Abstract
We aimed to design a multimodal intervention to improve adherence following first episode psychosis, consistent with current evidence. Existing literature identified medication attitudes, insight, and characteristics of support as important determinants of adherence to medication: we examined medication attitudes, self-esteem, and insight in an early psychosis cohort better to understand their relationships. Existing longitudinal data from 309 patients with early Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, nonaffective psychosis (83% first episode) were analyzed to test the hypothesis that medication attitudes, while meaningfully different from “insight,” correlated with insight and self-esteem, and change in each influenced the others. Rosenberg Self-Esteem Scale, Birchwood Insight Scale, and Positive and Negative Syndrome Scale insight were assessed at presentation, after 6 weeks and 3 and 18 months. Drug Attitudes Inventory (DAI) and treatment satisfaction were rated from 6 weeks onward. Structural equation models of their relationships were compared. Insight measures’ and DAI’s predictive validity were compared against relapse, readmission, and remission. Analysis found five latent constructs best fitted the data: medication attitudes, self-esteem, accepting need for treatment, self-rated insight, and objective insight. All were related and each affected the others as it changed, except self-esteem and medication attitudes. Low self-reported insight at presentation predicted readmission. Good 6-week insight (unlike drug attitudes) predicted remission. Literature review and data modeling indicated that a multimodal intervention using motivational interviewing, online psychoeducation, and SMS text medication reminders to enhance adherence without damaging self-concept was feasible and appropriate.
Key words: insight, self-esteem, schizophrenia, first episode, mHealth, web-based, adherence
Introduction
Poor adherence to antipsychotics is a major cause of psychotic relapse. Interventions to improve adherence are becoming more sophisticated1 as many of its determinants become clearer.2 Nevertheless, therapeutic successes have been limited and much of this complex, multidetermined behavior remains enigmatic. The argument that early psychosis forms a critical period that shapes later prognosis3,4—partly because attitudes to illness and treatment are still developing that will evolve to shape later behavior5–7—in turn suggests that intervention during this window might succeed better.
Recent evidence-based guidelines for developing such complex interventions8 emphasize the importance of literature review to provide some theoretical rationale, followed by “modeling” of key processes, the feasibility and acceptability of potential interventions’ elements (separately or combined), and a potential trial before trialing and further evaluation.
This article will examine (1) how far the complex frameworks developed to explain nonadherence in chronic schizophrenia are supported by recent studies in first episode psychosis, (2) how far and how quickly key processes related to nonadherence interact and change in an acutely presenting first episode cohort followed over the medium term, (3) why combining interventions, is likely to be the most effective approach to a randomized trial of intervention, and (4) feasibility of the interventions selected within the Optimization of Treatment and Management of Schizophrenia in Europe (OPTiMiSE) program.9
What is the Prevalence and Impact of Poor Adherence After First Episodes?
The definition of poor adherence varies, depending on the rigor of the assessment method,10 although one first episode survey found different methods had an intraclass correlation of .84 and similar predictive validity.11 Individuals’ adherence fluctuates: one group12 found 33%–44% were nonadherent sometime in any 6-month period and 53% sometime in the first 2 years, while another13 found 63% nonadherent for at least a week over a year. Another study14 estimated 45% took under 75% of antipsychotics in the first 6 months, while 42%–60% were rated as nonadherent at some point in first episode psychosis cohorts followed up for at least 1 year,12,13,15–17 particularly if the samples were juvenile or all had schizophrenia. Poor adherence predicts total discontinuation.13,18
Relapse is also variably defined.19 Nonetheless, irregular adherence consistently predicts similarly increased risk of relapse, readmission, and other adversity.20,21 Relapse risk multiplies 2- to 5-fold in the months after stopping medication across definitions of relapse, and both established and early illness22,23 (cf placebo’s effect on relapse risk compared with maintenance24). Decreased adherence leading to early readmission may be a particular risk after early initial discharge.20,25
In one first episode trial, 11% of patients judged adherent over 1 year relapsed, against 27% “nonadherent patients.”26 Twenty-two percent on maintenance antipsychotics in the MESIFOS (Medication Strategies In First Onset Schizophrenia) trial relapsed over 18 months, while 42% relapsed if allocated to the arm where careful, often incomplete attempts at discontinuation were made.27 Wunderink et al28 then found that the proportion relapsing in the “maintenance” arm caught up after 7 years’ follow-up. However, the proportions in each arm finishing on subtherapeutic dose or off antipsychotic had begun converging: 25% in the maintenance arm and 40% in the other. The discontinuation arm’s social recovery was substantially better27 but that arm had functioned better before randomization.27,29 In comparison to planned discontinuation, Gaebel’s group found that poor adherence led to poorer function and delayed remission in several studies.30
Determinants of Poor Adherence in Early Schizophrenia
There is increasing evidence, with some gaps, that explanatory frameworks for nonadherence derived from studies in chronic disorder apply to early schizophrenia. The most proximal predictors influence the act of taking medication by altering its delivery and availability, support for/monitoring of administration, or patients’ attitudes to medication.
1. Antipsychotic formulation. Long acting injectable (LAI) formulations have equivocal impact on outcome compared with oral medication in randomized trials31 although these often suffer methodological difficulties such as selection bias against the nonadherent.32 LAIs have as much as halved relapse hazard in large prospective cohort studies,33,34 including after first admission in a national case register study.35 Despite these advantages, they are not often used first line, in part because there are few trials after first episodes36 and because of concerns about acceptability.37
2. Social and professional support. Adherence in chronic schizophrenia is influenced by setting (eg, supported accommodation) and reminders (eg, from diaries, adherence aids, and staff).38–41 Information technology–based interventions are discussed below. Family support is important in young patients in first episode services,42–44 but little other recent work specific to early schizophrenia focuses on setting, support, and adherence.
3. Engagement with services. Integration of a psychotic episode into self-concept after early psychotic episodes, as opposed to “sealing over” or “isolation” that involves denying its future relevance,45 predicts better early engagement but not adherence.46,47 Supporting decision making enhances satisfaction and may provide a sense of control that supports integration (and may affect attitudes to medication) but again does not seem directly to impact adherence.48 However, disengagement will cause unmeasured nonadherence, data from such lost patients being missing, leaving its impact underestimated. Some cohort analyses exclude such patients14,43,49–51 and they are unlikely to enter trials.18,30,52,53 Difficulty accessing services has also been posited as affecting adherence and engagement in chronic54 and in early psychosis,20 but there is no evidence from existing studies, albeit mostly conducted in early intervention services where active engagement of services with patients is emphasized. This appears more important than access. Properly coordinated follow-up after discharge influences adherence38 and the early intervention model probably reduces relapse partly by improving adherence.55
4. Neurocognitive deficits. Their impact varies in early psychosis: some studies find low premorbid and executive cognitive function predicts early adherence,56 but others do not57; there is no consistent general effect.58 Neurocognitive deficits that predict medication mismanagement in chronic illness (eg, prospective, working memory, learning, and comprehension) predict more general self-management difficulty,59–61 so such deficits might therefore necessitate more support, improving adherence. Paradoxically, some out-patients with partial adherence might intend concordance but fail neurocognitively, while those not adhering with better cognition may have made a decision to stop antipsychotics.62 Of course, forgetting medication is common across disorders.
5. Attitudes to medication. These attitudes are consistently important predictors when assessed in early illness,20,42,63–65 even when potential proxies such as detention or initial medication refusal are included14,66,67: overall hostility predicts negative attitudes and poor early adherence.53,66 Occasional studies find that attitudes are not predictive.50,52 The timing of their “baseline” assessments may be critical for assessing their longitudinal effect because attitudes and adherence are dynamic in early psychosis.5,20,43,46
Because variables directly predicting attitudes to medication in early psychosis appear to be fluid and so potentially amenable to change, it is worth considering what influences them:
1. Perceived benefit of treatment. There is evidence in early illness that actual benefit58,68 is important and that patients test this with antipsychotic “holidays” to see if symptoms re-emerge.69 Reactance, the disposition to reverse perceived losses of autonomy, affects perceived benefit70 as does insight,70–72 especially recognizing symptoms during first episodes.52 However, there appears an independent effect of perceived benefit too.73 Impaired cognition might reduce the capacity to detect beneficial changes68 even where it does not directly determine adherence.58 Metacognition (the ability to cognitively “step back,” and in this context consider medication’s effect on symptoms) appears to affect insight after first episodes74,75 and later adherence.76–78
2. Perceived risk. Successful frameworks1,20 identify both fear of consequences of relapse (presumably related to awareness of current and past consequences of illness) and recognition of the likelihood of relapse.
3. Insight and self-concept. Scores on the Drug Attitudes Inventory (DAI),79 the most used instrument to assess attitudes to medication, are affected by key attitudes to symptoms, illness, and self. Many studies in early psychosis show insight to be a major predictor of nonadherence and discontinuation,20,51,53,58,80 but its effect may be indirect.68 Various studies find no direct effect of insight on adherence, but they include potential mediators in their models: eg, attitudes,30,42,63 detention, or initial medication refusal14,66,67; arguably, duration of untreated psychosis and continued substance misuse.15,50
Common social stereotypes shape attitudes to disorder and treatment, which appear important determinants of not only acceptance of illness, perceived risk, and hence adherence but also self-esteem, self-stigmatization, and depression in early psychosis.81–83 Lester’s qualitative study82 confirmed that awareness of a first episode could be integrated or isolated (“sealed over,” as above). This is consistent with data from chronic illness showing that negative stereotypes only affected morale if accepted as applicable to oneself,84 and some evidently have good insight without self-stigmatization,78 but these processes explain resistance to change in some patients.
Sealing over can be seen as a maladaptive response to cognitive dissonance between self-concept and awareness of illness that requires a change in schemata to resolve. Adherence attitudes have also been described in terms of the “stages of change” model.85 The same model is used for substance misuse, often a comorbid problem.21,49,50,67 Common themes of decision-making capacity and motivation to continue healthy behavior in the face of ambivalence are relevant to cognitive intervention in both types of disorder. Stopping cannabis was associated with improved adherence in one first episode cohort.67
4. Prescribing alliance and significant others. For adherence in chronic illness, relationship with prescribers seems to be by far the most important relationship with services.72 It in turn influences attitudes to medication,73 but internal locus of control may moderate the effect of significant others on attitudes and adherence. Highly autonomous individuals may be more or less adherent86 depending on their adherence style; their views are less influenced by others one way or the other. These processes are relevant in early illness: Haley et al87 confirmed that the more powerful others’ views were felt to be, the more positive medication attitudes 6 weeks and 18 months after first presentation. Montreuil et al49 found that key worker alliance also predicted later adherence, whether staff or patient rated.
Family and peer relationships might be important. Family members skeptical of medication and treatment may have a corrosive effect,54 which in early illness appears dynamic, depending on contact and family function.43 Consistent with this is the positive effect of family intervention on adherence and relapse in chronic illness,2 even with 8 weeks of family psychoeducation.88
5. Side effects. Although it is implausible that, say, erectile dysfunction in young men or weight gain in young women have no effect on adherence,10,30,89–91 overall ratings of objective or subjective adverse effects have failed consistently to predict adherence in early illness.12,22,58,63,68 The Health Beliefs Model92 suggests that given the seriousness of the illness, if one perceives antipsychotics’ value one will tolerate many adverse effects.
Existing literature therefore suggests that—apart from medication formulation and the nature of and relationships with support—neurocognitive deficits, attitudes to medication, and insight make natural targets for adherence interventions. However, understanding when and how to shift attitudes and insight without consequent demoralization and long-term dysfunction76,93–95 is critical.
Modeling Drug Attitudes and Insight in Early Schizophrenia
Much of the growing body of evidence about insight and treatment attitudes in early psychosis cohorts relies on samples assessed potentially weeks after presentation, when the peak of initial psychosis has passed. They often use methods unsuited to reveal the full complexity of potential interactions, important in understanding how interventions might be constructed to target multiple processes when they are at their most tractable.
We investigated attitudes to medication, insight, and self-esteem, though not adherence, in a cohort of 309 patients. They were recruited within 2 weeks of consecutive presentations with first (n = 257, 83%) or second (n = 52) episode nonaffective psychosis. They were originally randomized to Cognitive Behavioral Therapy for psychosis, supportive counseling or treatment as usual96,97 before repeated assessments over 18 months.
Insight, attitudes, and self-esteem can display trait-like characteristics or state-driven ones (eg, during acute episodes). Arguably intervention targets should display sufficient change to indicate malleability but sufficient stability to envisage lasting change. Review reveals medication attitudes to be dynamic, though some predictors are relatively stable. Some argue that insight is trait like,7 yet if first assessed sufficiently early one might expect it to improve as symptoms resolve.80 If insight and attitudes change, scores after response should be more predictive of relapse, readmission, and remission than initial attitudes, contrary to some previous data.52,64,80 Both trait-like and state-driven aspects were evidenced in a stable, early psychosis group.98
Self-esteem during acute initial psychosis is usually studied retrospectively.81–83 A prospective, longitudinal study starting from presentation ignored its trajectory and effect on attitudes.5 Notably, in later psychosis positive self-evaluation predicts integration rather than sealing over (which may nevertheless avoid depression).99
How far medication attitudes and different aspects of insight (eg, awareness of illness, recognizing symptoms as such, accepting a need for treatment100) reflect separate constructs rather than common schemata, even in the earliest stages of psychosis, is also important. Implicit in those studies that assess both,50,52,72 it has not been examined. Nor is it clear how far different aspects of insight differ in their effect on self-concept. If they differ and are separable, it might be possible to improve some aspects of concordance or insight without damaging self-concept.
We proposed to examine four hypotheses.
1. Attitudes to medication and the various aspects of insight reflect separate underlying constructs.
2. All aspects of insight, reflecting a common construct, cross-sectionally correlate with self-esteem and medication attitudes, good insight correlating with low self-esteem and concordant attitudes.
3. Trait high self-esteem promotes integration and predicts more concordant medication attitudes. Improving self-esteem, signaling integration, predicts improving concordance.
4. These variables all display rapid initial improvement and then stability, ie, state and trait characteristics.
Methods
Participants were recruited from consecutive presentations with first and second episodes (within 2 years of the first) of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, schizophreniform disorder, schizophrenia, schizoaffective, delusional disorders, and “psychosis not otherwise specified” from geographically defined catchments (procedures detailed elsewhere96,97). Self-esteem was assessed with the Rosenberg Self-Esteem Scale,101 insight with Birchwood’s self-rated Insight Scale (BIS)102 and the assessor rated Positive and Negative Syndrome Scale (PANSS)103 insight item, g12, at presentation, 6 weeks, 3 months, and 18 months later. The Insight Scale has three subscales: relabeling symptoms, awareness of illness, and recognizing the need for treatment.102 Satisfaction with treatment (rated on a 10-cm visual analog scale by participants) and Drug Attitude Inventory (DAI79) were rated at 6 weeks and each succeeding visit.
The pattern of missing data was analyzed by entering completion of each scale at each stage into a series of binary logistic regressions with baseline sex, age at onset, ethnic group, diagnosis,97 substance dependence, education, and center as independent variables.
These data were included in various structural equation models (with AMOS 20.0104). A series of models and assumptions were tested cross-sectionally (see supplementary methods and supplementary table 2). Based on these, longitudinal models were constructed: first to model the development of coherent elements and then interactions across the whole variable set. Sensitivity analyses for the effect of center, sex, episode, and therapy were planned.
Attitudes’ effect on outcome was assessed using receiver operating characteristic curves to predict relapse, readmission, and remission using SPSS 20.0.104 Readmission was recorded from case notes.97 Relapse was defined as a 2-week exacerbation of symptoms recorded in case notes and leading to a change in management97 and remission by applying the symptomatic criteria of Andreasen et al105 at 18 months, without the time criterion. Curves were assessed using the area under the curve, and Fisher’s exact test was used to compare performance of different cutoffs as predictors of outcomes. Potential cutoffs for comparison were selected as (1) furthest from the chance line, (2) nearest to coordinates of 100% sensitivity and 0% false negative, or (3) highest likelihood ratio.
Results
Patients were first assessed a median of 6 days after admission. Number followed-up, scores for the modeled variables, and PANSS are presented in table 1. Mean scores often changed little despite large variations in individuals’ scores over time: DAI change scores were mean 1.7 and then 1.7 (effect sizes: d = 0.15 and 0.15), but corresponding SDs were 9.8 and 10.6.
Table 1.
Mean (SD) Scores for Insight, Self-esteem, Drug Attitudes, and Symptoms
| Variable (Potential Range) | Baseline, n = 309 (100%) | 6 Weeks, n = 236 (76%) | 3 Months, n = 218 (71%) | 18 Months, n = 225 (73%) |
|---|---|---|---|---|
| PANSSa insight (1–7) | 4.41 (1.49) | 3.26*(1.58) | 3.10 (1.48) | 3.04 (1.45) |
| Relabeling symptoms (0–4) | 2.30 (1.32) | 2.85*(1.22) | 2.81 (1.23) | 2.71 (1.31) |
| Awareness of illness (0–4) | 1.96 (1.43) | 2.00 (1.38) | 2.00 (1.36) | 2.05 (1.40) |
| Need for treatment (0–4) | 2.68 (1.15) | 2.80 (1.12) | 2.84 (1.06) | 2.88 (1.19) |
| Self-Esteemb (10–40) | 27.16 (4.82) | 26.90 (4.71) | 27.66 (4.19) | 27.80 (4.42) |
| DAIc (−30 to +30) | — | 7.06(11.09) | 7.88(10.66) | 9.50(11.39) |
| Satisfactiond (0–10) | — | 6.55 (2.82) | 6.79 (2.40) | 6.90 (2.75) |
| PANSS positive (7–49) | 23.4 (4.8) | 15.1* (6.2) | 13.0 (5.1) | 13.7 (5.4) |
| PANSS total (30–210) | 88.0 (17.3) | 67.5* (19.2) | 62.2 (17.7) | 62.1 (18.2) |
Note: aPositive and Negative Symptom Scale.
bRosenberg Self-Esteem Scale.
cDrug Attitudes Inventory.
dMedication satisfaction.
*Significant change in score (paired t-test P < .05).
Logistic regression showed that while “center” frequently predicted that data for various scales were missing, no other variables did. Data were therefore “missing at random” in Little and Rubin’s106 classification (supplementary table 1).
Cross-sectional Models of Medication Attitudes, Self-esteem, and Insight
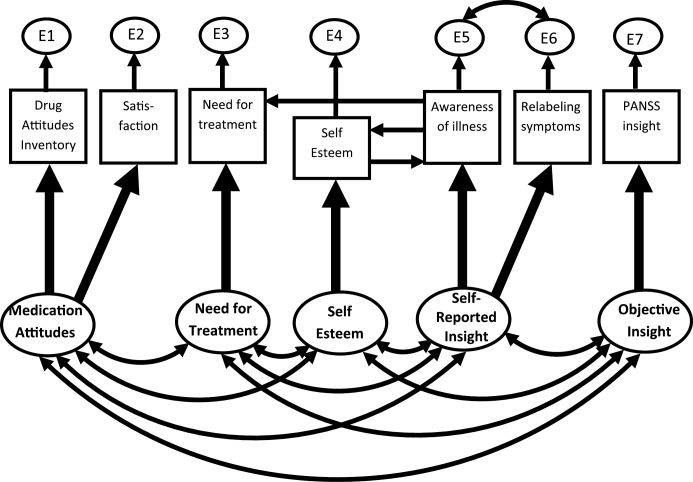
Models of scores for Rosenberg Self-Esteem, BIS subscales, DAI, and satisfaction at each stage of follow-up were compared against criteria for fit and parsimony described in table 2. Although models with three latent variables—Insight, Self-Esteem, and Medication Attitudes—fitted adequately (supplementary methods and supplementary table 2), longitudinal models (below) showed that five latent constructs—Objective Insight, Self-Reported Insight, Self-Esteem, Medication Attitudes, and Need for Treatment—were necessary to explain longitudinal changes in scores. Cross-sectional models based on longitudinal model M6 (below) also fitted adequately (table 2 and figure 1), apart from marginal fit at 18 months. Though the constructs were clearly separable, supporting Hypothesis 1, the aspects of insight neither reflected a common factor nor were equally related to self-esteem, disconfirmed Hypothesis 2.
Table 2.
Comparison of Structural Equation Models
| Model | χ2 | df | CFIa | NNFIb | RMSEAc | AICd |
|---|---|---|---|---|---|---|
| Baseline: four-factor cross-sectionale | 7 | 3 | 0.98 | 0.92 | 0.068 | 41 |
| 6 weeks: five-factor cross-sectionalf | 10 | 10 | 1.00 | 1.00 | 0.010 | 60 |
| 3 months: five-factor cross-sectionalf | 9 | 6 | 0.99 | 0.95 | 0.037 | 66 |
| 18 months: five-factor cross-sectionalf | 17 | 6 | 0.95 | 0.83 | 0.076 | 59 |
| L1: satisfaction, DAI, NFTg | 43 | 23 | 0.94 | 0.89 | 0.053 | 105 |
| L2: PANSS insight, RLS, AWIh | 83 | 46 | 0.95 | 0.92 | 0.051 | 171 |
| L3: Rosenbergi | 0.2 | 1 | 1.00 | 1.04 | 0 | 26 |
| M2: Combining L1, L2, L3 | 561 | 248 | 0.82 | 0.76 | 0.064 | 765 |
| M6: M2 modified (see text) | 385 | 229 | 0.91 | 0.87 | 0.047 | 627 |
| M7: M6 with nonsignificant relationships removed | 376 | 242 | 0.92 | 0.90 | 0.042 | 592 |
Note: Measures of fit and parsimony for various models of insight, self-esteem, and medication attitudes. Models for each cross-sectional stage, longitudinal growth curve models for specific groups of scales (L1–L3), and integrated longitudinal models combining all growth curves for all measures (M2, M6, and M7). CFI, Common Fit Index; RMSEA, root mean square error approximation; AIC, Akaike’s Information Criterion; AWI, Awareness of Illness, RLS, Relabeling Symptoms.
aCommon fit index: scores <0.90 indicate a substantial improvement is possible.
bNon-normed fit index: scores <0.90 indicate a substantial improvement is possible.
cRoot mean square error approximation: scores <0.100 adequate, <0.050 good.
dAkaike’s information criterion: low values indicate better fitted, more parsimonious models.
eFour latent variables: NFT, Self-Esteem, Self-Rated Insight, and Objective Insight (without Medication Attitudes because DAI and Satisfaction unmeasured at baseline).
fFive latent variables: Medication Attitudes, NFT, Self-Esteem, Self-Rated Insight, and Objective Insight.
gGrowth curve model, latent constructs Medication Satisfaction and NFT.
hGrowth curve model, latent constructs Self-Rated and Objective Insight.
iGrowth curve model, latent constructs Self-Esteem.
Fig. 1.
Cross-sectional model of scale scores. This represents underlying objective assessment of insight, self-report of insight, self-esteem, acceptance of the need for treatment, and concordance with medication as predicting scores on a range of scales at each stage of follow-up.
Simple Longitudinal Models
Growth curve models were fitted separately for Self-Esteem, Insight, and Medication Attitudes (supplementary figure 2). These included a latent constant (representing constant underlying Self-Esteem, Insight, or Attitudes) correlated with a latent change term (representing change across follow-up for each of these constructs). This fitted well for Self-Esteem (L3, table 2). Adequately fitting models for insight (L2) required latent “Objective Insight” constant and change variables to predict PANSS insight and latent “Self-Reported Insight” constant and change variables to predict Awareness of Illness (AWI) and Relabeling Symptoms (RLS). Latent Medication Attitudes constant and change variables predicted DAI and Satisfaction, but Need For Treatment (NFT) scores required separate “Need for Treatment” latent constant and change variables (L1). Self-Esteem and Self-Reported Insight (as Hypothesis 4 predicted, unlike other latent change variables) fitted better if change scores were modeled on the basis of rapid initial change decelerating over follow-up, rather than linear change (supplementary methods).
Overall Longitudinal Models
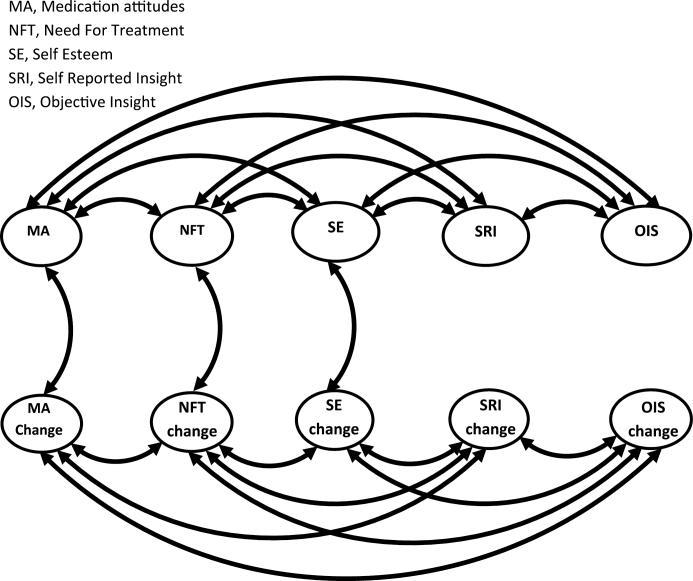
These growth curve models were combined into overall models predicting all scale scores throughout follow-up (M2, table 2). All latent constants (Self-Esteem, Self-Reported and Objective Insight, Mediation Attitudes, and Need for Treatment) correlated with each other and all latent change scores correlated with each other, but each constant only correlated with its own change variable. Serial modifications were made to a sequence of models (M3–M6) to improve fit (supplementary table 2). In summary: (a) Self-Esteem and Awareness of Illness each predicted the other, (b) AWI predicted NFT, and (c) Self-Esteem latent change predicted AWI at each follow-up (cf figure 1). To generate the final model, M7, nonsignificant relationships were removed from M6 in sequence, least significant first, until M7’s overall fit significantly worsened.
In model M7, Rosenberg scores no longer consistently predicted AWI, though rising latent Self-Esteem always predicted poorer Awareness, and better Awareness always directly predicted higher Rosenberg score. Three-month Relabeling Symptoms was unaffected by insight change. All latent constants intercorrelated, and all change scores intercorrelated except “Self-esteem” and “Medication attitudes” (figure 2), only partly supporting Hypothesis 3. Neither of the insight latent constants correlated with their latent change scores.
Fig. 2.
Relationships between latent variables in the longitudinal model M7. This represents relationships between latent constant and change variables within the model, for simplicity excluding their relationships to actual scale scores over follow-up.
Planned sensitivity analyses fitting M7 to various subpopulations (supplementary methods) produced common fit index scores >0.90 and root mean square error approximation score of 0.043–0.052, indicating adequate fit.
Performance of Scales as Predictors of Outcome
Readmission was significantly predicted by poor baseline Birchwood Insight Scale scores (but not 6-week scores), 6-week PANSS insight (but not baseline), and poor concordance on 6-week DAI scores (supplementary table 2). Insight Scale cutoff < 6 performed best: P < .001; positive predictive value (PPV) 61%; negative predictive value (NPV) 72%. Relapse was more poorly predicted, only baseline Insight Scale being even trend significantly predictive (P = .068) and only Insight Scale cutoff <6 being significantly predictive (PPV 73% and NPV 65%; P = .039; supplementary table 3). DAI cutoff <4 was significant where the whole scale was not (PPV for relapse 67%, NPV 56%, P = .048).
Cross-sectional remission at final follow-up (supplementary table 4) was predicted by insight and PANSS score but not the overall DAI scale, though the best individual DAI cutoff (score ≥ 0) was significantly associated with remission (PPV 69%, NPV 61%, P = .006). Objective PANSS insight was more predictive than Insight Scale at baseline and 6 weeks, though PANSS total was a better predictor at each of these stages.
Findings were very similar for first episode patients alone (available on request).
Discussion
Hypothesis 1 was largely supported: longitudinal modeling showed that Self-Esteem, Medication Attitudes, Need for Treatment, Objective, and Self-Reported Insight represented distinct but correlated constructs.
Hypotheses 2 and 3 were only partially supported. Although all latent variables were significantly correlated, Awareness of Illness scores emerged as most related to self-esteem. Paradoxically, falling latent self-esteem predicted greater Awareness of Illness at follow-up, but at each assessment point greater Awareness predicted higher Rosenberg scores. Perhaps the process of accepting the illness label was demoralizing, but those who had done so were then able to maintain a positive self-image. These findings, consistent across follow-up, add to existing models of self-stigmatization and insight.82,84,93,94,107
Contrary to Hypothesis 2, insight separated into three latent variables (need for treatment, objective, and self-reported insight) not one. The separation of objective and self-reported insight on longitudinal modeling might have been due to initial symptom severity influencing assessors’ rating of insight, producing artifactual improvement as symptoms reduced. This is consistent with 6-week, not baseline, PANSS insight predicting readmission, whereas baseline BIS scores were most predictive.
Hypothesis 4 is relevant here. Most measures’ raw scores did not support it: of all included in the model only changes from baseline to 6 weeks in g12 and Relabeling Symptoms were significant (table 1). Other measures’ mean remained consistent despite considerable change in scores from visit to visit for individuals. Growth curve modeling, however, suggested that self-esteem and self-reported insight had, buried in this variation, underlying tendencies toward rapid initial improvement (however limited) that then slowed. This implies that aspects of insight and self-esteem are affected by the initial illness episode before returning to more trait-like behavior. Medication Attitudes did not show this, but DAI and Satisfaction were not measured over the initial phase of rapid improvement. This perhaps deflated Attitudes’ change variables correlations with other change variables (eg, Self-Esteem’s) because they did not cover this period of greater change. However, this highlights the association of insight-related with Attitude change variables, confirming them as important determinants of change (figure 2).
Modeling insight and concordance as predictors of outcome indicated that readmission and remission were relatively well predicted but not relapse, which may not have been as reliably detected. Despite this, poor self-reported insight during acute psychosis predicted relapse, unlike objective insight. This was consistent with the findings of Heinrichs et al108 that poor insight during relapse predicted readmission, arguably by impairing help seeking during relapse. The current finding could be explained by relapse somehow reinstating an earlier state of poor insight best detected by self-report. Readmission was substantially more likely in the 22% with the least insight (scores <6), suggesting that this very poor initial insight group segregates itself as having a particularly high likelihood of readmission (61% over 18 months compared with 27% for the rest of the cohort: see supplementary table 3). On the other hand, PANSS insight after response was somewhat more predictive of outcome, arguably because objectively rated insight better reflected trait insight that influences ongoing concordance.71
Concordance (DAI) failed to predict remission, suggesting that adherence is necessary but insufficient, underlining another difference from insight. Other predictors include severity of illness and treatment response, as indicated by the performance of PANSS totals as predictors.
This cohort’s strength was its representativeness: recruited from consecutive presentations from defined catchments with a low refusal rate96 (13%) and the repeated assessments providing data from a short interval after admission (under 14 working days). Unfortunately, adherence itself was unmeasured. It was a limitation that second episode sufferers were included in order to provide sufficient participants, given the high noncompletion rate of the satisfaction measure and (to a lesser extent) the DAI in certain centers. However, sensitivity analyses showed good fit in the first episode patients. There were problems with missing data for some scales (the treatment satisfaction scale at 18 months, particularly, though this was remedied by its removal from the structural equation models), but these were largely explained by center differences that produced data “missing at random.”106 Sensitivity analysis removing centers with fewer completions showed that the models still fitted well and structural equation models fitted by full information maximum likelihood methods are relatively insensitive to this type of attrition. Depression was not included. Previous work in this cohort shows that it is not an important mediator of insight’s relationship with self-esteem.5
Implications for Interventions
In these models, change occurred rapidly without specific intervention in the early weeks of treatment, consistent with findings that good initial response drives adherence.58,68 This suggests that the extra impetus of intervention is best timed after this period. It should address attitudes to medication and knowledge of treatment without focusing on awareness of illness, which is intimately related to self-concept and likely to provoke resistance, whereas change in medication attitudes need not do so.
Rationale for Combining Online Psychoeducation, SMS Reminders, and Motivational Interviewing
The complexity of many successful interventions reveals that the interplay between different processes is often critical to intervention as well as understanding.2 This is the case for adherence interventions across a range of disorders: efficacious approaches are complex and include elements common to antipsychotic adherence interventions; none has a large effect on adherence or outcome.109 It is unsurprising that simple interventions for schizophrenia have poor efficacy: motivational interviewing alone fails to make it easier to take tablets, while reminders alone little affect attitudes. Meta-analysis by Pijnenborg et al110 of interventions improving psychotic insight found that simple interventions had moderate or small effect sizes (eg, psychoeducation—d = 0.42, CI: −0.13, 0.98; adherence therapy—d = 0.26, CI: −0.23, 0.76), but two studies of complex interventions had far larger effects (d = 0.69, CI: −0.15, 1.23). As discussed, individual characteristics that make natural, potentially synergistic, targets for intervention are attitudes to medication, insight into symptoms and the need for treatment, and neurocognitive deficits. Existing research indicates the feasibility and acceptability of three potential elements of a combined approach to them.
Motivational Interviewing.
This has been a highly influential approach to changing attitudes in substance misuse treatment.111 Despite initial optimism,112 motivational approaches alone have not continued their success in chronic schizophrenia.113,114 They may not address enough of the complex causes of poor adherence alone110 or perhaps adherence measures are failing to capture improvement because in Kemp’s trial112 readmission reduced and in another trial by Schulz et al,114 symptoms improved. In early illness, a mirror image study showed reduction in relapse after therapy.115 On the other hand, the trial by Staring et al116 showed substantial improvement in adherence (but not insight), d = 0.48, after a complex intervention involving motivational interviewing for those with negative attitudes (87% of the participants) but with other techniques for those with poor response or those who were chaotic. One such technique is use of reminders.
SMS Reminders.
Mobile phone–based “mHealth” approaches are natural in predominantly young early psychosis patients. Prompts may increase the proportion of psychosis sufferers attending clinic,117 including SMS reminders,118 which are capable of altering behavior across a range of disorders.119 Several studies have confirmed that SMS reminders are acceptable and feasible in schizophrenia sufferers.118,120 Spaniel et al121,122 showed an increase in adherence after automatic SMS texting was started (the Information Technology Aided Relapse Prevention in Schizophrenia [ITAREPS] system) in a mirror image design. An open randomized controlled trial of the ITAREPS system over a year in 45 Japanese schizophrenia sufferers found a just-significant decrease in hazard of relapse (0.21, CI: 0.04, 0.99), loosely defined, and a significant decrease in in-patient days from 88 to 19 days.123
Psychoeducation.
This is an obvious need after first episodes. Although one meta-analysis found psychoeducation’s effect on insight was appreciable but not significant because of the small number of trials,110 another124 found that psychoeducation improved adherence in the medium term and was likely to reduce relapse (relative risk, RR: 0.70, CI: 0.61, 0.81) and readmission (RR: 0.71; CI: 0.56, 0.89). Briefer interventions had less effect on nonadherence (RR: 0.63; CI: 0.41, 0.96) but no evidence of different effect on relapse (RR: 0.61; CI: 0.43, 0.89). Though using information technology to provide this education was less well supported, the review by Välimäki et al125 finds a significant decrease in the risk of nonadherence (RR: 0.45, CI: 0.27, 0.77) in one, probably open, randomized trial in 71 chronic sufferers.
Proposed Trial
Given the evidence concerning early adherence’s determinants and interventions, we proposed a combined intervention for a randomized controlled trial of a multimodal intervention within the OPTiMiSE program.9 The approach chosen includes SMS text reminders to take medication, psychoeducation by website, and motivational interviewing. Raters blind to allocation assess attitudes (DAI),79 knowledge (Knowledge About Psychosis Inventory126), insight (Insight Scale102), adherence (Compliance Rating Scale112), symptoms (PANSS103), and side effects at baseline, the end of the 6-week initial intervention, and after 3 months (after the final booster sessions) and 12 months. Relapse is the primary outcome and hospitalization a secondary one.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
European Commission via the European Community’s Seventh Framework Programme (Grant Agreement HEALTH-F2-2010-242114).
Supplementary Material
Acknowledgments
We acknowledge support from Dr Richard Emsley, Lecturer in Biostatistics, University of Manchester, and Dr Ankur Khanna, Manchester Mental Health & Social Care NHS Trust. C.A. has been a consultant to or has received honoraria or grants from Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Janssen Cilag, Lundbeck, Merck, Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Ministerio de Economía y Competitividad, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Takeda, and Schering Plough. B.G. is the leader of a Lundbeck Foundation Center of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), which is partially financed by an independent grant from the Lundbeck Foundation based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. She has nothing else to declare. In the last 3 years, S.L. has received honoraria for lectures from Abbvie, BristolMyersSquibb, ICON, EliLilly, Janssen, Johnson & Johnson, Roche, SanofiAventis, Lundbeck, Servier, and Pfizer and for consulting/advisory boards from Roche, EliLilly, Medavante, BristolMyersSquibb, Janssen, Johnson & Johnson, and Lundbeck. EliLilly has provided medication for a study with S.L. as primary investigator. No other authors acknowledged a conflict of interest.
References
- 1.Barkhof E, Meijer CJ, de Sonneville LM, Linszen DH, de Haan L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia—a review of the past decade. Eur Psychiatry. 2012;27:9–18. [DOI] [PubMed] [Google Scholar]
- 2.Beck EM, Cavelti M, Kvrgic S, Kleim B, Vauth R. Are we addressing the ‘right stuff’ to enhance adherence in schizophrenia? Understanding the role of insight and attitudes towards medication. Schizophr Res. 2011;132:42–49. [DOI] [PubMed] [Google Scholar]
- 3.Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172:53–59. [PubMed] [Google Scholar]
- 4.Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001;178:506–517. [DOI] [PubMed] [Google Scholar]
- 5.Drake RJ, Pickles A, Bentall RP, et al. The evolution of insight, paranoia and depression during early schizophrenia. Psychol Med. 2004;34:285–292. [DOI] [PubMed] [Google Scholar]
- 6.Crumlish N, Whitty P, Kamali M, et al. Early insight predicts depression and attempted suicide after 4 years in first-episode schizophrenia and schizophreniform disorder. Acta Psychiatr Scand. 2005;112:449–455. [DOI] [PubMed] [Google Scholar]
- 7.Ayesa-Arriola R, Moríñigo JD, David AS, Pérez-Iglesias R, Rodríguez-Sánchez JM, Crespo-Facorro B. Lack of insight 3 years after first-episode psychosis: an unchangeable illness trait determined from first presentation? Schizophr Res. 2014;157:271–277. [DOI] [PubMed] [Google Scholar]
- 8.OPTIMISE. http://www.optimisetrial.eu/ Accessed November 28, 2014.
- 9.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new Medical Research Council guidance. BMJ. 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32:724–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassidy CM, Rabinovitch M, Schmitz N, Joober R, Malla A. A comparison study of multiple measures of adherence to antipsychotic medication in first-episode psychosis. J Clin Psychopharmacol. 2010;30:64–67. [DOI] [PubMed] [Google Scholar]
- 12.Verdoux H, Lengronne J, Liraud F, et al. Medication adherence in psychosis: predictors and impact on outcome. A 2-year follow-up of first-admitted subjects. Acta Psychiatr Scand. 2000;102:203–210. [DOI] [PubMed] [Google Scholar]
- 13.Mojtabai R, Lavelle J, Gibson PJ, et al. Gaps in use of antipsychotics after discharge by first-admission patients with schizophrenia, 1989 to 1996. Psychiatr Serv. 2002;53:337–339. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovitch M, Béchard-Evans L, Schmitz N, Joober R, Malla A. Early predictors of nonadherence to antipsychotic therapy in first-episode psychosis. Can J Psychiatry. 2009;54:28–35. [DOI] [PubMed] [Google Scholar]
- 15.Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr Scand. 2002;106:286–290. [DOI] [PubMed] [Google Scholar]
- 16.Svedberg B, Mesterton A, Cullberg J. First-episode non-affective psychosis in a total urban population: a 5-year follow-up. Soc Psychiatry Psychiatr Epidemiol. 2001;36:332–337. [DOI] [PubMed] [Google Scholar]
- 17.Gearing RE, Mian I, Sholonsky A, et al. Developing a risk-model of time to first-relapse for children and adolescents with a psychotic disorder. J Nerv Ment Dis. 2009;197:6–14. [DOI] [PubMed] [Google Scholar]
- 18.Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83:53–63. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson JF, Alvarez-Jimenez M, Cotton SM, Parker AG, Hetrick S. A systematic review of relapse measurement in randomized controlled trials of relapse prevention in first-episode psychosis. Schizophr Res. 2010;119:79–88. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Baki A, Ouellet-Plamondon C, Malla A. Pharmacotherapy challenges in patients with first-episode psychosis. J Affect Disord. 2012;138(suppl):S3–S14. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Jimenez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139:116–128. [DOI] [PubMed] [Google Scholar]
- 22.Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. [DOI] [PubMed] [Google Scholar]
- 23.Caseiro O, Pérez-Iglesias R, Mata I, et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res. 2012;46:1099–1105. [DOI] [PubMed] [Google Scholar]
- 24.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–2071. [DOI] [PubMed] [Google Scholar]
- 25.Bodén R, Brandt L, Kieler H, Andersen M, Reutfors J. Early non-adherence to medication and other risk factors for rehospitalization in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;133:36–41. [DOI] [PubMed] [Google Scholar]
- 26.Crespo-Facorro B, Pérez-Iglesias R, Mata I, et al. Long-term (3-year) effectiveness of haloperidol, risperidone and olanzapine: results of a randomized, flexible-dose, open-label comparison in first-episode nonaffective psychosis. Psychopharmacology (Berl). 2012;219:225–233. [DOI] [PubMed] [Google Scholar]
- 27.Wunderink L, Nienhuis FJ, Sytema S, Slooff CJ, Knegtering R, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry. 2007;68:654–661. [DOI] [PubMed] [Google Scholar]
- 28.Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70:913–920. [DOI] [PubMed] [Google Scholar]
- 29.Wunderink L, Sytema S, Nienhuis FJ, Wiersma D. Clinical recovery in first-episode psychosis. Schizophr Bull. 2009;35:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaebel W, Riesbeck M, Wölwer W, et al. Rates and predictors of remission in first-episode schizophrenia within 1 year of antipsychotic maintenance treatment. Results of a randomized controlled trial within the German Research Network on Schizophrenia. Schizophr Res. 2014;152:478–486. [DOI] [PubMed] [Google Scholar]
- 31.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel MX, Taylor M, David AS. Antipsychotic long-acting injections: mind the gap. Br J Psychiatry Suppl. 2009;52:S1–S4. [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi-Bensouda L, Rouillon F, Astruc B, et al. Does long-acting injectable risperidone make a difference to the real-life treatment of schizophrenia? Results of the Cohort for the General study of Schizophrenia (CGS). Schizophr Res. 2012;134:187–194. [DOI] [PubMed] [Google Scholar]
- 34.Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. [DOI] [PubMed] [Google Scholar]
- 36.Emsley R, Chiliza B, Asmal L, Mashile M, Fusar-Poli P. Long-acting injectable antipsychotics in early psychosis: a literature review. Early Interv Psychiatry. 2013;7:247–254. [DOI] [PubMed] [Google Scholar]
- 37.Iyer S, Banks N, Roy MA, et al. A qualitative study of experiences with and perceptions regarding long-acting injectable antipsychotics: part II-physician perspectives. Can J Psychiatry. 2013;58:23S–29S. [DOI] [PubMed] [Google Scholar]
- 38.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909. [DOI] [PubMed] [Google Scholar]
- 39.Zygmunt A, Olfson M, Boyer CA, Mechanic D. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653–1664. [DOI] [PubMed] [Google Scholar]
- 40.Velligan DI, Diamond PM, Mintz J, et al. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008;34:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1–46; quiz 47–48. [PubMed] [Google Scholar]
- 42.Quach PL, Mors O, Christensen TØ, et al. Predictors of poor adherence to medication among patients with first-episode schizophrenia-spectrum disorder. Early Interv Psychiatry. 2009;3:66–74. [DOI] [PubMed] [Google Scholar]
- 43.Rabinovitch M, Cassidy C, Schmitz N, Joober R, Malla A. The influence of perceived social support on medication adherence in first-episode psychosis. Can J Psychiatry. 2013;58:59–65. [DOI] [PubMed] [Google Scholar]
- 44.Calvo A, Moreno M, Ruiz-Sancho A, et al. Intervention for adolescents with early-onset psychosis and their families: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53:688–696. [DOI] [PubMed] [Google Scholar]
- 45.McGlashan TH, Carpenter WJ. Does attitude toward psychosis relate to outcome? Am J Psychiatry. 1981;138:797–801. [DOI] [PubMed] [Google Scholar]
- 46.Tait L, Birchwood M, Trower P. Predicting engagement with services for psychosis: insight, symptoms and recovery style. Br J Psychiatry. 2003;182:123–128. [DOI] [PubMed] [Google Scholar]
- 47.Thompson KN, McGorry PD, Harrigan SM. Recovery style and outcome in first-episode psychosis. Schizophr Res. 2003;62:31–36. [DOI] [PubMed] [Google Scholar]
- 48.Hamann J, Cohen R, Leucht S, Busch R, Kissling W. Shared decision making and long-term outcome in schizophrenia treatment. J Clin Psychiatry. 2007;68:992–997. [DOI] [PubMed] [Google Scholar]
- 49.Montreuil TC, Cassidy CM, Rabinovitch M, et al. Case manager- and patient-rated alliance as a predictor of medication adherence in first-episode psychosis. J Clin Psychopharmacol. 2012;32:465–469. [DOI] [PubMed] [Google Scholar]
- 50.Hill M, Crumlish N, Whitty P, et al. Nonadherence to medication four years after a first episode of psychosis and associated risk factors. Psychiatr Serv. 2010;61:189–192. [DOI] [PubMed] [Google Scholar]
- 51.Steger KA, Cassidy C, Rabinovitch M, Joober R, Malla A. Impact of symptom resolution on medication adherence in first episode psychosis. Psychiatry Res. 2012;196:45–51. [DOI] [PubMed] [Google Scholar]
- 52.Drake RJ, Dunn G, Tarrier T, Bentall RP, Haddock G, Lewis SW. Insight as a predictor of the outcome of first episode non-affective psychosis. J Clin Psychiatry. 2007;68:81–86. [DOI] [PubMed] [Google Scholar]
- 53.Czobor P, Volavka J, Derks EM, et al. Insight and hostility as predictors and correlates of nonadherence in the European First Episode Schizophrenia Trial. J Clin Psychopharmacol. 2013;33:258–261. [DOI] [PubMed] [Google Scholar]
- 54.Weiden P, Rapkin B, Mott T, et al. Rating of medication influences (ROMI) scale in schizophrenia. Schizophr Bull. 1994;20:297–310. [DOI] [PubMed] [Google Scholar]
- 55.Petersen L, Thorup A, Øqhlenschlaeger J, et al. Predictors of remission and recovery in a first-episode schizophrenia spectrum disorder sample: 2-year follow-up of the OPUS trial. Can J Psychiatry. 2008;53:660–670. [DOI] [PubMed] [Google Scholar]
- 56.Robinson DG, Woerner MG, Alvir JMJ, Bilder RM, Hinrichsen GA, Lieberman JA. Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophr Res. 2002;57:209–219. [DOI] [PubMed] [Google Scholar]
- 57.Lepage M, Bodnar M, Joober R, Malla A. Is there an association between neurocognitive performance and medication adherence in first episode psychosis? Early Interv Psychiatry. 2010;4:189–195. [DOI] [PubMed] [Google Scholar]
- 58.Perkins DO, Gu H, Weiden PJ, McEvoy JP, Hamer RM, Lieberman JA. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69:106–113. [DOI] [PubMed] [Google Scholar]
- 59.Lam JWS, Lui SSY, Wang Y, Chan RCK, Cheung EFC. Prospective memory predicts medication management ability and correlates with non-adherence to medications in individuals with clinically stable schizophrenia. Schizophr Res. 2013;147:293–300. [DOI] [PubMed] [Google Scholar]
- 60.Heinrichs RW, Goldberg JO, Miles AA, McDermid Vaz S. Predictors of medication competence in schizophrenia patients. Psychiatry Res. 2008;157:47–52. [DOI] [PubMed] [Google Scholar]
- 61.Twamley EW, Woods SP, Zurhellen CH, et al. Neuropsychological substrates and everyday functioning implications of prospective memory impairment in schizophrenia. Schizophr Res. 2008;106:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jónsdóttir H, Opjordsmoen S, Birkenaes AB, et al. Predictors of medication adherence in patients with schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2013;127:23–33. [DOI] [PubMed] [Google Scholar]
- 63.Baloush-Kleinman V, Levine SZ, Roe D, Shnitt D, Weizman A, Poyurovsky M. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130:176–181. [DOI] [PubMed] [Google Scholar]
- 64.Gaebel W, Riesbeck M, von Wilmsdorff M, et al. Drug attitude as predictor for effectiveness in first-episode schizophrenia: results of an open randomized trial (EUFEST). Eur Neuropsychopharmacol. 2010;20:310–316. [DOI] [PubMed] [Google Scholar]
- 65.Fraguas D, Llorente C, Rapado-Castro M, et al. Attitude toward antipsychotic medication as a predictor of antipsychotic treatment discontinuation in first-episode early-onset psychosis. Rev Psiquiatr Salud Ment. 2008;1:10–17. [DOI] [PubMed] [Google Scholar]
- 66.de Haan L, van Amelsvoort T, Dingemans P, Linszen D. Risk factors for medication non-adherence in patients with first episode schizophrenia and related disorders; a prospective five year follow-up. Pharmacopsychiatry. 2007;40:264–268. [DOI] [PubMed] [Google Scholar]
- 67.Barbeito S, Vega P, Ruiz de Azúa S, et al. Cannabis use and involuntary admission may mediate long-term adherence in first-episode psychosis patients: a prospective longitudinal study. BMC Psychiatry. 2013;13:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McEvoy JP, Johnson J, Perkins D, et al. Insight in first-episode psychosis. Psychol Med. 2006;36:1385–1393. [DOI] [PubMed] [Google Scholar]
- 69.Ko NY, Yeh ML, Hsu ST, Chung HH, Yen CF. Investigation of insight formation using narrative analyses of people with schizophrenia in remission. J Nerv Ment Dis. 2006;194:124–127. [DOI] [PubMed] [Google Scholar]
- 70.Moore A, Sellwood W, Stirling J. Compliance and psychological reactance in schizophrenia. Br J Clin Psychol. 2000;39(pt 3):287–295. [DOI] [PubMed] [Google Scholar]
- 71.Rittmannsberger H, Pachinger T, Keppelmüller P, Wancata J. Medication adherence among psychotic patients before admission to inpatient treatment. Psychiatr Serv. 2004;55:174–179. [DOI] [PubMed] [Google Scholar]
- 72.Day JC, Bentall RP, Roberts C, et al. Attitudes toward antipsychotic medication: the impact of clinical variables and relationships with health professionals. Arch Gen Psychiatry. 2005;62:717–724. [DOI] [PubMed] [Google Scholar]
- 73.Klingberg S, Schneider S, Wittorf A, Buchkremer G, Wiedemann G. Collaboration in outpatient antipsychotic drug treatment: analysis of potentially influencing factors. Psychiatry Res. 2008;161:225–234. [DOI] [PubMed] [Google Scholar]
- 74.Koren D, Seidman LJ, Poyurovsky M, et al. The neuropsychological basis of insight in first-episode schizophrenia: a pilot metacognitive study. Schizophr Res. 2004;70:195–202. [DOI] [PubMed] [Google Scholar]
- 75.McLeod HJ, Gumley AI, Macbeth A, Schwannauer M, Lysaker PH. Metacognitive functioning predicts positive and negative symptoms over 12 months in first episode psychosis. J Psychiatr Res. 2014;54:109–115. [DOI] [PubMed] [Google Scholar]
- 76.Lysaker PH, Roe D, Yanos PT. Toward understanding the insight paradox: internalized stigma moderates the association between insight and social functioning, hope, and self-esteem among people with schizophrenia spectrum disorders. Schizophr Bull. 2007;33:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lysaker PH, McCormick BP, Snethen G, et al. Metacognition and social function in schizophrenia: associations of mastery with functional skills competence. Schizophr Res. 2011;131:214–218. [DOI] [PubMed] [Google Scholar]
- 78.Lysaker PH, Vohs J, Hasson-Ohayon I, Kukla M, Wierwille J, Dimaggio G. Depression and insight in schizophrenia: comparisons of levels of deficits in social cognition and metacognition and internalized stigma across three profiles. Schizophr Res. 2013;148:18–23. [DOI] [PubMed] [Google Scholar]
- 79.Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13:177–183. [DOI] [PubMed] [Google Scholar]
- 80.Parellada M, Fraguas D, Bombín I, et al. Insight correlates in child- and adolescent-onset first episodes of psychosis: results from the CAFEPS study. Psychol Med. 2009;39:1433–1445. [DOI] [PubMed] [Google Scholar]
- 81.Staring AB, Van der Gaag M, Van den Berge M, Duivenvoorden HJ, Mulder CL. Stigma moderates the associations of insight with depressed mood, low self-esteem, and low quality of life in patients with schizophrenia spectrum disorders. Schizophr Res. 2009;115:363–369. [DOI] [PubMed] [Google Scholar]
- 82.Lester H, Marshall M, Jones P, et al. Views of young people in early intervention services for first-episode psychosis in England. Psychiatr Serv. 2011;62:882–887. [DOI] [PubMed] [Google Scholar]
- 83.Evans-Lacko S, Brohan E, Mojtabai R, Thornicroft G. Association between public views of mental illness and self-stigma among individuals with mental illness in 14 European countries. Psychol Med. 2012;42:1741–1752. [DOI] [PubMed] [Google Scholar]
- 84.Corrigan PW, Rafacz J, Rüsch N. Examining a progressive model of self-stigma and its impact on people with serious mental illness. Psychiatry Res. 2011;189:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsang HWH, Fung KMT, Chung RCK. Self-stigma and stages of change as predictors of treatment adherence of individuals with schizophrenia. Schizophr Res. 2010;180:10–15. [DOI] [PubMed] [Google Scholar]
- 86.Jaeger S, Pfiffner C, Weiser P, et al. Adherence styles of schizophrenia patients identified by a latent class analysis of the Medication Adherence Rating Scale (MARS): a six-month follow-up study. Psychiatry Res. 2012;200:83–88. [DOI] [PubMed] [Google Scholar]
- 87.Haley CJ, Drake RJ, Bentall RP, Lewis SW. Health beliefs alter duration of untreated psychosis and attitudes to later treatment in early psychosis. Social Psychiatr Psychiatric Epidemiol. 2002;38:311–316. [DOI] [PubMed] [Google Scholar]
- 88.Pitschel-Walz G, Bäuml J, Bender W, Engel RR, Wagner M, Kissling W. Psychoeducation and compliance in the treatment of schizophrenia: results of the Munich Psychosis Information Project Study. J Clin Psychiatry. 2006;67:443–452. [DOI] [PubMed] [Google Scholar]
- 89.Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66:51–57. [DOI] [PubMed] [Google Scholar]
- 90.Olfson M, Uttaro T, Carson WH, Tafesse E. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry. 2005;66:331–338. [DOI] [PubMed] [Google Scholar]
- 91.Masi G, Liboni F. Management of schizophrenia in children and adolescents: focus on pharmacotherapy. Drugs. 2011;71:179–208. [DOI] [PubMed] [Google Scholar]
- 92.Perkins DO. Adherence to antipsychotic medications. J Clin Psychiatry. 1999;60(suppl 21):25–30. [PubMed] [Google Scholar]
- 93.Sibitz I, Unger A, Woppmann A, Zidek T, Amering M. Stigma resistance in patients with schizophrenia. Schizophr Bull. 2011;37:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cavelti M, Rüsch N, Vauth R. Is living with psychosis demoralizing? Insight, self-stigma, and clinical outcome among people with schizophrenia across 1 year. J Nerv Ment Dis. 2014;202:521–529. [DOI] [PubMed] [Google Scholar]
- 95.Shahar G, Davidson L. Depressive symptoms erode self-esteem in severe mental illness: a three-wave, cross-lagged study. J Consult Clin Psychol. 2003;71:890–900. [DOI] [PubMed] [Google Scholar]
- 96.Lewis SW, Tarrier N, Haddock G, et al. A randomised, controlled trial of cognitive-behaviour therapy in acute, early schizophrenia: the SOCRATES trial. Br J Psychiatry. 2002;181(suppl 43):91–98. [DOI] [PubMed] [Google Scholar]
- 97.Tarrier N, Lewis S, Haddock G, et al. Cognitive-behavioural therapy in first-episode and early schizophrenia: 18-month follow-up of a randomised controlled trial. Br J Psychiatry. 2004;184:231–239. [DOI] [PubMed] [Google Scholar]
- 98.Wiffen BD, Rabinowitz J, Lex A, David AS. Correlates, change and ‘state or trait’ properties of insight in schizophrenia. Schizophr Res. 2010;122:94–103. [DOI] [PubMed] [Google Scholar]
- 99.Tait L, Birchwood M, Trower P. Adapting to the challenge of psychosis: personal resilience and the use of sealing-over (avoidant) coping strategies. Br J Psychiatry. 2004;185:410–415. [DOI] [PubMed] [Google Scholar]
- 100.Amador XF, David AS, eds. Insight in Psychosis. 2nd ed. New York, NY: OUP; 2004. [Google Scholar]
- 101.Rosenberg M.Society and the Adolescent Self Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 102.Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62–67. [DOI] [PubMed] [Google Scholar]
- 103.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 104.SPSS. SPSS and AMOS 20.0. Chicago, IL: SPSS Inc; 2009. [Google Scholar]
- 105.Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. [DOI] [PubMed] [Google Scholar]
- 106.Little RJA, Rubin DB.Statistical Analysis With Missing Data. 2nd ed. New York, NY: John Wiley; 2002. [Google Scholar]
- 107.Cavelti M, Kvrgic S, Beck EM, Rüsch N, Vauth R. Self-stigma and its relationship with insight, demoralization, and clinical outcome among people with schizophrenia spectrum disorders. Compr Psychiatry. 2012;53:468–479. [DOI] [PubMed] [Google Scholar]
- 108.Heinrichs DW, Cohen BP, Carpenter WT., Jr Early insight and the management of schizophrenic decompensation. J Nerv Ment Dis. 1985;173:133–138. [DOI] [PubMed] [Google Scholar]
- 109.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. [DOI] [PubMed] [Google Scholar]
- 110.Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophr Res. 2013;144:109–117. [DOI] [PubMed] [Google Scholar]
- 111.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: The Guilford Press; 2002. [Google Scholar]
- 112.Kemp R, Kirov G, Everitt B, Hayward P, David A. Randomised controlled trial of compliance therapy: 18-month follow-up. Br J Psychiatry. 1998;172:413–419. [DOI] [PubMed] [Google Scholar]
- 113.Gray R, Leese M, Bindman J, et al. Adherence therapy for people with schizophrenia. European multicentre randomised controlled trial. Br J Psychiatry. 2006;189:508–514. [DOI] [PubMed] [Google Scholar]
- 114.Schulz M, Gray R, Spiekermann A, Abderhalden C, Behrens J, Driessen M. Adherence therapy following an acute episode of schizophrenia: a multi-centre randomised controlled trial. Schizophr Res. 2013;146:59–63. [DOI] [PubMed] [Google Scholar]
- 115.Brown E, Gray R, Jones M, Whitfield S. Effectiveness of adherence therapy in patients with early psychosis: a mirror image study. Int J Ment Health Nurs. 2013;22:24–34. [DOI] [PubMed] [Google Scholar]
- 116.Staring AB, Van der Gaag M, Koopmans GT, et al. Treatment adherence therapy in people with psychotic disorders: randomised controlled trial. Br J Psychiatry. 2010;197:448–455. [DOI] [PubMed] [Google Scholar]
- 117.Reda S, Rowett M, Makhoul S. Prompts to encourage appointment attendance for people with serious mental illness. Cochrane Database Syst Rev. 2001;2:CD002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pijnenborg G, Withaar F, Brouwer W, Timmerman M, Bosch R, Evans J. The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. Br J Clin Psychol. 2010;49:259–274. [DOI] [PubMed] [Google Scholar]
- 119.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36:165–173. [DOI] [PubMed] [Google Scholar]
- 120.Humphrey Beebe L, Smith K, Bennett C, et al. Cell phone use in people with schizophrenia spectrum disorders. J Psychosoc Nurs. 2010;48:32–37. [DOI] [PubMed] [Google Scholar]
- 121.Spaniel F, Vohlídka P, Hrdlicka J, et al. ITAREPS: information technology aided relapse prevention programme in schizophrenia. Schizophr Res. 2008;98:312–317. [DOI] [PubMed] [Google Scholar]
- 122.Spaniel F, Vohlídka P, Kozený J, et al. The Information Technology Aided Relapse Prevention Programme in Schizophrenia: an extension of a mirror-design follow-up. Int J Clin Pract. 2008;62:1943–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Komatsu H, Sekine Y, Okamura N, et al. Effectiveness of Information Technology Aided Relapse Prevention Programme in Schizophrenia excluding the effect of user adherence: a randomized controlled trial. Schizophr Res. 2013;150:240–244. [DOI] [PubMed] [Google Scholar]
- 124.Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database Syst Rev. 2011;6:CD002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Välimäki M, Hätönen H, Lahti M, Kuosmanen L, Adams CE. Information and communication technology in patient education and support for people with schizophrenia. Cochrane Database Syst Rev. 2012;10:CD007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barrowclough C, Tarrier N, Watts S, Vaughn C, Bamrah JS, Freeman HL. Assessing the functional value of relatives’ knowledge about schizophrenia: a preliminary report. Br J Psychiatry. 1987;151:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.