Abstract
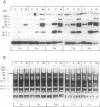
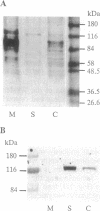
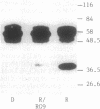
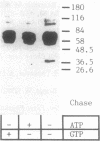
The fractionation of cells of a parsley suspension culture [Petroselinum crispum (Mill.) A. Hill] by protoplasting and subsequent removal of the vacuoles led to physiologically intact evacuolated protoplasts retaining light inducibility of chalcone synthase expression. Lysis of the evacuolated protoplasts permitted the isolation of a pure, highly concentrated cytosolic fraction containing major cytosolic membranes but only minor contamination by proplastids, mitochondria, and nuclei. Short-time irradiations of the cytosol with red or UV-containing white light resulted in very fast changes of the phosphorylation pattern of 18-, 40-, 48-, 55- to 70-, and 120-kDa proteins. Major differences were observed between the phosphorylation patterns obtained by red or UV-containing white light treatment, indicating a different primary action of the excited photoreceptors in vitro. Separation of the microsomal fraction from the cytosolic matrix established the localization of these proteins. Chase and photoreversibility experiments revealed that phytochrome in vitro regulates the phosphorylation of the 40-kDa protein by modifying a soluble cytosolic kinase/phosphatase system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M., Cashmore A. R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993 Nov 11;366(6451):162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Dec;82(4):1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992 Oct;17(10):408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Fallon K. M., Shacklock P. S., Trewavas A. J. Detection in Vivo of Very Rapid Red Light-Induced Calcium-Sensitive Protein Phosphorylation in Etiolated Wheat (Triticum aestivum) Leaf Protoplasts. Plant Physiol. 1993 Mar;101(3):1039–1045. doi: 10.1104/pp.101.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer H., Hahlbrock K., Schäfer E. A light-responsive in vitro transcription system from evacuolated parsley protoplasts. Plant J. 1994 Mar;5(3):437–449. doi: 10.1111/j.1365-313x.1994.00437.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Kaufman L. S. Transduction of Blue-Light Signals. Plant Physiol. 1993 Jun;102(2):333–337. doi: 10.1104/pp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini L., Vaeck M., van Montagu M. Conserved epitopes on plant H1 histones recognized by monoclonal antibodies. Eur J Biochem. 1989 Jan 2;178(3):779–787. doi: 10.1111/j.1432-1033.1989.tb14509.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Bowler C., Kern R., Chua N. H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993 Jun 4;73(5):937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Neumann D., Emmermann M., Thierfelder J. M., zur Nieden U., Clericus M., Braun H. P., Nover L., Schmitz U. K. HSP68--a DnaK-like heat-stress protein of plant mitochondria. Planta. 1993;190(1):32–43. doi: 10.1007/BF00195672. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Quail P. H. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Reymond P., Short T. W., Briggs W. R. Blue light activates a specific protein kinase in higher plants. Plant Physiol. 1992 Oct;100(2):655–661. doi: 10.1104/pp.100.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L. C., Biswal B., Song P. S. Protein phosphorylation in isolated nuclei from etiolated Avena seedlings. Effects of red/far-red light and cholera toxin. FEBS Lett. 1991 May 6;282(2):347–350. doi: 10.1016/0014-5793(91)80510-a. [DOI] [PubMed] [Google Scholar]
- Romero L. C., Lam E. Guanine nucleotide binding protein involvement in early steps of phytochrome-regulated gene expression. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1465–1469. doi: 10.1073/pnas.90.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. R., Song P. S. Phytochrome and protein phosphorylation. Photochem Photobiol. 1990 Jul;52(1):249–254. doi: 10.1111/j.1751-1097.1990.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Warpeha K. M., Hamm H. E., Rasenick M. M., Kaufman L. S. A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8925–8929. doi: 10.1073/pnas.88.20.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]