Abstract
When cells undergo replication stress, proper checkpoint activation and deactivation are critical for genomic stability and cell survival and therefore must be highly regulated. Although mechanisms of checkpoint activation are well studied, mechanisms of checkpoint deactivation are far less understood. Previously, we reported that chromatin remodeling factors Isw2 and Ino80 attenuate the S-phase checkpoint activity in Saccharomyces cerevisiae, especially during recovery from hydroxyurea. In this study, we found that Isw2 and Ino80 have a more pronounced role in attenuating checkpoint activity during late S phase in the presence of methyl methanesulfonate (MMS). We therefore screened for checkpoint factors required for Isw2 and Ino80 checkpoint attenuation in the presence of MMS. Here we demonstrate that Isw2 and Ino80 antagonize checkpoint activators and attenuate checkpoint activity in S phase in MMS either through a currently unknown pathway or through RPA. Unexpectedly, we found that Isw2 and Ino80 increase chromatin accessibility around replicating regions in the presence of MMS through a novel mechanism. Furthermore, through growth assays, we provide additional evidence that Isw2 and Ino80 partially counteract checkpoint activators specifically in the presence of MMS. Based on these results, we propose that Isw2 and Ino80 attenuate S-phase checkpoint activity through a novel mechanism.
Keywords: Isw2, Ino80, chromatin accessibility, S-phase checkpoint
FOR proper propagation of cells, DNA must be faithfully copied during replication in S phase . However, during replication, cells are prone to encountering nucleotide depletion or DNA damage, which generates replication stress and induces replication fork stalling. Upon replication stress, cells trigger a mechanism called the S-phase checkpoint to allow for proper completion of replication before exit out of S phase. Because this pathway is essential for genomic stability, as evidenced by the development of cancer or cell death in checkpoint mutants (Zeman and Cimprich 2014) and strict conservation in eukaryotic cells, mechanisms of S-phase checkpoint activation have been studied extensively (Branzei and Foiani 2009).
When replication forks stall in budding yeast cells, excess single-stranded DNA (ssDNA) is produced that is bound by replication protein A (RPA). Accumulation of the ssDNA-RPA complex recruits a central kinase Mec1 [ataxia telangiectasia and Rad3-related kinase (ATR) in mammalian cells] with its interacting partner Ddc2 [ATR interacting protein (ATRIP)], which activates the downstream effector kinases Rad53 (Chk2) and Chk1 (Chk1) to stabilize replication forks, up-regulate damage-inducible genes, and slow S-phase progression through the delay of late origin firing until DNA is replicated correctly (Rouse and Jackson 2002; Zou and Elledge 2003; Friedel et al. 2009). The S-phase checkpoint can be activated via two different pathways, the DNA damage checkpoint (DDC) and/or the DNA replication checkpoint (DRC), depending on the type of damage recognized by the cell (Crabbe et al. 2010). In the DDC of budding yeast, the clamp loader RFCRad24 (RFCRad17) and subsequently the PCNA-like 9-1-1 clamp Rad17-Ddc1-Mec3 (Rad9-Hus1-Rad1) are recruited to ssDNA–double-stranded DNA (dsDNA) junctions to facilitate activation of the checkpoint along with Dpb11 (TopBP1) and Rad9 (Gilbert et al. 2001; Kondo et al. 2001; Majka et al. 2006; Navadgi-Patil and Burgers 2008). In the DRC, Tof1 (TIM), Mrc1 (claspin), and Dna2 are recruited to replication forks to facilitate activation of the checkpoint (Katou et al. 2003; Kumar and Burgers 2013).
In contrast to S-phase checkpoint activation mechanisms, mechanisms that regulate checkpoint deactivation are far less understood. The best-characterized mechanism of checkpoint deactivation is via Rad53 phosphatases Pph3 and Ptc2. Deletion of Pph3 and Ptc2 results in elevated sensitivity to replication inhibitors and complete replication fork arrest (Szyjka et al. 2008). This result demonstrates that proper control of the amplitude and inactivation of the S-phase checkpoint is also essential for DNA replication control in the presence of replication stress. It also has been shown that other modes of checkpoint deactivation exist that have yet to be identified, highlighting the need for more studies on checkpoint deactivation mechanisms (Travesa et al. 2008).
We have recently proposed that the highly conserved chromatin remodeling factors Isw2 and Ino80 play roles in S-phase checkpoint attenuation (Au et al. 2011). Chromatin remodeling factors use the energy of ATP hydrolysis to alter chromatin structure and have been implicated in many DNA-dependent processes, including transcription, DNA replication, DNA repair, and checkpoint regulation (Morrison and Shen 2009). Isw2 has been demonstrated to slide nucleosomes in vivo (Fazzio and Tsukiyama 2003). Functionally, Isw2 has been shown to repress transcription of genes by sliding nucleosomes over transcriptional start sites (Goldmark et al. 2000; Whitehouse et al. 2007). In the mean time, Ino80 can replace the histone variant Htz1 within nucleosomes with canonical histone H2A (Papamichos-Chronakis et al. 2011). Ino80 is required for proper DNA damage response after DNA double-strand breaks (Shen et al. 2000; van Attikum et al. 2004), as well as replication stress responses such as maintaining fork stability and promoting recovery of stalled forks (Papamichos-Chronakis and Peterson 2008; Shimada et al. 2008). However, how this complex performs these functions remains largely unknown.
We previously reported that Isw2 and Nhp10, a subunit of the Ino80 complex, function together to attenuate the S-phase checkpoint after transient exposure to the DNA replication inhibitor hydroxyurea (HU) (Au et al. 2011). In this study, we show that Isw2 and Nhp10 exhibit a much stronger effect in attenuating checkpoint activity in the presence of the DNA alkylating agent methyl methanesulfonate (MMS) throughout late S phase. Through systematic genetic tests, we show that Isw2 and Nhp10 antagonize checkpoint activators and facilitate checkpoint attenuation in MMS either through a currently unknown pathway or through the ssDNA binding protein RPA. In investigating changes in chromatin structure in isw2nhp10 cells during replication stress, we unexpectedly found that Isw2 and Ino80 increase chromatin accessibility around replicating regions through a mechanism unrelated to their known biochemical activities. Consistent with checkpoint activity control, growth assays in the presence of MMS reveal that Isw2 and Ino80 partially oppose checkpoint activators specifically in the presence of MMS. Based on these results, we propose that Isw2 and Ino80 alter chromatin structure at replicating regions and attenuate the S-phase checkpoint through currently unknown mechanism(s).
Materials and Methods
Yeast strains and culture
All yeast strains are MATa and congenic to W303-1a with a correction for the weak rad5 allele in the original W303 (Thomas and Rothstein 1989; Zhao et al. 1998). Strains used for stable isotope labeling by amino acids in cell culture (SILAC) mass spectrometry were in a Δlys2 Δarg4CAN1+ background. Strains were constructed using standard gene knockout protocols and genetic crosses (Table 1).
Table 1. Summary of Yeast Strains Used in This Study.
| Strain | Genotype | Source |
|---|---|---|
| W1588-4c | MATa ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 RAD5+ | Zhao et al. 1998; Thomas and Rothstein 1989 |
| YTT3777 | MATa Δisw2::NatMX Δnhp10::Hyg | Au et al. 2011 |
| YTT4960 | MATa Pol1-3FLAG-KanMX | Rodriguez and Tsukiyama 2013 |
| YTT5031 | MATa Pol1-3FLAG-KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5787 | MATa Δexo1::KanMX | This study |
| YTT5805 | MATa Δexo1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5822 | MATa Δsgs1::KanMX | This study |
| YTT5852 | MATa Δsgs1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT2616 | MATa Δrad50::KanMX | Vincent et al. 2008 |
| YTT2653 | MATa Δrad50::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YTT5969 | MATa Δslx4::KanMX | This study |
| YTT5931 | MATa Δslx4::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5277 | MATa Δelg1::KanMX | This study |
| YTT5279 | MATa Δelg1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT3203 | MATa Δmrc1::KanMX | Vincent et al. 2008 |
| YTT3224 | MATa Δmrc1::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| Ssy072 | MATa Δmrc1::HIS5 pRS405-mrc1aq::LEU2 | Szyjka et al. 2005 |
| YTT5860 | MATa Δmrc1::HIS5 pRS405-mrc1aq::LEU2 Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5952 | MATa Δrad9::KanMX Δmrc1::HIS5 pRS405-mrc1aq::LEU2 | This study |
| YTT5956 | MATa Δrad9::KanMX Δmrc1::HIS5 pRS405-mrc1aq::LEU2 Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT3394 | MATa Δtof1::KanMX | Vincent et al. 2008 |
| YTT3409 | MATa Δtof1::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YTT5986 | MATa dna2-WY-AA | Modified from Kumar and Burgers 2013 |
| YTT5990 | MATa dna2-WY-AA Δddc1::KanMX | This study |
| YTT5994 | MATa dna2-WY-AA Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5996 | MATa dna2-WY-AA Δddc1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT3759 | MATa Δctf18::KanMX | This study |
| YTT5275 | MATa Δctf18::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT2883 | MATa Δddc1::KanMX | Vincent et al. 2008 |
| YTT2904 | MATa Δddc1::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YBP82 | MATa dpb11-600::Hyg | Pfander and Diffley 2011 |
| YTT5831 | MATa dpb11-600::Hyg Δisw2::NatMX Δnhp10::KanMX | This study |
| YTT5943 | MATa dpb11-600::Hyg Δddc1::URA3 | This study |
| YTT5948 | MATa dpb11-600::Hyg Δddc1::URA3 Δisw2::NatMX Δnhp10::KanMX | This study |
| YTT2953 | MATa Δrad17::KanMX | Vincent et al. 2008 |
| YTT2958 | MATa Δrad17::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YTT2938 | MATa Δrad24::KanMX | Vincent et al. 2008 |
| YTT2943 | MATa Δrad24::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YTT2885 | MATa Δrad9::KanMX | Vincent et al. 2008 |
| YTT2909 | MATa Δrad9::KanMX Δisw2::NatMX Δnhp10::Hyg | Vincent et al. 2008 |
| YTT4976 | MATa Δmec1::KanMX Δsml1::NatMX | Au et al. 2011 |
| YTT4967 | MATa Δmec1::KanMX Δsml1::NatMX Δisw2::LEU2 Δnhp10::Hyg | Au et al. 2011 |
| YTT4560 | MATa Δtel1::KanMX | This study |
| YTT4584 | MATa Δtel1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5327 | MATa Δchk1::KanMX | This study |
| YTT5329 | MATa Δchk1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| RDKY2218 | MATa rfa1-t11 | Umezu et al. 1998 |
| YTT6113 | MATa rfa1-t11 Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5293 | MATa ADE2 URA3::CUP1-Ub-Rfa1-8ala-CFP | Modified from Lisby et al. 2004 |
| YTT5683 | MATa ADE2 URA3::CUP1-Ub-Rfa1-8ala-CFP Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT3621 | MATa Δhtz1::KanMX | This study |
| YTT3636 | MATa Δhtz1::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6083 | MATa Δlys2 Δarg4::HIS3 CAN1+ Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6080 | MATa Δlys2 Δarg4::HIS3 CAN1+ | This study |
| YTT6184 | MATa Δrad51::KanMX | This study |
| YTT6192 | MATa Δrad51::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6188 | MATa Δrad52::KanMX | This study |
| YTT6196 | MATa Δrad52::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT5323 | MATa dpb11-1 | Masumoto et al. 2000 |
| YTT5325 | MATa dpb11-1 Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6277 | MATa yku70::KanMX | This study |
| YTT6282 | MATa yku70::KanMX Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6115 | MATa rad52-21 | Miles et al. 2013 |
| YTT6117 | MATa rad52-21 Δisw2::NatMX Δnhp10::Hyg | This study |
| YTT6355 | MATa orc2-1 | This study |
| YTT6357 | MATa orc2-1 Δisw2::NatMX Δnhp10::KanMX | This study |
| YTT2612 | MATa Δswr1::Hyg | This study |
| YTT6363 | MATa Δswr1::Hyg Δnhp10::KanMX | This study |
| YTT6365 | MATa Δswr1::Hyg Δisw2::NatMX | This study |
| YTT6367 | MATa Δswr1::Hyg Δisw2::NatMX Δnhp10::KanMX | This study |
| YTT3122 | MATa Δhtz1::Hyg | This study |
| YTT2189 | MATa Δisw2::NatMX Δies5::KanMX | This study |
| YTT6371 | MATa Δisw2::NatMX Δies5::KanMX Δhtz1::Hyg | This study |
| 06328 | MATa pol2-12 | Li et al. 2008 |
All strains are congenic to W1588-4c with a correction for the weak rad5 allele.
Cell synchronization and flow cytometry (FACS)
All yeast strains were grown at 30° to early log phase to a density of OD660 = 0.2–0.25 and then arrested in G1 phase with a final concentration of 5 µg/ml α factor. Cells were filtered on a 0.45-mm nitrocellulose membrane, washed twice with YPD, and released into half the volume of prewarmed YPD containing 0.02% MMS. Cells were harvested at each time point, stored in a final concentration of 66.7% (v/v) ethanol, and then processed for flow cytometry as described previously (Vincent et al. 2008).
Rad53 in situ autophosphorylation assay (ISA) and Rad53 Western blotting
Cells were arrested in G1 by α factor (5 µg/ml) and released into rich medium (YPD) containing 0.02% MMS, as described earlier. In the initial protein preparation method, ∼25 ml of cells was harvested for each time point. However, in the modified method accounting for cell density, 108 cells were harvested at each time point. Cells were washed once with water and then with 20% trichloroacetic acid (TCA). Protein samples were prepared as described previously and run on an 8% SDS–polyacrylamide gel (Pellicioli et al. 1999). Rad53 autophosphorylation was normalized by autophosphorylation of nonspecific proteins within the same sample that served as a loading control. For Rad53 Western blotting, goat polyclonal Rad53 (yC-19) antibody (sc-6749, Santa Cruz Biotech, Santa Cruz, CA) was used.
Chromatin fractionation and SILAC
Strains were grown in yeast culture (YC) medium with either 15 mg/liter l-arginine and 30 mg/liter l-lysine or 15 mg/liter [13C6]l-arginine and 30 mg/liter [13C6,15N2]l-lysine. Then 4 × 109 cells were harvested at mid-S phase in 0.02% MMS, and chromatin was prepared as described previously (Kubota et al. 2012). The chromatin pellet was resuspended in 1.5× Tris-glycine SDS sample buffer. An H2B Western blot using the H2B C-term antibody (Active Motif, Carlsbad, CA) was used for normalization. RPA polycolonal antibody (AS07 214, Agrisera, Vännäs, Sweden) was used to detect Rfa1. Equivalent amounts of protein were loaded onto an 8–16% Tris-glycine gel (Thermo Fisher Scientific, Rockford, IL), washed in sterile water, stained with GelCode Blue Safe Protein Stain (Thermo Fisher Scientific), and then destained in water. The lane was cut into 10 slices and subjected to mass spectrometry (MS).
Liquid chromatography–tandem mass spectrometry and data analysis
The 10 gel pieces were destained with 50% methanol, 5% acetic acid and washed consecutively with water twice and 50% acetonitrile. After rinsing once more with 100 mM ammonium bicarbonate in water, the gel pieces were dehydrated using acetonitrile. The protein was digested overnight at 37° with 12.5 ng/µl trypsin (Promega, Madison, WI) in 50 mM ammonium bicarbonate. After transferring excess solutions to separate vials, the peptides were first extracted using 0.1% trifluoroacetic acid in water for 30 min, and then acetonitrile was added to make 50% acetonitrile, 0.1% trifluoroacetic acid. The pooled extracts were dried in a speed vacuum and cleaned using ZipTip C18 (Millipore, Bedford, MA) before the subsequent MS analysis following the manufacture’s protocols. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis was performed with an Easy-nLC 1000 (Thermo Fisher Scientific) coupled to an Orbitrap Elite mass spectrometer (Thermo Scientific). The LC system configured in a vented format (Licklider et al. 2002) consisted of a fused-silica nanospray needle (PicoTip emitter, 50 µm ID, New Objective, Woburn, MA) packed in-house with Magic C18 AQ 100 Å reverse-phase medium (25 cm, Michrom BioResources, Auburn, CA) and a trap (IntegraFrit Capillary, 100 µm ID, New Objective) containing Magic C18 AQ 200 Å (2 cm). The peptide sample was diluted in 10 µl of 2% acetonitrile and 0.1% formic acid in water, and 8 µl was loaded onto the column and separated using a two-mobile-phase system consisting of 0.1% formic acid in water (A) and 0.1% acetic acid in acetonitrile (B). A 90-min gradient from 7 to 35% acetonitrile in 0.1% formic acid at a flow rate of 400 nl/min was used for chromatographic separations. The mass spectrometer was operated in a data-dependent MS/MS mode over the m/z range of 400–1800. The mass resolution was set at 120,000. For each cycle, the 15 most abundant ions from the scan were selected for MS/MS analysis using 35% normalized collision energy. Selected ions were dynamically excluded for 30 sec. Data analysis was performed using Proteome Discoverer 1.4 (Thermo Fisher Scientific). The data were searched against Saccharomyces cerevisiae strain S288C protein sequences that were updated on February 3, 2011, from the Saccharomyces Genome Database (SGD, http://www.yeastgenome.org/), which included common contaminants. Trypsin was set as the enzyme, with maximum missed cleavages set to 2. The precursor ion tolerance was set to 10 ppm, and the fragment ion tolerance was set to 0.6 Da. Variable modifications were set to methionine oxidation (+15.995 Da), 6 × 13C (+6.020 Da) on arginine, and 6 × 13C and 2 × 15N on lysine. Sequest (Eng et al. 1994) was used for search, and search results were run through Percolator (Kall et al. 2007) for scoring. The ratio between heavy and light amino acids was normalized to an average of all histone proteins detected by MS.
Genomic DNA profiles, microarray hybridization, and data analysis
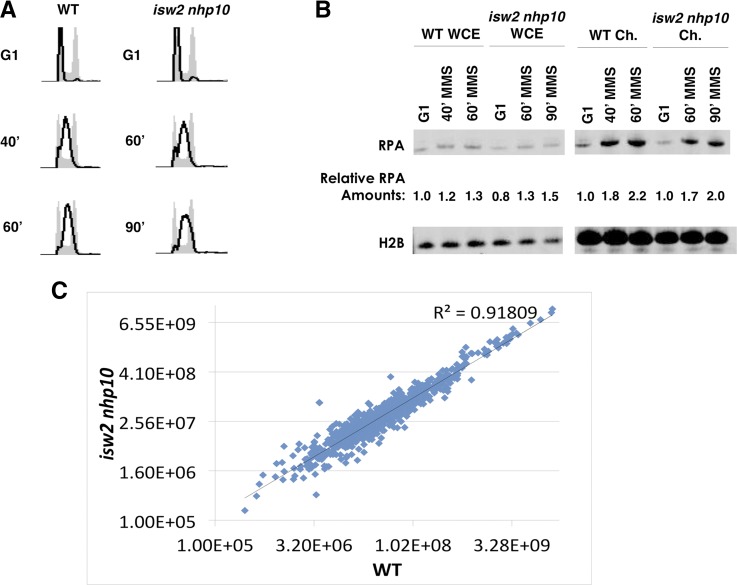
DNA samples were amplified, fragmented, and labeled as described previously (Rodriguez and Tsukiyama 2013). S-phase DNA samples were competitively hybridized to G1 DNA for replication profiling to custom-tiled arrays, as described previously (Rodriguez and Tsukiyama 2013). The data were smoothed using a previously established pseudomedian method (Royce et al. 2007) with a 50-bp moving window. Relative RPA levels were ranked based on increasing amounts of genomic DNA content for both wild-type (WT) and isw2nhp10 samples, where the average amounts of RPA were further smoothed with a 100-bp sliding window. The ribosomal DNA (rDNA) locus and outliers from either extremely low or saturated DNA signals were left out of the analysis.
Normalized chromatin accessibility to MNase (NCAM) assay
Cells were harvested from G1 and at an early time point S phase in 0.02% MMS, where FACS profiles were similar between WT and isw2nhp10 cells. Sample harvest, preparation, and analysis were performed as described previously (Rodriguez and Tsukiyama 2013; Rodriguez et al. 2014). Z-score normalization was performed as described previously (Rodriguez and Tsukiyama 2013) but based on digestion patterns at the +2 to +6 nucleosomes within ORFs genome-wide rather than transcriptional start sites (TSSs) because promoter regions tend to be targets for Isw2 and Ino80 remodeling (Whitehouse et al. 2007; Papamichos-Chronakis et al. 2011). The NCAM graphs were generated by integrating the signal over 40-kb regions surrounding three classes of origin: early high (n = 11), early low (n = 12), and late (n = 37). To determine S-phase-specific changes, NCAM from G1 was subtracted from S phase. NCAM was averaged over a 40-kb region surrounding the origin or a 3-kb region surrounding TSSs to quantitate relative NCAM signal.
Results
Isw2 and Ino80 attenuate the checkpoint on MMS treatment in middle-late S phase
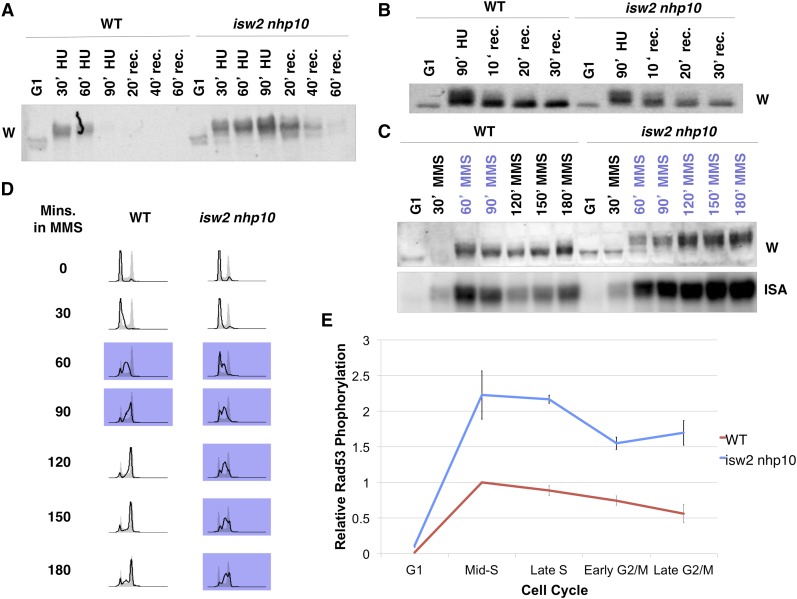
S. cerevisiae Isw2p is the ATPase subunit of the Isw2 chromatin remodeling factor involved in gene repression (Goldmark et al. 2000; Whitehouse et al. 2007). Nhp10p is a subunit of Ino80 that is known to exist exclusively within the Ino80 complex and facilitates interactions with chromatin (Morrison et al. 2004). We previously reported that the isw2nhp10 mutant exhibits two phenotypes in the presence of replication inhibitors: (1) slow growth due to a prolonged S phase without an increase in cell death and (2) overactivation of the checkpoint during S phase (Vincent et al. 2008; Au et al. 2011). Previously, we reported that isw2nhp10 elevates and prolongs checkpoint activity during S phase in the presence of replication inhibitors, especially after transient exposure to HU (Au et al. 2011). Our Rad53 ISA revealed rapid loss of checkpoint activity in WT cells but not in isw2nhp10 cells after transient treatment with HU (Au et al. 2011). However, on examining Rad53 checkpoint activity by Western blot, we noticed that the Rad53 protein could no longer be detected in later time points when samples were prepared according to the protocol used in our previous report (Au et al. 2011) (Figure 1A). Importantly, Rad53 protein was lost at much earlier time points in WT than in isw2nhp10 cells (Figure 1A). We found that the loss of Rad53 protein was attributed to an increase in cell density as the time course went on, which we did not adjust for previously, causing inefficient TCA precipitation of proteins. Because of the slower S-phase progression in isw2nhp10 cells, Rad53 is lost later in isw2nhp10 compared with WT cells (Figure 1A). Thus the apparent checkpoint hyperactivity in isw2nhp10 cells after HU treatment detected by ISA could be attributed to inefficient recovery of Rad53 protein in WT cells. We therefore prepared protein samples adjusting for the differences in cell numbers between WT and isw2nhp10 cells (see Materials and Methods for details) and repeated the ISA. This revealed that although Rad53 protein is still slightly more phosphorylated based on Western blotting in isw2nhp10 compared with WT cells after HU treatment (Figure 1B), the difference in phosphorylation is not to the same degree as what we previously reported using ISA (Figure 1A).
Figure 1.
isw2 nhp10 cells display stronger checkpoint activity throughout S phase and into G2/M phase in the presence of MMS. (A) Rad53 Western blot of WT and isw2 nhp10 cells using the protein preparation methods in our previous report (Au et al. 2011). Cells were arrested in G1 with α factor for 90 min and released into 200 mM HU for 90 min, and then HU was washed out. rec, recovery time in minutes. (B) Rad53 Western blot of protein preparations that used the amount of TCA normalized for cell volume. (C) A Western blot (W) and in situ autophosphorylation assay (ISA) of Rad53 of samples taken from indicated time points. Time points indicated in purple represent when cells are in S phase. (D) Cell cycle profiles of WT and isw2 nhp10 cells after release from α factor into medium containing MMS. Asynchronous profiles are shown in gray. FACS profiles shown in purple indicate time points in S phase. (E) Quantitation of Rad53 ISA time course. WT checkpoint activity was measured at 60 min for middle S, 90 min for late S, 120 min for early G2/M, and 150 min for late G2/M. isw2 nhp10 checkpoint activity was measured at 120 min for middle S, 180 min for late S, 240 min for early G2/M, and 300 min for late G2/M. The error bars denote the standard errors of the mean from two experiments using independently created strains.
These results prompted us to reexamine the checkpoint activity of WT and isw2nhp10 cells under various conditions using both Western blotting and Rad53 ISA while accounting for cell density. These tests revealed that the difference in Rad53 checkpoint activity between WT and isw2nhp10 cells is much more robust upon MMS treatment than HU treatment (Figure 1, B and C). To investigate how Isw2 and Ino80 attenuate checkpoint activity in MMS, we sought to determine when, during S phase, Isw2 and Ino80 perform their roles by defining the kinetics of checkpoint activity. To this end, we performed a time-course experiment in which cells were arrested in G1 and released into S phase in rich medium containing MMS, and we monitored cell cycle progression and checkpoint activity by FACS and Rad53 ISA, respectively (Figure 1D and Supporting Information, Figure S1). Consistent with our previous report (Vincent et al. 2008), isw2nhp10 cells have a delay in S-phase progression that is not a result of incomplete replication (Figure 1D and Figure S1B). The delay is associated with stronger and prolonged checkpoint activity in the presence of MMS (Figure 1E). This experiment revealed that the biggest difference in checkpoint activity occurs during middle-late S phase (Figure 1E). Consistent with the fact that early origin firing is not greatly affected in isw2nhp10 cells (Vincent et al. 2008), these results suggest that Isw2 and Ino80 function after replication has already been initiated. Once checkpoint activity is more strongly triggered in S phase, activation remains elevated via transition into G2/M phase, which coincides with the presence of a subpopulation of cells that continue to remain in S phase in isw2nhp10 cells (Figure 1, C and D). Taken together, our results suggest that Isw2 and Ino80 prevent hyperactivation of the checkpoint activity in middle to late S phase in the presence of MMS to allow for proper replication progression and transition into G2/M phase.
Isw2 and Ino80 function outside of DNA repair or fork protection pathways
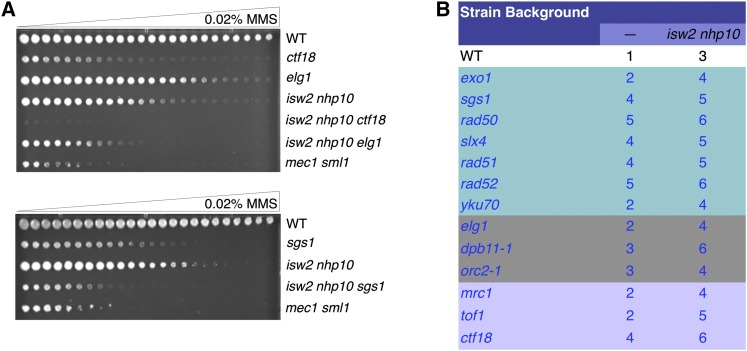
Because checkpoint activity is much more strongly activated in isw2nhp10 cells by MMS than by HU, we next tested whether ISW2 and NHP10 could function through DNA repair or replication fork protection pathways. We have shown previously, by genetic tests, that ISW2 and NHP10 do not function through several genes involved in replication fork protection and DNA repair pathways (Vincent et al. 2008), leading to the conclusion that they do not function via these pathways (Au et al. 2011). However, these tests were not very quantitative, and additional genes involved in DNA damage response and replication fork protection were identified after our previous tests (Crabbe et al. 2010; Engels et al. 2011; Hegnauer et al. 2012; Gonzalez-Prieto et al. 2013; Kubota et al. 2013). We therefore performed more comprehensive genetic tests to determine whether ISW2 and NHP10 function in these pathways. ISW2 and NHP10 were deleted in a given mutant to create a triple mutant, and the single- and triple-mutant growth phenotypes were compared on gradient plates of rich medium with increasing concentrations of MMS (Figure 2A). If ISW2 and NHP10 function through a pathway in which a DNA damage response or replication fork protection gene plays a central role, the single and triple mutants should exhibit similar MMS sensitivity. In contrast, if ISW2 and NHP10 function independently of a DNA damage response or replication fork protection gene, the phenotypes should be additive, and the triple mutant should be more sensitive than the single mutant. CTF18, ELG1, and SGS1 all influence checkpoint activity (Kanellis et al. 2003; Crabbe et al. 2010; Hegnauer et al. 2012). All the triple mutants within these mutant backgrounds show additive MMS sensitivity, suggesting that these genes are not required for ISW2 and NHP10 function (Figure 2A). Other genes involved in fork protection (lavender), replication (gray), and different DNA damage response pathways (teal) also show that Isw2 and Ino80 act independently of these pathways (Figure 2B). DNA polymerase ε (Pol ε) associates with Dpb11 to facilitate DNA replication and the S-phase checkpoint (Masumoto et al. 2000), and the Dpb4 subunit of Pol ε also exists in the Isw2 complex (Iida and Araki 2004; Mcconnell et al. 2004). Therefore, to test genetic interactions of Pol ε with Isw2 and Nhp10, we used a pol2-12 mutant that attenuates S-phase checkpoint activity (Li et al. 2008). However, the pol2-12 isw2nhp10 mutants exhibited extreme growth defects and readily picked up suppressor mutations (data not shown). While we were not able to measure MMS sensitivity of this mutant, the additive effect even in the absence of replication stress suggests that Isw2 and Nhp10 have functions independent of Pol ε. Together these results reinforce the conclusion that Isw2 and Ino80 do not function through DNA repair or replication fork protection pathways.
Figure 2.
Isw2 and Ino80 function is independent of DNA damage response or fork protection pathways. (A) Representative results of the spot tests on 0.02% MMS gradient plates performed as described previously (Au et al. 2011). The top panel shows that deletion of replication components such as ELG1 or CTF18 results in additive MMS sensitivity with isw2 nhp10. The bottom panel shows that deletion of a DNA resection factor SGS1 is additive with isw2 nhp10 as well. mec1 sml1 serves as a control to determine the severity of sensitivity on MMS. (B) A summary of the spot-test results with genes involved in DNA repair and replication fork protection. MMS sensitivity is ranked on a scale of 1–6, with 1 being the least sensitive (WT level) and 6 being the most sensitive. Repair genes are shown in teal, replication fork protection genes are shown in lavender, and other genes involved in replication are shown in gray.
Isw2 and Ino80 antagonize checkpoint activators and attenuate checkpoint activity independent of any single checkpoint factor downstream of RPA
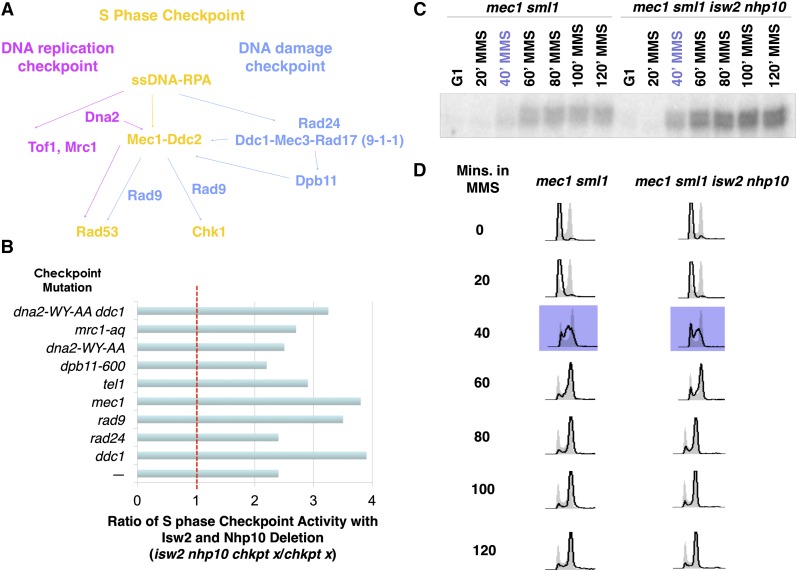
The preceding results are consistent with our model that Isw2 and Ino80 play a direct role in attenuating the S-phase checkpoint activity. Thus, to gain insight into the mechanism of checkpoint attenuation by Isw2 and Ino80, we performed systematic genetic tests to screen for checkpoint factors required for Isw2 and Ino80 checkpoint function using the Rad53 ISA. Based on a similar reasoning as earlier, if a checkpoint protein is required for Isw2 and Ino80 to attenuate checkpoint activity, we would expect deletion of ISW2 and NHP10 to not increase checkpoint activity in the relevant mutant background. Alternatively, if a given checkpoint protein is not required for Isw2 and Ino80 function, then deletion of ISW2 and NHP10 still would increase the deficient checkpoint activity in the checkpoint mutants. Through these analyses, we aimed to identify the most upstream checkpoint factor by which Isw2 and Ino80 attenuate checkpoint activity. We screened all major checkpoint factors known to be required for robust S-phase checkpoint activity (Figure 3A). Unless otherwise indicated, the checkpoint mutants tested were null mutations. The S-phase checkpoint cascade has two partially overlapping pathways (Figure 3A), the DRC and the DDC, that both function to activate the S-phase checkpoint. Although the DDC (blue) is activated primarily in the presence of MMS, the DRC (pink) can redundantly function to partially activate the S-phase checkpoint in the absence of DDC factors (Branzei and Foiani 2009). Some Checkpoint components (yellow) function in both the DDC and the DRC and thus are most critical for proper activation. Complete deletion of a DRC component resulted in severe MMS sensitivity because such components are also involved in DNA replication, in addition to the DRC (Figure 2B). Therefore, for checkpoint factors that also have roles in DNA replication, we used mrc1-aq (Szyjka et al. 2005), dpb11-600 (Pfander and Diffley 2011), and dna2-WY-AA (Kumar and Burgers 2013), which are alleles that were shown previously to be specifically deficient in checkpoint activation. Unexpectedly, we found that the isw2nhp10 mutation partially rescued checkpoint activity in all the checkpoint-deficient mutants tested, for both DRC and DDC mutants, as indicated by a checkpoint activity ratio that was significantly higher than one on ISW2 and NHP10 deletion (Figure 3B). These results suggest that Isw2 and Ino80 antagonize checkpoint activators in affecting S-phase checkpoint activity. Mec1 plays a central role in S-phase checkpoint activation, and the absence of the Mec1 kinase severely abrogates the S-phase checkpoint (Branzei and Foiani 2010). Perhaps most surprisingly, even in mec1sml1 cells, where checkpoint activity is mostly depleted, deletion of ISW2 and NHP10 still results in higher checkpoint activity during S phase, indicating that Isw2 and Ino80 function independent of Mec1 (Figure 3, C and D). Residual checkpoint activity in the mec1sml1 mutant is due to the partially redundant function of Tel1 (Mantiero et al. 2007). The mode of Tel1 activation in S phase is not well understood, but in the absence of Mec1, Tel1 can activate the checkpoint to a much lesser degree (Zou 2013). We attempted to examine the effects of Isw2 and Ino80 when the S-phase checkpoint is completely abolished by making a mec1tel1sml1isw2nhp10 mutant. However, tel1 deletion alone causes strong synthetic growth defects with the isw2nhp10 mutation independent of S-phase checkpoint function (Figure 6A). Because of this genetic interaction, the quintuple mutant had extreme growth defects and readily picked up suppressors (data not shown), making phenotype analyses impossible. The dna2-WY-AA ddc1 mutant, which abolishes Mec1 activation through both the DRC and DDC pathways (Kumar and Burgers 2013), also displayed higher checkpoint activity on ISW2 and NHP10 deletion (Figure 3B). This result is consistent with the partial rescue of the checkpoint activity of the mec1 mutant by isw2nhp10. These findings suggest that Isw2 and Ino80 deactivate checkpoint activity independent of both the DRC and DDC pathways. We were unable to test RPA because of the lack of an rpa mutant allele that solely affects checkpoint activity. Because RPA is essential for viability and has various roles in DNA replication, checkpoint activation, DNA damage response, and recombination, deleting ISW2 and NHP10 in rpa mutant backgrounds rfa1-degron and rfa1-t11 (Umezu et al. 1998; Lisby et al. 2004) causes severe growth defects (data not shown). While the strong synthetic growth defects of the rpa isw2nhp10 mutant were not unexpected, they made measuring the checkpoint activity in the mutant impossible. Nevertheless, our data demonstrate that no known S-phase checkpoint factor downstream of RPA is required for Isw2 and Ino80 to attenuate checkpoint activity. These results collectively suggest that Isw2 and Ino80 function either through currently unidentified checkpoint activator(s) or through RPA, which feeds into multiple independent checkpoint activation pathways. For MRC1, DPB11, and DNA2, we cannot rule out the possibility that the alleles we used did not inactivate their relevant checkpoint activation function.
Figure 3.
Isw2 and Ino80 function is independent of any single checkpoint factor downstream of RPA. (A) Schematic drawing of S-phase checkpoint pathway, which is divided into the DNA replication checkpoint (DRC) in pink, DNA damage checkpoint (DDC) in blue, and factors present in both pathways in yellow. (B) Bar graph showing the ratio of S-phase checkpoint activity in checkpoint mutants in the absence of ISW2 and NHP10 compared with the checkpoint mutant alone. The dashed red line indicates the expected S-phase checkpoint ratio if a given checkpoint protein is required for Isw2 and Ino80 function. (C) Rad53 ISA of mec1 sml1 and mec1 sml1 isw2 nhp10 cells released from G1 into S phase containing 0.02% MMS. Time points in S phase are denoted in purple. (D) Corresponding FACS profiles of time points from the ISA, with profiles in purple denoting time points in S phase.
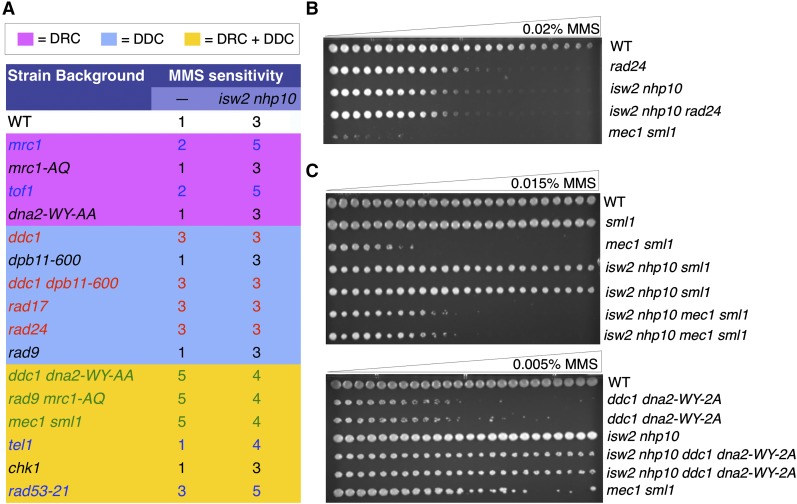
Figure 6.
Isw2 and Nhp10 antagonize checkpoint activators. (A) A summary of the spot-test results with checkpoint proteins. MMS sensitivity is ranked on a scale of 1–6, with 1 being the least sensitive (WT level) and 6 being the most sensitive. Mutated genes are grouped into colored boxes, with pink representing the DRC genes, blue representing the DDC genes, and yellow presenting components of both DRC and DDC genes. Results are color-coded, with blue indicating additive sensitivity, red indicating similar sensitivity, and green indicating a partial rescue in sensitivity with the deletion of ISW2 and NHP10. The mutations in black did not show detectable MMS sensitivity as single mutants and exhibited no genetic interactions with isw2 nhp10. (B) The gradient plate shows the lack of genetic interactions between ISW2, NHP10, and RAD24. (C) Both gradient plates show a partial rescue of varying degrees to MMS sensitivity of either the mec1 sml1 mutant (top panel) or the ddc1 dna2-WY-2A mutant (bottom panel) by isw2 nhp10 mutation.
Isw2 and Ino80 do not affect the abundance of checkpoint factors
We envision that Isw2 and Ino80 attenuate checkpoint activity via at least two possible mechanisms. One possibility is that Isw2 and Ino80 may facilitate removal of a checkpoint protein from replication forks. Alternatively, they may down-regulate the activity of a checkpoint protein. We first tested whether Isw2 and Ino80 alter the abundance of chromatin-bound RPA by chromatin fractionation from cells in G1 and S phase with 0.02% MMS (Figure 4A), followed by Western blotting. This analysis revealed that relative levels of chromatin-bound RPA were similar in both WT and isw2nhp10 cells in G1 and S phase, indicating that higher checkpoint activity in the mutant is not a result of RPA accumulation on bulk chromatin (Figure 4B). Consistent with this result, overexpression of Rad51 and Rad52, proteins involved in displacement of RPA from double-strand break (DSB) sites (Sugiyama and Kowalczykowski 2002), did not affect MMS sensitivity of isw2nhp10 cells (data not shown). In order to test whether Isw2 and Ino80 affect the abundance of other checkpoint proteins bound to chromatin, we performed stable SILAC mass spectrometry to compare differences in global levels of chromatin-bound proteins in WT and isw2nhp10 cells. This method was used successfully to find an abnormal increase in chromatin-bound replisome components in Δctf18 cells (Kubota et al. 2011). This analysis revealed no notable difference in the abundance of the vast majority of chromatin-bound proteins between WT and isw2nhp10 cells in the presence of MMS (r2 = 0.92), including DNA replication, replication checkpoint, and DNA repair proteins (Figure 4C). The outliers that increase the most in abundance in isw2nhp10 cells are Sen34p (∼20-fold), a subunit of the tRNA splicing endonuclease; Hsp26p (∼20-fold), a small heat-shock protein; and Gpx2 (∼7-fold), a phospholipid hydroperoxide glutathione peroxidase that is normally induced on replication stress (Tkach et al. 2012). Outliers that correspond to the most reduced abundance in isw2nhp10 cells include Sts1p (∼1/8-fold), which is involved in protein transport and ribosomal RNA stability; Ptc2p (∼1/5-fold), a Rad53 phosphatase; and not surprisingly, other Ino80 subunits such as Ies1 (∼1/4-fold). Other than Ptc2p, no outliers have been described previously to have a function in the S-phase checkpoint. No other Rad53 phosphatases were found to have different protein abundances in the SILAC data, and because of the redundancy of the Rad53 phosphatases, Ptc2 alone plays only a minor role in checkpoint recovery after MMS treatment (Szyjka et al. 2008). Furthermore, we have shown previously that Isw2 and Ino80 clearly function independent of the Rad53 phosphatases (Au et al. 2011). Taken together, our results show that Isw2- and Ino80-dependent checkpoint attenuation cannot be explained by changes in the abundance of chromatin-bound proteins, at least at the bulk level.
Figure 4.
Bulk levels of chromatin-bound checkpoint proteins are largely unchanged between WT and isw2 nhp10 cells. (A) FACS profiles of samples used for chromatin preparation in B during G1 and S phase in 0.02% MMS. (B) Western blot of RPA and H2B levels from whole-cell extracts and fractionated chromatin of WT and isw2 nhp10 cells. Levels of RPA were normalized to H2B, and the quantitation is shown. (C) Chart graphing the SILAC mass spectrometry data of nuclear proteins from WT vs. isw2 nhp10 cells in middle S phase in 0.02% MMS. Total protein was normalized based on the total amount of histones.
Chromatin accessibility around replication forks is decreased in isw2 nhp10 cells in the presence of MMS
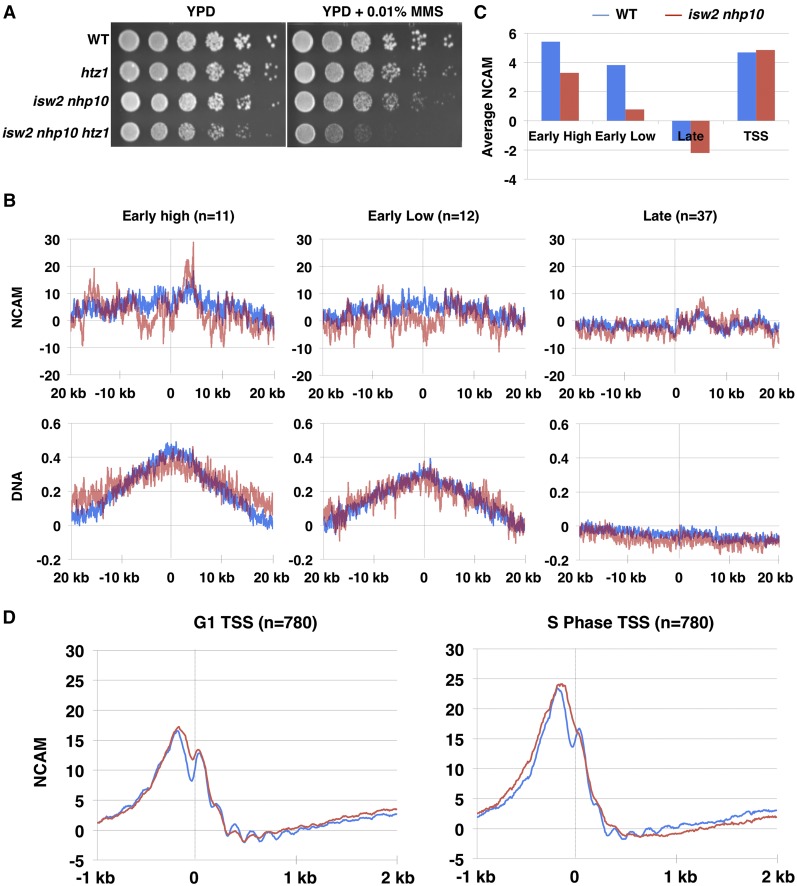
Previously, we (Vincent et al. 2008) and others (Papamichos-Chronakis and Peterson 2008; Shimada et al. 2008) have shown that both Isw2 and Ino80 are enriched at stalled replication forks. Therefore, we considered the possibility that Isw2 and Ino80 may affect chromatin structure around replication forks, thereby altering checkpoint activity. Ino80 functions in opposition to Swr1 and exchanges nucleosomal H2A.Z/H2B dimers for H2A/H2B dimers (Papamichos-Chronakis et al. 2006, 2011). Meanwhile, Isw2 alters chromatin structure by sliding nucleosomes away from coding regions or toward gene promoters (Fazzio and Tsukiyama 2003; Yadon et al. 2010). We sought to determine whether these known biochemical activities might be required for the S-phase functions of Isw2 and Ino80. If the important role of Nhp10 is in Htz1 exchange, we can expect to see partial rescue of the MMS sensitivity of isw2nhp10 cells by deleting HTZ1. However, htz1 mutation not only showed no rescue but also increased the MMS sensitivity of isw2nhp10 cells, suggesting that Htz1 exchange is not the relevant activity of Isw2 and Nhp10 in MMS (Figure 5A). To confirm that the genetic interaction with Htz1 was representative of the Ino80 complex rather than Nhp10 alone, we tested another subunit of Ino80, Ies5. The isw2ies5htz1 cells displayed an even stronger additive growth defect in the presence of MMS (Figure S2A). Deleting swr1 in isw2nhp10 cells also resulted in a stronger growth defect in the presence of MMS compared with either swr1 or isw2nhp10 (Figure S2B). These results collectively suggest that Htz1 deposition is not the relevant biochemical activity for growth in MMS.
Figure 5.
Isw2 and Ino80 increase chromatin accessibility at replicating regions. (A) Spot assay of htz1 and htz1 isw2 nhp10 cells grown at 30°, as described previously (Au et al. 2011), on YPD and YPD with 0.01% MMS. (B) Graphs represent NCAM and DNA profiles over a 40-kb window from three classes of origin on chromosomes III, VI, and XII: early high (n = 11), early low (n = 12), and late (n = 37). The dashed line indicates the origin midpoint. (C) Measurement of NCAM around replication origins in WT and isw2 nhp10 cells. WT cells are depicted in blue, while isw2 nhp10 cells are in red. NCAM has been Z-score normalized by the +2 to +6 ORF nucleosomes. To visualize S-phase-specific changes, S-phase NCAM was subtracted from G1 NCAM. The bar graph quantitates the average S-phase-specific differences in NCAM from 40 kb around origins in C and 3 kb around TSSs in D. (D) NCAM signal from all TSSs (n = 780) at G1 and S phase in MMS in a 3-kb window. The dashed line indicates the TSS midpoint.
We also mapped nucleosome positions around replicating regions in WT and isw2nhp10 cells in both G1 and S phase in MMS. For this experiment, harvesting cells in early S phase was necessary to achieve the best possible synchrony of replication forks. We first confirmed that nucleosome positions at known Isw2 targets shift in isw2nhp10 cells, as anticipated. However, we did not detect significant changes in nucleosome positioning at replicating regions in either G1 or early S phase in MMS (data not shown). Although this negative result has caveats that replication forks are not completely synchronous within cell population and that the resolution of our nucleosome mapping may not be high enough, it does not support the idea that a model that regulates nucleosome positions accounts for the mechanism behind checkpoint regulation by Isw2 and Ino80.
We next tested whether chromatin accessibility might be altered in isw2nhp10 cells around replication forks. We have recently developed a method to measure chromatin accessibility to micrococcal nuclease (MNase) in a way normalized for nucleosome occupancy called normalized chromatin accessibility to MNase (NCAM) (Rodriguez and Tsukiyama 2013; Rodriguez et al. 2014). To measure NCAM, we digest chromatin by MNase and purify monoucleosomal DNA, followed by hybridization of nucleosomal DNA to high-density tiled microarrays. We then quantify nucleosome signals, which can be affected by both nucleosome occupancy and the sensitivity of nucleosomes to MNase at any given site (Weiner et al. 2010; Rodriguez and Tsukiyama 2013). At the same time, we perform chromatin immunoprecipitation using anti-histone H3 antibody to measure nucleosome occupancy. By normalizing the strength of MNase signals to nucleosome occupancy, we can calculate NCAM, which measures the relative amount of MNase digestion per nucleosome (Rodriguez and Tsukiyama 2013). As expected for both WT and isw2nhp10 cells, NCAM increases at regions of active replication, which is depicted near early efficient and early inefficient origins that have fired in most cells at this time point. However, NCAM is significantly reduced at these regions in isw2nhp10 cells (Figure 5, B and C). It is unlikely that the lower NCAM at this time point is caused by inefficient replication initiation in the mutant because we have shown previously that isw2nhp10 cells do indeed efficiently fire early origins similarly to WT cells (Vincent et al. 2008). Indeed, WT and isw2nhp10 cells exhibit similar levels of DNA synthesis around early-firing origins at this time point (Figure 5B). In contrast, nonreplicating regions, which are represented by chromatin surrounding late origins that have not fired at this time point, are overall less accessible than the rest of the genome, as expected (Figure 5, B and C). We did detect minor decreases in NCAM in isw2nhp10 compared with WT cells at these nonreplicating regions, which is most likely attributed to passive replication occurring at these sites (Figure 5C). Taken together, these results suggest that Isw2 and Ino80 complexes affect NCAM around actively replicating regions. Furthermore, the reduction of NCAM in isw2nhp10 cells is not a result of global reduction of the NCAM signal because NCAM is very similar or even slightly increased in the mutant cells at transcriptional start sites in both G1 and S phase in MMS (Figure 5D). These results collectively show that Isw2 and Ino80 increase chromatin accessibility specifically around replication forks in the presence of MMS. Although the decrease in NCAM in isw2nhp10 cells at actively replicating regions can be attributed in part to differences in nucleosome occupancy, the changes are mainly driven by a decrease in nucleosome signal (Figure S2, C and D). We have confirmed the genome-wide decrease in nucleosome signals around replication forks in isw2nhp10 cells by deep sequencing nucleosomal DNA (data not shown).
isw2 nhp10 partially rescues MMS sensitivity of severe checkpoint-deficient mutants
Because isw2nhp10 can stimulate checkpoint activity even in the absence of the known checkpoint activators, we sought to determine whether deletion of isw2nhp10 in checkpoint-deficient mutants also could rescue cell growth in the presence of MMS. The DRC checkpoint-specific mutations yielded WT-like MMS sensitivity, consistent with previous reports that the DRC checkpoint components are dispensable for the checkpoint in MMS (Alcasabas et al. 2001; Crabbe et al. 2010) (Figure 6A). Deletion of ISW2 and NHP10 in these backgrounds did not cause detectable genetic interactions. However, the DDC mutants were more sensitive to MMS than the WT cells, as expected (Hustedt et al. 2013), and unexpectedly, deletion of ISW2 and NHP10 did not yield a rescue in growth despite a rescue in checkpoint activation, as shown earlier (Figure 6, A and B). These results show, for the first time, that checkpoint overactivation and growth in MMS are genetically separable because checkpoint overactivation does take place without affecting MMS sensitivity in DDC mutants (Figure 6, A and B). However, in the mutants that could not activate either the DRC or the DDC in S phase, isw2nhp10 partially rescued MMS sensitivity, which is consistent with the partial rescue in checkpoint activity (Figure 6, A and C). We previously failed to detect the rescue of MMS sensitivity of mec1 mutants by isw2nhp10 mutation (Au et al. 2011) because the rescue is slight and can be detected only after an extended period of time owing to very slow growth of the mutant. While the rescue of the mec1 phenotype by isw2nhp10 was modest, we saw a more prominent rescue in ddc1dna2-WY-AA cells (Figure 6, A and C). Because sensitivity of mec1 to MMS is a result of both an abrogated checkpoint and a replication fork protection defect, it is not surprising that isw2nhp10 only slightly rescues MMS sensitivity. In contrast, isw2nhp10 dramatically rescues ddc1dna2-WY-AA cells, which have been shown to be sensitive to replication inhibitors solely because of their defect in activating the checkpoint. To determine whether the rescue was specific to MMS, we also tested genetic interactions on other replication inhibitors, including HU and camptothecin (CPT) (Figure S3, A and B). Both CPT and HU exposure yielded either minimal or additive sensitivity when ISW2 and NHP10 were deleted in the checkpoint-deficient mutants (Figure S3, A and B). These results are consistent with the fact that Isw2 and Ino80 only minimally affect checkpoint activity on HU treatment (Figure 1B). While growth sensitivity to MMS does not always directly reflect the changes in checkpoint regulation, a partial rescue of the severe MMS sensitivity of these checkpoint-deficient mutants by isw2nhp10 is consistent with the notion that Isw2 and Ino80 oppose checkpoint activators in the presence of MMS.
Discussion
We have shown previously that Isw2 and Ino80 are important for attenuation of the S-phase checkpoint, especially after transient HU treatment (Au et al. 2011). However, in this study, we discovered that the previous method of protein preparation resulted in the loss of Rad53 protein. Using a method that can efficiently retain Rad53 protein, we found that Isw2 and Ino80 play much bigger roles in attenuating S-phase checkpoint activity in the presence of MMS than in the presence of HU. Consistent with this finding, growth assays also show that Isw2 and Ino80 antagonize checkpoint activators more profoundly in the presence of MMS compared with other replication inhibitors. Although isw2nhp10 cells are sensitive to other replication inhibitors (Vincent et al. 2008), our results suggest that Isw2 and Ino80 may play distinct roles in the presence of different replication inhibitors.
Based on previous findings (Vincent et al. 2008; Au et al. 2011) and this study, we believe that isw2nhp10 cells do not have increased replication fork problems or DNA damage response defects, leading to our model that Isw2 and Ino80 directly attenuate checkpoint activity. We cannot completely exclude the possibility that there is an increased uncoupling of the replicative helicase to DNA polymerase (Byun et al. 2005) in isw2nhp10 cells in MMS, which could be the cause of stronger checkpoint activation and slower S-phase progression. However, we do not favor this model. As we have shown previously (Au et al. 2011) and here, the dissociation of replicative helicase and DNA polymerases in the presence of MMS is detrimental in the absence of replication fork protection by the S-phase checkpoint. For example, we previously reported that deletion of CTF4 or TOF1, which are implicated in coupling MCM helicase with DNA polymerase during replication (Katou et al. 2003; Tanaka et al. 2009), in the mec1 background leads to strongly additive MMS sensitivity (Au et al. 2011). In contrast, isw2nhp10 mutations cause no increase but partially rescue the MMS sensitivity of severe checkpoint mutants such as mec1 and ddc1dna2-WY-AA.
How do Isw2 and Ino80 complexes function? We do not believe that Isw2 and Ino80 function by affecting transcription because there were no significant differences in transcription between MMS-treated WT and isw2nhp10 cells (Vincent et al., 2008, data not shown). Our checkpoint activity assays suggest that Isw2 and Ino80 attenuate checkpoint activity independent of all major checkpoint activators except RPA. Therefore, we propose that they function either through a currently unidentified checkpoint regulator(s) or through RPA. We show here that Isw2 and Ino80 do not affect the amounts of chromatin-bound checkpoint proteins including RPA at the bulk level, but it is possible that they affect the location of checkpoint proteins on chromatin. Isw2 has the ability to translocate on ssDNA (Fischer et al. 2009). Furthermore, chromatin remodeling factors have been shown to interact with nonhistone proteins, and Mot1, an ATPase that is highly related to chromatin remodeling factors, can displace TBP, a nonhistone protein, from DNA (Auble et al. 1994; Moreau et al. 2003; Au et al. 2011; Sugimoto et al. 2011). Because Isw2 and Ino80 physically interact with RPA (Au et al. 2011), we initially considered the possibility that they may be able to directly remove RPA from the ssDNA template using DNA translocase activity. However, the results of the direct test for this possibility in vitro have been negative so far (data not shown). We also have attempted RPA chromatin immunoprecipitation with DNA microarray (ChIP-chip) and chromatin immunoprecipitation with massively parallel DNA sequencing (ChIP-seq), which have yet to yield any conclusive results because of the difficulty of capturing RPA peaks on moving replication forks with imperfect synchrony (data not shown). While it is still possible that Isw2 and Ino80 could be affecting RPA localization at sites of stalled replication, we currently do not have adequate tools to address this scenario. Although it seems unlikely based on biochemical activities of chromatin remodeling factors, it is also possible that Isw2 and Ino80 directly affect the activity of a checkpoint kinase rather than localization of checkpoint proteins.
Most intriguingly, we demonstrate for the first time that Isw2 and Ino80 promote chromatin accessibility around actively replicating regions. Together with the fact that both Isw2 and Ino80 are enriched at stalled replication forks (Vincent et al. 2008), this is consistent with a model suggesting that the chromatin remodeling factors function at stalled replication forks. Multiple reports have suggested that chromatin remodeling factors can alter chromatin structure to facilitate replication fork progression (Fyodorov et al. 2004; Poot et al. 2004; Shimada et al. 2008; Vincent et al. 2008). Moreover, we showed previously that chromatin accessibility increases around replication forks to promote replication (Rodriguez and Tsukiyama 2013). Therefore, one possibility is that Isw2 and Ino80 may attenuate checkpoint activity by changing the way checkpoint protein(s) interact with chromatin at stalled forks, which, in turn, affects replication progression and checkpoint dynamics. Because we found that Isw2 and Ino80 likely do not function through their known biochemical activities, we speculate that Isw2 and Ino80 function in a currently unknown manner. This can be either by modifying how DNA and core histones interact or by changing histone turnover rate, which may influence other factors to bind and potentially affect checkpoint factor localization or activity. For example, weakening histone-DNA interactions around stalled replication forks could cause an increase in accessibility to recruit checkpoint repressors to facilitate removal of checkpoint factor(s) and promote resumption of DNA replication. Indeed, another ATP-dependent chromatin remodeling factor, Fun30, and its mammalian counterpart, SMARCAD1, were both found to be recruited to sites of DSBs to promote resection presumably by removing Rad9 from DSB ends (Chen et al. 2012; Costelloe et al. 2012). Alternatively, checkpoint hyperactivation in the chromatin-remodeling-factor mutant may be the cause of the observed decrease in chromatin accessibility. Interestingly, we have shown previously that a mutation in mec1 also results in decreased chromatin accessibility at replicating regions in S phase in HU (Rodriguez and Tsukiyama 2013). However, because Mec1 facilitates chromatin accessibility around stalled replication forks (Rodriguez and Tsukiyama 2013), it is difficult to explain the increased checkpoint activity in isw2nhp10 cells as the direct cause of the decreased chromatin accessibility in the mutant. Whether these changes in chromatin structure are related to the phenotypes of isw2nhp10 cells in MMS remains to be explored and will be investigated in future work. Finally, it is also possible that the changes in chromatin accessibility are separable from checkpoint activation.
Our findings suggest that Isw2 and Ino80 likely function through currently unknown mechanisms to regulate both chromatin accessibility and S-phase checkpoint attenuation. In the future, understanding the molecular mechanisms underlying Isw2- and Ino80-dependent chromatin accessibility at replication forks will be crucial in defining new biochemical activities of chromatin remodeling factors as well as elucidating a currently unknown mechanism of checkpoint control.
Supplementary Material
Acknowledgments
We thank Steve Elledge, Peter Burgers, Dave Toczyski, John Diffley, Hiroyuki Araki, Jac Nickoloff, Sue Biggins, Linda Breeden, Keiko Umezu, Rodney Rothstein, and Oscar Aparicio for plasmids and strains used in this study. We also thank Anne Donaldson for her advice on SILAC protocols and Phil Gafken for his help with mass spectrometry and data analysis. We are grateful to Tsukiyama Laboratory members and Sue Biggins for discussion and comments on this manuscript. This work is supported by Molecular and Cellular Biology Training Grant GM-07270 to L.L. and Grant R-01GM058465 to T.T.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.174730/-/DC1
Communicating editor: J. A. Nickoloff
Literature Cited
- Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P. J., et al. , 2001. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 3: 958–965. [DOI] [PubMed] [Google Scholar]
- Au T. J., Rodriguez J., Vincent J. A., Tsukiyama T., 2011. ATP-dependent chromatin remodeling factors tune S phase checkpoint activity. Mol. Cell. Biol. 31: 4454–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble D. T., Hansen K. E., Mueller C. G., Lane W. S., Thorner J., et al. , 1994. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 8: 1920–1934. [DOI] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2009. The checkpoint response to replication stress. DNA Repair 8: 1038–1046. [DOI] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2010. Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 11: 208–219. [DOI] [PubMed] [Google Scholar]
- Byun T. S., Pacek M., Yee M. C., Walter J. C., Cimprich K. A., 2005. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Cui D., Papusha A., Zhang X., Chu C. D., et al. , 2012. The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature 489: 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costelloe T., Louge R., Tomimatsu N., Mukherjee B., Martini E., et al. , 2012. The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489: 581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe L., Thomas A., Pantesco V., De Vos J., Pasero P., et al. , 2010. Analysis of replication profiles reveals key role of RFC-Ctf18 in yeast replication stress response. Nat. Struct. Mol. Biol. 17: 1391–1397. [DOI] [PubMed] [Google Scholar]
- Eng J. K., McCormack A. L., Yates J. R., 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5: 976–989. [DOI] [PubMed] [Google Scholar]
- Engels K., Giannattasio M., Muzi-Falconi M., Lopes M., Ferrari S., 2011. 14-3-3 Proteins regulate exonuclease 1–dependent processing of stalled replication forks. PLoS Genet. 7: e1001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio T. G., Tsukiyama T., 2003. Chromatin remodeling in vivo: evidence for a nucleosome sliding mechanism. Mol. Cell 12: 1333–1340. [DOI] [PubMed] [Google Scholar]
- Fischer C. J., Yamada K., Fitzgerald D. J., 2009. Kinetic mechanism for single-stranded DNA binding and translocation by S. cerevisiae Isw2. Biochemistry 48: 2960–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel A. M., Pike B. L., Gasser S. M., 2009. ATR/Mec1: coordinating fork stability and repair. Curr. Opin. Cell Biol. 21: 237–244. [DOI] [PubMed] [Google Scholar]
- Fyodorov D. V., Blower M. D., Karpen G. H., Kadonaga J. T., 2004. Acf1 confers unique activities to ACF/CHRAC and promotes the formation rather than disruption of chromatin in vivo. Genes Dev. 18: 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. S., Green C. M., Lowndes N. F., 2001. Budding yeast Rad9 is an ATP-dependent Rad53 activating machine. Mol. Cell 8: 129–136. [DOI] [PubMed] [Google Scholar]
- Goldmark J. P., Fazzio T. G., Estep P. W., Church G. M., Tsukiyama T., 2000. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103: 423–433. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Prieto R., Munoz-Cabello A. M., Cabello-Lobato M. J., Prado F., 2013. Rad51 replication fork recruitment is required for DNA damage tolerance. EMBO J. 32: 1307–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegnauer A. M., Hustedt N., Shimada K., Pike B. L., Vogel M., et al. , 2012. An N-terminal acidic region of Sgs1 interacts with Rpa70 and recruits Rad53 kinase to stalled forks. EMBO J. 31: 3768–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustedt N., Gasser S. M., Shimada K., 2013. Replication checkpoint: tuning and coordination of replication forks in S phase. Genes 4: 388–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T., Araki H., 2004. Noncompetitive counteractions of DNA polymerase epsilon and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L., Canterbury J. D., Weston J., Noble W. S., MacCoss M. J., 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4: 923–925. [DOI] [PubMed] [Google Scholar]
- Kanellis P., Agyei R., Durocher D., 2003. Elg1 forms an alternative PCNA-interacting RFC complex required to maintain genome stability. Curr. Biol. 13: 1583–1595. [DOI] [PubMed] [Google Scholar]
- Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., et al. , 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424: 1078–1083. [DOI] [PubMed] [Google Scholar]
- Kondo T., Wakayama T., Naiki T., Matsumoto K., Sugimoto K., 2001. Recruitment of Mec1 and Ddc1 checkpoint proteins to double-strand breaks through distinct mechanisms. Science 294: 867–870. [DOI] [PubMed] [Google Scholar]
- Kubota T., Hiraga S., Yamada K., Lamond A. I., Donaldson A. D., 2011. Quantitative proteomic analysis of chromatin reveals that Ctf18 acts in the DNA replication checkpoint. Mol Cell Proteomics 10: M110.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Nishimura K., Kanemaki M. T., Donaldson A. D., 2013. The Elg1 replication factor C-like complex functions in PCNA unloading during DNA replication. Mol. Cell 50: 273–280. [DOI] [PubMed] [Google Scholar]
- Kubota T., Stead D. A., Hiraga S., ten Have S., Donaldson A. D., 2012. Quantitative proteomic analysis of yeast DNA replication proteins. Methods 57: 196–202. [DOI] [PubMed] [Google Scholar]
- Kumar S., Burgers P. M., 2013. Lagging strand maturation factor Dna2 is a component of the replication checkpoint initiation machinery. Genes Dev. 27: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Tetzlaff M. T., Elledge S. J., 2008. Identification of MSA1, a cell cycle-regulated, dosage suppressor of drc1/sld2 and dpb11 mutants. Cell Cycle 7: 3388–3398. [DOI] [PubMed] [Google Scholar]
- Licklider L. J., Thoreen C. C., Peng J., Gygi S. P., 2002. Automation of nanoscale microcapillary liquid chromatography–tandem mass spectrometry with a vented column. Anal. Chem. 74: 3076–3083. [DOI] [PubMed] [Google Scholar]
- Lisby M., Barlow J. H., Burgess R. C., Rothstein R., 2004. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118: 699–713. [DOI] [PubMed] [Google Scholar]
- Majka J., Niedziela-Majka A., Burgers P. M., 2006. The checkpoint clamp activates Mec1 kinase during initiation of the DNA damage checkpoint. Mol. Cell 24: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantiero D., Clerici M., Lucchini G., Longhese M. P., 2007. Dual role for Saccharomyces cerevisiae Tel1 in the checkpoint response to double-strand breaks. EMBO Rep. 8: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Sugino A., Araki H., 2000. Dpb11 controls the association between DNA polymerases alpha and epsilon and the autonomously replicating sequence region of budding yeast. Mol. Cell. Biol. 20: 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A. D., Gelbart M. E., Tsukiyama T., 2004. Histone fold protein Dls1p is required for Isw2-dependent chromatin remodeling in vivo. Mol. Cell. Biol. 24: 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau J. L., Lee M., Mahachi N., Vary J., Mellor J., et al. , 2003. Regulated displacement of TBP from the PHO8 promoter in vivo requires Cbf1 and the Isw1 chromatin remodeling complex. Mol. Cell 11: 1609–1620. [DOI] [PubMed] [Google Scholar]
- Morrison A. J., Highland J., Krogan N. J., Arbel-Eden A., Greenblatt J. F., et al. , 2004. INO80 and γ-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119: 767–775. [DOI] [PubMed] [Google Scholar]
- Morrison A. J., Shen X., 2009. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat. Rev. Mol. Cell Biol. 10: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navadgi-Patil V. M., Burgers P. M., 2008. Yeast DNA replication protein Dpb11 activates the Mec1/ATR checkpoint kinase. J. Biol. Chem. 283: 35853–35859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Krebs J. E., Peterson C. L., 2006. Interplay between Ino80 and Swr1 chromatin remodeling enzymes regulates cell cycle checkpoint adaptation in response to DNA damage. Genes Dev. 20: 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Peterson C. L., 2008. The Ino80 chromatin-remodeling enzyme regulates replisome function and stability. Nat. Struct. Mol. Biol. 15: 338–345. [DOI] [PubMed] [Google Scholar]
- Papamichos-Chronakis M., Watanabe S., Rando O. J., Peterson C. L., 2011. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144: 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicioli A., Lucca C., Liberi G., Marini F., Lopes M., et al. , 1999. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 18: 6561–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander B., Diffley J. F., 2011. Dpb11 coordinates Mec1 kinase activation with cell cycle-regulated Rad9 recruitment. EMBO J. 30: 4897–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot R. A., Bozhenok L., van den Berg D. L., Steffensen S., Ferreira F., et al. , 2004. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat. Cell Biol. 6: 1236–1244. [DOI] [PubMed] [Google Scholar]
- Rodriguez J., McKnight J. N., Tsukiyama T., 2014. Genome-wide analysis of nucleosome positions, occupancy, and accessibility in yeast: nucleosome mapping, high-resolution histone ChIP, and NCAM. Curr. Protoc. Mol. Biol. 108: 28.21:28.21.1–21.28.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Tsukiyama T., 2013. ATR-like kinase Mec1 facilitates both chromatin accessibility at DNA replication forks and replication fork progression during replication stress. Genes Dev. 27: 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J., Jackson S. P., 2002. Lcd1p recruits Mec1p to DNA lesions in vitro and in vivo. Mol. Cell 9: 857–869. [DOI] [PubMed] [Google Scholar]
- Royce T. E., Carriero N. J., Gerstein M. B., 2007. An efficient pseudomedian filter for tiling microrrays. BMC Bioinformatics 8: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Mizuguchi G., Hamiche A., Wu C., 2000. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406: 541–544. [DOI] [PubMed] [Google Scholar]
- Shimada K., Oma Y., Schleker T., Kugou K., Ohta K., et al. , 2008. Ino80 chromatin remodeling complex promotes recovery of stalled replication forks. Curr. Biol. 18: 566–575. [DOI] [PubMed] [Google Scholar]
- Sugimoto N., Yugawa T., Iizuka M., Kiyono T., Fujita M., 2011. Chromatin remodeler sucrose nonfermenting 2 homolog (SNF2H) is recruited onto DNA replication origins through interaction with Cdc10 protein-dependent transcript 1 (Cdt1) and promotes pre-replication complex formation. J. Biol. Chem. 286: 39200–39210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kowalczykowski S. C., 2002. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 277: 31663–31672. [DOI] [PubMed] [Google Scholar]
- Szyjka S. J., Aparicio J. G., Viggiani C. J., Knott S., Xu W., et al. , 2008. Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev. 22: 1906–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyjka S. J., Viggiani C. J., Aparicio O. M., 2005. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell 19: 691–697. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Katou Y., Yagura M., Saitoh K., Itoh T., et al. , 2009. Ctf4 coordinates the progression of helicase and DNA polymerase alpha. Genes Cells 14: 807–820. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R., 1989. The genetic control of direct-repeat recombination in Saccharomyces: the effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptionally regulated gene. Genetics 123: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach J. M., Yimit A., Lee A. Y., Riffle M., Costanzo M., et al. , 2012. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nat. Cell Biol. 14: 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travesa A., Duch A., Quintana D. G., 2008. Distinct phosphatases mediate the deactivation of the DNA damage checkpoint kinase Rad53. J. Biol. Chem. 283: 17123–17130. [DOI] [PubMed] [Google Scholar]
- Umezu K., Sugawara N., Chen C., Haber J. E., Kolodner R. D., 1998. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics 148: 989–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Attikum H., Fritsch O., Hohn B., Gasser S. M., 2004. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 119: 777–788. [DOI] [PubMed] [Google Scholar]
- Vincent J. A., Kwong T. J., Tsukiyama T., 2008. ATP-dependent chromatin remodeling shapes the DNA replication landscape. Nat. Struct. Mol. Biol. 15: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A., Hughes A., Yassour M., Rando O. J., Friedman N., 2010. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 20: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse I., Rando O. J., Delrow J., Tsukiyama T., 2007. Chromatin remodelling at promoters suppresses antisense transcription. Nature 450: 1031–1035. [DOI] [PubMed] [Google Scholar]
- Yadon A. N., Van de Mark D., Basom R., Delrow J., Whitehouse I., et al. , 2010. Chromatin remodeling around nucleosome-free regions leads to repression of noncoding RNA transcription. Mol. Cell. Biol. 30: 5110–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman M. K., Cimprich K. A., 2014. Causes and consequences of replication stress. Nat. Cell Biol. 16: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Muller E. G., Rothstein R., 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2: 329–340. [DOI] [PubMed] [Google Scholar]
- Zou L., 2013. Four pillars of the S-phase checkpoint. Genes Dev. 27: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Elledge S. J., 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542–1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.