Abstract
Background
Obesity has been associated with significantly greater risk of sudden cardiac death (SCD); however, identifying the obese patient at highest risk remains a challenge. We evaluated the association between QRS fragmentation on the 12‐lead electrocardiogram and SCD, in obese/overweight subjects.
Methods and Results
In the ongoing prospective, community‐based Oregon Sudden Unexpected Death Study (population approximately 1 million), we performed a case‐control analysis, comparing obese/overweight SCD victims with obese/overweight controls from the same geographic region. Archived ECGs prior and unrelated to the SCD event were used for cases and all ECG measurements were assessed in blinded fashion. Fragmentation was defined as the presence of RSR’ patterns and/or notching of the R/S wave in at least 2 contiguous leads. Analysis was limited to ECGs with QRS duration <120 ms. Overall prevalence of fragmentation was higher in cases (n=185; 64.9±13.8 years; 67.0% male) compared with controls (n=405; 64.9±11.0 years; 64.7% male) (34.6% versus 26.9%, P=0.06). Lateral fragmentation was significantly more frequent in cases (8.1% versus 2.5%; P<0. 01), with non‐significant differences in anterior and inferior territories. Fragmentation in multiple territories (≥2) was also more likely to be observed in cases (9.7% versus 4.9%, P=0.02). In multivariable analysis with consideration of established SCD risk factors, lateral fragmentation was significantly associated with SCD (OR 2.84; 95% CI 1.01 to 8.02; P=0.05).
Conclusion
QRS fragmentation, especially in the lateral territory is a potential risk marker for SCD independent of the ejection fraction, among obese/overweight subjects in the general population.
Keywords: ECG, obesity, QRS fragmentation, sudden cardiac death
Introduction
Current estimates suggest that more than one‐third of the world population is overweight or obese. In the United States, with more than two‐thirds of the adult population overweight and one‐third obese, obesity has been upgraded from a “condition” to a “disease.”1–2 Obesity is closely associated with the metabolic syndrome3 and has been linked to adverse cardiovascular prognosis and mortality.4–5 A diagnosis of obesity predicts incident coronary artery disease (CAD) over and above the traditional coronary risk factors and has been reported to be additive to the Framingham risk score.6
Large cohort studies7–8 as well as population‐based analysis from the Oregon Sudden Unexpected Death Study (SUDS)9 have confirmed obesity/overweight as a risk factor for sudden cardiac death (SCD). The link between obesity and arrhythmia is likely multifactorial and continues to be an area of active research. Of particular interest is the adverse cardiac remodeling associated with obesity. Obesity has been linked to left atrial and ventricular dilatation10 as well as left ventricular hypertrophy and diastolic dysfunction.11–12 Recent animal13 as well as clinical14 studies have suggested that fatty infiltration of the myocardium may itself predispose to ventricular arrhythmia, potentially as a result of poor electrical conductivity of fat which could favor delayed impulse transmission and re‐entry.
Electrical disturbances arising as a result of fatty infiltration/fibrosis may thus represent an important mechanism of arrhythmogenesis in obesity. QRS fragmentation (fQRS) on the surface ECG has been reported to be a surrogate marker of inhomogeneous conduction and delayed activation in the myocardium.15 Fragmented QRS complexes, thought to reflect myocardial scarring, have been linked to adverse cardiac prognosis as well as cardiac mortality.16–17 Moreover, earlier studies have suggested that QRS fragmentation predicts risk of arrhythmia in a variety of cardiac conditions.18–21
However, a potential association between QRS fragmentation and SCD in the general population needs further evaluation. It would be of significant interest to assess whether this simple, non‐invasive marker on the surface ECG could help identify high‐risk sub‐groups within the obese/overweight population. In light of the available knowledge about the pathophysiology of obesity and SCD, especially with regard to the effect of obesity on cardiac structure, we postulated that QRS fragmentation may be a useful marker to identify those at risk of SCD among obese/overweight subjects in the community. We therefore undertook a systematic investigation of the potential utility of QRS fragmentation, among obese/overweight subjects in the general population.
Methods
Study Population and Definitions
This is a community‐based, observational, case‐control study. The Oregon SUDS is a prospective, population‐based study of out‐of‐hospital SCD. Detailed methods and descriptions have been published earlier.22–24 Briefly, cases of possible SCD are prospectively ascertained in the Portland, Oregon metropolitan area (population approximately 1 million) using multiple sources, which include first responders, local hospital emergency rooms, and the county medical examiner. After detailed review of available medical records, autopsy reports, and the circumstances of arrest, SCD cases are identified through a 3‐physician adjudication system. SCD is defined as an unexpected sudden, pulseless condition of cardiac etiology, occurring within 1 hour of symptom onset in witnessed cases and within 24 hours if unwitnessed. Survivors of sudden cardiac arrest are also included as SCD cases. Subjects with known terminal illnesses (such as cancer), non‐cardiac causes of sudden death (such as cerebrovascular accident), and drug overdose are excluded. In parallel, controls are recruited from the same geographical location. Controls are subjects with no history of cardiac arrest, and are chosen to achieve a mix of a majority of subjects with coronary artery disease (CAD) and a minority of healthy controls from the population. The study is designed for the majority of controls (80%) to have CAD since published studies have established that a similar majority of SCD cases in the community have associated CAD.25 CAD was defined as ≥50% stenosis in a major coronary artery. CAD controls are recruited from among subjects undergoing angiography at one of the region's major participating health systems, those who were transported by the emergency medical system (EMS) for symptoms of acute coronary ischemia or subjects with diagnosed CAD from the region's Kaiser Permanente system. SCD cases ≥50 years of age were assumed to have CAD based on >95% likelihood of CAD in such cases.26 Detailed demographic and clinical information was collected for cases and controls from available medical records. Left ventricular (LV) function was evaluated by measurement of the LV ejection fraction (EF) and categorized as normal LV function (≥55%), mild to moderate dysfunction (36–54%), or severe dysfunction (≤35%). Body mass index (BMI) was calculated for subjects using weight and height measurements. As per National Institute of Health Guidelines, overweight was defined as a BMI of 25 to 29.9 kg/m2, and obesity was defined as a BMI of ≥30 kg/m2.27. The present study compared obese/overweight SCD cases with obese/overweight controls from the same geographical area. Other than being from the same geographic location, no other specific matching was performed between cases and controls.
QRS Fragmentation and Other ECG Parameters
Parameters assessed from the 12‐lead ECG included heart rate, QRS duration, QT interval, presence of Q waves, and QRS fragmentation. ECGs prior but unrelated to the SCD event were used for cases. Taking into consideration previously established criteria for assessing fQRS,15 the present analysis was limited to ECGs with narrow QRS (QRS duration <120 ms). The QT interval was corrected using Bazett's formula28 and categorized as normal or abnormal using sex‐specific criteria (men: QT c ≤430 ms—normal, 431 to 450 ms—borderline, >450 ms—abnormal; women: QTc ≤450 ms—normal, 451 to 470 ms—borderline, >470 ms—abnormal).29 Pathologic Q waves were defined as Q waves ≥40 milliseconds in duration and >25% of the voltage of the following R wave. Q waves were classified according to territory, based on presence in at least 2 contiguous leads, as anterior (leads V1–V5), lateral (leads I, aVL, and V6), and inferior (leads II, III, and aVF). fQRS was identified as previously defined15 by the presence of various RSR′ patterns with or without Q wave, including an additional R wave (R′), notching of the R wave, notching in the nadir of the S wave, or the presence of more than 1 R′ (fragmentation) in at least 2 contiguous leads corresponding to a major coronary artery territory (Figure 1A and 1B). If notching was confined only to the terminal QRS accompanied by J point elevation of ≥0.1 mV, it was classified as early repolarization pattern rather than fragmentation. Fragmentation was classified by anatomic territory, similar to Q waves, into anterior (leads V1–V5), lateral (leads I, aVL, and V6), or inferior (leads II, III, and aVF). ECG assessment for fragmentation and Q waves were performed by 2 trained readers blinded to the case/control status of the subjects. The study was approved by the Institutional Review Boards of Cedars‐Sinai Medical Center, Oregon Health and Science University, and all participating hospitals.
Figure 1.
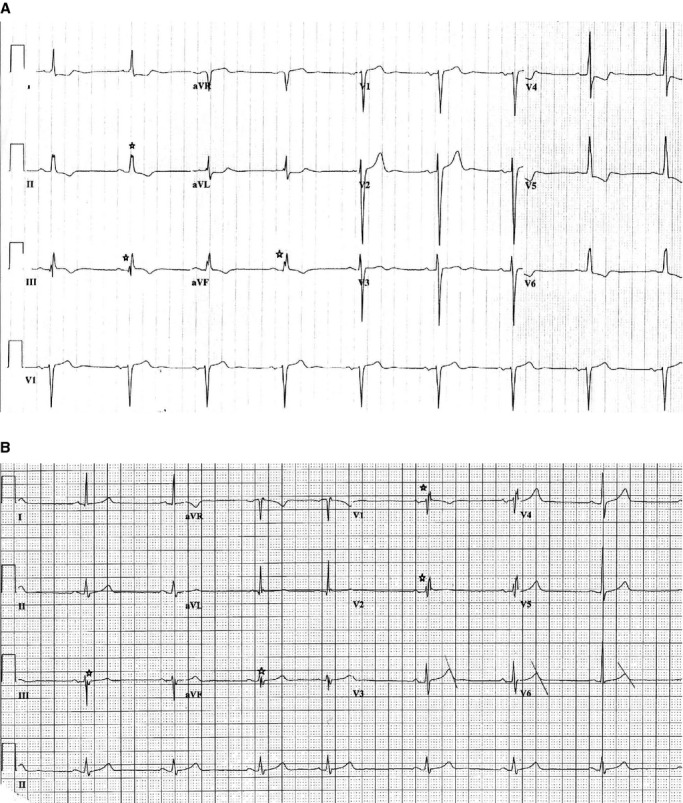
Examples of ECGs showing fragmentation. A, 12‐lead ECG with inferior fragmentation. B, 12‐lead ECG with anterior and inferior fragmentation. ECG indicates electrocardiogram.
Statistical Analysis
Student t tests and Pearson's χ2 tests were used for comparisons of continuous variables (presented as mean±standard deviation) and categorical variables (presented as numbers and percentages), respectively. Multivariable logistic regression was used to estimate odds ratios (OR) for the association of fQRS with SCD case status after adjusting for parameters that were significant in the univariate analyses. For the multivariable model, QTc (as described earlier) and heart rate (≥100 versus <100 bpm) were categorized based on accepted standards. Results were reported as 2‐tailed P values, with a P value of ≤0.05 being considered statistically significant. All analyses were performed using SPSS statistical software (version 20.0 for Windows, SPSS Inc, IBM Corporation, New York).
Results
Demographic and Clinical Characteristics
A total of 590 obese/overweight subjects (185 cases and 405 controls) were studied. Demographic and clinical characteristics of cases and controls are shown in Table 1. Age, sex, frequency of current/former smokers, and the relative proportions of overweight and obese were not significantly different between case and control groups. Severe LV dysfunction (LVEF ≤35%; 14.6% versus 4.7%; P<0.01), diabetes mellitus (43.8% versus 29.9%; P<0.01), and coronary artery disease (88.6% versus 77.5%; P<0.01) were all more frequent in cases. Use of angiotensin‐converting enzyme inhibitors (ACEI) and beta blockers was similar between cases and controls while use of angiotensin receptor blockers (ARBs) was higher among controls (6.8% versus 12.2%, P=0.05).
Table 1.
Demographic and Clinical Characteristics of Subjects
| Case (n=185) | Control (n=405) | P Value | |
|---|---|---|---|
| Age, y | 64.9±13.8 | 64.9±11.0 | 0.98 |
| Male | 124 (67.0) | 262 (64.7) | 0.58 |
| Body mass index | 0.53 | ||
| Overweight | 83 (44.9) | 193 (47.7) | |
| Obese | 102 (55.1) | 212 (52.3) | |
| Left ventricular function | <0.001 | ||
| Normal function | 71 (65.1) | 192 (73) | |
| Mild to moderate dysfunction | 22 (20.2) | 59 (22.4) | |
| Severe dysfunction | 15 (14.6) | 12 (4.7) | |
| Diabetes mellitus | 81 (43.8) | 121 (29.9) | <0.001 |
| Cholesterol, mg/dL | 182±44 | 177±48 | 0.34 |
| Current/former smoker | 116 (75.8) | 201 (67.4) | 0.07 |
| Hypertension | 143 (77.3) | 286 (70.6) | 0.09 |
| Coronary artery disease* | 164 (88.6) | 314 (77.5) | <0.001 |
| Use of angiotensin receptor blocker (ARB) | 12 (6.8) | 46 (12.2) | 0.05 |
| Use of angiotensin converting enzyme inhibitor (ACEI) | 76 (43.2) | 164 (43.6) | 0.92 |
| Use of beta blocker | 95 (53.7) | 226 (59.8) | 0.17 |
Results presented as mean±SD for continuous variables and n (%) for categorical variables. Body mass index categories: overweight (BMI 25 to 29.9 kg/m2), obese (BMI≥30 kg/m2). Left ventricular function categories: ejection fraction (EF) ≥55% (normal), EF 36–54% (mild‐moderate dysfunction), EF ≤35% (severe dysfunction); available for 109 cases and 263 controls. Cholesterol value available for 127 cases and 324 controls. Smoking status available for 153 cases and 298 controls. Data on use of angiotensin receptor blocker (ARB) and beta blocker were available for 177 cases and 226 controls. Data on use of angiotensin converting enzyme inhibitor (ACEI) were available for 176 cases and 376 controls. SCD indicates sudden cardiac death.
SCD cases ≥50 years age assumed to have CAD based on >95% likelihood of CAD in such cases.26
Prevalence of QRS Fragmentation and Other ECG Parameters
The ECGs were performed a median of 313 days prior to the SCD event (range 2 to 5094), with 75% being performed within 3 years of the SCD event. The prevalence of the specified ECG parameters among cases and controls is outlined in Table 2. QRS fragmentation in any territory was detected in 64 (34.6%) cases and 109 (26.9%) controls, with higher prevalence in cases (P=0.06). When stratified by coronary artery territory, fragmentation was most frequently seen in the inferior territory in cases as well as controls. While there was no significant difference between cases and controls with regard to inferior (28.1% versus 23.7%; P=0.25), and anterior territory fragmentation (9.7% versus 5.9%; P=0.10), lateral fQRS was significantly more likely to be seen in cases compared with controls (8.1% versus 2.5%; P<0.01; Figure 2). Cases were also significantly more likely than controls to have fragmentation in 2 or more anatomic territories (9.7% versus 4.9%; P=0.02; Figure 3). There were 3 (1.6%) cases with fragmentation in all 3 anatomic territories, compared with none in the control group. The frequency of pathologic Q waves was not significantly different between cases and controls (14.8% versus 10.0%; P=0.09).
Table 2.
Electrocardiographic Characteristics of Cases and Controls
| Case (n=185) | Control (n=405) | P Value | |
|---|---|---|---|
| fQRS distribution | |||
| Any fragmentation | 64 (34.6) | 109 (26.9) | 0.06 |
| Inferior territory | 52 (28.1) | 96 (23.7) | 0.25 |
| Anterior territory | 18 (9.7) | 24 (5.9) | 0.10 |
| Lateral territory | 15 (8.1) | 10 (2.5) | <0.01 |
| Q wave | 27 (14.8) | 40 (10.0) | 0.09 |
| QRS duration, milliseconds | 87.9±10.1 | 87.3±8.3 | 0.50 |
| Heart rate, beats per minute | 77.8±16.9 | 68.1±14.0 | <0.01 |
| Corrected QT interval, milliseconds | <0.01 | ||
| Normal | 112 (61.2) | 336 (83) | |
| Borderline | 28 (15.3) | 40 (9.9) | |
| Abnormal | 43 (23.5) | 29 (7.2) | |
Results presented as n (%).QT interval categories: men ≤430 ms (normal), 431 to 450 ms (borderline), and >450 ms (abnormal); women ≤450 ms (normal), 451 to 470 ms (borderline), and >470 ms (abnormal). fQRS indicates fragmented QRS.
Figure 2.
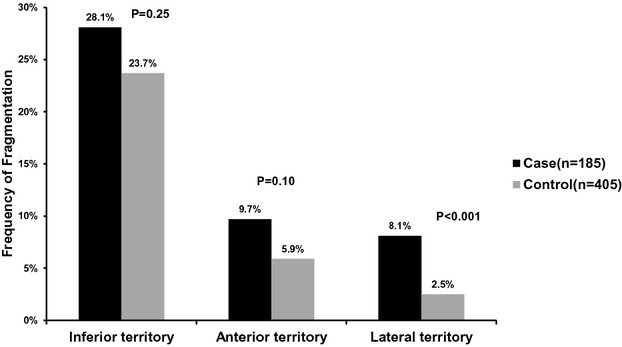
Prevalence of fragmented QRS complexes (fQRS) by coronary artery territory in cases and controls.
Figure 3.
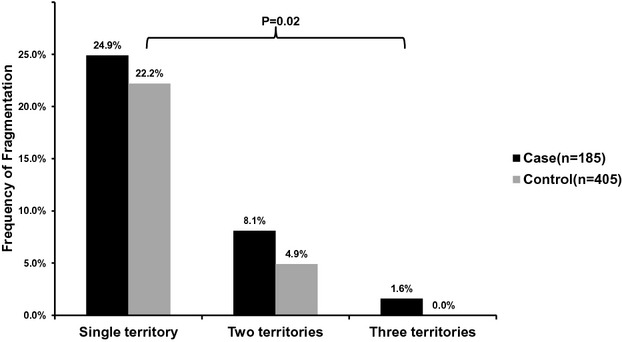
Number of anatomic territories with QRS fragmentation in cases and controls.
With regard to other ECG parameters, mean heart rate was significantly higher in cases compared with controls (77.8±16.9 versus 68.1±14.0 bpm; P<0.01). Cases were also more likely to have borderline (15.3% versus 9.9%) or abnormal QTc (23.5% versus 7.2%; both P<0.01) compared with controls (P<0.01). There was no significant difference in QRS duration (87.9±10.1 versus 87.3±8.3; P=0.50).
Adjusted Odds Ratios for SCD
In a multivariable logistic regression model, presence of lateral territory fragmentation was significantly associated with SCD (OR=2.84; 95% CI 1.01 to 8.02; P=0.05) after adjustment for covariates significant in the univariate analysis. Other significant parameters included severe LV dysfunction (OR=3.55; 95% CI 1.54 to 8.21; P<0.01), abnormal QTc (P<0.01), and abnormal heart rate (P=0.02; Table 3).
Table 3.
Multivariable Adjusted Odds Ratios for SCD
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Lateral fragmentation | 2.84 (1.01 to 8.02) | 0.05 |
| Severe left ventricular dysfunction | 3.55 (1.54 to 8.21) | <0.01 |
| Abnormal QT interval | 3.70 (1.97 to 6.96) | <0.01 |
| Abnormal heart rate | 3. 55 (1.24 to 10.14) | 0.02 |
| Age | 1.01 (0.99 to 1.03) | 0.25 |
| Diabetes mellitus | 1. 73 (1.05 to 2.85) | 0.03 |
| Coronary artery disease | 0. 73 (0.32 to 1.69) | 0.47 |
| Use of angiotensin receptor blocker | 0. 51 (0.21 to 1.28) | 0.15 |
95% CI=95% confidence interval. Severe Left ventricular dysfunction defined: EF ≤35%; abnormal QT interval categories: Men >450 ms and Women >470 ms; abnormal heart rate: heart rate >100 bpm. EF indicates ejection fraction; SCD, sudden cardiac death.
Discussion
To the best of our knowledge this is the first evaluation of the potential association between QRS fragmentation on ECG and SCD, in a community‐based obese/overweight population. The findings of this study could be important both from a mechanistic as well as risk stratification perspective. Firstly, there was a higher prevalence of fragmentation overall among SCD cases compared with controls. Secondly, fragmentation in the lateral coronary territory was significantly associated with increased SCD odds even after adjustment for reduced EF and other ECG parameters. Thirdly, SCD cases were also distinguished by a greater burden of fragmentation in that they were more likely to have fragmentation in 2 or more anatomic territories. Increased heart rate and QT interval were also significantly associated with increased SCD odds which is consistent with earlier reports.30–31 Importantly, presence of pathologic Q waves was not significantly different between cases and controls in this population. This is in concordance with prior studies which have shown that fragmented QRS complexes are more sensitive at detecting myocardial scar32 and predict cardiac events even after resolution of Q waves.33 Furthermore, since fragmentation, unlike Q waves owes its genesis to inhomogeneous myocardial conduction, it may reflect other causes of delayed conduction as well, such as myocardial fatty infiltration, which may be especially relevant in the obese/overweight.34 In arrhythmogenic right ventricular dysplasia (ARVD), characterized by fibrous/fatty replacement of right ventricular myocardium, fQRS has diagnostic value35 and also predicts arrhythmic events.36 Additionally, the utility of fQRS in conditions such as cardiac amyloidosis37 supports the concept that fQRS may be a broader marker of myocardial infiltration beyond CAD‐related scar.
Of note, fQRS remained significantly associated with SCD even after accounting for low LVEF. Reduced LVEF, while important, does not encompass the entire spectrum of adverse cardiac remodeling. For instance, fibrosis detected by cardiac magnetic resonance (CMR) has been reported to predict SCD beyond LVEF.38 In this context, fQRS has been shown to correlate with subclinical LV dysfunction and adverse cardiac events in patients with preserved LVEF.39 It has also been shown to correlate with ventricular dyssynchrony40 and regression of fQRS with response to cardiac resynchronization therapy.41 Thus, being a more sensitive marker of an adverse myocardial substrate, fQRS may have special relevance for SCD risk stratification in the obese/overweight. While the exact pathophysiologic pathways linking obesity to SCD remain to be elucidated, it is likely that the deleterious alterations in cardiac structure known to occur in obesity, including ventricular dilatation,10 cardiac hypertrophy,11–12 fibrofatty change,42 and myocardial lipid accumulation43 play an important role. Such changes may lead to increased cardiac irritability reflected by a greater burden of ventricular ectopy44 and a greater tendency to develop atrial fibrillation.45
In this study, lateral fragmentation was associated with SCD. Conversely, an analysis from the MADIT II population reported that inferior fQRS was more predictive of SCD.19 However, the latter study was conducted on a heart failure/defibrillator population, distinct from our evaluation in the general population. Another study assessing fragmentation in the general population also reported an association between lateral fQRS and arrhythmic mortality, similar to the present study.46 It is possible that different patterns of fragmentation may have relevance in different risk groups. Additionally, the quantitative burden of fragmentation, reflected by the number of leads with fragmentation, is likely to be important as well.47–48
Though fQRS appears promising as a risk marker, whether it can be used clinically to aid decision making for primary prevention of SCD needs further investigation. While there is accumulating evidence that fQRS predicts both arrhythmic events and appropriate ICD shocks,18–19 a large multisite study among patients with LVEF ≤35% found no evidence that fQRS would be useful in risk stratifying patients eligible for primary prevention ICD.49
Strengths of the present study include its population‐based nature, prospective ascertainment and adjudication of SCD cases, as well as availability of detailed lifetime clinical history for both cases and controls. However, any study of this nature is subject to certain limitations. The study was performed on obese/overweight subjects only and may not apply to the overall population. We had to restrict the analysis to those with appropriate ECGs available which could lead to some bias in selecting patients with higher cardiac risk. On the other hand, given the burgeoning obese population and the close association of obesity with cardiovascular risk, this group represents a potentially important target for aggressive prevention. The wide confidence intervals of the effect estimates likely reflect the limited number of subjects; however, these were identified from a population of approximately 1 million, highlighting the practical difficulties of studying such subjects in the general population. While adjustment for a more comprehensive list of variables could not be carried out, at present EF is the only parameter used for SCD risk stratification and fragmentation may help improve risk prediction beyond EF. Further studies will be needed to clarify the exact mechanisms linking fragmentation in specific territories to arrhythmogenesis. We could not precisely estimate the prevalence of fragmentation among subjects without CAD as a good proportion of cases without previously diagnosed CAD may in fact have underlying CAD, as has been demonstrated by prior autopsy studies of SCD in the general population.25 Finally, further prospective studies assessing the role of fQRS in a variety of high‐risk groups in the population will be needed prior to its application in the clinical arena.
Conclusion
In this population‐based study among obese/overweight subjects, QRS fragmentation in the lateral territory was associated with increased SCD risk even after adjustment for LVEF. Obese SCD subjects were also characterized by a greater likelihood of fragmentation in 2 or more coronary territories. The utility of fragmentation for SCD risk stratification in subjects with high BMI warrants further evaluation.
Acknowledgments
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments, and the Oregon State Medical Examiner's office.
Sources of Funding
Funded in part, by National Heart, Lung, and Blood Institute grants R01HL088416 and HL105170 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology Research at the Heart Institute, Cedars‐Sinai Medical Center, Los Angeles, CA.
Disclosures
None.
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014; 311:806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu‐Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi‐Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez‐Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang SX, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014; 384:766-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005; 365:1415-1428. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS, Investigators IS. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case‐control study. Lancet. 2005; 366:1640-1649. [DOI] [PubMed] [Google Scholar]
- Engeland A, Bjorge T, Sogaard AJ, Tverdal A. Body mass index in adolescence in relation to total mortality: 32‐year follow‐up of 227 000 Norwegian boys and girls. Am J Epidemiol. 2003; 157:517-523. [DOI] [PubMed] [Google Scholar]
- Mora S, Yanek LR, Moy TF, Fallin MD, Becker LC, Becker DM. Interaction of body mass index and framingham risk score in predicting incident coronary disease in families. Circulation. 2005; 111:1871-1876. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the framingham study. Am Heart J. 1988; 115:869-875. [DOI] [PubMed] [Google Scholar]
- Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999; 99:1978-1983. [DOI] [PubMed] [Google Scholar]
- Noheria A, Teodorescu C, Uy‐Evanado A, Reinier K, Mariani R, Gunson K, Jui J, Chugh SS. Distinctive profile of sudden cardiac arrest in middle‐aged vs. older adults: a community‐based study. Int J Cardiol. 2013; 168:3495-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001; 321:225-236. [DOI] [PubMed] [Google Scholar]
- Morse SA, Bravo PE, Morse MC, Reisin E. The heart in obesity‐hypertension. Expert Rev Cardiovasc Ther. 2005; 3:647-658. [DOI] [PubMed] [Google Scholar]
- Crisostomo LL, Araujo LM, Camara E, Carvalho C, Silva FA, Vieira M, Rabelo A., Jr Comparison of left ventricular mass and function in obese versus nonobese women <40 years of age. Am J Cardiol. 1999; 84:1127-1129. [DOI] [PubMed] [Google Scholar]
- Reig J, Domingo E, Segura R, Tovar JL, Vinallonga M, Borrell M. Rat myocardial tissue lipids and their effect on ventricular electrical activity: influence on dietary lipids. Cardiovasc Res. 1993; 27:364-370. [DOI] [PubMed] [Google Scholar]
- Molinari G, Sardanelli F, Zandrino F, Parodi RC, Bertero G, Richiardi E, Di Donna P, Gaita F, Masperone MA. Adipose replacement and wall motion abnormalities in right ventricle arrhythmias: evaluation by MR Imaging. Retrospective evaluation on 124 patients. Int J Card Imaging. 2000; 16:105-115. [DOI] [PubMed] [Google Scholar]
- Das MK, Saha C, El Masry H, Peng J, Dandamudi G, Mahenthiran J, McHenry P, Zipes DP. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007; 4:1385-1392. [DOI] [PubMed] [Google Scholar]
- Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009; 6:S8-S14. [DOI] [PubMed] [Google Scholar]
- Das MK, Suradi H, Maskoun W, Michael MA, Shen C, Peng J, Dandamudi G, Mahenthiran J. Fragmented wide QRS on a 12‐lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008; 1:258-268. [DOI] [PubMed] [Google Scholar]
- Das MK, Maskoun W, Shen C, Michael MA, Suradi H, Desai M, Subbarao R, Bhakta D. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010; 7:74-80. [DOI] [PubMed] [Google Scholar]
- Brenyo A, Pietrasik G, Barsheshet A, Huang DT, Polonsky B, McNitt S, Moss AJ, Zareba W. QRS fragmentation and the risk of sudden cardiac death in MADIT II. J Cardiovasc Electrophysiol. 2012; 23:1343-1348. [DOI] [PubMed] [Google Scholar]
- Morita H, Kusano KF, Miura D, Nagase S, Nakamura K, Morita ST, Ohe T, Zipes DP, Wu J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008; 118:1697-1704. [DOI] [PubMed] [Google Scholar]
- Kang KW, Janardhan AH, Jung KT, Lee HS, Lee MH, Hwang HJ. Fragmented QRS as a candidate marker for high‐risk assessment in hypertrophic cardiomyopathy. Heart Rhythm. 2014; 11:1433-1440. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large U.S. Community. J Am Coll Cardiol. 2004; 44:1268-1275. [DOI] [PubMed] [Google Scholar]
- Havmoeller R, Reinier K, Teodorescu C, Uy‐Evanado A, Mariani R, Gunson K, Jui J, Chugh SS. Low rate of secondary prevention ICDs in the general population: multiple‐year multiple‐source surveillance of sudden cardiac death in the Oregon Sudden Unexpected Death Study. J Cardiovasc Electrophysiol. 2013; 24:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population‐based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two‐year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006; 47:1161-1166. [DOI] [PubMed] [Google Scholar]
- Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010; 159:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: population at risk. Can J Cardiol. 1990; 6:439-444. [PubMed] [Google Scholar]
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National Institutes of Health. Obes Res. 1998; 6Suppl 2:51S-209S. [PubMed] [Google Scholar]
- Bazett HC. An analysis of time relations of the electrocardiograms. Heart. 1920; 7:353-370. [Google Scholar]
- Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006; 47:362-367. [DOI] [PubMed] [Google Scholar]
- Teodorescu C, Reinier K, Uy‐Evanado A, Gunson K, Jui J, Chugh SS. Resting heart rate and risk of sudden cardiac death in the general population: influence of left ventricular systolic dysfunction and heart rate‐modulating drugs. Heart Rhythm. 2013; 10:1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh SS, Reinier K, Singh T, Uy‐Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009; 119:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006; 113:2495-2501. [DOI] [PubMed] [Google Scholar]
- Pietrasik G, Goldenberg I, Zdzienicka J, Moss AJ, Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol. 2007; 100:583-586. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007; 101:759-767. [DOI] [PubMed] [Google Scholar]
- Peters S, Trummel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm. 2008; 5:1417-1421. [DOI] [PubMed] [Google Scholar]
- Canpolat U, Kabakci G, Aytemir K, Dural M, Sahiner L, Yorgun H, Sunman H, Baris Kaya E, Baris Kaya L, Oto A. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol. 2013; 24:1260-1266. [DOI] [PubMed] [Google Scholar]
- Perlini S, Salinaro F, Cappelli F, Perfetto F, Bergesio F, Alogna A, Mussinelli R, Boldrini M, Raimondi A, Musca F, Palladini G, Merlini G. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol. 2013; 167:2156-2161. [DOI] [PubMed] [Google Scholar]
- Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013; 309:896-908. [DOI] [PubMed] [Google Scholar]
- Yan GH, Wang M, Yiu KH, Lau CP, Zhi G, Lee SW, Siu CW, Tse HF. Subclinical left ventricular dysfunction revealed by circumferential 2D strain imaging in patients with coronary artery disease and fragmented QRS complex. Heart Rhythm. 2012; 9:928-935. [DOI] [PubMed] [Google Scholar]
- Basaran Y, Tigen K, Karaahmet T, Isiklar I, Cevik C, Gurel E, Dundar C, Pala S, Mahmutyazicioglu K, Basaran O. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011; 28:62-68. [DOI] [PubMed] [Google Scholar]
- Yang XW, Hua W, Wang J, Liu ZM, Ding LG, Chen KP, Zhang S. Regression of fragmented QRS complex: a marker of electrical reverse remodeling in cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2015; 20:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amad KH, Brennan JC, Alexander JK. The cardiac pathology of chronic exogenous obesity. Circulation. 1965; 32:740-745. [DOI] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004; 18:1692-1700. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Nunez BD, Ventura HO, Snyder DW. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987; 147:1725-1728. [DOI] [PubMed] [Google Scholar]
- Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity–results of a meta‐analysis. Am Heart J. 2008; 155:310-315. [DOI] [PubMed] [Google Scholar]
- Terho HK, Tikkanen JT, Junttila JM, Anttonen O, Kentta TV, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Prevalence and prognostic significance of fragmented QRS complex in middle‐aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014; 114:141-147. [DOI] [PubMed] [Google Scholar]
- Torigoe K, Tamura A, Kawano Y, Shinozaki K, Kotoku M, Kadota J. The number of leads with fragmented QRS is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012; 59:36-41. [DOI] [PubMed] [Google Scholar]
- Ozcan F, Turak O, Canpolat U, Avci S, Tok D, Isleyen A, Cebeci M, Yuzgecer H, Gurel OM, Topaloglu S, Aras D, Basar FN, Aydogdu S. Fragmented QRS predicts the arrhythmic events in patients with heart failure undergoing ICD implantation for primary prophylaxis: more fragments more appropriate ICD shocks. Ann Noninvasive Electrocardiol. 2014; 19:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema A, Khalid A, Wimmer A, Bartone C, Chow T, Spertus JA, Chan PS. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm Electrophysiol. 2010; 3:339-344. [DOI] [PubMed] [Google Scholar]


