Summary
Bacterial pathogens commonly show intra-species variation in virulence factor expression and often this correlates with pathogenic potential. The group A Streptococcus (GAS) produces a small regulatory RNA (sRNA), FasX, which regulates the expression of pili and the thrombolytic agent streptokinase. As GAS serotypes are polymorphic regarding (a) FasX abundance, (b) the fibronectin, collagen, T-antigen (FCT) region of the genome, which contains the pilus genes (nine different FCT-types), and (c) the streptokinase-encoding gene (ska) sequence (two different alleles), we sought to test whether FasX regulates pilus and streptokinase expression in a serotype-specific manner. Parental, fasX mutant, and complemented derivatives of serotype M1 (ska-2, FCT-2), M2 (ska-1, FCT-6), M6 (ska-2, FCT-1), and M28 (ska-1, FCT-4) isolates were compared. While FasX reduced pilus expression in each serotype, the molecular basis differed, as FasX bound, and inhibited the translation of, different FCT-region mRNAs. FasX enhanced streptokinase expression in each serotype, although the degree of regulation varied. Finally, we established that the regulation afforded by FasX enhances GAS virulence, assessed by a model of bacteremia using human plasminogen-expressing mice. Our data are the first to identify and characterize serotype-specific regulation by an sRNA in GAS, and to show an sRNA directly contributes to GAS virulence.
Keywords: sRNA, Streptococcus pyogenes, regulation, variation, virulence factors, humanized mice
Introduction
The group A Streptococcus (GAS; S. pyogenes) is a human-specific pathogen capable of instigating a wide array of diseases, ranging from the mild and typically self-limiting streptococcal pharyngitis (a.k.a. strep throat) to the severely invasive and often fatal necrotizing fasciitis (a.k.a. the flesh-eating syndrome) (Cunningham, 2008). The ability of GAS to infect and cause disease at different anatomical sites is promoted by an array of virulence factor-encoding genes accessible for expression (Kreikemeyer et al., 2003, Olsen & Musser, 2010). Importantly, the disease potential of GAS is thought to be attributable to the variable expression of distinctive combinations of virulence factors, rather than any one particular virulence factor. Controlling the regulation of gene expression in GAS are 13 two-component systems (Ribardo et al., 2004), more than 60 “stand-alone” transcriptional regulators (McIver, 2009), and approximately 40 small regulatory RNAs (sRNAs) (Miller et al., 2014). While the majority of protein-based regulators are present in all GAS isolates, recent data is consistent with serotype-specific differences in the functionality of some of these systems (Cao et al., 2014, Lynskey et al., 2013, Luo et al., 2008). These serotype-specific differences in regulatory activity lead to altered virulence factor expression, which is hypothesized to be a contributing factor in epidemiologically-identified serotype-specific differences in disease potential (Colman et al., 1993, O'Brien et al., 2002). GAS strains are placed into different serotypes based upon the sequence of the 5’ end of the M-protein-encoding (emm) gene, and can be further categorized by T-typing, a serological-based assay that characterizes a variable surface antigen termed the T-antigen (Lancefield, 1946).
To date, the only bona fide GAS sRNA that has been characterized is the fibronectin / fibrinogen-binding / hemolytic-activity / streptokinase-regulator-X (FasX) (Kreikemeyer et al., 2001, Ramirez-Pena et al., 2010, Liu et al., 2012). In serotype M1 GAS, the only GAS serotype in which the molecular basis of FasX-mediated regulation has been characterized, FasX negatively regulates the expression of pili (Liu et al., 2012). Pili are cell-wall anchored filamentous structures that protrude from the cell surface and allow attachment of bacteria to host cells, as well as promoting biofilm development (Manetti et al., 2007, Abbot et al., 2007). The negative regulation of pilus expression by FasX is a consequence of the inhibition of target mRNA translation. Specifically, FasX base-pairs to M1.cpa mRNA, which is the initial gene in the M1 GAS pilus biosynthesis locus, encoding a collagen-binding protein that forms the pilus tip (Quigley et al., 2009), and this base-pairing inhibits the access of ribosomes to the M1.cpa mRNA ribosome-binding site (Liu et al., 2012). Of importance to this manuscript, the pilus biosynthesis locus is located in a pathogenicity island termed the fibronectin, collagen, T-antigen (FCT) region (Bessen & Kalia, 2002). As the name suggests, the FCT region also includes the gene encoding for the T-antigen, the protein serologically categorized by T-typing. Recently, it has been determined the T-antigen is the major pilus protein, which covalently polymerizes into the long pilus shafts (Mora et al., 2005). The FCT region is highly polymorphic such that it can be used to type GAS strains into one of nine currently recognized FCT-types (Koller et al., 2010, Kratovac et al., 2007). Given the significant variation in gene content and gene order of the FCT regions of different GAS serotypes it remains to be tested whether FasX negatively regulates pilus expression in serotypes other than M1.
In addition to negatively regulating pilus expression, FasX also acts as a positive regulator of streptokinase expression (Ramirez-Pena et al., 2010, Kreikemeyer et al., 2001). Streptokinase is a secreted thrombolytic agent that catalyzes the activation of human plasminogen into the protease plasmin (Cook et al., 2012). Plasmin has multiple enzymatic activities, including the ability to degrade blood clots and to activate host metalloproteases and collagenases (Syrovets et al., 2012). To enhance streptokinase expression FasX base-pairs to the 5’ untranslated region (5’-UTR) of streptokinase-encoding (ska) mRNA, forming a secondary structure which enhances the stability of the mRNA (Ramirez-Pena et al., 2010). GAS strains are polymorphic with respect to the sequence of the ska gene such that they can be grouped into two distinct sequence clusters, designated type-1 and type-2 (Kapur et al., 1995, Kalia & Bessen, 2004). The streptokinase variants display altered plasminogen activation characteristics, which directly affect the ability of GAS to cause disease (McArthur et al., 2011). As FasX regulatory activity has only been determined in M1 GAS, which harbor the ska-2 allele, it is unknown whether FasX can also positively regulate streptokinase expression in GAS isolates that harbor the ska-1 cluster-type.
Herein, we compared representative serotype M1 (ska-2, FCT-2), M2 (ska-1, FCT-6), M6 (ska-2, FCT-1), and M28 (ska-1, FCT-4) GAS isolates for the ability of FasX to regulate pilus and streptokinase expression. We found that while FasX negatively regulated pilus expression in each of the serotype backgrounds tested, the targets of this regulation differed in an FCT-type dependent manner, as FasX bound discrete transcripts in different sero-/FCT-types. Despite the targeting of different transcripts, the molecular mechanism of inhibiting translation of the target mRNA is conserved. Consistent with FasX inhibiting pilus expression, fasX mutant derivatives of each serotype showed greater adherence than the parental strains in a tissue culture-based assay. With respect to streptokinase, the enhanced expression of this virulence factor by FasX was observed in all of the tested strains, regardless of ska allele cluster type. We have also established that FasX promotes GAS virulence, as evidenced by a fasX mutant strain having reduced lethality in a bacteremia model of infection using transgenic mice expressing human plasminogen. This data significantly expands our previous insights into the FasX regulon, and for the first time shows an sRNA’s ability to directly contribute to the virulence of this prevalent human bacterial pathogen.
Results
Variation in the FCT pathogenicity island between GAS serotypes
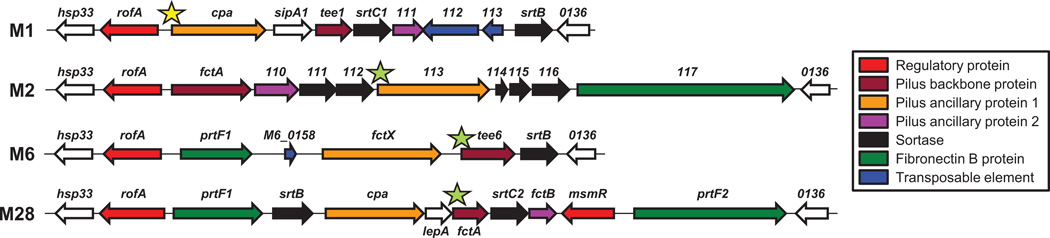
The FCT region includes the pilus biosynthesis genes that are post-transcriptionally regulated by FasX in serotype M1 GAS (Liu et al., 2012). Nine diverse FCT types have been described based upon differences in gene content and gene order (Koller et al., 2010, Kratovac et al., 2007). Given the extensive variation between different FCT regions, it is unclear whether FasX regulates pilus expression in GAS isolates that do not share the same FCT-type as M1 GAS (FCT-2). To initiate our investigation, we compared the FCT regions from four GAS serotypes, each of which harbored a different FCT-type: serotypes M1 (FCT-2), M2 (FCT-6), M6 (FCT-1), and M28 (FCT-4) (Figure 1). To regulate pilus expression in M1 GAS, FasX base-pairs to the 5’-UTR of M1.cpa mRNA (highlighted by a yellow star in Figure 1). Genes encoding proteins functionally equivalent to the collagen-binding Cpa are also present in the other FCT regions under investigation (genes M2.113, M6.fctX, and M28.cpa; orange genes in Figure 1). However, there is significant nucleotide diversity between these genes such that M2.113 is only 52% identical, M6.fctX is only 52% identical, and M28.cpa is only 67% identical to M1.cpa. Furthermore, these genes occupy disparate loci within their respective FCT regions and, most importantly, their 5’-UTRs differ. Therefore, if FasX base-pairs to the mRNAs of these genes in order to regulate pilus expression in M2, M6, and M28 isolates, it must do so via nucleotides distinct from those observed in M1 GAS.
Figure 1. Schematic of the FCT regions from representative serotype M1, M2, M6, and M28 GAS isolates.
Genes are represented by block arrows facing the direction of transcription. Gene naming and color-coding is consistent with that presented previously (Koller et al., 2010). The yellow star highlights the location of nucleotides within the 5’-UTR of M1. cpa mRNA that, in M1 GAS, base-pair with FasX to negatively influence pilus expression (Liu et al., 2012). The green stars highlight the location of nucleotides within the 5’-UTR of pilus mRNAs in serotype M2, M6, and M28 GAS that base-pair with FasX to negatively influence pilus expression (see results section).
FasX sRNA abundance alters FCT mRNA levels in representative serotype M1, M2, M6, and M28 strains
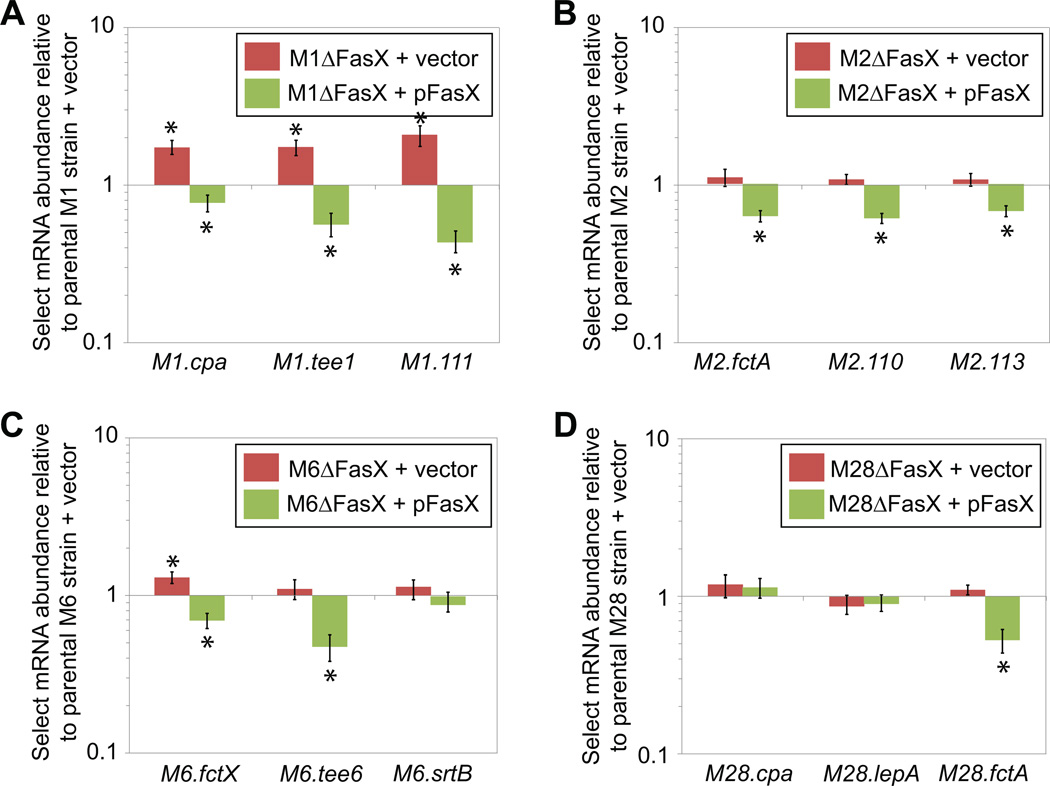
FasX predominately regulates pilus expression in M1 GAS isolates by inhibiting M1.cpa mRNA translation (Liu et al., 2012). However, base-pairing of FasX to M1.cpa mRNA also results in a small but reproducible decrease in the abundance of not only M1.cpa mRNA but also mRNAs from downstream FCT region genes (Liu et al., 2012). To test whether FasX decreases the abundance of mRNAs from the FCT region in types other than FCT-2, we selected three pilus genes from the FCT regions of representative M1, M2, M6, and M28 isolates for analysis via quantitative RT-PCR. Mutant and complemented mutant derivatives of the parental M2, M6, and M28 GAS strains were created for these assays, supplementing our previously constructed M1 GAS derivatives (Ramirez-Pena et al., 2010). Unlike the statistically significant increase observed for the three tested mRNAs in the M1 fasX mutant background (Figure 2A), only the abundance of M6.fctX mRNA differed between the other parental and fasX mutant strains (Figures 2B–D). In part, this may be a consequence of the fact that basal FasX sRNA levels are higher (at least two-fold) in M1 isolates relative to M2, M6, and M28 isolates (Perez et al., 2009). The complemented mutant strains, which over-express FasX due to the multi-copy nature of the complementation plasmid pFasX (Ramirez-Pena et al., 2010), had statistically significantly lower mRNA levels for 9 of the 12 genes assayed (green bars in Figure 2). The data are consistent with FasX potentially regulating the abundance of many of the assayed FCT region mRNAs, but for the M2, M6, and M28 isolates, only under conditions that increase FasX levels. Thus, if FasX regulates pilus expression in M2, M6, and/or M28 GAS isolates under the conditions tested, the mechanism must be almost exclusively at the level of inhibiting mRNA translation.
Figure 2. FasX negatively regulates the abundance of mRNAs encoding pilus biosynthesis proteins in serotype M1, M2, M6, and M28 GAS.
Parental, fasX mutant, and complemented mutant strains of serotype (A) M1, (B) M2, (C) M6, and (D) M28 GAS were grown to the mid-exponential phase of growth, RNA isolated, and the RNA used in quantitative RT-PCR analysis. Three genes from each FCT region were analyzed. Statistical significance was tested by T-test with significant (P < 0.05) data points highlighted by asterisks. Shown is the average (± standard deviation) of three independent experiments.
Deletion of fasX results in enhanced cell surface pilus expression in at least M1 and M2 GAS strains
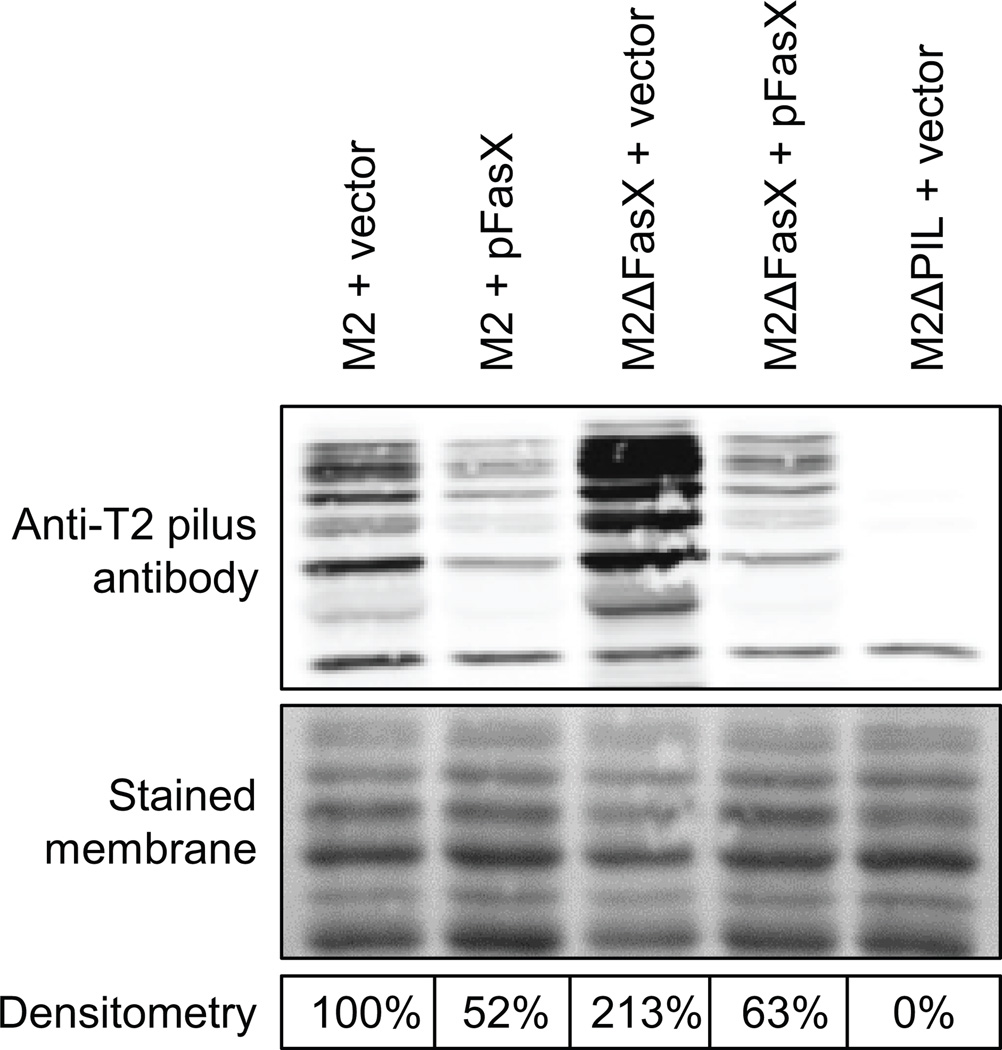
To further investigate whether FasX negatively regulates pilus expression in GAS serotypes other than M1 we performed Western blot analysis. Unfortunately, T-typing sera was only available for the detection of the M2 (FCT-6) GAS pilus, and not for the pili of our other tested serotypes (M6 and M28). Therefore, only cell wall protein fractions from M2 isolates were assayed. A total of five M2 isolates were compared, the parental strain containing empty vector or pFasX, the fasX mutant derivative containing empty vector or pFasX, and a derivative that lacks pilus expression (strain M2ΔPIL + vector). The fasX mutant strain containing empty vector (M2ΔFasX + vector) produced pili in greater abundance than the other tested strains, consistent with our hypothesis that FasX inhibits pilus formation (Figure 3). Similarly, the complementation plasmid pFasX reduced pilus expression in both the parental and fasX mutant derivative background, a finding we propose is due to the higher level of FasX expressed from pFasX relative to the chromosomally-encoded gene (Ramirez-Pena et al., 2010). These data are concordant with FasX negatively regulating pilus expression in at least GAS isolates containing FCT types 2 (M1) and 6 (M2). Given the significant differences between the four tested FCT regions (Figure 1) we set out to determine the molecular basis of the FasX-mediated regulation of pilus expression in non-M1 GAS isolates.
Figure 3. The FasX sRNA negatively regulates the abundance of pili on the surface of serotype M2 GAS.
Western blot analysis showing FasX inhibition of pilus expression in M2 GAS. Cell wall proteins from exponential phase cultures were isolated from the indicated strains, separated by SDS-PAGE, and used in Western blot analysis with sera that reacts against the major M2 pilus protein. The membrane was stained for use as a loading control. Densitometry analysis of the Western data was performed and is presented as percent pilus expression relative to the parental strain containing empty vector.
Known and putative interactions between FasX and pilus biosynthesis genes
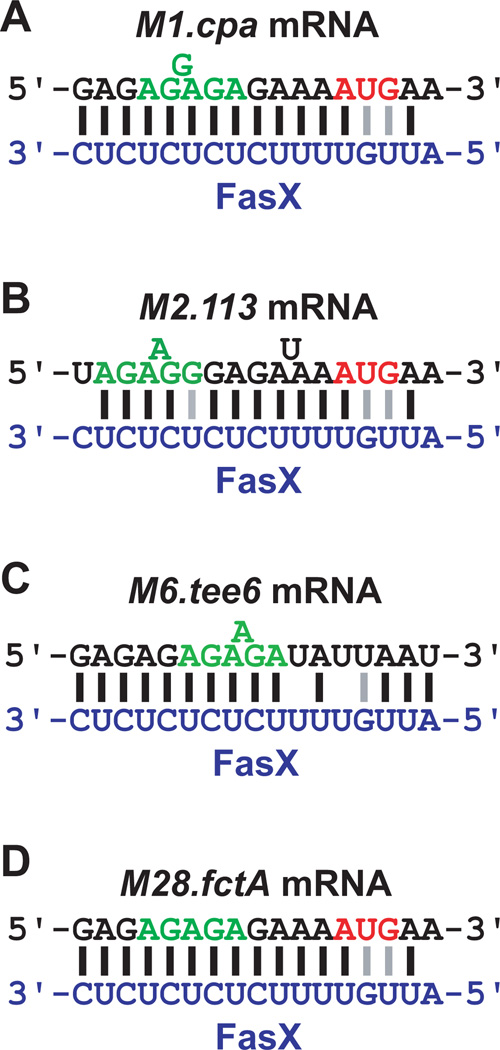
The regulation of pilus expression in M1 GAS isolates requires base-pairing between FasX and M1.cpa mRNA (Liu et al., 2012). Therefore, we hypothesized that FasX also base-pairs to mRNAs from the pilus biosynthesis genes of other serotypes. Regions of complementarity between FasX and the pilus biosynthesis mRNAs of our four tested GAS isolates were manually curated, and in each case sequences were identified with varying levels of complementarity (Figure 4). We previously employed RNA:RNA electrophoretic mobility shift assays (EMSAs) to confirm the FasX: M1.cpa mRNA interaction (Liu et al., 2012). For the M2 strain, the site most complementary to FasX within the FCT region was in the 5’-UTR of M2.113 mRNA, which is the M1.cpa homologue of the FCT-6 (M2) region; it is therefore possible that FasX base-pairs to the same gene in the FCT regions of M1 and M2 GAS isolates, even though they are situated in disparate locations within the FCT region (Figure 1), and that the M2.113 mRNA nucleotides that base-pair to FasX differ to those from M1.cpa mRNA (Figures 4A and 4B). For both the M6 and M28 strains, FasX was complementary to the 5’-UTRs of transcripts encoding for the major pilus protein: the tee6 gene in M6 GAS and the fctA gene in M28 GAS (Figures 1, 4C, and 4D). Thus, our sequence analysis is congruent with FasX hybridizing to different mRNAs (in sequence or in context) in each serotype, as a prerequisite to repressing pilus expression.
Figure 4. Known and putative interactions between FasX and FCT region genes in serotype M1, M2, M6, and M28 GAS.
FasX bases-pairs with the 5’-UTR of M1.cpa mRNA in M1 GAS isolates (A). For other tested serotypes putative regions of hybridization between FasX (blue nucleotides) and the 5′ end of FCT region mRNAs (black nucleotides) are shown for (B) M2 GAS, (C) M6 GAS, and (D) M28 GAS. The start codons (red nucleotides) and ribosome-binding sites (green nucleotides) of the mRNAs are highlighted. Lines between nucleotides of the mRNA and FasX highlight standard (Watson-Crick; black) and non-standard (G–U wobble; grey) base-pairing interactions.
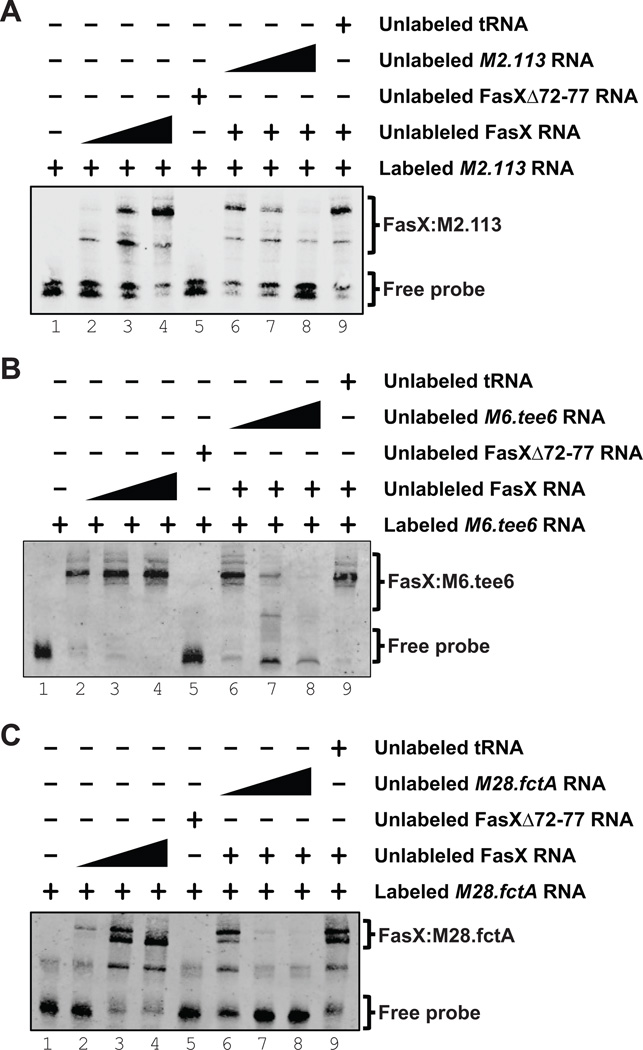
FasX complexes with pilus mRNAs in multiple GAS serotypes
To explore whether FasX base-pairs to the 5’ ends of the pilus mRNA transcripts as suggested by the data presented in Figure 4, we performed RNA:RNA EMSAs. Biotin-labeled target RNA, corresponding to the 5’ ends of M2.113, M6.tee6, or M28.fctA, was incubated with increasing concentrations of FasX RNA, which resulted in an increasing shift of the probe to higher molecular weight complexes (Figure 5, lanes 1–4 of each panel). The interactions between FasX and the target RNA probes were specific, as evidenced by the reduction in shifting when increasing concentrations of unlabeled free probe RNA were added to the samples (Figure 5, Lanes 6–8 of each panel), but not when unrelated RNA was added (yeast tRNA; Figure 5, lane 9 of each panel). Additional evidence for the proposed FasX:mRNA binding sites (Figure 4) was gained through use of a FasX derivative, FasXΔ72–77, in which six of the nucleotides hypothesized to base-pair with the mRNAs had been deleted (Liu et al., 2012). Use of FasXΔ72–77 failed to shift any of the probes (Figure 5, lane 5 of each panel), as expected. These data are consistent with FasX selectively base-pairing with M1.cpa mRNA in serotype M1 GAS isolates, M2.113 mRNA in M2 isolates, M6.tee6 mRNA in M6 isolates, and with M28.fctA mRNA in M28 isolates.
Figure 5. FasX base-pairs to the 5’ regions of M2.113 mRNA in serotype M2 GAS M6.tee6 mRNA in M6 GAS, and M28.fctA mRNA in M28 GAS.
RNA:RNA electrophoretic mobility shift assays confirming base-pairing between FasX and the 5′ end of (A) M2.113 mRNA in M2 GAS, (B) M6.tee6 mRNA in M6 GAS, and (C) M28.fctA mRNA in M28 GAS. A biotin-labeled RNA probe was incubated with wild-type FasX (FasX RNA; lanes 2–4 and 6–9), a FasX mutant in which six of the complementary nucleotides had been deleted (FasXΔ72–77 RNA; lane 5), unlabeled RNA probe (lanes 6–8), or unlabeled yeast tRNA (lane 9).
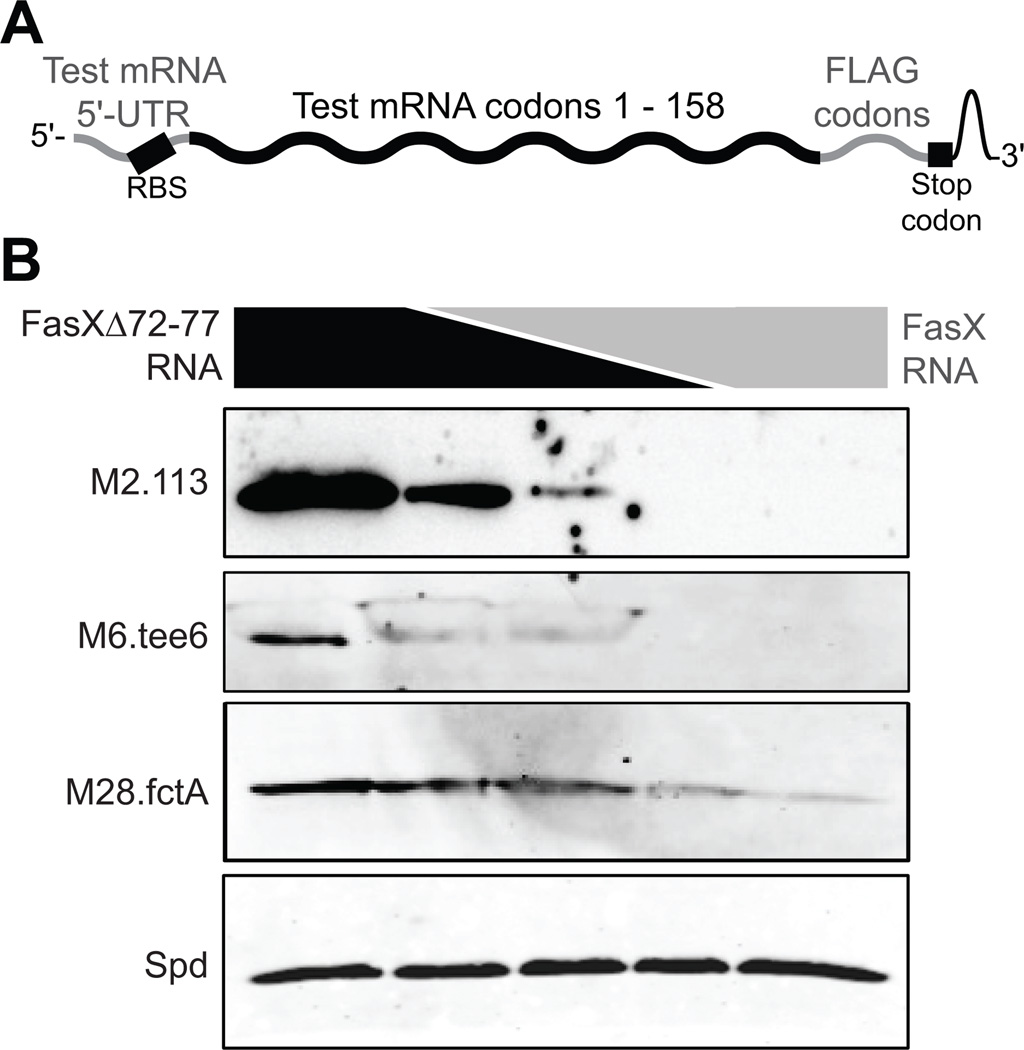
FasX:mRNA duplex formation inhibits translation of the pilus mRNAs in multiple GAS serotypes
The regions of base-pairing between FasX and M2.113, M6.tee6, and M28.fctA mRNAs include the putative Shine-Dalgarno ribosome binding sites of the mRNAs (green nucleotides in Figure 4). In the cases of M2.113 and M28.fctA mRNAs the region of interaction also includes the ATG start codon (red nucleotides in Figure 4), similar to that observed between FasX and M1.cpa mRNA. As the FasX:M1.cpa sRNA:mRNA complex formation inhibits translation of the M1.cpa mRNA by blocking ribosome access (Liu et al., 2012), we hypothesized a similar mechanism may be occurring following FasX binding to the M2.113, M6.tee6, and M28.fctA mRNAs, despite differences between the exact base-pairing nucleotides. To test our hypothesis, we performed in vitro translation assays. RNA substrates for these assays consisted of the 5’-UTRs and first ∼158 codons of each of the tested mRNAs (M2.113, M6.tee6, M28.fctA, and spd mRNA as a negative control), to which the sequence for a FLAG-tag had been fused at the 3’ end to enable subsequent detection of translated proteins with an anti-FLAG antibody (Figure 6A). For each of the chimeric pilus mRNAs, but not the chimeric control spd mRNA, the inclusion of FasX RNA in the in vitro translation reaction inhibited mRNA translation in a dose-dependent manner (Figure 6B). In contrast, the inclusion of the FasX mutant RNA FasXΔ72–77 did not inhibit translation in any of the reactions, as expected. The data are consistent with FasX negatively regulating pilus production in serotypes M1, M2, M6, and M28 by inhibiting the translation of an mRNA required for pilus formation, although the specific mRNA targeted differs in a serotype-specific manner.
Figure 6. Binding of FasX to M2.113 M6.tee6 and M28.fctA mRNAs inhibits their translation.
(A) Schematic showing the individual components of each of the four constructed chimeric mRNAs. (B) Western blot analyses of in vitro translation reactions. A total of 50 pmol of fctA.FLAG, 113.FLAG, tee6.FLAG, or spd.FLAG mRNA were pre-incubated with 150 pmol of RNA composed of different ratios of FasX and the deletion mutant derivative FasXΔ72–77. Following the in vitro translation reactions the products were used in Western blot analysis. In lanes 1–5, the pmol of FasXΔ72–77:FasX present in the reactions were 150:0, 100:50, 50:50, 50:100, and 0:150 respectively. The spd.FLAG mRNA was used as a negative control for FasX-mediated regulation.
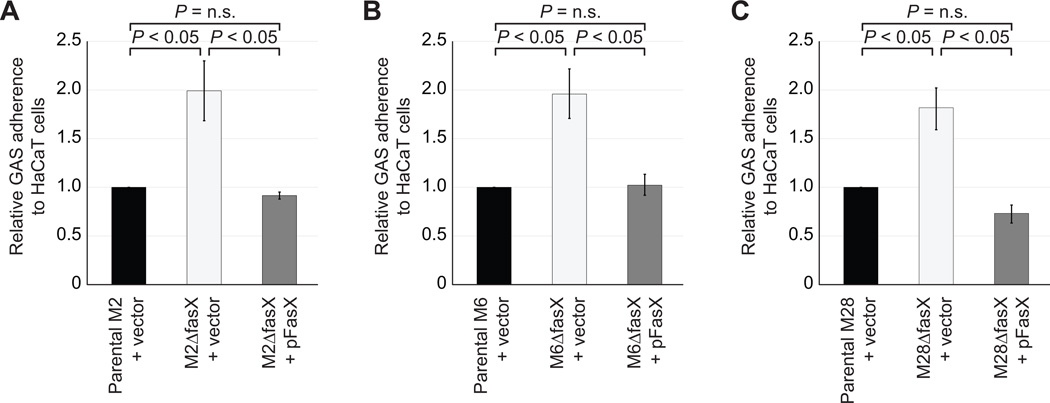
FasX reduces GAS adherence across multiple serotypes
Previously, we showed that by inhibiting pilus expression, FasX reduces the ability of serotype M1 GAS isolates to adhere to a human keratinocyte cell line (Liu et al., 2012). To test whether a FasX-mediated reduction in adherence is observed in other serotypes we tested our parental, fasX mutant, and complemented mutant derivatives of serotypes M2, M6, and M28 in tissue culture-based adherence assays. In each case the fasX mutant adhered at approximately twice the rate of the parental strain (Figure 7), while the complemented strain adhered at or slightly below that of the parental strain. These data reveal that FasX, presumably as a result of repressing pilus expression, reduces GAS adherence across multiple serotypes, regardless of FCT-type.
Figure 7. FasX inhibits the ability of serotype M2, M6 and M28 GAS isolates to adhere to host cells.
The indicated strains were added to lawns of HaCaT keratinocytes cells present in 12-well plates. The wells were washed extensively to remove non-adhered GAS before recovering the adhered GAS and determining the percent of GAS inoculum that bound the HaCaT cells. Data is displayed as level of adherence relative to that observed for the parental strains containing empty vector. Experiment was performed in triplicate with mean values (± standard deviation) shown. P-values were generated using Student’s T-test.
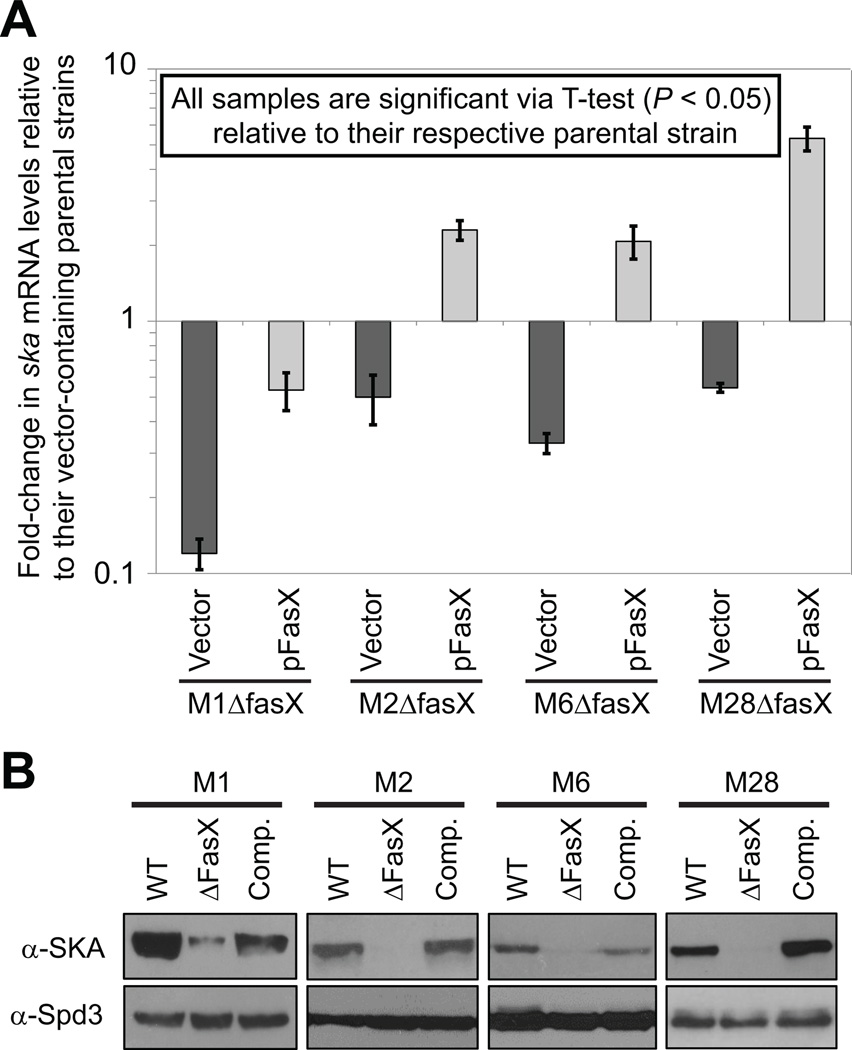
The positive regulation of streptokinase expression by FasX sRNA is not restricted to serotype M1 GAS
Whether the FasX-mediated regulation of streptokinase expression that we previously observed in the serotype M1 isolate MGAS2221 (Ramirez-Pena et al., 2010), which has a type-2 ska gene, also occurs in GAS serotypes that contain type-1 ska genes was investigated. We hypothesized that regulation would be maintained as the 5’-UTRs of the type-1 and type-2 ska genes, to which FasX base-pairs in serotype M1 GAS (Ramirez-Pena et al., 2010), are identical even though the coding regions of the genes are only ∼ 83% identical (Figure S1). Parental, fasX mutant, and complemented mutant strains of serotype M1 (ska-2), M2 (ska-1), M6 (ska-2), and M28 (ska-1) were compared for ska mRNA abundance via quantitative RT-PCR (Figure 8A). All four fasX mutant strains containing empty vector had lower ska mRNA abundance relative to their parental equivalents (dark grey bars in Figure 8A). Replacing the empty vector with the complementation plasmid pFasX restored ska mRNA levels in each strain (light grey bars in figure 8A), although the degree of restoration differed between the strains.
Figure 8. FasX positively regulates streptokinase expression in serotype M1, M2, M6, and M28 GAS.
Parental serotype M1, M2, M6, and M28 GAS strains containing empty vector were compared with fasX mutant derivatives containing either empty vector or a plasmid that harbors a wild-type fasX allele (pFasX). Strains were grown in triplicate to the mid-exponential phase of growth prior to comparison. (A) Quantitative RT-PCR analysis of ska mRNA levels. Experiment was run in triplicate with mean values (± standard deviation) shown. (B) Western blot analysis of streptokinase and Spd3 protein levels. Data shown is representative of that gained from the three independent cultures of each strain. The Spd3 Western is shown as a loading control as this secreted deoxyribonuclease is not FasX regulated.
To investigate the FasX-mediated regulation of streptokinase expression at the protein level we performed Western blot analysis using secreted protein samples of the parental, mutant, and complemented mutant strains. As expected, the fasX mutant strains all expressed lower levels of streptokinase, and this phenotype was complemented by plasmid pFasX (Figure 8B). Together, the quantitative RT-PCR and Western blot data confirm FasX positively regulates ska mRNA abundance in multiple GAS serotypes, regardless of ska allele status.
The FasX sRNA contributes to GAS virulence
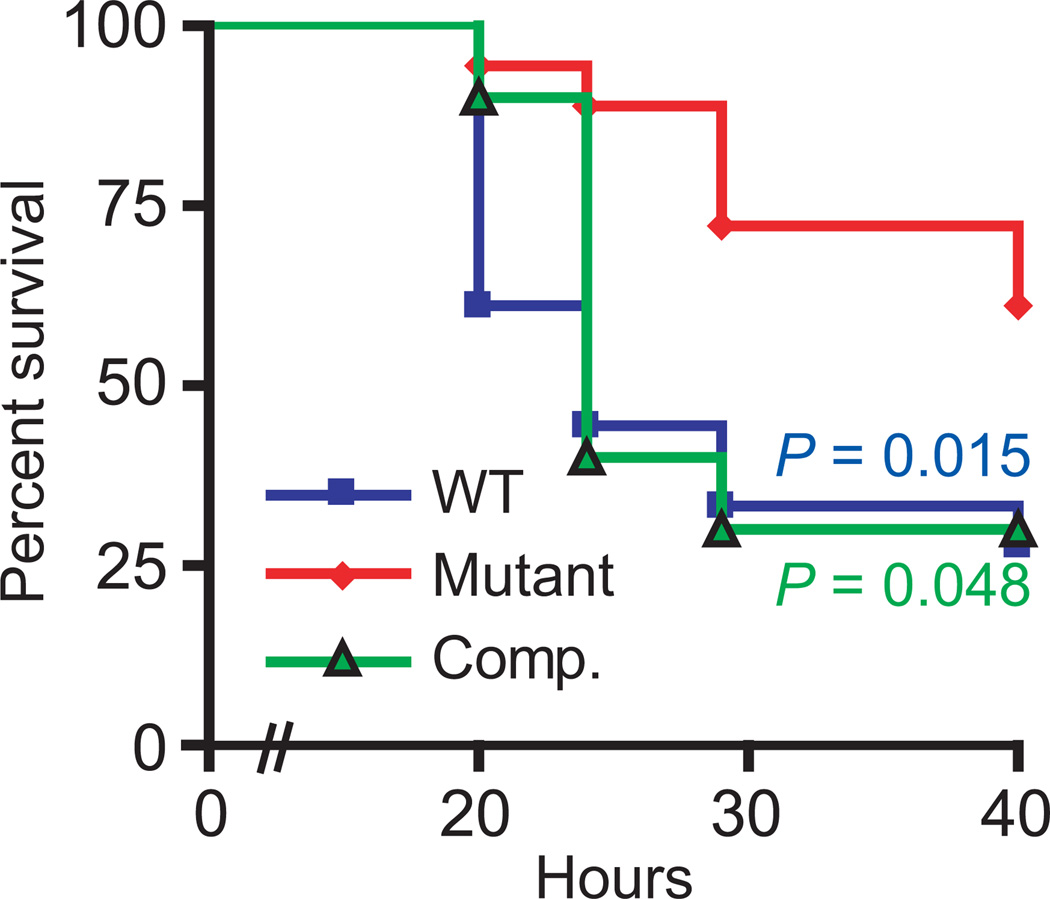
While FasX regulates streptokinase and pilus expression levels, it has not been investigated whether the regulation accorded by this sRNA contributes to the ability of GAS to cause disease. To test the hypothesis that FasX promotes GAS virulence, we compared the parental, fasX mutant, and complemented mutant serotype M1 strains in a murine model of bacteremia infection. As streptokinase is specific for human plasminogen, and hence has no activity against murine plasminogen, we used humanized transgenic mice that express human plasminogen (Sun et al., 2004). Animals infected with the fasX mutant strain survived at a significantly higher level than those animals infected with either the parental or complemented strains (Figure 9). Consequently, the FasX sRNA positively influences GAS virulence.
Figure 9. FasX enhances GAS virulence in a model of bacteremia infection using humanized plasminogen mice.
Data showing the survival rates for mice infected intraperitoneally with 1 × 106 CFUs of wild-type (MGAS2221; blue squares), mutant (2221ΔFasX; red diamonds), and complemented mutant (2221ΔFasX+pFasX; green triangles) GAS strains. Significance was tested by Log Rank test.
Discussion
An emerging theme in GAS pathogenesis research is that variations in virulence factor regulatory systems are a major contributor to observed strain- and serotype-specific differences in virulence (Cao et al., 2014, Lynskey et al., 2013, Sumby et al., 2006, Olsen et al., 2012, Vahling & McIver, 2005). Given that the pilus and streptokinase-encoding genes are polymorphic between different GAS serotypes, and that both of these important virulence factors are regulated by FasX in serotype M1 GAS, we set out to determine whether this sRNA exhibits serotype-specific variability in its regulatory targets. We identified that FasX negatively regulates the expression of pili regardless of tested FCT-type (FCT-1, FCT-2, FCT-4, FCT-6) or tested serotype (M1, M2, M6, or M28) (Figures 3 and 6). However, details of the molecular basis underlying the negative regulation of pilus expression differed in an FCT-type / serotype-specific manner. While the gene encoding the collagen-binding minor pilus protein is targeted for regulation by FasX in serotype M1 (FCT-2) and M2 (FCT-6) GAS, the M1.cpa and M2.113 genes, it is the gene encoding the major pilus protein which is targeted by FasX in serotype M6 (FCT-1) and M28 (FCT-4) GAS, the M6.tee6 and M28.fctA genes. Despite the different 5’-UTR sequences of the four FasX-targeted genes (Figure 4), the mechanism by which FasX inhibits pilus expression is through the inhibition of mRNA translation (Figure 6). We have also identified that FasX positively regulates the expression of streptokinase regardless of allele cluster type (ska-1 or ska-2) or tested serotype (M1, M2, M6, or M28) (Figure 8).
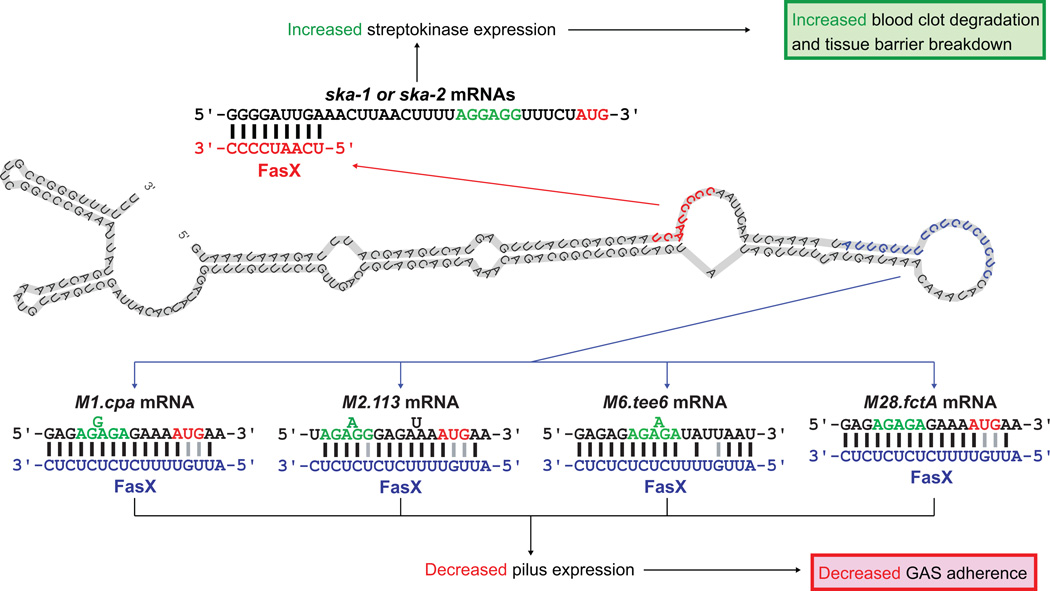
The ability of FasX to reduce expression of extracellular matrix-binding pili (Figure 3), thereby reducing the attachment of GAS to host cells and tissues (Figure 7) (Manetti et al., 2007), while at the same time enhancing expression of the thrombolytic agent streptokinase (Figure 8), thereby enhancing blood clot degradation and metalloprotease/collagenase activation (McArthur et al., 2012), is consistent with this sRNA contributing to the switch between colonization and dissemination (Figure 10). Our data presented here identify that this regulation occurs in multiple GAS serotypes (Figures 7 and 8), which together represent both ska alleles and four different FCT-types.
Figure 10. Model of how FasX may regulate the transition from colonization to dissemination by multiple GAS serotypes.
The putative FasX secondary structure is shown with nucleotides involved in the regulation of ska (red) and pilus (blue) mRNAs highlighted. The FasX:mRNA duplexes that form to regulate mRNA stability (ska-1 and ska-2) or translation (M1.cpa, M2.113, M6.tee6, and M28.fctA) are shown.
To inhibit the translation of the targeted pilus biosynthesis mRNAs in M1, M2, M6, and M28 GAS isolates, FasX base-pairs to different 5’-UTR sequences (Figure 4). While the 5’-UTR sequences upstream of the M1.cpa, M2.113, M6.tee6, and M28.fctA mRNAs differ, the same 17 nucleotides of FasX base-pair to these 5’ regions (Figure 10). Thus, these 17 nucleotides, which are predicted in part to be located within a single-stranded loop region of FasX, can be viewed as the pilus-inhibition region of FasX. The nucleotides within this region include five tandem UC dinucleotide repeats (Figure 10). Given that ribosome-binding sites are rich in A and G nucleotides, the UC repeats of FasX make it highly suited to efficiently bind and block mRNA ribosome binding sites, with specificity of interaction being provided by adjacent nucleotides.
The main mechanism by which FasX reduces pilus expression in M1 GAS is through inhibition of M1.cpa mRNA translation, although a modest reduction in M1.cpa mRNA stability is also observed (Liu et al., 2012). Unlike our previous data from M1 GAS, which was confirmed in Figure 2A, the fasX mutant strain derivatives of our serotype M2, M6, and M28 GAS isolates for the most part did not show an increase in the abundance of mRNAs from the pilus biosynthesis genes (red bars in Figure 2). This likely is explained by our previous finding that the basal level of FasX RNA is higher in the M1 background relative to the M2, M6, and M28 backgrounds (Perez et al., 2009). Thus, it is perhaps not surprising that a greater difference was observed between the M1 GAS parental and fasX mutant strains than those of the other investigated serotypes (Figure 2).
FasX transcription is primarily controlled through the activity of the FasBCA proteins, the genes for which are located immediately upstream of fasX (Kreikemeyer et al., 2001). While not studied in significant detail, the FasB and FasC proteins have homology to membrane-spanning sensor kinases, while FasA is homologous to response regulators. All three Fas proteins are required for efficient fasX transcription (Sumby, unpublished) (Kreikemeyer et al., 2001). Possible explanations for the lower basal levels of FasX previously detected in our M2, M6, and M28 isolates are that the M2 and M6 isolates both harbor three single nucleotide polymorphisms (SNPs) in the fasX promoter region, while the M28 isolate has a non-synonymous SNP in fasC (Perez et al., 2009) (data not shown). Whether these SNPs are responsible for the reduced FasX transcription, and whether they are present in a serotype-specific manner, remains to be investigated.
While FasX is the only sRNA known to regulate the expression of GAS pilus biosynthesis proteins, these proteins are regulated at the transcriptional level by the RofA/Nra and MsmR regulatory networks (Kreikemeyer et al., 2007, Luo et al., 2008, Nakata et al., 2005). These multiple layers of regulation provide evidence the expression of pilus is tightly controlled, suggesting this virulence factor may play a critical role during infection under certain conditions, and potentially a negative role under other conditions. This variable finely-tuned control is consistent with the finding that while the GAS pilus promotes both adhesion and biofilm formation (Manetti et al., 2007), it can also negatively affect virulence during invasive infections, due to greater entanglement and killing of pilus-expressing GAS by neutrophil extracellular traps (NETs) (Crotty Alexander et al., 2010).
The pilus mRNAs targeted by FasX in M6 and M28 GAS isolates are the M6.tee6 and M28.fctA mRNAs, which encode for the major pilus protein of the respective isolate. The major pilus protein forms covalently linked multimers, which generate the shaft of the pilus (Alegre-Cebollada et al., 2010, Linke-Winnebeck et al., 2014, Nakata et al., 2011, Young et al., 2014). The reduction in expression of the major pilus protein in M6 and M28 GAS isolates by FasX (Figure 6) provide straightforward explanations as to how this mechanism reduces pilus expression. However, the mechanism is more complicated in M1 GAS where FasX inhibits expression of the collagen-binding minor pilus subunit M1.Cpa (Liu et al., 2012), since reducing M1.Cpa levels should not impact the ability of the major pilus protein to polymerize and form the pilus shafts (Quigley et al., 2009). These shafts are what is recognized by the T-typing sera used in our studies. To attempt to reconcile this issue, we previously hypothesized that at least some of the M1.cpa mRNA transcripts are co-transcribed with the downstream pilus genes, especially the M1.sipA1 gene which is adjacent to M1.cpa, and that the reduction in M1.cpa mRNA translation by FasX would also lead to a reduction in M1.sipA1 mRNA translation due to fewer ribosomes translocating across the mRNAs (Liu et al., 2012). As the M1.SipA1 protein is required for polymerization of the major pilus protein in M1 GAS (Young et al., 2014), this provides a possible explanation for the reduction in pili. We conjecture a similar mechanism may be occurring in M2 GAS isolates, as the FasX-targeted M2.113 mRNA also encodes the collagen-binding minor pilus subunit. However, given the different order of genes in the FCT-6 region as compared to the FCT-2 type, any potential effect on the translation of genes downstream of M2.113 would require one or more of the putative sortases encoded by M2.114, M2.115, and M2.116 to be required for the polymerization and/or attachment of pili to the GAS cell wall. While not studied in M2 GAS, sortases encoded within the M1, M3, and M6 GAS FCT regions are required for pilus production by these serotypes (Kang et al., 2011, Nakata et al., 2011, Zahner & Scott, 2008).
The ability of FasX to regulate pilus expression across multiple FCT-types, despite the variation in gene order and content between types, is consistent with strong selection for maintaining this level of regulation. Interestingly, five genes located within FCT-6 from serotype M2 GAS strains, genes M2.fctA through M2.113 (Figure 1), are significantly more related to genes present in the pilus biosynthesis regions of several group B Streptococcus (GBS) isolates than in other GAS isolates (Falugi et al., 2008, Beres & Musser, 2007). For example, the M2.113 protein is 94% identical to a GBS pilus protein but no more than 28% identical to any known GAS protein. GBS isolates do not contain fasBCAX genes within their genomes; consequently, the expression of pili in GBS strains is not FasX regulated. It is therefore surprising, perhaps, that FasX is complementary to the 5’-UTR of the M2.113 transcript. A single SNP distinguishes the M2.113 mRNA 5’-UTR from the analogous GBS mRNA 5’-UTRs, with respect to the nucleotides shown in Figure 4B. This SNP is not thought to enhance the ability of FasX to bind to the M2.113 mRNA however, due to the fact that the SNP covers the M2.113 uracil nucleotide that is flipped out from the mRNA and does not base-pair with FasX (Figure 4B). Thus, the complementarity between FasX and the M2.113 mRNA 5’-UTR may in large part simply be a consequence of the inherent complementarity between the UC repeats of FasX and mRNA ribosome binding sites in general.
The FCT regions of the tested M2, M6, and M28 isolates all contain one or more genes encoding for fibronectin-binding proteins (green genes in Figure 1). These proteins function independently from the collagen-binding pilus (Bessen & Kalia, 2002, Jaffe et al., 1996). We are currently investigating the possibility that FasX also regulates the expression of these adhesins. Supporting this possibility is the previous finding that a serotype M49 GAS strain had an increased capacity to bind to fibronectin following fasX mutation (Kreikemeyer et al., 2001). Hence, FasX may negatively regulate expression of fibronectin-binding proteins in a similarly serotype-specific manner. If this hypothesis is correct, it would expand our knowledge of how FasX may inhibit GAS adherence and promote dissemination.
Prior to the completion of this work only two genes had been confirmed as being post-transcriptionally regulated by FasX, the ska-2 and M1.cpa genes (Liu et al., 2012, Ramirez-Pena et al., 2010). Now, we are able to include four additional genes in this group, ska-1, M2.113, M6.tee6, and M28.fctA. This study highlights new targets of FasX-mediated regulation that occur in clinically significant GAS serotypes, and provides insight into how an sRNA can regulate the expression of genetically polymorphic regions through serotype-specific mechanisms. Furthermore, we have demonstrated the regulation afforded by FasX contributes to GAS virulence in a humanized transgenic mouse model of bacteremia (Figure 9), representing the first such data for an sRNA from the pyogenic streptococci (e.g. GAS and GBS). The observations collected thus far provide strong incentive to further study this class of regulatory RNA molecule in GAS and other Gram-positive pathogens, which are, as a group, understudied with regard to the underlying molecular mechanisms and functions of sRNAs.
Experimental Procedures
Bacterial strains and culture conditions
The GAS strains used in this study are listed in Table S1. Routine growth of liquid GAS cultures made use of Todd-Hewitt broth with 0.2% yeast extract (THY broth), and cultures were incubated at 37°C (5% CO2). Chloramphenicol (4 µg/ml) and/or spectinomycin (150 µg/ml) were added when required.
Creation of serotype M2, M6, and M28 fasX mutant strains
Serotype M2, M6, and M28 GAS strains in which the fasX gene has been replaced with a spectinomycin resistance cassette were created using the primers listed in Table S2. Using construction of strain M2ΔfasX as an example, primers FASXA/M2FASXB and primers FASXC/M2FASXD were used to amplify 1kb regions upstream and downstream of the fasX gene. In addition, primers M2FASXSF/FASXSR were used to amplify a spectinomycin resistance cassette from plasmid pSL60 (Lukomski et al., 2000). All three PCR products were joined together via overlap-extension PCR using primers FASXA/M2FASXD. The resultant 3kb PCR product was cloned into vector pCR2.1 (Life Technologies) and sequenced to ensure the absence of mutations. The plasmid insert was amplified by PCR and transformed into parental M2 isolate MGAS10270. Replacement of the fasX gene with the spectinomycin resistance cassette was confirmed via PCR and sequencing.
Complementation of fasX mutant strains
Complementation of fasX mutant strains was through use of plasmid pFasX (Ramirez-Pena et al., 2010), a fasX containing derivative of the chloramphenicol-resistant vector pDC123 (Chaffin & Rubens, 1998).
Creation of the serotype M2 pilus locus mutant strain M2ΔPIL
A serotype M2 GAS strain that produces no cell surface pili was created using the primers listed in Table S2. Briefly, primers M2PILA/B and primers M2PILC/D were used to amplify 1kb regions upstream and downstream of the pilus biosynthesis locus. These two PCR products were joined together via over-lap extension PCR using primers M2PILA/D. The resultant PCR product was BamHI digested and cloned into similarly digested vector pBBL740 (Zhu et al., 2009). A 1 kb spectinomycin resistance cassette was inserted in the PvuII restriction site located near the middle of the cloned insert. The 3kb insert was amplified by PCR and used to transform parental M2 isolate MGAS10270. Deletion of the pilus genes, and insertion of the spectinomycin cassette, was confirmed via PCR and sequencing.
Quantitative RT-PCR analysis
RNA was isolated from exponential phase cultures of GAS strains (OD600 0.5), triple DNase-treated (TURBO-DNase; Life Technologies), and used in cDNA synthesis reactions. cDNA was synthesized from total GAS RNA using the reverse transcriptase Superscript III (Life Technologies) per the manufacturer’s instructions. TaqMan quantitative RT-PCR was performed using the CFX Connect Real Time System (Bio-Rad). Gene transcript levels present in different strains were compared to that of parental strains using the ΔΔCT method as described (Shelburne et al., 2008). TaqMan primers and probes for the genes of interest, and the internal control gene tufA, are listed in Table S2.
Isolation of cell wall protein fractions
GAS strains were grown to the exponential phase (OD600 of 0.5) of growth in THY broth. 40 ml aliquots were recovered and the cells were pelleted by centrifugation (5,000 g for 11 min). The bacterial cell pellets were washed once with 10 ml TE buffer before re-suspending in 1 ml of TE-sucrose buffer (47 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.55 M sucrose, 1 mg/mL lysozyme, 250 ug/mL mutanolysin, 250 ug/mL hyaluronidase). Samples were incubated at 37°C with end-to-end rotation in 1.5 ml tubes for two hours, centrifuged 15,000 g for 5 min to pellet the protoplasts, and the supernatants were removed to clean 1.5 ml tubes. The centrifugation was repeated to pellet any insoluble material carried over, and the supernatant was analyzed by Western blot analysis.
Western blot analysis of GAS cell-wall proteins
Protein samples were separated on a 6% SDS-PAGE gel. For use as a loading control the membrane was stained using the MemCode Reversible Protein Stain Kit (Pierce). Subsequently, the membrane was used in Western blot analysis using monoclonal T2 sera (TransEurope Chemicals; used at a 1:500 dilution) as the primary antibody. Monoclonal T2 sera reacts against the major pilus protein of serotype M2 GAS strains. After washing, Alexa Fluor 680 donkey anti-rabbit IgG secondary antibody (Molecular Probes) was used (1:10,000 dilution), and the fluorescent signal was detected using a Li-Cor Odyssey system. For densitometric analysis of the Western data we utilized Li-cor Image Studio Lite Software.
In vitro Transcription Reactions
In vitro transcribed RNAs for use in the RNA:RNA EMSA experiments (Fig. 6) and the in vitro translation experiments (Fig. 7) were created using the MEGAshortscript (FasX, FasXΔ72–77) and MEGAscript (M2.113.FLAG, M28.fctA.FLAG, M6.tee6.FLAG and spd.FLAG mRNAs) kits (Life Technologies), as per the manufacturer’s instructions. Template DNA for use in in vitro transcription reactions were generated by PCR, with a T7 promoter sequence embedded in the 5′ primer to allow subsequent transcription using T7 polymerase (see Table S2 for primer sequences). Following in vitro transcription reactions, the template DNA was removed using TURBO DNase (Life Technologies) and the RNA purified using the RNA Clean and Concentrator-25 kit (Zymo Research). The quantity and quality of purified RNA was assessed utilizing an RNA6000 NANO chip on the Agilent Bioanalyzer 2100 system.
RNA:RNA EMSA
In vitro transcribed RNAs consisting of ∼100 nucleotides of the M2.113, M6.tee6, and M28.fctA coding sequences, FasX (FasX RNA; 205 nt) and FasXΔ72–77 (FasXΔ72–77 RNA; 199 nt) were created. To generate the mRNA probes, an aliquot of the in vitro transcribed RNA was labeled with the Pierce RNA 3′ End Biotinylation Kit (Thermo Sci.), cleaned using an RNA Clean and Concentrator-5 column (Zymo Research), and the quantity and quality of the labeled RNA was assessed using an RNA6000 NANO chip on the Agilent Bioanalyzer 2100 system. To perform the EMSA reactions, labeled mRNA (15 nM) was incubated in the presence or absence of FasX RNA (0, 0.84, 8.4, and 84 nM), FasXΔ72–77 RNA (0 or 31 nM), unlabeled mRNA (0, 6, 63, 630 nM), and/or unlabeled yeast RNA (0 or 1000 nM). Reactions were performed as previously described (Liu, 2012). Samples were electrophoresed through a 5% TBE mini-gel, transferred by semi-dry electroblotter to a positively charged nylon membrane, and UV-crosslinked. The membrane was then blocked for an hour (Odyssey Blocking Buffer, Li-Cor), incubated (RT, 20 min) with Streptavidin IRDye (Molecular Probes), washed, and the labeled RNA was detected using a Li-Cor Odyssey system.
In vitro Translation
Test mRNAs were in vitro transcribed, and consist of 550 nucleotides that encompass the 5’-UTRs and 5’ coding regions of the spd, M2.113, M6.tee6, and M28.fctA genes. All test mRNAs were designed to include the sequence for a FLAG-tag that will be incorporated at the C-terminus if the encoded protein. Inclusion of a FLAG-tag was to enable subsequent analysis of the in vitro translation products via Western blot analysis using an anti-FLAG antibody. Briefly, 50pmol of fctA.FLAG, 113.FLAG, tee6.FLAG, or spd.FLAG mRNA was pre-incubated with 250 pmol of RNA composed of different ratios of FasX and the deletion mutant derivative FasXΔ72–77. The ratio of FasXΔ72–77 to FasX present in the reactions were 250:0, 200:50, 50:50, 50:200, and 0:250 respectively. In vitro translation reactions were then performed using the E. coli S30 Extract System for Linear Templates as previously described (Liu, 2012). Proteins were separated on a 12% SDS-PAGE gel, transferred to nitrocellulose membranes by electro-blotting, and probed with rabbit anti-FLAG (Sigma, 1:2000 dilution). To enable signal generation an Alexa Fluor 680 donkey anti-rabbit secondary antibody (1:10,000, Life Technologies) was used and the signal captured using the Li-Cor Odyssey system.
Tissue culture adherence assay
Our adherence assay was based upon a previously described protocol (Liu et al., 2012), but with some modifications. The human keratinocyte cell line HaCaT was cultured in Dulbecco's modified Eagle's medium: Nutrient Mixture F12 (DMEM: F12; ATCC) supplemented with 10% Fetal Bovine serum (ATCC) and Penicillin-Streptomycin Solution (Life Technologies) that contained 50 µg/ml Streptomycin and 50U/ml Penicillin. For adherence assays, cells were resuspended at a concentration of approximately 1 × 106 cells per ml in DMEM:F12 without any antibiotics, and 900µl seeded into each well of a 12-well tissue culture plate, which was then incubated for 24 h at 37°C with 5% CO2 before proceeding with the adherence experiment. GAS were prepared by growing each strain of a particular serotype to an O.D.600 of 0.4 before pelleting the bacteria from 1 ml of culture and resuspending the pellets in 1 ml of Hyclone Dulbecco’s Phosphate Buffered Saline without Calcium or Magnesium (DPBS; ThermoScientific). Each GAS strain suspension was diluted 100-fold with DPBS and 100µl of the diluted suspension was added to separate wells of the 12-well tissue culture plate (two wells per strain) containing lawns of HaCaT cells. After a 1 min incubation at 37°C (5% CO2) the liquid in the wells was removed, and the wells were washed five times (twice with 1 ml DPBS, followed by twice with 2 ml DPBS, followed by one wash with 1 ml DPBS) to remove non-adherent GAS. Following washing, 1 ml of DPBS containing 1% Saponin (Sigma-Aldrich) was added to each well and the plate was incubated for 15 min at room temperature to allow for lysis of the HaCaT cells. GAS were recovered from each well by scraping the cells from the bottom of each well. The number of adherent bacteria were enumerated following plating of 100 µl of cell suspension containing bacteria from each well onto blood agar plates in duplicate. For the inoculum, the GAS stocks were titered by making 10−6 dilutions of the aforementioned pellet suspension of 1 ml bacterial culture in DPBS. 100 µl of the diluent was used for plating blood agar plates in quadruplicate. The average number of bacteria recovered per ml (cfu/ml) was determined for each GAS strain, and the percentage of adhering bacteria versus the number of bacteria in the initial inoculum was calculated. The experiment was performed in triplicate with the mean (± standard deviation) shown.
Transgenic mouse model of bacteremia infection
The study protocol was approved by the animal care and use committee of the Houston Methodist Research Institute. The humanized transgenic mouse line B6.Cg-Tg(Alb-PLG)1Dgi/J, which expresses human plasminogen (Sun et al., 2004), was purchased from the Jackson Laboratory. Verification of human plasminogen expression by this mouse line was gained via ELISA (data not shown). The three GAS strains used in the animal study were grown in THY broth to the exponential phase of growth, washed twice with PBS, and titered. Groups of 10 mice were infected with 1 × 106 CFUs of individual GAS strains via intraperitoneal injection. The experiment was performed twice and the cumulative data used to generate a Kaplan-Meier survival curve, which was then analyzed for statistical significance using the Log Rank test.
Supplementary Material
Acknowledgments
This research was funded in part by grant R01AI087747 from the National Institute of Allergy and Infectious Diseases (to P.S.). Additional support was provided by the School of Medicine, University of Nevada, and by the Houston Methodist Hospital.
References
- Abbot EL, Smith WD, Siou GP, Chiriboga C, Smith RJ, Wilson JA, Hirst BH, Kehoe MA. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cellular microbiology. 2007;9:1822–1833. doi: 10.1111/j.1462-5822.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Alegre-Cebollada J, Badilla CL, Fernandez JM. Isopeptide bonds block the mechanical extension of pili in pathogenic Streptococcus pyogenes. The Journal of biological chemistry. 2010;285:11235–11242. doi: 10.1074/jbc.M110.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PloS one. 2007;2:e800. doi: 10.1371/journal.pone.0000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen DE, Kalia A. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infection and immunity. 2002;70:1159–1167. doi: 10.1128/IAI.70.3.1159-1167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TN, Liu Z, Cao TH, Pflughoeft KJ, Trevino J, Danger JL, Beres SB, Musser JM, Sumby P. Natural disruption of two regulatory networks in serotype M3 group A Streptococcus isolates contributes to the virulence factor profile of this hypervirulent serotype. Infection and immunity. 2014;82:1744–1754. doi: 10.1128/IAI.01639-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin DO, Rubens CE. Blue/white screening of recombinant plasmids in Gram-positive bacteria by interruption of alkaline phosphatase gene (phoZ) expression. Gene. 1998;219:91–99. doi: 10.1016/s0378-1119(98)00396-5. [DOI] [PubMed] [Google Scholar]
- Colman G, Tanna A, Efstratiou A, Gaworzewska ET. The serotypes of Streptococcus pyogenes present in Britain during 1980–1990 and their association with disease. Journal of medical microbiology. 1993;39:165–178. doi: 10.1099/00222615-39-3-165. [DOI] [PubMed] [Google Scholar]
- Cook SM, Skora A, Gillen CM, Walker MJ, McArthur JD. Streptokinase variants from Streptococcus pyogenes isolates display altered plasminogen activation characteristics - implications for pathogenesis. Mol Microbiol. 2012;86:1052–1062. doi: 10.1111/mmi.12037. [DOI] [PubMed] [Google Scholar]
- Crotty Alexander LE, Maisey HC, Timmer AM, Rooijakkers SH, Gallo RL, von Kockritz-Blickwede M, Nizet V. M1T1 group A streptococcal pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. Journal of molecular medicine (Berlin, Germany) 2010;88:371–381. doi: 10.1007/s00109-009-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections and their sequelae. Advances in experimental medicine and biology. 2008;609:29–42. doi: 10.1007/978-0-387-73960-1_3. [DOI] [PubMed] [Google Scholar]
- Falugi F, Zingaretti C, Pinto V, Mariani M, Amodeo L, Manetti AG, Capo S, Musser JM, Orefici G, Margarit I, Telford JL, Grandi G, Mora M. Sequence variation in group A Streptococcus pili and association of pilus backbone types with lancefield T serotypes. The Journal of infectious diseases. 2008;198:1834–1841. doi: 10.1086/593176. [DOI] [PubMed] [Google Scholar]
- Jaffe J, Natanson-Yaron S, Caparon MG, Hanski E. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Molecular microbiology. 1996;21:373–384. doi: 10.1046/j.1365-2958.1996.6331356.x. [DOI] [PubMed] [Google Scholar]
- Kalia A, Bessen DE. Natural selection and evolution of streptococcal virulence genes involved in tissue-specific adaptations. J Bacteriol. 2004;186:110–121. doi: 10.1128/JB.186.1.110-121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Coulibaly F, Proft T, Baker EN. Crystal structure of Spy0129, a Streptococcus pyogenes class B sortase involved in pilus assembly. PloS one. 2011;6:e15969. doi: 10.1371/journal.pone.0015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur V, Kanjilal S, Hamrick MR, Li LL, Whittam TS, Sawyer SA, Musser JM. Molecular population genetic analysis of the streptokinase gene of Streptococcus pyogenes: mosaic alleles generated by recombination. Mol Microbiol. 1995;16:509–519. doi: 10.1111/j.1365-2958.1995.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Koller T, Manetti AG, Kreikemeyer B, Lembke C, Margarit I, Grandi G, Podbielski A. Typing of the pilus-protein-encoding FCT region and biofilm formation as novel parameters in epidemiological investigations of Streptococcus pyogenes isolates from various infection sites. Journal of medical microbiology. 2010;59:442–452. doi: 10.1099/jmm.0.013581-0. [DOI] [PubMed] [Google Scholar]
- Kratovac Z, Manoharan A, Luo F, Lizano S, Bessen DE. Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. Journal of bacteriology. 2007;189:1299–1310. doi: 10.1128/JB.01301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol. 2001;39:392–406. doi: 10.1046/j.1365-2958.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- Kreikemeyer B, McIver KS, Podbielski A. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends in microbiology. 2003;11:224–232. doi: 10.1016/s0966-842x(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Kreikemeyer B, Nakata M, Koller T, Hildisch H, Kourakos V, Standar K, Kawabata S, Glocker MO, Podbielski A. The Streptococcus pyogenes serotype M49 Nra-Ralp3 transcriptional regulatory network and its control of virulence factor expression from the novel eno ralp3 epf sagA pathogenicity region. Infection and immunity. 2007;75:5698–5710. doi: 10.1128/IAI.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke-Winnebeck C, Paterson NG, Young PG, Middleditch MJ, Greenwood DR, Witte G, Baker EN. Structural model for covalent adhesion of the Streptococcus pyogenes pilus through a thioester bond. The Journal of biological chemistry. 2014;289:177–189. doi: 10.1074/jbc.M113.523761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Trevino J, Ramirez-Pena E, Sumby P. The small regulatory RNA FasX controls pilus expression and adherence in the human bacterial pathogen group A Streptococcus. Mol Microbiol. 2012;86:140–154. doi: 10.1111/j.1365-2958.2012.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukomski S, Hoe NP, Abdi I, Rurangirwa J, Kordari P, Liu M, Dou SJ, Adams GG, Musser JM. Nonpolar inactivation of the hypervariable streptococcal inhibitor of complement gene (sic) in serotype M1 Streptococcus pyogenes significantly decreases mouse mucosal colonization. Infect Immun. 2000;68:535–542. doi: 10.1128/iai.68.2.535-542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Lizano S, Bessen DE. Heterogeneity in the polarity of Nra regulatory effects on streptococcal pilus gene transcription and virulence. Infection and immunity. 2008;76:2490–2497. doi: 10.1128/IAI.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey NN, Goulding D, Gierula M, Turner CE, Dougan G, Edwards RJ, Sriskandan S. RocA truncation underpins hyper-encapsulation, carriage longevity and transmissibility of serotype M18 group A streptococci. PLoS pathogens. 2013;9:e1003842. doi: 10.1371/journal.ppat.1003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti AG, Zingaretti C, Falugi F, Capo S, Bombaci M, Bagnoli F, Gambellini G, Bensi G, Mora M, Edwards AM, Musser JM, Graviss EA, Telford JL, Grandi G, Margarit I. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Molecular microbiology. 2007;64:968–983. doi: 10.1111/j.1365-2958.2007.05704.x. [DOI] [PubMed] [Google Scholar]
- McArthur JD, Cook SM, Venturini C, Walker MJ. The role of streptokinase as a virulence determinant of Streptococcus pyogenes - potential for therapeutic targeting. Curr Drug Targets. 2011 doi: 10.2174/138945012799424589. [DOI] [PubMed] [Google Scholar]
- McArthur JD, Cook SM, Venturini C, Walker MJ. The role of streptokinase as a virulence determinant of Streptococcus pyogenes--potential for therapeutic targeting. Current drug targets. 2012;13:297–307. doi: 10.2174/138945012799424589. [DOI] [PubMed] [Google Scholar]
- McIver KS. Stand-alone response regulators controlling global virulence networks in streptococcus pyogenes. Contrib Microbiol. 2009;16:103–119. doi: 10.1159/000219375. [DOI] [PubMed] [Google Scholar]
- Miller EW, Cao TN, Pflughoeft KJ, Sumby P. RNA-mediated regulation in Gram-positive pathogens: an overview punctuated with examples from the group A Streptococcus. Molecular microbiology. 2014;94:9–20. doi: 10.1111/mmi.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, Maggi T, Taddei AR, Grandi G, Telford JL. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15641–15646. doi: 10.1073/pnas.0507808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M, Kimura KR, Sumitomo T, Wada S, Sugauchi A, Oiki E, Higashino M, Kreikemeyer B, Podbielski A, Okahashi N, Hamada S, Isoda R, Terao Y, Kawabata S. Assembly mechanism of FCT region type 1 pili in serotype M6 Streptococcus pyogenes. The Journal of biological chemistry. 2011;286:37566–37577. doi: 10.1074/jbc.M111.239780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M, Podbielski A, Kreikemeyer B. MsmR, a specific positive regulator of the Streptococcus pyogenes FCT pathogenicity region and cytolysin-mediated translocation system genes. Molecular microbiology. 2005;57:786–803. doi: 10.1111/j.1365-2958.2005.04730.x. [DOI] [PubMed] [Google Scholar]
- O'Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, Schuchat A. Epidemiology of invasive group a streptococcus disease in the United States, 1995–1999. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35:268–276. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- Olsen RJ, Laucirica DR, Watkins ME, Feske ML, Garcia-Bustillos JR, Vu C, Cantu C, Shelburne SA, 3rd, Fittipaldi N, Kumaraswami M, Shea PR, Flores AR, Beres SB, Lovgren M, Tyrrell GJ, Efstratiou A, Low DE, Van Beneden CA, Musser JM. Polymorphisms in regulator of protease B (RopB) alter disease phenotype and strain virulence of serotype M3 group A Streptococcus. The Journal of infectious diseases. 2012;205:1719–1729. doi: 10.1093/infdis/jir825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RJ, Musser JM. Molecular pathogenesis of necrotizing fasciitis. Annual review of pathology. 2010;5:1–31. doi: 10.1146/annurev-pathol-121808-102135. [DOI] [PubMed] [Google Scholar]
- Perez N, Trevino J, Liu Z, Ho SCM, Babitzke P, Sumby P. A Genome-Wide Analysis of Small Regulatory RNAs in the Human Pathogen Group A Streptococcus. PloS one. 2009;4:e7668. doi: 10.1371/journal.pone.0007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley BR, Zahner D, Hatkoff M, Thanassi DG, Scott JR. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Molecular microbiology. 2009;72:1379–1394. doi: 10.1111/j.1365-2958.2009.06727.x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Pena E, Trevino J, Liu Z, Perez N, Sumby P. The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol. 2010;78:1332–1347. doi: 10.1111/j.1365-2958.2010.07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribardo DA, Lambert TJ, McIver KS. Role of Streptococcus pyogenes two-component response regulators in the temporal control of Mga and the Mga-regulated virulence gene emm. Infect Immun. 2004;72:3668–3673. doi: 10.1128/IAI.72.6.3668-3673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS pathogens. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Science. Vol. 305. New York, N.Y.: 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection; pp. 1283–1286. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. Journal of leukocyte biology. 2012;92:509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- Vahling CM, McIver KS. Identification of residues responsible for the defective virulence gene regulator Mga produced by a natural mutant of Streptococcus pyogenes. Journal of bacteriology. 2005;187:5955–5966. doi: 10.1128/JB.187.17.5955-5966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PG, Proft T, Harris PW, Brimble MA, Baker EN. Structure and activity of Streptococcus pyogenes SipA: a signal peptidase-like protein essential for pilus polymerisation. PloS one. 2014;9:e99135. doi: 10.1371/journal.pone.0099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner D, Scott JR. SipA is required for pilus formation in Streptococcus pyogenes serotype M3. Journal of bacteriology. 2008;190:527–535. doi: 10.1128/JB.01520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Liu M, Sumby P, Lei B. The secreted esterase of group a streptococcus is important for invasive skin infection and dissemination in mice. Infection and immunity. 2009;77:5225–5232. doi: 10.1128/IAI.00636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.