Abstract
Learning processes in Drosophila have been studied through the use of Pavlovian associative memory tests, and these paradigms have been extremely useful in identifying both genetic factors and neuroanatomical structures that are essential to memory formation. Whether these same genes and brain compartments also contribute to memory formed from nonassociative experiences is not well understood. Exposures to environmental stressors such as predators are known to induce innate behavioral responses and can lead to new memory formation that allows a predator response to persist for days after the predator threat has been removed. Here, we utilize a unique form of nonassociative behavior in Drosophila where female flies detect the presence of endoparasitoid predatory wasps and alter their oviposition behavior to lay eggs in food containing high levels of alcohol. The predator-induced change in fly oviposition preference is maintained for days after wasps are removed, and this persistence in behavior requires a minimum continuous exposure time of 14 hr. Maintenance of this behavior is dependent on multiple long-term memory genes, including orb2, dunce, rutabaga, amnesiac, and Fmr1. Maintenance of the behavior also requires intact synaptic transmission of the mushroom body. Surprisingly, synaptic output from the mushroom body (MB) or the functions of any of these learning and memory genes are not required for the change in behavior when female flies are in constant contact with wasps. This suggests that perception of this predator that leads to an acute change in oviposition behavior is not dependent on the MB or dependent on learning and memory gene functions. Because wasp-induced oviposition behavior can last for days and its maintenance requires a functional MB and the wild-type products of several known learning and memory genes, we suggest that this constitutes a paradigm for a bona fide form of nonassociative long-term memory that is not dependent on associated experiences.
Keywords: Drosophila melanogaster, learning and memory, long-term memory, behavior
A fundamental trait that higher-order organisms possess is the ability to remember and recall past experiences. This wonderful ability of higher organisms to remember past experiences is observed in all animals and therefore drives intense interest to elucidate the molecular underpinning of learning and memory in model systems, such as Drosophila. Insight into the biology of memory has been gained with a wide array of experimental approaches ranging from behavioral phenomena and underlying physiological correlates to experimental interventions such as pharmacological, biochemical, and anatomical perturbations in model genetic systems. Genetic manipulations also provide key insight into behavioral plasticity, as this approach allows us to understand the genetic components regulating this plasticity while providing clues into the extent of evolutionary conservation among the different molecular mechanisms governing this plasticity (Greenspan 1995).
Drosophila melanogaster is an important model system for understanding the genetic basis of memory (Davis 2005; Margulies et al. 2005; McGuire et al. 2005). Almost all work in this area has taken advantage of the associative memory paradigm (Tully 1987; McGuire et al. 2005), whereby flies are conditioned to associate a particular innocuous smell with a stressful experience such as electric shock or with a pleasant experience such as a desirable food. More recent work on olfactory habituation has studied a form of nonassociative olfactory memory (Das et al. 2011; McCann et al. 2011; Ramaswami 2014). Although these studies have been critical in elucidating both genetic and physiological mechanisms of learning, memory, and olfactory perception, these paradigms do not use natural, ecologically pertinent experiences where the memories formed and assayed are not always particularly robust. These represent only a very small fraction of the range of learning and memory phenomena evidenced in the insect brain. Thus, it is valuable to define and mechanistically analyze ecologically relevant learning processes that are different from those probed by laboratory conditioning regimens. More importantly, an understanding of how nonassociative behaviors persist over time first requires the development of new approaches that are independent of conditioning regimens.
Fruit flies in the genus Drosophila are regularly attacked by endoparasitoid wasps. In natural D. melanogaster populations, upward of 90% of fly larvae are found to be infected by wasps, suggesting that they exert extremely strong selection pressures on Drosophila populations in nature (Driessen et al. 1990; LaSalle 1993; Fleury et al. 2004). Once infected, fruit fly larvae can mount an immune response against wasp eggs, termed “melanotic encapsulation” (Carton and Nappi 1997). However, if the immune response is unsuccessful, a hatched wasp larva begins to consume internal fly tissues before eventually killing the fly and eclosing from the fly pupal case.
Drosophila have ways beyond that of a cellular immune response to protect themselves. D. melanogaster can alter its egg-laying behavior when encountering endoparasitoid wasps. This behavioral change entails at least two very different and quantifiable behavioral responses. First, Drosophila females will depress their oviposition rate, possibly to allow for time to search and discover a new egg-laying environment that is not wasp-infested (Lefevre et al. 2012). Second, if high-ethanol-containing food is made available to adult Drosophila, then female flies in the presence of wasps will not depress oviposition and instead will actively prefer to lay eggs on ethanol-laden food (Kacsoh et al. 2013). Thus, from the same visual input, a parasitoid wasp, two possible responses can be elicited in the adult Drosophila. One entails a physiological modulation of the reproductive system manifesting as oviposition depression. The second involves a change in substrate preference, if ethanol is made available, that is independent of physiological changes in the reproductive system. These observations indicate that the two behavioral changes are managed by two different neuronal circuits, suggesting that the stress of a predatory wasp can elicit a suite of different behavioral outputs that are dependent on environmental context.
Adult Drosophila are not infected by these wasps, thus making the change in reproductive behavior beneficial only to an anticipated threat to their offspring. Both the changes in oviposition depression and ethanol food preference elicited by the wasp require an intact Drosophila visual system (Kacsoh et al. 2013). Drosophila larvae and adults in particular derive benefit from consumption of low concentrations of ethanol by converting ethanol to energy stores, increasing development speed, and increasing overall longevity (Chawla et al. 1981; Parsons 1981; Geer et al. 1985). However, at higher ethanol concentration (>4%), D. melanogaster larval development is protracted and flies suffer from increased mortality (McKenzie and McKechnie 1978; Chawla et al. 1981; Parsons 1981; Geer et al. 1985). D. melanogaster has evolved a high resistance to ethanol, including compared to other Drosophila species, and can grow in artificial media with ethanol concentrations upward of 10% by volume (David 1983; Mercot et al. 1994; Kacsoh et al. 2013). In contrast, it has been found that some endoparasitoid wasps are sensitive to high levels of ethanol (Milan et al. 2012). D. melanogaster adults are capable of prophylactically medicating their offspring through the use of ethanol and anticipatory oviposition. There has been some evidence to suggest the involvement of long-term memory in this oviposition change to alcohol-laden food (Kacsoh et al. 2013); however, it is not known if many of the traditional learning and memory genes or the mushroom body (MB) compartment of the brain are required for maintaining this behavioral change.
Four genetically distinct types of memory have been described in Drosophila based on the length of time that the memory persists: short-term memory (0–1 hr), middle-term memory (0–4 hr), anesthesia-resistant memory (0–24 hr), and long-term memory (5–indefinite hours) (Margulies et al. 2005). Previous work using the wasp system has demonstrated the role of at least one long-term memory gene (Alcohol Dehydrogenase Transcription Factor 1), where wasp-induced changes in oviposition behavior can persist for days (Kacsoh et al. 2013). Here we use this natural wasp predator system to explore long-term memory formation within D. melanogaster, further defining the genetic factors for this specific memory and anatomical components necessary for this response. We test the hypothesis that the persistence of the wasp-induced switch in fly oviposition behavior requires multiple long-term memory gene functions and continual neuronal functions of the adult mushroom body.
Materials and Methods
Insect species and strains
The D. melanogaster strains Canton-S (CS), Oregon-R (OR), and w1118 were used as wild-type strains for oviposition preference after wasp exposure. rutabaga lines rut1 and rut2080, amnesiac lines amn1 and amnX8, and dunce lines dnc1 and dncML were kindly provided by Leslie Griffith (Brandeis University). The mushroom body Gene-Switch line was kindly provided by Greg Roman (Baylor College of Medicine) (Supporting Information, Table S1, Table S3, Table S3).
The figitid larval endoparasitoid Leptopilina heterotoma (strain Lh14) was used in all memory experiments, specifically during the training period. The L. heterotoma strain used in this study (strain name Lh14) was originally collected from single females in Winters, California (2002), and was kindly provided by Todd Schlenke (Schlenke et al. 2007). To culture wasps, adult flies (strain Canton-S) were allowed to lay eggs in standard Drosophila vials containing standard Drosophila medium for 4 days. After 4 days, flies were removed and replaced with adult wasps (10 females, 6 males), which then attacked the developing fly larvae. Wasp vials were supplemented with ∼500 µl of a 50% honey/water solution applied to the inside of the cotton vial plugs. Wasps aged 3–7 days post-eclosion were used for all experiments.
Fly oviposition
Field cage preparation:
For all experiments utilizing 300 flies, collapsible field cages (24 × 24 × 24 in.) (60.9 cm) and dark green, UV-resistant polyester mesh netting from BioQuip were used (catalog no. 1451D). We first washed the mesh netting using the Fisher Brand Sparkleen powder (catalog no. 04-320-4) using the ratio of 4 g of Sparkleen to 2 gallons of water. The mesh was allowed to soak in the cleaning solution for at least 2 min and then rinsed with warm water. The mesh was rinsed again with cold, distilled water and allowed to dry overnight. The field cage frames were cleaned by spraying the frame with ∼70% ethanol and wiping down several times, followed by wiping down the cage with ∼10% bleach. The frames were allowed to dry overnight. After the overnight dry, the frame was placed into the mesh netting carefully. This protocol was followed both upon first receiving the cages and after every experiment to ensure a sterile environment for the assay.
Food preparation instructions for field cages:
For preparation of the oviposition plates, we measured 4 g of flaky Instant Blue Drosophila medium (S22315C, Biological Resource Center, no. 22315C) into the bottom part of a Corning Cell Culture Dish (catalog no. 430167) (do not crush food up). For control (0% ethanol) food, we pipetted 16 ml of distilled water onto the instant food in 1-ml increments.
For 6% ethanol dishes, we pipetted 14.992 ml of distilled water onto the instant food in 0.937-ml increments. After pipetting water, we pipetted 1.008 ml of 95% ethanol [190 proof (95%); KOPTEC UN1170, ethyl alcohol, 3, PG II; part no. V1101] onto the dish in four increments of 0.252 ml (Figure S4A). We found that mixing the food by pipetting ethanol first followed by water does not elicit the phenotype, presumably because the ethanol becomes too diluted to be detectable by the flies; thus the order in which the food is mixed is critical for the experiment. After mixing the liquid and the food, we immediately placed one dish containing ethanol and one dish containing no ethanol into cage ∼15.5 in. apart from the center of the dish and 12 in. apart from the edge of the dish (Figure S4B).
Fly preparation for field cage experiments to acute response:
Flies to be used for experiments were kept in bottles containing standard cornmeal/molasses Drosophila medium with appropriate fungicides and kept at 25°. Bottles were prepared by inserting three KimWipes (Kimberly-Clark catalog no. 34155) rolled together directly into the Drosophila medium. Flies were aged together and were 3–5 days old before the start of the experiment.
For flies to be used to measure acute response, 300 female flies were anesthetized and placed together in an empty bottle. For flies cohabitating with wasps, 300 female flies and 150 female wasps were anesthetized and placed together in an empty bottle. The flies were then transported in the empty bottles to the center of the field cage. The bottle was then opened and the flies, or flies and wasps, were released from the middle of the cage (12-in. mark). The flies were allowed to oviposit for 24 hr at room temperature (∼23°) at 40% humidity for 24 hr. Fifteen hours of overhead light were allowed daily. After 24 hr, plates were removed and replaced with freshly prepared plates (prepared in the exact manner in which the original plates were made). Once plates were removed, the number of eggs on both the ethanol plate and the control plate were counted. All treatments were run in quadruplicate.
Fly preparation for field cage experiments to assay memory:
Flies to be used for experiments were kept in bottles containing standard cornmeal/molasses Drosophila medium with appropriate fungicides and kept at 25°. Bottles were prepared by inserting three KimWipes (as above) rolled together directly into the Drosophila medium. Flies were aged together and were 3–5 days old before the start of the experiment.
For exposures, 3- to 5-day-old flies were anesthetized with CO2 by placing them on a CO2 pad. Batches of 100 female flies and 20 male flies were placed into bottles that had three KimWipes rolled together and placed directly into the medium. One replicate of the egg-lay experiment requires three of these bottles for a total of 300 female flies and 60 male flies. Fifty female wasps were placed into each of the three bottles for exposure. For sham exposure (control) flies, 3- to 5-day-old flies were anesthetized by placing them on a CO2 pad; batches of 100 female flies and 20 male flies were placed into bottles that had three KimWipes rolled together, and these were placed directly into the medium. One replicate of the oviposition choice experiments requires three of these bottles for a total of 300 female flies and 60 male flies. When using the CO2 pad, we cleaned it with ∼70% ethanol before putting flies on it. After placing wasps on the pad, the pad was again cleaned with ∼70% ethanol. Any surface that made contact with the wasps was cleaned before it came into contact with flies.
Batches of exposed and unexposed flies were placed at room temperature (∼23°) for 24 hr. A constant overhead light source was present. After 24 hr, we anesthetized the insects. In bottles containing flies and wasps, we placed the insects on a CO2 pad and removed all of the wasps. We then combined batches of three exposed bottles into an empty fly bottle and allowed the flies to wake up. Before anesthetizing unexposed flies, we cleaned the surface of the fly pad with ∼70% ethanol and allowed it to dry. We then combined batches of three unexposed bottles in an empty fly bottle and allowed the flies to wake up. The flies were then transported in the empty bottles to the center of the cage. The bottle was then opened, and the flies were released from the middle of the cage (12-in. mark). The flies were allowed flies to oviposit for 24 hr at room temperature (∼23°), at 40% humidity, for 24 hr. Fifteen hours of overhead light were allowed daily. After 24 hr, plates were removed and replaced with freshly prepared plates (prepared in the exact manner in which the original plates were made). Once plates were removed, the number of eggs on both the ethanol plate and the control plate were counted. All treatments were run in quadruplicate.
For titration experiments, the above protocol was followed, but the time of exposure changed based on treatment. To avoid circadian rhythm confounding the results, all titration assays were started at 9:00 am and terminated when the training period ended (2 1/2, 7, 14, and 24 hr), at which point wasps were removed and the flies were placed in field cages as described above.
Fly corral preparation:
For single-fly and five-fly memory assays, we made smaller cages, termed “fly corrals,” using petri dishes. Holes were drilled into the petri dish where the centers of the two holes were 6 cm apart, and the edge of the two holes were 7.2 cm apart (Figure S5, A–C). The diameter of each hole was ∼1.2 cm. We melted a nitex nylon mesh covering onto the lid of the petri dish that had 120-µm openings (Genesee Scientific catalog no. 57-102). Dishes were cleaned using the Fisher Brand Sparkleen powder (catalog no. 04-320-4) using the ratio of 4 g of Sparkleen to 2 gallons of water. The plate was allowed to soak in the cleaning solution for at least 2 min and then rinsed with warm water and rinsed in cold distilled water. Plates were then cleaned with ∼10% bleach and soaked in water again. Plates were then allowed to air-dry for 24 hr. This cleaning protocol was followed both before and after an experiment was performed.
Food preparation instructions for fly corrals:
For preparation of the oviposition caps in the fly corrals, we measured 0.375 g of flaky Instant Blue Drosophila medium into the caps of 15-ml Falcon Tubes (S22315C, Biological Resource Center, no. 22315C) (do not crush food up). Caps from 15-ml Polypropylene Conical Tubes (Falcon 352097) had measurements of 2 cm diameter and 1 cm height (2.5 ml total interior volume). For control (0% ethanol) food, we pipetted 2250 µl of distilled water directly onto the instant food.
For 6% ethanol dishes, we pipetted 1966.5 µl of distilled water onto the instant food. After pipetting water, we pipetted 141.75 µl of 95% ethanol [190 proof (95%), USP/NF/FCC/EP/BP/JP] onto the dish. After mixing the liquid and the food, we immediately placed one cap containing ethanol and one cap containing no ethanol onto the cage with lab tape (VWR).
Fly preparation for fly corral experiments:
Flies to be used for experiments were kept in bottles containing standard cornmeal/molasses Drosophila medium with appropriate fungicides at 25°, as stated above.
For single-fly exposures, 3- to 5-day-old flies were anesthetized with CO2 by placing them on a CO2 pad. Single females were isolated and placed into vials containing standard Drosophila media. For exposures, a single female wasp was placed into the vial with the single female fly. Batches of exposed and unexposed flies were placed at room temperature (∼23°) for 24 hr. A constant light source was present. After 24 hr, we anesthetized the insects. In vials containing flies and wasps, insects were placed onto a CO2 pad, with all wasps being removed. We then placed the single females into an empty vial to be allowed to wake up. Before anesthetizing unexposed flies, we cleaned the surface of the fly pad with ∼70% ethanol and allowed it to dry. We then anesthetized single sham (control)-treated flies and placed each female into an individual empty vial. The flies were then transported in the empty vials to the petri dish cage, upon which they were aspirated into the cage. Caps with food were then attached to the plate. Fly corrals were kept in a climate-controlled room maintained at 25° and 40% humidity, with direct overhead lighting and a 12-hr light/dark cycle. After 24 hr, caps were removed and replaced with freshly prepared caps (prepared in the exact manner the original caps were made). Once caps were removed, the number of eggs on both the ethanol cap and the control cap were counted both at the 24- and 48-hr time points. Ten replicates were performed.
For experiments utilizing five flies in the Fly Corral, 50 female flies that were 3–5 days old were co-incubated with 20 female Lh14 wasps for 24 hr in 2.25-cm diameter vials or mock-exposed. Flies were anesthetized and placed into fly corrals after the 24-hr exposure period with 5 females and one male fly placed per dish. Fly corrals were kept in a climate-controlled room maintained at 25° and 40% humidity, with direct overhead lighting and a 12-hr light/dark cycle. After 24 hr, caps were removed and replaced with freshly prepared caps (prepared in the exact manner in which the original caps were made). Once caps were removed, the number of eggs on both the ethanol cap and the control cap were counted at both the 24- and 48-hr time points. Ten replicates were performed.
For experiments utilizing five flies in the fly corrals to measure the acute response, flies were anesthetized and placed into fly corrals with five females and one male fly placed per dish for control (unexposed) rooms and five females and one male fly with three female Lh14 wasps for exposed subjects. Fly corrals were kept in a climate-controlled room maintained at 25° and 40% humidity with direct overhead lighting and a 12-hr light/dark cycle. After 24 hr, caps were removed and replaced with freshly prepared caps (prepared in the exact manner in which the original caps were made). Once caps were removed, the number of eggs on both the ethanol cap and the control cap were counted at both the 24- and 48-hr time points. Ten replicates were performed.
Fly oviposition experiments:
Fly oviposition rates were as described previously (B. Z. Kacsoh et al., unpublished results). Briefly, oviposition rates were measured by using The Fly Condo (Genesee Scientific catalog no. 59-110), which contains 24 independent chambers. Each chamber is 7.5 cm long by 1.5 cm diameter. Each condo has a bottom 24-well food plate with ∼2 mL of standard molasses cornmeal media per chamber. Mesh wire is along the top of the condo, allowing air transfer. To assay egg retention of flies in the presence of wasps (acute exposure), five female flies and one male fly were placed into one chamber of The Fly Condo in the control, while three female Lh14 wasps were placed with the flies in the experimental setting. The oviposition plate from control and experimental condos was made 24 hr later. All treatments were run at 25° in 24 replicates. Fly condos and oviposition plates were bleached thoroughly with 10% bleach and rinsed with distilled water after every use. Egg plates were coded, and scoring was blind as the individual who was counting eggs was not aware of treatments or genotypes.
Experimental coding:
All experimental oviposition plates from field cages were scored after being double blinded in the manner described here. First, the treatments (exposed, unexposed) were coded by a lab member (G.B.). Second, these coded treatments would then be given to another lab member (L.B.) who would place the flies into cages and give the flies a choice between 6% ethanol food and control food. After 24 hr, plates would be removed and replaced. Removed plates would be coded so that whether or not they contained ethanol would be known only by the coder. These coded plates would then be given to another lab member (B.Z.K.) to count eggs. Coded plate numbers and corresponding egg values would be sent to the lab member mixing the food and switching out plates (L.B.) to decode whether or not the plates had ethanol. These data would then be sent to the treatment coder (G.B.) for full genotypic information and treatment decoding.
All experimental oviposition caps from fly corrals were scored after being blinded in the manner described here. First, the treatments (exposed, unexposed) were set up and coded by a lab member (S.H. or L.B.). For these coded treatments flies would then be placed into fly corrals and given a choice between 6% ethanol food and control food. After 24 hr, plates would be removed and replaced. Coded caps would then be given to another lab member for counting (B.K.). These data would then be sent to the initial coder (S.H. or L.B.) for the full genotypic and treatment decoding.
RU486 feeding
RU486 (Mifepristone) was used from Sigma (lot #SLBG0210V). Petri dishes were prepared in the exact manner as described above, but instead of using distilled water, an RU486 solution was used. This was prepared by dissolving 3.575 mg of RU486 in 800 µl methanol (Fisher Scientific lot no. 141313). This solution was added to 15.2 ml of distilled water. The total solution (16 mL) was thoroughly mixed and pipetted onto the instant food. For plates containing ethanol, the total solution of RU486 was changed to 14.992 ml of RU486 solution pipetted onto the instant food. After pipetting the RU486 solution, we pipetted 1.008 ml of 95% ethanol as described before.
Brain immunofluorescence
To assay whether feeding flies RU486 in the cages would be sufficient to turn on the mushroom body gene switch construct, we placed flies into cages containing RU846+ food. Flies had the mushroom body switch construct as well as a UAS GFP nuclear localization signal construct, such that if the mushroom body switch is activated, it should fluoresce with GFP. After a 24-hr period in the cage, adults were removed and fixed in 4% methanol-free formaldehyde in PBS with 0.001% triton X overnight at 4°. The samples were then washed in PBS with 0.1% triton X and stained with DNA staining with 4′, 6-diamidino-2-phenylindole (DAPI) for 10 min.
Imaging
A Nikon A1R SI confocal microscope was used for brain imaging. Images of the fly corral were made using an iPad 2 operating with ISO 64 (Figure S5, B and C). Images of the fly corral were color-enhanced in iPhoto (Figure S5, B and C).
Statistical analysis
Statistical tests were preformed in Microsoft Excel. Welch’s two-tailed t-tests were preformed for all egg-count data. P-values reported were calculated for comparisons between paired treatment group and unexposed.
Results
Flies oviposit on ethanol-laden food following wasp exposure
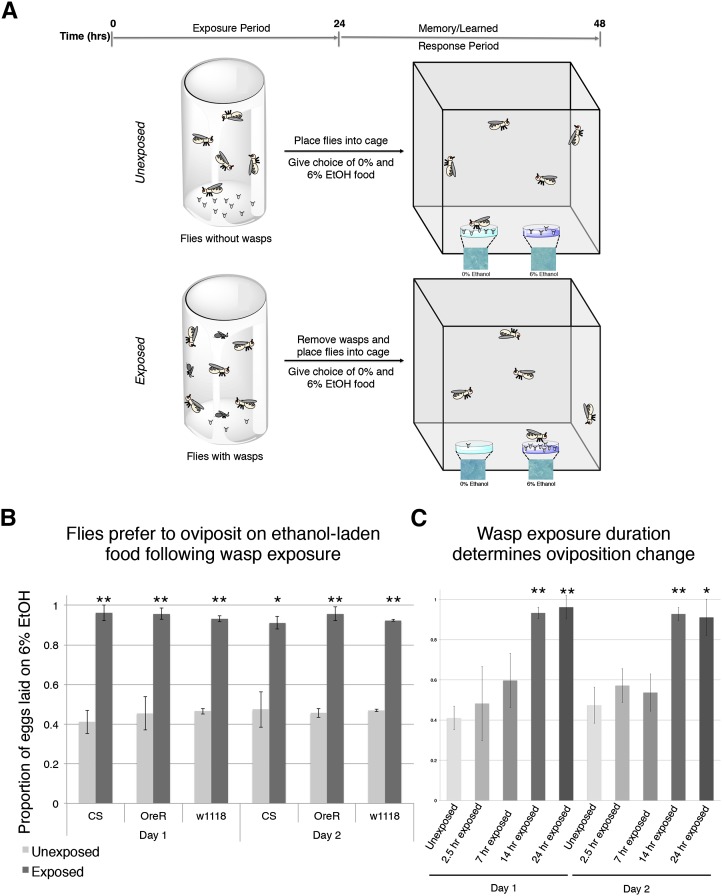
Previous work has found that fruit flies alter oviposition in the presence of wasps to actively oviposit on ethanol-laden food and has also demonstrated a role for memory in the persistence of changed oviposition behavior (Kacsoh et al. 2013). We decided to test whether different strains of adult D. melanogaster exposed to wasps continue to prefer to oviposit on ethanol-laden food or on food containing no ethanol in a 2-day period after wasps were removed from their environment. We tested this oviposition change of adult female D. melanogaster flies by placing batches of 100 female and 20 male flies into bottles containing standard cornmeal molasses Drosophila media (control). For wasp-exposed (Lh-14-exposed) bottles, batches of 100 female and 20 male flies were placed in bottles containing standard Drosophila media and 50 female wasps, as previously described (Kacsoh et al. 2013). Following a 24-hr exposure period, three bottles of control flies were anesthetized and pooled. For wasp-exposed flies, three bottles of flies and wasps were anesthetized, and wasps were separated from flies. The pooled flies were then placed in 0.6-m3 population cages with one cage containing control (unexposed) flies and the second containing exposed flies. The population cages contained two petri dishes filled with fly food (Figure 1A), one of which also contained 6% ethanol. Both control and ethanol food dishes contained a total 4 g of instant fly food. Control dishes were hydrated by pipetting 16 ml of water directly onto the food. To make ethanol-containing food dishes, food was first hydrated by pipetting 14.992 ml of water onto the instant food. After pipetting water, we pipetted 1.008 ml of 95% ethanol directly on top of the hydrated food (see Materials and Methods and Supporting Information for details). Fly eggs in each petri dish were counted at 24 hr, at which point new petri dishes were placed in the same configuration and fly eggs were counted again at 48 hr. All egg counts were performed in a double-blind fashion (see Materials and Methods). When no wasp exposure took place (control), flies preferred to oviposit on dishes containing no ethanol (Figure 1B). However, when exposed to females of the generalist wasp L. heterotoma (strain Lh14), a common parasite of D. melanogaster in nature, flies chose to lay a significantly greater proportion of their eggs on the ethanol dishes at both the 24- and 48-hr mark (Figure 1B). These results were consistent across multiple fly strains tested, demonstrating that genetic background is not causative for the behavioral changes observed (CS, OR, w1118). Previous work has shown that visual cues from wasps are essential to trigger a change in oviposition behavior (Kacsoh et al. 2013); however, it is not known if other important information about wasp presence could be perceived through auditory, gustatory, and/or olfactory sensory cues.
Figure 1.
Wasp exposure changes fly oviposition preference in multiple genetic backgrounds that persists for multiple days. The duration of wasp exposure determines the change in oviposition preference. (A) Diagram of the standard oviposition preference setup in field cages. (B) Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure after two time points in CS, OR, and w1118. (C) Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure after two time points in CS exposed to wasps for varying durations of 2 1/2, 7, 14, and 24 hr. All error bars represent 95% confidence intervals. (n = 4 biological replicates; *P < 0.01, **P < 0.001.)
Duration of wasp exposure determines behavioral output
Classical conditioning experiments demonstrate that long-term memory appears after training periods, which are temporally controlled (Tully et al. 1994). Long-lasting memories can be formed after spaced or massed training (Tully et al. 1994; Dubnau and Tully 1998). To see if behavioral changes depended on varying lengths of exposure or training, we modulated our protocol to expose flies to wasps for varying lengths of time and assay if the flies preferentially choose to oviposit on alcohol food throughout our 2-day oviposition choice assay. We found that a 2 1/2- and 7-hr exposure period was not enough to elicit a persistent behavioral change upon removal of wasps. However, 14- and 24-hr exposure periods elicited the change in oviposition preference for the duration of our 2-day assay, demonstrating that a training threshold exists for the change in behavior to persist even after wasps were removed (Figure 1C). These data also suggest that to have a long-lasting behavioral change flies must be in the constant presence of wasps for an extended period of time. Because the ratio of flies to wasps and the volume in which they interact is held constant, we presume that encounters between fly and wasp mixed in food bottles occur at equal frequency for the different exposure duration experiments (Figure 1C). The only variable for this experimental setup is the duration of time in which flies and wasps are allowed to cohabitate during the exposure phase. Thus, it is worth noting that at a population level this protocol could be viewed as massed training, but for the individual fly the exposure period may be more similar to repeated spaced training (Tully et al. 1994).
Maintenance of oviposition change depends on multiple canonical long-term memory genes
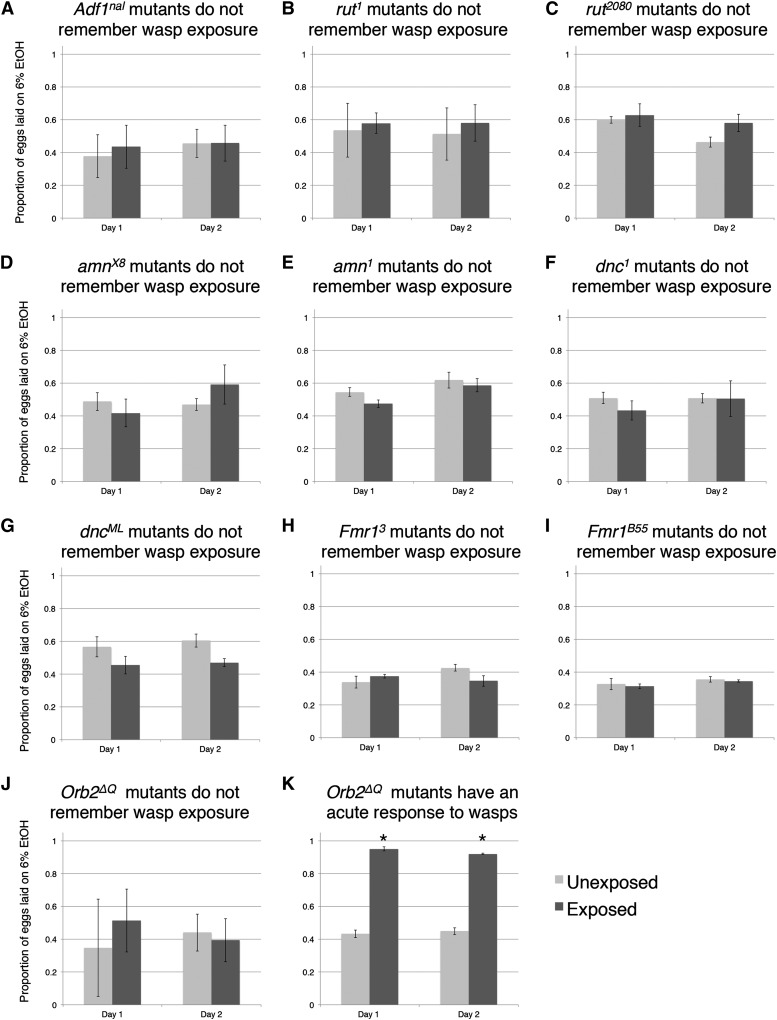
We hypothesized that the switch in oviposition preference that flies undergo when exposed to wasps would be controlled by long-term memory formation, given that this change in oviposition behavior lasted at least 2 days after removal of wasps (Figure 1B). To further dissect this persistent behavior, we decided to test six well-characterized genes known to have deficiencies in long-term memory formation: Alcohol Dehydrogenase Transcription Factor 1 (Adfl), rutabaga, amnesiac, dunce, FMR1, and Orb2.
The D. melanogaster gene Adf1 is known to be required for long-term memory formation and is also responsible for up-regulating alcohol response genes such as Adh, and a specific allele (Adf1nal) has been tested using this paradigm before (England et al. 1990; DeZazzo et al. 2000; Kacsoh et al. 2013). We tested Adf1nal, an Adf1 mutant that has normal early memory but lacks long-term memory (DeZazzo et al. 2000). When Adf1nal flies were pre-exposed to wasps and then put into oviposition preference cages without wasps, we found that the flies did not preferentially choose to oviposit on alcohol food throughout our 2-day oviposition choice assay (Figure 2A). This experiment recapitulates previously found results, further validates the behavioral assays used in the rest of the study, and suggests that other learning and memory gene products could also be responsible for memory of wasp presence. We emphasize that the Adf1nal flies are able to perceive wasps and change their oviposition behavior when in constant contact with wasps (Kacsoh et al. 2013; B. Z. Kacsoh et al., unpublished results); therefore, the Adf1nal mutant is deficient in maintenance of the oviposition behavior.
Figure 2.
Canonical memory genes are responsible for maintained oviposition depression. Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure in (A) Adf1nal, (B) rut1, (C) rut2080, (D) amnX8, (E) amn1, (F) dnc1, (G) dncML, (H) FMR13, (I) FMR1B55, and (J) Orb2ΔQ. Proportion of eggs laid on the 6% ethanol oviposition dish during active wasp exposure in (K) Orb2ΔQ. All error bars represent 95% confidence intervals. (n = 4 biological replicates; *P < 0.001.)
To test the possible role of other known learning and memory genes in this memory paradigm, we tested five additional genetic mutants. One of the most well-studied proteins in Drosophila memory formation is rutabaga (rut). The rut gene encodes for a calcium-calmodulin-dependent adenylyl cyclase (Han et al. 1992; Levin et al. 1992) as well as DCO that codes for a protein kinase (Drain et al. 1991; Skoulakis et al. 1993). The calcium-calmodulin-dependent adenylyl cyclase encoded by rut1 has been shown to be a detector of sensory information through the use of olfactory discrimination memory retention assays using electric shock reinforcement (Kandel et al. 1983). We tested two rut alleles (rut1, rut2080) and found that, when the flies were pre-exposed to wasps and then put into oviposition preference cages without wasps, flies did not preferentially choose to oviposit on ethanol-laden food throughout our 2-day oviposition choice assay (Figure 2, B and C).
Another well-studied protein in Drosophila memory formation is amnesiac (amn), which encodes a peptide regulator of adenylyl cyclase (Waddell et al. 2000). We tested two amn alleles (amnx8, amn1). We found that flies with either amn mutant allele did not preferentially choose to oviposit on ethanol-laden food throughout our 2-day oviposition choice assay (Figure 2, D and E). Additionally, we tested another well-studied protein in Drosophila memory formation, dunce (dnc), which encodes a cAMP-specific phosphodiesterase (Byers et al. 1981; Chen et al. 1986). We found that both mutant alleles tested (dnc1, dncML) were not able to maintain a preference for oviposition for ethanol-laden food throughout our 2-day oviposition choice assay (Figure 2, F and G).
To further demonstrate the role of long-term memory formation in our paradigm, we tested a human disease model gene, the Drosophila fragile X mental retardation protein (FMRP), in our memory assay. In humans, FMRP and Ataxin-2 (Atx2) are triplet expansion disease- and stress granule-associated proteins implicated in neuronal translational control and microRNA function (Oberle et al. 1991; Verkerk et al. 1991). The Drosophila FMRP (dFMR1) has been shown to be required for long-term associative memory (Dockendorff et al. 2002; McBride et al. 2005; Banerjee et al. 2010). This phenomenon has been shown to be dependent on Atx2-dependent potentiation of inhibitory transmission from local interneurons (McCann et al. 2011; Sudhakaran et al. 2014). We tested two alleles of the mutant Drosophila dFmr1 gene (dFmr13, dFmr1B55) and found that both mutants demonstrated defects in maintenance of behavior such that the flies did not preferentially choose to oviposit on alcohol food throughout our 2-day oviposition choice assay (Figure 2, H and I).
Finally, we tested a mutant in the gene Orb2, specifically the allele Orb2ΔQ. The ΔQ mutation was specifically selected as it leaves all essential functions of the Orb2 neuronal regulator intact, but deletes a Gln-rich prion domain exclusively required for persistent long-term memory, such that the flies have normal short-term memory formation, but defective long-term memory formation (Kruttner et al. 2012). This Gln-rich domain has been speculated to enable an Orb2 conformational change leading to active synaptic translation (Si et al. 2003; Keleman et al. 2007; Majumdar et al. 2012). We found that Orb2ΔQ mutant flies were unable to maintain oviposition preference on ethanol-laden food throughout the 2-day assay following wasp exposure (Figure 2J). This lack of maintenance of the oviposition behavior in Orb2ΔQ mutants cannot be explained by their inability to perceive or otherwise respond to wasp presence. This is because Orb2ΔQ mutants exhibit a robust acute oviposition behavioral response when they are in the presence of wasps (Figure 2K) and then lose this behavior once the wasps are removed (Figure 2J).
These data suggest that mutations in any of the above genes disrupt Drosophila’s ability to maintain the ethanol food preference after the predator threat has been removed. We emphasize that none of the mutants tested fail to perceive and respond to wasps as each of the mutants tested depress oviposition when in the presence of wasps (B. Z. Kacsoh et al., unpublished results) (Figure S1). Therefore, it is likely that failure to establish and/or maintain an ethanol food oviposition preference after wasp removal requires learning and memory consolidation functions of Adf1, rut, amn, dnc, orb2, and dFmr1 genes. It is worth noting that here we employ a definition of learning that merely requires experience-induced change in behavior. Since the wasp-induced change in oviposition behavior persists after removal of the wasps, and its maintenance does not require a wasp-associated cue, we suggest that this is an example of nonassociative learning.
Synaptic transmission in the fly mushroom body mediates long-term memory formation
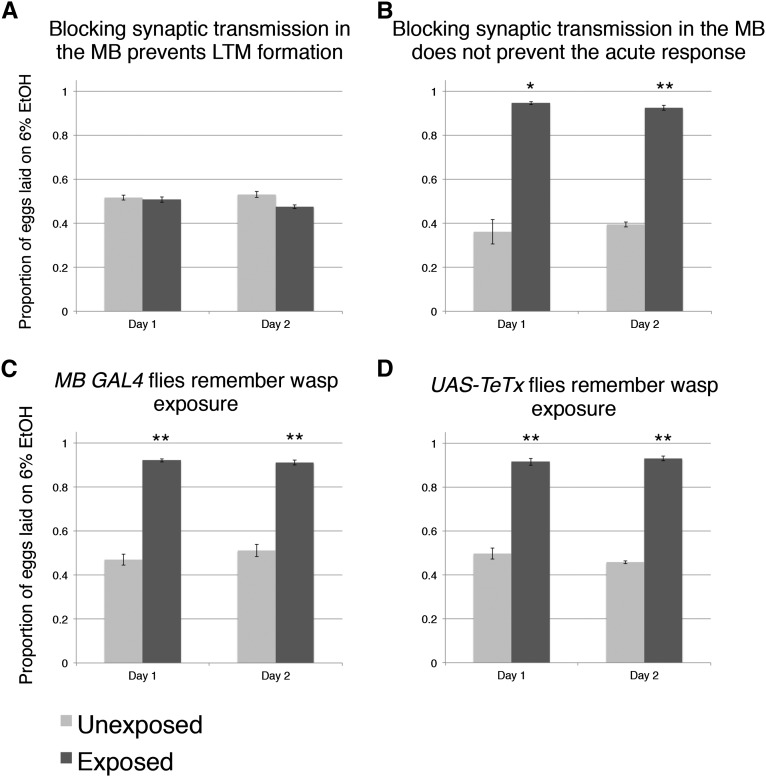
The MB of the adult brain is thought to be required for behaviors that are dependent on learning and memory (Schwaerzel et al. 2003; Aso et al. 2009; Claridge-Chang et al. 2009; Masse et al. 2009). A critical question that arose after testing multiple genes was whether these gene products were required in the MB for memory of wasp exposure to form. We hypothesized that the MB specifically plays a role in ethanol food preference memory after wasp exposure and thus set out to test this using the GAL4/UAS system (Brand and Dormand 1995). We used the GAL4/UAS system to express tetanus toxin light chain (UAS-TeTx) in conjunction with a MB driver (OK-107) (Aso et al. 2009). The tetanus toxin light chain works by catalytically inhibiting synaptic transmission once present in the cytosol by cleaving synaptobrevin, syntaxin, or SNAP-25 (Poulain et al. 1988; Bittner et al. 1989; Mochida et al. 1990; Kurazono et al. 1992; McMahon et al. 1993; Martin et al. 2002; Sweeney et al. 1995). Expression by the OK-107 > GAL4 transgene ensures that inhibition of synaptic transmission is restricted to the MB. We found that in the 24- and 48-hr period after wasp removal in which ethanol oviposition preference normally persists, the flies expressing UAS-TeTx in the MB no longer showed an oviposition preference for ethanol (Figure 3A). However, in the presence of wasps, flies expressing UAS-TeTx in the MB could perceive and respond to wasps by showing an oviposition preference for food containing ethanol (Figure 3B). Control parental lines with either OK-107 > GAL4 or UAS-TeTx transgenes (but not both) functioned as wild type (Figure 3, C and D). These data suggest that, although wasp presence is sensed by the visual system to elicit an acute response (Kacsoh et al. 2013), proper MB functions are likely to be required to maintain a persistent change in oviposition preference, lasting for the entirety of our assay (2 days) after the predator is removed from the environment. It is likely that some subset of the mushroom body neurons are responsible for the persistence of ethanol food preference, and additional studies will be necessary to clearly identify which of these neurons contribute to maintenance of this oviposition site selection behavior.
Figure 3.
Synaptic transmission in the fly mushroom body mediates long-term memory formation, but not the acute response. Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure in flies expressing tetanus toxin (UAS-TeTx) in mushroom body (A) and during constant wasp exposure (B). Proportion of eggs laid on the 6% ethanol oviposition following wasp exposure GAL4 (C) and UAS control (D) parental strains from UAS-TeTx expression. All error bars represent 95% confidence intervals. (n = 4 biological replicates; *P < 0.01, **P < 0.001.)
Inhibition of a canonical long-term memory gene in the mushroom body eliminates long-term memory formation
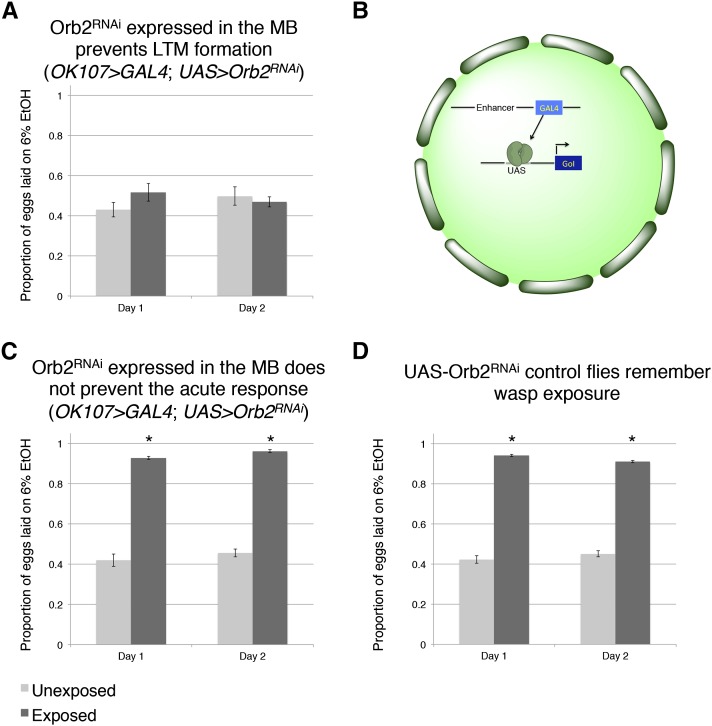
Mutants in orb2 exhibited a defect in persistence of the ethanol food oviposition preference assay (Figure 2J), but these experiments could not exclude the possibility that the orb2 gene product was required in non-neural tissues. Similarly, orb2 may have been necessary for early neuronal development and mutant phenotypes simply reflected developmental defects that precluded proper adult MB functions. Given that inhibiting synaptic transmission in the MB with TeTx eliminated a long-term behavioral response to wasp exposure (Figure 3A), we tested the hypothesis that the gene products of known learning and memory genes (like orb2) may also be required to function in this anatomical region of the brain. To test this, we used the GAL4/UAS system as above: In this case the MB driver (OK107 > GAL4) drove expression of an RNA-hairpin targeting orb2 messsenger RNA (mRNA). We found that RNA interference (RNAi) depletion of Orb2 in the MB produced the same phenotype as the orb2ΔQ mutant tested in the memory assay (Figure 4, A and B; Figure 2J). We also found that RNAi depletion of orb2 in the MB elicited the same phenotype as the orb2ΔQ mutant tested in the presence of wasps, where flies preferred to oviposit on ethanol-laden food (Figure 4C and Figure 2K). This result highlights that flies deficient in orb2 in the MB are able to perceive and respond to wasps, but not remember exposure once wasps are removed. Control parental lines with either just the OK107 > GAL4 or UAS > Orb2-hairpin transgenes (but not both) functioned as wild type as they exhibited no defects in behavior persistence (Figure 3C and Figure 4D). This suggests that orb2 is required in MB neuronal circuits for wasp-induced ethanol food oviposition behavior to persist, and it further suggests that persistence of this behavior likely requires long-term memory formation in the MB.
Figure 4.
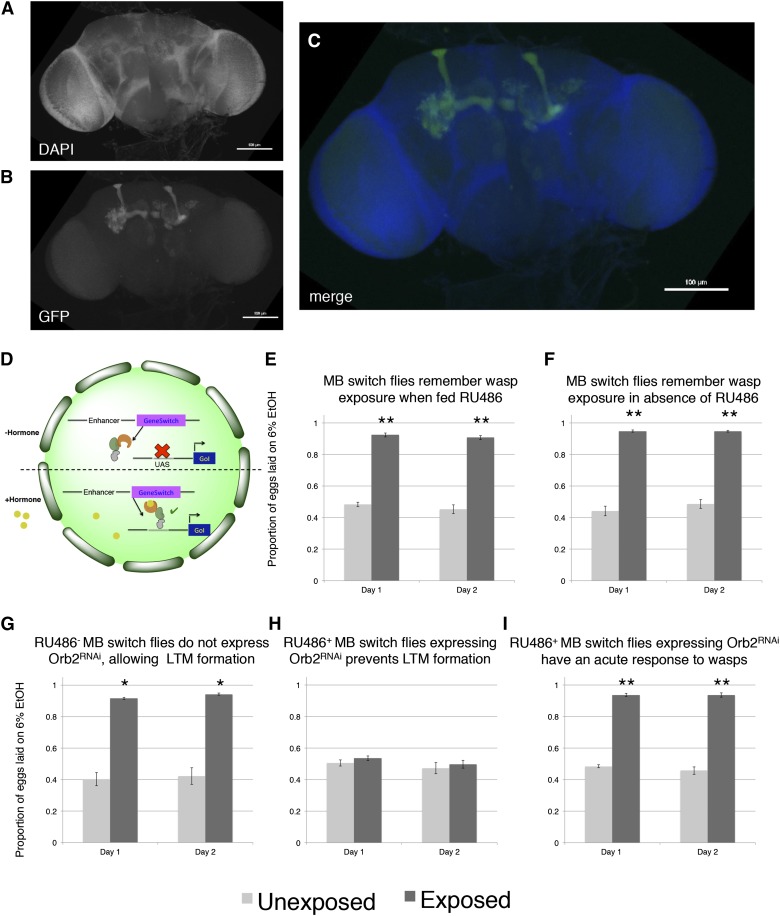
Inhibition of a canonical long-term memory gene in the mushroom body through RNAi eliminates long-term memory formation. Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure shown in A, C, and D. (A) Orb2 RNAi knockdown in the MB (OK107 > GAL4) memory assay (OK107 > GAL4;UAS > Orb2RNAi). (B) Cartoon diagram illustrating the GAL4/UAS system (GoI: Gene of Interest). (C) Orb2 RNAi knockdown in the MB (OK107 > GAL4) in the presence of wasps showing intact acute response (OK107 > GAL4;UAS > Orb2RNAi). (D) UAS control parental line from Orb2 RNAi-knockdown cross. All error bars represent 95% confidence intervals. (n = 4 biological replicates; *P < 0.001.)
These data, however, cannot distinguish between two possible roles for orb2. First, the orb2 gene product could be required for normal development of the MB and other parts of the nervous system that interface with the MB. Therefore, because the OK107 > GAL4 driver begins expression of GAL4 in the larvae it remains possible that RNAi depletion of Orb2 in the larvae could cause developmental defects that then indirectly cause behavioral phenotypes in adults. A second possibility is that persistence of ethanol food oviposition preference requires orb2 function in the adult MB, regardless of its possible function during MB development. To address this question, we turned to the GAL4-based Gene-Switch system where the GAL4 transcription factor is fused to the human progesterone ligand-binding domain (Burcin et al. 1999). We used flies expressing the Gene-Switch transgene specifically in the MB where only an administration of the pharmacological Gene-Switch ligand RU486 could activate the GAL4 transcription factor (Mao et al. 2004) (Figure 5). To confirm that our feeding protocol could work in our field cages, we used the MB Gene-Switch line to express a nuclear-localized GFP. Flies were placed into field cages containing a control and an ethanol food dish. Instead of using water, a mixture of RU486 dissolved in methanol and water were used to hydrate the food. Ethanol was added as in all other experiments described above. We found that flies placed in our field cages where the food dishes contain RU486 induce GFP signal specifically localized to the MB, whereas dishes lacking RU486 do not induce GFP after 24 hr (Figure 5, A–D; Figure S2, A–C). We highlight that the addition of ethanol onto the food does not prevent RU486 induction. We fed flies expressing the MB Gene-Switch transgene RU486 for 24 hr during the wasp exposure period and an additional 24 hr after the wasps were removed (Figure S2D; see Materials and Methods). Egg counts for the 0- to 24- and 24 to 48-hr period when wasps have been removed clearly show a preference for ethanol food oviposition, while unexposed sibling controls under the same conditions exhibited no such preference (Figure 5E). Given the successful feeding protocol, the MB Gene-Switch construct specificity, and that the addition of ethanol onto the food does not prevent RU486 induction, we used the MB Gene-Switch to express an RNA-hairpin targeting mRNA for Orb2. Induction of the RNA-hairpin through RU486 feeding in the MB was expected to occur within the same window of time as the GFP expression (Figure 5, A–D). When we perform the same wasp exposure with flies expressing the MB Gene-Switch transgene, but do not feed them RU486, we see a preference for ethanol food oviposition in wasp-exposed flies (Figure 5F). This observation again demonstrates that RU486 does not perturb Drosophila’s ability to perceive and respond to wasp presence by changing their oviposition behavior, preferentially laying eggs on ethanol containing food. Flies expressing the MB Gene-Switch and carrying the UAS > Orb2-RNA-hairpin construct, which were not fed RU486, showed normal, wild-type memory of the ethanol food oviposition preference behavior (Figure 5G). Flies expressing the MB Gene-Switch and carrying the UAS > Orb2-RNA-hairpin construct, which were fed RU486, showed impaired memory formation and did not preferentially oviposit on ethanol-laden food (Figure 5H). These two data points suggest that the UAS > Orb2-RNA-hairpin construct is driven only in flies expressing the MB Gene-Switch when fed RU486 only. When we use the MB Gene-Switch and carry the UAS > Orb2-RNA-hairpin construct that fed RU486 in the constant presence of wasps over a 2-day assay, we find wild-type behavior and preference for ethanol-laden food (Figure 5I). The MB Gene-Switch parental control line elicited wild-type memory formation with and without RU486 feeding, demonstrating that the Gene-Switch ligand (RU486) alone is not responsible for memory impairment (Figure 5, E and F). This observation again demonstrates that RU486 does not perturb Drosophila’s ability to perceive and respond to wasp presence and that orb2 function is required for formation of a long-term memory of wasp exposure and not perception of, and an acute response to, wasps.
Figure 5.
Inhibition of a canonical long-term memory gene in the mushroom body through use of Gene-Switch eliminates long-term memory formation. Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure shown in E–I. Brains from flies expressing the Gene-Switch construct in the mushroom body along with a GFP nuclear localization signal showing (A) DAPI, (B) GFP expression, and (C) the merged image. (D) Cartoon diagram illustrating the Gene-Switch system (GoI: Gene of Interest). Gene-Switch control parental line from Orb2 MB Gene-Switch RNAi-knockdown crosses fed RU486 (E) and not fed RU486 (F). (G) Orb2 RNAi knockdown in the MB (Gene-Switch) not fed Ru486. (H) Orb2 RNAi knockdown in the MB (Gene-Switch) fed Ru486. (I) Orb2 RNAi knockdown in the MB (Gene-Switch) fed Ru486 in the presence of wasps show acute response. For E–I, error bars represent 95% confidence intervals. (n = 4 biological replicates; *P < 0.01, **P < 0.001.)
Collectively, these data indicate that normal orb2 function is required in the adult MB for normal long-term memory formation and behavioral changes that persist over our 2-day assay. Use of the MB Gene-Switch construct provides strong evidence to delimit temporal and spatial expression requirements for orb2 function in the context of this memory assay. Importantly, that Orb2-RNAi knock-down in the MB using either OK107 > GAL4 or MB Gene-Switch did not prevent this food oviposition preference from occurring when flies were in the presence of wasps also demonstrates that loss or diminution of orb2 function in the MB does not affect perception and acute response to this predator (Figure 4C and Figure 5I).
Presence of conspecifics does not determine induction of memory
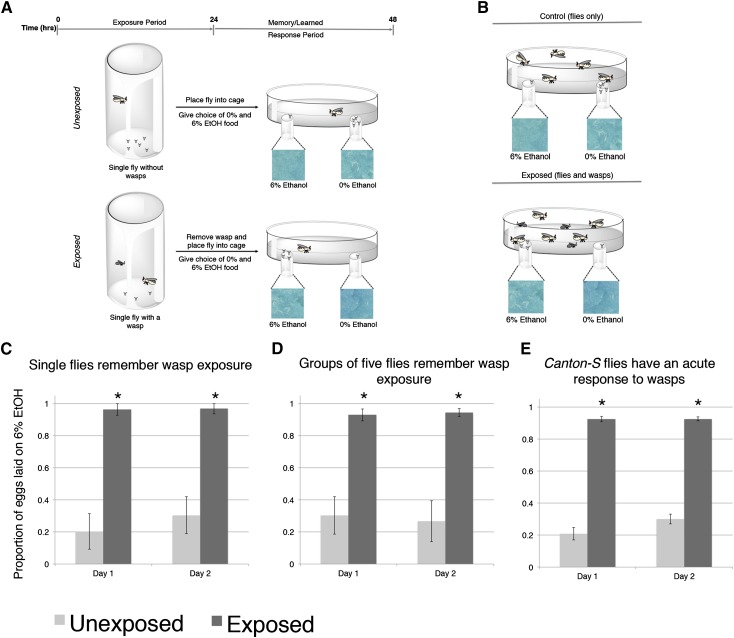
Given that our long-term memory assay utilizes groups of 300 female flies, we wondered whether the number of flies in the assay could be influencing the formation of the memory through social communication. Food choice preferences has been shown to be influenced by group behavior (Battesti et al. 2012). Previous work from our lab has demonstrated that experienced (wasp-exposed) flies, which reduce oviposition in the presence of wasps, can transmit this information to naive, unexposed flies, which results in depressed oviposition in both teacher and student fly (B. Z. Kacsoh et al., unpublished results). To account for such a variable, we redesigned our behavior assay: instead of using 300 females in a population cage, we used a single female, either exposed or unexposed, placed in a petri dish with a mesh covering, to allow for ventilation, and two food plates, one of which contained ethanol (Figure 6A). These scaled-down cages were our fly corrals (Figure S5). We also utilized our fly corral memory units to assay whether flies could still respond to the presence of wasps (Figure 6B). We found that our single-fly memory assay recapitulated the same result as observed in the field cages containing 300 females, where our single flies preferred to oviposit on ethanol-laden food throughout our 2-day oviposition choice assay (Figure 6C). We emphasize that individual flies in each fly corral cannot see, touch, and presumably cannot smell or hear other individuals in other cages since each cage is kept a minimum of 5 cm away from the next and never stacked. These data suggest that the long-term memory of the ethanol food oviposition preference behavior was maintained independently of other individuals. To further validate this method, we performed a memory assay in fly corrals again, but this time using five females instead of just one female. Five female flies in the assay yielded the same result as the single-fly assay (Figure 6D). Taken together, these data demonstrate that maintenance of long-term memory is individually independent and that the memory assay can be scaled down to make it feasible to test not just known memory genes, but novel ones as well. We also wanted to see if our fly corrals elicited a comparable acute response of flies in the presence of wasps. We find that, when we pair five female flies with wasps, flies prefer to oviposit on ethanol food to the same degree as in the field cages (Figure 2K and Figure 6E). Collectively, these data demonstrate the use of a scaled-down version of the field cage, using our fly corrals, to further elucidate components of this behavior. Using our scaled-down fly corrals, we decided to revisit the possibility that perhaps some of our memory mutants, which failed to show a memory phenotype, could not respond to wasps by altering oviposition to ethanol-laden food. We tested mutants in Adf1, rut, amn, dnc, orb2, and dFmr1 genes by using five female flies paired with either no wasp (control) or with three female wasps. We find that each of the mutants tested do indeed have an intact acute response to wasps, where they actively oviposit on food containing ethanol (Figure S3, A–J). The acute response of the orb2 mutant in the fly corral (Figure S3E) mirrored its acute response observed in the field cages (Figure 2K), demonstrating fly corral’s viability as an alternative to the field cages.
Figure 6.
Presence of conspecifics does not determine induction of memory. (A) Diagram of the standard oviposition preference setup using a single fly utilizing the fly corral. For assays using five flies, oviposition preference is the same as in A but with five flies. (B) Diagram of the standard oviposition preference setup using five flies under constant wasp exposure utilizing the fly corral to measure acute response. Proportion of eggs laid on the 6% ethanol oviposition dish following wasp exposure in the single-fly assay (C) and utilizing the five-fly assay (D). Proportion of eggs laid on the 6% ethanol oviposition dish during constant wasp exposure in the assay utilizing five flies (E). Error bars represent 95% confidence intervals. (n = 10 biological replicates; *P < 0.001.)
Discussion
In this study we have shown that Drosophila exhibit a behavioral change following wasp exposure, where, upon wasp removal, ethanol oviposition preference persists over multiple days in flies with intact learning and memory functions. Loss of memory gene functions, such as those of Adf1, amn, dnc, dFmr1, and rut, or inhibition of mushroom body synaptic transmission had no effect on the ability to change oviposition behavior in the presence of wasp; however, in each of these cases persistence of this behavior after wasp removal was abolished. Additionally, inhibition of orb2 using the GAL4/UAS and Gene-Switch systems suggests that maintenance of the change in oviposition state requires neural signaling mediated by a memory component of the adult brain.
Our observations document and describe a particularly robust form of memory in Drosophila and establish several fundamental features. First, learning and long-term memory consolidation in this nonassociative paradigm require genes known to be involved in associative learning and memory. Second, persistence of the predator response requires a neural input from the memory component of the brain (MB). Third, the long-term memory phenotype observed is conspecifically independent, where single flies can exhibit ethanol oviposition preference and persistence of this behavior does not seem to require interactions between different individuals. A scaled-down version of this behavioral assay, using one to five female flies, recapitulates both the initial response to wasps and the persistent memory component of their behavioral response. We propose that this specific assay will now make it possible to perform large-scale screens for genetic and environmental factors that contribute to memory formation and maintenance.
In sum, we have shown that extrinsic inputs modify synaptic signaling in the mushroom body of the fly brain to implement a behavioral change and that experiments based around wasp exposure can serve as a simple and robust memory paradigm in future D. melanogaster research. The genes that we tested and found to be involved in this long-term memory paradigm are conserved across many animal species (Bolduc and Tully 2009) and thus serve as an excellent approach to modeling cellular and neuronal network functions that may be relevant to vertebrate brain function. Although the vertebrate brain is vastly more complex than that of the fly, additional genes, gene families, and pharmacological effects can be elucidated in Drosophila and may identify core mechanisms that are used in all species. These conserved components provide starting points in vertebrate animals for further vertical integration. In this way, mechanisms that are unique to vertebrates can also be inferred, and we suggest that the memory paradigm and assay presented here will prove to be a useful discovery tool. We believe that this study establishes a new robust model in Drosophila for understanding the molecular basis of memory formation and consolidation of an ecologically relevant behavior with possible far-reaching implications for neurobiology and Darwinian selection and evolution.
Supplementary Material
Acknowledgments
We thank Leslie Griffith, Daniela Zarnescu, FlyBase, and the Bloomington Drosophila Stock Center for Drosophila stocks; Todd Schlenke for wasp stocks; the Dartmouth Department of Biological Sciences Light Microscopy Facility; and Huy Nguyen and Heather Wallace for helpful comments on the manuscript. We acknowledge grants from the Geisel School of Medicine at Dartmouth and National Institute of General Medical Sciences (K18GM097732 to G.B.) and Science Foundation Ireland (M.R.).
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172221/-/DC1.
Communicating editor: H. J. Bellen
Literature Cited
- Aso Y., Grubel K., Busch S., Friedrich A. B., Siwanowicz I., et al. , 2009. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 23: 156–172. [DOI] [PubMed] [Google Scholar]
- Banerjee P., Schoenfeld B. P., Bell A. J., Choi C. H., Bradley M. P., et al. , 2010. Short- and long-term memory are modulated by multiple isoforms of the fragile X mental retardation protein. J. Neurosci. 30: 6782–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battesti M., Moreno C., Joly D., Mery F., 2012. Spread of social information and dynamics of social transmission within Drosophila groups. Curr. Biol. 22: 309–313. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., DasGupta B. R., Holz R. W., 1989. Isolated light chains of botulinum neurotoxins inhibit exocytosis. Studies in digitonin-permeabilized chromaffin cells. J. Biol. Chem. 264: 10354–10360. [PubMed] [Google Scholar]
- Bolduc F. V., Tully T., 2009. Fruit flies and intellectual disability. Fly (Austin) 3: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Dormand E. L., 1995. The GAL4 system as a tool for unravelling the mysteries of the Drosophila nervous system. Curr. Opin. Neurobiol. 5: 572–578. [DOI] [PubMed] [Google Scholar]
- Burcin M. M., Schiedner G., Kochanek S., Tsai S. Y., O’Malley B. W., 1999. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA 96: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Davis R. L., Kiger J. A., Jr, 1981. Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 289: 79–81. [DOI] [PubMed] [Google Scholar]
- Carton Y., Nappi A. J., 1997. Drosophila cellular immunity against parasitoids. Parasitol. Today 13: 218–227. [DOI] [PubMed] [Google Scholar]
- Chawla S. S., Perron J.-M., Raouco-Thomas C., 1981. Effects of ingested ethanol on adult Drosophila melanogaster (Diptera:Drosophilidae). Can. Entomol. 113: 315–323. [Google Scholar]
- Chen C. N., Denome S., Davis R. L., 1986. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc. Natl. Acad. Sci. USA 83: 9313–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A., Roorda R. D., Vrontou E., Sjulson L., Li H., et al. , 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Sadanandappa M. K., Dervan A., Larkin A., Lee J. A., et al. , 2011. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. USA 108: E646–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R., Van Herrewege J., 1983. Adaptation to alcoholic fermentation in Drosophila species: relationship between alcohol tolerance and larval habitat. Comp. Biochem. Physiol. Part A. Physiol. 74: 283–288. [Google Scholar]
- Davis R. L., 2005. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28: 275–302. [DOI] [PubMed] [Google Scholar]
- DeZazzo J., Sandstrom D., de Belle S., Velinzon K., Smith P., et al. , 2000. nalyot, a mutation of the Drosophila myb-related Adf1 transcription factor, disrupts synapse formation and olfactory memory. Neuron 27: 145–158. [DOI] [PubMed] [Google Scholar]
- Dockendorff T. C., Su H. S., McBride S. M., Yang Z., Choi C. H., et al. , 2002. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34: 973–984. [DOI] [PubMed] [Google Scholar]
- Drain P., Folkers E., Quinn W. G., 1991. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron 6: 71–82. [DOI] [PubMed] [Google Scholar]
- Driessen G., Hemerik L., van Alphen J. J. M., 1990. Drosophila species breeding in the stinkhorn (Phallus impudicus Pers.) and their larval parasitoids. Neth. J. Zool. 40: 409–427. [Google Scholar]
- Dubnau J., Tully T., 1998. Gene discovery in Drosophila: new insights for learning and memory. Annu. Rev. Neurosci. 21: 407–444. [DOI] [PubMed] [Google Scholar]
- England B. P., Heberlein U., Tjian R., 1990. Purified Drosophila transcription factor, Adh distal factor-1 (Adf-1), binds to sites in several Drosophila promoters and activates transcription. J. Biol. Chem. 265: 5086–5094. [PubMed] [Google Scholar]
- Fleury F., Ris N., Allemand R., Fouillet P., Carton Y., et al. , 2004. Ecological and genetic interactions in Drosophila-parasitoids communities: a case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica 120: 181–194. [DOI] [PubMed] [Google Scholar]
- Geer B. W., Langevin M. L., McKechnie S. W., 1985. Dietary ethanol and lipid synthesis in Drosophila melanogaster. Biochem. Genet. 23: 607–622. [DOI] [PubMed] [Google Scholar]
- Greenspan R. J., 1995. Flies, genes, learning, and memory. Neuron 15: 747–750. [DOI] [PubMed] [Google Scholar]
- Han P. L., Levin L. R., Reed R. R., Davis R. L., 1992. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron 9: 619–627. [DOI] [PubMed] [Google Scholar]
- Kacsoh B. Z., Lynch Z. R., Mortimer N. T., Schlenke T. A., 2013. Fruit flies medicate offspring after seeing parasites. Science 339: 947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., et al. , 1983. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb. Symp. Quant. Biol. 48(Pt 2): 821–830. [DOI] [PubMed] [Google Scholar]
- Keleman K., Kruttner S., Alenius M., Dickson B. J., 2007. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat. Neurosci. 10: 1587–1593. [DOI] [PubMed] [Google Scholar]
- Kruttner S., Stepien B., Noordermeer J. N., Mommaas M. A., Mechtler K., et al. , 2012. Drosophila CPEB Orb2A mediates memory independent of its RNA-binding domain. Neuron 76: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurazono H., Mochida S., Binz T., Eisel U., Quanz M., et al. , 1992. Minimal essential domains specifying toxicity of the light chains of tetanus toxin and botulinum neurotoxin type A. J. Biol. Chem. 267: 14721–14729. [PubMed] [Google Scholar]
- LaSalle, J. G., I.D., 1993 Parasitic Hymenoptera, biological control and biodiversity, pp. 197–215 in Hymenoptera and Biodiversity edited by J. LaSalle and I. D. Gauld. CAB International, Wallingford, UK. [Google Scholar]
- Lefevre T., de Roode J. C., Kacsoh B. Z., Schlenke T. A., 2012. Defence strategies against a parasitoid wasp in Drosophila: Fight or flight? Biol. Lett. 8: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L. R., Han P. L., Hwang P. M., Feinstein P. G., Davis R. L., et al. , 1992. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell 68: 479–489. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Cesario W. C., White-Grindley E., Jiang H., Ren F., et al. , 2012. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell 148: 515–529. [DOI] [PubMed] [Google Scholar]
- Mao Z., Roman G., Zong L., Davis R. L., 2004. Pharmacogenetic rescue in time and space of the rutabaga memory impairment by using Gene-Switch. Proc. Natl. Acad. Sci. USAb 101: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C., Tully T., Dubnau J., 2005. Deconstructing memory in Drosophila. Curr. Biol. 15: R700–R713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Keller A., Sweeney S. T., 2002. Targeted expression of tetanus toxin: a new tool to study the neurobiology of behavior. Adv. Genet. 47: 1–47. [DOI] [PubMed] [Google Scholar]
- Masse N. Y., Turner G. C., Jefferis G. S., 2009. Olfactory information processing in Drosophila. Curr. Biol. 19: R700–R713. [DOI] [PubMed] [Google Scholar]
- McBride S. M., Choi C. H., Wang Y., Liebelt D., Braunstein E., et al. , 2005. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45: 753–764. [DOI] [PubMed] [Google Scholar]
- McCann C., Holohan E. E., Das S., Dervan A., Larkin A., et al. , 2011. The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc. Natl. Acad. Sci. USA 108: E655–E662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Deshazer M., Davis R. L., 2005. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog. Neurobiol. 76: 328–347. [DOI] [PubMed] [Google Scholar]
- McKenzie J. A., McKechnie S. W., 1978. Ethanol tolerance and the Adh polymorphism in a natural population of Drosophila melanogaster. Nature 272: 75–76. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., et al. , 1993. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364: 346–349. [DOI] [PubMed] [Google Scholar]
- Mercot H., Defaye D., Capy P., Pla E., David J. R., 1994. Alcohol tolerance, ADH activity, and ecological niche in Drosophila species. Evolution 48: 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan N. F., Kacsoh B. Z., Schlenke T. A., 2012. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr. Biol. 22: 488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Poulain B., Eisel U., Binz T., Kurazono H., et al. , 1990. Exogenous mRNA encoding tetanus or botulinum neurotoxins expressed in Aplysia neurons. Proc. Natl. Acad. Sci. USA 87: 7844–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle I., Rousseau F., Heitz D., Kretz C., Devys D., et al. , 1991. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Parsons P. A., Spence G. E., 1981. Ethanol utilization: threshold differences among three Drosophila species. Am. Nat. 117: 568–571. [Google Scholar]
- Poulain B., Tauc L., Maisey E. A., Wadsworth J. D., Mohan P. M., et al. , 1988. Neurotransmitter release is blocked intracellularly by botulinum neurotoxin, and this requires uptake of both toxin polypeptides by a process mediated by the larger chain. Proc. Natl. Acad. Sci. USA 85: 4090–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M., 2014. Network plasticity in adaptive filtering and behavioral habituation. Neuron 82: 1216–1229. [DOI] [PubMed] [Google Scholar]
- Schlenke T. A., Morales J., Govind S., Clark A. G., 2007. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 3: 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., et al. , 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23: 10495–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K., Giustetto M., Etkin A., Hsu R., Janisiewicz A. M., et al. , 2003. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell 115: 893–904. [DOI] [PubMed] [Google Scholar]
- Skoulakis E. M., Kalderon D., Davis R. L., 1993. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron 11: 197–208. [DOI] [PubMed] [Google Scholar]
- Sudhakaran I. P., Hillebrand J., Dervan A., Das S., Holohan E. E., et al. , 2014. FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc. Natl. Acad. Sci. USA 111: E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney S. T., Broadie K., Keane J., Niemann H., O’Kane C. J., 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14: 341–351. [DOI] [PubMed] [Google Scholar]
- Tully T., 1987. Drosophila learning and memory revisited. Trends Neurosci. 10: 330–335. [Google Scholar]
- Tully T., Preat T., Boynton S. C., Del Vecchio M., 1994. Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., et al. , 1991. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914. [DOI] [PubMed] [Google Scholar]
- Waddell S., Armstrong J. D., Kitamoto T., Kaiser K., Quinn W. G., 2000. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 103: 805–813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.