Abstract
Lack or downregulation of the dopamine D2 receptor (D2R) increases the vulnerability to renal inflammation independently of blood pressure in mice. Common single nucleotide polymorphisms (SNPs) rs 6276, 6277 and 1800497 in the human D2R gene are associated with decreased receptor expression/function, and hypertension. Human renal proximal tubule cells from subjects carrying these SNPs have decreased D2R expression and increased expression of pro-fibrotic factors and production of extracellular matrix proteins. We tested the hypothesis that the D2R mediates these effects by regulating microRNA expression. In cells carrying D2R SNPs, microRNAs (miRs)-217, -224, -335 and -1265 were downregulated while miR-1290 was upregulated more than 4-fold compared with those carrying D2R wild-type (WT) alleles. However, only miR-217 was directly regulated by D2R expression. In cells carrying D2R WT, miR-217 inhibitor increased the expression of TGFβ1, MMP3, FN1, and Col1a while miR-217 mimic had the opposite effect. In cells carrying D2R SNPs, miR-217 mimic also decreased the expression of TGFβ1 and its targets. Wnt5a, a miR-217 target, was increased in cells carrying D2R SNPs, and decreased by miR-217 mimic but increased by miR-217 inhibitor in both cell types. In cells carrying D2R WT, Wnt5a treatment increased TGFβ1 while silencing Ror2, a Wnt5a receptor, decreased TGFβ1 and blunted the Wnt5a-induced increase in cells carrying D2R WT. Our results show that renal proximal tubule cells from subjects carrying D2R SNPs resulting in D2R downregulation have increased TGFβ1 that is mediated by decreased regulation of the miR-217-Wnt5a-Ror2 pathway.
Keywords: renal proximal tubule cell, dopamine D2 receptor, microRNA, TGFβ1, Wnt5a
Introduction
Besides its actions in the maintenance of normal renal function and blood pressure the renal dopaminergic system has remarkable effects in the regulation of inflammatory reaction and tissue injury (1,2). In mice, intrarenal dopamine (DA) deficiency leads to increased infiltration of inflammatory cells and oxidative stress (3). Conversely, increased intrarenal DA prevents the increase in the expression of profibrotic pathways and decreases glomerular and tubular interstitial injury resulting from chronic angiotensin Ⅱ (Ang Ⅱ) infusion (4). In diabetic mice, increased intrarenal DA levels decreased markers of oxidative stress, inhibited macrophage infiltration, and reduced renal damage whereas decreased intrarenal DA production had the opposite effect (5).
The protective effects of DA on the development of renal inflammation and tissue damage are mediated, at least in part, by the dopamine D2 receptor (D2R) that acts as a negative regulator of inflammation (1,6). Mice with lack or reduced level of D2R expression and function in the kidney have increased renal expression of pro-inflammatory and decreased expression of anti-inflammatory cytokines/chemokines, as well as histological and functional evidence of renal inflammation and injury, indicating that impaired function of the D2R results in renal inflammation and organ damage (1). The protective effects of D2R are not restricted to renal inflammation. Lack of D2R expression in astrocytes results in a marked inflammation in multiple brain regions and treatment of Drd2 wild-type (WT) mice with a selective D2R agonist partially suppressed 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated inflammation in nigral dopaminergic neurons (7).
In humans, the DRD2 gene is highly polymorphic. The presence of some common single nucleotide polymorphisms (SNPs; rs 6276, rs6277, and rs1800497) in the DRD2 gene results in decreased D2R expression and function attributable to decreased D2R mRNA stability and synthesis of the receptor, and are associated with elevated blood pressure and even hypertension (6,8,9). We have shown that renal proximal tubule cells (RPTCs) of subjects bearing these SNPs have decreased expression of the D2R, express a proinflammatory phenotype with increased TNFα, cytokines, and chemokines and a profibrotic phenotype with increased TGFβ1, markers of epithelial mesenchymal transition (EMT) such as vimentin and snail, and synthesize the extracellular matrix proteins collagen 1a (Col1a) and fibronectin 1 (FN1) (6). These alterations were reproduced when RPTCs carrying the DRD2 WT gene were treated with TGFβ1 (6). These results suggest that subjects bearing these D2R SNPS may have increased susceptibility to chronic kidney disease. Consistent with our data, a study in an Asian Indian population with type 2 diabetes found that a D2R polymorphism (rs1799732) resulting in decreased expression of the receptor, conferred susceptibility to chronic diabetic nephropathy (10).
MicroRNAs (miRNAs) are short single-stranded RNAs that post-transcriptionally repress gene expression via degradation or translational inhibition of their target mRNAs. Several miRNAs are associated with the regulation of chemokines, fibrosis, extracellular matrix remodeling, and cell adhesion (11,12). We hypothesized that regulation of miRNA expression is one of the mechanisms involved in the protective effects of D2R. To test this hypothesis we studied miRNA expression in RPTCs with or without D2R SNPs.
Materials and methods
Cells
RPTCs were isolated from human kidney specimens from patients who had unilateral nephrectomy due to renal carcinoma or trauma. Only the visually and histologically normal pole, distal from the affected part of the kidney, was used to isolate RPTCs and immortalized, as previously described (13). A University of Virginia institutional review board-approved protocol was used according to the Declaration of Helsinki. The RPTCs were genotyped for the presence of rs6276, rs6277, and rs1800497 SNPs. Four cell lines from subjects not carrying SNPs (D2R-WT), 3 from subjects bearing rs6276 and rs6277 alleles, and 2 from subjects bearing rs6276 and rs1800497 alleles were studied. These 3 SNPs occur within and adjacent to the DRD2 gene and because of their close proximity are likely to be in linkage disequilibrium which limits the possibility of identifying cell lines bearing only one of the SNPs. The cell lines carrying SNPs were termed RPTC-D2R-SNPs and those not carrying SNPs were called RPTC-D2R-WT. These quences, chromosomal loci, and frequencies of these D2R-SNPs are shown in Table S1 and Figure S1.
Transfections and assays
Cells were studied untransfected and transfected with plasmid harboring human DRD2 WT, miScript miRNA mimics, inhibitors targeting hsa-miR-217 or hsa-miR-224-5p, or siRNA targeting human DRD2 or Ror2 as described in Supplemental Methods.
Cells were collected and lysates were prepared for purification of miRNA and total RNA for miRNA expression profiling and quantitative real-time PCR as described in Supplemental Methods and Table S2. Cell lysates were also prepared for protein extraction and immunoblotting (Supplemental Methods)
miRNA expression profiling
The miRNA expression profiling in human RPTCs in both groups was carried out using a human miScript miRNA PCR Array (SABiosciences-Qiagen) which profiles the expression of the 372 most abundantly expressed miRNAs. Real-time PCR was performed following the manufacturer's protocol. Quality controls were all within the recommended range and the data were analyzed using the manufacturer's software.
Immunofluorescence and confocal microscopy
Human RPTCs, grown on poly-d-lysine-coated coverslips to 50-70% confluence, were immunostained using a rabbit polyclonal anti-TGFβ1 antibody (Santa Cruz), followed by Alexa Fluor 488 goat anti-rabbit IgG antibody (Molecular Probes, Grand Island, NY). DAPI was used to visualize the nuclei.
Statistical analysis
The data are expressed as mean±SEM. Comparisons between 2 groups used the Student's t test. One-way factorial ANOVA followed by Holm-Sidak test was used to assess significant differences in more than two groups. P<0.05 was considered significant.
Results
Presence of D2R SNPs increases expression of TGFβ1 and its target genes in human RPTCs
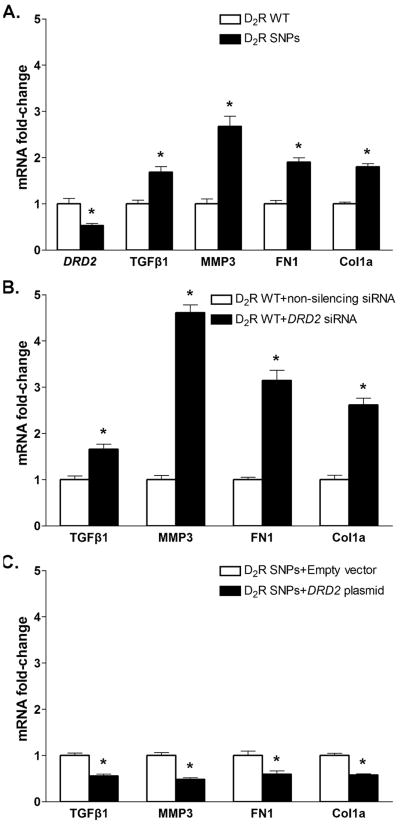
The expression of D2R mRNA was lower in RPTC-D2R-SNPs than in RPTC-D2R-WT (Figure 1A), in agreement with our previous report (6). Because the degree of reduction in D2R expression was similar in RPTCs bearing the rs6276 and rs6277 alleles and those bearing the rs6276 and rs1800497 alleles (6), the samples were pooled for further analysis. The mRNA expression of TGFβ1 and the EMT markers MMP3, FN1, and Col1a was increased in RPTC-D2R-SNPs compared with RPTC-D2R-WT (Figure 1A). Decreasing D2R expression in RPTC-D2R-WT with DRD2 siRNA downregulated D2R mRNA and protein (Figure S2) and increased the expression of TGFβ1, MMP3, FN1 and Col1a (Figure 1B). Conversely, increasing D2R expression in RTPC-D2R-SNPs by transfection of a DRD2 plasmid which increased D2R mRNA expression and protein (Figure S2) decreased the mRNA expression of TGFβ1, MMP3, FN1 and Col1a (Figure 1C). These effects are observed in the absence of ligand added to the medium because both RPTCs and microdissected proximal tubules, in culture, produce significant amounts of dopamine from L-DOPA present in the serum added to the medium (14) and the endogenous D2R is constitutively active (15).
Figure 1.
Expression of TGFβ1 and the EMT markers MMP3, FN1, and Col1a in human renal proximal tubule cells (RPTCs) from subjects carrying DRD2 wild-type (D2R-WT) or D2R-SNPs.
A. mRNA expressions of DRD2, TGFβ1, MMP3, FN1, and Col1a in RPTC-D2R-WT or RPTC-D2R-SNPs were quantified by qRT-PCR; GAPDH mRNA was used for normalization of the data, n = 4-5/ group. *P< 0.05 vsD2R-WT, t-test.
B. mRNA expressions of TGFβ1, MMP3, FN1, and Col1a in RPTC-D2R-WT after siRNA mediated DRD2 silencing were quantified as above, n = 4/group. * P< 0.05 vs non-silencing siRNA, t-test.
C. mRNA expressions of TGFβ1, MMP3, FN1, and Col1a in RPTC-D2R-SNPs after transfection of a DRD2 plasmid were quantified as above, n = 5/group. * P< 0.05 vs empty vector, t-test.
Several miRNAs are differentially expressed in RPTC-D2R-SNPs
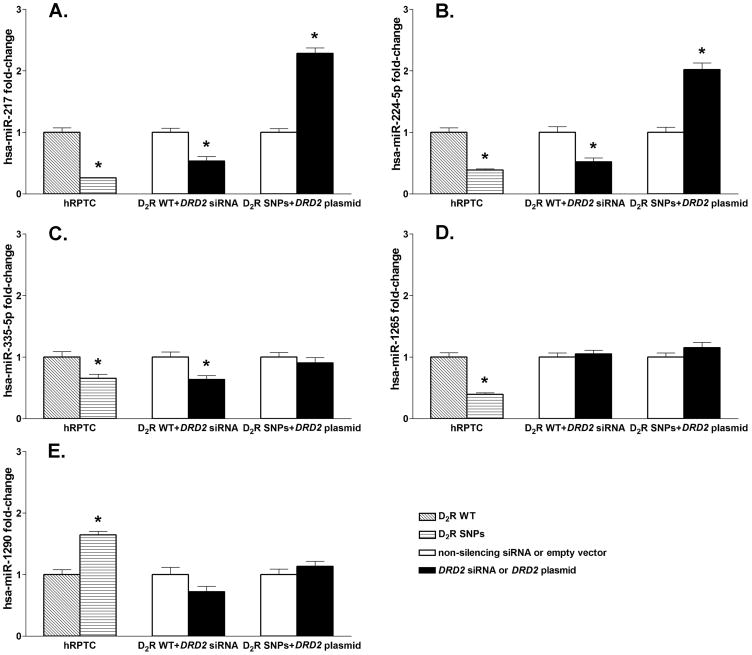
To determine the mechanism underlying the effects of the D2R SNPs, we studied the expression of miRNAs that are associated with the transcriptional regulation of target genes. The expression of 372 miRNAs was analyzed in RPTC-D2R-WT and RPTC-D2R-SNPs using a qRT-PCR array. Fifty miRNAs were differentially expressed by more than 2-fold in RPTC-D2R-SNPs in comparison with RPTC-D2R-WT; 36 miRNAs were downregulated and 14 were upregulated (Table S3). The expressions of 4 miRNAs (miR-217, miR-224-5p, miR-335-5p, and miR-1265) were downregulated more than 4-fold and that of one miRNA (miR-1290) was upregulated also more than 4-fold in RPTC-D2R-SNPs compared with RPTC-D2R-WT. These miRNAs were selected for further experiments.
D2R function regulates the expression of miR-217 and miR-224-5p
The decreased expression of miR-217 (Figure 2A), miR-224-5p (Figure 2B), miR-335-5p (Figure 2C), and miR-1265 (Figure 2D) and increased expression of miR-1290 (Figure 2E) in RPTC-D2R-SNPs was confirmed by qRT-PCR of the miRNA expression. To determine the effects of D2R expression/function on the expression of the selected miRNAs, we downregulated D2R expression in RPTC-D2R-WT by treatment with DRD2 siRNA and upregulated its expression in RPTC-D2R-SNPs by transfection of a DRD2 plasmid. D2R downregulation in RPTC-D2R-WT decreased the expression of miR-217, miR-224-5p, and miR-335-5p mimicking the effect of the presence of D2R SNPs but had no significant effect on the expression of miR-1265 or miR-1290. Increasing D2R expression in RPTC-D2R-SNPs increased miR-217 and miR-224-5p expression but had no effect on the expression miR-335-5p, miR-1265, and miR-1290. These results indicate that miR-217 and miR-224-5p but not miR-335-5p, miR-1265, and miR-1290 are regulated by D2R expression/function.
Figure 2.
Expressions of miR-217 (A), miR-224-5p (B), miR-335-5p (C), miR-1265 (D), and miR-1290 (E) in human renal proximal tubule cells (RPTCs) from subjects carrying (D2R-WT) or D2R-SNPs. miRNA expression was measured in RPTC-D2R-WT, RPTC-D2R-SNPs, RPTC-D2R-WT transfected with DRD2 siRNA, and RPTC-D2R-SNPs transfected with DRD2 WT plasmid. Rnu6-2 was used for normalization of the data; n=4-5/group. *P<0.05 vs corresponding control, t-test.
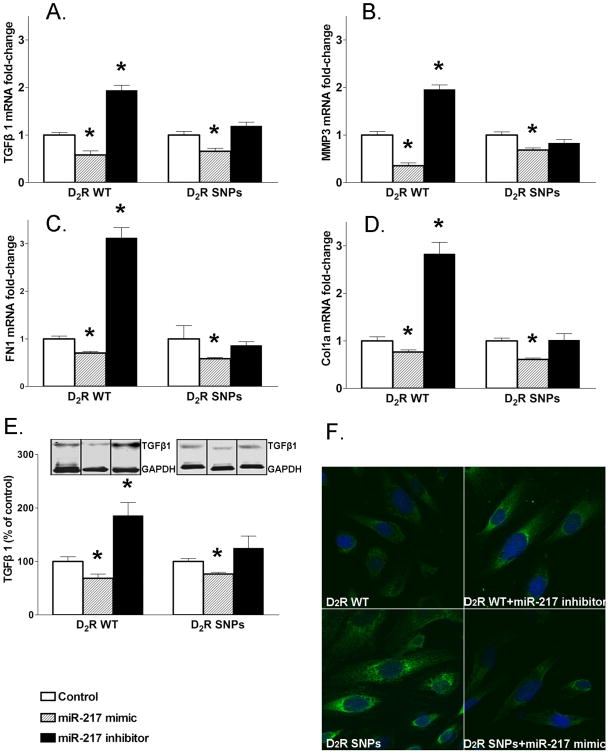
miR-217 regulates the expression of TGFβ1 and EMT markers
To determine whether or not miR-217 and miR-224-5p are involved in the phenotypic alterations of RPTC-D2R-SNPs, we treated RPTC-D2R-WT and RPTC-D2R-SNPs with mimics and inhibitors of both miRNAs. Treatment of RPTC-D2R-SNPs with miR-217 mimic increased miR-217 expression (Figure S3) and decreased the expression of TGFβ1 and EMT markers MMP3, FN1, and Col1a and decreased TGFβ1 immunofluorescence signal (Figures 3A-F). Treatment of RPTC-D2R-WT with miR-217 mimic also decreased the expression of TGFβ1 and EMT markers MMP3, FN1, and Col1a. Treatment with miR-217 inhibitor decreased miR-217 expression (Figure 3S) and increased expression of TGFβ1 and EMT markers MMP3, FN1, and Col1a and increased TGFβ1 immunofluorescence signal in RPTC-D2R-WT (Figures 3A-F), but had no effect in RPTC-D2R-SNPs (Figure 3A-E), probably because miR-217 is already decreased in RPTC-D2R -SNPs (Figure 2A, Table S3).
Figure 3.
Effect of miR-217 on the expression of TGFβ1 and EMT markers in human renal proximal tubule cells (RPTCs) from subjects carrying D2R-WT or D2R-SNPs.
A-D. mRNA expressions of TGFβ1, MMP3, FN1,and Col1a in RPTC-D2R-WT or RPTC-D2R-SNPs before and after transfection of miR-217 mimic or inhibitor were quantified by qRT-PCR as above, n=4-5/group. *P<0.05 vs control; ANOVA followed by Holm-Sidak test.
E. Protein expression of TGFβ1 in RPTC-D2R-WT or RPTC-D2R-SNPs treated as above of the data. n=4-5/group. *P<0.05 vs control; ANOVA followed by Holm-Sidak test
F. Laser-scanning confocal images of immunofluorescence staining of TGFβ1 in RPTC-D2R-WT treated with control or miR-217 inhibitor and in RPTC-D2R-SNPs treated with control or miR-217 mimic. DAPI was used to immunostain the nuclei.
By contrast, treatment of RPTC-D2R-WT or RPTC-D2R-SNPs with miR-224-5p mimic or inhibitor had no significant effect on TGFβ1 and EMT markers (Figure S4) although the mimic increased miR-224-5p and the inhibitor decreased miR-224-5p expression (Figure S3). These results indicate that the effects of D2R SNPs on TGFβ1 and the EMT markers are mediated by miR-217 but not by miR-224-5p.
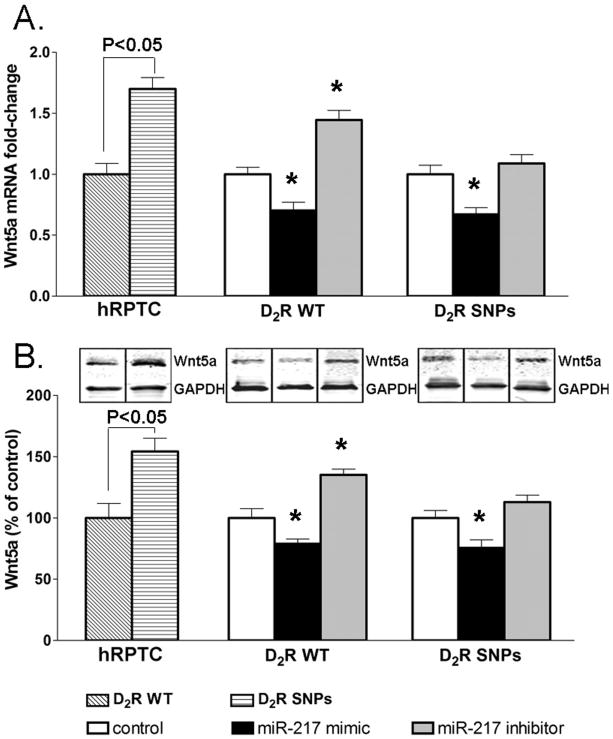
Wnt 5a mediates the miR-217 effects onTGFβ1 and EMT markers
To elucidate the mechanism by which miR-217 regulates the expression of TGFβ1 and its target genes, we used TargetScanHuman6.2 to predict miR-217 targets. This analysis showed Wnt5a as one of the targets of miR-217. Consistent with the decreased miR-217 expression in RPTC-D2R-SNPs in comparison with RPTC-D2R-WT, we found increased Wnt5a mRNA and protein expression in RPTC-D2R-SNPs. In RPTC-D2R-WT, treatment with miR-217 mimic decreased while miR-217 inhibitor increased Wnt5a mRNA and protein expression (Figure 4A-B). In RPTC-D2R-SNPs, miR-217 mimic also decreased the mRNA and protein expression of Wnt5a but transfection of miR-217 inhibitor had no significant effect (Figure 4A-B), similar to those obtained for TGFβ1 and EMT markers, probably because the expression of miR-217 is already decreased in these cells (Figure 2A, Table S3), as indicated earlier. These results indicate that Wnt5a is negatively regulated by miR-217.
Figure 4.
Effect of miR-217 on Wnt5a expression in human renal proximal tubule cells (RPTCs) from subjects carrying D2R-WT or D2R-SNPs before and after treatment with miR-217 mimic or inhibitor.
A-B. mRNA (A) and protein (B) expression of Wnt5a in RPTCs. mRNA expression was quantified by qRT-PCR, protein expression was measured by immunoblotting, GAPDH mRNA or protein was used for normalization of the data, n=4-5/group. *P<0.05 vs corresponding controls; ANOVA followed by Holm-Sidak test.
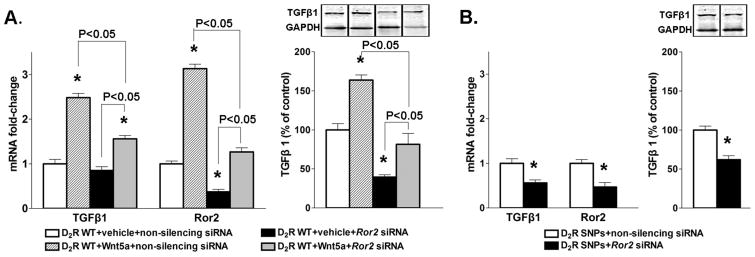
To determine if Wnt5a mediates the inhibitory effects of miR-217 on TGFβ1 we treated RPTC-D2R-WT with human recombinant Wnt5a and determined the expression of TGFβ1. Both mRNA and protein expressions of TGFβ1 were increased in cells treated with Wnt5a (Figure 5A). Wnt5a signals though the receptor tyrosine kinase-like orphan receptor 2 (Ror2) activating the non-canonical Wnt pathway (16). In RPTC-D2R-WT, the mRNA expression of Ror2 was increased by treatment with Wnt5a and decreased by treatment with Ror2 siRNA; treatment with both Wnt5a and Ror2 siRNA blunted the increase in Ror2 expression elicited by Wnt5a demonstrating that Wnt5a positively regulates the expression of Ror2 (Figure 5A). Silencing Ror2 decreased TGFβ1 expression and blunted significantly the increase induced by treatment with Wnt5a (Figure 5A). In RPTC-D2R-SNPs which have increased expression of Wnt5a silencing Ror2 decreased the expression of both Ror2 and TGFβ1 (Figure 5B) indicating that the stimulatory effect of D2R SNPs on TGFβ1 is mediated by the Wnt5a-Ror2 pathway.
Figure 5.
Effect of Wnt5-Ror2 pathway onTGFβ1 in human renal proximal tubule cells (RPTCs) from subjects carrying D2R-WT or D2R SNPs.
A. mRNA expressions of TGFβ1 and Ror2 and protein expression of TGFβ1 in RPTC-D2R-WT treated with Wnt5a or Ror2 siRNA or the combination were quantified as above. GAPDH mRNA or protein was used for normalization of the data, n=4-5/group. *P<0.05 vs treatment with vehicle and non-silencing siRNA. ANOVA followed by Holm-Sidak test.
B. mRNA expressions of TGFβ1 and Ror2 and protein expression of TGFβ1 in RPTC-D2R-SNPs treated with Ror2 siRNA were quantified as above. GAPDH mRNA or protein was used for normalization of the data, n=5/group. *P<0.05 vs non-silencing siRNA. t-test.
Discussion
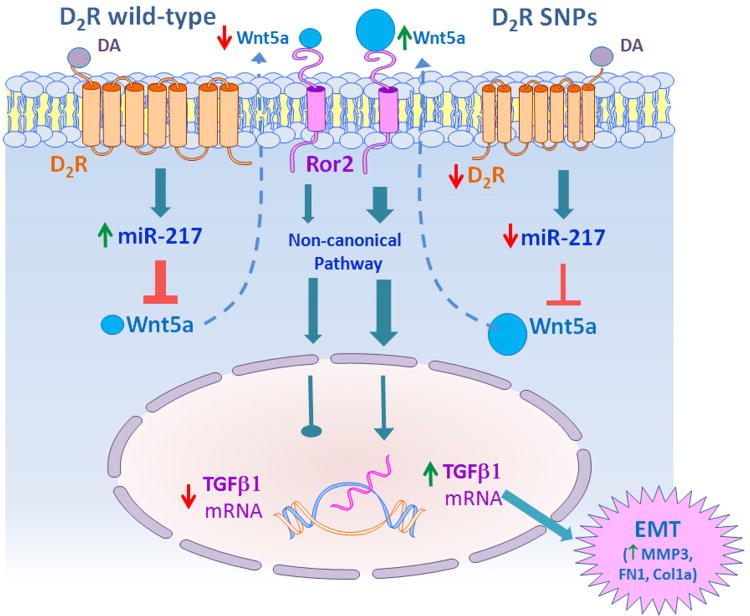
These results show that in human RPTCs the presence of common allelic variants (SNPs rs 6276, rs6277, and rs800497) in the D2R that decrease its expression and function results in increased expression of TGFβ1 and an EMT phenotype. This phenotype is mimicked by silencing D2R in RPTCs bearing D2R-WT and reversed by rescuing D2R expression in RPTCs with D2R-SNPs, indicating that the D2R has protective effects in these cells. We show that the protective effects of the D2R are mediated by regulation of miR-217 expression, as D2R downregulation decreases miR-217 expression while D2R overexpression has the opposite effect. A decrease in miR-217 expression or the inhibition of its function increases the expression of TGFβ1 and EMT markers. This is mediated by releasing the repressor effect of miR-217 on Wnt5a and its receptor Ror2 (Figure 6).
Figure 6.
Schema of the protective effect of the D2R on fibrosis in human renal proximal tubule cells (RPTCs).
The effect of the D2R is mediated by regulation of miR-217 expression. Decreased D2R expression/function, as observed in RPTCs, bearing D2R rs6276, rs6277, and rs1800497 alleles, results in decreased miR-217 expression which releases the repressor effect of miR-217 on Wnt5a. Increased expression of Wnt5a and its receptor Ror2 increases the expression of TGFβ1 through the non-canonical pathway, resulting in epithelial mesenchymal transition (EMT), demonstrated by the presence of the EMT markers MMP3, FN1, and Col1a.
miRNAs may induce or protect from fibrosis by targeting TGFβ or its downstream pathways (17). Out of the five miRNAs that were up- or downregulated more than 4-fold in RPTC-D2R -SNPs only miR-217 and miR-224-5p were directly regulated by the D2R and only miR-217 negatively regulated TGFβ1 and EMT markers. miR-217 has been shown to have a role in the development and progression of renal fibrosis. In patients with chronic kidney disease, the degree of tubulointerstitial fibrosis and proteinuria, but not glomerulosclerosis, negatively correlated with the amount of urinary miR-217 that is derived most likely from tubular epithelial cells and podocytes (18). Chronic pancreatitis and pancreatic cancer tissues demonstrated active EMT process and decreased miR-217 expression while ectopic expression of miR-217 inhibited TGFβ1-induced EMT (19). However, miRNAs may have a cell type-specific pattern of expression and in human liver cancer tissues an EMT phenotype was associated with increased expression of miR-216a/217 and overexpression of miR-216a/217 in hepatic cancer cells induced EMT by activating the TGFβ and PI3K/AKT signaling pathways through downregulation of PTEN and SMAD7 (20). In mouse mesangial cells treated with TGFβ and in the glomeruli of diabetic mice, miR-216a/miR-217 cluster levels were increased and mediated the downstream effects of TGFβ including the expression of genes related to production of extracellular matrix (21). In RPTC-D2R-SNPs, miR-216a was not consistently changed, indicating species and cell-type differences in the regulation of these miRNAs.
Wnt5a is a member of the Wnt family of secreted glycoproteins that play important roles in development, cell proliferation, and cell migration. A major receptor for Wnt5a is Ror2 which mediates Wnt5a cellular functions through the non-canonical Wnt pathway (16). Most studies on the role of the Wnt5a-Ror2 pathway have been performed in cancer cells in which upregulation of the pathway either promotes tumor formation and progression or acts as a tumor suppressor depending on the cell type (22). The non-canonical Wnt5a pathway has been implicated in the molecular mechanisms underlying TGFβ-induced expression of extracellular matrix in airway smooth muscle cells (23). Other studies have also implicated Wnt5a expression and signaling in renal and hepatic fibrosis (24,25). Wnt5a increases the proliferation and viability of fibroblasts in the lungs of patients with interstitial pneumonia (26). In epithelial Madin-Darby canine kidney cells undergoing EMT, Wnt5a expression was elevated and transient Wnt5a silencing attenuated both cell migration and invasion in these cells (27). Moreover, Wnt5a and Ror2 are induced in kidneys after unilateral ureteral obstruction where Ror2 was expressed in tubular epithelial cells exhibiting EMT characteristics (24). Our results show that the increase in TGFβ1 in human RPTCs with D2R SNPs is mediated by the miR-217-Wnt5a-Ror2 pathway.
miRNAs that are located in intergenic regions such as miR-217 carry their own promoters. One possible mechanism of the regulation of miR-217 expression by the D2R is through regulation of transcription factors binding to the miR-217 promoter. Nurr1 is a nuclear receptor transcription factor belonging to the NR4A family of orphan nuclear receptors and functions as a constitutively active transcription factor. Gene expression profiling of cells overexpressing Nurr1 has shown that miR-217 is a target of Nurr1 (28). The D2R interacts with Nurr1 via ERK signaling and is critical for its transcription (29). The deletion of D2R in dopaminergic neurons results in decreased Nurr1 expression (29). Preliminary data from our laboratory show that decreasing D2R expression selectively in the mouse kidney also reduces renal cortical Nurr1 expression. Thus the presence of allelic variants of the D2R that reduce its expression and function of may also decrease Nurr1 expression which in turn decreases miR-217 transcription. Experiments to test this possibility are currently being performed.
Perspectives
Genetic factors contribute to the susceptibility to renal disease associated with essential hypertension, and hypertension may cause progressive kidney disease only in genetically susceptible individuals (30,31). The genetic predisposition to chronic kidney disease is polygenic, but so far only a few genes have been shown to be contributory (32-34). These results show that renal proximal tubule cells from human subjects carrying common DRD2 allelic variants that are associated with hypertension and result in decreased dopamine D2R mRNA and protein expression have increased TGFβ1, a profibrotic phenotype, and markers of EMT that are mediated by the miR-217-Wnt5a-Ror2 pathway. Individuals carrying these D2R allelic variants could be more vulnerable to renal injury. Genetic testing can help to identify the individuals at risk and pharmacological treatment tailored to prevent or ameliorate the insult may decrease the prevalence of renal injury and chronic kidney disease.
Supplementary Material
Novelty and Significance.
1) What Is New?
We show that the increased TGFβ1 expression and markers of epithelial mesenchymal transition in renal proximal tubule cells from individuals carrying polymorphisms of the dopamine D2 receptor that downregulate its expression are mediated by activation of the miR-217-Wnt5a-Ror2 pathway.
2) What Is Relevant?
Individuals carrying these D2R SNPs could be more vulnerable to renal injury when challenged with an insult such as hypertension. Genetic testing can help to identify the individuals at risk and pharmacological treatment targeting miR-217-Wnt5a-Ror2 pathway tailored to prevent or ameliorate the insult could decrease the prevalence of renal injury and chronic kidney disease.
Summary.
This article shows miR-217, by its repressor effect on Wnt5a-Ror2 pathway, mediates the protective effects of the dopamine D2 receptor on fibrosis in human renal proximal tubule cells.
Acknowledgments
Source(s) of Funding: This work was funded by grants from the US National Institutes of Health, R01DK090918, P01HL074940, P01HL068686, R01HL092196, R37HL023081 and R01DK039308.
Footnotes
Disclosures: The authors declare nothing to disclose.
References
- 1.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One. 2012;7:e38745. doi: 10.1371/journal.pone.0038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar M, Chugh G, Lokhandwala MF. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol. 2009;297:F1543–F1549. doi: 10.1152/ajprenal.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol. 2012;302:F742–F749. doi: 10.1152/ajprenal.00583.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang MZ, Yao B, Yang S, Yang H, Wang S, Fan X, Yin H, Fogo AB, Moeckel GW, Harris RC. Intrarenal dopamine inhibits progression of diabetic nephropathy. Diabetes. 2012;61:2575–2584. doi: 10.2337/db12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, Felder RA, Jose PA, Armando I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension. 2014;63:e74–80. doi: 10.1161/HYPERTENSIONAHA.113.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao W, Zhang SZ, Tang M, et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αβ-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GN, Tomlinson B, Critchley JA. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension. 2000;36:177–182. doi: 10.1161/01.hyp.36.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 10.Prasad P, Kumar KM, Ammini AC, Gupta A, Gupta R, Thelma BK. Association of dopaminergic pathway gene polymorphisms with chronic renal insufficiency among Asian Indians with type-2 diabetes. BMC Genet. 2008;9:26. doi: 10.1186/1471-2156-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pottier N, Cauffiez C, PerraisM, Barbry P, Mari B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol Sci. 2014;35:119–126. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Castro NE, Kato M, Park JT, Natarajan R. Transforming growth factor β1 (TGF-β1) enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem. 2014;289:29001–29013. doi: 10.1074/jbc.M114.600783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gildea JJ, McGrath HE, Van Sciver RE, Wang DB, Felder RA. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. Methods Mol Biol. 2013;945:329–345. doi: 10.1007/978-1-62703-125-7_20. [DOI] [PubMed] [Google Scholar]
- 14.Ibarra FR, Armando I, Nowicki S, Carranza A, De Luca Sarobe V, Arrizurieta EE, Barontini M. Dopamine is metabolised by different enzymes along the rat nephron. Pflugers Arch. 2005;450:185–191. doi: 10.1007/s00424-005-1386-6. [DOI] [PubMed] [Google Scholar]
- 15.Kozell LB, Neve KA. Constitutive activity of a chimeric D2/D1 dopamine receptor. Mol Pharmacol. 1997;52:1137–1149. doi: 10.1124/mol.52.6.1137. [DOI] [PubMed] [Google Scholar]
- 16.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor-tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signaling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Vettori S, Gay S, Distler O. Role of microRNAs in fibrosis. Open Rheumatol J. 2012;6:130–139. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune LF, Cheung-Lung CP, Gang W, Kai-Ming C, Kam-Tao LP. Micro-RNA expression in the urinary sediment ofpatients with chronic kidney diseases. Dis Markers. 2012;33:137–144. doi: 10.3233/DMA-2012-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng S, Zhu S, Wang B, et al. Chronic pancreatitis and pancreatic cancer demonstrate active epithelial–mesenchymal transition profile, regulated by miR-217-SIRT1 pathway. Cancer Lett. 2014;355:184–191. doi: 10.1016/j.canlet.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013;58:629–641. doi: 10.1002/hep.26369. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-β activates Akt kinase via a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–354. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Kumawat K, Menzen MH, Bos IS, Baarsma HA, Borger P, Roth M, Tamm M, Halayko AJ, Simoons M, Prins A, Postma DS, Schmidt M, Gosens R. Noncanonical WNT-5A signaling regulates TGF-β-induced extracellular matrix production by airway smooth muscle cells. FASEB J. 2013;27:1631–1643. doi: 10.1096/fj.12-217539. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Yamagata K, Nishita M, Endo M, Arfian N, Rikitake Y, Emoto N, Hirata K, Tanaka Y, Minami Y. Activation of Wnt5a-Ror2 signaling associated with epithelial-to-mesenchymal transition of tubular epithelial cells during renal fibrosis. Genes Cells. 2013;18:608–619. doi: 10.1111/gtc.12064. [DOI] [PubMed] [Google Scholar]
- 25.Rashid ST, Humphries JD, Byron A, Dhar A, Askari JA, Selley JN, Knight D, Goldin RD, Thursz M, Humphries MJ. Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res. 2012;11:4052–4064. doi: 10.1021/pr3000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, Kaminski N. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol. 2009;41:583–589. doi: 10.1165/rcmb.2008-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Xu L, Ye X, Shen S, Li Z, Niu X, Lu S. Polymorphisms of microRNA sequences or binding sites and lung cancer: a meta-analysis and systematic review. PLoS One. 2013;8:e61008. doi: 10.1371/journal.pone.0061008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MM, Michelhaugh SK, Bouhamdan M, Schmidt CJ, Bannon M. The transcription factor NURR1 exerts concentration-dependent effects on target genes mediating distinct biological processes. Front Neurosci. 2011;5:135. doi: 10.3389/fnins.2011.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY, Choi KC, Chang MS, Kim MH, Kim SY, Na YS, Lee JE, Jin BK, Lee BH, Baik JH. The dopamine D2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J Neurosci. 2006;26:4567–4576. doi: 10.1523/JNEUROSCI.5236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKnight AJ, Currie D, Maxwell AP. Unravelling the genetic basis of renal diseases; from single gene to multifactorial disorders. J Pathol. 2010;220:198–216. doi: 10.1002/path.2639. [DOI] [PubMed] [Google Scholar]
- 31.Garrett MR, Pezzolesi MG, Korstanje R. Integrating human and rodent data to identify the genetic factors involved in chronic kidney disease. J Am Soc Nephrol. 2010;21:398–405. doi: 10.1681/ASN.2009080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma RC, Tam CH, Wang Y, Luk AO, Hu C, Yang X, Lam V, Chan AW, Ho JS, Chow CC, Tong PC, Jia W, Ng MC, So WY, Chan JC. Genetic variants of the protein kinase C-beta 1 gene and development of end-stage renal disease in patients with type 2 diabetes. JAMA. 2010;304:881–889. doi: 10.1001/jama.2010.1191. [DOI] [PubMed] [Google Scholar]
- 33.Xia Y, Entman ML, Wang Y. Critical Role of CXCL16 in Hypertensive Kidney Injury and Fibrosis. Hypertension. 2013;62:1129–1137. doi: 10.1161/HYPERTENSIONAHA.113.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köttgen A, Hwang SJ, Rampersaud E, Coresh J, North KE, Pankow JS, Meigs JB, Florez JC, Parsa A, Levy D, Boerwinkle E, Shuldiner AR, Fox CS, Kao WH. TCF7L2 variants associate with CKD progression and renal function in population-based cohorts. J Am Soc Nephrol. 2008;19:1989–1999. doi: 10.1681/ASN.2007121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.