments indicate involvement of CaMKII and casein kinase II (De Felice et al., 2009). These receptor pathologies discovered in cell culture have been confirmed in tg-mouse models and human AD brain (Cisse et al., 2011; Moloney et al., 2010).
A second prediction based on altered Arc expression is that oligomers would induce aberrant dendritic spine morphology, and this also has been confirmed in culture and in situ (Lacor et al., 2007; Ma et al., 2009). Spines after short-term exposure to oligomers are long and spindly, resembling immature spines that express low levels of AMPA-Rs, as found early in development. Such aberrant spines also are seen in traumatic brain injury and prionoses (Fiala et al., 2002). Eventually, spines deteriorate and synapses are lost, consistent with low levels of Arc found in mature tg-mice (Chin et al., 2005). Another actin binding protein found in spines, drebrin, also becomes greatly diminished in culture (Lacor et al., 2007) and in tg-mice (Calon et al., 2004). Synapse deterioration in tg-mice is prevented by ICV injections of oligomer-specific antibodies (Chauhan, 2007; Oddo et al., 2006).
Underlying the mechanism of synapse deterioration is hyperactive glutamatergic signaling. Excessive Ca++ caused by Aβ oligomers is partially inhibited by NMDA-R antagonists, including the clinically approved AD drug Namenda (De Felice et al., 2007). This reduction is sufficient to inhibit synapse deterioration (Lacor et al., 2007). Interestingly, protection also is afforded by antagonists of metabotropic mGluR5 receptors (Rammes et al., 2011; Renner et al., 2010; Wang et al., 2004). Consistent with action upstream in the mechanism, mGluR5 stimulation is known to enhance NMDA-R responses (Blaabjerg et al., 2001). The profound impact of oligomers on mGluR5 mobility and the induced formation of mGluR5 clusters appears germane to the mechanism by which Ca++ is elevated to synapto-toxic levels. Clustering can be elicited by mGluR5 antibodies, and antibody-induced clustering mimics the effects of oligomers in causing elevated Ca++ and synapse deterioration. Whether oligomers act directly on mGluR5 receptors, like antibodies, or indirectly through interactions with other scaffolding molecules, it appears that it is the clustering that causes synapto-toxic mGluR5 hyperactivity. This mechanism is in many respects analogous to the clustering of receptors at immunological synapses during T cell activation (Douglass and Vale, 2005), with the fundamental difference that oligomer-induced clustering is toxic.
Comparison with another disorder of learning and memory: AD and Fragile X Syndrome show striking similarities
Alzheimer’s drastically affects learning and memory in older individuals. Its emerging molecular mechanisms, however, show parallels with those of the leading heritable cause of mental retardation in childhood, Fragile X Syndrome. Fragile X is a developmental disorder caused by inability to express the regulatory protein FMRP. FMRP participates in the trafficking and expression of mRNAs that target spines. Its control of Arc is a prime example (Iacoangeli et al., 2008). As in oligomer-exposed neurons, neurons in Fragile X ectopically overexpress Arc (Zalfa et al., 2003). Additional synaptic pathologies instigated by oligomers also are present in Fragile X, including aberrant spine morphology, loss of NMDA and AMPA receptors, and elevated LTD (Bear et al., 2004). Fragile X also leads to hyperactivity in the protein phosphatase PP2a (Kim et al., 2008) which inactivates MAPK, an enzyme with impaired activity in oligomer-exposed neurons (Tong et al., 2001).
A particularly intriguing commonality between Fragile X and oligomer synaptotoxicity is the involvement of mGluR5 receptors. In healthy neurons, Ca++ mobilization by mGluR5 leads to dephosphorylation of FMRP, relieving FMRP repression of translation (Bear et al., 2004). Excessive mGluR5 activity is prevented by FMRP itself through a negative feedback loop. In the absence of FMRP, mGluR5 is hyperactive, with the consequences detailed above, and mGluR5 antagonists have been suggested as an approach to Fragile X treatment. Hyperactivity of mGluR5 receptors caused by oligomers is due to a clustering mechanism, as described earlier, and this receptor state hypothetically could be insensitive to negative feedback by FMRP. The consequent chronic mGluR5 activity would keep FMRP dephosphorylated and eliminate the ability of FMRP to repress mRNA translation. This impact of oligomers would be functionally equivalent to eliminating FMRP protein.
Intriguingly, the relationship between mGluR5 hyperactivity and FMRP may also be related to the accumulation of oligomers in AD. mGluR5, through its physiological inactivation of FMRP, has been reported to stimulate translation of APP (Westmark and Malter, 2007). Oligomer-induced hyperactivity of mGluR5 in Alzheimer’s disease would thus promote production of even more Aβ and oligomers. Coupled with the potential of dendrites for activity-stimulated exocytosis (Kennedy et al., 2010), such a phenomenon could account for the “perineuronal deposits” of oligomers seen in Figure 1 (Lacor et al., 2004). A positive feedback loop in dendrites theoretically could provide a basis for a progressive self-stimulating disease mechanism at the level of individual neurons.
Although by no means identical, the behavioral and cognitive abnormalities in AD and Fragile X appear to have common roots at the molecular and synaptic level. Mechanistic similarities may even be sufficient to regard AD theoretically as a type of autism spectrum disorder that shows age-onset and, conversely, to ask if Fragile X might be a very early manifestation of oligomer-induced disease. While there is no direct evidence to support this speculation, the absence of FMRP and excessive mGluR5 activity in Fragile X hypothetically could stimulate overexpression of APP and local accumulation of toxic oligomers.
Promise of therapeutics
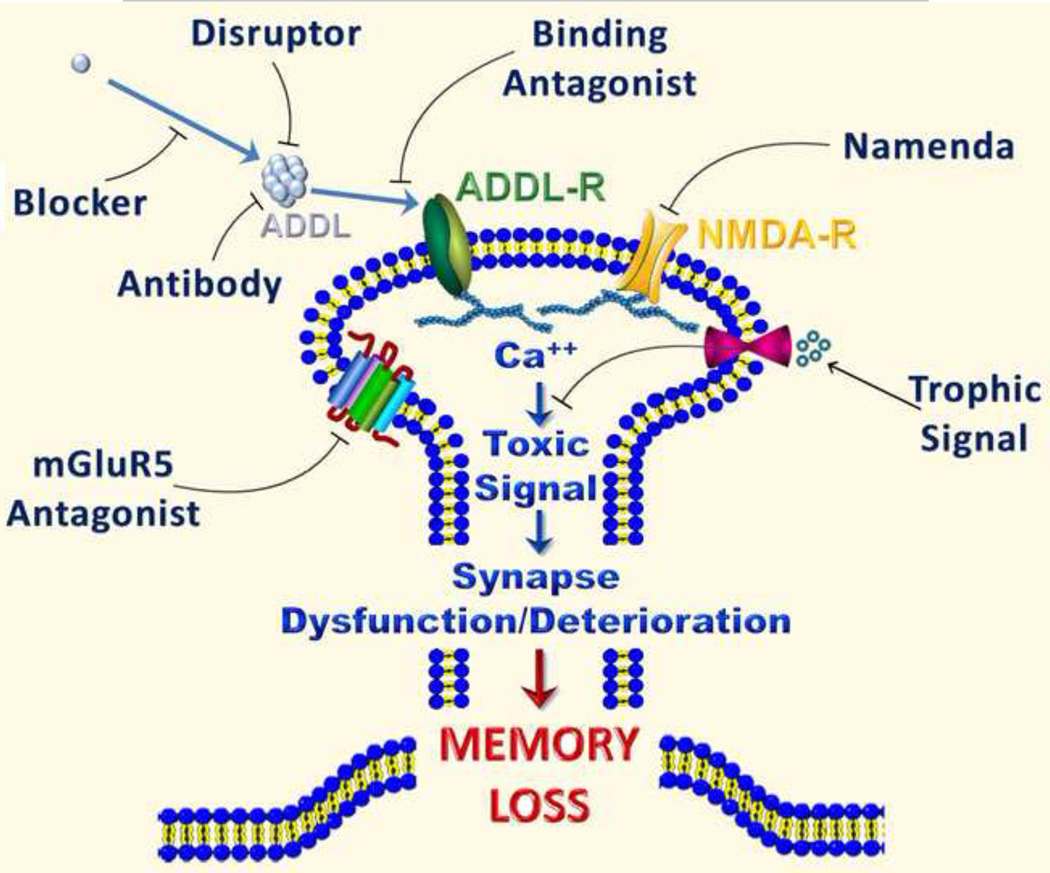
Studies reviewed above suggest a number of targets that might be exploited towards the development of novel and effective therapeutics for AD (Figure 2) or the mild cognitive impairment that is often a prodrome to AD (Allain et al., 2007). In particular, the discovery that insulin resistance plays an important role in the pathogenesis of AD suggests that strategies aimed to bolster brain insulin signaling might be beneficial in preventing synapse failure and neuronal damage in AD. Also of interest, a connection has recently been established between dopaminergic signaling and AD, with demonstration that selective activation of D1/D5 dopamine receptors prevents pathological changes in synapse composition and function (including inhibition of LTP) induced by Aβ oligomers (Jurgensen et al., 2011). One of the implications of this finding is that D1/D5 receptors may constitute a target for the development of novel approaches to combat synapse failure in AD. In addition, the recent finding that mGluR5 is implicated in the toxic clustering of Aβ oligomers at synapses and the ensuing aberrant calcium signaling (Renner et al., 2010) suggests that selective mGluR5 inhibitors may hold potential to block oligomer-induced synapse dysfunction. While these and other possible pharmacological targets are being explored, there is also reason to believe that non-pharmacological approaches based on these novel findings might be beneficial to ameliorate AD symptoms (Schaeffer et al., 2009). For example, exercise and a balanced diet might have positive effects on both peripheral and brain insulin signaling. In addition, dopaminergic neurotransmission, centrally implicated in the brain reward system and known to decline with aging (De Keyser et al., 1990), plays important roles in non-pharmacological strategies aimed to improve age-related cognitive decline, such as environmental enrichment and physical exercise (van Praag et al., 2000). In the current absence of effective pharmacological treatments, keeping a physically and mentally active, stimulating lifestyle may be the best approach to warding off Alzheimer’s.
Figure 2.
Drug targets emerging from the oligomer hypothesis for Alzheimer' disease.
Highlights.
Abeta oligomers comprise a variety species ranging from dimers to 12mers or higher. -The molecular identity of the oligomer species that cause synapse failure is still a matter of debate. -Oligomers target excitatory synapses and bind to a receptor complex that involves NMDA and mGluR5 receptors. -Oligomers instigate AD pathology and provide a unifying basis for elucidating pathogenesis and developing therapeutics.
Acknowledgements
WLK is co-founder of Acumen Pharmaceuticals, which has been licensed by Northwestern University to target Aβ oligomers for Alzheimer’s therapeutics and diagnostics. Work in the authors’ laboratories has been funded by grants from NIH, NSF, the American Health Assistance Foundation, and the Alzheimer’s Association (WLK), and by the Brazilian agencies Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Fundacao de Amparo `a Pesquisa do Estado do Rio de Janeiro and National Institute of Translational Neuroscience (STF). We wish to acknowledge Kirsten L. Viola for her assistance with preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sergio T Ferreira, Institute of Medical Biochemistry, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941-590, Brazil, ferreira@bioqmer.ufrj.br.
William L Klein, Department of Neurobiology, Cognitive Neurology and Alzheimer’s Disease Center, Northwestern University, Evanston, IL 60208, wklein@northwestern.edu.
Reference List
- Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J.Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajit D, Udan ML, Paranjape G, Nichols MR. Amyloid-beta(1–42) fibrillar precursors are optimal for inducing tumor necrosis factor-alpha production in the THP-1 human monocytic cell line. Biochemistry. 2009;48:9011–9021. doi: 10.1021/bi9003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Cavaliere F, Perez-Samartin A, Zugaza JL, Trullas R, Domercq M, Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Allain H, Bentue-Ferrer D, Akwa Y. Treatment of the mild cognitive impairment (MCI) Hum.Psychopharmacol. 2007;22:189–197. doi: 10.1002/hup.838. [DOI] [PubMed] [Google Scholar]
- Allsop D, Wong CW, Ikeda S, Landon M, Kidd M, Glenner GG. Immunohistochemical evidence for the derivation of a peptide ligand from the amyloid beta-protein precursor of Alzheimer disease. Proc.Natl.Acad.Sci.U.S.A. 1988;85:2790–2794. doi: 10.1073/pnas.85.8.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol.Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2010 Alzheimer's Disease Facts and Figures. Alzheimer's Association. 2010 doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. What is Alzheimer's. Alzheimer's Association. 2011 [Google Scholar]
- Apostolova LG, Cummings JL. The pathogenesis of Alzheimer's disease: General Overview. In: Uversky VN, Fink A, editors. Protein misfolding, aggregation, and conformational diseases. New York: Springer Science + Business Media, LLC; 2007. pp. 3–29. [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- Arispe N. Architecture of the Alzheimer's A beta P ion channel pore. J.Membr.Biol. 2004;197:33–48. doi: 10.1007/s00232-003-0638-7. [DOI] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1–40)] in bilayer membranes. Proc.Natl.Acad.Sci.U.S.A. 1993a;90:10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc.Natl.Acad.Sci.U.S.A. 1993b;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc.Natl.Acad.Sci.U.S.A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer's disease. J.Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J.Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, Bowers MT. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nat.Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti V, Osorio RS, Mosconi L, Li Y, De SS, de Leon MJ. Early detection of Alzheimer's disease with PET imaging. Neurodegener.Dis. 2010;7:131–135. doi: 10.1159/000289222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Lin H, Lal R. Fresh and globular amyloid beta protein (1–42) induces rapid cellular degeneration: evidence for AbetaP channel-mediated cellular toxicity. FASEB J. 2000;14:1233–1243. doi: 10.1096/fasebj.14.9.1233. [DOI] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc.Natl.Acad.Sci.U.S.A. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaabjerg M, Kristensen BW, Bonde C, Zimmer J. The metabotropic glutamate receptor agonist 1S,3R-ACPD stimulates and modulates NMDA receptor mediated excitotoxicity in organotypic hippocampal slice cultures. Brain Res. 2001;898:91–104. doi: 10.1016/s0006-8993(01)02148-5. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. PGH2-derived levuglandin adducts increase the neurotoxicity of amyloid beta1–42. J.Neurochem. 2006;96:917–923. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Jansen EN, Bratzke H, Braak E. Neuropathological hallmarks of Alzheimer's and Parkinson's diseases. Prog.Brain Res. 1998;117:267–285. doi: 10.1016/s0079-6123(08)64021-2. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr.Opin.Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Brito-Moreira J, Paula-Lima AC, Bomfim TR, Oliveira FB, Sepulveda FJ, De Mello FG, Aguayo LG, Panizzutti R, Ferreira ST. Abeta Oligomers Induce Glutamate Release from Hippocampal Neurons. Curr.Alzheimer Res. 2011 doi: 10.2174/156720511796391917. [DOI] [PubMed] [Google Scholar]
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem.J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Bush AI, Tanzi RE. Therapeutics for Alzheimer's disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, LaFontaine MA. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer's disease and Huntington's disease. Curr.Med.Chem. 2001;8:815–828. doi: 10.2174/0929867013373048. [DOI] [PubMed] [Google Scholar]
- Caetano FA, Beraldo FH, Hajj GN, Guimaraes AL, Jurgensen S, Wasilewska-Sampaio AP, Hirata PH, Souza I, Machado CF, Wong DY, De Felice FG, Ferreira ST, Prado VF, Jane RR, Martins VR, Prado MA. Amyloid-beta oligomers increase the localization of prion protein at the cell surface. J.Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07225.x. [DOI] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol.Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr., Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J.Biol.Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J.Alzheimers Dis. 2010;20:711–722. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, Farias GG, Godoy JA, Fuenzalida M, Bonansco C, Inestrosa NC. Wnt-5a occludes Abeta oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol.Neurodegener. 2010;5:3. doi: 10.1186/1750-1326-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafekar SM, Hoozemans JJ, Zwart R, Baas F, Scheper W. Abeta 1–42 induces mild endoplasmic reticulum stress in an aggregation state-dependent manner. Antioxid.Redox.Signal. 2007;9:2245–2254. doi: 10.1089/ars.2007.1797. [DOI] [PubMed] [Google Scholar]
- Chang L, Bakhos L, Wang Z, Venton DL, Klein WL. Femtomole immunodetection of synthetic and endogenous amyloid-beta oligomers and its application to Alzheimer's disease drug candidate screening. J.Mol.Neurosci. 2003;20:305–313. doi: 10.1385/JMN:20:3:305. [DOI] [PubMed] [Google Scholar]
- Chauhan NB. Intracerebroventricular passive immunization with anti-oligoAbeta antibody in TgCRND8. J.Neurosci.Res. 2007;85:451–463. doi: 10.1002/jnr.21110. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Sandoval J, Lambert M, Klein W. ICV Anti-ADDL Antibody Prevented Early Synaptic Deficits in TgCRND8 Mouse Model of Alzheimer's Disease. 2008 [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J.Biol.Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Chiang HC, Wang L, Xie Z, Yau A, Zhong Y. PI3 kinase signaling is involved in Abeta-induced memory loss in Drosophila. Proc.Natl.Acad.Sci.U.S.A. 2010;107:7060–7065. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J.Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL. Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, Ho K, Yu GQ, Mucke L. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George HP, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat.Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat.Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J.Biol.Chem. 2008;283:29621–29625. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann.N.Y.Acad.Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K. Insulin metabolism in Alzheimer's disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70:146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol.Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J.Biol.Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc.Natl.Acad.Sci.U.S.A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J, Acton PJ, Shughrue PJ, Chen-Dodson E, Kinney GG, Klein WL. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol.Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser J, Ebinger G, Vauquelin G. D1-dopamine receptor abnormality in frontal cortex points to a functional alteration of cortical cell membranes in Alzheimer's disease. Arch.Neurol. 1990;47:761–763. doi: 10.1001/archneur.1990.00530070055011. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Insulin resistance and Alzheimer's disease. BMB.Rep. 2009;42:475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J.Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat.Rev.Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, Epstein AL, De Felice FG, Jerusalinsky D, Ferreira ST. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-beta peptide oligomers. J.Neurochem. 2010a;115:1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J.Neurosci. 2010b;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J.Biol.Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G. Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J.Neurosci.Res. 2010;88:2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat.Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Doi Y, Mizuno T, Maki Y, Jin S, Mizoguchi H, Ikeyama M, Doi M, Michikawa M, Takeuchi H, Suzumura A. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer's disease. Am.J.Pathol. 2009;175:2121–2132. doi: 10.2353/ajpath.2009.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J.Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez dM, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer's transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fa M, Orozco IJ, Francis YI, Saeed F, Gong Y, Arancio O. Preparation of oligomeric beta-amyloid 1–42 and induction of synaptic plasticity impairment on hippocampal slices. J.Vis.Exp. 2010 doi: 10.3791/1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB.Life. 2007;59:332–345. doi: 10.1080/15216540701283882. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res.Brain Res.Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Fifre A, Sponne I, Koziel V, Kriem B, Yen Potin FT, Bihain BE, Olivier JL, Oster T, Pillot T. Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis during soluble amyloid beta-peptide-induced neuronal apoptosis. Synergistic involvement of calpain and caspase-3. J.Biol.Chem. 2006;281:229–240. doi: 10.1074/jbc.M507378200. [DOI] [PubMed] [Google Scholar]
- Finch CE, Longo V, Miyal A, Morgan TE, Rozovsky I, Soong Y, Wei M, Xie Z, Zanjani H. Inflammation in Alzheimer's disease. In: Chesselet MF, editor. Molecular Mechanisms of Neurodegenerative Diseases. Totowa: Humana Press; 2001. pp. 87–110. [Google Scholar]
- Frackowiak J, Zoltowska A, Wisniewski HM. Non-fibrillar beta-amyloid protein is associated with smooth muscle cells of vessel walls in Alzheimer disease. J.Neuropathol.Exp.Neurol. 1994;53:637–645. doi: 10.1097/00005072-199411000-00011. [DOI] [PubMed] [Google Scholar]
- Freir DB, Holscher C, Herron CE. Blockade of long-term potentiation by beta-amyloid peptides in the CA1 region of the rat hippocampus in vivo. J.Neurophysiol. 2001;85:708–713. doi: 10.1152/jn.2001.85.2.708. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J.Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Riederer P, Hoyer S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Ann.N.Y.Acad.Sci. 1999;893:290–293. doi: 10.1111/j.1749-6632.1999.tb07839.x. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010;24:2716–2726. doi: 10.1096/fj.09-150359. [DOI] [PubMed] [Google Scholar]
- Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, Zuckermann RN, Connolly MD, Hansson O, Minthon L, Zetterberg H, Blennow K, Fedynyshyn JP, Allauzen S. Abeta40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer's disease. PLoS.One. 2010;5:e15725. doi: 10.1371/journal.pone.0015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc.Natl.Acad.Sci.U.S.A. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerozissis K, Orosco M, Rouch C, Nicolaidis S. Basal and hyperinsulinemia-induced immunoreactive hypothalamic insulin changes in lean and genetically obese Zucker rats revealed by microdialysis. Brain Res. 1993;611:258–263. doi: 10.1016/0006-8993(93)90511-k. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Tagliavini F, Linoli G, Bouras C, Frigerio L, Frangione B, Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci.Lett. 1989;97:232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J.Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J.Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleckman AM, Evans RJ, Bell MD, Smith TW. Optic nerve damage in shaken baby syndrome: detection by beta-amyloid precursor protein immunohistochemistry. Arch.Pathol.Lab Med. 2000;124:251–256. doi: 10.5858/2000-124-0251-ONDISB. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem.Biophys.Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J.Clin.Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Niidome T, Akaike A, Kihara T, Sugimoto H. Amyloid beta-peptide preconditioning reduces glutamate-induced neurotoxicity by promoting endocytosis of NMDA receptor. Biochem.Biophys.Res.Commun. 2006;351:259–265. doi: 10.1016/j.bbrc.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog.Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J.Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haes AJ, Chang L, Klein WL, Van Duyne RP. Detection of a biomarker for Alzheimer's disease from synthetic and clinical samples using a nanoscale optical biosensor. J.Am.Chem.Soc. 2005;127:2264–2271. doi: 10.1021/ja044087q. [DOI] [PubMed] [Google Scholar]
- Hampel H, Broich K, Hoessler Y, Pantel J. Biological markers for early detection and pharmacological treatment of Alzheimer's disease. Dialogues.Clin.Neurosci. 2009;11:141–157. doi: 10.31887/DCNS.2009.11.2/hhampel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K. Biological markers of amyloid beta-related mechanisms in Alzheimer's disease. Exp.Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wchmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Wlliams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat.Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20(Suppl):268–273. [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch.Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, Kinney G, Joyce JG. Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry. 2006;45:15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- Hillen H, Barghorn S, Striebinger A, Labkovsky B, Muller R, Nimmrich V, Nolte MW, Perez-Cruz C, van der Auwera I, van Leuven F, van Gaalen M, Bespalov AY, Schoemaker H, Sullivan JP, Ebert U. Generation and therapeutic efficacy of highly oligomer-specific beta-amyloid antibodies. J.Neurosci. 2010;30:10369–10379. doi: 10.1523/JNEUROSCI.5721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int.J.Dev.Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Risk factors for Alzheimer's disease during aging. Impacts of glucose/energy metabolism. J.Neural Transm.Suppl. 1998;54:187–194. doi: 10.1007/978-3-7091-7508-8_18. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc.Natl.Acad.Sci.U.S.A. 2008;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Chiang HC, Hearn SA, Hakker I, Gatt A, Shenton C, Granger L, Leung A, Iijima-Ando K, Zhong Y. Abeta42 mutants with different aggregation profiles induce distinct pathologies in Drosophila. PLoS.One. 2008;3:e1703. doi: 10.1371/journal.pone.0001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid {beta}-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc.Natl.Acad.Sci.U.S.A. 2011 doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Graham DI, Stewart JE, Praestgaard AH, Smith DH. A neprilysin polymorphism and amyloid-beta plaques after traumatic brain injury. J.Neurotrauma. 2009;26:1197–1202. doi: 10.1089/neu.2008.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen S, Antonio LL, Mussi GE, Brito-Moreira J, Bomfim TR, De Felice FG, Garrido-Sanabria ER, Cavalheiro EA, Ferreira ST. Activation of D1/D5 Dopamine Receptors Protects Neurons from Synapse Dysfunction Induced by Amyloid-{beta} Oligomers. J.Biol.Chem. 2011;286:3270–3276. doi: 10.1074/jbc.M110.177790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc.Natl.Acad.Sci.U.S.A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J.Biol.Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J.Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466:E3–E4. doi: 10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat.Rev.Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kim SH, Markham JA, Weiler IJ, Greenough WT. Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc.Natl.Acad.Sci.U.S.A. 2008;105:4429–4434. doi: 10.1073/pnas.0800257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL. Cytotoxic intermediates in the fibrillation pathway: Abeta oligomers in Alzheimer's disease as a case study 2761. In: Uversky VN, Fink AL, editors. Protein Misfolding, Aggregation, and Conformational Diseases. New York: Kluwer Academic/Plenum; 2006. pp. 289–309. [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ, Anwyl R, Walsh DM, Rowan MJ. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J.Neurosci. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander GL, Ishikawa C, Massa SM, Wyss-Coray T, Longo FM. The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo. J.Neurosci. 2009;29:10627–10637. doi: 10.1523/JNEUROSCI.0620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc.Natl.Acad.Sci.U.S.A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf D, Frolich L. Risk of incident Alzheimer's disease in diabetic patients: a systematic review of prospective trials. J.Alzheimers Dis. 2009;16:677–685. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J.Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall NW, Beal MF, Busciglio J, Duffy LK, Yankner BA. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc.Natl.Acad.Sci.U.S.A. 1991;88:7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasinski A, Grieb P. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer's disease. Acta Neurochir.Suppl. 2010;106:177–181. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- Lacor PN. Advances on the understanding of the origins of synaptic pathology in AD. Current Genomics. 2007;8:486–508. doi: 10.2174/138920207783769530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J.Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J.Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc.Natl.Acad.Sci.U.S.A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Abeta. J.Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Viola KL, Klein WL. Targeting generation of antibodies specific to conformational epitopes of amyloid beta-derived neurotoxins. CNS.Neurol.Disord.Drug Targets. 2009;8:65–81. doi: 10.2174/187152709787601876. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J.Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J.Biol.Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol.Aging. 2009;30:377–387. doi: 10.1016/j.neurobiolaging.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol.Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, DeWilde A, Vercauteren F, Atifeh R, Ducatenzeiler A, Klein W, Szyf M, Alhonen L, Cuello AC. A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J.Alzheimers.Dis. 2010;20:113–126. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J.Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J.Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Bhatia R, Lal R. Amyloid beta protein forms ion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 2001;15:2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc.Natl.Acad.Sci.U.S.A. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur.J.Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J.Neurosci. 2009;29:9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Zimin PI, Wulff H, Jin LW. Amyloid-{beta} Protein Oligomer at Low Nanomolar Concentrations Activates Microglia and Induces Microglial Neurotoxicity. J.Biol.Chem. 2011;286:3693–3706. doi: 10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J.Biol.Chem. 2008;283:9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdesian MH, Nery AA, Martins AH, Juliano MA, Juliano L, Ulrich H, Ferreira ST. Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid beta. J.Biol.Chem. 2005;280:31085–31090. doi: 10.1074/jbc.M502406200. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau in Alzheimer's disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr., Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- Martins SM, Frosoni DJ, Martinez AM, De Felice FG, Ferreira ST. Formation of soluble oligomers and amyloid fibrils with physical properties of the scrapie isoform of the prion protein from the C-terminal domain of recombinant murine prion protein mPrP-(121–231) J.Biol.Chem. 2006;281:26121–26128. doi: 10.1074/jbc.M605367200. [DOI] [PubMed] [Google Scholar]
- Martins VR, Beraldo FH, Hajj GN, Lopes MH, Lee KS, Prado MM, Linden R. Prion protein: orchestrating neurotrophic activities. Curr.Issues Mol.Biol. 2010;12:63–86. [PubMed] [Google Scholar]
- Marx J. Alzheimer's disease. Fresh evidence points to an old suspect: calcium. Science. 2007;318:384–385. doi: 10.1126/science.318.5849.384. [DOI] [PubMed] [Google Scholar]
- Masters CL, Kril JJ, Halliday GM, Pamphlett R, Collins S, Hill AF, McLean C. Overview and recent advances in neuropathology. Part 2: Neurodegeneration. Pathology. 2011;43:93–102. doi: 10.1097/PAT.0b013e3283426eee. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and antiinflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J.Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol.Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J.Alzheimers Dis. 2008;15:29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mrak RE. Neuropathology and the neuroinflammation idea. J.Alzheimers Dis. 2009;18:473–481. doi: 10.3233/JAD-2009-1158. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J.Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J.Neuropathol.Exp.Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, Bruehl C. Amyloid beta oligomers (A beta(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J.Neurosci. 2008;28:788–797. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitsuji K, Tomiyama T, Ishibashi K, Ito K, Teraoka R, Lambert MP, Klein WL, Mori H. The E693Delta mutation in amyloid precursor protein increases intracellular accumulation of amyloid beta oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am.J.Pathol. 2009;174:957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogalska A, D'Agostino C, Engel WK, Klein WL, Askanas V. Novel demonstration of amyloid-beta oligomers in sporadic inclusion-body myositis muscle fibers. 2010 doi: 10.1007/s00401-010-0737-3. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE, Collinge J, Walsh DM. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J.Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J.Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Pasinetti GM, Osterburg HH, Anderson C, Johnson SA, Finch CE. Purification and characterization of brain clusterin. Biochem.Biophys.Res.Commun. 1994;204:1131–1136. doi: 10.1006/bbrc.1994.2580. [DOI] [PubMed] [Google Scholar]
- Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, Rozovsky I, Stine WB, Snyder SW, Holzman TF, Krafft GA, Finch CE. Clusterin (Apoj) Alters the Aggregation of Amyloid Beta-Peptide (A-Beta(1–42)) and Forms Slowly Sedimenting A-Beta Complexes That Cause Oxidative Stress. Experimental Neurology. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J.Biol.Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, Zhang H, Wang L, Kim TW, Duff KE, Wenk MR, Arancio O, Di Paolo G. Phospholipase d2 ablation ameliorates Alzheimer's disease-linked synaptic dysfunction and cognitive deficits. J.Neurosci. 2010;30:16419–16428. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, Yan SS, Domenici L. Microglial receptor for advanced glycation end product-dependent signal pathway drives beta-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J.Neurosci. 2010;30:11414–11425. doi: 10.1523/JNEUROSCI.2127-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Paula-Lima AC, Adasme T, Sanmartin C, Sebollela A, Hetz C, Carrasco MA, Ferreira ST, Hidalgo C. Amyloid beta-Peptide Oligomers Stimulate RyR-Mediated Ca(2+) Release Inducing Mitochondrial Fragmentation in Hippocampal Neurons and Prevent RyR-Mediated Dendritic Spine Remodeling Produced by BDNF. Antioxid.Redox.Signal. 2011 doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer's disease. Neurobiol.Dis. 2004;17:500–506. doi: 10.1016/j.nbd.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, Mount HT, Mufson EJ, Salehi A, Fahnestock M. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J.Neurosci. 2009;29:9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JL, Carrero I, Gonzalo P, Arevalo-Serrano J, Sanz-Anquela JM, Ortega J, Rodriguez M, Gonzalo-Ruiz A. Soluble oligomeric forms of beta-amyloid (Abeta) peptide stimulate Abeta production via astrogliosis in the rat brain. Exp.Neurol. 2010;223:410–421. doi: 10.1016/j.expneurol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Perl DP. Neuropathology of Alzheimer's disease. Mt.Sinai J.Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc.Natl.Acad.Sci.U.S.A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur.J.Pharmacol. 1991a;207:367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991b;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Pitt J, Roth W, Lacor P, Smith AB, III, Blankenship M, Velasco P, De Felice F, Breslin P, Klein WL. Alzheimer's-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol.Appl.Pharmacol. 2009;240:189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Kopitz J, Siegelin M, Schliebs R, Salkovic-Petrisic M, Riederer P, Hoyer S. Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbetaPP-overexpressing mice. J.Alzheimers Dis. 2010;19:691–704. doi: 10.3233/JAD-2010-1270. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol.Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Lee G, Selkoe DJ. Gene dosage of the amyloid beta precursor protein in Alzheimer's disease. Science. 1987;238:669–671. doi: 10.1126/science.2960019. [DOI] [PubMed] [Google Scholar]
- Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW, Jeon NL, Cotman CW. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol.Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011 doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J.Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Hasenjager A, Sroka-Saidi K, Deussing JM, Parsons CG. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of beta-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 2011;60:982–990. doi: 10.1016/j.neuropharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer's disease. Age Ageing. 1994;23:396–399. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Insulin signaling effects on memory and mood. Curr.Opin.Pharmacol. 2007;7:633–637. doi: 10.1016/j.coph.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J.Alzheimers Dis. 2008a;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008b;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Bartl J, Laux G, Grunblatt E. Diabetes type II: a risk factor for depression-Parkinson-Alzheimer? Neurotox.Res. 2011;19:253–265. doi: 10.1007/s12640-010-9203-1. [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J.Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG. Early neuronal dysfunction by amyloid beta oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol.Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida OF. Soluble beta-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J.Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva LM, Seixas da Silva GS, Galina A, da Silva WS, Klein WL, Ferreira ST, De Felice FG. Amyloid-beta triggers the release of neuronal hexokinase 1 from mitochondria. PLoS One. 2010;5:e15230. doi: 10.1371/journal.pone.0015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EL, Forlenza OV, Gattaz WF. Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berl) 2009;202:37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp.Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat.Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc.Natl.Acad.Sci.U.S.A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid beta protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989;58:611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The genetics and molecular pathology of Alzheimer's disease: roles of amyloid and the presenilins. Neurol.Clin. 2000;18:903–922. doi: 10.1016/s0733-8619(05)70232-2. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J.Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat.Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Kawahara K, Kajizono M, Sawada M, Nakayama H. IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1–42) by rat primary type 2 microglia. J.Immunol. 2008;181:6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- Shipton OA, Leitz JR, Dworzak J, Acton CE, Tunbridge EM, Denk F, Dawson HN, Vitek MP, Wade-Martins R, Paulsen O, Vargas-Caballero M. Tau Protein Is Required for Amyloid {beta}-Induced Impairment of Hippocampal Long-Term Potentiation. J.Neurosci. 2011;31:1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P. The postsynaptic density: a possible role in long-lasting effects in the central nervous system. Proc.Natl.Acad.Sci.U.S.A. 1985;82:3494–3498. doi: 10.1073/pnas.82.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat.Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem.Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Sponne I, Fifre A, Drouet B, Klein C, Koziel V, Pincon-Raymond M, Olivier JL, Chambaz J, Pillot T. Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J.Biol.Chem. 2003;278:3437–3445. doi: 10.1074/jbc.M206745200. [DOI] [PubMed] [Google Scholar]
- Sponne I, Fifre A, Koziel V, Oster T, Olivier JL, Pillot T. Membrane cholesterol interferes with neuronal apoptosis induced by soluble oligomers but not fibrils of amyloid-beta peptide. FASEB J. 2004;18:836–838. doi: 10.1096/fj.03-0372fje. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J.Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc.Natl.Acad.Sci.U.S.A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J.Neurosci. 2008;28:5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am.J.Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Okamoto Y, Kawarabayashi T, Yokoseki T, Shibata M, Mouri A, Nabeshima T, Sun H, Abe K, Urisu T, Yamamoto N, Shoji M, Yanagisawa K, Michikawa M, Matsubara E. Extracellular and intraneuronal HMW-AbetaOs represent a molecular basis of memory loss in Alzheimer's disease model mouse. Mol.Neurodegener. 2011;6:20. doi: 10.1186/1750-1326-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Haines JL, Watkins PC, Stewart GD, Wallace MR, Hallewell R, Wong C, Wexler NS, Conneally PM, Gusella JF. Genetic linkage map of human chromosome 21. Genomics. 1988;3:129–136. doi: 10.1016/0888-7543(88)90143-7. [DOI] [PubMed] [Google Scholar]
- Terry RD, Katzman R, Bick KL. Alzheimer's Disease. New York: Raven Press; 1994. [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann.Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, Guntert A, Proitsi P, Powell J, Causevic M, Killick R, Lunnon K, Lynham S, Broadstock M, Choudhry F, Howlett DR, Wlliams RJ, Sharp SI, Mitchelmore C, Tunnard C, Leung R, Foy C, O'Brien D, Breen G, Furney SJ, Ward M, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Hodges A, Murphy DG, Parkins S, Richardson JC, Resnick SM, Ferrucci L, Wong DF, Zhou Y, Muehlboeck S, Evans A, Francis PT, Spenger C, Lovestone S. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch.Gen.Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, Nishitsuji K, Ito K, Shimada H, Lambert MP, Klein WL, Mori H. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J.Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Thornton PL, Balazs R, Cotman CW. Beta -amyloid-(1–42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival Is not compromised. J.Biol.Chem. 2001;276:17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J.Alzheimers Dis. 2009;17:827–844. [PMC free article] [PubMed] [Google Scholar]
- Toro P, Schonknecht P, Schroder J. Type II diabetes in mild cognitive impairment and Alzheimer's disease: results from a prospective population-based study in Germany. J.Alzheimers Dis. 2009;16:687–691. doi: 10.3233/JAD-2009-0981. [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J.Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol.Aging. 2008;29:1607–1618. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T, Tomiyama T, Sakama N, Tanaka S, Lambert MP, Klein WL, Mori H. Intraneuronal amyloid beta oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J.Neurosci.Res. 2011;89:1031–1042. doi: 10.1002/jnr.22640. [DOI] [PubMed] [Google Scholar]
- Unger JW, Betz M. Insulin receptors and signal transduction proteins in the hypothalamo-hypophyseal system: a review on morphological findings and functional implications. Histol.Histopathol. 1998;13:1215–1224. doi: 10.14670/HH-13.1215. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat.Rev.Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc.Natl.Acad.Sci.U.S.A. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Nallar L, Grabowski CP, Alfaro IE, Alvarez AR, Inestrosa NC. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Dev. 2009;4:41. doi: 10.1186/1749-8104-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese K, Molnar P, Das M, Bhargava N, Lambert S, Kindy MS, Hickman JJ. A new target for amyloid beta toxicity validated by standard and high-throughput electrophysiology. PLoS.One. 2010;5:e8643. doi: 10.1371/journal.pone.0008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. J.Neurosci. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, SantAngelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc.Natl.Acad.Sci.U.S.A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer's disease and avenues for therapeutic intervention. Biochem.Soc.Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J.Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zheng W, Xie JW, Wang T, Wang SL, Teng WP, Wang ZY. Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Mol.Neurodegener. 2010;5:46. doi: 10.1186/1750-1326-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS.Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS.Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen BM, Selkoe DJ, Hartley DM. Small non-fibrillar assemblies of amyloid beta-protein bearing the Arctic mutation induce rapid neuritic degeneration. Neurobiol.Dis. 2005;20:254–266. doi: 10.1016/j.nbd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- White JA, Manelli AM, Holmberg KH, Van Eldik LJ, Ladu MJ. Differential effects of oligomeric and fibrillar amyloid-beta 1–42 on astrocyte-mediated inflammation. Neurobiol.Dis. 2005;18:459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Wsniewski T, Sigurdsson EM. Murine models of Alzheimer's disease and their use in developing immunotherapies. Biochim.Biophys.Acta. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J.Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Small DH, Seydel U, Smith JD, Hallmayer J, Gandy SE, Martins RN. Apolipoprotein E promotes the binding and uptake of beta-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 1999;90:1217–1226. doi: 10.1016/s0306-4522(98)00561-2. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di PA, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pan BS, Zhao B, Zhang LM, Huang YL, Sun FY. Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of beta-secretase activation and beta-amyloid generation in rat brains. Neuroscience. 2009;161:1045–1056. doi: 10.1016/j.neuroscience.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol.Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur.J.Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Lacor PN, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta} J.Biol.Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, Kinney GG, Shughrue PJ, Ray WJ. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J.Biol.Chem. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]