Abstract
Sulforaphane is a natural product found in broccoli which is known to exert many different molecular effects in the cell, including inhibition of histone deacetylase (HDAC) enzymes. Here we examine for the first time the potential for sulforaphane to inhibit HDACs in HaCaT keratinocytes and compare our results with those found using HCT116 colon cancer cells. Significant inhibition of HDAC activity in HCT116 nuclear extracts required prolonged exposure to sulforaphane in the presence of serum. Under the same conditions HaCaT nuclear extracts did not exhibit reduced HDAC activity with sulforaphane treatment. Both cell types displayed down-regulation of HDAC protein levels by sulforaphane treatment. Despite these reductions in HDAC family member protein levels, acetylation of marker proteins (acetylated Histone H3, H4 and tubulin) was decreased by sulforaphane treatment. Timecourse analysis revealed that HDAC6, HDAC3 and acetylated histone H3 protein levels are significantly inhibited as early as 6hr into sulforaphane treatment. Transcript levels of HDAC6 are also suppressed after 48hr of treatment. These results suggest that HDAC activity noted in nuclear extracts is not always translated as expected to target protein acetylation patterns, despite dramatic inhibition of some HDAC protein levels. In addition, our data suggest that keratinocytes are at least partially resistant to the nuclear HDAC inhibitory effects of sulforaphane which is exhibited in HCT116 and other cells.
Keywords: skin, chemoprevention, isothiocyanate
Introduction
Sulforaphane (SF) is a natural product found in broccoli which has gained interest as a chemopreventive agent [1]. We and others have shown that SF effectively inhibits non-melanoma skin cancer in UVB-irradiated hairless mice [2, 3]. SF has shown efficacy at inhibiting other mouse models of cancer and is the subject of several clinical trials, including topical applications examining the activation of protective enzymes in the skin and prevention of UV-induced erythema [4–8]. Among other mechanisms, SF has been shown to inhibit Activator Protein-1 (AP-1) activity and stimulate antioxidant and cytoprotective genes through activation of the Nf-E2-related factor 2 (Nrf2) transcription factor [9, 10] [11, 12]. However, the effects of SF treatment seem to vary greatly depending upon the cell type, dosage, and stressor conditions used [1].
Histone deacetylases (HDACs) have been implicated as a target for SF. Myzak et al. [13] demonstrated that treatment of HCT116 colon cancer cells with SF resulted in inhibition of HDAC activity in a dose-dependent manner. SF has also been shown to inhibit HDAC activity in a variety of models, including prostate cell lines, mouse colon tissue, and human peripheral blood mononuclear cells (PBMCs) [14–16]. In vitro studies and in silico modeling suggest that SF binds to cysteine in the cell in order to fit within the HDAC active site and inhibit HDAC activity. This interaction, mediated by metabolism through the mercapturic acid pathway, is hypothesized to create the correct spacing in order for cysteine to interact with zinc in the active site of an HDAC, while SF blocks accessibility to the binding pocket [13, 17].
We are interested in determining whether sulforaphane would be a good chemopreventive agent if topically applied to the skin. While there is good evidence that sulforaphane blocks UV-induced tumorigenesis in vivo [2, 3], the mechanism of that inhibition is poorly understood. Therefore, this paper seeks to examine whether sulforaphane acts as an HDAC inhibitor in HaCaT keratinocytes.
Materials and Methods
Reagents
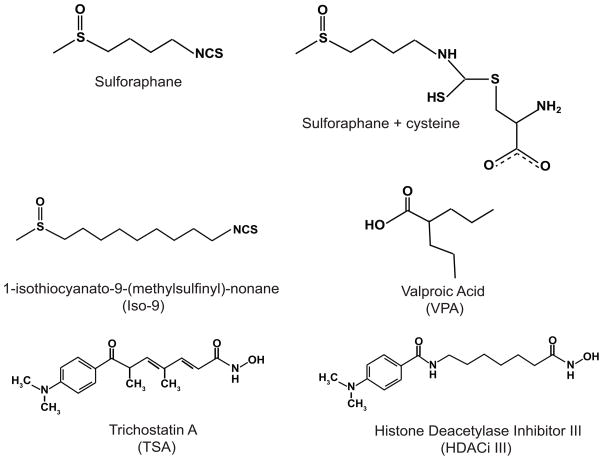
R,S-sulforaphane and 1-Isothiocyanato-9-(methylsulfinyl)-heptane (Iso-9) were purchased from LKT laboratories and dissolved in AcN. Trichostatin A (TSA), a classical pan-HDAC inhibitor, was purchased from Sigma-Aldrich. Histone Deacetylase Inhibitor III (HDACi III) and Valproic Acid (VPA) were purchased from EMD Chemicals. HDACi III is an amide analogue of TSA. VPA is a class I and IIa HDAC inhibitor. Structures of the agents of interest are shown in Figure 1.
Figure 1.
Chemical structures of compounds described in this study.
Cell Culture
Human HaCaT keratinocytes and human HCT116 cells (a kind gift of Dr. Jesse Martinez, The University of Arizona) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 100U/mL penicillin/streptomycin at 37°C with 5% CO2.
HDAC activity assay
Histone deacetylase activity was assayed using a fluorometric HDAC activity assay kit (Enzo Life Sciences). For non-starved conditions, cells were plated at a density of 2.8×106 cells/100mm dish and grown for 24 hr. SF, Iso-9 or vehicle were then added for a 48 hr treatment period. Cells were exposed to TSA or VPA for five hours prior to harvest. For experiments requiring starvation, cells were plated at a density of 1.8×106 cells/100mm dish, grown for 24 hr, and then starved in serum-free medium for 24 hr before addition of the drugs as described for a 48 hr (SF, Iso-9) or 5 hr (TSA, VPA) incubation. Media was not changed after addition of drugs. Nuclear proteins were extracted as described [11]. Five μg of nuclear protein were used in the HDAC assays, performed in triplicate in 96-well plates, per manufacturer’s directions. Plates were incubated with substrate at 37°C for 20 min, followed by incubation with developer at room temperature for 10 min. Plates were then read at an excitation wavelength of 365nm and emission wavelength of 450nm using a Spectra Max Gemini (Molecular Devices).
Western Blots
Cells were seeded in 60mm dishes at a density of 1×106 cells/dish and treated as described for the HDAC activity assay. Cells were harvested in radioimmunoprecipitation buffer [18] and protein concentration was determined using the Dc assay (BioRad). Thirty μg of protein per sample were separated on SDS-PAGE gels and transferred to PVDF membranes. Blots were visualized and quantified using a ChemiDoc system with ImageLab software (BioRad). Antibodies were purchased from the following companies: Abcam (Nrf2 #62352), Cell Signaling (acetylated histone H3 #9677, acetylated histone H4 #2594, HDAC2 #2540, HDAC4 #2072), Santa Cruz Biotechnology (acetylated tubulin sc-23950, HDAC3 sc-11417, HDAC6 sc-11420), Upstate (HDAC1 #05-614) and Sigma (β-actin, A5441).
Quantitative Real-Time PCR Analysis
Cells were seeded as described above for Western blots. Twenty-four hours later, cells were treated with 15μM SF or vehicle and harvested at the indicated times. RNA was extracted using Qiagen RNeasy Mini kit (#74104) and reverse transcribed using Quanta qScript cDNA Synthesis kit (#95047). TaqMan primer/probe set Hs00195869_m1 and Hs99999905_m1 was used to amplify HDAC6 and GAPDH using TaqMan Universal PCR Master Mix (#4324018), respectively using an Applied Biosystems (ABI) 7000 SDS machine as described previously [19].
Statistical Analysis
For all figures, statistical significance was calculated using student’s t test. * indicates p ≤ 0.05, ** indicates p ≤ 0.001.
Results
Sulforaphane does not inhibit nuclear HDAC activity in the absence of serum
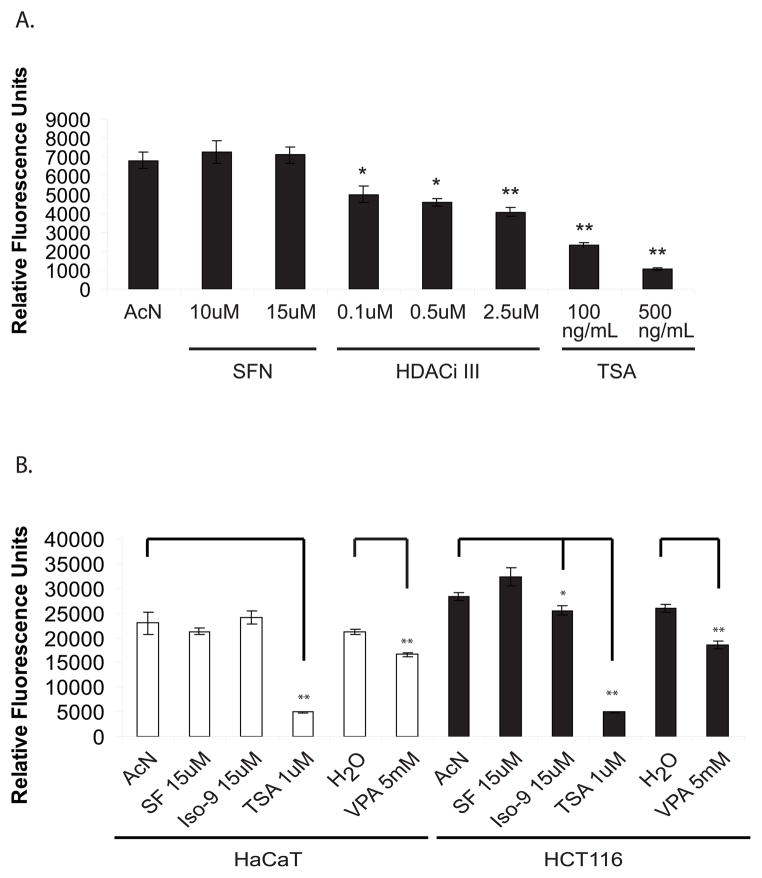
Previous studies have shown that SF inhibits HDAC activity in nuclear and whole cell lysates [13, 17, 20]. In HaCaT cells, treatment with SF for 8hr at concentrations of up to 15μM failed to inhibit HDAC activity, whereas commercially-available inhibitors successfully suppressed HDAC activity in a dose-dependent manner (Fig 2A). We therefore increased the treatment time to 48 hr to match experimental conditions reported in the literature and also performed parallel experiments in HCT116 colon cancer cells as a positive control. HCT116 cells have previously been shown to respond to SF-induced HDAC inhibition at a dose as low as 3μM using this assay, although not under serum starvation conditions [13]. We also included an SF analogue, Iso-9 (Fig. 1), which was shown to be a more efficient inhibitor of HDAC activity than SF in HCT116 cells [17]. Long-term treatment (48hr) with SF during serum starvation failed to inhibit HDAC activity in both cell lines, although treatment with Iso-9 yielded 10% inhibition in HCT116 cells (Fig 2B). The pan-HDAC inhibitor, TSA, inhibited HDAC activity to a greater extent than did the Class I-selective HDAC inhibitor, VPA, in both cell lines, although both agents yielded significant inhibition.
Figure 2.
Sulforaphane does not inhibit HDAC activity under serum-depleted conditions. (A) Sub-confluent HaCaT cells were serum starved for 24 hr prior to addition of vehicle (AcN) or the indicated drugs, and nuclear proteins were extracted 8 hr later for the fluorometric HDAC activity assay. (B) HaCaT and HCT116 cells were serum starved for 24 hr prior to addition of vehicle or drugs (AcN, SF or Iso-9) and nuclear extracts were harvested 48 hr later for the same assay. VPA, TSA or water control were added 5 hr prior to harvest. Results representative of n = 3.
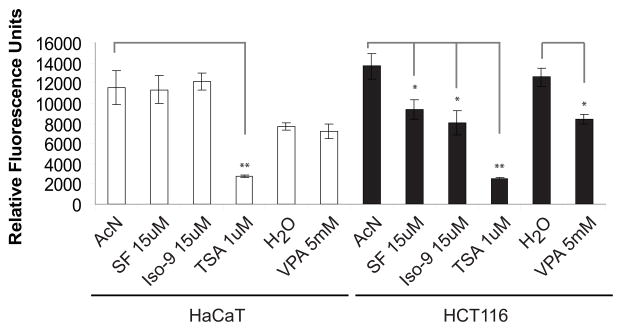
In the presence of serum, sulforaphane blocks HDAC activity in HCT116 cells but not in HaCaT cells
We subsequently repeated the HDAC inhibitory assay with cells that had not been serum-deprived in order to fully replicate conditions documented in the literature [13, 17, 20]. Under these conditions, HCT116 cells exhibited significant HDAC inhibition after exposure to SF, Iso-9, VPA and TSA when compared to vehicle controls (Fig. 3). In HaCaT cells, however, only TSA inhibited HDAC activity under these conditions. VPA failed to inhibit HDACs in HaCaT cells, while it significantly inhibited activity in HCT116 cells.
Figure 3.
Presence of serum allows for HDAC inhibition by sulforaphane in HCT116 cells but not HaCaT cells. Twenty-four hr after seeding, cells were treated with vehicle or drugs for 48 hr or 5 hr in the presence of serum as described in Figure 2 and samples were subjected to the HDAC activity assay. Results representative of n = 3.
Sulforaphane-mediated reduction in HDAC levels does not translate to increased acetylation of reporter proteins
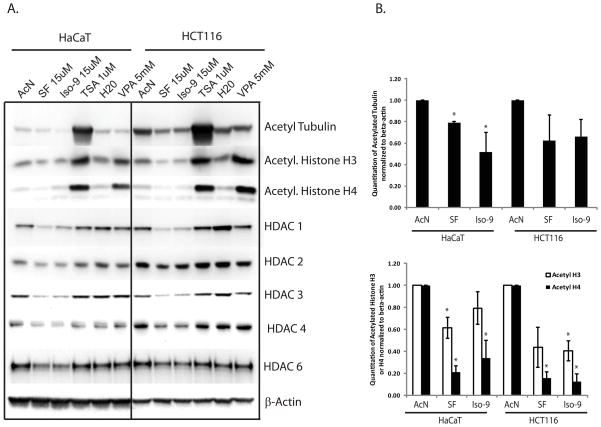
Western blots were performed on total cell lysates from cells which had been treated in the presence of serum as described in Figure 3. We hypothesized that inhibition of HDAC activity would result in increased acetylation of target proteins as measured with acetylation-specific antibodies. As expected, histones H3 and H4 as well as α-tubulin were all strongly acetylated in response to TSA treatment in both cell lines. Also as expected, VPA treatment resulted in strong acetylation of histones H3 and H4 but not α-tubulin. It also slightly reduced protein levels of HDACs 1–3, as supported by the literature [21]. However, cells treated with SF or Iso-9 did not show the expected increase in histone or α-tubulin acetylation when compared to AcN control (Figure 4A). Acetylation was instead decreased in these reporter proteins (quantified in Figure 4B).
Figure 4.
Western blots indicate decreased levels of HDAC proteins and decreased acetylation of reporter proteins. A) HaCaT and HCT116 cells were treated as described for Figure 3, but whole cell lysates were harvested and used for Western blot analysis. β-actin is used as a loading control. Results representative of n = 3. B) The levels of acetylated histone H3, H4 and tubulin from the three replicates were quantified and summarized.
Recent literature suggests that SF may modulate protein acetylation by decreasing the levels of cellular HDAC proteins. We examined the protein levels of HDACs 1,2 and 3 (class I), HDAC 4 (class IIa) and HDAC 6 (class IIb). Class I HDACs are primarily nuclear, acetylating histones and other nuclear proteins. Class IIa HDACs are both nuclear and cytoplasmic, and are known to regulate transcription factors and other proteins. Class IIb HDACs are primarily cytoplasmic. HDAC 6 is strongly associated with acetylation of tubulin and HSP90 [22, 23]. Our data show that long-term (48hr) treatment with SF and Iso-9 resulted in reduced levels of all the HDACs tested (HDACs 1,2,3,4, and 6). Thus, in our hands, levels of both class I and class II HDAC proteins are down-regulated by SF treatment. Treatment with Iso-9 followed the trend seen with SF.
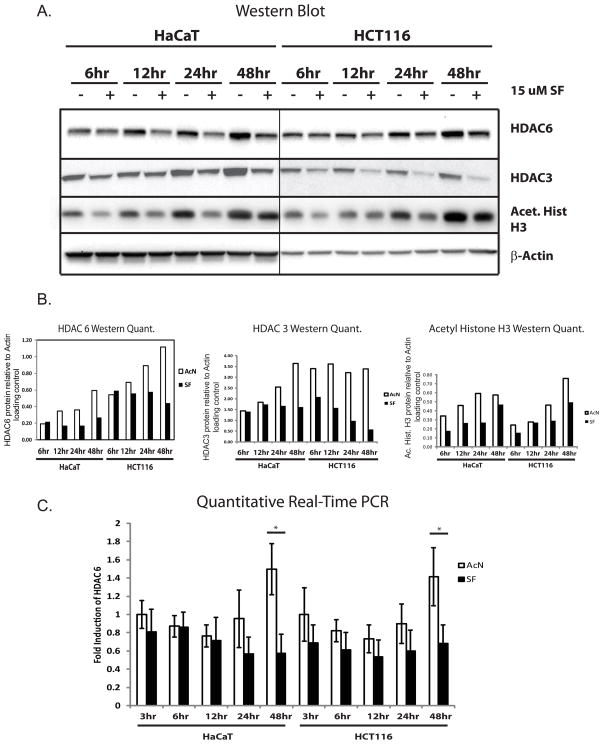
Levels of HDAC 3 and 6 protein, HDAC 6 RNA and Histone H3 acetylation, are reduced early after sulforaphane treatment
HDAC6 has been implicated as a key target for degradation during SF treatment in cancer cells, and is most sensitive to SF treatment in transformed cells compared to initiated/normal cells [24–26]. We therefore performed a kinetic analysis of HDAC6 and HDAC3 expression as well as histone H3 acetylation after SF treatment. As shown in Figure 5A and 5B, protein levels of HDAC6 begin responding to SF treatment by 12 hr of exposure, while HDAC3 responds as early as 6 hr. SF treatment blocks accumulation of HDAC 3 and 6 proteins seen in control cells over time and significantly reduces HDAC 6 transcription only after 48 hr (Figure 5C). Levels of acetylated Histone H3 levels are reduced by SF treatment as early as 6 hr after exposure to SF, but mildly recover by 48 hr.
Figure 5.
Timecourse of response of HDAC 6 and acetylated Histone H3 to sulforaphane treatment. Twenty-four hours after seeding, cells were treated with SF or vehicle and harvested at the indicated timepoints. Cells were then processed for either Western blot analysis (A) and quantified relative to β-actin levels (B), or were processed for quantitative real-time PCR analysis (C). Results representative of n = 3.
Discussion
SF has been shown to inhibit HDACs in a variety of cell types. Our study sought to determine whether SF acts as an HDAC inhibitor in skin-derived HaCaT keratinocytes. Our results show that 1) SF does not reduce nuclear HDAC activity in keratinocytes but does reduce levels of HDAC proteins 1–4 and 6 and 2) SF decreases, rather than increases, acetylation of three reporter proteins in HaCaT cells. These results suggest that SF may have a distinct impact on the human keratinocyte acetylome relative to that of other cell types.
Using an assay that largely detects the activity of Zn-dependent HDACs, we found that SF treatment failed to show any effect on HDAC activity in HaCaT cells in the presence or absence of serum. In contrast, the potent HDAC inhibitor TSA dramatically reduced nuclear HDAC activity in the HaCaT cells under all conditions tested. These results strongly suggest that both cell type and growth conditions can have a significant impact on whether SF and its derivatives act as HDAC inhibitors, consistent with reports in the literature. [26, 27]. A study of normal to transformed prostate cancer cell lines indicated that SF had a greater impact on HDAC activity in transformed lines relative to non-transformed lines [26]. In addition, keratinocytes are known to exhibit cell-specific responses to xenobiotics and stress events which are different than those noted in other epithelial cell types [28, 29]. Therefore, adaptation to conditions found in the stratified epithelia of the skin may result in resistance to certain HDAC regulatory stimuli. HaCaT cells are also mutant for p53, and the role of p53 in the response of keratinocytes to SF is unknown.
The requirement for serum to enact the HDAC inhibitory activity of SF in HCT116 cells could be due to several factors. First, serum may upregulate cysteine metabolism. An interaction of SF with cysteine is required for HDAC inhibition in vitro [13, 17] while HDAC inhibitors TSA and VPA bind directly to HDACs without a requirement for metabolism. A 48 hr incubation is required for inhibition of HDACs in HCT116 cells in our model and the literature [13, 26], while clinical studies indicate that SF metabolites peak 2 hr post ingestion and HDACs are maximally inhibited 3 hr post ingestion [16, 30]. These inconsistencies may be due to differences in cysteine availability between cell culture and in vivo models. The rate of removal of the SF-cysteine moiety may be affected by serum starvation as well. Second, SF is known to block cell cycle progression [31]. It is therefore possible that HCT116 cells must be actively proliferating in order for inhibition of HDACs to be measurable. Finally, serum may have distinct effects on signaling in the transformed HCT116 cells that influence the effect of SF on HDACs. As noted above, there is precedence for SF’s effects to be stronger against transformed cells compared to normal counterparts, and traditional HDAC inhibitors are more toxic to transformed cells than to normal cells [17, 26].
In spite of having no effect on nuclear HDAC activity in HaCaT cells, SF does have a significant impact on levels of HDAC proteins. Consistent with other reports [25, 32], SF treatment caused a decrease in levels of HDACs 3 and 6 in HCT116 cells (as well as on HDAC proteins 1,2 and 4). We observed similar reductions in HaCaT cells. Levels of HDACs 3 and 6 are reduced within 12 h of SF treatment in both cell lines, despite the fact that nuclear HDAC activity is unaffected at 8 and 48 h of SF treatment in HaCaT cells. HDAC6 is largely a cytoplasmic deacetylase and is likely to make only a small contribution to total nuclear HDAC activity relative to the Class I deacetylases, HDACs 1–3. Given the sizable decrease in the levels of HDACs 1 and 3 in HaCaT cells by 48 h treatment, it is surprising that there is no loss in nuclear HDAC activity. Recently it was discovered that Class I HDACs require inositol phosphates such as inositol-tetraphosphate [Ins(1,4,5,6)P4 or IP4] for maximal activity [33]. The absolute levels of HDAC proteins in the cell may not correlate with activity if cellular concentrations of IP4 are limiting.
Interestingly, we found that SF treatment decreases levels of acetylated α-tubulin and histones H3 and H4 in both HaCaT and HCT116 cells. This loss of acetylation is unexpected given that nuclear HDAC activity does not change in HaCaT cells and decreases in HCT116 cells in response to SF treatment. A decrease in overall H3 and H4 acetylation could be due to an increase in nuclear sirtuin activity, which has not been explored in relation to SF. Alternatively, SF may downregulate the activity of key HATs that acetylate histones, a subject of ongoing studies in our laboratory. We also observed a loss of α-tubulin acetylation in response to SF treatment in HaCaT and HCT116 cells despite a relatively rapid and significant down-regulation of HDAC6. Previous studies showed that SF caused increased tubulin acetylation in HCT116 cells [32]. It is unclear why our results differ but it should be noted that decreases in HDAC6 levels in breast and prostate cancer cell lines have not always correlated with tubulin acetylation [26, 27].
While SF does not exhibit HDAC inhibitory activity in HaCaT keratinocytes, our results show that it does impact the acetylome because it changes the overall acetylation status of three HDAC target proteins that are localized in distinct cellular compartments. The decreased acetylation of these proteins in response to SF is unexpected and strongly suggests that SF influences protein acetylation through mechanisms other than HDAC inhibition. Both HDACs and HATs are known to be regulated through metabolites such as NAD+, IP4, and Acetyl-CoA. The impact of SF on the cellular metabolome is largely unknown. However, a recent study showed that activation of Nrf-2 by SF in fibroblasts caused increased glucose uptake and metabolism [34]. Thus it is possible that SF may impact the cellular acetylome through a shift in the metabolites that regulate HATs and HDACs.
SF has long been examined as an excellent prospect for treatment and prevention of various forms of cancer. The finding that SF is also an HDAC inhibitor only adds to its appeal, since HDAC inhibitors have proven viable candidates for treatment of several forms of cancer in humans [35]. The results described above do not diminish our enthusiasm for the potential of SF to be successful in future clinical trials. They do, however, suggest that cell or tissue-specific targets should be considered when designing future trials of HDAC inhibitors.
Acknowledgments
This work was supported by NIH grants P30CA23074, P01CA27502, R25T CA78447, K07CA132956, and K07CA132956-02S1.
We dedicate this manuscript to the memory of Jadrian Jon Rusche, who was instrumental for its completion. Jadrian’s positive attitude, love of teaching and passion for learning will be greatly missed by those who knew him.
Abbreviations
- AcN
Acetonitrile
- SF
sulforaphane
- HDAC
histone deacetylase
- AP-1
activator protein-1
- Nrf2
Nf-E2 related factor 2
- UVB
ultraviolet B light
- PBMC
peripheral blood mononuclear cell
- Iso-9
1-Isothiocyanato-9-(methylsulfinyl)-heptane
- TSA
Trichostatin A
- VPA
Valproic Acid
- HDACi III
HDAC inhibitor III
- TRE
TPA response element
References
- 1.Jeffery EH, Keck AS. Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S7–17. doi: 10.1002/mnfr.200700226. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson SE, Melton TF, Olson ER, Zhang J, Saboda K, Bowden GT. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: implications for chemoprevention of UVB-induced skin cancer. Cancer Res. 2009;69(17):7103–10. doi: 10.1158/0008-5472.CAN-09-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240(2):243–52. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, Zeng Y, Hahm ER, Marynowski SW, Bommareddy A, Desai D, Amin S, Parise RA, Beumer JH, Chambers WH. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69(5):2117–25. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, Reddy BS, Huang MT, Newmark HL, Kong AN. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67(20):9937–44. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 6.Kanematsu S, Yoshizawa K, Uehara N, Miki H, Sasaki T, Kuro M, Lai YC, Kimura A, Yuri T, Tsubura A. Sulforaphane inhibits the growth of KPL-1 human breast cancer cells in vitro and suppresses the growth and metastasis of orthotopically transplanted KPL-1 cells in female athymic mice. Oncol Rep. doi: 10.3892/or.2011.1311. [DOI] [PubMed] [Google Scholar]
- 7.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104(44):17500–5. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev. 2007;16(4):847–51. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 9.Jakubikova J, Sedlak J, Bod’o J, Bao Y. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J Agric Food Chem. 2006;54(5):1656–62. doi: 10.1021/jf052717h. [DOI] [PubMed] [Google Scholar]
- 10.Jeong WS, Keum YS, Chen C, Jain MR, Shen G, Kim JH, Li W, Kong AN. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Biochem Mol Biol. 2005;38(2):167–76. doi: 10.5483/bmbrep.2005.38.2.167. [DOI] [PubMed] [Google Scholar]
- 11.Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT. Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol Carcinog. 2004;41(3):179–86. doi: 10.1002/mc.20052. [DOI] [PubMed] [Google Scholar]
- 12.Shibata A, Nakagawa K, Yamanoi H, Tsuduki T, Sookwong P, Higuchi O, Kimura F, Miyazawa T. Sulforaphane suppresses ultraviolet B-induced inflammation in HaCaT keratinocytes and HR-1 hairless mice. J Nutr Biochem. 21(8):702–9. doi: 10.1016/j.jnutbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64(16):5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 14.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27(4):811–9. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. Faseb J. 2006;20(3):506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232(2):227–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27(2):344–9. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson ER, Melton T, Dong Z, Bowden GT. Stabilization of quercetin paradoxically reduces its proapoptotic effect on UVB-irradiated human keratinocytes. Cancer Prev Res (Phila) 2008;1(5):362–8. doi: 10.1158/1940-6207.CAPR-08-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Bowden GT. UVB irradiation regulates Cox-2 mRNA stability through AMPK and HuR in human keratinocytes. Mol Carcinog. 2008;47(12):974–83. doi: 10.1002/mc.20450. [DOI] [PubMed] [Google Scholar]
- 20.Rajendran P, Delage B, Dashwood WM, Yu TW, Wuth B, Williams DE, Ho E, Dashwood RH. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14–3–3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 10:68. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong LH, Cheng S, Zheng Z, Wang L, Shen Y, Shen ZX, Chen SJ, Zhao WL. Histone deacetylase inhibitor potentiated the ability of MTOR inhibitor to induce autophagic cell death in Burkitt leukemia/lymphoma. J Hematol Oncol. 2013;6:53. doi: 10.1186/1756-8722-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 23.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–9. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A. 2009;106(39):16663–8. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajendran P, Kidane AI, Yu TW, Dashwood WM, Bisson WH, Lohr CV, Ho E, Williams DE, Dashwood RH. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics. 2013;8(6):612–23. doi: 10.4161/epi.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55(7):999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6(3):1013–21. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 28.Durchdewald M, Beyer TA, Johnson DA, Johnson JA, Werner S, auf dem Keller U. Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo. J Invest Dermatol. 2007;127(3):646–53. doi: 10.1038/sj.jid.5700585. [DOI] [PubMed] [Google Scholar]
- 29.Beyer TA, Auf dem Keller U, Braun S, Schafer M, Werner S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007;14(7):1250–4. doi: 10.1038/sj.cdd.4402133. [DOI] [PubMed] [Google Scholar]
- 30.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82(6):1283–91. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 31.Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD. Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS One. 2012;7(12):e51251. doi: 10.1371/journal.pone.0051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajendran P, Delage B, Dashwood WM, Yu TW, Wuth B, Williams DE, Ho E, Dashwood RH. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14–3–3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011;10:68. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV, Fairall L, Schwabe JW. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell. 2013;51(1):57–67. doi: 10.1016/j.molcel.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heiss EH, Schachner D, Zimmermann K, Dirsch VM. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 2013;1(1):359–65. doi: 10.1016/j.redox.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res. 2011;3(2):166–79. [PMC free article] [PubMed] [Google Scholar]