Abstract
Fetal hypoxia triggers compensatory angiogenesis and remodeling through mechanisms not fully elucidated. In response to hypoxia, hypoxia inducible factor drives expression of cytokines that exert multiple effects on cerebral structures. Among these, the artery wall is composed of a heterogeneous cell mix, and exhibits distinct patterns of cellular differentiation and reactivity. Governing these patterns are the vascular endothelium, smooth muscle (SM), adventitia, sympathetic perivascular nerves (SPN) and the parenchyma. Whereas an extensive literature details effects of non-neuronal factors on cerebral arteries, the trophic role of perivascular nerves remains unclear. Hypoxia increases sympathetic innervation with subsequent release of norepinephrine (NE), neuropeptide-y (NPY) and adenosine triphosphate (ATP), which exert motor and trophic effects on cerebral arteries and influence dynamic transitions among smooth muscle phenotypes. Our data also suggests that the cerebrovasculature reacts very differently to hypoxia in fetuses and adults, and we hypothesize that these differences arise from age-related differences in arterial smooth muscle phenotype reactivity and proximity to trophic factors, particularly of neural origin. We provide an integration of recent literature focused on mechanisms by which SPN mediate hypoxic remodeling. Our recent findings suggest that trophic effects of SPN on cerebral arteries accelerate functional maturation through shifts in SM phenotype in an age-dependent manner.
Keywords: Cerebral Arteries, Chronic Hypoxia, Neuropeptide Y, Perivascular Sympathetic Innervation, Smooth Muscle Phenotype, Vasotrophic Effects
INTRODUCTION
Rates of premature births are increasing globally owing to numerous different causes that vary from country to country (1, 2). Despite this heterogeneity, a common feature among causes of premature birth, including gestational diabetes, preeclampsia (3) and placental insufficiency (4) involves varying severities of hypoxia (5). Exposure to reduced levels of oxygen induces multiple intrinsic compensatory mechanisms geared towards preserving oxygen delivery, particularly to the fetal brain and heart (6–8). Persistent exposure to hypoxia eventually overwhelms these intrinsic compensations and subsequently results in pathophysiological changes in the structure and function of many different tissues (9–11). In many cases, fetuses survive initial hypoxic insults but acquire increased long-term risks for altered cerebral and / or cardiovascular homeostasis (12–15). Increased long-term vulnerabilities to coronary, cerebrovascular, and even metabolic diseases secondary to such in utero fetal insults has been defined as fetal programming (14, 16).
Given that the brain has a high oxygen and metabolic demand with no commensurate reserves, its vasculature promptly undergoes angiogenesis and remodeling during hypoxic episodes (17–19). Several structural (18) and functional (20–22) changes in the cerebral vascular network define these remodeling processes. In addition, immaturity of the fetal cerebral vasculature increases the extent of remodeling upon exposure to decreased oxygen tension (10, 23, 24). Because of the highly heterogeneous mix of cells present in the medial layer of the artery wall and their innate plasticity, their reactivity to hypoxia varies significantly with artery type, size, and location (25, 26). Several studies have further suggested that shifts in smooth muscle phenotype are critically important components of vascular remodeling (27–31).
Under normoxic conditions HIF-1α is synthesized but rapidly ubiquitinated and targeted for proteosomal degradation (32). However, during hypoxia, the oxygen regulated HIF-1α-subunit gets stabilized, accumulates and dimerizes with the constitutively expressed HIF-1β-subunit. The HIF dimer then triggers a cascade of events that culminate in the transcription of multiple genes that encode numerous proteins including several angiogenic cytokines (33) (34). Coupling between HIF and angiogenic factors such as erythropoietin (EPO), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), fibroblast growth factor (FGF) and their respective receptors serve to maintain the supply of oxygen and fuels to all cells (34). Cellular metabolic status and survival during hypoxia depend heavily on how successfully these compensatory changes increase vascular density, oxygen delivery and metabolic adaptation to hypoxia.
Previous work characterizing the various cell types in the artery wall reveals that these cells are tightly and uniquely organized into distinct phenotypic categories. In turn, the characteristics of these cells vary in relation to their relative distances to sources of various trophic factors coming from either the parenchyma or the lumen (35). Given that fetal vascular smooth muscle cells are largely immature and subject to high rates of differentiation, smooth muscle cells in all major phenotypic categories including migratory, proliferative, synthetic, and contractile can be found in the fetal arterial wall (Figure 1). In contrast, adult smooth muscle cells are more highly differentiated, at least under normoxic and non-pathologic conditions (29). Extravascular tissue cells, such as neurons and glia in the brain parenchyma, serve as a major source of vasotrophic factors that influence vascular differentiation and adaptation to hypoxia (35–38).
Figure 1. The Continuum of Vascular Smooth Muscle Phenotypes.
The medial layer of arteries consists of a highly heterogeneous mix of cells of diverse origins. Many but not all smooth muscle cells begin as adventitial fibroblasts. These fibroblasts initially differentiate into myofibroblasts and then into smooth muscle myocytes that migrate through the medial layer. Migratory myocytes can then transform into proliferative, synthetic and contractile smooth muscle in response to growth factor stimulation. These patterns of differentiation are not terminal, and can be reversed when certain growth and stress factors are introduced in the local environment. In this manner, the artery wall is highly dynamic and heterogeneous in terms of both its structural and functional characteristics.
Another major source of vasotrophic factors is the vascular endothelium, which can exert both autocrine and paracrine effects on cerebrovascular smooth muscle and thereby contribute to vascular adaptation to chronic hypoxia (39–41). In addition, vasotrophic factors such as VEGF can exert direct angiogenic and remodeling effects on vascular smooth muscle cells during exposure to low oxygen tensions (42, 43). During hypoxic insults or vessel injury, adventitial fibroblasts can also migrate into the intima as myoblasts, which transform into myocytes and finally differentiate into smooth muscle cells (44, 45). Additional progenitor cells have also been suggested to migrate into the vascular medial layer during hypoxic exposure where they transform and differentiate into smooth muscle cells (46, 47).Other fetal stresses, such as maternal food restriction, also can exert trophic influences that alter the structural and functional characteristics of cerebral arteries (48). During maternal food restriction in particular, stress-induced maternal glucocorticoid release can downregulate mediators of fetal angiogenesis and remodeling, including VEGF and its receptors (49). Further studies have implicated glucocorticoids as mediators of smooth muscle redifferentiation toward a non-contractile phenotype, as indicated by an increased colocalization of smooth muscle alpha actin with non-muscle myosin heavy chain II (48).
In addition to humoral mechanisms, a broad variety of evidence supports a major role for the sympathetic autonomic nervous in hypoxic cerebrovascular remodeling (50–52). Sympathetic nerves express receptors for VEGF (53) that stimulate proliferation and differentiation of neural cells consequently increasing the release of vasotrophic factors (53) (54). This review focuses on the role of the sympathetic autonomic system in hypoxic remodeling, particularly in the fetal cerebral vasculature. The main hypothesis addressed here is that the sympathetic perivascular innervation contributes to both structural and functional hypoxic remodeling of the fetal cerebrovasculature through a combination of adrenergic and non-adrenergic mechanisms.
Neuronal Pathways of Hypoxic Remodeling
Hypoxia upregulates sympathetic perivascular innervation in fetal cerebral arteries
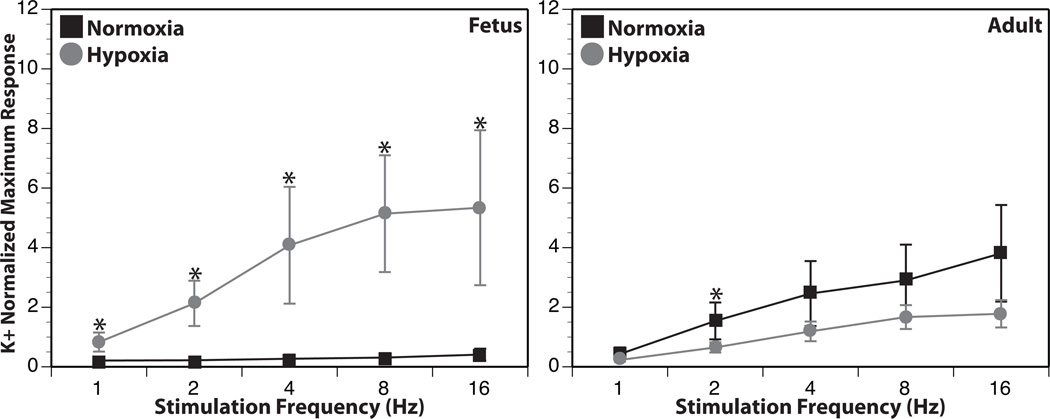
Cerebral blood flow is regulated by numerous factors, including locally produced metabolites, circulating neurohormones, intrinsic myogenic mechanisms, and perivascular nerves (55–57). Under hypoxic conditions, the contribution from perivascular sympathetic nerves can become increasingly important, particularly at the limits of cerebral autoregulation (58). Correspondingly, hypoxic acclimatization can significantly increase the density of the sympathetic innervation (50, 59–61). Consistent with these and other findings (62–64), reactivity to electrical nerve stimulation after 110 days of altitude hypoxic acclimatization was significantly increased in fetal cerebral arteries, even though it was significantly depressed in adult cerebral arteries (Figure 2). This high degree of age-dependence could be accounted for by differences in sympathetic nerve density, neurotransmitter content, quantal release (64), reuptake capacity, cleft width or rates of neurotransmitter degradation (65). Alternatively, dynamic transitions between synthetic and contractile phenotypes during hypoxia may also be age-dependent (29, 66–69). In support of this latter possibility, adult vascular smooth muscle is more resistant to extraneous factors that induce phenotypic transformation, whereas fetal smooth muscle typically exhibits enhanced sensitivity to vasotrophic factors (70–72). Closely related to this concept is the finding that sympathetic nerve endings are typically more diffusely distributed throughout the medial smooth muscle layer in fetal than in adult cerebral (73) and non-cerebral (74) arteries.
Figure 2. Chronic hypoxia modulates contractile responses to transmural nerve stimulation in an age-dependent manner.
Following 110 days of hypoxic acclimatization, reactivity to electrical nerve stimulation in ovine fetal cerebral arteries was significantly enhanced compared to normoxic controls. Conversely, hypoxic acclimatization modestly depressed contractile reactivity to nerve stimulation in adult cerebral arteries. Results are presented as mean ± SEM. For fetal normoxic (FN), fetal hypoxic (FH), and adult normoxic (SNC) groups, N=16. For the adult hypoxic group (SHC), N=21.
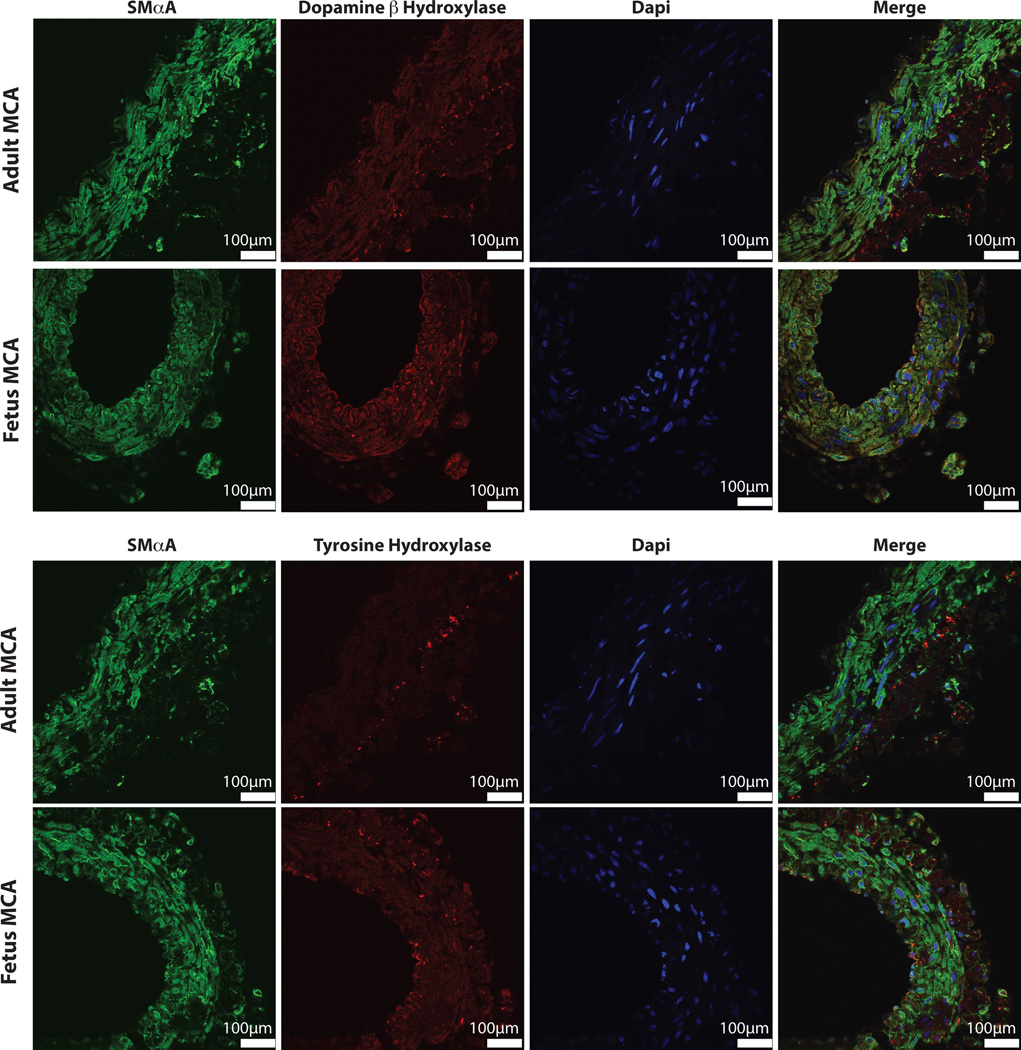
To further explore the effects of postnatal maturation and chronic hypoxia on the characteristics of the sympathetic cerebrovascular innervation, we have recently examined immunoreactivity for tyrosine hydroxylase (TH) and dopamine beta hydroxylase (DβH), the rate-limiting enzyme in the biosynthesis of norepinephrine (Figure 3). In adult cerebral arteries, immunostaining for both TH and DβH revealed well-developed nerve terminals at the medial-adventitial junction with long axes of medial smooth muscle nuclei oriented circumferentially around the lumen. In contrast, immunostaining of fetal arteries demonstrated an adrenergic innervation that was diffuse, was less well organized, and extended significantly into the medial layer. Although smooth muscle cells were more abundant in the fetal arteries, they were more poorly organized and their nuclei were more heterogeneously shaped and oriented than in adult arteries. In the adventitial layer, the density of cell nuclei, which presumably includes fibroblasts and other smooth muscle precursor cells, was relatively sparse in adult arteries but much greater in fetal arteries (Figure 3). These age-related differences in the patterns of cell size and organization in the artery wall emphasize the important effects of maturation on arterial structure and function. These differences also reinforce the view, particularly in fetal arteries, that smooth muscle cells of all major phenotypes including migratory, proliferative, synthetic and contractile, (29) are simultaneously present together with adrenergic nerves in both mature and immature cerebral arteries (Figure 1). Together, these results are highly consistent with the findings that activation of perivascular adrenergic nerves is far more efficacious in adult than in fetal cerebral arteries (75).
Figure 3. Dopamine βhydroxylaseand tyrosine hydroxylase staining demonstrate major developmental differences.
Immunofluorescent staining for both dopamine β hydroxylase (DβH) and tyrosine hydroxylasein ovine middle cerebral arteries revealed distinct and well-developed neuronal terminals at the medial-adventitial border. In adult arteries, long axes of smooth muscle cell nuclei were oriented circumferentially and adventitial cells were relatively sparse. In contrast, within fetal arteries the neuronal terminals were much more diffuse with extensions well into the medial layer. In addition, adventitial cell density was much greater than in adult arteries and smooth muscle nuclei were more abundant but less organized in fetal arteries.
Preliminary studies recently undertaken in our laboratory have examined the effects of chronic hypoxia on the abundance and organization of the sympathetic perivascular innervation in ovine fetal cerebral arteries. These studies suggest, through measurements of both immunostaining and immunoblotting for DβH, that chronic hypoxia dramatically increases the density of sympathetic innervation in ovine cerebral arteries, as reported in other preparations (50). Whereas our model is unique in that hypoxia is imposed in a large mammal by acclimatization at high altitude (3820m) for 110 days (43, 68), the results add further support to the concept that chronic hypoxia induces expansion, and presumably efficacy, of the perivascular sympathetic innervation.
Sympathetic perivascular nerves influence cerebrovascular structure and function
The sympathetic autonomic nervous system is a key regulator of cerebral blood flow (76), particularly under conditions of hypoxia and hypercapnia (77). Sympathetic activation during hypoxia can significantly increase contractile tone, decrease arterial diameter, and enhance wall thickness (78). Such increases in wall thickness may afford protection against elevated wall stress in cerebral blood vessels (79). Importantly, the character and extent of the sympathetic innervation pattern is highly dynamic (80) and subject to modulation during the physiological processes of ageing and development as well as pathophysiological processes such as hypertension and chronic hypoxia (64, 81). In turn, sympathectomy promotes remodeling of the extracellular matrix and promotes redifferentiation of smooth muscle toward non-contractile phenotypes (82). These findings are highly consistent with our preliminary findings that chronic hypoxia upregulated contractile responses to sympathetic activation in fetal cerebral arteries (Figure2).
Sympathetic nerves can also exert potent long-term trophic effects on arterial structure and function (78, 83–85). Some of these changes include increases in arterial wall stiffness secondary to either surgical or chemical sympathectomy in multiple models (86, 87) suggesting that sympathetic nerves mediate preferential decreases in the ratio of collagen to elastin (88). Whereas adult subjects appear relatively resistant to effects of surgical or chemical denervation, fetuses respond more robustly via marked changes in arterial wall composition (71) and distensibility (86). Importantly, most changes in arterial structure and function resulting from sympathectomy are directly attributable to changes in the release, reuptake and degradation of the multiple neurotransmitters released by the sympathetic perivascular innervation (89).
Norepinephrine released from sympathetic nerves exerts trophic effects on arterial smooth muscle
Norepinephrine is the main neurotransmitter released from post-ganglionic sympathetic nerve terminals that innervate vascular smooth muscle cells. In turn, hypoxic upregulation of sympathetic nerve activity increases the local release and activity of norepinephrine (89, 90). The immediate effects of norepinephrine involve induction of contraction in most artery types, including cerebral arteries (91, 92). In contrast, the long-term effects of sympathetic nerve activation include trophic effects of arterial smooth muscle that alter the function of contractile proteins (93) and electrical behavior of the smooth muscle membrane (94). Not surprisingly, the magnitude of norepinephrine induced trophic effects on the artery wall correlate with mass of norepinephrine released (90), access to its receptors (95), adrenergic receptor type and density (92, 96, 97), rates of reuptake, and synaptic cleft width (98)} (64, 99, 100). As indicated by denervation studies, the sympathetic perivascular innervation also influences artery wall thickness, rates of hyperplasia and hypertrophy, and remodeling of the adventitial matrix (72). Norepinephrine released from sympathetic nerves also can exert paracrine trophic effects and induce secretion of other trophic factors (101) that alter arterial phenotype in the cerebral vasculature (102). Together, these results highlight the important role of the sympathetic perivascular innervation, and norepinephrine in particular, on the maturation and differentiation of arterial smooth muscle (54).
Neuropeptide-Y (NPY) released from sympathetic nerve terminals exerts trophic effects on arterial smooth muscle
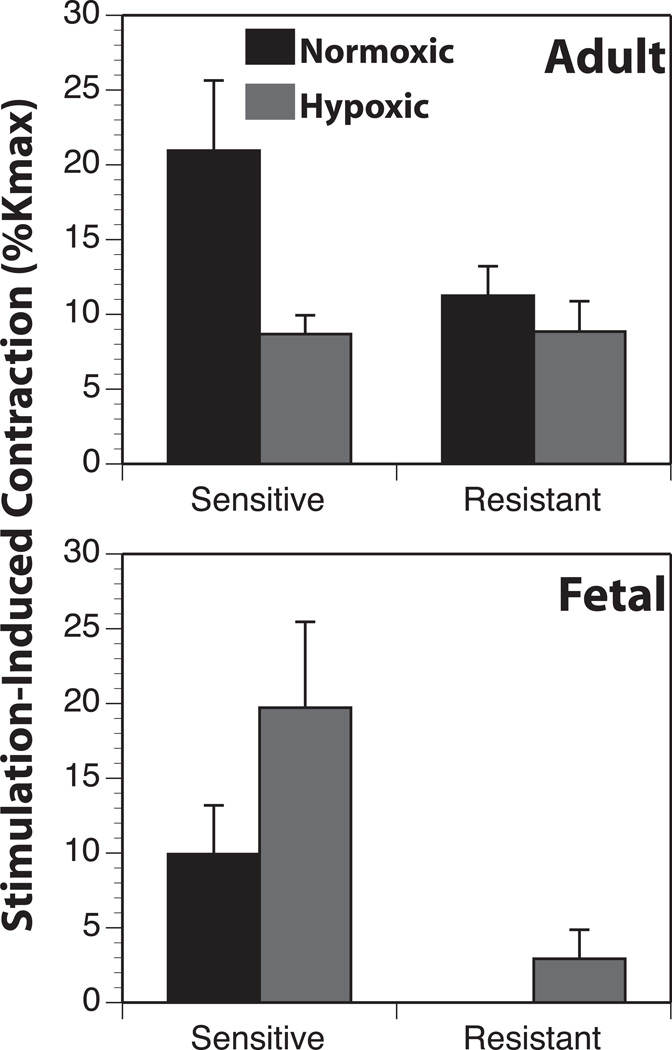
In addition to norepinephrine, sympathetic perivascular nerves release other vasoactive compounds, and careful analysis reveals that adrenergic receptors cannot mediate all the effects of sympathetic nerve activation (103–105). To probe the potential involvement of molecules other than norepinephrine in the effects of sympathetic stimulation on cerebral arteries, we recently examined the effects of transmural stimulation on cerebral artery contractions before and after depletion of norepinephrine with guanethidine. Our results demonstrated a significant guanethidine, non-adrenergic component that in fetal but not adult arteries was significantly greater than the norepinephrine component and was enhanced by hypoxia (Figure4). These findings suggest, at least in fetal cerebral arteries, that non-adrenergic factors released from sympathetic nerves may play an important role in hypoxic cerebrovascular remodeling.
Figure 4. Chronic hypoxia enhances guanethidine-resistant contractions in fetal arteries.
To test the possible involvement of non-adrenergic factors in modulation of arterial reactivity during hypoxic acclimatization, electrical nerve stimulation was applied before and after catecholamine depletion with guanethidine. The guanethidine-sensitive (GS) component was an index of the adrenergic contribution to arterial reactivity whereas the guanethidine resistant (GR) component was an index of the sympathetic release of a contractile and potentially trophic molecule other than NE. In adult arteries, hypoxia decreased only the GS component. Conversely, in fetal arteries hypoxia increased both the GR and GS components, suggesting that hypoxia preferentially enhanced release of a non-adrenergic transmitter from sympathetic perivascular nerves in fetal arteries. Results are presented as mean ± SEM.
Aside from norepinephrine, Neuropeptide-Y (NPY) is the most potent vasoconstrictor released from sympathetic post-ganglionic nerve terminals (106–108). Composed of 36 amino acids NPY exerts direct vasoconstrictor effects through activation of Y1 receptors (109) on many blood vessel types (103, 110), including cerebral arteries (103, 106) (110–113). Although NPY does not initiate phasic contractions or induce spontaneous rhythm contractility, it can enhance the tone and frequency of phasic contractions produced by norepinephrine (114). NPY also potentiates the vasoconstrictor effects of norepinephrine (115) and is an important mediator of the contractile response to high frequencies of sympathetic nerve stimulation (116).
In addition to its acute contractile effects, NPY also has potent trophic and mitogenic effects on arterial smooth muscle (104). Of the 6 known subtypes of NPY-Y receptors, NPY exerts its most potent trophic effects through activation of its Y-1 receptors, which appear the subtype preferentially expressed by vascular smooth muscle (109, 117). Activation of Y1 receptors, in turn, promotes both angiogenesis and remodeling via cellular adhesion, migration, proliferation, and differentiation in the cerebral vasculature during periods of hypoxia (106). In addition, however, activation of Y2 and Y5 receptors can also promote angiogenesis (117). Through actions on these receptors, NPY can promote phenotypic transformation of arterial smooth muscle in both mature and immature arterial smooth muscle (118).
Adenosine Triphosphate (ATP) released from sympathetic nerve terminals exerts trophic effects on arterial smooth muscle
Together with norepinephrine and NPY, sympathetic nerves also release adenosine triphosphate (ATP) (105, 119–121). The idea that norepinephrine, NPY and ATP are released simultaneously (119, 122, 123) has evolved over the years into the more recent concept that ATP is released much earlier than norepinephrine (124, 125). In this way, ATP induces the initial phasic vasoconstriction and norepinephrine initiates more slowly developing and longer-lasting tonic contractions (124–127). ATP effects are largely mediated by the P2 class of purinergic receptors, which are coupled to the smooth muscle interior through either ion channels (P2X) or G-protein coupled receptors (P2Y), both of which can be found in cerebral arteries (52, 128). In smooth muscle, both classes of P2 receptors can mediate short-term effects on contractile tone, and also potent long-term mitogenic and trophic effects (104). Depending on the vessel type and receptor activated, ATP can promote cellular proliferation, migration or apoptosis (52, 121). These effects must be transduced very rapidly, however, because ATPases (124, 125, 129) and ADPases (130) are concurrently released with ATP and truncate its duration of effects. Even so, the characteristics of ATP render it a strong candidate for contribution to cerebrovascular remodeling, particularly under conditions of hypoxia that promote expansion of the sympathetic perivascular innervation. The exact role played by ATP in this capacity, however, remains largely unexplored in cerebral arteries of any age.
Overview
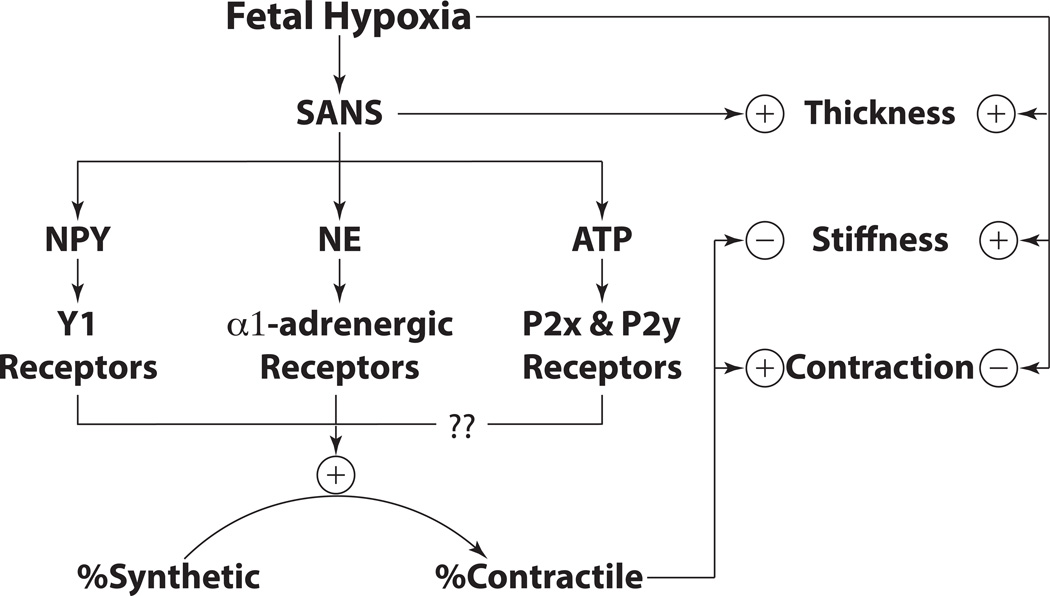
Vasotrophic factors that govern the growth and differentiation of cerebrovascular smooth muscle arise from many sources, including the neurons and glia of the brain parenchyma (131, 132), smooth muscle progenitors (133–137) and immune cells (138, 139) that reside in the adventitia, smooth muscle cells acting through autocrine and paracrine mechanisms in the medial layer (140), and the vascular endothelium (141). Adding to this rich mixture are perivascular nerve endings at the adventitial-medial border that release an abundance of vasotrophic factors including norepinephrine, NPY and ATP (52, 106, 142). The release of all these factors is enhanced by fetal hypoxia, due to concomitant increases in VEGF, which has potent growth-promoting effects on the sympathetic innervation (54) (Figure 5). The combined trophic actions of norepinephrine on α1-adrenergic receptors, NPY on Y1 receptors, and ATP on P2X/P2Y receptors all promote the contractile differentiation of vascular smooth muscle toward the contractile phenotype, particularly in immature arteries that are phenotypically highly plastic (101, 104, 143, 144). These effects can enhance fetal cerebral artery contractility, but also attenuate artery stiffness (87), probably through mechanisms that depress the ratio of collagen to elastin (88). Independent of the sympathetic innervation, fetal hypoxia can also increase wall thickness, increase stiffness, and depress contractility (6), indicating that the fetal cerebrovascular response is an integration of many different and highly dynamic influences. Chief among these is the sympathetic nervous system, which acts in a highly age-dependent manner to influence the structural and functional maturation of fetal cerebral vasculature, particularly under conditions of hypoxia.
Figure 5. Overview Schematic.
Hypoxic acclimatization induces the release of trophic factors such as VEGF, which stimulates growth and expansion of the sympathetic perivascular innervation. These nerves, in turn release NE, NPY and ATP, all of which act through their respective receptors to promote contractile differentiation of smooth muscle. These phenotypic changes enhance contractility but depress stiffness, as shown by denervation experiments. Independent of the sympathetic nerves, hypoxia can increase wall thickness and stiffness while also depressing contractility. Owing to these opposing effects, the final influence of hypoxia on artery structure and function depends on the balance between nerve-dependent and nerve-independent mechanisms.
ACKNOWLEDGMENTS
The work reported in this manuscript was supported by USPHS Grants HL54120, HD31266, HL64867, NS076945 and the Loma Linda University School of Medicine.
REFERENCES
- 1.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE Born Too Soon preterm prevention analysis g. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013 Jan 19;381(9862):223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013 May;131(5):855–860. doi: 10.1542/peds.2012-2471. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert JS, Nijland MJ, Knoblich P. Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: making the connections. Expert Rev Cardiovasc Ther. 2008 Nov;6(10):1367–1377. doi: 10.1586/14779072.6.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Jan 7;24(1):24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohsin M, Bauman AE, Jalaludin B. The influence of antenatal and maternal factors on stillbirths and neonatal deaths in New South Wales, Australia. J Biosoc Sci. 2006 Sep;38(5):643–657. doi: 10.1017/S002193200502701X. [DOI] [PubMed] [Google Scholar]
- 6.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol. 1993 Jan;264(1 Pt 2):R65–R72. doi: 10.1152/ajpregu.1993.264.1.R65. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000 Jan 1;59(1):47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 8.Neerhof MG, Thaete LG. The fetal response to chronic placental insufficiency. Semin Perinatol. 2008 Jun;32(3):201–205. doi: 10.1053/j.semperi.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol. 1992 Feb;262(2 Pt 2):H399–H405. doi: 10.1152/ajpheart.1992.262.2.H399. [DOI] [PubMed] [Google Scholar]
- 10.Pearce WJ, Butler SM, Abrassart JM, Williams JM. Fetal cerebral oxygenation: the homeostatic role of vascular adaptations to hypoxic stress. Advances in experimental medicine and biology. 2011;701:225–232. doi: 10.1007/978-1-4419-7756-4_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2013 Dec;305(12):H1772–H1780. doi: 10.1152/ajpheart.00592.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong W, Zhang L. Fetal hypoxia and programming of matrix metalloproteinases. Drug discovery today. 2012 Feb;17(3–4):124–134. doi: 10.1016/j.drudis.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7(2):e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Gonzalez P, Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Progress in neurobiology. 2012 May 22; doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodson RB, Rozance PJ, Fleenor BS, Petrash CC, Shoemaker LG, Hunter KS, Ferguson VL. Increased arterial stiffness and extracellular matrix reorganization in intrauterine growth-restricted fetal sheep. Pediatric research. 2013 Feb;73(2):147–154. doi: 10.1038/pr.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey KM, Barker DJ. Fetal programming and adult health. Public health nutrition. 2001 Apr;4(2B):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MR. Apoptosis of vascular smooth muscle cells in vascular remodelling and atherosclerotic plaque rupture. Cardiovascular research. 1999 Feb;41(2):361–368. doi: 10.1016/s0008-6363(98)00212-0. [DOI] [PubMed] [Google Scholar]
- 18.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006 Sep 29;99(7):675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 19.Boroujerdi A, Welser-Alves JV, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in beta1 integrins. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012 Sep;32(9):1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis NC, Messinger L, Monteleone B, Ainslie PN. Effect of acute hypoxia on regional cerebral blood flow: effect of sympathetic nerve activity. Journal of applied physiology. 2014 May 1;116(9):1189–1196. doi: 10.1152/japplphysiol.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu XQ, Longo LD, Gilbert RD, Zhang L. Effects of long-term high-altitude hypoxemia on alpha 1-adrenergic receptors in the ovine uterine artery. Am J Physiol. 1996 Mar;270(3 Pt 2):H1001–H1007. doi: 10.1152/ajpheart.1996.270.3.H1001. [DOI] [PubMed] [Google Scholar]
- 22.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, Cornfield DN. Hypoxia-inducible factor-1alpha in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ Res. 2013 Apr 26;112(9):1230–1233. doi: 10.1161/CIRCRESAHA.112.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gleason CA, Hamm C, Jones MD., Jr Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol. 1990 Apr;258(4 Pt 2):H1064–H1069. doi: 10.1152/ajpheart.1990.258.4.H1064. [DOI] [PubMed] [Google Scholar]
- 24.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. Journal of applied physiology. 2006 Jan;100(1):225–232. doi: 10.1152/japplphysiol.00221.2005. [DOI] [PubMed] [Google Scholar]
- 25.Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- 26.Frid MG, Dempsey EC, Durmowicz AG, Stenmark KR. Smooth muscle cell heterogeneity in pulmonary and systemic vessels. Importance in vascular disease. Arterioscler Thromb Vasc Biol. 1997 Jul;17(7):1203–1209. doi: 10.1161/01.atv.17.7.1203. [DOI] [PubMed] [Google Scholar]
- 27.Cook CL, Weiser MC, Schwartz PE, Jones CL, Majack RA. Developmentally timed expression of an embryonic growth phenotype in vascular smooth muscle cells. Circ Res. 1994 Feb;74(2):189–196. doi: 10.1161/01.res.74.2.189. [DOI] [PubMed] [Google Scholar]
- 28.Worth NF, Rolfe BE, Song J, Campbell GR. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell motility and the cytoskeleton. 2001 Jul;49(3):130–145. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 29.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15(3):100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinert PM, Marekov LN, Parry DA. Diversity of intermediate filament structure. Evidence that the alignment of coiled-coil molecules in vimentin is different from that in keratin intermediate filaments. J Biol Chem. 1993 Nov 25;268(33):24916–24925. [PubMed] [Google Scholar]
- 31.Valgeirsdottir S, Claesson-Welsh L, Bongcam-Rudloff E, Hellman U, Westermark B, Heldin CH. PDGF induces reorganization of vimentin filaments. Journal of cell science. 1998 Jul 30;111(Pt 14):1973–1980. doi: 10.1242/jcs.111.14.1973. [DOI] [PubMed] [Google Scholar]
- 32.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997 Sep 5;272(36):22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 33.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Molecular pharmacology. 2006 Nov;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007 Oct 9;2007(407):cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 35.Butler SM, Abrassart JM, Hubbell MC, Adeoye O, Semotiuk A, Williams JM, Mata-Greenwood E, Khorram O, Pearce WJ. Contributions of VEGF to age-dependent transmural gradients in contractile protein expression in ovine carotid arteries. American journal of physiology Cell physiology. 2011 Sep;301(3):C653–C666. doi: 10.1152/ajpcell.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 37.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15(7 Pt 1):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends in cardiovascular medicine. 2007 May;17(4):140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53(23):5822–5827. [PubMed] [Google Scholar]
- 40.McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms [published errata appear in Am J Physiol 1995 Feb;268(2 Pt 2):section H following table of contents and 1995 Jun;268(6 Pt 3):section H following table of contents] Am J Physiol. 1994;267(5 Pt 2):H1921–H1927. doi: 10.1152/ajpheart.1994.267.5.H1921. [DOI] [PubMed] [Google Scholar]
- 41.Brogi E, Schatteman G, Wu T, Kim EA, Varticovski L, Keyt B, Isner JM. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996;97(2):469–476. doi: 10.1172/JCI118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambalavanan N, Bulger A, Philips IJ. Hypoxia-induced release of peptide growth factors from neonatal porcine pulmonary artery smooth muscle cells. Biol Neonate. 1999 Nov;76(5):311–319. doi: 10.1159/000014173. [DOI] [PubMed] [Google Scholar]
- 43.Adeoye OO, Butler SM, Hubbell MC, Semotiuk A, Williams JM, Pearce WJ. Contribution of increased VEGF receptors to hypoxic changes in fetal ovine carotid artery contractile proteins. American journal of physiology Cell physiology. 2013 Apr 1;304(7):C656–C665. doi: 10.1152/ajpcell.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 45.Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: implications for venous neointimal hyperplasia in hemodialysis access. Journal of vascular and interventional radiology : JVIR. 2010 Jun;21(6):896–902. doi: 10.1016/j.jvir.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogawa S, Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2005 May;127(5):1793–1798. doi: 10.1378/chest.127.5.1793. [DOI] [PubMed] [Google Scholar]
- 47.Daniel JM, Sedding DG. Circulating smooth muscle progenitor cells in arterial remodeling. J Mol Cell Cardiol. 2011 Feb;50(2):273–279. doi: 10.1016/j.yjmcc.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Durrant LM, Khorram O, Buchholz JN, Pearce WJ. Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: effects of metyrapone. American journal of physiology Regulatory, integrative and comparative physiology. 2014 Mar 15;306(6):R401–R410. doi: 10.1152/ajpregu.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khorram O, Ghazi R, Chuang TD, Han G, Naghi J, Ni Y, Pearce WJ. Excess maternal glucocorticoids in response to in utero undernutrition inhibit offspring angiogenesis. Reproductive sciences. 2014 May;21(5):601–611. doi: 10.1177/1933719113508819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruijtenbeek K, le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE, De Mey JG. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation. 2000 Dec 5;102(23):2892–2897. doi: 10.1161/01.cir.102.23.2892. [DOI] [PubMed] [Google Scholar]
- 51.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 52.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66(1):102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 53.Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2646–H2652. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- 54.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. Am J Physiol Heart Circ Physiol. 2005 Jun;288(6):H2785–H2791. doi: 10.1152/ajpheart.00354.2004. [DOI] [PubMed] [Google Scholar]
- 55.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain and language. 2007 Aug;102(2):141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Experimental physiology. 2010 Feb;95(2):251–262. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- 57.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. Journal of applied physiology. 2006 Feb;100(2):725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- 58.Kato K, Yokoyama T, Yamaguchi-Yamada M, Yamamoto Y. Short-term hypoxia transiently increases dopamine beta-hydroxylase immunoreactivity in glomus cells of the rat carotid body. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2013 Jan;61(1):55–62. doi: 10.1369/0022155412464639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003 Feb 1;546(Pt 3):921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respiratory physiology & neurobiology. 2010 Apr 15;171(1):36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T, Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. American journal of physiology Regulatory, integrative and comparative physiology. 2011 Aug;301(2):R456–R464. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- 62.Custer JR, Hales CA. Chemical sympathectomy decreases alveolar hypoxic vasoconstriction in lambs but not in sheep. Journal of applied physiology. 1986 Jan;60(1):32–37. doi: 10.1152/jappl.1986.60.1.32. [DOI] [PubMed] [Google Scholar]
- 63.Davy KP, Jones PP, Seals DR. Influence of age on the sympathetic neural adjustments to alterations in systemic oxygen levels in humans. Am J Physiol. 1997 Aug;273(2 Pt 2):R690–R695. doi: 10.1152/ajpregu.1997.273.2.R690. [DOI] [PubMed] [Google Scholar]
- 64.Buchholz J, Edwards-Teunissen K, Duckles SP. Impact of development and chronic hypoxia on NE release from adrenergic nerves in sheep arteries. Am J Physiol. 1999 Mar;276(3 Pt 2):R799–R808. doi: 10.1152/ajpregu.1999.276.3.R799. [DOI] [PubMed] [Google Scholar]
- 65.Rorie DK, Tyce GM. Effects of hypoxia on norepinephrine release and metabolism in dog pulmonary artery. Journal of applied physiology: respiratory, environmental and exercise physiology. 1983 Sep;55(3):750–758. doi: 10.1152/jappl.1983.55.3.750. [DOI] [PubMed] [Google Scholar]
- 66.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Pahlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002 May 14;99(10):7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jogi A, Vallon-Christersson J, Holmquist L, Axelson H, Borg A, Pahlman S. Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp Cell Res. 2004 May 1;295(2):469–487. doi: 10.1016/j.yexcr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Hubbell MC, Semotiuk AJ, Thorpe RB, Adeoye OO, Butler SM, Williams JM, Khorram O, Pearce WJ. Chronic Hypoxia and VEGF Differentially Modulate Abundance and Organization of Myosin Heavy Chain Isoforms in Fetal and Adult Ovine Arteries. American journal of physiology Cell physiology. 2012 Sep 19;303(10):C1090–C1103. doi: 10.1152/ajpcell.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horackova M, Slavikova J, Byczko Z. Postnatal development of the rat intrinsic cardiac nervous system: a confocal laser scanning microscopy study in whole-mount atria. Tissue & cell. 2000 Oct;32(5):377–388. doi: 10.1054/tice.2000.0126. [DOI] [PubMed] [Google Scholar]
- 70.Sadoshima S, Busija DW, Heistad DD. Mechanisms of protection against stroke in stroke-prone spontaneously hypertensive rats. Am J Physiol. 1983 Mar;244(3):H406–H412. doi: 10.1152/ajpheart.1983.244.3.H406. [DOI] [PubMed] [Google Scholar]
- 71.Bloom GD, Carlsoo B, Danielsson A, Hellstrom S, Henriksson R. Trophic effect of the sympathetic nervous system on the early development of the rat parotid gland: a quantitative ultrastructural study. Anat Rec. 1981 Dec;201(4):645–654. doi: 10.1002/ar.1092010409. [DOI] [PubMed] [Google Scholar]
- 72.Bevan RD. Trophic effects of peripheral adrenergic nerves on vascular structure. Hypertension. 1984 Nov-Dec;6(6 Pt 2):III19–III26. doi: 10.1161/01.hyp.6.6_pt_2.iii19. [DOI] [PubMed] [Google Scholar]
- 73.Duckles SP, Banner W., Jr Changes in vascular smooth muscle reactivity during development. Annual review of pharmacology and toxicology. 1984;24:65–83. doi: 10.1146/annurev.pa.24.040184.000433. [DOI] [PubMed] [Google Scholar]
- 74.Smolich JJ, Cox HS, Eisenhofer G, Esler MD. Pulmonary clearance and release of norepinephrine and epinephrine in newborn lambs. Am J Physiol. 1997 Jul;273(1 Pt 1):L264–L274. doi: 10.1152/ajplung.1997.273.1.L264. [DOI] [PubMed] [Google Scholar]
- 75.Pearce WJ, Duckles SP, Buchholz J. Effects of maturation on adrenergic neurotransmission in ovine cerebral arteries. Am J Physiol. 1999 Oct;277(4 Pt 2):R931–R937. doi: 10.1152/ajpregu.1999.277.4.R931. [DOI] [PubMed] [Google Scholar]
- 76.ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. British journal of anaesthesia. 2013 Sep;111(3):361–367. doi: 10.1093/bja/aet122. [DOI] [PubMed] [Google Scholar]
- 77.Faucher DJ, Laptook AR, Porter JC, Rosenfeld CR. Effects of acute hypercapnia on maternal and fetal vasopressin and catecholamine release. Pediatric research. 1991 Oct;30(4):368–374. doi: 10.1203/00006450-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Angouras DC, Dosios TJ, Dimitriou CA, Chamogeorgakis TP, Rokkas CK, Manos TA, Sokolis DP. Surgical thoracic sympathectomy induces structural and biomechanical remodeling of the thoracic aorta in a porcine model. The Journal of surgical research. 2012 Jan;172(1):68–76. doi: 10.1016/j.jss.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 79.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension. 1980 Jul-Aug;2(4):419–423. doi: 10.1161/01.hyp.2.4.419. [DOI] [PubMed] [Google Scholar]
- 80.Bleys RL, Cowen T. Innervation of cerebral blood vessels: morphology, plasticity, age-related, and Alzheimer's disease-related neurodegeneration. Microsc Res Tech. 2001 Apr 15;53(2):106–118. doi: 10.1002/jemt.1075. [DOI] [PubMed] [Google Scholar]
- 81.Eichmann A, Brunet I. Arterial Innervation in Development and Disease. Science translational medicine. 2014 Sep 3;6(252):252ps9. doi: 10.1126/scitranslmed.3008910. [DOI] [PubMed] [Google Scholar]
- 82.Hachani R, Dab H, Sakly M, Sercombe R, Callebert J, Vicaut E, Kacem K. The profile of the extracellular matrix changes in the aorta after sympathectomy in the hypercholesterolemic rats. Auton Neurosci. 2011 Oct 28;164(1–2):67–73. doi: 10.1016/j.autneu.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Bevan RD, Tsuru H. Functional and structural changes in the rabbit ear artery after sympathetic denervation. Circ Res. 1981 Aug;49(2):478–485. doi: 10.1161/01.res.49.2.478. [DOI] [PubMed] [Google Scholar]
- 84.Southwell BR, Chamley-Campbell JH, Campbell GR. Tropic interactions between sympathetic nerves and vascular smooth muscle. Journal of the autonomic nervous system. 1985 Aug;13(4):343–354. doi: 10.1016/0165-1838(85)90022-0. [DOI] [PubMed] [Google Scholar]
- 85.Todd ME, Gowen B. Arterial wall and smooth muscle cell development in young Wistar rats and the effects of surgical denervation. Circ Res. 1991 Aug;69(2):438–446. doi: 10.1161/01.res.69.2.438. [DOI] [PubMed] [Google Scholar]
- 86.Lacolley P, Glaser E, Challande P, Boutouyrie P, Mignot JP, Duriez M, Levy B, Safar M, Laurent S. Structural changes and in situ aortic pressure-diameter relationship in long-term chemical-sympathectomized rats. Am J Physiol. 1995 Aug;269(2 Pt 2):H407–H416. doi: 10.1152/ajpheart.1995.269.2.H407. [DOI] [PubMed] [Google Scholar]
- 87.Kahrstrom J, Hardebo JE, Owman C. Neonatal chronic sympathectomy in normotensive rats affects pial arteries: enhanced stiffness and reduced capacity to dilate. Acta physiologica Scandinavica. 1996 Jun;157(2):217–224. doi: 10.1046/j.1365-201X.1996.d01-743.x. [DOI] [PubMed] [Google Scholar]
- 88.Baumbach GL, Heistad DD, Siems JE. Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J Physiol. 1989 Sep;416:123–140. doi: 10.1113/jphysiol.1989.sp017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abel PW, Hermsmeyer K. Sympathetic cross-innervation of SHR and genetic controls suggests a trophic influence on vascular muscle membranes. Circ Res. 1981 Dec;49(6):1311–1318. doi: 10.1161/01.res.49.6.1311. [DOI] [PubMed] [Google Scholar]
- 90.Bevan RD, Purdy RE, Su C, Bevan JA. Evidence for an increase in adrenergic nerve function in blood vessels from experimental hypertensive rabbits. Circ Res. 1975 Oct;37(4):503–508. doi: 10.1161/01.res.37.4.503. [DOI] [PubMed] [Google Scholar]
- 91.Crawford RA, Gregory PC, Griffiths IR. The response of feline spinal pial arterioles to norepinephrine. J Neurosurg. 1980 Jan;52(1):60–63. doi: 10.3171/jns.1980.52.1.0060. [DOI] [PubMed] [Google Scholar]
- 92.Edvinsson L, Nielsen KC, Owman C, West KA. Sympathetic neural influence on norepinephrine vasoconstriction in brain vessels. Archives of neurology. 1972 Dec;27(6):492–495. doi: 10.1001/archneur.1972.00490180028007. [DOI] [PubMed] [Google Scholar]
- 93.Puzdrova VA, Kudryashova TV, Gaynullina DK, Mochalov SV, Aalkjaer C, Nilsson H, Vorotnikov AV, Schubert R, Tarasova OS. Trophic action of sympathetic nerves reduces arterial smooth muscle Ca sensitivity during early post-natal development in rats. Acta Physiol (Oxf) 2014 Jun 19; doi: 10.1111/apha.12331. [DOI] [PubMed] [Google Scholar]
- 94.Aprigliano O, Hermsmeyer K. Trophic influence of the sympathetic nervous system on the rat portal vein. Circ Res. 1977 Aug;41(2):198–206. doi: 10.1161/01.res.41.2.198. [DOI] [PubMed] [Google Scholar]
- 95.Sato A, Sato Y, Suzuki H, Trzebski A. Reflex responses in adrenal sympathetic nerves to stimulation of glucoreceptors and chemoreceptors in aging rats. Journal of the autonomic nervous system. 1991 Jan;32(1):63–68. doi: 10.1016/0165-1838(91)90236-v. [DOI] [PubMed] [Google Scholar]
- 96.Vanhoutte PM, Rimele TJ. Calcium and alpha-adrenoceptors in activation of vascular smooth muscle. J Cardiovasc Pharmacol. 1982;4(Suppl 3):S280–S286. [PubMed] [Google Scholar]
- 97.Kluck P. The autonomic innervation of the human urinary bladder, bladder neck and urethra: a histochemical study. Anat Rec. 1980 Nov;198(3):439–447. doi: 10.1002/ar.1091980306. [DOI] [PubMed] [Google Scholar]
- 98.Bevan JA, Su C. Variation of intra- and perisynaptic adrenergic transmitter concentrations with width of synaptic cleft in vascular tissue. The Journal of pharmacology and experimental therapeutics. 1974 Jul;190(1):30–38. [PubMed] [Google Scholar]
- 99.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiological reviews. 1990 Oct;70(4):921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 100.Buchholz J, Duckles SP. Chronic hypoxia alters prejunctional alpha(2)-receptor function in vascular adrenergic nerves of adult and fetal sheep. American journal of physiology Regulatory, integrative and comparative physiology. 2001 Sep;281(3):R926–R934. doi: 10.1152/ajpregu.2001.281.3.R926. [DOI] [PubMed] [Google Scholar]
- 101.Majesky MW, Daemen MJ, Schwartz SM. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta. J Biol Chem. 1990 Jan 15;265(2):1082–1088. [PubMed] [Google Scholar]
- 102.Todd ME. Trophic interactions between rat nerves and blood vessels in denervated peripheral arteries and in anterior eye chamber transplants. Circ Res. 1986 May;58(5):641–652. doi: 10.1161/01.res.58.5.641. [DOI] [PubMed] [Google Scholar]
- 103.Vu HQ, Budai D, Duckles SP. Neuropeptide Y preferentially potentiates responses to adrenergic nerve stimulation by increasing rate of contraction. The Journal of pharmacology and experimental therapeutics. 1989 Dec;251(3):852–857. [PubMed] [Google Scholar]
- 104.Erlinge D, Yoo H, Edvinsson L, Reis DJ, Wahlestedt C. Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am J Physiol. 1993;265(4 Pt 2):H1089–H1097. doi: 10.1152/ajpheart.1993.265.4.H1089. [DOI] [PubMed] [Google Scholar]
- 105.Burnstock G. Purinergic regulation of vascular tone and remodelling. Autonomic & autacoid pharmacology. 2009 Jul;29(3):63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 106.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998 Jul 27;83(2):187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 107.Stjernquist M, Owman C, Sjoberg NO, Sundler F. Coexistence and cooperation between neuropeptide Y and norepinephrine in nerve fibers of guinea pig vas deferens and seminal vesicle. Biology of reproduction. 1987 Feb;36(1):149–155. doi: 10.1095/biolreprod36.1.149. [DOI] [PubMed] [Google Scholar]
- 108.Ohlen A, Persson MG, Lindbom L, Gustafsson LE, Hedqvist P. Nerve-induced nonadrenergic vasoconstriction and vasodilatation in skeletal muscle. Am J Physiol. 1990 May;258(5 Pt 2):H1334–H1338. doi: 10.1152/ajpheart.1990.258.5.H1334. [DOI] [PubMed] [Google Scholar]
- 109.Abounader R, Elhusseiny A, Cohen Z, Olivier A, Stanimirovic D, Quirion R, Hamel E. Expression of neuropeptide Y receptors mRNA and protein in human brain vessels and cerebromicrovascular cells in culture. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999 Feb;19(2):155–163. doi: 10.1097/00004647-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 110.Pellieux C, Sauthier T, Domenighetti A, Marsh DJ, Palmiter RD, Brunner HR, Pedrazzini T. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crnkovic S, Egemnazarov B, Jain P, Seay U, Gattinger N, Marsh LM, Balint Z, Kovacs G, Ghanim B, Klepetko W, Schermuly RT, Weissmann N, Olschewski A, Kwapiszewska G. NPY/Y1 receptor-mediated vasoconstrictory and proliferative effects in pulmonary hypertension. British journal of pharmacology. 2014 Aug;171(16):3895–3907. doi: 10.1111/bph.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hokfelt T, Lundberg JM, Tatemoto K, Mutt V, Terenius L, Polak J, Bloom S, Sasek C, Elde R, Goldstein M. Neuropeptide Y (NPY)- and FMRFamide neuropeptide-like immunoreactivities in catecholamine neurons of the rat medulla oblongata. Acta physiologica Scandinavica. 1983 Feb;117(2):315–318. doi: 10.1111/j.1748-1716.1983.tb07214.x. [DOI] [PubMed] [Google Scholar]
- 113.Blessing WW, Howe PR, Joh TH, Oliver JR, Willoughby JO. Distribution of tyrosine hydroxylase and neuropeptide Y-like immunoreactive neurons in rabbit medulla oblongata, with attention to colocalization studies, presumptive adrenaline-synthesizing perikarya, and vagal preganglionic cells. The Journal of comparative neurology. 1986 Jun 8;248(2):285–300. doi: 10.1002/cne.902480211. [DOI] [PubMed] [Google Scholar]
- 114.Prieto D, Hernandez M, Rivera L, Garcia-Sacristan A, Simonsen U. Distribution and functional effects of neuropeptide Y on equine ureteral smooth muscle and resistance arteries. Regulatory peptides. 1997 Apr 30;69(3):155–165. doi: 10.1016/s0167-0115(97)00003-7. [DOI] [PubMed] [Google Scholar]
- 115.Fabi F, Argiolas L, Ruvolo G, del Basso P. Neuropeptide Y-induced potentiation of noradrenergic vasoconstriction in the human saphenous vein: involvement of endothelium generated thromboxane. British journal of pharmacology. 1998 May;124(1):101–110. doi: 10.1038/sj.bjp.0701808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pernow J, Lundberg JM. Release and vasoconstrictor effects of neuropeptide Y in relation to non-adrenergic sympathetic control of renal blood flow in the pig. Acta physiologica Scandinavicas. 1989 Aug;136(4):507–517. doi: 10.1111/j.1748-1716.1989.tb08696.x. [DOI] [PubMed] [Google Scholar]
- 117.Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Current topics in medicinal chemistry. 2007;7(17):1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- 118.Fan XM, Hendley ED, Forehand CJ. Enhanced vascular neuropeptide Y-immunoreactive innervation in two hypertensive rat strains. Hypertension. 1995 Nov;26(5):758–763. doi: 10.1161/01.hyp.26.5.758. [DOI] [PubMed] [Google Scholar]
- 119.Stjarne L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Reviews of physiology, biochemistry and pharmacology. 1989;112:1–137. doi: 10.1007/BFb0027496. [DOI] [PubMed] [Google Scholar]
- 120.Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. The Journal of pharmacology and experimental therapeutics. 2002 Nov;303(2):439–444. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- 121.Burnstock G. Purinergic signalling in the reproductive system in health and disease. Purinergic Signal. 2014 Mar;10(1):157–187. doi: 10.1007/s11302-013-9399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kennedy C, McLaren GJ, Westfall TD, Sneddon P. ATP as a co-transmitter with noradrenaline in sympathetic nerves--function and fate. Ciba Foundation symposium. 1996;198:223–235. doi: 10.1002/9780470514900.ch13. discussion 35–8. [DOI] [PubMed] [Google Scholar]
- 123.Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. British journal of pharmacology. 1999 Jan;126(1):11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Todorov LD, Mihaylova-Todorova S, Craviso GL, Bjur RA, Westfall DP. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J Physiol. 1996 Nov 1;496(Pt 3):731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mihaylova-Todorova S, Todorov LD, Westfall DP. Correlation between the release of the sympathetic neurotransmitter ATP and soluble nucleotidases from the guinea pig vas deferens. The Journal of pharmacology and experimental therapeutics. 2001 Jan;296(1):64–70. [PubMed] [Google Scholar]
- 126.Sneddon P, Westfall DP, Fedan JS. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- 127.Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochemistry international. 1990;17(2):357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- 128.Lewis CJ, Ennion SJ, Evans RJ. P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J Physiol. 2000 Sep 1;527(Pt 2):315–324. doi: 10.1111/j.1469-7793.2000.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Westfall TD, Kennedy C, Sneddon P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. British journal of pharmacology. 1996 Mar;117(5):867–872. doi: 10.1111/j.1476-5381.1996.tb15273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gayle RB, 3rd, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, Brasel K, Price V, Drosopoulos JH, Islam N, Alyonycheva TN, Broekman MJ, Marcus AJ. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J Clin Invest. 1998 May 1;101(9):1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brunelli A, Dimauro I, Sgro P, Emerenziani GP, Magi F, Baldari C, Guidetti L, Di Luigi L, Parisi P, Caporossi D. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Medicine and science in sports and exercise. 2012 Oct;44(10):1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- 132.He XY, Chen ZZ, Cai YQ, Xu G, Shang JH, Kou SB, Li M, Zhang HT, Duan CZ, Zhang SZ, Ke YQ, Zeng YJ, Xu RX, Jiang XD. Expression of cytokines in rat brain with focal cerebral ischemia after grafting with bone marrow stromal cells and endothelial progenitor cells. Cytotherapy. 2011 Jan;13(1):46–53. doi: 10.3109/14653249.2010.510505. [DOI] [PubMed] [Google Scholar]
- 133.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda, Md) 2006 Apr;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 134.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovascular research. 2007 Sep 1;75(4):659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001 Dec 7;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 136.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 138.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovascular research. 2007 Sep 1;75(4):679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 139.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012 Mar 16;110(6):889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu R, Leslie KL, Martin KA. Epigenetic regulation of smooth muscle cell plasticity. Biochimica et biophysica acta. 2014 Jun 15; doi: 10.1016/j.bbagrm.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hayden MR, Tyagi SC. Arterial vascular remodeling: the endothelial cell's central role. Missouri medicine. 1998 May;95(5):213–217. [PubMed] [Google Scholar]
- 142.Hasan W, Pedchenko T, Krizsan-Agbas D, Baum L, Smith PG. Sympathetic neurons synthesize and secrete pro-nerve growth factor protein. Journal of neurobiology. 2003 Oct;57(1):38–53. doi: 10.1002/neu.10250. [DOI] [PubMed] [Google Scholar]
- 143.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiological reviews. 1995 Jul;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 144.Spin JM, Maegdefessel L, Tsao PS. Vascular smooth muscle cell phenotypic plasticity: focus on chromatin remodelling. Cardiovascular research. 2012 Jul 15;95(2):147–155. doi: 10.1093/cvr/cvs098. [DOI] [PMC free article] [PubMed] [Google Scholar]