Abstract
Staphylococcus aureus (S. aureus) is a virulent bacterium that abundantly colonizes inflammatory skin diseases. Since S. aureus infections occur in an impaired skin barrier, it is important to understand the protective mechanism through cutaneous immune responses against S. aureus infections and the interaction with Staphylococcal virulence factors. In this review, we summarize not only the pathogenesis and key elements of S. aureus skin infections, but also the cutaneous immune system against its infections and colonization. The information obtained from this area may provide the groundwork for further immunomodulatory therapies or vaccination strategies to prevent S. aureus infections.
Keywords: Staphylococcus aureus, Skin infection, Cutaneous immune system
INTRODUCTION
Staphylococcus aureus (S. aureus) most commonly causes skin infections where the skin barriers are impaired from multiple factors, including wounding [1]. S. aureus infections show minor symptoms without spreading to other sites and are easily treated with antibiotics. However, these infections can become more invasive and cause life-threatening infections such as bloodstream infections, pneumonia, abscesses, endocarditis, and surgical site infections [2–4]. Moreover, methicillin-resistant staphylococcus aureus (MRSA) is a problem to communities and healthcare settings [5]. The rates of infections linked to MRSA are soaring, and MRSA is currently the leading cause of invasive illness, resulting in more fatalities worldwide. A recent study in the US demonstrated that 76% of all bacterial infections in emergency rooms were identified as MRSA stains [5].
It is well accepted that barrier functions of the skin localize to the stratum corneum (SC), the outer-most layer of the skin [6]. The SC deploys various barrier functions to defend an organism from external insults, and pathogenic microbes, including S. aureus, and to maintain internal homeostasis. Although the composition of SC, e.g., pH, contents of water, lipids, and antimicrobial peptides, is optimized to prevent bacterial infection and growth, changes in these factors due to barrier disruption increase the susceptibility to infection [7–9].
In this review, we summarize the pathogenesis of the most virulent bacteria S. aureus infections, and cutaneous immune responses against their infections and colonization. The information obtained from this area will provide the groundwork for further immunomodulatory therapies or vaccination strategies, which help to prevent S. aureus infections.
Staphylococcus aureus (S. aureus) AND ITS VIRULENCE FACTORS
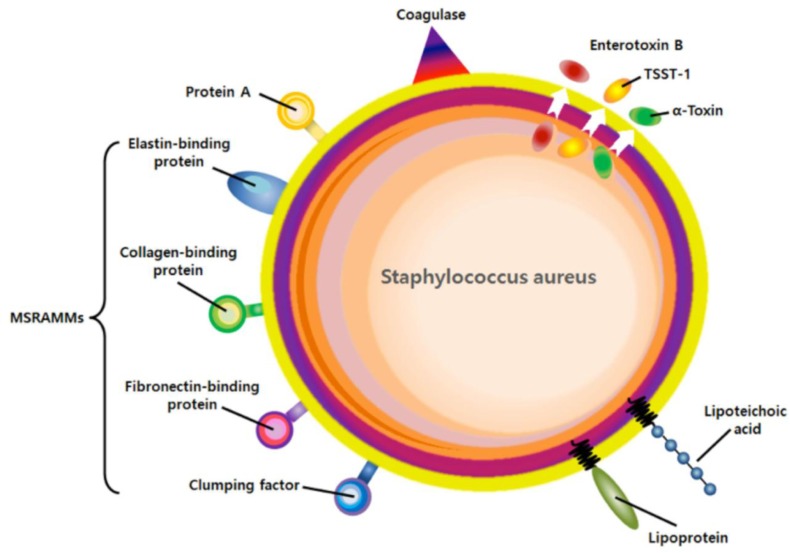
S. aureus is a gram-positive opportunistic pathogen that is normally found on the skin and nose of appropriately 25–30% of healthy adults and in 25% of hospital workers [9,10]. Colonization provides a reservoir from which bacteria can be introduced, usually resulting in a localized collection of pus, known as an abscess or furuncle, cellulitis, impetigo, and scalded skin syndrome [11]. Once S. aureus disseminate into the bloodstream and spread to the organs, the organism spreads widely to peripheral sites in the distant organs, leading to serious illnesses known as bacteremia, sepsis, osteomyelitis, and infective endocarditis [12,13]. The prevalence of these infections has apparently increased owing to higher rates of colonization, immunosuppression, a greater use of surgical implants, and dramatic increases in antibiotic resistance [3]. It is critical to understand the mechanisms of skin S. aureus colonization, which is an important risk factor for subsequent infection (Fig. 1).
Fig. 1.
Virulent factors of S. aureus.
The antibiotic resistance of S. aureus is due to the acquisition of a new penicillin-binding protein, penicillin-binding protein 2a (PBP2a), which has cross-resistance to all β-lactam antibiotics [14]. A common drug-resistance strain, methicillin resistant S. aureus (MRSA) is resistant not only to antibiotic methicillin, but also to other drugs in the same class, including penicillin, amoxicillin, and oxacillin [15]. MRSA infections normally occur in patients in hospitals and other health facilities, especially in the elderly, the very sick, and those with an open wound or catheter in the body (health care-associated MRSA) [15,16]. Until the early 20th century, MRSA rarely caused infections among community members, and not in hospital settings. The first outbreak of community acquired MRSA infections was reported at 1991 and aggressive MRSA infections started to be reported in the late 1990s associated with necrotizing pneumonia or pulmonary abscess and sepsis [17]. The U.S. Centers for Disease Control and Prevention (CDC) estimated in 2008 that about 12% of MRSA infections are now community-associated [18,19]. The strain responsible for these infections is the USA400 clonal type strain (also called MW2 strain) [20]. Subsequently, other clonal outbreaks of skin infection by MRSA were also reported among prison inmates, soldiers, and athletes. The strain responsible for these infections was USA300 (LAC strain) [21]. More recently, some clonal strains of S. aureus have been identified to be resistant to the antibiotic vancomycin, the last drug to which the organism had been uniformly sensitive [22]. These organisms are known to be vancomycin-intermediate-resistant S. aureus (VISA) and vancomycin-resistant Staph aureus (VRSA).
1. Microbial surface components recognizing adhesive matrix molecules
The extracellular matrix (ECM) is the extracellular part of the multicellular structure that provides physical structural and biochemical support to the surrounding cells, such as cell adhesion, migration, and differentiation [23]. In addition, ECM also provides the attachment of colonizing micro-organisms [24]. Many microorganisms express cell surface adhesions that mediate microbial adhesion to the ECM of the host tissue. Over the last 20 years, the genomes of many isolates of S. aureus have been sequenced and different assays have been employed to identify virulence factors associated with colonization or invasion [25]. When S. aureus colonization occurs in the skin, human extracellular matrix proteins, e.g., fibronectin, fibrinogen, laminin, elastin, and collagen recognize invaded S. aureus [23,26]. This ECM binding adhesin family is called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) [27] (Fig. 1).
MSCRAMMs are cell-wall anchored proteins and exhibit specific, high-affinity binding of collagen, fibronectin, fibrinogen, elastin, laminin, von Willebrand factor, vitronectin, and thrombospondin. MSCRAMMs were identified by their ability to bind ECM such as elastin (EbpS), fibrinogen (ClfA, ClfB), fibronectin (FnbpA, FnbpB), and Collagen (Cna) [27]. More than twenty MSCRAMMs have been identified, but the contribution of individual MSCRAMMs to the infection and colonization process is not clear. This ambiguity might be due to the functional redundancy with regard to the host-bacterial protein recognition. For example, S. aureus express at least seven fibrinogen-binding proteins with higher affinity to fibrinogen. Moreover, individual MSCRAMMs might only be important in particular pathological conditions that are not fully addressed by current experimental models.
2. Secreted Staphylococcal virulence factors
S. aureus secrete a number of molecules to kill host immune cells, to stimulate host immune cells, and to inhibit neutrophil recruitment (chemotaxis inhibitory protein) [26, 28]. Pore-forming toxins of S. aureus have the capacity to lyse host cells, including α-hemolysin, β-hemolysin, γ-hemolysin, Panton-Valentine leukocidin (PVL), leukocidin E/D, and leukocidin M/F-PV-like [29]. PVL encoded in a prophage is a cytotoxin and has initially been epidemiologically linked with CA-MRSA cutaneous infections [30]. These are soluble monomers prior to engagement of the target cell membrane with subsequent formation of an aqueous membrane pore. Membrane pore formation is responsible for not only immediate host cell lysis, but also contributes to the penetration of epithelial barriers and evasion of the immune system, thus creating survival niches for pathogens [29,31]. Pore-forming toxins can also contribute to the induction of inflammation and the manifestation of local inflammation and systemic infections [29,31]. Clearly, pore-forming toxins are not the sole factors that drive sepsis progression, but they often act in combination with other bacterial virulent factors, such as lipoteichoic acid, peptidoglycan, or MSCRAMMs.
3. Immunoglobulin binding molecules of S. aureus
S. aureus produces a virulence factor, protein A (SpA), which is a LPxTG mediated cell-wall anchoring protein binding tightly to the Fcγ portion of IgG [32]. In addition, SpA has an ability to stimulate B lymphocyte proliferation, provoking their clonal expansion [33]. The binding of Fcγ on SpA on the cell surface of S. aureus could mask the underlying surface antigens and inhibit opsonophagocytic death by neutrophils.
Staphylococcal binder of immunoglobulin (Sbi) is a secreted protein containing two IgBD-like modules that share amino acid sequence homology to immunoglobulin binding domain of SpA [34]. In addition, Sbi encodes two conserved domains responsible for binding of complement factor C3d and factor H. However, it provides only for Fcγ binding to inhibit opsonophagocytsis, but not stimulate a B cell unlike SpA. Instead, its higher binding affinity to complement factors (C3d and Factor H) leads to an accumulation of C3 to inhibit the activation of complement cascade.
4. Lipoproteins and lipoteichoic acid
Lipoproteins, which are found in both staphylococcal cell walls, are a functionally diverse class of membrane bacterial proteins characterized by an N-terminal lipid moiety. The lipid moiety serves to anchor these proteins to the cell surface [35]. Typically, between 1% and 3% of bacterial genomes encode lipoproteins. Lipoproteins may be divided into five general groups according to their function: adhesion and invasion, cell wall synthesis, nutrient uptake, degradative processes, and sensing and transmembrane signaling. While synthetic lipopeptides have proven to be potential toll like receptor 2 (TLR2) ligands, it is still unclear whether real bacterial lipoproteins function the same as synthetic lipopeptides in Gr+ inflammation and infection [36].
Lipoteichoic acid (LTA) forms another group of TLR2 ligands. LTA is a polyphosphate polymer linked to a glycerol backbone with two acyl chains and accounts for 5% of the cell wall [36,37]. Like lipoproteins, LTA is anchored on the cell membrane via a lipid moiety. Unlike lipoprotein, however, LTA is found only on Gram-positive bacteria, including S. aureus. Additionally, since LTA shares many of its biochemical and physiological properties with lipopolysaccharide (LPS), which is a major inflammatory component in Gram-negative bacteria, it plays an important role in the process of staphylococcal infection and inflammation as LPS does in Gr- inflammation and infection [36–38]. Note that the strains deleted in LTA synthesis genes showed a dramatic reduction in staphylococcal skin and/or systemic infections.
DEFENSIVE SKIN BARRIER
Skin has three structure layers: 1) epidermis, the outermost layers of skin, provides multiple defensive barrier and creates our skin tone; 2) dermis contains hair follicles and sweat glands; and 3) the subcutaneous tissue is made of fat and connextive tissue [39]. Moreover, the outer layer of skin, the epidermis, consists of four separate layers, stratum basale (SB), stratum spinosum (SP), stratum granulosum (SG) and stratum corneum (SC). Because the epidermis, particularly the SC, is positioned at an interface between the internal and external environment, the primary role of the SC is to protect our body from external stresses, including infectious microbes [39].
The composition of the skin is optimized to prevent the growth pathogenic microbes, e.g, low pH and structural sphingolipids [39]. In particular, the skin contains multi-functional, specialized peptides that provide cutaneous immune defense through their activity against pathogenic microorganisms [39,40].
CUTANEOUS IMMUNE DEFENSES
The cutaneous immune system develops two distinct defense mechanisms, innate and adaptive immune systems [39–41]. The first-line defense system is cutaneous innate immunity, which prevents invasion of microbes through physical or chemical barriers. The SC consists of keratinocytes linked by desmosomes and small bridges, and resembles spines that provide intercellular adhesion complexes, thereby creating a physical barrier against pathogenic damages. In addition to the physical barrier, epidermal keratinocytes regulates cutaneous innate immunity through cytokine production, regulating cellular signaling pathways, complement cascade, recruiting other immune cells, and the production of host defense peptides, antimicrobial peptides (AMPs) [40,42]. Importantly, AMPs are a critical innate immune element for immune response of the skin to microbial infection. Two major AMPs in human skin are cathelicidin antimicrobial peptide (CAMP) and β-defensins (hBDs) [43,44].
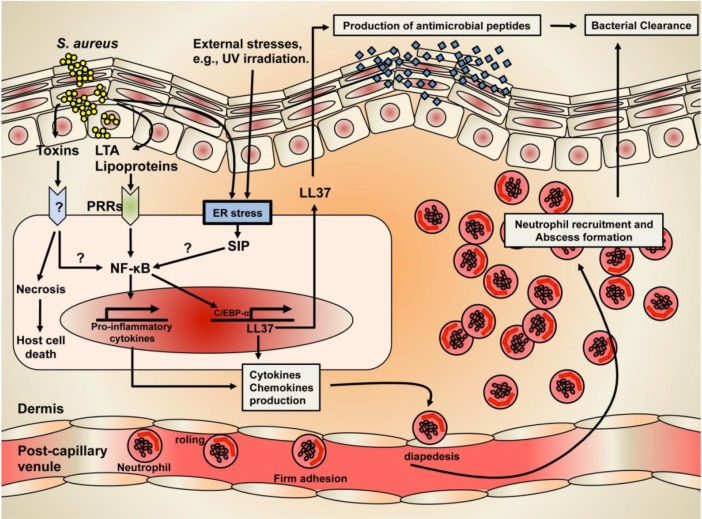
Of these AMPs, CAMP is the first AMP found in the skin. The Vitamin D3-mediated Vitamin D Receptor (VDR) activation is the primary transcriptional regulatory mechanism of CAMP generation in the skin [44,45]. Alternatively, we recently demonstrated that subtoxic external stresses such as UVB irradiation that induce endoplasmic reticulum (ER) stress and increase cellular ceramide production in parallel with stimulated metabolic conversion of sphingosine to sphingosine-1-phosphate (S1P) lead to enhanced CAMP production via an NF-κB activation, independent of the VDR pathway [46,47]. Although a detailed mechanism of how the ER stress-mediated increase in S1P activates NF-B still remains unresolved, our recent studies further demonstrated that CAMP generation likely occurs by an S1P receptor independent mechanism (Fig. 2). CAMP has potent, broad-spectrum antimicrobial activities against virulent S. aureus. It is generally accepted that CAMP disrupts the integrity of the cell membrane of S. aureus, which accounts for their ability to kill invaded S. aureus [40,48]. In addition to antimicrobial activity, CAMP is known to have other functions, e.g., cytokine production, cellular differentiation, and adaptive immunity [49]. Interestingly, CAMP expression is highly increased in skin wounds, and decreased after wound closure, suggesting an important role of CAMP in wound healing [50].
Fig. 2.
The mechanism of Staphylococcal skin infection and cutaneous immune defense.
Another major epidermal AMP is hBDs, which have a wide range of antimicrobial activity and keratinocytes expressing hBD-1, hBD-2, and hBD-3 [43,51]. While hBD-1 is constitutively expressed in the skin, both hBD-2 and hBD-3 are upregulated by membrane receptors, cytokines, or external stresses such as UVB irradiation. Toll-like receptors (TLR) 4, which are evolutionarily conserved pattern recognition receptors, can recognize a bacterial virulence factor, lipopolysaccharides (LPS), increasing the hBD-2 expression in the skin [52]. Moreover, IL-1 regulates hBD-2 production, whereas hBD-3 induction is influenced primarily by IL-6, suggesting that different inducible factors or regulatory mechanisms are responsible for the generation of various hBDs [43,51,53]. Although hBD-2 has no antimicrobial activity against Gram-positive S. aureus, which is the virulent bacteria that colonizes approximately 90% of atopic dermatitis (AD) patients, recent studies revealed that hBD-2 combined with another major AMP, hBD-3 and/or CAMP, has synergistic antimicrobial activity by effectively killing S. aureus [43,51].
In addition to innate immunity, prior studies have demonstrated that the formyl peptide receptor-like (FPRL)1, which is a receptor of CAMP, activates the chemotaxis of neutrophils, monocytes, and CD4 T cells [51,54]. Moreover, hBDs induce the migration of adaptive immune cells via the CC chemokine receptors (CCR) on memory T cells and immature dendritic cells (CCR6), as well as on monocytes and mast cells (CCR2), suggesting that both epidermal AMPs, CAMP and hBDs can link to adaptive immune response [51,54] (Fig. 2).
CONCLUSIONS
Healthy human skin is optimized to prevent the infection/colonization of S. aureus, the most common species of staphylococcus on the skin. However, S. aureus infections of the skin occur in impaired skin caused by numerous factors, including certain skin diseases or wounds. To provide cutaneous immune defense against these infection/colonization, major epidermal AMPs initially function to kill S. aureus. In addition to antimicrobial ability, another role of AMPs is adaptive immune response via activating chemotaxis and/or migration of immune cells, such as T cells, neutrophils, and monocytes (Fig. 2).
Acknowledgments
This study was supported by Nuclear R&D program of Ministry of Education, Science and Technology (MEST), Republic of Korea. The authors thank to Dr. Taj Mann superb editorial assistance.
REFERENCES
- 1.Whitman TJ. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Dis Mon. 2008;54:780–6. doi: 10.1016/j.disamonth.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Belthur MV, Birchansky SB, Verdugo AA, Mason EO, Jr, Hulten KG, Kaplan SL, Smith EO, Phillips WA, Weinberg J. Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J Bone Joint Surg Am. 2012;94:34–42. doi: 10.2106/JBJS.J.01915. [DOI] [PubMed] [Google Scholar]
- 3.Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis. 2010;23:346–58. doi: 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 4.Montanaro L, Testoni F, Poggi A, Visai L, Speziale P, Arciola CR. Emerging pathogenetic mechanisms of the implant-related osteomyelitis by Staphylococcus aureus. Int J Artif Organs. 2011;34:781–8. doi: 10.5301/ijao.5000052. [DOI] [PubMed] [Google Scholar]
- 5.Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: a review of epidemiology, clinical features, management, and prevention. Int J Dermatol. 2007;46:1–11. doi: 10.1111/j.1365-4632.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- 6.Elias PM. Skin barrier function. Curr Allergy Asthma Rep. 2008;8:299–305. doi: 10.1007/s11882-008-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2011;34:261–80. doi: 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery CP, Daniels MD, Zhao F, Spellberg B, Chong AS, Daum RS. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS One. 2013;8:e69508. doi: 10.1371/journal.pone.0069508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble WC. Skin bacteriology and the role of Staphylococcus aureus in infection. Br J Dermatol. 1998;139(Suppl 53):9–12. doi: 10.1046/j.1365-2133.1998.1390s3009.x. [DOI] [PubMed] [Google Scholar]
- 10.Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121:310–5. doi: 10.1016/j.amjmed.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S368–77. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 12.Austin TW, Wallace JF. Staphylococcus aureus bacteremia: A critical review of its treatment and association with infective endocarditis. Infection. 1973;1:214–7. doi: 10.1007/BF01639652. [DOI] [PubMed] [Google Scholar]
- 13.Walpot J, Shivalkar B, Pasteuning WH, Hokken R. Staphylococcus aureus infective endocarditis mimicking a hydatid cyst. Echocardiography. 2010;27:E80–2. doi: 10.1111/j.1540-8175.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 14.Lemaire S, Fuda C, Van Bambeke F, Tulkens PM, Mobashery S. Restoration of susceptibility of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics by acidic pH: role of penicillin-binding protein PBP 2a. J Biol Chem. 2008;283:12769–76. doi: 10.1074/jbc.M800079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemer T, Schneider T, Pinho MG. Auxiliary factors: a chink in the armor of MRSA resistance to beta-lactam antibiotics. Curr Opin Microbiol. 2013;16:538–48. doi: 10.1016/j.mib.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Chavez TT, Decker CF. Health care-associated MRSA versus community-associated MRSA. Dis Mon. 2008;54:763–8. doi: 10.1016/j.disamonth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Bowman C, Mitchell J, Tillotson G. Methicillin-resistant Staphylococcus aureus (MRSA) in the community. Lancet. 1995;346:513–4. [PubMed] [Google Scholar]
- 18.Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: Not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA - its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis. 2011;52:99–114. doi: 10.1093/cid/ciq067. [DOI] [PubMed] [Google Scholar]
- 19.Elias AF, Chaussee MS, McDowell EJ, Huntington MK. Community-based intervention to manage an outbreak of MRSA skin infections in a county jail. J Correct Health Care. 2010;16:205–15. doi: 10.1177/1078345810366679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golding GR, Levett PN, McDonald RR, Irvine J, Quinn B, Nsungu M, Woods S, Khan M, Ofner-Agostini M, Mulvey MR. High rates of Staphylococcus aureus USA400 infection, Northern Canada. Emerg Infect Dis. 2011;17:722–5. doi: 10.3201/eid1704.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchini A, Spitoni MG, Monaco M, Raglio A, Grigis A, Petro W, Menchini M, Pesenti A, Goglio A, Pantosti A. Outbreak of skin and soft tissue infections in a hospital newborn nursery in Italy due to community-acquired meticillin-resistant Staphylococcus aureus USA300 clone. J Hosp Infect. 2013;83:36–40. doi: 10.1016/j.jhin.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Moravvej Z, Estaji F, Askari E, Solhjou K, Naderi Nasab M, Saadat S. Update on the global number of vancomycin-resistant Staphylococcus aureus (VRSA) strains. Int J Antimicrob Agents. 2013;42:370–1. doi: 10.1016/j.ijantimicag.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci. 2012;125(Pt 13):3051–60. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol Mol Biol Rev. 2009;73:407–50. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faghri J, Shahbazzadeh D, Pooshang Bagheri K, Moghim S, Ghasemian Safaei H, Nasr Esfahani B, Fazeli H, Yazdani R, Mirmohammad Sadeghi H. Two Dimensional Structural Analysis and Expression of a New Staphylococcus aureus Adhesin Based Fusion Protein. Iran J Basic Med Sci. 2012;15:725–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez V, Liang X, Horndahl JK, Ganesh VK, Smeds E, Foster TJ, Hook M. Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp) J Biol Chem. 2011;286:29797–805. doi: 10.1074/jbc.M110.214981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palma M, Haggar A, Flock JI. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J Bacteriol. 1999;181:2840–5. doi: 10.1128/jb.181.9.2840-2845.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuMont AL, Torres VJ. Cell targeting by the Staphylococcus aureus pore-forming toxins: it’s not just about lipids. Trends Microbiol. 2014;22:21–7. doi: 10.1016/j.tim.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer CC, Apfalter P, Daxboeck F, Bachhofner N, Stadler M, Blacky A, Diab-Elschahawi M, Assadian O. Prevalence of Panton-Valentine leukocidin genes in methicillin-resistant Staphylococcus aureus isolates phenotypically consistent with community-acquired MRSA, 1999–2007, Vienna General Hospital. Eur J Clin Microbiol Infect Dis. 2009;28:909–12. doi: 10.1007/s10096-009-0721-9. [DOI] [PubMed] [Google Scholar]
- 31.Dhakal BK, Mulvey MA. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe. 2012;11:58–69. doi: 10.1016/j.chom.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harraghy N, Hussain M, Haggar A, Chavakis T, Sinha B, Herrmann M, Flock JI. The adhesive and immunomodulating properties of the multifunctional Staphylococcus aureus protein Eap. Microbiology. 2003;149:2701–7. doi: 10.1099/mic.0.26465-0. [DOI] [PubMed] [Google Scholar]
- 33.Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol. 2008;40:355–61. doi: 10.1016/j.biocel.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch TK, Reuter M, Barthel D, Bohm S, van den Elsen J, Kraiczy P, Zipfel PF, Skerka C. Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b. PLoS One. 2012;7:e47638. doi: 10.1371/journal.pone.0047638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuda S, Tokuda H. Lipoprotein sorting in bacteria. Annu Rev Microbiol. 2011;65:239–59. doi: 10.1146/annurev-micro-090110-102859. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006;18:355–62. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 37.Reichmann NT, Grundling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–84. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 39.Meyer W, Seegers U. Basics of skin structure and function in elasmobranchs: a review. J Fish Biol. 2012;80:1940–67. doi: 10.1111/j.1095-8649.2011.03207.x. [DOI] [PubMed] [Google Scholar]
- 40.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565–73. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R13–8. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Ménard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol. 2013;11:701–12. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 43.Park K, Lee S, Lee YM. Sphingolipids and Antimicrobial Peptides: Function and Roles in Atopic Dermatitis. Biomol Ther (Seoul) 2013;21:251–7. doi: 10.4062/biomolther.2013.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallo RL. In defense of skin: antimicrobial peptides have their day. Interview by Hannah Branch. Future Microbiol. 2013;8:829–31. doi: 10.2217/fmb.13.62. [DOI] [PubMed] [Google Scholar]
- 45.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 46.Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, Uchida Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–30. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, Gallo RL, Saba J, Holleran WM, Uchida Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33:752–62. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park K, Elias PM, Hupe M, Borkowski AW, Gallo RL, Shin KO, Lee YM, Holleran WM, Uchida Y. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol. 2013;133:1942–9. doi: 10.1038/jid.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brogden KA, Johnson GK, Vincent SD, Abbasi T, Vali S. Oral inflammation, a role for antimicrobial peptide modulation of cytokine and chemokine responses. Expert Rev Anti Infect Ther. 2013;11:1097–113. doi: 10.1586/14787210.2013.836059. [DOI] [PubMed] [Google Scholar]
- 50.Merres J, Hoss J, Albrecht LJ, Kress E, Soehnlein O, Jansen S, Pufe T, Tauber SC, Brandenburg LO. Role of the cathelicidin-related antimicrobial Peptide in inflammation and mortality in a mouse model of bacterial meningitis. J Innate Immun. 2014;6:205–18. doi: 10.1159/000353645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974–80. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hostanska K, Melzer J, Amon A, Saller R. Suppression of interleukin (IL)-8 and human beta defensin-2 secretion in LPS-and/or IL-1beta-stimulated airway epithelial A549 cells by a herbal formulation against respiratory infections (BNO 1030) J Ethnopharmacol. 2011;134:228–33. doi: 10.1016/j.jep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Rupec RA, Boneberger S, Ruzicka T. What is really in control of skin immunity: lymphocytes, dendritic cells, or keratinocytes? facts and controversies. Clin Dermatol. 2010;28:62–6. doi: 10.1016/j.clindermatol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Prat C, Haas PJ, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP. A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J Immunol. 2009;183:6569–78. doi: 10.4049/jimmunol.0801523. [DOI] [PubMed] [Google Scholar]