Abstract
The mosquito Aedes aegypti is a potent vector of the chikungunya, yellow fever, and dengue viruses, responsible for hundreds of millions of infections and over 50,000 human deaths per year. Mutagenesis in Ae. aegypti has been established with TALENs, ZFNs, and homing endonucleases, which require the engineering of DNA-binding protein domains to provide genomic target sequence specificity. Here, we describe the use of the CRISPR-Cas9 system to generate site-specific mutations in Ae. aegypti. This system relies on RNA-DNA base-pairing to generate targeting specificity, resulting in efficient and flexible genome-editing reagents. We investigate the efficiency of injection mix compositions, demonstrate the ability of CRISPR-Cas9 to generate different types of mutations via disparate repair mechanisms, and report stable germ-line mutations in several genomic loci. This work offers a detailed exploration into the use of CRISPR-Cas9 in Ae. aegypti that should be applicable to non-model organisms previously out of reach of genetic modification.
Introduction
As a primary vector of the serious and sometimes fatal chikungunya, yellow fever and dengue viruses, the mosquito Aedes aegypti (Ae. aegypti) is responsible for hundreds of millions of human infections annually (Bhatt et al., 2013). To transmit disease, a female mosquito must bite an infected individual, and, after a period of viral incubation within the mosquito, bite and infect another human. Female mosquitoes use cues such as odor, carbon dioxide, and temperature to locate a host and obtain a blood-meal (McMeniman et al., 2014), which is used to produce a clutch of approximately 100 eggs. Once a mosquito has developed mature eggs, she uses volatile and contact cues to locate and evaluate a body of water at which to lay her eggs. Our long-term goal is to use genome-engineering techniques coupled with quantitative behavioral analysis to investigate the genetic and neural basis of innate chemosensory behaviors in this important disease vector.
Clustered regularly interspaced palindromic repeats (CRISPR) and CRISPR associated (Cas) genes are components of an adaptive immune system that are found in a wide variety of bacteria and archaea (Doudna and Charpentier, 2014). Beginning in late 2012 (Jinek et al., 2012), the bacterial type II CRISPR-Cas9 system was adapted as a genome-engineering tool in a wide variety of organisms and in vitro preparations, dramatically expanding the ability to modify genomes (Doudna and Charpentier, 2014). The ease of designing and generating these reagents at the bench has opened the door for studies of gene function in non-traditional model organisms.
The genome of Ae. aegypti is relatively large and incompletely mapped (Juneja et al., 2014; Nene et al., 2007; Timoshevskiy et al., 2014), making it difficult to recover mutations generated by traditional forward genetics. Ae. aegypti has a recent history of genetic modification, including transposon-mediated transgenesis (Coates et al., 1998; Lobo et al., 2002) and loss-of-function gene editing with zinc-finger nucleases (ZFNs) (DeGennaro et al., 2013; Liesch et al., 2013; McMeniman et al., 2014), TAL-effector nucleases (TALENS) (Aryan et al., 2013a, 2014), and homing endonuclease genes (HEGs) (Aryan et al., 2013b). ZFNs and TALENs are modular DNA-binding proteins tethered to a non-specific FokI DNA nuclease (Carroll, 2014), while HEGs are naturally occurring endonucleases that can be reengineered to target novel sequences (Stoddard, 2014). Targeting specificity by these reagents is conferred by context-sensitive protein-DNA binding interactions and these proteins can be difficult to engineer.
Here we describe methods for site-directed mutagenesis in Ae. aegypti using RNA-guided endonucleases based on the type II CRISPR-Cas9 system. The double-stranded endonuclease Cas9 derived from Streptococcus pyogenes uses RNA-DNA Watson-Crick base pairing to target to specific genomic locations. This system has been adapted for precision genome-engineering in dozens of organisms from bacteria to primates (Doudna and Charpentier, 2014; Peng et al., 2014). In particular, two studies in the vinegar fly Drosophila melanogaster (Bassett et al., 2013) and the zebrafish Danio rerio (Hwang et al., 2013) were important in guiding our early attempts to adapt CRISPR-Cas9 to Ae. aegypti.
A detailed bench manual with step-by-step guidance for designing, generating, and testing these reagents in mosquitoes is available as Supplemental Data S1. Given the proven flexibility of this system, we believe that the protocols and procedures outlined here and by numerous other laboratories will continue to be optimized and modified for use in many organisms for which precision genome-engineering has not yet been employed.
Experimental Procedures
Detailed procedures are available as Supplemental Experimental Procedures.
Cas9 mRNA and protein
Cas9 mRNA was transcribed from pMLM3613 (Addgene #42251) (Hwang et al., 2013) using mMessage mMachine T7 Ultra Transcription kit (AM1345, Life Technologies). Recombinant Cas9 protein was obtained commercially (CP01, PNA Bio).
sgRNA design and construction
sgRNAs were designed by manually searching genomic regions for the presence of protospacer-adjacent motifs (PAMs) with the sequence NGG, where N is any nucleotide. We required that sgRNA sequences be 17–20 bp in length, excluding the PAM, and contain one or two 5′ terminal guanines to facilitate transcription by T7 RNA polymerase. sgRNA sequences were checked for potential off-target binding using the following two web tools: http://zifit.partners.org/ZiFiT/ and http://crispr.mit.edu. See Table S1 for sgRNA sequences and predictions of off-target binding.
Extraction of genomic DNA
Genomic DNA was extracted from individual or pools of mosquitoes using either the DNEasy Blood and Tissue Kit (69581, Qiagen) or a 96-well plate extraction protocol (Holleley and Sutcliffe, 2014).
Sequencing and analysis of CRISPR-Cas9 induced mutations
A two-step PCR protocol was used to amplify amplicons surrounding the putative CRISPR-Cas9 cut site from genomic DNA of G0 or G1 animals using primers in Table S2. Libraries were sequenced on an Illumina MiSeq, aligned to the wild-type reference sequence and examined for the presence of insertions, deletions, or other polymorphisms. Scripts developed for the analysis of this data are available at https://github.com/bnmtthws/crispr_indel.
Donor construction for homology-directed repair
Single-stranded DNA oligodeoxynucleotides (ssODNs) were synthesized as 200 bp ‘Ultramers’ (IDT Inc.). Homology arms for plasmid donors were PCR-amplified from LVP-IB12 genomic DNA and cloned with In-Fusion HD cloning (Clontech) into PSL1180polyUBdsRED (Addgene #49327) or pSL1180-HR-PUbECFP (Addgene #47917). Annotated sequences of oligonucleotides and plasmids used for homology-directed repair are available in as Supplemental Data S2.
Molecular genotyping of stable germ line alleles by PCR
To verify the presence of exogenous sequences inserted by homology-directed-repair, or the presence of insertions and deletions, PCR amplicons surrounding the putative cut site were generated from genomic DNA (see Table S2 for primer sequences). Purified amplicons were Sanger sequenced (Genewiz), or used as a template for a restriction digest using BamHI (R0136, New England Biolabs [NEB]) or PacI (R0547, NEB).
Statistics
Summary data were plotted using the python packages matplotlib (boxplots) and seaborn (means ± 95% confidence intervals).
Genotyping stable alleles by fluorescence
Larvae or pupae were immobilized on a piece of moist filter paper and examined under a dissection microscope (SMZ1500, Nikon) with a fluorescent light source and ECFP and dsRed filter sets.
Results
Outline of CRISPR-Cas9 system and injection components
The core of the CRISPR-Cas9 system has two components: 1) a synthetic single guide RNA (sgRNA), which is a small RNA containing 17–20 bases of complementarity to a specific genomic sequence, and 2) the Cas9 nuclease derived from Streptococcus pyogenes (SpCas9). SpCas9 forms a complex with the sgRNA and induces double-stranded DNA breaks at sequences of the genome that are directly 5′ to a protospacer-adjacent motif (PAM) and are complementary to the sgRNA recognition site. The PAM sequence for SpCas9 is ‘NGG’ which occurs approximately once every 17 bp in the Ae. aegypti genome, making it possible to target essentially any locus.
To generate stable germ-line mutations, CRISPR-Cas9 reagents are injected into pre-blastoderm stage embryos composed of a syncytium of nuclei prior to cellularization that offers access of genome-editing reagents to the nuclei of both somatic and germ-line cells. Embryos are microinjected 4–8 hours after egg-laying, and allowed to develop for 3 days before being hatched in a deoxygenated hatching solution (Lobo et al., 2006). G0 pupae are collected for sequencing to determine genome modification rates, or are allowed to emerge as adults and outcrossed to wild-type LVP-IB12 mosquitoes. Following blood-feeding, G1 eggs are collected from these outcrosses to screen for germ-line transmission of stable mutations.
When faced with a double-stranded DNA break, DNA repair machinery can resolve this break in one of two ways: non-homologous end-joining, which can result in small insertions and deletions, or, less frequently, homology-directed repair, which uses exogenous sequence containing regions of homology surrounding the cut site as a template for repair. Cutting with multiple sgRNAs can result in large deletions between the two cut sites. In this paper, we will discuss stable germ-line transmission of all three types of alleles in Ae. aegypti.
Identifying optimal injection mixes for CRISPR-Cas9 mutagenesis
Insertions and deletions resulting from non-homologous end-joining are a proxy for the activity of a particular sgRNA/Cas9 combination and can be detected by Surveyor or T7 Endonuclease I (T7E1; Reyon et al., 2012), high-resolution melting point analysis (HRMA) (Dahlem et al., 2012), Sanger sequencing (Brinkman et al., 2014), or deep sequencing (Gagnon et al., 2014). Each of these techniques evaluates the level of polymorphism in a short PCR-generated amplicon surrounding the sgRNA target site. With the exception of deep sequencing, these approaches provide only semi-quantitative estimates of the mutagenesis in each sample and furthermore, HRMA and Surveyor/T7E1 are prone to false positives as the Ae. aegypti genome is highly polymorphic.
We used deep sequencing of barcoded PCR amplicons surrounding putative CRISPR-Cas9 cut sites from small pools of injected animals to accurately determine the rates of cutting at different genomic loci. Sequencing libraries were prepared using a two-step PCR process that incorporates adapter and barcode sequences necessary for Illumina sequencing (Figure 1A). To minimize the underestimation of mutagenesis rate due to deleted primer binding sequences, we designed a forward primer 50–100bp from the predicted sgRNA cut site and a reverse primer >50bp on the opposite side. We estimate that 10,000–100,000 reads per sample are ample for this analysis, so sequencing of amplicons from 3 sgRNAs per gene, for 10 different genes costs approximately $70 per gene at current pricing (MiSeq v3 reagents, 150-cycle flowcell, item MS-102-3001). In our judgment this method is cost-effective and provides high resolution relative to all other techniques.
Figure 1. Deep sequencing to quantify CRISPR-Cas9 efficiency.
(a) Schematic of PCR amplicon barcoding. (b–c) amplicons generated from adults reared from embryos injected with 333ng/μL Cas9 protein and 40ng/μL sgRNA (AAEL004091-sgRNA1) using primers AAEL004091-1-F and AAEL004091-1-R. (b) Visualization of a subset of alignments to the amplicon reference sequence (top). (c) Quantification of three replicate libraries as percentage of reads aligned to a given base that contain an insertion or deletion at that base. (d–e) Summary of sequencing data from animals injected with two different sgRNAs in combination with Cas9 mRNA (left) or Cas9 recombinant protein (right), at the indicated concentrations. n=7–18 libraries (d) and n=2 libraries (e). Data are plotted as mean (circle) and 95% confidence intervals (line).
Following sequencing, reads were aligned to a reference sequence using the GSNAP short read aligner (Wu and Nacu, 2010), and analyzed with the python package pysamstats. This procedure reveals the number of polymorphisms, including insertions and deletions, found in reads that span each nucleotide of a reference sequence (Figure 1B). In injections containing a sgRNA and Cas9, a pattern of elevated insertions and deletions can be observed with a peak 3 bp 5′ of the beginning of the PAM, exactly the position at which Cas9 is known to make a double-stranded break (Figure 1C). Importantly, there was high concordance in the mutagenesis rates between multiple biological replicates (Figure 1C).
We next varied the delivery method and concentration of Cas9, and the concentration of a given sgRNA, to determine an optimal injection mix. A DNA plasmid that expresses Cas9 under the control of the Ae. aegypti polyubiquitin (PUb) promoter did not induce detectable rates of insertions or deletions with a validated sgRNA (data not shown). When included at 500ng/μL, both Cas9 mRNA and recombinant Cas9 protein induced detectable mutagenesis at two distinct guide RNA sites. However, Cas9 protein induced mutagenesis at rates 5–10× higher than Cas9 mRNA (Figure 1D). To test whether the concentration of sgRNA or Cas9 mRNA or protein was limiting in these earlier injections, we tried four additional injection mixes with a single validated sgRNA (Figure 1E), and determined that mixes containing 400ng/μL Cas9 recombinant protein induced the highest rates of mutagenesis. Increasing sgRNA concentration did not dramatically increase mutagenesis rates.
Identifying active sgRNAs for a given genomic target
We reasoned that sgRNAs causing higher somatic cut rates would be more likely to result in stable germ-line mutations. We manually searched 6 different genes for sgRNAs composed of 17–20 bases (Fu et al., 2014) adjacent to a PAM and beginning with GG or G to facilitate in vitro transcription. We then selected 3 sgRNAs per gene with a low probability of off-target binding (Figure 2A and Table S1).
Figure 2. Identifying active sgRNAs.
(a) Schematic of two target genes in the Ae. aegypti genome indicating the three sgRNAs designed in the first exon of each gene. (b) Schematic of the workflow for a small injection (approx. 150 embryos) of Cas9 protein and a pool of three sgRNAs against three distinct target genes. (c) Sequencing results from 6 small injections (sgRNA sequences can be found in Table S1). n=3 sequencing libraries per sgRNA. Data are presented as means and 95% confidence intervals.
To test the efficiency of these sgRNAs, we performed a series of 6 small test injections of recombinant Cas9 protein at 333ng/μL and a pool of three sgRNAs (40ng/μL each), each targeting a different gene, into 145–168 Ae. aegypti embryos (Figure 2B). Survival rates were very high for these injections (ranging from 46.1%–63.3%, as compared to 18.6% average survival with 500ng/μL Cas9 protein or mRNA). We attribute this marked increase in survival to the reduction in Cas9 concentration. Surviving embryos were reared to pupal stages and collected for sequencing of PCR amplicons (Figure 2B).
All 18 sgRNAs induced detectable levels of mutagenesis, which varied within and between different genomic targets (Figure 2C), reflecting sequence- or context-dependent effects on sgRNA efficiency that are not yet fully understood. Designing and testing 3 sgRNAs resulted in the identification of at least one highly active sgRNA for the 6 genomic targets tested here. Users are strongly advised to test multiple sgRNAs per gene before undertaking large-scale mutagenesis injections.
Germ-line transmission of mutant alleles
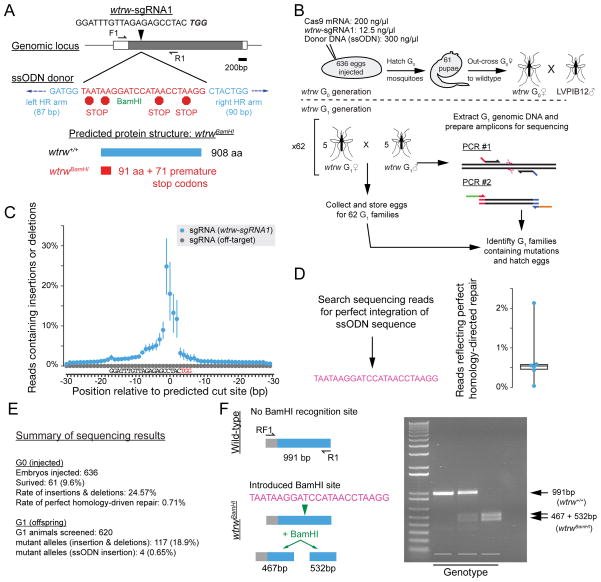
We next examined whether somatic mutagenesis detected in adults reared from injected embryos (G0 animals) resulted in transmission of stable mutant alleles through the germline to their offspring (G1 animals). We designed an sgRNA near the 5′ end of Aaeg-wtrw, and included 300ng/μL of a 200 bp ssODN donor as a template for homology-directed repair. The ssODN had homology arms of 87–90 bases on either side of the Cas9 cut site, flanking an insert with stop codons in all three frames of translation and a restriction enzyme site. Successful integration of this template would truncate the full-length protein of 908 amino acids at 91 amino acids (Figure 3A).
Figure 3. Germ-line transmission of mutant alleles.
(a) Schematic of the Ae. aegypti wtrw locus detailing the sgRNA binding site, single-stranded oligonucleotide (ssODN) donor, and modified locus. (b) Schematic of injection performed to isolate mutations. (c) Summary sequencing data from G0 adults. n=6 (wtrw) and n=3 (off-target), presented as means (circle) and 95% confidence intervals (lines). (d) Exogenous ssODN sequence used to as a query for the unix tool grep (left) and reads containing perfect homology-directed repair (right) presented as a boxplot (box represents median and 1st and 3rd quartiles; whiskers represent data range). (e) Summary of G0 and G1 sequencing results (f) Restriction enzyme diagnostic of ssODN insertion.
636 embryos were injected with a mixture of 200ng/μL Cas9 mRNA and 12.5ng/μL sgRNA (these injections were performed prior to the optimization of injection mixes described above). We performed amplicon sequencing on 6 pools of 5–6 adult G0 animals after they were outcrossed and allowed to lay eggs (Figure 3B). These samples contained a maximum mutation rate of 24.87% centered on the Cas9 cut site (Figure 3C). Amplicons derived from animals injected with an sgRNA targeting a different region of the genome contained no detectable insertions or deletions at the Aaeg-wtrw locus (Figure 3C). On average 0.71% of aligned reads from six samples contained sequences corresponding to the single-stranded DNA donor (Figure 3D), indicating that the ssODN template could drive homology-directed repair in somatic tissue, albeit at a much lower frequency than insertions or deletions mediated by non-homologous end-joining.
To determine whether these mutations were stably transmitted through the germline, we sequenced PCR amplicons derived from 62 pools each containing 5 male and 5 female G1 offspring. Analysis of resulting insertions and deletions using Genome Analysis Toolkit [GATK; (DePristo et al., 2011)] revealed at least 117 mutant chromosomes spread across 50 pools. Given that each G1 individual can only carry a single mutant chromosome, we conclude that the G1 mutation rate was at least 117/620, or 18.9% (Figure 3E). Four of these alleles corresponded perfectly to the sequence of the ssODN, so our rate of stable germline transmission of alleles generated by homology-driven repair is at least 0.6% (Figure 3E). This is similar to rates in G0 animals (Figure 3E), suggesting that somatic mutagenesis can predict the efficiency of germ-line mutagenesis.
We hatched F2 eggs from a single family containing an allele generated by homology-directed repair. Sequencing of single-pair crosses allowed us to isolate a stable mutant line that can be genotyped by the ssODN-introduced restriction site (Figure 3F). This line was outcrossed for 8 generations to wild-type mosquitoes to increase genetic diversity and reduce the possibility of retaining off-target mutations. The presence of the mutant allele was verified in individual female mosquitoes at each generation by molecular genotyping, and eggs were hatched from heterozygous mutant females only.
Deletions induced by multiplexed sgRNAs
Double-stranded breaks induced at multiple sgRNA sites can induce large deletions between the two cut sites in D. melanogaster (Ren et al., 2013). We performed a series of five injections into small numbers of embryos using sgRNAs targeting 3 different genes. All but one of these sgRNAs (AAEL000926-sgRNA4) was previously validated (Figure 2C) and injection mixes also included ssODN donors (Figure 4A and Supplemental Data S2). G1 embryos were hatched and screened as individual families derived from a single female G0. (Figure 4B). We identified mutant families by screening PCR amplicons generated from pools of G1 male pupae for size-shifted bands. Following out-crossing and egg-collection, individual G1 females were similarly genotyped by PCR amplicon size (Figure 4C–E) to estimate mutation rates.
Figure 4. Deletions generated by multiple sgRNAs.
(a) Schematic of the AAEL010779 genomic locus detailing the design of two sgRNAs and an ssODN donor. (b) Injection strategy to identify deletion events in G1 animals with the injection of a small (125–150) number of embryos. (c) Example agarose gel of 9 G1 female offspring of a single G0 female, revealing 4 wild-type and 5 heterozygous individuals. (d) Per cent of mutant AAEL010779 G1 females from the 10 G0 families identified as containing at least one mutant allele presented as a boxplot (box represents median and 1st and 3rd quartiles; whiskers represent data range). (e) Summary data of G1 mutagenesis from 3 injections of this type.
We found a wide range of mutant transmission rates in families derived from single G0 individuals (Figure 4D). Sanger sequencing of some bands revealed that mutations ranged from simple deletions, to homology-directed repair from the ssODN donor, to more complex modifications, including polymorphisms, inversions, and duplications. Additionally, we were successful in obtaining germ-line mutations at high rates in each of 3 small injections (Figure 4E), making this a cost-effective and efficient way to generate loss-of-function mutant alleles. The relatively large size of deletions generated by this method simplifies sized-based molecular genotyping of females at each generation.
Integration and transmission of large fluorescent cassettes
Finally, we asked whether CRISPR-Cas9 could be used to introduce gene cassettes via homology-dependent repair. Previously, zinc-finger nucleases were used to introduce large cassettes into Ae. aegypti from a plasmid DNA donor with homology arms of at least 800 bp on either side (Liesch et al., 2013; McMeniman et al., 2014), generating a null mutant by the insertion of a visible fluorescent reporter. We performed injections with Cas9 protein, a validated sgRNA, and a plasmid donor containing homology arms of 799 bp and 1486 bp. This plasmid (Addgene #47917) contains a cassette comprising the constitutive PUb promoter driving the expression of the fluorescent reporter ECFP (Figures 5A–B). Arms were cloned from wild-type LVP-IB12 mosquitoes and were designed to avoid repetitive sequences such as transposable elements. Following injection, individual female G0 animals were outcrossed to wild-type mosquitoes and G1 eggs were collected (Figure 5A). Because we previously observed that successful homology-directed repair occurs primarily, if not exclusively, in female G0 Ae. aegypti, we discarded G0 males and screened G1 families generated from females.
Figure 5. Insertion of fluorescent cassettes by homology-directed repair.
(a) Schematic of injection and screening strategy to obtain alleles with an insertion of an ECFP cassette (blue). (b) Design of the plasmid donor. (c) At left, brightfield and ECFP fluorescence images of two pupae: wild-type (top) and AAEL000582ECFP/ECFP (bottom). Scale bar is 1mm. At right and below, PCR strategy to verify directed insertion of the PUb-ECFP cassette. (d) PCR strategy to identify homozygous individuals. See also Table S3.
G1 larvae at 3–5 days post-hatching were screened under a fluorescence dissecting microscope for the fluorescent protein expressed under control of the PUb promoter (Figure 5C). Fluorescent individuals were collected, reared to adulthood, and crossed to wild-type animals to establish stable lines. To verify gene-specific insertion of our cassette, we designed PCR primers spanning both homology arms (Figure 5C). It is critical that these primers are designed outside each arm and that bands obtained are sequenced to verify junctions between genomic and exogenous sequence on each end of the insertion.
Lines containing verified targeted insertions were out-crossed to wild-type mosquitoes for 8 generations by selecting fluorescent larvae and pupae. A homozygous line was established by mating heterozygotes. Putative homozygous mosquitoes were selected by increased fluorescence as larvae, separated by sex, and used to establish single-pair matings. The genotype of these single-pair matings was verified by PCR (Figure 5D).
We generated verified targeted insertions in two of four genomic loci with the Ae. aegypti PUb promoter driving the expression of ECFP (Figure 5 and Table S3) or dsRed (Table S3), suggesting that homology-directed repair with large plasmid donors occurs at a relatively low frequency compared to other forms of CRISPR-Cas9-mediated genome modification. A drastic variance in the efficiency noted in two injections (Table S3) suggests that simple modifications of injection mix component concentration may increase integration rates, perhaps at the expense of embryo survival. The identification of stably transmitting integration events at non-targeted genomic loci underscores the necessity of verifying all lines generated by this technique by PCR or other molecular methods.
Discussion
We have demonstrated that the CRISPR-Cas9 system is a highly effective tool for precision genome-editing in the mosquito Ae. aegypti. Compared to the relatively low throughput and high cost of ZFN- and TALEN-mediated mutagenesis, the ease of designing and producing CRISPR-Cas9 reagents has allowed us to generate stable and precise loss-of-function mutations in 5 genes described here. A variety of mutant alleles can be recovered, including frame-shift mutations caused by insertions or deletions, deletion of a region between two sgRNA target sites, and integration of exogenous sequences from a single-stranded oligonucleotide or a double-stranded plasmid DNA donor. This protocol provides a step-by-step manual to mutagenesis in Ae. aegypti and also provides general principles that will be useful when translated to other species.
Optimal injection mix
We recommend recombinant Cas9 protein for its reproducibility, increased rates of mutagenesis, and embryo survival. It is likely more stable than mRNA, both at the bench and in injected embryos, and may form a complex with sgRNA in vitro prior to injection. This can stabilize sgRNA/Cas9 complexes (Jinek et al., 2014) and ensures mutagenesis without the delay of translation of Cas9 mRNA in the embryo. The specific concentrations suggested here represent a good trade-off between survival and efficiency in our hands. However, further modifications to this protocol may result in significant increases in certain types of repair.
We recommend the following injection mix for Ae. aegypti embryos. This may be a good starting point for other insect embryos.
300ng/μL recombinant Cas9 protein
40ng/μL sgRNA (each)
200ng/μL single stranded ssODN or 500ng/μL double stranded plasmid DNA (optional)
Designing active sgRNAs
As in other organisms and cell lines (Fu et al., 2014; Ren et al., 2014), we observed success with sgRNAs ranging in length from 17–20 bp. However, different sgRNAs varied significantly in effectiveness, even when targeted to a small region of the same gene (Figure 2). Additionally, a single genomic target (AAEL001123) was resistant to mutagenesis with 6 different sgRNAs. Further experiments will determine whether sgRNA base composition (such as GC content; Ren et al., 2014) or underlying chromatin-state (Wu et al., 2014) influence efficacy, To maximize the chance of successful mutagenesis, we recommend designing and testing multiple sgRNAs targeting a given gene before committing to large-scale injections.
Off-target effects
Off-target effects are a concern with any genome-editing technology, and we address these concerns in our experiments in four ways. First, we check sgRNA specificity using publicly available bioinformatic tools (Hsu et al., 2013; Hwang et al., 2013; Sander et al., 2010), selecting the most specific sgRNAs within the region we wish to target. No obvious correlation was detected between the cut rate and the predicted specificity of the sgRNA (Figure 2C and Table S1), with the caveat that we did not screen for mutagenesis at predicted off-target binding sites. Second, we can generate mutant alleles with different sgRNAs and test phenotypes in heteroallelic combination, reducing the likelihood of shared off-target mutations. Third, we have successfully used truncated (<20 bp) sgRNAs, which have been shown in cell culture to reduce the likelihood of off-target modifications (Fu et al., 2014). Finally, all lines are outcrossed to wild-type mosquitoes for at least 8 generations to reduce co-inheritance of all but the most tightly linked off-target mutations. While these guidelines reduce the likelihood of off-target mutagenesis, there is a need for continued efforts to improve and verify the specificity of all genome-engineering technologies.
Enhancing the efficiency of homology-directed repair
In our experiments, insertions and deletions mediated by non-homologous end-joining occur at much higher frequency than by homology-directed repair. This is similar to what has been observed in Ae. aegypti with other genome-editing tools, such as ZFNs (Liesch et al., 2013; McMeniman et al., 2014), and in other organisms such as D. melanogaster (Gratz et al., 2014).
Several approaches have been developed to increase rates of homology-directed repair. These include injections in the background of a DNA ligase 4 mutation (Beumer et al., 2013b, 2013a), or schemes that linearize a double-stranded donor template in vivo. Finally, many laboratories working with D. melanogaster have developed transgenic strains that express Cas9 protein under ubiquitous or germ-line promoters, generally improving the efficiency of mutagenesis and specifically increasing rates of homology-directed repair (Gratz et al., 2014; Ren et al., 2014). It remains to be seen whether transgenic Cas9 delivery or alternative integration approaches can be effectively implemented in Ae. aegypti.
We note that we have observed a single round of injection that resulted in high (>30%) rates of homology-directed repair but extremely low survival (Table S3). This suggests that we might achieve improvements in insertion efficiency by continuing to modulate the composition of the injection mix. If the rates of homology-directed-repair can be sufficiently improved, CRISPR-Cas9 coupled with homology-directed repair will likely prove to be a versatile tool to tag gene products and introduce transgenes into specific genomic loci, enabling the study of identified neural circuits and other subsets of cells.
Conclusion
Precision genome-engineering in mosquitoes holds great promise for studies on the genetic basis of behavior (DeGennaro et al., 2013; Liesch et al., 2013; McMeniman et al., 2014), and for genetic strategies to control vector population or disease competence (Alphey, 2014). Ongoing efforts to increase the specificity and efficiency of these technologies is critical to their adaptation as routine techniques, and we believe that the protocols outlined here have met those criteria for the generation of loss-of-function mutations in the mosquito Ae. aegypti. Reagents based on CRISPR-Cas9 have been used successfully in organisms from bacteria to primates. This suggests that the techniques described here can likely be adapted to many other non-model organisms, as long as efficient methods for introducing the reagents into the germline and screening for mutations can be developed.
Supplementary Material
Acknowledgments
We thank Rob Harrell and the Insect Transformation Facility/University of Maryland for expert mosquito embryo microinjection, Gloria Gordon and Libby Mejia for mosquito rearing, and members of the Vosshall laboratory for helpful comments and critiques. We thank Román Corfas and Conor McMeniman for feedback on an earlier version of this manuscript. This work was supported in part by contract HHSN272200900039C from the National Institute of Allergy and Infectious Diseases and grant UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program). B.J.M. was a Jane Coffin Childs Postdoctoral Fellow and L.B.V is an investigator of the Howard Hughes Medical Institute.
Footnotes
Accession Number
Sequencing data are available from NCBI under Bioproject accession number PRJNA272452.
Author Contributions
K.E.K. and B.J.M. designed the study, performed experiments, and analyzed the data. All authors together designed the figures and wrote the manuscript.
Conflict of interest statement:
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- Aryan A, Anderson MAE, Myles KM, Adelman ZN. TALEN-based gene disruption in the dengue vector Aedes aegypti. PLoS ONE. 2013a;8:e60082. doi: 10.1371/journal.pone.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A, Anderson MAE, Myles KM, Adelman ZN. Germline excision of transgenes in Aedes aegypti by homing endonucleases. Sci Rep. 2013b;3:1603. doi: 10.1038/srep01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A, Myles KM, Adelman ZN. Targeted genome editing in Aedes aegypti using TALENs. Methods. 2014;69:38–45. doi: 10.1016/j.ymeth.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Tibbit C, Ponting CP, Liu J-L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer KJ, Trautman JK, Mukherjee K, Carroll D. Donor DNA utilization during gene targeting with zinc-finger nucleases. G3. 2013a;3:657–664. doi: 10.1534/g3.112.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer KJ, Trautman JK, Christian M, Dahlem TJ, Lake CM, Hawley RS, Grunwald DJ, Voytas DF, Carroll D. Comparing zinc finger nucleases and transcription activator-like effector nucleases for gene targeting in Drosophila. G3. 2013b;3:1717–1725. doi: 10.1534/g3.113.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem. 2014;83:409–439. doi: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8:e1002861. doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, Angel G, del Rivas MA, Hanna M, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279–284. doi: 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Ahkmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-Scale assessment of single-guide RNAs. PLoS ONE. 2014;9:e98186. doi: 10.1371/journal.pone.0098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O’Connor-Giles KM. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 2014;196:961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol. 2014;32:577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleley C, Sutcliffe A. 2014 Methods in Anopheles Research. Centers for Disease Control; Atlanta, GA: 2014. [Accessed 2/19/2015]. http://www.mr4.org/AnophelesProgram/TrainingMethods.aspx. [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh J-RJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. doi: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja P, Osei-Poku J, Ho YS, Ariani CV, Palmer WJ, Pain A, Jiggins FM. Assembly of the genome of the disease vector Aedes aegypti onto a genetic linkage map allows mapping of genes affecting disease transmission. PLoS Negl Trop Dis. 2014;8:e2652. doi: 10.1371/journal.pntd.0002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesch J, Bellani LL, Vosshall LB. Functional and genetic characterization of neuropeptide Y-like receptors in Aedes aegypti. PLoS Negl Trop Dis. 2013;7:e2486. doi: 10.1371/journal.pntd.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo NF, Hua-Van A, Li X, Nolen BM, Fraser MJ. Germ line transformation of the yellow fever mosquito, Aedes aegypti, mediated by transpositional insertion of a piggyBac vector. Insect Mol Biol. 2002;11:133–139. doi: 10.1046/j.1365-2583.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- Lobo NF, Clayton JR, Fraser MJ, Kafatos FC, Collins FH. High efficiency germ-line transformation of mosquitoes. Nat Protocols. 2006;1:1312–1317. doi: 10.1038/nprot.2006.221. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Development. 2014;141:4042–4054. doi: 10.1242/dev.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Sun J, Housden BE, Hu Y, Roesel C, Lin S, Liu L-P, Yang Z, Mao D, Sun L, et al. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc Natl Acad Sci USA. 2013;110:19012–19017. doi: 10.1073/pnas.1318481110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Yang Z, Xu J, Sun J, Mao D, Hu Y, Yang S-J, Qiao H-H, Wang X, Hu Q, et al. Enhanced specificity and efficiency of the CRISPR/Cas9 system with optimized sgRNA parameters in Drosophila. Cell Rep. 2014;9:1151–1162. doi: 10.1016/j.celrep.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Maeder ML, Reyon D, Voytas DF, Joung JK, Dobbs D. ZiFiT (Zinc Finger Targeter): an updated zinc finger engineering tool. Nucleic Acids Res. 2010;38:W462–W468. doi: 10.1093/nar/gkq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard BL. Homing endonucleases from mobile group I introns: discovery to genome engineering. Mob DNA. 2014;5:7. doi: 10.1186/1759-8753-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoshevskiy VA, Kinney NA, Debruyn BS, Mao C, Tu Z, Severson DW, Sharakhov IV, Sharakhova MV. Genomic composition and evolution of Aedes aegypti chromosomes revealed by the analysis of physically mapped supercontigs. BMC Biol. 2014;12:27. doi: 10.1186/1741-7007-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat Biotechnol. 2014;32:670–676. doi: 10.1038/nbt.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.