Summary
Phospholipase D (PLD) is capable of hydrolyzing membrane phospholipids, producing phosphatidic acid. To alter phospholipid profiles in soybean seed, we attenuated PLD enzyme activity by an RNA interference construct using the partial sequence from a soybean PLDα gene. Two transgenic soybean lines were established by particle inflow gun bombardment by co-bombarding with pSPLDi and pHG1 vectors. The lines were evaluated for the presence and expression of transgenes thoroughly through the T4 generation. PLD-suppressed soybean lines were characterized by decreased PLDα enzyme activity and decreased PLDα protein both during seed development and in mature seeds. There was no change in total phospholipid amount; however the PLD-attenuated transgenic line, SW, had higher levels of di18:2 (dilinoleoyl)-phosphatidylcholine (PC) and -phosphatidylethanolamine (PE) in seeds than the non-transgenic lines. The increased polyunsaturation was at the expense of PC and PE species containing monounsaturated or saturated fatty acids. In addition to increased unsaturation in the phospholipids, there was a decrease in unsaturation of the triacylglycerol (TAG) fraction of the soybean seeds. Considering recent evidence for the notion that desaturation of fatty acids occurs in the PC fraction and that the PC → DAG (diacylglycerol) → TAG pathway is the major route of TAG biosynthesis in developing soybean seed, the current data suggest that PLDα suppression slows the conversion of PC to TAG. This would be consistent with PLD playing a positive role in that conversion. The data indicate that soybean PLD attenuation is a potentially useful approach to altering properties of edible and industrial soybean lecithin.
Keywords: genetic transformation, phospholipase D, soybean lecithin
Introduction
Modifying soybean oil composition is one goal in soybean breeding programs (Burton, 2005). To improve food and other applications of soybean oil, it is desirable to reduce saturated and polyunsaturated fatty acid and increase monounsaturated fatty acid composition (Wilson, 2004). Several soybean genotypes with altered fatty acid compositions have been established by conventional breeding, mutation breeding, and transgene application (Sandhu et al., 2007; Burton et al., 2005; Singh and Hymowitz, 1999).
To improve the functionality of the phospholipid mixtute, soybean lecithin, in a variety of applications, modification of its composition is another breeding goal. Soybean lecithin has both hydrophobic and hydrophilic properties, allowing it to serve as a surface tension-reducing agent or emulsifier (Krog, 1997), and it is a widely used in the food, cosmetic, technical product, and pharmaceutical sectors (Wendel, 1995). De-oiled soybean lecithin, obtained as a byproduct of the soy oil extraction process, typically contains 23% PC, 20% PE, 14% phosphatidylinositol (PI), 8% phosphatidic acid (PA), 8% minor phospholipids, 15% glycolipids, 8% complex sugars, and 3% neutral lipids (Cherry and Kramer, 1989). Soybean lecithin contains more polyunsaturated fatty acid than egg yolk lecithin, but data suggest that soybean lecithin is still quite stable to lipid oxidation (Wu and Wang, 2003; Palacios and Wang 2005).
Phospholipase D in plants has been implicated as being involved in the anabolism and catabolism of phospholipids, including the degradation of membrane lipids during senescence and necrosis. Other functions of PLD in plants, animals, and microbes have been revealed by cloning PLD genes. In higher plants, individual PLD isoenzymes play specific and different roles in membrane remodeling, cell signaling during development, stress response, and programmed cell death (Zhang et al., 2003; Taylor and Low, 1997). PLDs in the PLDα group encode the “conventional” type of PLDs in plants. PLDα1 has high activity at millimolar concentrations of Ca2+ and is PIP2-independent. PLDα1 is relatively highly expressed in a broad range of plant cell types, and its enzymatic activity tends to decline as plants mature, while soluble and membrane-bound, PIP2-dependent PLD activities, such as those encoded by PLDβ and PLDγ, peak at the end of seed maturation in Brassica napus seeds (Novotna et al. 2000; Pappan et al. 2004; Wang 2005). Recent soybean genome sequencing and annotation (www.phytozome.net) have identified 18 full-length soybean PLD sequences. Sequence analysis indicates that these include 6 PLDα-type, 3 PLDβ-type, 2 PLDγ-type, 3 PLDδ-type, 4 PLDε-type, and 0 PLDζ-type genes.
At the time that we started to work on PLD from soybean, a full-length soybean PLD gene had not been cloned, although 45 PLD expressed sequence tags (ESTs) from soybean were detectable in the NCBI nucleotide database. Abousalham et al. (1995) had purified a 92-kD soybean PLD from a soybean cell suspension culture using hydrophobic affinity chromatography. The activity of the soybean PLD was affected by millimolar Ca2+ concentrations, in a manner similar to PLDα gene products (Abousalham et al., 1995). The sequence of the 15 amino acid residues at the N-terminus of the 92-kD soybean PLD was similar to the sequence in cabbage and castor bean PLDs.
The effect of PLDα activity in soybean seeds on the quality and quantity of soybean lecithin and storage lipid is not known. In this study, we established transgenic soybeans with attenuated PLDα activity in the seed and analyzed changes in lipid profiles of phospholipids and TAG in the seeds. Understanding and interpreting compositional changes in soybean PL and TAG molecular species requires an understanding of the metabolic relationships among the lipid classes. Recently, Bates et al. (2009) analyzed acyl fluxes in developing soybean embryos. A major pathway of TAG synthesis in developing seeds includes acylation of the sn-1 and sn-2 positions of glycerol-3-phosphate (G3P) to form PA and then DAG, conversion of DAG to PC, conversion of PC to a separate pool of DAG, and then 3-acylation of this DAG to form TAG. Bates and co-workers determined that PC plays a key role in the TAG synthesis pathway, both as the molecule on which fatty acid desaturation occurs and as the major site for incorporation of newly synthesized and recycled fatty acids by acyl editing (Bates et al., 2009). Their data indicate that most TAG in soybean seeds is made from DAG derived from PC.
We report here that the suppression of soybean PLD resulted in alteration of phospholipid profiles. Furthermore, the composition of storage lipid, TAG, was changed in PLD-attenuated soybean seeds. These results indicate that soybean PLDα is a candidate gene for creation of value-added soybean cultivars for the seed industry. We also propose that PLD may play a biochemical role in conversion of PC to TAG in soybean seed.
Results
Recovering PLDα-attenuated soybean transformants
Soybean cultivars “Fayette” and “Flyer” were co-transformed with pHG1 and pSPLDi via particle inflow gun bombardment and regenerated under hygromycin selection (Figure 1). The presence of pSPLDi transgenes was confirmed in all transformed embryogenic calli and leaves by PCR throughout the regeneration procedures and all transgenic generations. To confirm the presence of the transgene in the presence of the endogenous PLDα gene, PCR was performed by amplifying the 3’ end of β-conglycinin promoter fragment to loop fragment (800 bp) and loop fragment to the 5’ end of β-conglycinin terminator fragment. Primers from β-conglycinin promoter and terminator were used for PCR as an additional verification of transgene integration. Amplicons from PCR reactions were of the predicted size from each of the positive transgenic events. One hundred forty-three putative tissues were recovered after various bombardments and tested using gene of interest (GOI) and hygromycin PCR analyses. Twelve transgenic soybean events were recovered and fully grown in the greenhouse. Leading transgenic events, SW and SI, were selected from twelve transgenic events based on agronomic traits, such as fertility and yield, and molecular analysis, including PCR, Southern blotting, western blotting, and PLDα enzyme assay. All T0 individual transgenic lines were PCR-positive with amplification from two different primer sets. Nomenclature to describe the transgenic progeny used a prefix based on the name of transformants (‘SW’ for Fayette background and ‘SI’ for Flyer background) followed by one or more numbers separated by dashes. The first number after the prefix indicated the seed harvested from an individual plant in the T0 generation, the second number indicated the seed from an individual plant in the T1 generation, etc. Null transgenic lines for each event were selected in T1 generation by PCR and Southern blot analysis.
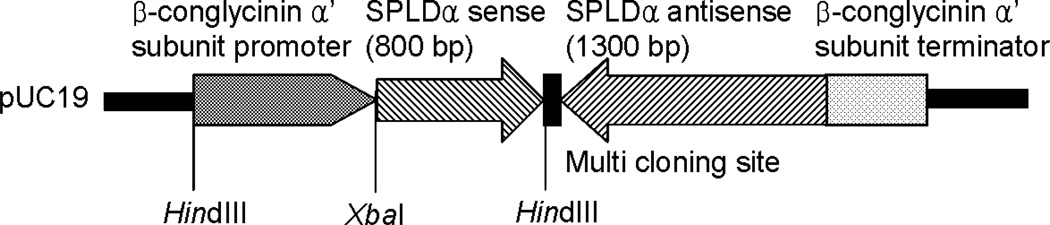
Figure 1.
Restriction map of pSPLDi plant expression vector.
Southern blot analyses of SW transgenic soybean lines
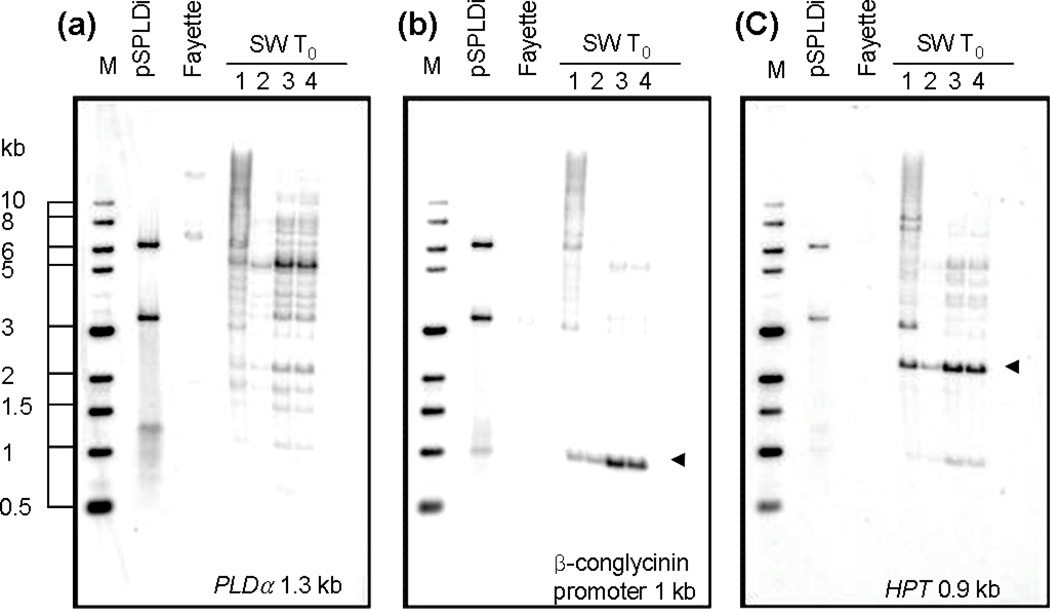
Southern blot analyses were executed to detect the presence of transgenes and to determine the copy number and integration pattern of exogenous and endogenous PLDα. Southern blot analyses were performed using three different probes; a partial SPLDα ORF fragment, a β-conglycinin promoter fragment, and a HPT ORF fragment. Genomic DNA was digested with HindIII, digesting two sites in pSPLDi, which are at the 5’ end of β-conglycinin promoter sequence and at the end of the PLD sense fragment, with double-cuts in pHG1. The hybridization pattern using a β-conglycinin probe showed that the β-conglycinin promoter was digested by HindIII at both ends. HPT hybridizing banding patterns confirmed the presence of the HPT transgene in all T0 SW individual plants (Figure 2). The banding patterns also demonstrated that the pSPLDi and pHG1 plasmids were co-integrated into genomic DNA.
Figure 2.
Southern blot analysis of PLDα-suppressed T0 soybean lines with negative controls. Fifteen µg of genomic DNA were digested with HindIII. Digested genomic DNA from SW T0 young leaves was hybridized with a PLDα probe (a), beta-conglycinin promoter probe (b), and HPT probe (c). The probe used for DNA-DNA hybridization was indicated on the bottom of each gel image. pSPLDi transgene was linearized by HindIII digestion (a), whereas the β-conglycinin promoter (b) and HPT gene (c) were digested by HindIII. Triangles indicate the bands corresponding to the β-conglycinin promoter (b) and HPT gene (c) in the transgene in individual PLDα-suppressed T0 soybean, SW1 to 4.
Two copies of intrinsic PLDα were detected in the background soybean cultivar, Fayette, with different restriction enzymes. The banding patterns of the T0 to T4 progenies of the same transgenic event, detected using HindIII digestion, showed a single band (Figure 2). SW1–2–8-1, SW1–3–8-2, and SW1–3–8-3 lines showed a polymorphic single band with XbaI digestion (Figure S1). The results implied that integration of the transgene is highly linked in clusters at a locus in the soybean genome.
PLDα gene expression in PLD-attenuated transgenic soybean, null transgenic soybean, and background cultivar seeds
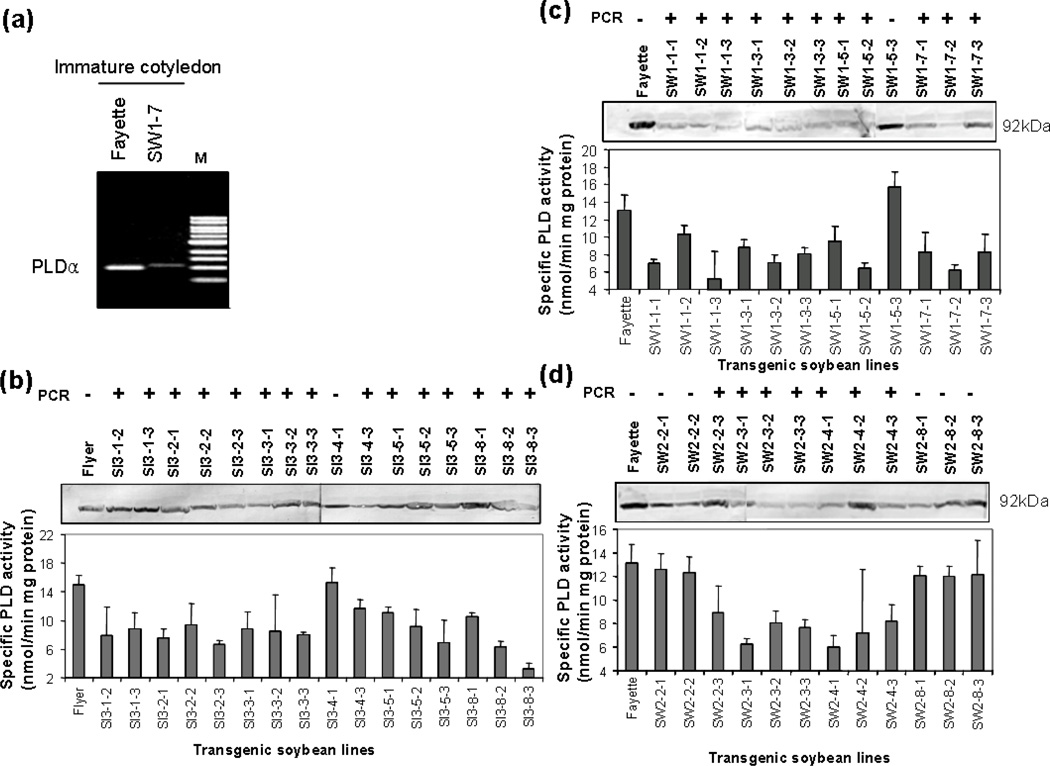
T1 seedlings were screened for the presence of the transgene by planting T1 seeds on MS medium with 100 mg/L hygromycin for 10 days; putative pSPLDi transgenic seedlings were identified based on lack of hygromycin-derived necrotic symptoms. Hygromycin-tolerant plants were further screened by GOI PCR. T2 transgenic soybean seeds were collected at approximately 28 DAF. Each seed was divided into thirds for DNA, RNA, and protein analyses. The presence of pSPLDi transgene was detected by gDNA PCR with pSPLDi-specific primer sets, mentioned above. Different levels of PLDα transcript were detected in immature cotyledons from pSPLDi transgenic and background cultivar soybean by RT-PCR. Fewer PLDα transcripts accumulated in immature cotyledons from the SW1–7 line than in the background soybean cultivar (Figure 3a). Immunoblotting with Arabidopsis PLDα1 antibody, binding to a 92 kD PLDα protein, showed that different levels of PLDα accumulation were detected in transgenic, null transgenic, and background cultivar seeds (Figure 3b, c, and d). PLDα enzyme activity in immature cotyledons of transgenic lines was observed to be approximately 20 to 80% lower than the activity in the background cultivars. Among the transgenic progeny, SI3–8-3 showed the lowest relative PLD activity with 21.5% the activity of the background cultivar.
Figure 3.
PCR, RT-PCR, immunoblot and enzyme assay of PLDα in immature cotyledons from heterozygous T2 seed from SW1, SW2, and SI3 lines. (a); Changes in PLDα mRNA in the immature cotyledon from SW1–7 and background cultivar, Fayette. One microgram of total RNA was used for a reverse transcriptase reaction, and one twentieth of the aliquot was taken for cDNA PCR with a PLDα internal primer. The gel was stained with ethidium bromide after 25 cycles. M = markers. (b), (c), and (d); PCR, immunoblot and enzyme assay of PLDα from SI3, SW1, and SW2 lines respectively. Immature cotyledons were split with a razor blade into three parts. One part of the tissue was used for PCR analysis to confirm the presence of the modified PLDα transgene (top of each panel). Immunoblot analysis of PLDα was performed with AtPLDα antibody, and western blots of the PLDα protein at 92 kD position (based on protein molecular-weight markers) are shown in each panel. At the bottom of each panel, the PLDα enzyme activity, expressed as nmoles of phosphatidylcholine min−1 mg−1 total protein, is shown. Error bars indicate ± SD of three independent seed measurements.
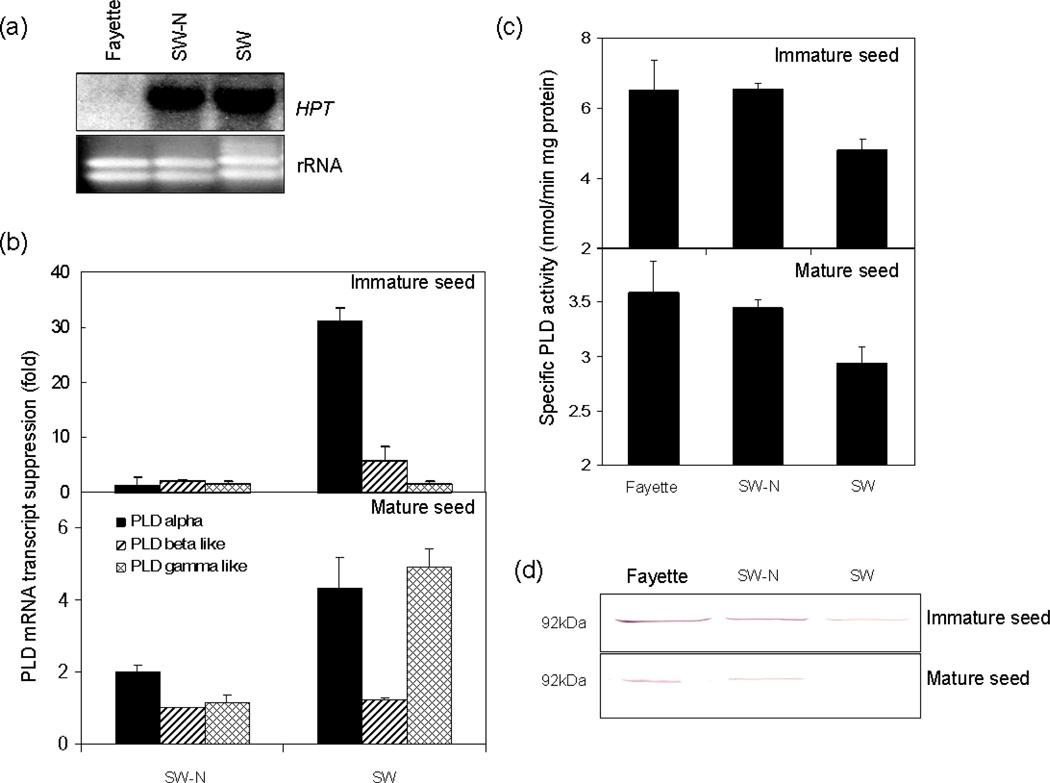
The transcript of HPT in PLDα-attenuated transgenic, null transgenic, and background soybean lines, observed by RNA blot analysis (Figure 4a), suggested that the pHG1 transgene was stably over-expressed in the T3 generation. The endogenous PLDα transcript was barely detected by RNA blotting analysis. To confirm changes in the level of endogenous PLDα mRNA, quantitative PCR was performed with primers that amplified 130 base pairs from the left arm of the PLDα mRNA sequence which was supposed to be amplifying endogenous PLDα mRNA. The amount of endogenous PLDα in immature and mature SW transgenic soybean seeds was reduced 30 and 4.5 fold respectively relative to the background cultivar. No suppression of PLDα transcript was detected in the null transgenic, SW-N, seed (Figure 4b). PLDα expression was suppressed strongly by the transgene in immature seeds, whereas PLDγ expression was not affected and PLDβ was somewhat affected. However, in mature seeds, suppression of PLDγ expression as well as PLDα expression was observed. PLDβ expression was not affected by the presence of the transgene in mature seeds. Reduced amounts of PLDα protein were accumulated and PLDα enzyme tended to exhibit reduced activity in SW seed compared to null transgenic (SW-N) and background soybean seed (Figure 4c and d). Although the data suggest that other PLDs such as PLDγ may be suppressed in addition to PLDα, by the transgene, the results indicate that PLDα gene expression, protein, and enzyme activity were attenuated by the expression of the RNAi construct, and that PLDα suppression occurs throughout seed development.
Figure 4.
Levels of mRNA and PLDα protein in the immature and mature cotyledons from SW progeny and the background cultivar ‘Fayette’. The SW progeny line, SW-N, was characterized by PCR and Southern blot analysis as a homozygous, non-transgenic line, segregated from the T2 progeny of SW. (a); RNA blot analysis of steady-state levels for HPT in transgenic soybean lines and background cultivar. Total RNA are from the immature soybean seeds that were background cultivar (Fayette), null transgenic line (SW-N), and PLDα suppressed line (SW). (b); Quantitative PCR analysis of PLDαβ-like, and γ-like mRNAs from immature and mature cotyledon respectively. The levels of endogenous of PLDαβ-like, and γ-like mRNAs were measured by real-time PCR from null transgenic line (SW-N) and PLDα suppressed line (SW). Changes in PLD mRNA transcription were represented suppression compared to the level of PLD mRNA transcription in Fayette. PCR primers of soybean PLDβ-like and γ-like were designed from soybean EST clones, gi:21888889 and gi:17401471, which showed sequence similarity to 5’UTR region in AtPLDβ and AtPLDγ respectively. (c); Changes in PLDα activities in immature and mature cotyledons from null transgenic line (SW-N) and PLDα suppressed line (SW). The PLDα enzyme activity was expressed as nmoles of phosphatidylcholine min−1 mg−1 total protein. Error bars indicates ± SE with three independent measurements. (d); Immunoblot analysis of cytosolic protein from immature and mature cotyledon with polyclonal anti-AtPLDα antibody. Proteins (40 µg per lane) were separated in SDS-PAGE in 12% polyacylamide gel and PLDα protein was visualized by alkaline phosphatase.
Changes in lipid profiles in PLDα- attenuated soybean seed
The molecular species of PC, PE, PG, PI, PS, PA, lysoPC, lysoPE, and TAG were characterized by ESI tandem mass spectrometry (ESI-MS/MS). ESI-MS/MS was able to detect minor lipid classes such as PG and PA as well as plastidically produced galactolipids, monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), in soybean seeds. ESI-MS/MS data provided quantitative information on molecular species of phospholipid and triacylglycerol as a result of suppressing PLDα activity in soybean seeds.
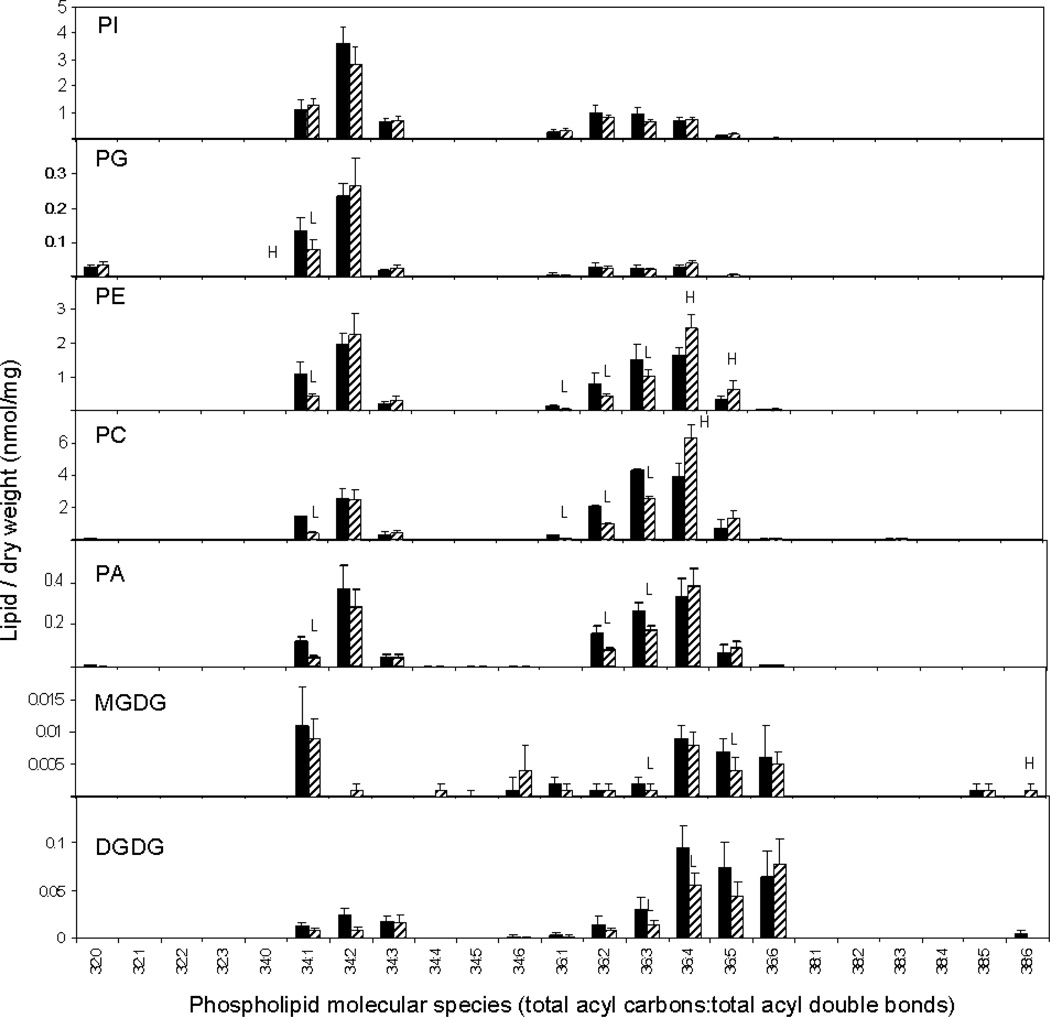
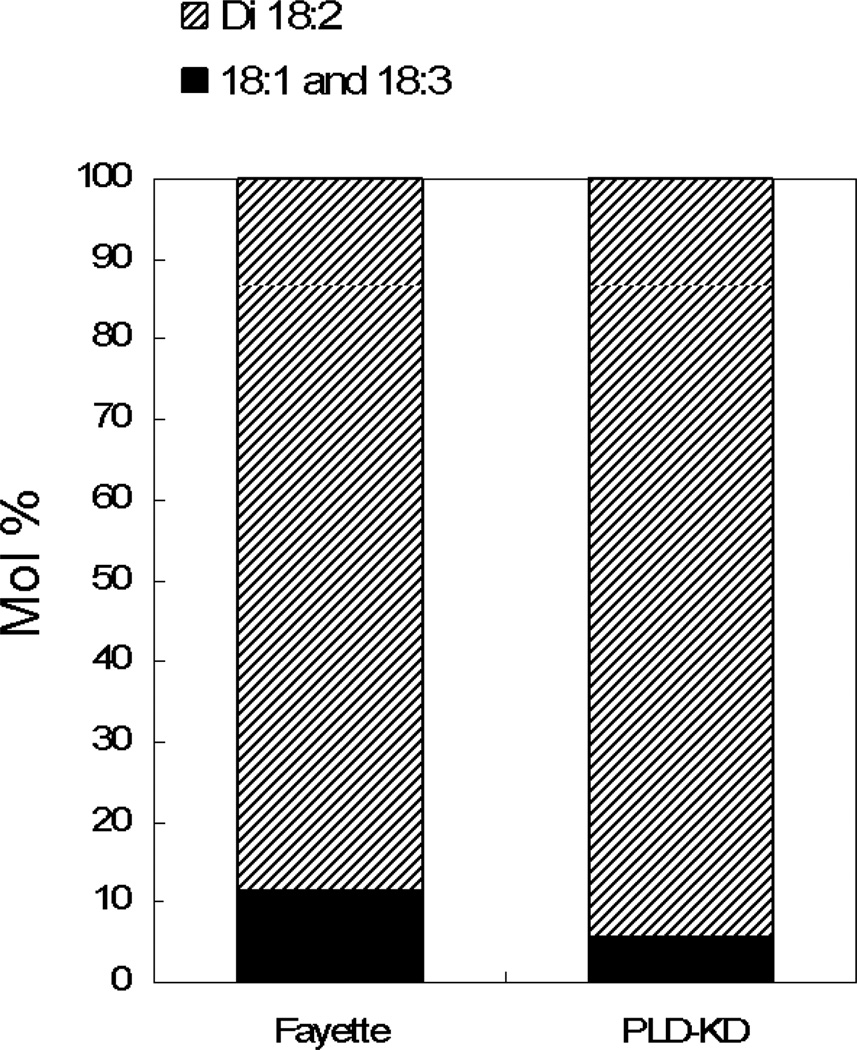
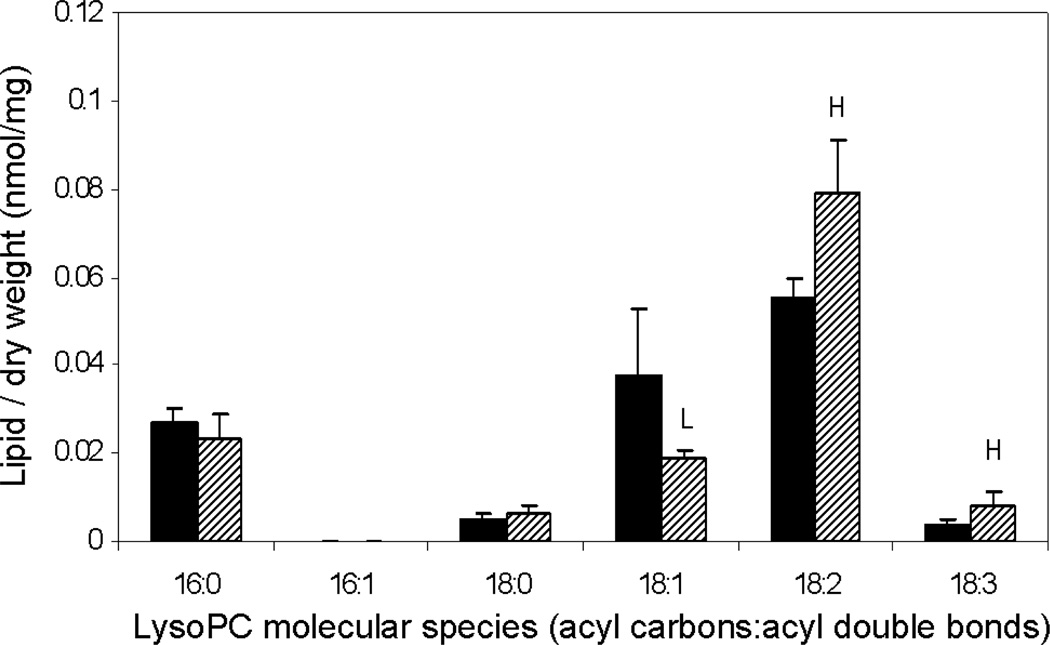
Analysis of polar lipid MS data indicated that total amounts of phospholipid in each head group class were not significantly different in Fayette and PLDα-attenuated soybean (SW) seeds (Table 1). However, PLDα attenuation in soybean seeds did produce significant changes in phospholipid and lysophospholipid molecular species. PC and PE both showed a shift toward more unsaturated molecular species, with significant increases in PC and PE species containing 36 carbons and 4 double bonds and significant decreases in less unsaturated molecular species, including 34:1, 36:1, 36:2, and 36:3 (Figure 5). PG also exhibited a decrease in its 34:1 species. Changes in the molecular species profile of PA, the product of PLD activity, were similar to those of PC, PE, and PG which are known to be in vitro substrates of PLDα (Pappan et al., 1998). Product ion analysis of the elevated lipid molecular species, 36:4-PC, revealed that it is primarily composed of di18:2-PC in both Fayette and in PLDα-suppressed seed (Figure 6). The data indicate that PLDα suppression produced an overall increase in unsaturation among the phospholipids and that the diacyl PC molecular species that was most increased in the PLDα-suppressed seed contained the polyunsaturated fatty acid 18:2. PLD-suppressed soybean seeds also contained higher levels of 18:2-lyso PC and 18:3-lysoPC than Fayette, whereas the level of 18:1-lysoPC was significantly lower in the transgenic seed compared to Fayette (Figure 7). These data are again consistent with decreased PL unsaturation in the PLDα-suppressed soybean seeds.
Table 1.
Total amount of lipid in each head group class in Fayette and PLDα-attenuated soybean seed.
| Polar lipid | Fayette | SW |
|---|---|---|
| Lipid per dry weight (nmol/mg) | ||
| PS | 0.26 ± 0.04 | 0.23 ± 0.03 |
| PI | 8.38 ± 1.52 | 7.54 ± 0.80 |
| PG | 0.52 ± 0.80 | 0.52 ± 0.09 |
| PE | 7.75 ± 1.27 | 7.67 ± 1.18 |
| PC | 15.9 ± 3.09 | 14.9 ± 1.79 |
| PA | 1.35 ± 0.24 | 1.11 ± 0.20 |
| LysoPE | 0.07 ± 0.02 | 0.08 ± 0.13 |
| LysoPC | 0.13 ± 0.02 | 0.14 ± 0.02 |
| MGDG | 0.04 ± 0.00 | 0.04 ± 0.01 |
| DGDG | 0.34 ± 0.11 | 0.23 ± 0.06 |
| Triacylglycerol | Fayette | SW |
| Lipid per dry weight (normalized mass spectral signal/mg) |
||
| 16:0-containing | 14.7 ± 4.5 | 9.7 ± 1.6* |
| 18:0-containing | 5.0 ± 1.3 | 3.6 ± 3.0 |
| 18:1-containing | 38.7 ± 11.6 | 40.0 ± 5.6 |
| 18:2-containing | 31.0 ± 1.5 | 20.9 ± 4.0* |
| 18:3-containing | 5.4 ± 0.4 | 4.7 ± 1.0 |
indicates a significant difference at p<0.05 between non-transgenic background cultivar and SW line
Figure 5.
Lipid molecular species of mature soybean cotyledons as revealed by ESI-MS/MS. The black bars represent non-transgenic soybean cultivar, Fayette, and the hatched bars represent PLDα-suppressed transgenic soybean, SW. The values are the mean + SD (n = 5). An H or L indicates that the value is higher or lower than that of non-transgenic soybean at p<0.05 level, respectively. PI; phosphatidylinositol, PG; phosphatidylglycerol, PE; phosphatidylethanolamine, PC; phosphatidylcholine, PA; phosphatidic acid, MGDG; monogalactosyldiacylglycerol, DGDG; digalactosyldiacylglycerol.
Figure 6.
Product ion analysis of 36:4-PC from Fayette and PLDα-suppressed seeds. Bars represent relative amount of di18:2-PC and 18:1–18:3-PC making up 36:4-PC. (No positional information in attachment to the glycerol is implied for the fatty acids in 18:1–18:3-PC).
Figure 7.
Molecular species of lysophosphatidylcholine in PLDα-suppressed mature soybean cotyledon. The black bars represent non-transgenic soybean cultivar, Fayette, and the hatched bars represent PLDα-attenuated soybean, SW. The values are mean + SD (n=5). An H or L indicates that the value is higher or lower than that of non-transgenic soybean at P<0.05 level determined by student’s t-test, respectively.
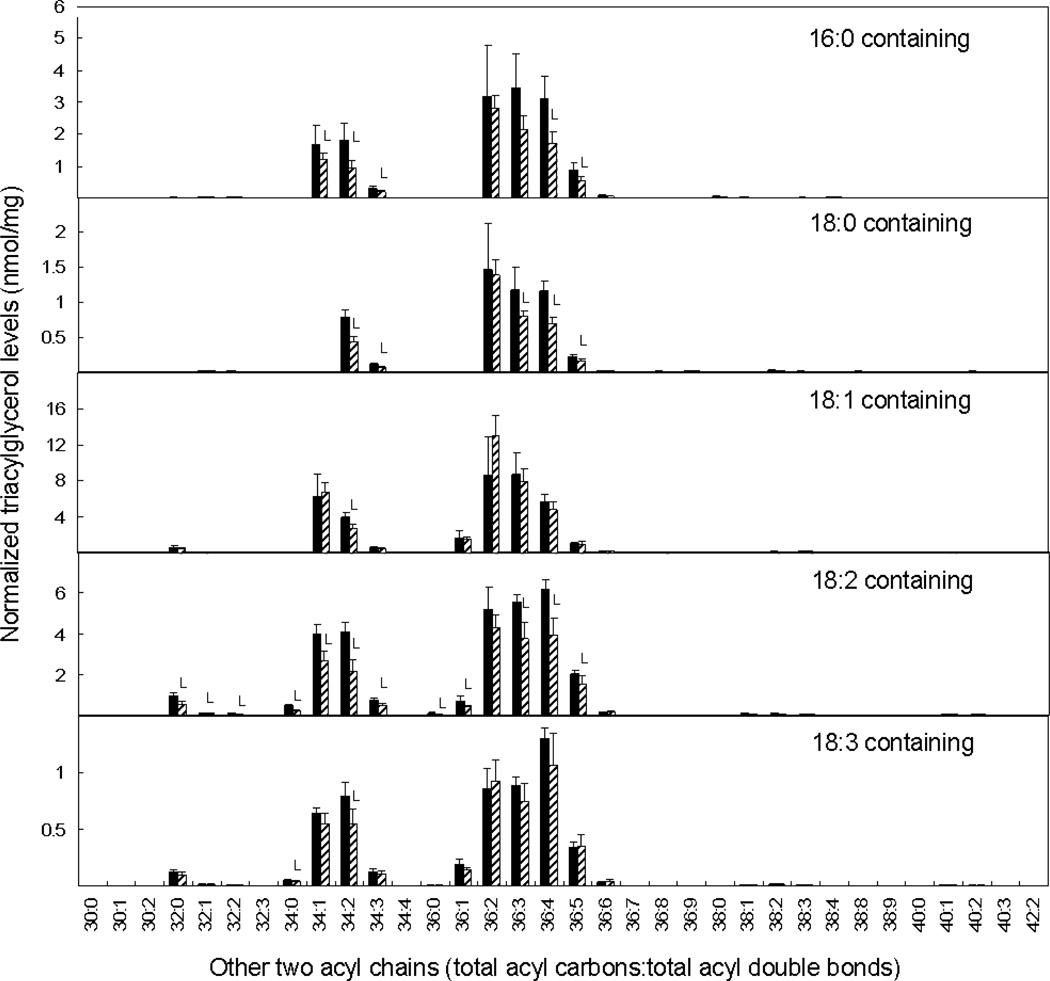
PLD suppression produced a different alteration in the TAG fraction. The amounts of mass spectral signal associated with TAG molecular species containing palmitic (16:0) and linoleic (18:2) acids were reduced in PLDα-attenuated soybean seeds (Table 1). In particular, TAG species containing two fatty acids with 36 carbons and 4 double bonds declined or tended to decline upon PLDα suppression in every subset of TAGs examined (i.e., regardless of what the third fatty acid was) (Figure 8). These data show that the PLD-suppressed line has reduced levels of TAG containing 36:4, while this polyunsaturated diacyl combination (mainly di18:2) is increased in the PC and PE classes. The TAG data are consistent with the observed decrease in the fraction of 18:2 in the overall fatty acid composition of PLDα-suppressed seeds (Figure S2).
Figure 8.
Triacylglycerol molecular species in PLDα-attenuated soybean seeds. The black bars represent non-transgenic soybean seeds, and the hatched bars represent PLDα-suppressed transgenic soybean seeds. The values are mean + SD (n=5). An L indicates that the value is lower than that of non-transgenic soybean seeds at P<0.05 level. Each panel depicts amounts (normalized to an internal standard and to dry weight) of a subset of triacylglycerol species containing a particular fatty acid. The y-axis indicates the identity of the other acyl chains in the TAG molecular species as total acyl carbons and total double bonds, combined for the other two acyl chains. Note that the same molecular species may be detected in more than one panel.
Agricultural traits, protein and oil content in PLD-suppressed seeds
Transgenic field tests of PLD-KD and null transgenic were performed in 2005 and 2006. The plant height of PLD-KD and the null transgenic line tended to be significantly greater than the wild type cultivar in 2005 (Table 2). PLD-KD and the null transgenic line also tended to lodge more than the wild type cultivar. In PLD-KD and the null transgenic line, maturity was observed to be retarded in 2006, whereas maturity in 2005 tended to earlier than the background cultivar. Seed weight and seed quality do not appear to be affected by transformation or the presence of the transgene, but plot weight and lodging were significantly affected by transformation. Protein content and oil content were not significantly different in transgenic, null transgenic, and wild type seeds. However, leaf maturity was retarded in PLD-KD (data not shown), and leaf drop in PLDα-suppressed plants was retarded by average of 6 to 8 days in the field during two years of field trial, compared to those of wild type and null-transgenic lines.
Table 2.
Agronomic traits of T3 (2005) and T4 (2006) pSPLDi transgenic soybean seeds from field experiments in 2005 and 2006.
| Year | Entry1 | Ht2 | Lod3 | Sw4 | Sq5 | Plotwt6 | Protein content | Oil content |
|---|---|---|---|---|---|---|---|---|
| 2005 | Fayette | 34.0 | 1.8 | 16.7 | 2.6 | 314.4 | na7 | na |
| Null transgenic | 33.3 | 4.5** | 16.0 | 3.8 | 65.5** | na | na | |
| PLD-KD | 40.3*†† | 3.3**†† | 19.0 | 3.0 | 135.5** | na | na | |
| 2006 | Fayette | 26.0 | 2.3 | 13.5 | 3.0 | 657.0 | 45.7 | 16.8 |
| Null transgenic | 17.3** | 4.7** | 13.3 | 3.0 | 7.9** | 44.4 | 16.4 | |
| PLD-KD | 28.3† | 3.3*† | 13.8 | 3.0 | 150.3** | 44.5 | 15.2 | |
Nomenclature reflects transgenic lines and transgenic events
Plant height: the distance from soil surface to end of main stem (inch)
Lodging rating: the range of rating, 1 to 5
Seed weight: grams per 100 seeds
Seed quality: the range of rating, 1 to 5
Plot weight: total weight of seed harvested from two central rows of plot
na: not analyzed
indicates a significant difference of Tukey or Tukey-Kramer tests at p<0.05
indicates a significant difference at p<0.01 between non-transgenic background cultivar and transgenic lines.
† and †† indicate a significant difference at p<0.05 and p<0.01 between null transgenic lines and transgenic lines, respectively.
Discussion
Twelve independent transgenic soybean lines with pSPLDi and pHG1 were generated via co-bombardment using a particle inflow gun. Most transgenic events were fully fertile. Based on the background cultivar and pod-setting ability, two elite lines, SW and SI, were chosen for further examination of the characteristics of PLDα-suppressed soybeans. By transferring endogenous DNA using particle inflow gun bombardment, more than 18 copies of the modified PLD transgene were integrated into both transgenic events. In T1 seed, the segregating ratio was best fit to 3:1 (data not shown). Banding patterns generated by different restriction enzymes were fairly consistent through the generations. Only two SW and SI T4 progeny lines out of total 20 lines showed polymorphic banding patterns, consisting of either an additional or deleted band using Southern blot analysis (Figure 2 and Figure S1). These results indicated that arrangement of pSPLDi and HPT transgenes within the host genome traced to two events. It has been reported that the transgene locus is predominantly organized as a transgene cluster containing multiple copies of transgene in oat and rice (Pawlowski and Somers, 1998; Kohli et al., 1998). The multimerization of transgene results in repeated transgene with direct or indirect orientation (De Neve et al., 1997).
Soybean PLDα protein can be detected using the AtPLDα1 antibody and the size of soybean PLDα was consistent with previous reports (Abousalham et al., 1995; Ryu et al., 1996). In this study, no seed with a complete lack of PLDα expression was obtained by insertion of a PLDα RNAi construct driven by a seed-specific promoter; instead, PLDα activity in SI and SW lines ranged from 20 to 80% of the non-trangenic controls (Figure 3). Decreases in PLDα enzyme activity and protein level corresponding to reduction of PLDα transcript accumulation appeared to be caused by the presence of the pSPLDi transgene. It has been proposed that the pldα1-knockdown Arabidopsis seed are more tolerant to aging than pldα1-ablated mutant seed, suggesting that at least some PLDα1 activity is required for optimal seed functionality (Devaiah et al., 2007). Here, the PLDα-knockdown transgenic soybean, with 20 – 80% of non-transgenic PLD activity, exhibited normal germination and seedling growth in the greenhouse.
Ryu et al. (1996) observed that PLD mRNA exists at low levels during soybean seed development. In fact, PLDα transcript was hardly detected during seed maturation using northern blot analysis, so we used real time RT-PCR with PLDα specific primers for more sensitive detection. Based on relative Ct values of PLD genes against soybean actin1 gene with real time RT-PCR in mature soybean seeds, the Ct value for PLDα was lower by around 14 cycles compared to that of soybean actin1. When we assume that the primer efficiencies of PLDα and actin1 polymerase chain reaction are 2, quantitative RT-PCR data suggest that PLDα mRNA levels theoretically were as low as 214 times of actin1 mRNA in mature seeds. Co-suppression caused by pSPLDi transgene may coincidently suppress the level of PLDβ and PLDγ mRNA as well as the level of PLDα. Soybean EST clones, gi:21888889 and gi:17401471, are known as premature PLD cDNA from soybean seedlings. Based on sequence homology with Arabidopsis PLDs, soybean ESTs, gi:21888889 and gi:17401471, showed sequence similarity to the 5’UTR region in AtPLDβ and AtPLDγ, respectively. Indeed, the expression of PLDβ-like mRNA was somewhat suppressed in SW immature seeds, whereas the level of PLDγ-like mRNA was suppressed in mature seeds (Figure 4c). Co-suppression of a conserved domain, the HKD2 domain, presumably results in some suppression of PLDβ and PLDγ, along with PLDα, by an RNAi mechanism. The expression level of PLDβ and PLDγ was as low as 16 (24) times of PLDα in mature seeds (data not shown). While PLDα is clearly expressed and suppressed on the mRNA and protein levels, we cannot rule out the possibility that some of the observed changes in lipid composition are due to co-suppression of other PLD isoforms.
PLD suppression changed the lipid profile of the soybean seeds. Soybean is low in saturated fatty acids with a content of about 15%, high in unsaturated fatty acids with 61% polyunsaturated and 24% monounsaturated fatty acids. Based on ESI MS/MS data, some TAG species tended to decrease by PLD suppression, in particular those containing diacylglycerols with 36 carbons and 4 double bonds and the subsets containing 16:0 and 18:2. Recent work by Bates et al. (2009) indicates that the major pathway for triacylglycerol formation in developing soybean is through conversion of PC to DAG and acylation of DAG to produce TAG. Fatty acids can be incorporated into glycerolipids by acylation of G3P to produce PA, which is then converted to DAG and PC. However, more frequently, fatty acids are incorporated directly into PC by acyl editing. PC is also the molecule on which desaturases act. The data of Bates et al. (2009) thus indicate that once PC is formed, it becomes more unsaturated, and the DAG portion of PC is combined with a third acyl chain to form most TAG species by acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) or phospholipid:diacylglycerol acyltransferase 1 (PDAT1) (Bates et al., 2009; Zhang et al., 2009). Our data indicate that PLD suppression increased PC unsaturation while decreasing the unsaturation of TAG molecular species. The greater unsaturation of PC may mean that PC molecules are spending longer in the PC pool, thus increasing the action of desaturases on PC fatty acids. The decrease in polyunsaturated species in TAGs in the PLD-suppressed line may thus indicate a positive role for a PLD in conversion of PC into TAG. Certainly the first candidate for this potential role is PLDα1, which has been shown previously to affect seed lipid metabolism (Devaiah et al., 2007) and which is shown here to be suppressed in the PLD attenuated transgenic seeds. However, PLDγ, which was also suppressed in mature seeds (Figure 4) is another potential candidate for this role. The current data do not suggest any specific mechanism for conversion of PC to TAG, but the difference in composition of PLD-suppressed and wild type plants suggests that PLD is somehow involved in this conversion for at least some fraction of the TAG formed. One possibility is that PLD (or its PA product) might regulate one of the acyl transferases or acyl exchange reactions. Alternatively, PLD could be involved in catalyzing the conversion of PC (to PA) to DAG. The current data do not allow us to differentiate between the alternatives of PLD being a regulator or PLD being catalytically involved in the conversion of PC to DAG. It also is clear that the multiple potential routes for formation of TAG from PC might be, to some extent, redundant, and this might account for the lack of a stronger effect of PLD suppression on physiological function.
Poor seed and pod set of both PLD-KD and null transgenic lines due to lodging results in lower yields than wild type, suggesting that the procedure of plant regeneration and recovery may affect stem elongation and lodging. There was no effect of PLDα suppression on protein and oil content of seeds. The delayed leaf senescence observed in PLDα-KD plants may have been due to the PLDα gene action reducing leaf apoptosis or programmed cell death. In the detached leaves, PLDα-deficient Arabidopsis exhibited retarded phytohormone-promoted senescence in comparison to wild-type, implying that PLDα may mediate ABA- and ethylene-promoted senescence events (Fan et al., 1997).
PLDα and its product PA have been implicated as increasing membrane instability by increasing phospholipid nonbilayer phase under certain conditions. Reduced levels of PA and increased levels of PC correlated with freezing injury tolerance in PLDα-deficient Arabidopsis (Welti et al. 2002). Suppression of PLDα in Arabidopsis also led to enhanced seed quality and viability after storage and after accelerated aging (Devaiah et al. 2007). Our unpublished data suggest that a reduced level of PLDα and it product PA in soybean also enhance low temperature tolerance and increase tolerance to heat-shock stress.
Soybean lecithin has advantages over egg yolk lecithin in having a higher polyunsaturated fatty acid content, while retaining its stability. PLD suppression decreased polyunsaturation of the soybean TAG to some extent, and this is considered to be a desirable change in properties (Krog, 1997), although dietary soybean lecithin with its current level of polyunsaturation is able to decrease the plasma cholesterol concentration and induce reduction in the plasma levels of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) (Jimenez et al., 1990). We propose that PLD-attenuated seed holds promise for production of a stable oil with desirable health effects and that it may be useful for preparation of soybean lecithin for pharmaceutical purposes.
Experimental Procedures
Plasmid construction for transformation
The 1.3 Kb partial sequence of soybean PLDα (SPLDα) was cloned by Suqin Zheng of Xuemin Wang’s laboratory by screening a soybean cDNA library using Arabidopsis thaliana PLDα1 cDNA as a probe; the cloned cDNA was kindly donated. The plasmids used for soybean transformation via particle inflow gun (PIG) bombardment originated from pUC19, and the expression cassette was subcloned from pCong (4.1 Kb) harboring soybean β-conglycinin α’ subunit promoter and terminator unit (Doyle et al., 1986). A pSPLDanti (β-conglycinin promoter::SPLDanti) plasmid was constructed using a 1.3 Kb partial sequence of SPLDα. SPLDα was digested with BamHI and ligated between the β-conglycinin promoter and terminator in the antisense orientation. To construct pSPLDi, an 820 bp of SPLDα partial sequence was subcloned into pGEM-T Easy vector (Promega), and a multi-cloning site was added at the 3’ end of the SPLDα fragment. The subcloned fragment, digested by XbaI and SalI, was ligated between the β-conglycinin promoter and the SPLD anti-sequence in the pSPLDanti-vector (Figure 1). The vector pHG1 (Finer et al, 1992), containing the selectable marker gene hygromycin phosphotrasferase (HPT, 1.0 Kb), was co-bombarded to provide hygromycin resistance to the transgenic tissue. The HPT gene served as a selectable marker during transformation and regeneration. Null transgenic lines were selected in the T1 generation; these lines had lost the SPLDi transgene by segregation.
Plant material and tissue culture
Soybean cultivars ‘Flyer’ (Bernard et al., 1987) and ‘Fayette’ (Bernard et al., 1988) were grown in a greenhouse with a 25 ± 2°C/ 18 h day and 20 ± 2°C/ 6 h night photoperiod. Immature pods were excised at growth stage R5 and surface-sterilized with 1.05% sodium hypochlorite (NaOCl) and 0.02% Tween-20 for 15 min with agitation, followed by three washes with sterile, distilled water in a hood. Methods for culture initiation and proliferation were as described by Finer (1988) and modified by Trick et al. (1997). Briefly, approximately three to six months after initiation of somatic embryo cultures on D40 medium, the embryos were proliferated on D20 medium, prepared for bombardment and then transformed using a particle inflow gun (Finer and McMullen, 1991). For co-bombardment, one microgram of pSPLDi plasmid and pHG1 plasmid were mixed in a 1:1 ratio. Putative transformed tissues were maintained on D20H7.5, D20H3, and D20H15 media containing 7.5 mg/l, 3 mg/l, and 15 mg/l hygromycin, respectively. Throughout the selection process, the hygromycin concentration was either increased or decreased depending upon the health of the tissue. After 3 to 6 months, putative transgenic clumps were tested by PCR for the presence of pSPLDi and HPT transgenes. Transgene-positive tissues were then transferred and allowed to mature on M6 medium. After embryos matured to the ‘torpedo’ stage of development and an apical meristem was visible, embryos were desiccated for 1 to 2 days and rooted in vermiculite saturated with 1/2-strength OSM medium. Recovered plants possessing two trifoliate leaves were transplanted to peat moss, hardened at 23°C under 24 h light then transferred to 18.9 liter pots and grown to maturity in the greenhouse with a 16-h photoperiod.
PCR, Southern blotting, and northern blotting
Genomic DNA was extracted from young soybean leaves and immature seeds via a modified CTAB DNA extraction method by Saghai-Maroof et al. (1984). For PCR reactions, 50 ng of genomic DNA in a 5 µl aliquot was used as a template in a 50 µl PCR reaction containing 1X NH4 buffer, 1.5 mM MgCl2, 0.2 mM of deoxynucleotide triphosphate (dNTPs), 20 pmol each of forward and reverse primers, and 1.25 U Taq DNA polymerase (New England BioLabs, MA, USA). The transgene-specific primer sequences of left arm (forward: 5’-TATTTCAACACCCGTCA-3’ and reverse: 5’-CCACCATGACATGCGGAAACC-3’), right arm (forward: 5’-TTTCCGCATGTCATTGTGGTA-3’ and reverse: TAGCCCGATACTTTCCT-3’), and HPT (forward: 5’-GCACAATCCCACTATCCTTCGCAA-3’ and reverse: 5’-CTTCTACACAGCCATCGGTCCAG-3’) were designed by primer3 software (http://frodo.wi.mit.edu) and used in each reaction.
For Southern blot analysis, 20 µg of genomic DNA was digested overnight using the appropriate restriction enzyme (50 U) at the designated temperature. Quantification of DNA was performed using a Nanodrop ND1000 spectrophotometer (Nanodrop, DE, USA). Digested DNA samples were separated by electrophoresis on a 0.8% TAE agarose gel and denatured with a 0.4 M NaOH solution for 12 to 16 h. Using the semi-dry and wet capillary transfer methods, DNA was blotted onto a Hybond N+-XL membrane (Amersham Biochech Ltd., Piscataway, NJ, USA).
A 1.2 Kb SPLDα fragment, a 1.0 Kb HPT fragment, and a 1.1 Kb β-conglycinin promoter fragment were used for probe synthesis; the fragments were digested from pSPLDi and pHG1 plasmids. All DNA probes (50 ng) were labeled with [αP−32]dCTP (sp. ACT. 3000 Ci/mM) using the random priming method (Promega, WI, USA). A radioactively labeled probe was purified by Nick column (Amersham Biochech Ltd., Piscataway, NJ, USA) to remove the unlabeled [αP−32]dCTP and non-specific small fragments. The purified probe DNA was denatured at 95° C for 10 min and placed on ice for 10 min. The membrane was hybridized overnight with each DNA probe at 65° C in hybridization solution. After hybridization, the membrane was washed twice in 2X SSC with 0.1% SDS for 15 min and twice in 0.2X SSC with 0.01% SDS at 65° C for 30 min.
For RNA analysis, total RNA was isolated from soybean tissues (>200 mg dry weight) using the TRIZOL reagent (Invitrogen, Canada) extraction method. Fifteen µg of total RNA were separated on a 1.0% agarose gel containing 1.5% formaldehyde and transferred onto a nylon membrane using the semi-wet capillary method. The membrane was hybridized at 42° C in 10 ml of ULTRAhyb™ Solution (Ambion, CA, USA) with 1.1 Kb HPT probe containing [αP−32]dCTP labeled by random priming; the membrane was washed twice in 1X SSC with 0.1% SDS for 15 min at 42° C and twice in 0.1X SSC with 0.01% SDS for 30 min at 42° C. The hybridizing signals were detected using the Storm840 phosphorimager (Molecular Dynamics, CA, USA).
Real time RT-PCR
To eliminate genomic DNA contamination, RNA samples were treated with 1 µl of RNase free RQ1 DNase (Promega, WI, USA) for 20 min. Two µg total RNA were used for cDNA synthesis with the AMV reverse transcription system according to the manufacturer’s protocol (Promega, WI, USA) with a random hexamer for amplifying transgenic and oligo-dT primer for amplifying endogenous PLD mRNA. After cDNA synthesis, DNase free water was added to a final volume of 100 µl. To quantify gene expression of PLD transcripts from various soybean seeds, 20 µl aliquots from reverse transcription were diluted in 80 µl of nuclease-free water and used as a template for real time RT-PCR following the manufacturer’s instructions (Bio-Rad, CA, USA). Primers for quantitative RT-PCR were designed by Beacon designer 6 (Premier Biosoft International, CA, USA) with a Tm value of approximately 55 to 60° C. Primer sequences of PLDα (forward: 5’-AGGTCAATGGATGGTGCTAGGG-3’ and reverse: 5’-TCACTTTCTGGCTGGAGGAAGG-3’), PLDβ-like (forward: 5’-TTGGGCTGAACATACgggTACG-3’ and reverse: 5’-ACCGGGTACTTGAGCAGATGC-3’), and PLDγ-like (forward: 5’-AGGAACCAGAGAGCCTTGAATG-3’ and reverse: 5’-AGAGGCTTCACTTTGCCCTTTG-3’) were designed for real time RT-PCR using Beacon designer 6.0. The PCR reaction was performed in 50 µl of 1X iQ SYBR Green Supermix, 200 nM of each primer, cDNA template and sterile water via Bio-Rad iCycler (Bio-Rad, CA, USA). Each reaction had 3 biological replications with 2 technical replications. The gene expression fold was technically calculated by 2-ΔΔCt=(CtGOI for Tr-CtGOI for CK)-(CtHKG for Tr-CtHKG for CK). When PCR efficiencies for each primer set were not 100%, [(1+EGOI)-ΔCtGOI]/[(1+EHKG)-ΔCtHKG] (where Ct: threshold cycle; GOI: gene of interest; HKG: house-keeping gene; Tr: cDNA samples from transgenic soybean; CK: cDNA samples from non-transgenic control; and E: primer efficiency in %) was substituted for ΔΔCt value.
PLDα enzyme assay and western blot analysis
Total protein was extracted by grinding immature and mature soybean seed with a liquid N2-chilled screwdriver in a 1.5 ml microcentrifuge tube with homogenization buffer containing 50 mM Tris-HCl, pH 7.5, 10 mM KCl, 1 mM EDTA, 0.5 mM phenyl methylsulfonyl fluoride, and 2 mM DTT at 4° C (Fan et al., 1997). Protein concentration was measured using a Nanodrop ND1000 spectrophotometer. Aliquots of 20 µg of native protein were used for the PLD enzyme assay as described previously (Fan et al., 1997). Briefly, the reaction mixture contained 100 mM MES, pH 6.5, 25 mM CaCl2, 0.5 mM SDS, 1% (v/v) ethanol, and 2 mM phosphatidylcholine (egg yolk) containing 1,2-dipalmitoyl glycerol-3-phosphate-(methyl-3H)-choline in a final volume of 200 µl. Substrate preparation, reaction conditions, and product separation were based on the procedure used by Wang et al. (1993). The release of free head group, 3H-labeled choline, into the aqueous phase was quantified using a liquid scintillation counter (Beckman Couter, Inc, CA, USA).
Proteins were extracted from immature or mature soybean seed (Zhang et al., 2003). Total native proteins were incubated at 95° C for 15 min with SDS-PAGE loading buffer which was 100 µl of 50 mM Tris-HCl, pH 6.8, 10 mM DTT, 2% SDS, 0.01% bromophenol blue, and 10% glycerol. Twenty µg of denatured proteins were fractionated by 10% SDS-PAGE gel at 100 V until the tracking dye reached the bottom of the PAGE gel. The membrane containing denatured proteins was blotted with Arabidopsis PLDα1 antibodies as described by Zhang et al. (2003). Briefly, gels were transferred to an ImmobilonTM-P transfer membrane (Millipore, Bedford, MA, USA) using a semi-dry blotting apparatus (Bio-Rad, CA, USA) according to the manufacturer’s instructions. The membranes were blocked with non-fat milk for 1 h and soaked in the first antibody overnight. The PLDα polyclonal antibody was diluted to 1:1000 in TBST (10 mM Tris, 140 mM NaCl, pH 7.5 and 0.05% Tween 20). The blots were washed in 1 × TBST 3 times, 5 min/wash. Goat anti-rabbit IgG (H+L) horseradish peroxide (HRP) conjugate (Bio-Rad, CA, USA) was used as the second antibody at a dilution of 1:1000 in TBST. The blots were incubated in a second antibody for 1 h and washed two times in TBST followed by one wash in TBS (10 mM Tris, 140 mM NaCl, pH 7.5). The protein bands recognized by the antibody were visualized with a HRP color development reagent (Bio-Rad).
Polar lipid and triacylglycerol analyses
To extract lipids from mature seed, a single seed was smashed and transferred immediately to 3 ml of hot isopropanol with 0.01% butylated hydroxytoluene. After 15 min incubation at 75° C, 1.5 ml of chloroform and 0.6 ml of water was added, followed by agitation for 1 h. Each seed was extracted with chloroform/methanol (2:1) with 0.01% butylated hydroxytoluene four more times, each time followed by 30 min agitation at 25° C. The remaining tissue was dried at 105° C for overnight and weighed. The extract was washed with 1 ml of 1 M KCl and then 2 ml water. The solvent of the lower phase was evaporated under nitrogen. The dry lipid extract was dissolved in 1 ml of chloroform.
The molecular species of triacylglycerols (TAG) and PLs were analyzed by electrospray ionization (ESI) triple quadrupole mass spectrometry (API 4000, Applied Biosystems, CA, USA). The molecular species of polar lipid were defined by the presence of a head-group fragment and the mass/charge of the intact lipid ion formed by ESI (Welti et al., 2002; Devaiah et al., 2006). Such tandem ESI-MS/MS precursor and product ion scanning, based on head group fragment, do not determine the individual fatty acyl species. Instead, polar lipids are identified at the level of class, total acyl carbons, and total number of acyl carbon-carbon double bonds. Polar lipids were quantified in comparison of a series of polar lipid internal standards. This procedure allows accurate quantification of polar lipids (Han and Gross, 1994; Brügger et al., 1997). Acyl ions (product ions) derived from 36:4-PC species were identified in a separate experiment following collision induced dissociation of the 36:4-PC [M + OAc]- ion.
TAGs were defined by the presence of one acyl fragment and the mass/charge of the ion formed from the intact lipid. This allows identification of one TAG acyl species and the total acyl carbons and total number of acyl double bonds in the other two chains. The procedure does not allow identification of the other two fatty acids individually, nor the positions (sn-1, sn-2, or sn-3) that individual acyl chains occupy on the glycerol. TAGs were quantified in a manner similar to the polar lipids, including background subtraction, smoothing, integration, isotope deconvolution, and comparison of sample peaks with those of the internal standard. However, whereas polar lipids within a class exhibit similar mass spectral response factors, the mass spectral responses of various TAG species are variable, due to differential ionization of individual molecular TAG species. In the data shown herein, no response corrections were applied to the data. The data were normalized to the internal standard, such that one unit is equal to the signal of 1 nmol of the internal standard (tri17:1). The data were further normalized to the dry weight of the sample. Five replicates of non-transgenic and PLDα-attenuated seeds were analyzed. The Student’s t-test at the p<0.05 level was employed to determine statistical significance.
Fatty acid composition was determined by gas-liquid chromatography on a Supelco 30-meter Omegawax 250 capillary column (Sigma-Aldrich, St. Louis, MO, USA) after derivitization of the fatty acids to methyl esters in 1.5 M methanolic HCl, followed by addition of water, and extraction with pentane.
Transgenic soybean field test and analysis of seed oil and protein content
Agricultural traits of PLDα-suppressed soybean lines were evaluated in 2005 by planting T3 transgenic soybean and in 2006 by planting T4 transgenic soybean, bulked from the progeny tested in the T3 generation. Null transgenic line and wild type cultivar, Fayette, were planted along with the transgenic lines at the Kansas State University Ashland Agronomy Farm, Manhattan, Kansas in 2005 and 2006. Soybeans were planted on 21 July 2005 and on 26 June 2006. The experimental design in the 2005 field test was a randomized incomplete block design with 1 to 4 replications per entry and was a randomized complete block design with 3 replications per entry in 2006. Weeds were controlled with pre-emergence herbicide application and hand weeding.
The center two rows of each plot were evaluated for plant maturity, plant height, lodging, 100-seed weight, seed quality, and plot yield. Maturity was recorded as the date when 95% of pods had reached mature color. Height was measured in cm as the distance from the soil surface to the top of main stem. Lodging was rated on a scale of 1 to 5, where 1 equaled almost all plants erect, and 5 equaled almost all plants prostrate. Seed weight was measured as weight in grams per hundred seeds. Seed quality was rated according to a 1 to 5 score, considering the amount and degree of wrinkling, defective seed coats, greenishness, and moldy or rotten seeds, where 1 equaled excellent and 5 equaled poor. Plot yield was recorded as the weight in grams of seed from the center two rows of the plot. Oil and protein content were measured using the nondestructive NIR method for whole grain analysis (American Association of Cereal Chemist (AACC) method 39–21, 1998). Fifteen to 20 seeds were used for oil and protein analysis. Seed samples were submitted to the USDA-ARS National Center for Agricultural Utilization Research, Peoria, Illinois.
Acknowledgements
We thank Juliane S. Essig and Marcy L. Main for their technical support with soybean genetic transformations, Mary Roth and Dr. Richard Jeannotte for lipid analysis, Dr. Xuemin Wang at Donald Danforth Center for critical review, Suqin Zheng for donating soybean PLD cDNA, and Dr. Shivakumar Devaiah for PLD enzyme analysis. The lipid analyses were performed at the Kansas Lipidomics Research Center Analytical Laboratory with funding from KSU’s Targeted Excellence program. Equipment acquisition and method development at the Kansas Lipidomics Research Center were funded by National Science Foundation (EPS 0236913, MCB 0455318, DBI 0521587), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20RR16475), and Kansas State University. We also thank USDA-ARS National Center for Agricultural Utilization Research, Peoria, Illinois for oil and protein analysis. This research was supported by Kansas State University and the Kansas Soybean Commission. This article is contribution no. 10–294-J from the Kansas Agricultural Experimental Station, Kansas State University, Manhattan, Kansas.
References
- Abousalham A, Teissere M, Gardies AM, Verger R, Noat G. Phospholipase D from soybean (Glycine max L.) suspension-cultured cells: purification, structural and enzymatic properties. Plant Cell Physiol. 1995;36(6):989–996. doi: 10.1093/oxfordjournals.pcp.a078871. [DOI] [PubMed] [Google Scholar]
- Bates PD, Durrette TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard RL, Juvik GA, Nelson RL. International Agricultural Publications. Vol. 2. International Agriculture Publications: University of Illinois; 1987. USDA soybean germplasm collection inventory. INTSOY Series Number 31. [Google Scholar]
- Bernard RL, Noel GR, Anand SC, Shannon JG. Registration of Fayette soybean. Crop Sci. 1988;28:1028–1029. [Google Scholar]
- Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl Acad. Sci. USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JW. Recent developments in breeding soybeans for improved oil quality. Fett Wissenschaft Techonolgies/Fat Science Technology. 2006;93(4):121–128. [Google Scholar]
- Burton JW, Wilson RF, Rebetzke GJ, Pantalone VR. Registration of N98–4445A mid-oleic soybean germplasm line. Crop Sci. 2005;46:1010–1012. [Google Scholar]
- Cherry JP, Kramer WH. In: Plant sources of lecithin, in lecithins: Sources, manufacture and uses. Szuhaj BF, editor. Champaign, IL: American Oil Chemists’ Society; 1989. pp. 16–31. [Google Scholar]
- De Neve M, De Buck S, Jacobs A, Van Montagu M, Depicker A. T-DNA integration patterns in co-transformed plant cells suggest that T-DNA repeats originate from co-integration of separate T-DNAs. Plant J. 1997;11:15–29. doi: 10.1046/j.1365-313x.1997.11010015.x. [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Pan X, Hong Y, Roth M, Welti R, Wang X. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. Plant J. 2007;50:950–957. doi: 10.1111/j.1365-313X.2007.03103.x. [DOI] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Schuler MA, Godette WD, Zenger V, Beachy RN. The glycosylated seed storage proteins of Glycine max and Phascolus vulgaris; structural homologies of genes and proteins. J. Biol. Chem. 1986;261:9228–9238. [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase Dα retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer JJ. Apical proliferation of embryogenic tissue of soybean [Glycine max (L.) Merrill] Plant Cell Reports. 1988;7:238–241. doi: 10.1007/BF00272532. [DOI] [PubMed] [Google Scholar]
- Finer JJ, McMullen MD. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell Develop. Biol. - Plant. 1991;27:175–182. [Google Scholar]
- Finer JJ, Vain P, Jones MW, McMullen MD. Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Reports. 1992;11:323–328. doi: 10.1007/BF00233358. [DOI] [PubMed] [Google Scholar]
- Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc. Natl Acad. Sci. USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez MA, Scarino ML, Vignolini F, Mengheri E. Evidence that polyunsaturated lecithin induces a reduction in plasma cholesterol level and favorable changes in lipoprotein composition in hypercholesterolemic rats. J. Nutr. 1990;120:659–667. doi: 10.1093/jn/120.7.659. [DOI] [PubMed] [Google Scholar]
- Kohli A, Leech M, Vain P, Laurie DA, Christou P. Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot spots. Proc. Natl Acad. Sci. USA. 1998;95:7203–7208. doi: 10.1073/pnas.95.12.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krog NJ. Food emulsifiers and their chemical and physical properties. In: Friberg SE, Lrsson K, editors. Food Emulsions. New York: Marcel Dekker ; 1997. pp. 141–188. [Google Scholar]
- Novotna Z, Valentova O, Martinec J, Feltl T, Nokhrina K. Study of phospholipases D and C in maturing and germinating seeds of Brassica napus. Biochem. Soc. Trans. 2000;28(6):817–818. [PubMed] [Google Scholar]
- Palacios L, Wang T. Extraction of egg-yolk lecithin. J. Am. Oil. Chem. Soc. 2006;82:565–569. [Google Scholar]
- Pappan K, Austin-Brown S, Chapman KD, Wang X. Substrate selectivities and lipid modulation of plant phospholipase D alpha, -beta, and -gamma. Arch. Biochem. Biophys. 1998;353(1):131–140. doi: 10.1006/abbi.1998.0640. [DOI] [PubMed] [Google Scholar]
- Pappan K, Zheng L, Krishnamoorthi R, Wang X. Evidence for and characterization of Ca2+ binding to the catalytic region of Arabidopsis thaliana phospholipase Dβ. J. Biol. Chem. 2004;279:47833–47839. doi: 10.1074/jbc.M402789200. [DOI] [PubMed] [Google Scholar]
- Pawlowski WP, Somers DA. Transgenic DNA integrated into the oat genome is frequently interspersed by host DNA. Proc. Natl Acad. Sci. USA. 1998;95:12106–12110. doi: 10.1073/pnas.95.21.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Zheng L, Wang X. Changes in phospholipase D expression in soybeans during seed development and germination. J. Am. Oil. Chem. Soc. 1996;79:1171–1176. [Google Scholar]
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl Acad. Sci. USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu D, Alt IL, Scherder CW, Fehr WR, Bhattacharyya MK. Enhanced oleic acid content in the soybean mutant M23 is associated with the deletion in the Fad2-1a gene encoding a fatty acid desaturase. J. Am. Oil. Chem. Soc. 2007;84:229–235. [Google Scholar]
- Singh RJ, Hymowitz T. Soybean genetic resources and crop improvement. Genome. 1999;42:605–616. [Google Scholar]
- Taylor ATS, Low PS. Phospholipase D involvement in the plant oxidative burst. Biochemical and Biophys Res. Comm. 1997;237:10–15. doi: 10.1006/bbrc.1997.6965. [DOI] [PubMed] [Google Scholar]
- Trick HN, Dinkins RD, Santarem ER, Di R, Samoylov VM, Meurer C, Walker D, Parrott WA, Finer JJ, Collins GB. Recent advances in soybean transformation. Plant Tiss. Cult. Biotechnol. 1997;3:9–26. [Google Scholar]
- Wang X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005;139:566–573. doi: 10.1104/pp.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- Wendel R. In: Lecithin, in Kirk-Othmer Encyclopedia of Chemical Technology. 14th edn. Howe-Grant M, editor. Vol. 15. New York: John Wiley and Sons; 1995. pp. 192–209. [Google Scholar]
- Wilson RF. In: Soybeans: Improvement, production, and uses. 3rd ed. Boerma HR, Specht JE, editors. Madison, WI: ASA, CSSA and SSSA; 2004. pp. 621–677. [Google Scholar]
- Wu Y, Wang T. Soybean lecithin fractionation and functionality. J. Am. Oil. Chem. Soc. 2003;80:319–326. [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferase have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009;21:3885–3901. doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang C, Qin C, Wood T, Olafsdottir G, Welti R, Wang X. The oleate-stimulated phospholipase D, PLDδ and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell. 2003;15:2285–2295. doi: 10.1105/tpc.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]