Abstract
Background
An inverse association between Parkinson disease (PD) and total vitamin D levels has been reported but it is unknown whether vitamin D from different sources, i.e. 25(OH)D2 (from diet and supplements) and 25(OH)D3 (mainly from sunlight exposure), all contribute to the association.
Methods
Plasma total 25(OH)D, 25(OH)D2, and 25(OH)D3 levels were measured by liquid chromatography-tandem mass spectrometry in PD patients (N=478) and controls (N=431). Total 25(OH)D was categorized by clinical insufficiency or deficiency, 25(OH)D2 and 25(OH)D3 were analyzed in quartiles.
Results
Vitamin D deficiency (total 25(OH)D < 20 ng/mL) and vitamin D insufficiency (total 25(OH)D < 30 ng/mL) are associated with PD risk [Odds Ratio (OR)=2.6 (deficiency) and 2.1 (insufficiency), P<0.0001], adjusting for age, sex and sampling season. Both 25(OH)D2 and 25(OH)D3 levels are inversely associated with PD (Ptrend<0.0001). The association between 25(OH)D2 and PD risk is largely confined to individuals with low 25(OH)D3 levels (Ptrend=0.0008 and 0.12 in individuals with 25(OH)D3 < 20 ng/mL and 25(OH)D3 >= 20 ng/mL, respectively)
Conclusions
Our data confirm the association between vitamin D deficiency and PD, and for the first time demonstrate an inverse association of 25(OH)D2 with PD. Given that 25(OH)D2 concentration is independent of sunlight exposure, this new finding suggests that the inverse association between vitamin D levels and PD is not simply due to lack of sunlight exposure PD patients with impaired mobility. The current study, however, cannot exclude the possibility that gastrointestinal dysfunction, a non-motor PD symptom, contributes to the lower vitamin D2 levels in PD patients.
Keywords: vitamin D, Parkinson disease, diet, vitamin D2
Introduction
Parkinson disease (PD) is a common complex neurodegenerative disorder for which genetic studies have identified more than a dozen susceptibility loci (1, 2). Epidemiologic studies have identified several lifestyle factors that are associated with PD. Among them, the most consistent evidence has been reported for cigarette smoking, coffee drinking, the use of anti-inflammatory drugs (NSAIDs), and pesticide application, with less compelling evidence being found for exercise, education, occupation, and diet (3–14). In addition, studies have suggested that genetic variation may modulate the effect of these lifestyle factors, pointing to an additional level of complexity (15, 16). With known genetic and environmental factors not completely accounting for the risk of PD, efforts to uncover additional contributing factors and the complex interplay between them are needed.
Recently, epidemiologic studies have suggested an inverse association between circulating vitamin D concentration and risk of PD. It was reported that people with lower serum 25(OH)D (a stable metabolite of vitamin D that has been used as a measure of vitamin D status) concentration at baseline had significantly higher risk of developing PD during a 29 years of follow up(17). The study, however, was conducted in Finland where the prevalence of vitamin D deficiency is higher than other parts of the world; whether the Finnish findings are generalizable to other populations remains untested in other longitudinal studies. A few case-control studies in Japan and United States have reported lower 25(OH)D levels in PD patients compared to age-matched controls (18–20). These reports utilized relatively small sample sizes and may have limited accuracy of estimated strength of the association between 25(OH)D and PD risk. Therefore, studies with larger sample sizes are needed to refine the estimate of effect size and further establish the inverse relationship between vitamin D and PD. In addition, it remains a concern that lower 25(OH)D levels in PD patients are a consequence of reduced mobility (and therefore reduced sunlight exposure, the major source of vitamin D in humans) rather than a contributing factor to the development of PD. Additional studies are warranted to clarify the cause and effect relationship between lower vitamin D level and risk of PD.
Circulating total 25(OH)D concentrations include 25(OH)D2 and 25(OH)D3, metabolites of vitamin D2 and vitamin D3, respectively. Vitamin D3 can either be endogenously synthesized upon sunlight exposure in the skin or acquired from dietary or supplement intake. In contrast, vitamin D2 only naturally exists in some plants (notably, mushrooms) and therefore can only be acquired from dietary and supplement intake (21–23). Sunlight exposure is the major source for vitamin D: 5 to 10 min of direct sunlight exposure to arms and legs produce 3000IU of vitamin D3, compared to 100 IU of vitamin D3 per 8 ounces of fortified milk and ~ 100 IU of vitamin D2 per 3.5 ounces of fresh mushroom, and 400 IU of vitamin D2 or D3 in general multivitamin supplement daily (21). Because 25(OH)D2 level is independent of sunlight exposure, an inverse relationship between 25(OH)D2 and PD would suggest that lower vitamin D concentrations are not simply the result of reduced mobility in PD patients. Prior publications have not addressed the issue of vitamin D source, mainly because immunoassay-based vitamin D measuring assays used do not distinguish 25(OH)D2 and 25(OH)D3.
To address these limitations in previous investigations, the current study was designed 1) using a large case-control sample of over 900 individuals and 2) using liquid chromatography tandem mass spectrometry (LC-MS/MS) technology to measure 25(OH)D2, 25(OH)D3 and total 25(OH)D.
Methods
Participant Recruitment
We have previously reported results of a genome-wide association study (GWAS) in PD using 635 PD cases and 478 controls (24). Of these, 478 cases and 431 controls had stored plasma samples for vitamin D assessment and were included in the current study. Details on participant enrollment and clinical assessment were previously described (24). Briefly, most subjects were ascertained by the Morris K. Udall Parkinson Disease Research Center of Excellence (PDRCE) at Duke University, the University of Miami (J.M. Vance, PI), and the 13 centers of the Parkinson Disease Genetics Collaboration. Controls were ascertained from community outreach efforts or were spouses of individuals with PD or Alzheimer disease (from a genetic study on Alzheimer disease) (25). PD cases possess at least two of three cardinal signs of PD (resting tremor, bradykinesia and rigidity). Rigorous clinical assessment was performed by all participating clinicians to provide a clear diagnosis of PD and to exclude any individuals that displayed atypical features of Parkinsonism. All controls are cognitively normal with no PD symptoms by self-reported symptom questionnaire (26). All subjects in this study are non-Hispanic Caucasians by self-report (confirmed by principal component analysis of population structure included in the initial GWAS report (24)). All ascertainment protocols were approved by each contributing center’s institutional review board.
Laboratory assessment of 25(OH)D2 and 25(OH)D3
Plasma samples were collected between 1996–2003 and stored at -80°C. Stored plasma samples were analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS) at the Emory Clinical Translational Research Laboratory in four batches with a balanced number of cases and controls in random order. Laboratory technicians were blinded to affection status. Each batch of samples was analyzed using a Waters (Milford, MA) Acquity UPLC coupled to a Xevo mass spectrometer in 7 to 12 continuous runs. We used the method outlined by Lensmeyer, et al (27) with some modifications (See Supplementary Methods). d6-25(OH)D3 (Cerilliant, Round Rock, TX) and d6-25(OH)D2 (Medical Isotopes, Pelham, NH) were mixed with vitamin D free serum (Golden West Bio, Temecula, CA) to be used as quality control (QC). Three levels of duplicate QC (approximately 6, 21, 62 ng/mL) were included at the beginning and end of each run. The assay was linear up to 200 ng/mL and had a limit of detection (LOD) of 1 ng/mL. Total imprecision ranged from 10.8% to 17.1% over the 48 runs done for this study. The assay was validated using the NIST 972a standards. The laboratory participates in the DEQAS proficiency scheme. DEQAS specimens run with study samples all passed proficiency (06/2012 through 06/2013).
Statistical Analysis
Three vitamin D concentrations - 25(OH)D2, 25(OH)D3 and total 25(OH)D (the sum of 25(OH)D2 and 25(OH)D3) - were analyzed for association with PD diagnosis. Since we hypothesized that PD patients have lower vitamin D levels, the distribution of the vitamin D measurements was evaluated in cases and controls separately. Samples with concentrations beyond three standard deviations (SD) from the mean were regarded as outliers and excluded from association analysis. In total, 5 cases and 5 controls were excluded as outliers.
Logistic regression analysis was used to evaluate the association between vitamin D concentrations and PD, adjusting for age at sample draw, sex, and sampling season. Total 25(OH)D was analyzed categorically, using the established clinical criteria for vitamin D deficiency (25(OH)D<20 ng/mL) and vitamin D insufficiency (25(OH)D<30 ng/mL) (28). 25(OH)D2 and 25(OH)D3 concentrations were analyzed in quartiles delimited by their distributions in the control samples. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated for each quartile individually using the highest quartile as the reference. A linear test for trend was also conducted, coding each quartile as an ordinal value. Stratification analysis was performed to evaluate the association of PD with 25(OH)D2 in individuals with low 25(OH)D3 (<20ng/mL) and high 25(OH)D3(>=20ng/mL) levels. Linear regression analysis was used to evaluate the vitamin D association with symptom duration. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina).
Results
Table 1 shows several demographic characteristics of the subjects. The PD cases had a higher percentage of men (63%) than the control sample (35%), consistent with prior observations of increased prevalence of PD in men. To reduce the possibility of including controls that would develop PD after ascertainment, we intentionally recruited controls at older ages, resulting in an older mean age at sample draw in controls than in cases (70±8 years vs 64±12 years). In our data set, neither age nor sex is associated with vit D deficiency (data not shown). There is no significant difference on sampling season between cases and controls.
Table 1.
Characteristics of Patients with Parkinson Disease and Control Participants:
| PD Patients (N=478) | Controls (N=431) | P-Value | |
|---|---|---|---|
| Gender (men %) | 63% | 35% | <0.0001 |
| Age-at-sampling (years, mean ± SD) | 64±12 | 70±8 | <0.0001 |
| Age-at-onset (years, mean ± SD) | 56±13 | N/A | N/A |
| Symptom duration (years, mean ± SD) | 7.60±6.24 | N/A | N/A |
| Sampling Season (Jul-Dec; %) | 231 (48.33%) | 211 (48.96%) | 0.8496 |
| Plasma 25(OH)D total (ng/mL, mean ± SE) | 25.59±0.56 | 30.14±0.56 | <0.0001 |
| Plasma 25(OH)D3 (ng/mL. mean ± SE) | 23.79±0.54 | 27.38±0.58 | <0.0001 |
| Plasma 25(OH)D2 (ng/mL, mean ± SE) | 1.72±0.19 | 2.88±0.23 | <0.0001 |
| Prevalence of vit D deficiency | 36% | 17% | <0.0001 |
| Prevalence of vit D insufficiency | 71% | 54% | <0.0001 |
Both vitamin D deficiency (total 25(OH)D<20ng/mL) and vitamin D insufficiency (total 25(OH)D<30ng/mL) are significantly associated with PD (P<0.0001) (Table 2). Individuals with PD were 2.64 times as likely to be vitamin D deficient as controls (95% CI, 1.88–3.71) and 2.13 times as likely as controls to be vitamin D insufficient (95% CI, 1.58–2.89).
Table 2.
Association between vitamin D from different sources and PD risk
| Odds Ratio | 95% CI | P-Value | |
|---|---|---|---|
| clinical cutoff analysis | |||
| Vit D deficiency ( total 25(OH)D <20 ng/mL) | 2.64 | (1.88,3.71) | <0.0001 |
| Vit D insufficiency (total 25(OH)D<30 ng/mL) | 2.13 | (1.58,2.89) | <0.0001 |
| quartile analysis | |||
| Total 25(OH) D | |||
| Q1 (4.3–21.8 ng/mL) | 2.66 | (1.76,4.03) | <0.0001 (trend test) |
| Q2 (21.8–29 ng/mL) | 1.89 | (1.23,2.91) | |
| Q3 (29–37 ng/mL) | 0.92 | (0.58,1.46) | |
| Q4 (>37 ng/mL) | Reference | ||
| 25(OH) D2 | |||
| Q1 (<1 ng/mL) | 2.04 | (1.24,3.37) | <0.0001 (trend test) |
| Q2 (1–3.25 ng/mL) | 1.84 | (1.02,3.33) | |
| Q3 (3.25–8 ng/mL) | 1.46 | (0.80,2.69) | |
| Q4 (>8ng/mL) | Reference | ||
| 25(OH) D3 | |||
| Q1 (6–19.3 ng/mL) | 2.39 | (1.60,3.58) | <0.0001 (trend test) |
| Q2 (19.3–26.0 ng/mL | 1.42 | (0.92,2.18) | |
| Q3 (26.0–34.1 ng/mL) | 1 | (0.65,1.56) | |
| Q4 (>34.1 ng/mL) | Reference | ||
Next, we evaluated the association of PD and concentration of vitamin D metabolites from different sources: 25(OH)D2 (obtained from dietary and supplement intake) and 25(OH)D3 (obtained mainly from sunlight exposure). The analysis revealed a strong inverse association between 25(OH)D2 and 25(OH)D3 concentrations and PD diagnosis (Ptrend <0.0001 for both metabolites), again in a dose-response manner (Table 2). The lowest quartile of concentration was significantly associated with PD (relative to the highest quartile) for both 25(OH)D2 (OR=2.04; 95%CI=1.24–3.37) and 25(OH)D3 (OR=2.39; 95%CI=1.60–3.58). The second lowest quartile of 25(OH)D2 (OR=1.84 (1.02–3.33) but not 25(OH)D3 was significantly associated with PD, with a weaker effect. The third quartiles of both vitamin D metabolites were not associated with PD, suggesting that the association is saturating with increasing vitamin D concentration.
We detected a significant interaction between 25(OH)D2 and 25(OH)D3 on PD risk (data not shown), suggesting that the association between 25(OH)D2 and PD is modified by 25(OH)D3 concentration. Stratification analysis revealed that the inverse association between 25(OH)D2 and PD is largely confined to individuals with low 25(OH)D3 levels (Ptrend=0.0008 in individuals with 25(OH)D3 < 20 ng/mL vs Ptrend=0.12 in individuals with 25(OH)D3 >= 20 ng/mL, Table 3)
Table 3.
Association of PD with 25(OH)D2 in the presence of low and high 25(OH)D3 levels.
| Odds Ratio | 95% CI | P-Value for trend test | ||
|---|---|---|---|---|
| Low 25 (OH)D3 stratum (25(OH)D3<20ng/mL) | ||||
| Q1 | 3.58 | (1.72,7.42) | 0.0008 | |
| Q2 | 2.66 | (1.01,7.01) | ||
| Q3 | 2.26 | (0.95,5.41) | ||
| Q4 | Reference | |||
| High 25 (OH)D3 stratum (25(OH)D3>=20ng/mL) | ||||
| Q1 | 1.56 | (0.77,3.15) | 0.12 | |
| Q2 | 1.60 | (0.72,3.55) | ||
| Q3 | 1.05 | (0.44,2.50) | ||
| Q4 | Reference | |||
25(OH)D2 quartiles: <1, 1–3.25, 3.25–8, >8 ng/mL
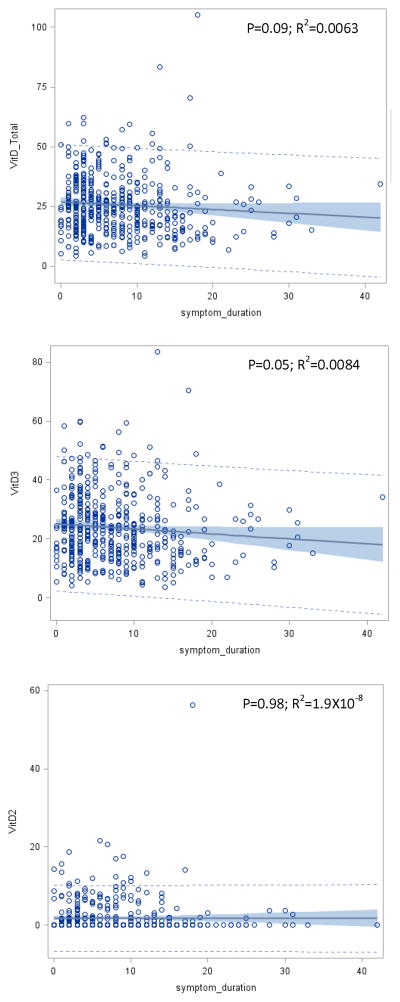
Finally, we examined the relationship between symptom duration and vitamin D levels. Our data showed that total 25(OH)D and 25(OH)D2 concentrations were not correlated with symptom duration (P=0.09 and 0.98, respectively) while 25(OH)D3 was marginally significantly decreased with prolonged symptom duration (P=0.05). This correlation, however, explained very little of the overall variance in 25(OH)D3 concentrations ( R2 =0.0084) (Figure 1).
Figure 1. Correlation between vitamin D from different source with symptom duration.
Correlation between total vitamin D (upper panel), 25(OH)D3 (middle panel) 25(OH)D2 (lower panel), and Parkinson symptom duration are displayed. X-axis displays symptom duration in years, and Y-axis displays plasma vitamin D levels. Solid line represents fitted correlation between vitamin D levels and symptom duration, shadows represent 95% confidence of the fitted correlation, and the dotted lines represent the predication limits of the fitted correlation.
Discussion
Several studies have reported an association of PD with lower plasma 25(OH)D concentrations (18–20). The current study is a much larger case-control sample than the previous reports. Our data revealed a stronger association of PD with vitamin D deficiency compared to vitamin D insufficiency, suggesting a potential dosage effect of circulating 25(OH)D concentration on risk of PD. Importantly, our study is the first to separately analyze vitamin D metabolites from dietary and supplement sources [25(OH)D2] and those mainly from sunlight exposure [25(OH)D3]. Our data demonstrated that both sources of vitamin D were inversely associated with when considered individually. As a modest source of total vitamin D, the inverse association between 25(OH)D2 and PD was largely confined to individuals who have low 25(OH)D3 levels.
Because the initial association of vitamin D concentration with PD was observed in case-control samples, it was not clear whether the reduction in vitamin D concentration preceded the development of PD symptoms, or was rather an effect of the disease process (18, 19). This arises because most vitamin D is produced upon sunlight exposure, and as a result of limited mobility, individuals with PD may have less sunlight exposure than age-matched controls. To address this potential “reverse causation,” subsequent studies have taken different approaches to dissect this question. Post-hoc analysis of blood samples from the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) study, which included a well-characterized cohort of patients with early, non-disabling and untreated PD, revealed that PD subjects with relatively normal mobility already have high prevalence of hypovitaminosis D (29). However, it remains to be tested if fatigue, an early symptom in PD, reduces time spent outside in the sunlight even when patients remain fully mobile. In the current study, we detected an inverse association between PD diagnosis and, 25(OH)D2 that is obtained independently from sunlight exposure. Therefore, this association is unlikely to be confounded by the mobility of the subjects, providing additional support against “reverse causation” caused by the reduced mobility in PD patients. However, the inverse association between 25(OH)D2 and PD could result from another form of “reverse causation”. Gastrointestinal dysfunction is a commonly observed non-motor symptom in PD, sometimes occurring years before motor symptom onset. The most frequently documented gastrointestinal symptom is impaired motility, such as delayed gastric emptying resulting from stomach dysmotility and constipation resulting from colonic dysmotility (36). Delayed gastric emptying can lead to abdominal discomfort and early satiety, reducing food intake (and potentially reducing vitamin D2 intake). Very few studies have described higher prevalence of small intestinal bacterial overgrowth (SIBO), possibly due to abnormal small intestine motility, in PD patients (37). It is possible that severe SIBO could lead to malabsorption of nutrients including vitamin D. In addition, the major food source of 25(OH)D2 is mushrooms. If individuals with PD are less likely to eat mushrooms, possibly due to more conservative eating habits somehow related to PD susceptibility, the observed association could also occur.
Other evidence against “reverse causation” comes from the analysis of vitamin D concentration and PD symptom duration. If lower vitamin D levels in PD patients compared to controls are due to reduced mobility, it is expected that vitamin D concentration is even lower in patients who have had PD for longer time. We found a modest inverse association of PD symptom duration with 25(OH)D3 (p=0.05), which probably reflects reduced mobility in patients as the disease progresses, but is a small effect: for every 10 years of symptom duration, circulating 25(OH)D3 concentration is estimated to decrease 1.7 ng/mL in our dataset. No association was found between 25(OH)D2 and symptom duration (P=0.98) Given the mean symptom duration of 7.6 years for the PD patients in this study, reduced mobility is unlikely to be an explanation for the observed inverse association, especially for 25(OH)D2.
Circulating 25(OH)D concentrations in our study are much higher than in the longitudinal Finnish study: the prevalence of vitamin D deficiency in controls is 18% in our study compared to more than 70% in the Finnish study. Consistent with the higher prevalence of vitamin D deficiency, the age-adjusted PD incidence rate (per 100,000) was 15.7 (in 1971) and 14.9 (in 1992) in Southwestern Finland, which is slightly higher than the age and gender-adjusted incidence rate of 12.9, 13.6, and 14.0 in studies conducted in Northern Manhattan (during 1989–1991), Northern California (during 1994–1995), and Minnesota (during 1976~1990), respectively. However, many factors affect the estimation of PD incidence rates and these findings should be interpreted cautiously (30, 31). Despite the differences on vitamin D deficiency prevalence and study design, the estimated effect size comparing the lowest and highest quartiles of total vitamin D concentration is similar in the two studies. In the Finnish study, people in the lowest quartile at baseline had about three times the risk of developing PD compared to people in the highest quartile (17). In our case-control study, cases are 2.66 times as likely as controls to be in the lowest quartile compared to the highest quartile. The odds ratio estimate of PD for vitamin D deficiency in our study (OR=2.64) is similar to the Emory study (OR=2.66) and higher than the Harvard Biomarker Study (OR=2.1) (19, 20). The statistical significance of the association is much stronger in our study (P<0.0001) than in the other two US-based case-control studies (P=0.008 and 0.002), likely attributable to a larger sample size in the current study.
Interestingly, several studies have previously reported a positive association between dairy intake (a good source of vitamin D) and PD risk, which seemed to be in conflict with the inverse association between vitamin D and PD reported here. However, in-depth analysis suggested that increased PD risk in individuals with higher dairy intake is unlikely due to vitamin D (32, 33).
Through its receptor VDR (vitamin D receptor), vitamin D regulates expression of many genes, exerting pleiotropic effects. Several candidate gene studies, including ours, have reported an association between VDR gene polymorphism and PD risk (34, 35), which provides a different line of evidence supporting a role of vitamin D in PD. It is plausible that genetic variants, especially in vitamin D related genes, modify the effects of vitamin D on PD. Gene-vitamin D interaction studies are needed to fully understand the effects of vitamin D on PD and facilitate identifying individuals who are likely to benefit more from vitamin D supplementation to achieve an optimal vitamin D status.
The strength of this study comes from the large case-control sample and use of LC-MS/MS to measure different vitamin D metabolites separately such that we did not have to rely on dietary recall to estimate vitamin D2 intake. We found that individuals with PD have lower 25(OH)D2 levels than controls, which is consistent with other studies reporting that PD patients have less vitamin D supplementation and dietary intake of vitamin D (18, 20). While our case-control cross-sectional study design limits causal inference and is subject to bias due to unknown confounds, the inverse association observed here corresponds well with the prior longitudinal cohort study (17). Similarly, lack of longitudinal clinical assessment, including multiple vitamin D measurements, of cases and controls prevents evaluation of the relationship between vitamin D status (and its change over time) and disease progression. Additional studies in longitudinal cohorts followed through different periods of life are necessary to provide valuable information on whether there is a time window that is critical for adequate vitamin D exposure to prevent or delay onset of PD. Also, the control set lacks data on certain potential confounders such as smoking, education, physical activity, alcohol consumption, and therefore we cannot control for them. However, in the previous study, these factors have negligible effect on the association between vitamin D levels and PD risk (17).
In summary, our study detected an inverse association of PD with circulating total 25(OH)D concentration as well as its two components, (25(OH)D2 and 25(OH)D3. These results confirm prior associations and provide additional evidence against the previous concern that reduced mobility is the main explanation for higher prevalence of vitamin D deficiency in PD patients.
Acknowledgments
Funding Sources: This study was sponsored by National Institute of Health grant 2P50NS071674.
We are grateful to the families and staff who participated in this study. This study was sponsored by National Institute of Health grant 2P50NS071674.
Footnotes
Financial Disclosure: None
All authors concur with the submission of the work and there are no conflicts of interest.
Author Roles:
LW: conception, organization, and execution of study; writing the first draft of manuscript;
MLE: interpretation of results; critical reviewing and editing manuscript;
LGM and WRP: execution of statistical analysis;
JCR: acquisition of data, critical reviewing and editing manuscript;
GW and ER: design and supervision of statistical analysis;
JLH and MPV: organization and execution of study;
JMV: organization and execution of study, critical reviewing and editing manuscript
WKS: conception, organization and execution of study, design and supervision of statistical analysis, interpretation of results, critical reviewing and editing manuscript.
References
- 1.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;R1:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 2.Pankratz N, Beecham GW, DeStefano AL, et al. Meta-analysis of Parkinson’s disease: identification of a novel locus, RIT2. Ann Neurol. 2012;3:370–384. doi: 10.1002/ana.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbaz A, Clavel J, Rathouz PJ, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;4:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005;6:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 5.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 6.Scott WK, Zhang F, Stajich JM, Scott BL, Stacy MA, Vance JM. Family-based case-control study of cigarette smoking and Parkinson disease. Neurology. 2005:442–447. doi: 10.1212/01.WNL.0000150905.93241.B2. [DOI] [PubMed] [Google Scholar]
- 7.Hancock DB, Martin ER, Mayhew GM, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock DB, Martin ER, Stajich JM, et al. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson’s disease. Arch Neurol. 2007;4:576–580. doi: 10.1001/archneur.64.4.576. [DOI] [PubMed] [Google Scholar]
- 9.Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010:S58–62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;4:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Diet and Parkinson’s disease: a potential role of dairy products in men. Ann Neurol. 2002;6:793–801. doi: 10.1002/ana.10381. [DOI] [PubMed] [Google Scholar]
- 12.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;10:1575–1583. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 13.Miyake Y, Tanaka K, Fukushima W, et al. Lack of association of dairy food, calcium, and vitamin D intake with the risk of Parkinson’s disease: a case-control study in Japan. Parkinsonism Relat Disord. 2011;2:112–116. doi: 10.1016/j.parkreldis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol. 2013;1:67–77. doi: 10.1007/s10654-012-9760-0. [DOI] [PubMed] [Google Scholar]
- 15.Hamza TH, Chen H, Hill-Burns, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS Genet. 2011;8:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock DB, Martin ER, Fujiwara K, et al. NOS2A and the modulating effect of cigarette smoking in Parkinson’s disease. Ann Neurol. 2006:366–373. doi: 10.1002/ana.20915. [DOI] [PubMed] [Google Scholar]
- 17.Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;7:808–811. doi: 10.1001/archneurol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Abnormal bone and calcium metabolism in immobilized Parkinson’s disease patients. Mov Disord. 2005;12:1598–1603. doi: 10.1002/mds.20658. [DOI] [PubMed] [Google Scholar]
- 19.Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;10:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Dhima K, Lockhart KC, et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology. 2013;17:1531–1537. doi: 10.1212/WNL.0b013e3182a95818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;3:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Cashman KD, Kinsella M, McNulty BA, Walton J, Gibney MJ, Flynn A, Kiely M. Dietary vitamin D2 - a potentially underestimated contributor to vitamin D nutritional status of adults? Br J Nutr. 2014:1–10. doi: 10.1017/S0007114514000725. [DOI] [PubMed] [Google Scholar]
- 23.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;3:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards TL, Scott WK, Almonte C, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;2:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beecham GW, Martin ER, Li YJ, Slifer MA, Gilbert JR, Haines JL, Pericak-Vance MA. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. Am J Hum Genet. 2009;1:35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca WA, Maraganore DM, McDonnell SK, Schaid DJ. Validation of a telephone questionnaire for Parkinson’s disease. J Clin Epidemiol. 1998:517–523. doi: 10.1016/s0895-4356(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 27.Lensmeyer G, Poquette M, Wiebe D, Binkley N. The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J Clin Endocrinol Metab. 2012;1:163–168. doi: 10.1210/jc.2011-0584. [DOI] [PubMed] [Google Scholar]
- 28.Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;1:111–148. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evatt ML, DeLong MR, Kumari M, Auinger P, McDermott MP, Tangpricha V Parkinson Study Group DATATOP Investigators. High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch Neurol. 2011;3:314–319. doi: 10.1001/archneurol.2011.30. [DOI] [PubMed] [Google Scholar]
- 30.Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Changing epidemiology of Parkinson’s disease in southwestern Finland. Neurology. 1999;52:302–308. doi: 10.1212/wnl.52.2.302. [DOI] [PubMed] [Google Scholar]
- 31.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, O’Reilly E, McCullough ML, et al. Consumption of dairy products and risk of Parkinson’s disease. Am J Epidemiol. 2007;165:998–1006. doi: 10.1093/aje/kwk089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Diet and Parkinson’s disease: A potential role of dairy products in men. Ann Neurol. 2002;52:793–801. doi: 10.1002/ana.10381. [DOI] [PubMed] [Google Scholar]
- 34.Kim JS, Kim YI, Song C, et al. Association of vitamin D receptor gene polymorphism and Parkinson’s disease in Koreans. J Korean Med Sci. 2005;3:495–498. doi: 10.3346/jkms.2005.20.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler MW, Burt A, Edwards TL, et al. Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet. 2011;2:201–210. doi: 10.1111/j.1469-1809.2010.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 37.Fasano A, Bove F, Gabrielli M, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;9:1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]