Abstract
Introduction
Several inhibitors of histone deacetylase have been shown to enhance chemotherapy induced apoptosis and reduce sarcoma tumor volume in preclinical models. We sought to determine the MTD, PK/PD, safety and toxicity of the histone deacetylase inhibitor (HDACi) abexinostat (PCI-24781) when administered with doxorubicin in patients with metastatic sarcomas.
Methods
Participants were enrolled in a standard 3+3 dose escalation phase I study design. Abexinostat was administered on days 1–5 with 75 mg/m2 of doxorubicin administered on day 4 of every 21 day cycle until disease progression, drug intolerance, or a cumulative lifetime dose of 450 mg/m2 of doxorubicin was reached. G-CSF support was provided at physician discretion on Arm A or provided to all participants in Arm B. 3–6 participants were initially administered abexinostat at 30 mg/m2 BID, and then subsequent cohorts were administered doses of 15, 45, or 60 mg/m2 BID. All patients without progressive disease after receiving a cumulative lifetime dose of 450 mg/m2 of doxorubicin were given the option to continue with abexinostat as a single agent until disease progression.
Results
22 participants (10 with prior tumor growth after doxorubicin therapy) were enrolled (6 in Arm A, 14 in Arm B), 20 were evaluable for DLT, and 17 were evaluable for radiologic response. In Arm A, participants were administered abexinostat at 15 or 30 mg/m2 BID. DLTs of grade 3 and 4 ANC were observed in two out of three participants dosed at 30 mg/m2 BID. Neither of these patients received G-CSF prophylaxis. In Arm B, participants were administered abexinostat at 30, 45, or 60 mg/m2 BID, all with mandated G-CSF support. Two DLTs were observed on the 60 mg/m2 BID dose (grade 3 infection and grade 4 thrombocytopenia). The pharmacokinetics of abexinostat was not affected by doxorubicin. HDAC activity, as measured by histone acetylation in PBMC, was maximally inhibited at 30 mg/m2 BID. In the 17 participants evaluable for radiologic response there was 1 PR, 9 SD, and 7 PD as best response, with 8 participants completing 5 cycles or more. 3 of those participants remain in SD as their last disease status when this abstract was submitted. 4 participants who continued on monotherapy remained in SD for a median of 9.8 weeks after completing doxorubicin. The most common toxicities were fatigue, thrombocytopenia, and anemia. No study related deaths were observed.
Conclusion
The MTD for abexinostat is 45 mg/m2 BID when administered on days 1–5 when doxorubicin is given at 75 mg/m2 on day 4 of a 3-week cycle and G-CSF support is mandated. Toxicities were manageable and tumor responses were seen. Additional studies are needed to further define the specific contributions of HDAC inhibition for patients receiving doxorubicin to treat metastatic sarcoma.
Introduction
Sarcoma is a heterogeneous family of cancers that arise in the body’s connective tissues which represent a diverse group of histologic subtypes, with 80% of soft tissue origin, and the remainder of bone origin1. There will be approximately 11,410 new cases of sarcoma this year in the United States, and 4,390 deaths1,2. The aggressive sarcomas frequently present or recur as metastatic or inoperable disease and are difficult to cure with conventional therapies. These tumors can have modest rates of response to doxorubicin, the current standard therapy for metastatic sarcomas, but more than one-half of treated patients are refractory at the onset and exhibit progressively lower rates of response with subsequent lines of therapy, leading to eventual and certain death3.
Available therapies for sarcomas include cytotoxic chemotherapy, given either as single agent or combined with one or more other agents, surgery and/or radiotherapy, as clinically appropriate4. Once patients with sarcoma recur or present with distant metastasis, the median survival is approximately 12 months5. Treatment with the most effective chemotherapeutic agents for sarcomas yields an objective response rate of 20–30%, with an average time-to-progression of less than six months. Most sarcomas, therefore, either do not respond or quickly develop resistance to chemotherapy. Several strategies have been used or are being explored to enhance cytotoxic therapies or to reverse drug resistance in other types of human cancers, include small molecule drugs or nanoparticles that target specific cellular signaling proteins or genes4. Among these targets are histone deacetylase (HDAC) inhibitors6–14. Classic HDAC inhibitors, such as vorinostat6 and valproate7 were observed to exert a growth inhibitory effect sarcoma cell lines. These HDAC inhibitors were also observed to sensitize fibrosarcoma11, osteosarcoma13, and chondrosarcoma14 cell lines to chemotherapy.
Abexinostat (PCI-24781) is a hydroxamic acid–based HDAC inhibitor that was developed based on in vivo efficacy and overall benign therapeutic index15. More direct evidence shows that abexinostat enhances cytotoxicity induced by doxorubicin in sarcoma cell lines. Yang et al. observed that combination abexinostat and doxorubicin is effective in inhibiting proliferation of sarcoma cell lines that are resistant to doxorubicin alone16,17. Lopez et al observed superior anti-proliferative effects when abexinostat is used in combination with chemotherapy18. When abexinostat was administered parenterally to tumor-bearing mice, significant tumor growth inhibition (~ 50–80%) was demonstrated at abexinostat doses of 20 to 80 mg/kg (60–240 mg/m2). A dose-escalation study in humans was designed to determine the MTD and elucidate the safety of abexinostat when given in combination with doxorubicin in patients with sarcoma.
Methods and Statistical Design
The study protocol was approved by the institutional review board of Dana Farber / Harvard Cancer Center and registered on clinicaltrials.gov under the NLM identifier NCT01027910. The treatment regimen consisted of abexinostat given orally twice daily for 5 days in combination with doxorubicin given intravenously on day 4 of every 21 day cycle. A starting dose of abexinostat at 30 mg/m2 BID was escalated or decreased in subsequent cohorts in increments of 15 mg/m2 to a maximum of 60 mg/m2 BID. Participants continued with combination doxorubicin and abexinostat up to a maximum of 6 cycles, until 450 mg/m2 lifetime cumulative dose of doxorubicin was administered.
Administration of abexinostat with doxorubicin was discontinued for the following reasons: Disease progression as determined RECIST 1.1, participant reaches cumulative lifetime anthracycline dose of 450 mg/m2, intercurrent illness that prevented further administration of study drug, DLT(s) during Cycle 1, unacceptable adverse event(s), participant decides to withdraw from the study, or general or specific changes in the participant’s condition with render the participant unacceptable for further administration of study drug in the judgment of the investigator. All patients (including those who discontinued early from protocol therapy) were followed for at least 30 days after removal from study or until death. Participants who were removed from study for unacceptable adverse events were followed until resolution or stabilization of the adverse event.
Study participants were enrolled into two arms. Arm A administered abexinostat and doxorubicin with optional G-CSF support. Arm B administered abexinostat and doxorubicin with required G-CSF support to all participants. The study uses the standard 3 + 3 phase I dose escalation design. Three cohorts of 3–6 participants were enrolled in each arm and separate inter-cohort dose escalations were performed in up to three cohorts of 3–6 participants enrolled sequentially until the maximum tolerated dose (MTD) of the combination abexinostat with doxorubicin, without (Arm A) or with (Arm B) mandatory G-CSF support was established.
Dose-limiting toxicities (DLT) were evaluated on Day 1 of Cycle 2, and included all adverse events experienced through Cycle 1. A DLT was defined as any of the following events occurring during cycle 1: grade 3 or 4 toxicities involving prolongation of the QTc interval; vomiting not controlled by maximal support care measures; thrombocytopenia lasting more than 7 days or associated with bleeding; or non-hematologic toxicity except for alopecia, nausea, or vomiting; grade 4 toxicities involving thrombocytopenia, neutropenia lasting more than 5 days on growth factors; or any other toxicities to cause failure to restart PCI-24781 administration within 2 weeks of first missed dose. Participants had to complete at least two cycles of abexinostat administration in order to be evaluable for response.
Participants removed from the study during cycle 1 for reasons other than a pre-defined DLT were replaced for the purpose of DLT evaluation. The algorithm-based design of the Phase I part of the study was specified because of its practical simplicity and not because of power considerations. A dose level was determined to be tolerable if none of the first 3 patients in that cohort experienced a DLT. A dose level was determined to have surpassed MTD if 2 or more of a cohort of 6 patients experienced a DLT.
Given that the study is at a specific cohort, the probabilities of declaring that dose level intolerable, given various true occurrence rates for DLTS, is presented in Table 1.
Table 1.
True Occurrence Rate of DLTs
| Declare Cohort: | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | 0.40 | 0.50 | 0.60 |
| Intolerable | 0.09 | 0.19 | 0.29 | 0.40 | 0.51 | 0.69 | 0.83 | 0.92 |
| Tolerable | 0.91 | 0.81 | 0.71 | 0.60 | 0.49 | 0.31 | 0.17 | 0.08 |
Blood samples for pharmacokinetics assessment were obtained and plasma was collected by centrifugation and shipped to Pharmacyclics for analysis. The plasma concentrations of abexinostat were determined by high-performance liquid chromatography (HPLC) with tandem mass spectrometric (MS/MS) using validated assays.
PBMC pellets for pharmacodynamics assessment were obtained from centrifugation of blood samples, frozen, and shipped to Pharmacyclics for analysis. Proteins from the frozen PBMC pellets were extracted by using Qiagen AllPrep. (Qiagen, Valencia, CA) Total protein (50 µg) was subjected to SDS-PAGE gel (Invitrogen, Grand Island, NY). The gel was then blotted, and acetylated histones, acetylated tubulin, total tubulin levels were detected by standard western blots. Acetylated histone western blots were performed by using rabbit anti-acetylated lysine antibody (Cell Signaling Technologies, Beverly, MA), mouse anti-acetylated tubulin antibody (Sigma-Aldrich, St. Louis, MO), mouse anti-alpha-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-actin (Cell Signaling Technology), and IR-DYE conjugated goat anti-mouse and IRDYE conjugated goat anti rabbit (LICOR, Lincoln, NE). Bands were imaged and quantified in the linear range using the Odyssey Infrared Imaging System (LICOR).
Results
This study was opened to accrual on January 28, 2010, and closed to accrual on April 16, 2012. 22 participants (10 with prior tumor growth after doxorubicin therapy) were enrolled, 20 were evaluable for DLT (6 in Arm A, 14 in Arm B), and 17 were evaluable for radiologic response (Figure 1) as patients who experienced a DLT were removed from the study before completing cycle 1. One patient withdrew consent two days after starting treatment, and therefore the patient’s data were removed from analysis.
Figure 1. Pharmacokinetics and Pharmacodynamics.
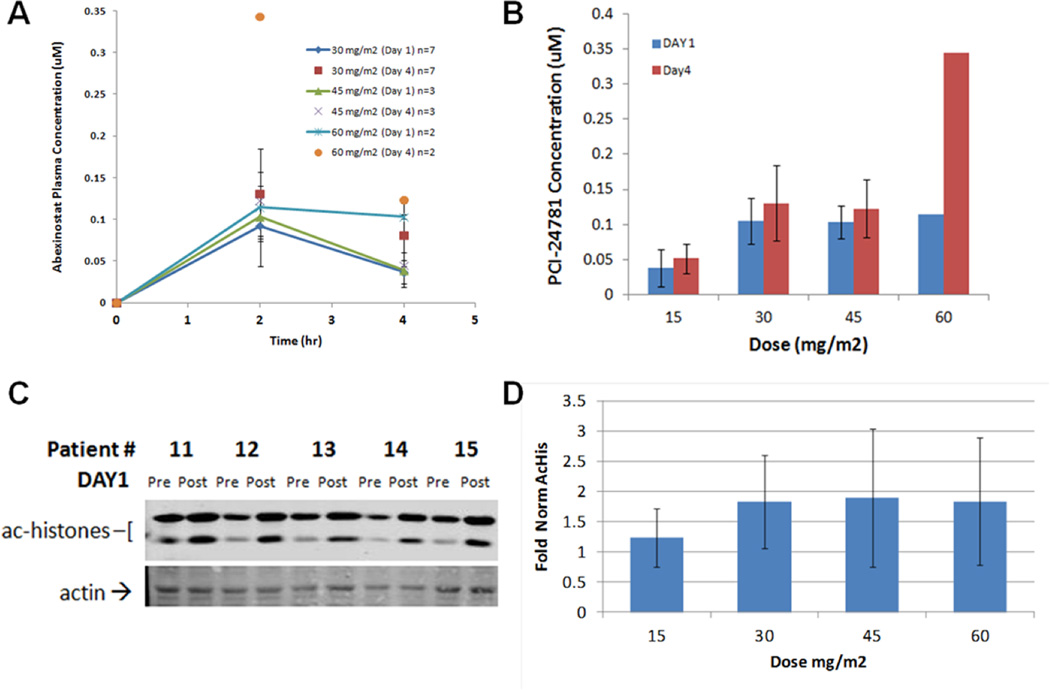
A & B: Pharmacokinetics of abexinostat. A: Time dependence of plasma levels on Day 1 and Day 4. B. Plasma levels at 2 hrs (near-maximal levels) compared at Day 1 and Day 4. The plasma concentrations at studied time-points on Day 1 were consistent with historical data; while Day 4 plasma levels appeared to increase, no significant conclusions could be made due to limited data collection.
C & D: Pharmacodynamics measured by histone acetylation in PBMC on Day 1. C. Exemplar Western blots of acetylated histones and actin at 4 hrs post-dose for five patients. D. Normalized acetyl-histone/actin levels showed maximal inhibition of HDAC activity was achieved at the 30 mg/m2 BID dose level, with little increase at higher doses. Pharmacodynamic responses were consistent with maximum plasma concentrations measured on Day 1.
Table 2 displays patient demographics and other characteristics at baseline. The median age was 54 years (range 21 to 77 years). 95.2% of subjects were white, 4.8% (1 subject) was in the category of other, and 12 (57.1%) were Male, while 9 (42.9%) were Female. Histologic subtype of tumor among subjects was distributed as follows: 1 subject (4.8%) with Liposarcoma, 5 (23.8%) with Leiomyosarcoma, 2 (9.5%) with Fibrosarcoma, 2 (9.5%) with Synovial sarcoma, and 11 (52.4%) with “Other”. 100% of participants had metastatic sarcoma.
Table 2.
Baseline Characteristics
| Characteristic | n=21 |
|---|---|
| Age years (median, range) | 54, 21–77 |
| Race | |
| White | 20 (95.2%) |
| Other | 1 (4.8%) |
| Ethnicity | |
| Not Hispanic or Latino | 19 (90.5%) |
| Other | 2 (9.5%) |
| Gender | |
| Male | 12 (57.1%) |
| Female | 9 (42.9%) |
| Institution | |
| DFCI | 13 (61.9%) |
| MGH | 8 (38.1%) |
| Performance Status | |
| 0 | 11 (52.4%) |
| 1 | 10 (47.6%) |
| Dose Level | |
| 0 | 3 |
| 1 | 7 |
| 2 | 6 |
| 3 | 5 |
| Histologic Subtype of Tumor | |
| Liposarcoma | 1 (4.8%) |
| Leiomyosarcoma | 5 (23.8%) |
| Fibrosarcoma | 2 (9.5%) |
| Synovial sarcoma | 2 (9.5%) |
| Other | 11 (52.4%) |
| Disease Status | |
| Metastatic sarcoma | 21 (100%) |
| Prior Doxorubicin Therapy | |
| No | 11 (52.4%) |
| Yes | 10 (47.6%) |
| Prior Doxorubicin Response among those with prior doxorubicin therapy (n=10) | |
| Response with Subsequent Relapse | 3 (30%) |
| Progressive Disease | 7 (70%) |
| Lifetime Cumulative Maximum Doxorubicin Dose among those with prior doxorubicin therapy (mg/m2) (mean (SD), median, range) | 121.6 (73.32), 97.5, 36–300 |
| Total number of prior chemotherapy regimens per patient (mean (SD), median, range) | 1.2 (1.0), 1, 0–5 |
Because HDAC inhibitors, as a class, are known to prolong QTc, ECGs were performed before and after abexinostat administration to measure its effects on QTc.
Table 3 shows results for Baseline and Treatment ECGs. Patients with QTc ≥ 450 msecs were excluded from the study, as were patients with other significant ECG abnormalities defined as 2nd degree AV block type II, 3rd degree AV block, or bradycardia (ventricular rate less than 55 beats/min)." Patients with any other ECG abnormalities that were determined to be minor and clinically insignificant were allowed to enroll. No clinically significant abnormalities were seen in this study. Compared with 81% of Baseline ECGs having a normal result, 62.7% of all pre-abexinostat ECGs had a normal result, 53.5% of all ECGs performed 1–2 hours after the first dose of abexinostat were normal, and 73.9% of all ECGs performed 1–2 hours after the second dose were normal. The mean pre-dose QTc Interval rose to 428 msec (from a baseline mean of 425 msec), 437 msec at 1–2 hours after the first dose, and 427 at 1–2 hours after the second dose.
Table 3.
Baseline and Treatment ECG
| n=21 | |
|---|---|
| Baseline | |
| Normal | 17 (81.0%) |
| Abnormal | 4 (19.0%) |
| 1–2 Hours Post first dose ECG, within cycle | |
| Normal | 54 (53.5%) |
| Abnormal | 47 (46.5%) |
| 1–2 Hour Post second dose ECG, within cycle | |
| Normal | 17 (73.9%) |
| Abnormal | 6 (26.1%) |
| Baseline QTc Interval (msec) (n, mean (SD), median, range) | 21, 425.1 (15.0), 423, 396–446 |
| Pre-dose QTc Interval (msec), performed in triplicate (n, mean (SD), median, range) | 133, 428.3 (18.1), 427, 388–474 |
| 1–2 Hour Post first dose QTc Interval (msec), within cycle (n, mean (SD), median, range) | 101, 436.5 (18.6), 438, 399–484 |
| 1–2 Hours Post second dose QTc Interval (msec), within cycle (n, mean (SD), median, range) | 23, 427.3 (18.8), 431, 391–458 |
A summary of grade 3 or higher treatment-related adverse events is given in Table 4 (counts by worst grade, out of all 21 treated patients). Four participants experienced DLTs, as protocol-defined. In Arm A, participants were administered abexinostat at 15 or 30 mg/m2 BID. DLTs of grade 3 and 4 neutropenia were observed in two out of three participants dosed at 30 mg/m2 BID. Neither of these patients received GCSF prophylaxis. No DLTs were observed in participants dosed at 15 mg/m2 BID. In Arm B, participants were administered abexinostat at 30, 45, or 60 mg/m2 BID, all with mandated GCSF support. Two DLTs were observed on the 60 mg/m2 BID dose (grade 3 infection and grade 4 thrombocytopenia). No DLTs were observed in participants dosed at 45 mg/m2 BID. Therefore the MTD of abexinostat when administered with 75mg/m2 of doxorubicin and with required G-CSF support is 45 mg/m2 BID.
Table 4.
Grade 3 or Higher Adverse Events
| Arm | |||||
|---|---|---|---|---|---|
| A | B | Total | |||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | ||
| Hemoglobin | 0 | 0 | 1 | 0 | 1 |
| Leukocytes | 2 | 0 | 1 | 0 | 3 |
| Lymphopenia | 1 | 0 | 3 | 0 | 4 |
| Neutrophils | 1 | 1 | 3 | 2 | 7 |
| Platelets | 0 | 0 | 1 | 2 | 3 |
| Alopecia | 0 | 0 | 1 | 0 | 1 |
| Diarrhea w/o prior colostomy | 0 | 0 | 1 | 0 | 1 |
| GI-other | 0 | 0 | 1 | 0 | 1 |
| Infection-Grade 0–2 Neutrophils, urinary tract | 0 | 0 | 1 | 0 | 1 |
| ALT, SGPT | 1 | 0 | 0 | 0 | 1 |
| Hyperglycemia | 0 | 0 | 1 | 0 | 1 |
| Tearing | 0 | 0 | 1 | 0 | 1 |
| Sexual/Reproductive function-other | 0 | 0 | 2 | 0 | 2 |
| Thrombosis/thrombus/embolism | 0 | 1 | 1 | 0 | 2 |
| Total | 5 | 2 | 18 | 4 | 29 |
The pharmacokinetics of abexinostat was not affected by doxorubicin. HDAC activity, as measured by histone acetylation in PBMC, was maximally inhibited at 30 mg/m2 BID (see Figure 1). In the 17 participants evaluable for radiologic response, a determination of SD as best response was made if the response at the end of the 2nd cycle showed SD unless the patient subsequently developed a PR. There were 1 PR, 9 SD, and 7 PD as best responses (Tables 5 and 6), with 8 participants completing 5 cycles or more (see Appendix 1). 4 participants who continued on monotherapy remained in SD for a median of 9.8 weeks after completing doxorubicin. The most common toxicities were fatigue, thrombocytopenia, and anemia. No study related deaths were observed.
Table 5.
Response
| Best Response | n=21 |
|---|---|
| Partial Response | 1 (4.8%) |
| Stable Disease | 9 (42.9%) |
| Progressive Disease | 7 (33.3%) |
| Unevaluable * | 4 (19.0%) |
Patients came off treatment prior to completing cycle 1 due to a Dose-Limiting Toxicity
Table 6.
Diagnosis and Response
| Case # |
Histology | # Prior Chemo Regimens |
Prior Doxorubicin? |
Response to Prior Doxorubicin |
Best Response |
Time to Disease Progression (Weeks) |
|---|---|---|---|---|---|---|
| 1 | Synovial sarcoma | 1 | Yes | Subsequent Relapse | Unevaluable* | |
| 2 | Other | 1 | Yes | Progressive Disease | PD | 3.3 |
| 3 | Fibrosarcoma | 2 | Yes | Progressive Disease | PD | 5.4 |
| 4 | Other | 0 | No | N/A | PD | 6 |
| 5 | Leiomyosarcoma | 0 | No | N/A | SD | 14.9 |
| 6 | Other | 1 | No | N/A | SD | 31.9 |
| 7 | Other | 1 | Yes | Subsequent Relapse | SD | 20.3 |
| 8 | Leiomyosarcoma | 1 | No | N/A | PR | 32.7 |
| 9 | Other | 1 | No | N/A | Unevaluable* | |
| 10 | Other | 1 | No | N/A | SD | 19.4 (censored, no progression) |
| 11 | Fibrosarcoma | 0 | No | N/A | SD | 20 |
| 12 | Other | 1 | No | N/A | PD | 5.6 |
| 13 | Other | 2 | Yes | Progressive Disease | SD | 6 |
| 14 | Synovial sarcoma | 1 | Yes | Subsequent Relapse | Unevaluable* | |
| 15 | Leiomyosarcoma | 0 | No | N/A | SD | 13.6 (censored, no progression) |
| 16 | Other | 5 | Yes | Progressive Disease | SD | 13 |
| 17 | ** | |||||
| 18 | Other | 1 | Yes | Progressive Disease | PD | 6 |
| 19 | Leiomyosarcoma | 1 | Yes | Progressive Disease | Unevaluable* | |
| 20 | Liposarcoma | 1 | No | N/A | SD | 23.9 (censored, no progression) |
| 21 | Other | 1 | Yes | Progressive Disease | PD | 5.9 |
| 22 | Leiomyosarcoma | 1 | No | N/A | PD | 7.3 |
Patient came off treatment prior to completing Cycle 1 due to a Dose-Limiting Toxicity.
Patient’s data removed from analysis as patient withdrew consent 2 days after starting treatment.
Discussion
Acetylation and deacetylation of histones by HDACs and HATs (histone acetyltransferases) modulate chromatin structure and regulate gene transcription19. HDAC inhibitors are being developed as anticancer drugs because of their ability to inhibit tumor cell growth, induce differentiation, and lower apoptotic threshold in transformed cells20. HDAC inhibitors can increase accessibility of cytotoxic agents to DNA by altering chromatin structure, thereby allowing DNA alkylating and intercalating agents to interact with DNA more directly. HDAC inhibition has been shown in vitro to enhance the toxicity of several anticancer drugs that target DNA (e.g., etoposide, doxorubicin, cisplatin)16–18. Several studies suggest that inhibition of DNA repair may also be the key mechanism for treatment sensitization by some HDAC inhibitors21. Abexinostat has been observed to downregulate several enzymes required for DNA repair22. Preclinical results have established growth inhibitory concentrations for abexinostat in several tumor cell lines, as well as tumor growth inhibition in three xenograft models using different treatment schedules15.
Abexinostat is currently undergoing testing for safety, tolerability, and pharmacokinetics in several phase I and II trials. This is the first study to evaluate the clinical parameters of abexinostat when administered to patients in combination with doxorubicin. We found that the MTD of abexinostat and doxorubicin was significantly higher when patients were supported by GCSF. Interestingly, maximal histone acetylation was seen at 30 mg/m2 dose levels, and the administration of doxorubicin did not appear to alter this pharmacokinetic profile. Fortunately, the MTD of abexinostat and doxorubicin exceeded doses for maximal histone acetylation, so the effectiveness of this combination would not be limited by inadequate dosing. We observed clinical benefit of abexinostat and doxorubicin (7 of 17 evaluable participants maintained stable disease for at least 5 cycles of chemotherapy) in this cohort of 21 sarcoma patients that also included 10 patients who had previously developed disease progression on prior treatment with doxorubicin. However, in this nonrandomized and uncontrolled study, the sample size is too small to determine if these participants would have enjoyed the same rate of clinical benefit with doxorubicin alone. Fortunately, we did not observe any significant QTc abnormalities or any other life-threatening complications that could be attributed to abexinostat or otherwise unexplained or unexpected from patients receiving doxorubicin alone.
In conclusion, the MTD for abexinostat is 45 mg/m2 BID when administered on days 1–5 when doxorubicin is given at 75 mg/m2 on day 4 of a 3 week cycle and G-CSF support is required at this dose and schedule. This phase I study serves as the clinical basis to perform a more extensive phase II or III study to better define the contributions of abexinostat to the clinical benefit for sarcoma patients receiving doxorubicin.
Acknowledgements
This research was supported by funds from Pharmacyclics, Inc. Additionally, the study was also supported by funding from the Ludwig Institute for Cancer Research, Jennifer Hunter Yates Foundation, Brian MacIsaac Sarcoma Foundation, the KL2 Medical Research Investigator Training (MeRIT) grant awarded to E.C. through Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Institutes of Health grant 1KL2RR025757), and the National Cancer Institute of the National Institutes of Health under Award Number U54CA168512. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix 1
Treatment Modifications Detail View
| PCI-24781 Dose Levels: 0 = 30 mg/m2 1 = 60 mg/m2 2 = 90 mg/m2 3 = 120 mg/m2 |
Doxorubicin Dose Levels: 1 = 75 mg/m2 2 = 60mg/m2 3 = 45 mg/m2 |
||||
|---|---|---|---|---|---|
| PCI-24781 Treatment | Doxorubicin Treatment | ||||
| Dose Level |
Dose Modifications |
Number of cycles |
Dose Level |
Dose Modifications |
Number of cycles |
| 0 | No modifications | 2 | 1 | No modifications | 3 |
| 0 | No modifications | 5 | 1 | No modifications | 5 |
| 0 | No modifications | 10 | 1 | No modifications | 6 |
| 1 | No modifications | 1 | 1 | No modifications | 1 |
| 1 | 1 dose withheld | 1 | 1 | No modifications | 1 |
| 1 | No modifications | 2 | 1 | No modifications | 2 |
| 1 | 1 dose modifcation to dose level 0, 10 doses withheld | 6 | 1 | No modifications | 4 |
| 1 | 3 doses withheld | 10 | 1 | No modifications | 6 |
| 1 | No modifications | 1 | 1 | No modifications | 1 |
| 1 | 1 dose modification to dose level 0 | 6 | 1 | 1 dose modification to dose level 2 | 6 |
| 2 | No modifications | 6 | 1 | No modifications | 6 |
| 2 | 1 dose modification | 3 | 1 | 1 dose modification | 3 |
| 2 | 1 dose missed | 2 | 1 | No modifications | 2 |
| 2 | No modifications | 7 | 1 | No modifications | 6 |
| 2 | 1 dose withheld | 2 | 1 | No modifications | 2 |
| 2 | No modifications | 2 | 1 | No modifications | 2 |
| 3 | No modifications | 1 | 1 | No modifications | 1 |
| 3 | 9 doses withheld and 1 dose modification to dose level 2 | 5 | 1 | 2 doses withheld and 1 dose modification in dose level 2 | 5 |
| 3 | No modifications | 4 | 1 | No modifications | 4 |
| * | |||||
| 3 | No modifications | 2 | 1 | No modifications | 2 |
| 3 | No modifications | 1 | 1 | No modifications | 1 |
| Summary: |
| Dose Level 0: No Modifications for 3 subjects |
| Dose Level 1: Modifications/holds for 4 out of 7 subjects |
| Dose Level 2: Modifications/holds for 3 out of 6 subjects |
| Dose Level 3: Modifications/holds for 1 out of 5 subjects |
Patient’s data removed from analysis as patient withdrew consent 2 days after starting treatment.
Footnotes
Author Disclosures: E. Choy: Amgen. S. Balasubramanian: Pharmacyclics. J. Butrynski: None. J. Morgan: None. M. Sirisawad:; Pharmacyclics. C. Mani:; Pharmacyclics. S. George: None. A. Wagner: None. D. Harmon: None. Y. Rubinstein: None. Z. Duan: None. F. Hornicek: None. G. Demetri:; Novartis.; Pfizer.; Sanofi-Aventis; Johnson & Johnson; Merrimack Pharma; Foundation Medicine; Merck; Ariad; ZioPharm; Glaxo-Smith-Kline.; Koltan Pharmaceuticals; Blueprint Medicines; G-1 Therapeutics; Champions Biotechnology; N-of-One
References
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Ehnert S, Zhao J, Pscherer S, et al. Transforming growth factor beta1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 10:101. doi: 10.1186/1741-7015-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003;(3):CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 38(Suppl 3):S19–S29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17(1):150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 6.Hrzenjak A, Moinfar F, Kremser ML, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol Cancer. 9:49. doi: 10.1186/1476-4598-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hrzenjak A, Moinfar F, Kremser ML, et al. Valproate inhibition of histone deacetylase 2 affects differentiation and decreases proliferation of endometrial stromal sarcoma cells. Mol Cancer Ther. 2006;5(9):2203–2210. doi: 10.1158/1535-7163.MCT-05-0480. [DOI] [PubMed] [Google Scholar]
- 8.Lubieniecka JM, de Bruijn DR, Su L, et al. Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer Res. 2008;68(11):4303–4310. doi: 10.1158/0008-5472.CAN-08-0092. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco M, Nielsen TO. Histone deacetylase 1 and 2 in mesenchymal tumors. Mod Pathol. 25(2):222–230. doi: 10.1038/modpathol.2011.157. [DOI] [PubMed] [Google Scholar]
- 10.Sakimura R, Tanaka K, Yamamoto S, et al. The effects of histone deacetylase inhibitors on the induction of differentiation in chondrosarcoma cells. Clin Cancer Res. 2007;13(1):275–282. doi: 10.1158/1078-0432.CCR-06-1696. [DOI] [PubMed] [Google Scholar]
- 11.Sampson ER, Amin V, Schwarz EM, O'Keefe RJ, Rosier RN. The histone deacetylase inhibitor vorinostat selectively sensitizes fibrosarcoma cells to chemotherapy. J Orthop Res. 29(4):623–632. doi: 10.1002/jor.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell Death Differ. 2005;12(1):10–18. doi: 10.1038/sj.cdd.4401507. [DOI] [PubMed] [Google Scholar]
- 13.Wittenburg LA, Bisson L, Rose BJ, Korch C, Thamm DH. The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemother Pharmacol. 67(1):83–92. doi: 10.1007/s00280-010-1287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, Tanaka K, Sakimura R, et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 2008;28(3A):1585–1591. [PubMed] [Google Scholar]
- 15.Buggy JJ, Cao ZA, Bass KE, et al. CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther. 2006;5(5):1309–1317. doi: 10.1158/1535-7163.MCT-05-0442. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Choy E, Hornicek FJ, et al. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother Pharmacol. 67(2):439–446. doi: 10.1007/s00280-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Choy E, Hornicek FJ, et al. Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Res. 31(4):1115–1123. [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez G, Liu J, Ren W, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15(10):3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 19.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 20.Cote GM, Choy E. Role of Epigenetic Modulation for the Treatment of Sarcoma. Curr Treat Options Oncol. doi: 10.1007/s11864-013-0239-3. [DOI] [PubMed] [Google Scholar]
- 21.Karagiannis TC, Kn H, El-Osta A. The epigenetic modifier, valproic acid, enhances radiation sensitivity. Epigenetics. 2006;1(3):131–137. doi: 10.4161/epi.1.3.2896. [DOI] [PubMed] [Google Scholar]
- 22.Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci U S A. 2007;104(49):19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]


