Abstract
A method for the rapid diagnosis of early dengue virus (DENV) infection is highly needed. Here, a prototype reverse transcription-recombinase polymerase amplification (RT-RPA) assay was developed. The assay detected DENV RNA in <20 min without the need for thermocycling amplification. The assay enabled the detection of as few as 10 copies of DENV RNA. The designed RT-RPA primers and exo probe detected the DENV genome of at least 12 genotypes of DENV circulating globally without cross-reacting with other arboviruses. We assessed the diagnostic performance of the RT-RPA assay for the detection of DENV RNA in 203 serum samples of patients with clinically suspected dengue. The sera were simultaneously tested for DENV using a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay, quantitative RT-PCR (qRT-PCR), and IgM- and IgG-capture enzyme-linked immunosorbent assays (ELISA). Acute DENV infection was confirmed in 130 samples and 61 of the samples (46.9%) were classified as viremic with qRT-PCR. The RT-RPA assay showed good concordance (κ of ≥0.723) with the RT-LAMP and qRT-PCR assays in detecting the dengue viremic samples. When used in combination with ELISA, both the RT-RPA and RT-LAMP assays increased the detection of acute DENV infection to ≥95.7% (≥45/47) in samples obtained within 5 days of illness. The results from the study suggest that the RT-RPA assay is the most rapid molecular diagnostic tool available for the detection of DENV. Hence, it is possible to use the RT-RPA assay in a laboratory to complement routine serology testing for dengue.
INTRODUCTION
Dengue is one of the most prevalent mosquito-borne viral diseases in the tropics and subtropics. It is estimated that at least 3.6 billion people are at risk of contracting the infection (1). The spectrum of the illness of dengue ranges from mild dengue fever (DF) to severe and fatal dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (2). Dengue viruses (DENV) are the causative agents of dengue (3). There are at least four known antigenically distinct DENV serotypes, DENV-1, DENV-2, DENV-3, and DENV-4 (4), and each serotype contains several phylogenetically distinct genotypes (5). The virus is transmitted following mosquito bites of viremic febrile patients; the viremia phase usually corresponds to the first 3 days of illness (6). Therefore, the early detection of viremic individuals is important not only for patient management but also for early and immediate implementation of appropriate vector-control measures (7).
Molecular techniques to detect the presence of DENV genomic RNA sequences are gradually being accepted as routine procedures for the early detection of DENV infection. The reverse transcription-PCR (RT-PCR) and real-time quantitative RT-PCR (qRT-PCR) are the two most commonly used methods (8–12). However, the requirement for costly nucleic acid amplification instruments and the high level of skill needed for these methods have limited their use to well-equipped laboratories. The introduction of the isothermal nucleic acid amplification method, which obviates the need for any major instrumentation, could change this situation, hence allowing greater access to the nucleic acid amplification method and its wider application in regions where dengue is endemic and resources may be limited (13).
We have previously demonstrated the use of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) for the detection of DENV RNA in a clinical setting (14). More recently, the recombinase polymerase amplification (RPA) assay has emerged as a novel alternative isothermal amplification method for the detection of nucleic acid (15). The RPA assay was reported to take <20 min to perform and requires a constant temperature of only 37°C to 42°C. Both the RPA and RT-RPA assays have been used for the detection of various other infectious agents (15–36), but they have not been implemented for the detection of DENV. In the present study, we describe the development and application of the RT-RPA assay for the detection of DENV infection in a diagnostic laboratory setting using freshly obtained samples from dengue-suspected patients.
MATERIALS AND METHODS
Dengue viruses.
Twenty-two reference DENV strains were used in this study: 11 isolates from Malaysia and 11 isolates from Spain. The Malaysian DENV strains included four genotypes of DENV-1 (genotypes I, II, and III and sylvatic) (37, 38), two genotypes of DENV-2 (Asian I and Cosmopolitan) (39), three genotypes of DENV-3 (genotypes I, II, and III) (40), and two subgenotypes of DENV-4 (subgenotypes IIa and IIb) (10). All of the Malaysian dengue viruses were archived in the Department of Medical Microbiology repository; the 11 dengue viruses obtained from Spain were from the Arbovirus and Imported Viral Diseases, National Centre for Microbiology, Institute of Health Carlos III. The DENV strains from Spain included three isolates of DENV-1 (all genotype III or American/African) (41), three isolates of DENV-2 (Cosmopolitan, American/Asian, and African sylvatic) (42), three isolates of DENV-3 (one genotype I and two genotype III), and two isolates of DENV-4 (all genotype II). These viruses were from collections from Brazil, Paraguay, Venezuela, Thailand, Pakistan, India, South America, Senegal, and the Philippines (see Table S1 in the supplemental material).
Clinical samples.
The study was approved by the University of Malaya Medical Center (UMMC) Medical Ethics Committee (Ethics Committee/Institutional Review Board [IRB] reference number 908.11). A total of 203 serum samples from 203 patients clinically suspected to be infected with DENV at the UMMC during the period from June to August 2013 were obtained for this study. The majority of the patients from this cohort were adults, and the median age of the patients was 29 years (range, 3 to 79 years). The serum samples were divided in the laboratory for simultaneous testing by the serology and nucleic acid amplification assays. A retrospective study was performed to review the patients' medical records for the date of illness onset.
RNA extraction.
The total RNA was extracted from 140 μl of infected culture supernatant or patient serum samples using the QIAamp viral RNA minikit (Qiagen, Germany). All of the RNA extractions were performed according to the manufacturer's protocol. The RNA was eluted in 60 μl of nuclease-free water and stored at −80°C until needed.
Design of DENV-specific RT-RPA assay primers and exo probe.
The DENV-specific primers and exo probe used for the RT-RPA assay were designed from the highly conserved 3′-untranslated region (UTR) consensuses of the genomes of all four DENV serotypes according to the criteria described previously (43). Each 3′-UTR consensus was derived from all of the genotypes of each DENV serotype (see Fig. S1 in the supplemental material). The coverage of the RT-RPA primers and exo probe was validated by evaluating the assay using viral RNA extracted from the different reference DENV strains, as described above.
RT-RPA assay.
A DENV-specific TwistAmp RT exo lyophilized kit was supplied as strips of eight reactions in vacuum-sealed pouches (TwistDx, Ltd., Cambridge, United Kingdom). The RT-RPA was performed in a final reaction volume of 50 μl containing a lyophilized pellet dissolved with 45 μl of TwistAmp RT exo rehydration buffer and 5 μl of the extracted RNA template. The lyophilized RPA pellet contained an optimized blend of enzymes and primers and a fluorescence probe. The enzyme concentrations were based on standard commercial RPA pellets for exonuclease detection (TwistAmp Exo) (15) containing Moloney murine leukemia virus reverse transcriptase for combined RT-RPA (18). All of the enzyme levels were optimized for performance with dengue-specific oligonucleotides. Each reconstituted reaction mixture contained 0.63 mM DenMPF19, 0.84 mM DenMPR11, 0.63 mM DenMPR32, 0.12 mM DenMPExoP9FAM, and 5 U RNase inhibitor (Fermentas, USA). The customized rehydration buffer contained magnesium acetate [Mg(OAc)2], potassium acetate [KOAc], Tris-acetate (pH 8.3), and polyethylene glycol (PEG) (35,000) and was engineered for a 5-μl input volume per 50-μl reaction.
A positive control using 1,000 copies (determined by qRT-PCR) of DENV RNA extracted from the culture supernatant and a negative control (nuclease-free water) were included in each run. The RT-RPA reaction mixtures were incubated at 40°C for 20 min with a brief mixing of the reaction mixtures at 230 s after the start of the incubation. The 6-carboxyfluorescein (FAM) fluorescence of RT-RPA was generated via the endonuclease-mediated cleavage of the TwistAmp exo probe at the tetrahydrofuran abasic site mimic. The fluorescence signal was measured using a Twista real-time fluorometer (TwistDx, Ltd.). In this study, the Twista real-time fluorometer was obtained at a cost almost 4 and 6 times lower than the potential market costs of devices used for RT-LAMP and qRT-PCR, respectively. Twista Studio software, version 2.06.06 (TwistDx, Ltd.) was used to interpret the fluorescence data with a combined threshold and signal slope analysis. The threshold limit was the mean fluorescence intensity (mV) plus 3 standard deviations of that mean. The slope limit was set at 70 mV/min. The RT-RPA reaction was deemed positive when the measured data were higher than the threshold and slope limit within the defined measurement time (adaptable).
Detection limit and cross-reactivity of RT-RPA assay.
The detection limit of the RT-RPA assay for all of the four DENV serotypes was assessed using a panel of serially diluted viral RNAs (1,000, 100, 50, and 10 copy numbers) extracted from the culture supernatant. The RT-RPA detection limit test was repeated eight times. The viral RNA was quantified using the genesig real-time qRT-PCR DENV detection kit (PrimerDesign, Ltd., United Kingdom) as previously described (14, 44). The cross-reactivity of the DENV RT-RPA primers and exo probe was evaluated using the Japanese encephalitis virus (JEV), Chikungunya virus (CHIKV), and Sindbis virus (SINV), all of which were obtained from the Department of Medical Microbiology repository (45, 46). The CHIKV and SINV were isolated from human and mosquito, respectively. No record was available for the origin of the JEV.
Evaluation of RT-RPA assay.
The single-tube RT-RPA assay for the detection of DENV RNA was clinically assessed in 203 serum samples freshly obtained from dengue-suspected patients. The samples were also screened for the presence of DENV RNA using the RT-LAMP and qRT-PCR assays as previously described (14). The qRT-PCR assay was used as a reference for the detection of DENV RNA in the samples. In addition, the samples were simultaneously screened for the presence of anti-DENV IgM and IgG using dengue IgM- and IgG-capture enzyme-linked immunosorbent assay (ELISA) kits (Standard Diagnostics Inc., Republic of Korea), respectively. The test results for RT-RPA, RT-LAMP, qRT-PCR, and ELISA were analyzed and compared. Acute DENV infection was confirmed by the positive detection of DENV RNA with qRT-PCR or the presence of anti-DENV IgM detected with an ELISA. The presence of anti-DENV IgG concurrent with the positive detection of the DENV RNA genome indicated secondary acute DENV infection. The serum sample that tested positive for anti-DENV IgG only was identified as a reflection of a previous DENV infection. The samples that tested positive by the RT-RPA or RT-LAMP assays but negative by qRT-PCR were considered false-positive tests.
Statistical analysis.
All of the statistical analyses were performed using IBM SPSS Statistics, version 21 (IBM Corporation, NY, USA). A probit analysis was performed to calculate the detection limit of the RT-RPA assay at a 95% probability level. The degrees of agreement among the RT-RPA, RT-LAMP, and qRT-PCR test results were measured with the kappa value (κ). A chi-square test (McNemar's exact test, two-tailed) was performed to compare the sensitivities of all of the molecular and serological methods used. In this study, a P value of <0.001 was used to suggest significant results. The diagnostic performances of the RT-RPA and RT-LAMP assays compared with that the qRT-PCR assay were calculated using the web-based Centre for Evidence-Based Medicine Statistics Calculator (http://ktclearinghouse.ca/cebm/toolbox/statscalc).
RESULTS
Design of DENV-specific RT-RPA assay primers and exo probe.
Three RT-RPA primers and one exo probe were designed to allow the simultaneous detection of all four DENV serotypes in a single-tube reaction (Table 1; see also Fig. S1 in the supplemental material). The developed RT-RPA primers and exo probe detected all of the 22 reference DENV strains obtained in Malaysia (see Fig. S2A to D in the supplemental material) and those from the collection available at the Arbovirus and Imported Viral Diseases, National Centre for Microbiology, Institute of Health Carlos III, Spain (see Fig. S2E to G in the supplemental material).
TABLE 1.
RT-RPA primers and exo probe used for the rapid detection of DENV
| Name | Sequence (5′ to 3′)a |
|---|---|
| DenMPF19PS | CAGCATATTGACGCTGGGAGAGACCAGAGATC*C |
| DenMPR11PS | GAACCTGTTGATTCAACAGCACCATTCCATTT*T |
| DenMPR32PS | GAACCTGTTGGATCAACAACACCAATCCATC*T |
| DenMPExoP9FAM | CCATTTTCTGGCGTTCTGTGCCTGGAATGATG(dT-FAM)TG(dSpacer)(dT-BHQ1)GAGACAGCAGGAT-3′C3-Spacer |
Asterisks indicate a phosphothioate link in the backbone to inhibit exonuclease degradation; dT-FAM, thymidine nucleotide carrying fluorescein; dSpacer, tetrahydrofuran basic site mimic; dT-BHQ1, thymidine nucleotide carrying black hole quencher 1; 3′C3-Spacer, C3 spacer at the 3′ end to block elongation.
Detection limit and cross-reactivity of the RT-RPA assay.
The detection limits of the RT-RPA assay for all of the four DENV serotypes were determined using a panel of serially diluted viral RNAs extracted from infected cell culture supernatants with known copy numbers of 1,000, 100, 50, and 10 (quantified by qRT-PCR). The detection limit of the RT-RPA assay at the 95% probability level was 11 DENV genome copies (probit analysis, P ≤ 0.05) (see Fig. S3 and Table S2 in the supplemental material). The RT-RPA assay detected all of the replicates of up to 10 viral RNA copies for DENV-1, DENV-2, and DENV-3 (see Fig. S4A to C in the supplemental material), whereas it tested negative in one of the replicates of the 10 viral RNA copies for DENV-4 (see Fig. S4D in the supplemental material). No amplification of the Japanese encephalitis virus (JEV), Chikungunya virus (CHIKV), or Sindbis virus (SINV) was observed (see Fig. S4E in the supplemental material).
Evaluation of the RT-RPA assay.
The RT-RPA assay for the detection of DENV RNA was evaluated by performance of the assay on 203 freshly obtained serum samples from patients with clinically suspected dengue. The performance of the RT-RPA assay was compared to that of the RT-LAMP and qRT-PCR assays. Out of the 203 samples, acute DENV infection was confirmed in 130 samples (64.0%) by qRT-PCR, a dengue IgM-capture ELISA, or both of these methods (Table 2). Sixty-one of the 130 samples (46.9%) tested viremic by qRT-PCR, and their DENV RNA copies were quantified. Twenty-two of the 130 samples (16.9%) were identified as secondary DENV infections, as viral RNA and dengue-specific IgG were detected concurrently. Out of the 22 samples, anti-dengue IgM was detected in 14 samples but was absent in 8 samples. Sixteen samples were identified as prior DENV infections, as only dengue IgG tested positive.
TABLE 2.
Summary of dengue detection in serum samples (n = 203) from patients with clinically suspected dengue at UMMC using RT-RPA, RT-LAMP, and qRT-PCR assays and IgM and IgG ELISA
| Assay and results (no. of tests) | IgM ELISA results (no. [%]): |
IgG ELISA results (no. [%]) |
||
|---|---|---|---|---|
| Positive (n = 91) | Negative (n = 112) | Positive (n = 96a) | Negative (n = 107) | |
| RT-RPA | ||||
| Positive (50b) | 17 (34.0) | 33 (66.0) | 16 (32.0) | 34 (68.0) |
| Negative (153) | 74 (48.4) | 79 (51.6) | 80 (52.3) | 73 (47.7) |
| RT-LAMP | ||||
| Positive (51c) | 13 (25.5) | 38 (74.5) | 14 (27.5) | 37 (72.5) |
| Negative (152) | 78 (51.3) | 74 (48.7) | 82 (53.9) | 70 (46.1) |
| qRT-PCR | ||||
| Positive (61) | 22 (36.1)d | 39 (63.9)d | 22 (36.1)e | 39 (63.9) |
| Negative (142) | 69 (48.6)d | 73 (51.4) | 74 (52.1) | 68 (47.9) |
Sixteen IgG-positive dengue samples tested negative by qRT-PCR and dengue IgM ELISA.
Three RT-RPA-positive samples tested negative by qRT-PCR.
Five RT-LAMP-positive samples tested negative by qRT-PCR.
Acute DENV infection was confirmed in 130 samples (dengue IgM- or qRT-PCR-positive samples).
Eight dengue IgG- and qRT-PCR-positive samples tested negative by dengue IgM ELISA.
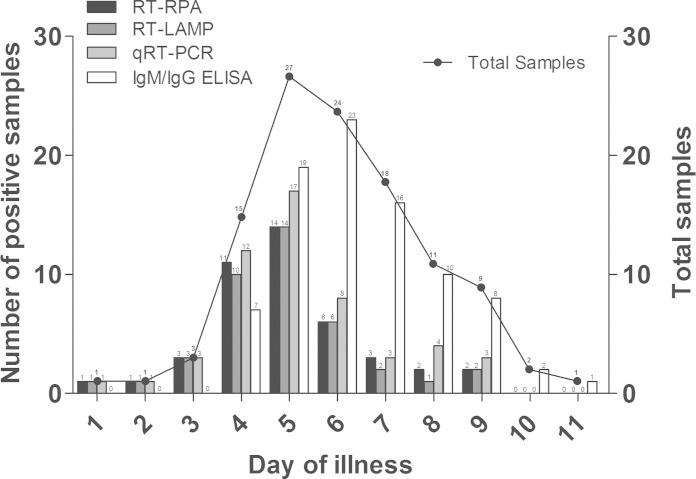
The performance of all of the nucleic acid amplification methods (RT-RPA, RT-LAMP, and qRT-PCR) and serological assays (IgM/IgG ELISA) was analyzed based on the day of illness when the samples were obtained (Fig. 1). The date of illness onset was available for 112 (out of 130) dengue-confirmed cases, and the samples ranged from day 1 to day 11 of illness (mean at day 6.0). Of the samples, 75% (84/112) were obtained between day 4 and day 7 of illness. The nucleic acid amplification assays tested positive in 75.0 to 85.0% (15 to 17 out of 20) of the samples obtained before day 5 of illness; the proportion decreased to 51.9 to 63.0% (14 to 17 out of 27) for samples obtained on day 5 of illness and to 16.9 to 27.7% (11 to 18 out of 65) for those obtained after day 5 of illness. In contrast, the IgM/IgG ELISA tested positive in 35.0% (7/20), 70.4% (19/27), and 92.3% (60/65) of the samples obtained before, during, and after day 5 of illness, respectively.
FIG 1.
Comparison of the performance of various dengue diagnostic methods against laboratory-confirmed dengue samples according to the day of illness (n = 112).
DENV was detectable in patients ranging from day 1 to day 9 of illness; the viral loads ranged from 2.97 to 6.67 log10 DENV RNA copies/ml of serum (Fig. 2). Viral loads of <2.93 log10 RNA copies/ml in viremic patients were beyond the detection limit of qRT-PCR (10 RNA copies, equivalent to 2.93 log10 RNA copies/ml of serum). The mean viral load was 6.04 log10 RNA copies/ml on day 3 of illness and decreased to 5.73 log10 RNA copies/ml on day 4, 5.57 log10 RNA copies/ml on day 5, 4.27 log10 RNA copies/ml on day 6, 4.58 log10 RNA copies/ml on day 7, 3.91 log10 RNA copies/ml on day 8, and 5.11 log10 RNA copies/ml on day 9. The mean viral loads were not available for day 1 and day 2 of illness, as only one sample that tested positive by qRT-PCR for each of these days was obtained.
FIG 2.
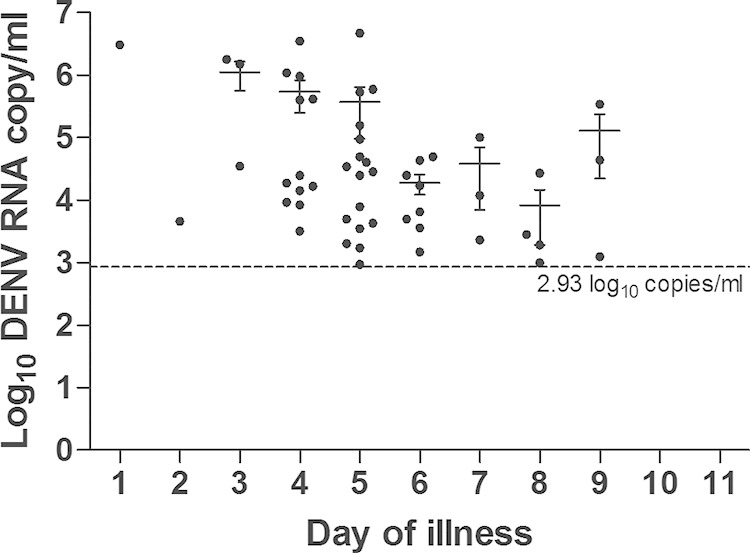
Viral loads of the patients' sera that tested positive by qRT-PCR according to the day of illness (n = 52). The dashed line indicates the detection limit of qRT-PCR. The error bars indicate the standard errors of the viral loads from the mean.
The performance of the RT-RPA and RT-LAMP assays in comparison to the qRT-PCR assay is summarized in Table 3. Kappa (κ) values of 0.790 (P < 0.001) and 0.723 (P < 0.001) were obtained when the RT-RPA assay was compared with the qRT-PCR and RT-LAMP assays, respectively. Using the results obtained by qRT-PCR as a reference, 14 and 15 viremic samples were misdetected by the RT-RPA and RT-LAMP assays, respectively; 92.9% (13/14) and 60.0% (9/15) of the misdetected samples had viral loads of <50 RNA copies (Fig. 3). Using the present parameters, 3 and 5 samples were positively detected by the RT-RPA and RT-LAMP assays, respectively, but these samples were found to be negative by qRT-PCR (Table 3).
TABLE 3.
Diagnostic performance of the RT-RPA and RT-LAMP assays against that of the qRT-PCR assay in serum samples (n = 203) from patients with clinically suspected dengue at UMMC
| Assay and results | qRT-PCR results (no.)a |
Sensitivity (% [95% CIb]) | Specificity (% [95% CI]) | PPVc (% [95% CI]) | NPVd (% [95% CI]) | |
|---|---|---|---|---|---|---|
| Pos | Neg | |||||
| RT-RPAe,f | ||||||
| Pos | 47 | 3g | 77.0 (65.1–85.8) | 97.9 (94.0–99.3) | 94.0 (83.8–97.9) | 90.8 (85.2–94.5) |
| Neg | 14 | 139 | ||||
| RT-LAMPh | ||||||
| Pos | 46 | 5i | 75.4 (63.3–84.5) | 96.5 (92.0–98.5) | 90.2 (79.0–95.7) | 90.1 (84.4–93.9) |
| Neg | 15 | 137 | ||||
Pos, positive; Neg, negative.
CI, confidence interval.
PPV, positive predictive value.
NPV, negative predictive value.
Agreement of results between RT-RPA and qRT-PCR: κ = 0.790, P < 0.001.
Agreement of results between RT-RPA and RT-LAMP: κ = 0.723, P < 0.001.
One RT-RPA false-positive sample tested positive by dengue IgM ELISA.
Agreement of results between RT-LAMP and qRT-PCR: κ = 0.754, p < 0.001.
One RT-LAMP false-positive sample tested positive by the dengue IgM ELISA.
FIG 3.
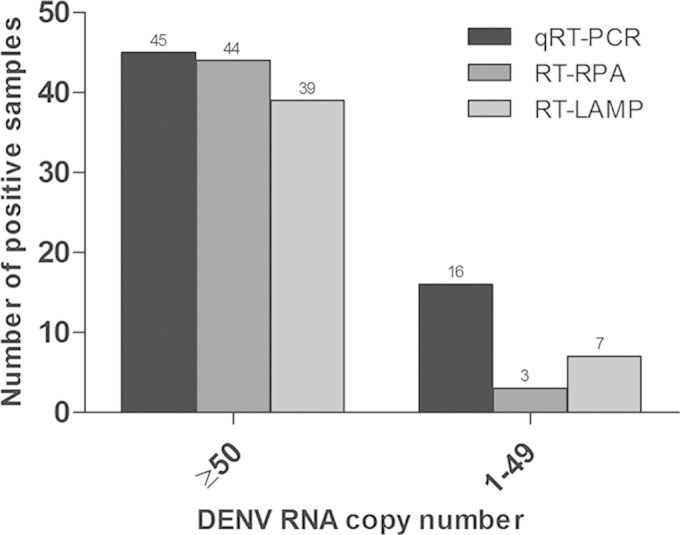
Numbers of viremic samples that tested positive by the qRT-PCR, RT-RPA, and RT-LAMP assays.
The RT-RPA assay detected the DENV genome in 63.8% (30/47) of the acute dengue samples obtained within 5 days of illness, compared with 72.3% (34/47) by the qRT-PCR assay and 61.7% (29/47) by the RT-LAMP assay (Fig. 4). When used in combination with the dengue IgM and IgG ELISA, both the RT-RPA and RT-LAMP assays showed significant increases (P < 0.001) in positive detection to 95.7% (45/47) and 97.9% (46/47), respectively, in comparison with use of the dengue IgM and IgG ELISA alone, which had a positive detection of only 55.3% (26/47).
FIG 4.
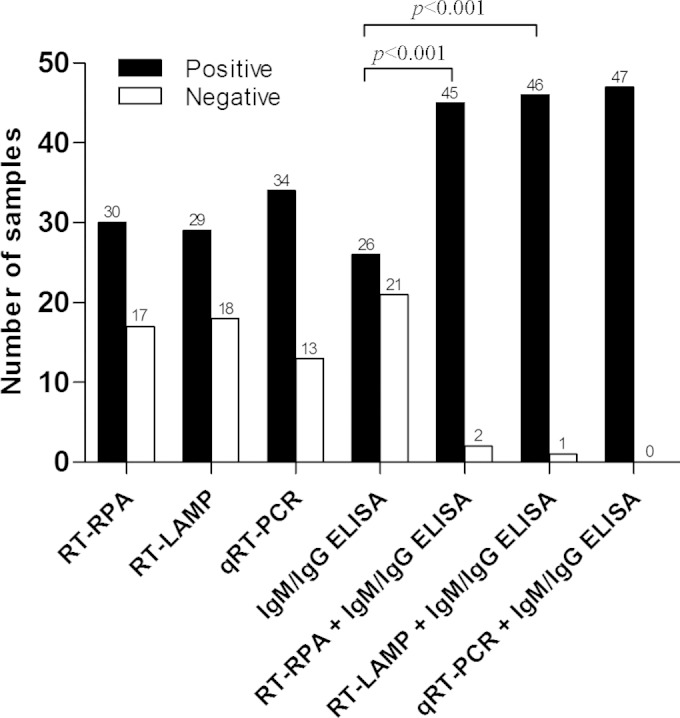
Sensitivity of various dengue diagnostic methods against laboratory-confirmed dengue samples obtained within 5 days of illness (n = 47). The sensitivity between groups was compared using McNemar's exact test (two-tailed).
DISCUSSION
In the present study, a rapid single-tube RT-RPA assay was developed for real-time isothermal detection of DENV genomic RNA in clinical specimens. We demonstrated that the RT-RPA assay detected a panel of 22 globally distributed DENV strains. There was no cross-reactivity of the RT-RPA assay with a number of other common arboviruses. The RT-RPA assay was as sensitive as the RT-LAMP and qRT-PCR assays for DENV detection in viremic patient serum samples.
This is the first study describing the application of the RT-RPA assay for the detection of DENV infection in clinically diagnosed patient samples. The detection limit of the RT-RPA assay (50 copies of DENV RNA) was similar to that of RT-RPA assays previously reported for other RNA viruses; the detection limits ranged from 10 to 100 copies of viral RNA (18, 21, 23, 24, 26, 34). The performance of the RT-RPA assay was superior to that of the RT-LAMP assay we previously developed for the detection of DENV (14), which had a detection limit of 100 DENV RNA copies. It is worth mentioning that the sensitivity of the RT-LAMP assay for DENV detection varied when it was evaluated on different patient cohorts. In this study, the sensitivity of the RT-LAMP assay was only 75.4%, which was lower than that of the previously assessed RT-LAMP assay (92.5%). The variation could be due to random effects in the target amplification that occurred when the viral titer of the samples was below the detection limit of the assay. As previously mentioned, 100% reproducibility was only achieved in samples with at least 100 copies of viral RNA (14).
In the current study, the RT-RPA assay was superior to the RT-LAMP assay for the detection of DENV in patient serum samples. The qRT-PCR assay, however, was still the most sensitive method, especially for detecting a low viral RNA titer (<50 copies). The possibility of false-positive detections, especially in those samples with low copy numbers, was unlikely, as the qRT-PCR used in this study used the TaqMan probe to increase the specificity of the assay (47). Most of the viremic samples that were misdetected by the RT-RPA and/or RT-LAMP assays had viral loads of <50 RNA copies. We showed here that the low viral load was accompanied with a rise in the antibody titers. More than 80% of the misdetected viremic samples were positive when detected by IgM/IgG ELISA, and these samples were mainly among those obtained beyond day 4 of illness (data not shown). When used in combination with an ELISA, the RT-RPA and RT-LAMP assays still performed as well as qRT-PCR for increasing the diagnostic coverage of febrile dengue patients. However, the false-positive detection of the RT-RPA and RT-LAMP assays could reflect the false-negative detection of qRT-PCR, possibly due to mismatches of qRT-PCR primers and probes for certain DENV strains. This possibility needs to be verified using virus isolation.
The application of the molecular detection of DENV genomes primarily depends on the time of sample collection following the onset of disease. In this study, we demonstrated that the qRT-PCR, RT-RPA, and RT-LAMP assays were useful for the diagnosis of dengue in febrile patients during the first 5 days of illness. The use of nucleic acid amplification assays, however, is not promoted for samples obtained after day 5 of illness, which comprised 58% of the total samples used in our study. At this stage of the infection, there is already a significant rise in the antibody levels. Therefore, knowing the exact day of illness is desirable for choosing the appropriate method for dengue diagnosis to be cost-effective, especially in low-resource countries where dengue is endemic.
Although the nucleic acid-based diagnostics for DENV infection are more widely available in referral hospitals than in clinics, the majority of the febrile patients in countries where dengue is endemic prefer to seek early medical care from community clinics at their own convenience (48). Patients are usually only referred to hospitals for the confirmation of DENV infection in severe cases. It is shown in our study that >80% of the dengue patients presenting to the hospital were already beyond day 4 of illness. Most of the dengue-suspected patients from the community near the UMMC therefore came to the hospital long after the onset of fever (49). Many of these patients were already in the convalescence phase of dengue. Alternatively, the low percentage of early-phase samples obtained could be a reflection of the unavailability of molecular diagnostics for dengue at the hospital, and, therefore, blood samples were not taken from patients exhibiting fever for <4 days. Hence, the implementation of nucleic acid amplification assays such as RT-RPA for routine dengue diagnosis in hospitals and clinics could improve the early detection of viremic dengue patients. The early detection of DENV infection would in turn improve the recognition of severe dengue warning signs, such as impending intravascular leakages (49).
During the viremic phase of DENV infection, patients harbor tremendous amounts of viruses in their blood. The virus can be transmitted following mosquito bites. It was reported that the 50% mosquito infectious dose (MID50) for DENV ranged from 6.27 to 7.52 log10 RNA copies/ml of human plasma (50). In our study, 50 and 100 DENV RNA copies were equivalent to 3.63 and 3.93 log10 RNA copies/ml of serum, respectively. The RT-RPA (detection limit of 50 RNA copies) and RT-LAMP (detection limit of 100 RNA copies) assays (14) can therefore still be useful for detecting the potential viremic patients who are contagious. This information is crucial for the immediate implementation of preventive measures and precautionary behavior to prevent mosquito bites. In our sample cohort, high viral loads (up to 5.53 log10 RNA copies/ml) were found in some patients up to day 9 of illness, which was comparable to earlier findings (51, 52). This also implies that it is possible for DENV to be transmitted by patients even after the defervescence phase. However, the DENV RNA detected in these convalescent patients did not necessarily reflect the presence of infectious viruses; it may have been just the residual genome nucleic acid.
Although it is still in an early stage of development, the RT-RPA assay reported here has several advantages compared to the RT-LAMP assay, including (i) faster assay run time (<20 min for RT-RPA instead of ≥60 min for RT-LAMP), (ii) relative ease of performance (the freeze-dried RT-RPA ready-to-use reaction mixture was designed to simplify the operator workload and minimize pipetting errors), and (iii) lower energy consumption (40°C for RT-RPA versus 63°C for RT-LAMP). These advantages make the RT-RPA assay an attractive diagnostic test for the routine detection of DENV RNA, especially in resource-limited health care centers, as it can be easily performed even by less-skilled staff members. In addition, the RT-RPA assay is more amenable to scaling down for point-of-care applications than the RT-LAMP assay because it requires less energy input (53, 54).
It is possible that the performance of the DENV RPA assay could be improved from that observed in this study. The presence of divalent cations such as Mg2+ and how they are added to the reaction could be optimized to limit the opportunity for spurious amplification, which would improve the assay performance and perhaps eliminate the false-negative results, particularly in samples with low titers. However, this is beyond the scope of this investigation, as in its present form the assay was proven to be as good as or comparable in sensitivity to other methodologies. The encouraging findings from this study, however, justify further improvement of the RT-RPA assay for eventual clinical evaluations and applications.
In summary, the RT-RPA assay developed in our study is specific and simple to perform. The RT-RPA assay has a rapid run time (<20 min) and can detect diverse DENV strains at viral titers up to as few as 10 RNA copies. The RT-RPA assay can greatly enhance the diagnostic coverage of suspected dengue cases, complementing serological assays and improving the diagnosis of dengue in rural clinics and health care facilities in regions where dengue is endemic and resources are limited.
Supplementary Material
ACKNOWLEDGMENTS
This study was financially supported by the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement 282589 (DengueTools).
David Brooks, Olaf Piepenburg, and Oliver Nentwich are employees of TwistDx, Ltd., the owner and producer of the RPA technology.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02648-14.
REFERENCES
- 1.Gubler DJ. 2012. The economic burden of dengue. Am J Trop Med Hyg 86:743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. 1998. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11:480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henchal EA, Putnak JR. 1990. The dengue viruses. Clin Microbiol Rev 3:376–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell PK, Nisalak A. 1967. Dengue virus identification by the plaque reduction neutralization test. J Immunol 99:291–296. [PubMed] [Google Scholar]
- 5.Holmes EC, Burch SS. 2000. The causes and consequences of genetic variation in dengue virus. Trends Microbiol 8:74–77. doi: 10.1016/S0966-842X(99)01669-8. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2009. Clinical management and delivery of clinical services, p. 25−54. In Dengue guidelines for diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 7.Wilder-Smith A, Renhorn KE, Tissera H, Abu Bakar S, Alphey L, Kittayapong P, Lindsay S, Logan J, Hatz C, Reiter P, Rocklov J, Byass P, Louis VR, Tozan Y, Massad E, Tenorio A, Lagneau C, L'Ambert G, Brooks D, Wegerdt J, Gubler D. 2012. DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action 5. doi: 10.3402/gha.v5i0.17273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 30:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seah CL, Chow VT, Tan HC, Can YC. 1995. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J Virol Methods 51:193–200. doi: 10.1016/0166-0934(94)00104-O. [DOI] [PubMed] [Google Scholar]
- 10.AbuBakar S, Wong PF, Chan YF. 2002. Emergence of dengue virus type 4 genotype IIA in Malaysia. J Gen Virol 83:2437–2442. [DOI] [PubMed] [Google Scholar]
- 11.Laue T, Emmerich P, Schmitz H. 1999. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J Clin Microbiol 37:2543–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, Lin TH, Huang JH. 2003. Development of group- and serotype-specific one-step SYBR green I-based real-time reverse transcription-PCR assay for dengue virus. J Clin Microbiol 41:2408–2416. doi: 10.1128/JCM.41.6.2408-2416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill P, Ghaemi A. 2008. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 27:224–243. doi: 10.1080/15257770701845204. [DOI] [PubMed] [Google Scholar]
- 14.Teoh BT, Sam SS, Tan KK, Johari J, Danlami MB, Hooi PS, Md-Esa R, AbuBakar S. 2013. Detection of dengue viruses using reverse transcription-loop-mediated isothermal amplification. BMC Infect Dis 13:387. doi: 10.1186/1471-2334-13-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piepenburg O, Williams CH, Stemple DL, Armes NA. 2006. DNA detection using recombination proteins. PLoS Biol 4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz S, Weber P, Focke M, Faltin B, Hoffmann J, Muller C, Mark D, Roth G, Munday P, Armes N, Piepenburg O, Zengerle R, von Stetten F. 2010. Microfluidic lab-on-a-foil for Nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 10:887–893. doi: 10.1039/b921140c. [DOI] [PubMed] [Google Scholar]
- 17.Shen F, Davydova EK, Du W, Kreutz JE, Piepenburg O, Ismagilov RF. 2011. Digital isothermal quantification of Nucleic acids via simultaneous chemical initiation of recombinase polymerase amplification reactions on SlipChip. Anal Chem 83:3533–3540. doi: 10.1021/ac200247e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Euler M, Wang Y, Nentwich O, Piepenburg O, Hufert FT, Weidmann M. 2012. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J Clin Virol 54:308–312. doi: 10.1016/j.jcv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Euler M, Wang Y, Otto P, Tomaso H, Escudero R, Anda P, Hufert FT, Weidmann M. 2012. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J Clin Microbiol 50:2234–2238. doi: 10.1128/JCM.06504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrman BA, Richards-Kortum RR. 2012. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip 12:3082–3088. doi: 10.1039/c2lc40423k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Euler M, Wang Y, Heidenreich D, Patel P, Strohmeier O, Hakenberg S, Niedrig M, Hufert FT, Weidmann M. 2013. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. J Clin Microbiol 51:1110–1117. doi: 10.1128/JCM.02704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, Parker M, Piepenburg O, Overbaugh J. 2013. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. mBio 4. doi: 10.1128/mBio.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amer HM, Abd El Wahed A, Shalaby MA, Almajhdi FN, Hufert FT, Weidmann M. 2013. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. J Virol Methods 193:337–340. doi: 10.1016/j.jviromet.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd El Wahed A, El-Deeb A, El-Tholoth M, Abd El Kader H, Ahmed A, Hassan S, Hoffmann B, Haas B, Shalaby MA, Hufert FT, Weidmann M. 2013. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS One 8:e71642. doi: 10.1371/journal.pone.0071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krõlov K, Frolova J, Tudoran O, Suhorutsenko J, Lehto T, Sibul H, Mager I, Laanpere M, Tulp I, Langel U. 2014. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. J Mol Diagn 16:127–135. doi: 10.1016/j.jmoldx.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Abd El Wahed A, Patel P, Heidenreich D, Hufert FT, Weidmann M. 2013. Reverse transcription recombinase polymerase amplification assay for the detection of Middle East respiratory syndrome coronavirus. PLoS Curr 5. doi: 10.1371/currents.outbreaks.62df1c7c75ffc96cd59034531e2e8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, Maquieira A. 2014. Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal Chim Acta 811:81–87. doi: 10.1016/j.aca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 28.del Rio JS, Yehia Adly N, Acero-Sanchez JL, Henry OY, O'Sullivan CK. 2014. Electrochemical detection of Francisella tularensis genomic DNA using solid-phase recombinase polymerase amplification. Biosens Bioelectron 54:674–678. doi: 10.1016/j.bios.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Daher RK, Stewart G, Boissinot M, Bergeron MG. 2014. Isothermal recombinase polymerase amplification assay applied to the detection of group B Streptococci in vaginal/anal samples. Clin Chem 60:660–666. doi: 10.1373/clinchem.2013.213504. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Park J, Kim CJ, Cho YK. 2014. Fully integrated lab-on-a-disc for Nucleic acid analysis of food-borne pathogens. Anal Chem 86:3841–3848. doi: 10.1021/ac403971h. [DOI] [PubMed] [Google Scholar]
- 31.Aebischer A, Wernike K, Hoffmann B, Beer M. 2014. Rapid genome detection of Schmallenberg virus and bovine viral diarrhea virus by use of isothermal amplification methods and high-speed real-time reverse transcriptase PCR. J Clin Microbiol 52:1883–1892. doi: 10.1128/JCM.00167-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersting S, Rausch V, Bier FF, von Nickisch-Rosenegk M. 2014. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar J 13:99. doi: 10.1186/1475-2875-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crannell ZA, Castellanos-Gonzalez A, Irani A, Rohrman B, White AC, Richards-Kortum R. 2014. Nucleic acid test to diagnose cryptosporidiosis: lab assessment in animal and patient specimens. Anal Chem 86:2565–2571. doi: 10.1021/ac403750z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escadafal C, Faye O, Sall AA, Weidmann M, Strohmeier O, von Stetten F, Drexler J, Eberhard M, Niedrig M, Patel P. 2014. Rapid molecular assays for the detection of yellow fever virus in low-resource settings. PLoS Negl Trop Dis 8:e2730. doi: 10.1371/journal.pntd.0002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murinda SE, Ibekwe AM, Zulkaffly S, Cruz A, Park S, Razak N, Md Paudzai F, Ab Samad L, Baquir K, Muthaiyah K, Santiago B, Rusli A, Balkcom S. 2014. Real-time isothermal detection of shiga toxin-producing Escherichia coli using recombinase polymerase amplification. Foodborne Pathog Dis 11:529–536. doi: 10.1089/fpd.2013.1663. [DOI] [PubMed] [Google Scholar]
- 36.Hill-Cawthorne GA, Hudson LO, El Ghany MF, Piepenburg O, Nair M, Dodgson A, Forrest MS, Clark TG, Pain A. 2014. Recombinations in Staphylococcal cassette chromosome mec elements compromise the molecular detection of methicillin resistance in Staphylococcus aureus. PLoS One 9:e101419. doi: 10.1371/journal.pone.0101419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh BT, Sam SS, Abd-Jamil J, AbuBakar S. 2010. Isolation of ancestral sylvatic dengue virus type 1, Malaysia. Emerg Infect Dis 16:1783–1785. doi: 10.3201/eid1611.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teoh BT, Sam SS, Tan KK, Johari J, Shu MH, Danlami MB, Abd-Jamil J, MatRahim N, Mahadi NM, AbuBakar S. 2013. Dengue virus type 1 clade replacement in recurring homotypic outbreaks. BMC Evol Biol 13:213. doi: 10.1186/1471-2148-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chee HY, AbuBakar S. 2003. Phylogenetic investigation of dengue virus type-2 isolated in Malaysia. Dengue Bull 27:100–107. [Google Scholar]
- 40.Kobayashi N, Thayan R, Sugimoto C, Oda K, Saat Z, Vijayamalar B, Sinniah M, Igarashi A. 1999. Type-3 dengue viruses responsible for the dengue epidemic in Malaysia during 1993-1994. Am J Trop Med Hyg 60:904–909. [DOI] [PubMed] [Google Scholar]
- 41.Kurolt IC, Betica-Radic L, Dakovic-Rode O, Franco L, Zelena H, Tenorio A, Markotic A. 2013. Molecular characterization of dengue virus 1 from autochthonous dengue fever cases in Croatia. Clin Microbiol Infect 19:E163−E165. doi: 10.1111/1469-0691.12104. [DOI] [PubMed] [Google Scholar]
- 42.Franco L, Palacios G, Martinez JA, Vazquez A, Savji N, De Ory F, Sanchez-Seco MP, Martin D, Lipkin WI, Tenorio A. 2011. First report of sylvatic DENV-2-associated dengue hemorrhagic fever in West Africa. PLoS Negl Trop Dis 5:e1251. doi: 10.1371/journal.pntd.0001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TwistDx. 2012. TwistAmp DNA amplification kits: combined instruction manual. TwistDx, Cambridge, United Kingdom. [Google Scholar]
- 44.Tan KK, Johari J, Abd-Jamil J, Zulkifle NI, Sulaiman S, Zainal N, Ab-Rahman HA, Sam SS, Teoh BT, AbuBakar S. 2013. Comparison of real time reverse transcription-polymerase chain reaction assays for the detection of dengue virus. JUMMEC 16:16. [Google Scholar]
- 45.Johari J, Kianmehr A, Mustafa MR, Abubakar S, Zandi K. 2012. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int J Mol Sci 13:16785–16795. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sam IC, Loong SK, Michael JC, Chua CL, Wan Sulaiman WY, Vythilingam I, Chan SY, Chiam CW, Yeong YS, AbuBakar S, Chan YF. 2012. Genotypic and phenotypic characterization of chikungunya virus of different genotypes from Malaysia. PLoS One 7:e50476. doi: 10.1371/journal.pone.0050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland PM, Abramson RD, Watson R, Gelfand DH. 1991. Detection of specific polymerase chain reaction product by utilizing the 5′→3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A 88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao XT, Ngo TN, Wills B, Kneen R, Nguyen TT, Ta TT, Tran TT, Doan TK, Solomon T, Simpson JA, White NJ, Farrar JJ. 2002. Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Trop Med Int Health 7:125–132. doi: 10.1046/j.1365-3156.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 49.Sam SS, Omar SF, Teoh BT, Abd-Jamil J, AbuBakar S. 2013. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS Negl Trop Dis 7:e2194. doi: 10.1371/journal.pntd.0002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, Dui le T, Nguyen HL, Farrar JJ, Holmes EC, Rabaa MA, Bryant JE, Nguyen TT, Nguyen HT, Nguyen LT, Pham MP, Luong TT, Wills B, Nguyen CV, Wolbers M, Simmons CP. 2013. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erra EO, Korhonen EM, Voutilainen L, Huhtamo E, Vapalahti O, Kantele A. 2013. Dengue in travelers: kinetics of viremia and NS1 antigenemia and their associations with clinical parameters. PLoS One 8:e65900. doi: 10.1371/journal.pone.0065900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. 2011. Kinetics of viremia and NS1 antigenemia are shaped by immune status and virus serotype in adults with dengue. PLoS Negl Trop Dis 5:e1309. doi: 10.1371/journal.pntd.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asiello PJ, Baeumner AJ. 2011. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 11:1420–1430. doi: 10.1039/c0lc00666a. [DOI] [PubMed] [Google Scholar]
- 54.Craw P, Balachandran W. 2012. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12:2469–2486. doi: 10.1039/c2lc40100b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.