Abstract
The Epstein–Barr virus (EBV)-encoded oncoprotein latent membrane protein 1 (LMP1) constitutively activates nuclear factor κB (NFκB) from intracellular membranes to promote cell growth and survival. LMP1 associates with CD63 in intracellular membranes and is released via exosomes. Whether tumour necrosis factor (TNF) receptor-associated factors (TRAFs) mediate LMP1 NFκB signalling from endosomes and modulate exosomal sorting is unknown. In this article, we show that LMP1–TRAF2 signalling complexes accumulate at endosomes in a palmitoylation-dependent manner, thereby driving LMP1-dependent oncogenicity. Palmitoylation is a reversible post-translational modification and is considered to function as a membrane anchor for proteins. Mutagenesis studies showed that LMP1–TRAF2 trafficking to endosomes is dependent on one single cysteine residue (C78), a known palmitoylation site of LMP1. Notably, growth assays in soft agar revealed that oncogenic properties of the palmitoylation-deficient LMP1 mutant C78A were diminished compared to wild-type LMP1. Since LMP1 recruitment of TRAF2 and downstream NFκB signalling were not affected by a disturbance in palmitoylation, the specific localization of LMP1 at endosomal membranes appears crucial for its transforming potential. The importance of palmitoylation for trafficking to and signalling from endosomal membranes was not restricted to LMP1, as similar observations were made for the cellular oncoproteins Src and Fyn. Despite abundant LMP1–TRAF2 association at endosomal membranes TRAF2 could not be detected in exosomes by Western blotting or proteomics. Interestingly, point mutations that prevented TRAF binding strongly promoted the sorting and release of LMP1 via exosomes. These observations reveal that LMP1–TRAF2 complexes at endosomes support oncogenic NFκB activation and suggest that LMP1 dissociates from the activated signalling complexes upon sorting into intraluminal vesicles. We propose that “signalling endosomes” in EBV-infected tumour cells can fuse with the plasma membrane, explaining LMP1 release via exosomes.
Keywords: LMP1, oncogenic signalling, endosomes, exosomes, palmitoylation
Endocytosis of signalling receptors has been traditionally viewed as the main pathway for the attenuation of signal transduction (1). Recently, endosomes themselves are increasingly considered as integral platforms for signal prolongation, initiation, and cellular transformation (1,2). For example, the signalling activity of the receptor tyrosine kinase receptor (RTK) c-Met results from activation of the receptor by ligand binding at the plasma membrane (PM). However, oncogenic Met prolongs signalling after endocytosis from the PM by accumulating at endosomal membranes (2). This may have other consequences as well since exosomes that carry activated Met promote metastatic niche formation in mice and have been found in circulation of cancer patients (3). In addition, a naturally occurring epidermal growth factor receptor (EGFR) mutant, EGFRvIII, expressed in some cancer cycles between the PM and recycling endosomes, escapes down-regulation due to impaired trafficking and sorting to lysosomes (4). Apart from RTKs, the signalling of oncogenic Src family kinases (SFKs), originally identified in Rous sarcoma virus in chickens, is strongly linked to endosomal trafficking (5).
The first discovered human tumour virus, Epstein–Barr virus (EBV), encodes for the constitutively activated oncoprotein latent membrane protein 1 (LMP1), a signalling homolog of human CD40 that is expressed in EBV-associated tumours. LMP1 can escape degradation through association with CD63, which is required for its release via exosomes (6) and may thus control non-cell-autonomous functions of LMP1 (7). In newly EBV-infected proliferating B cells, LMP1 provides essential survival signals during normal B-cell development into memory B cells (8). LMP1 is unique in that it can signal without a ligand, which explains its oncogenic properties caused by chronic NFκB activation (9). Indeed, studies in transgenic mice have shown that human CD40 without the control of an external ligand causes lymphomagenesis (10,11). Because EBV essentially infects the entire world population, in healthy EBV carriers, the NFκB signalling activity of LMP1 must be tightly constrained to prevent lymphomagenesis (12).
The LMP1 protein has 6 transmembrane domains that are involved in trafficking and self-aggregation through recruitment of tumour necrosis factor (TNF) receptor-associated factors (TRAFs) at cytoplasmic C-terminal activating regions (CTARs) (13–15). Immunoprecipitation (IP) studies in various cell lines of different species show binding of LMP1 to TRAF1, 2, 3, 5, and 6 (16,17). In contrast, only small interfering RNA (siRNA) inhibition of endogenous TRAF2 seems to abolish NFκB activation and concomitantly increases apoptosis in various human lymphoma and lymphoblastic cell lines (LCLs) (18). Thus, TRAF2 seems crucial for LMP1-mediated NFκB activation, which does not exclude binding of other TRAFs per se. It should be noted, however, that many IP results have not been confirmed in intact cells by localization studies, for example using confocal microscopy.
To control the downstream signalling activity of cellular surface receptors such as EGF and MET, these proteins are endocytosed from the PM upon external ligand binding, and recycled back to the PM or degraded in lysosomes (2,19). LMP1 lacks ligand control, and previously we discovered that LMP1 escapes lysosomal degradation via association with CD63 and sorting into exosomes (6). Exosomes are extracellular vesicles (EVs) produced through inward budding of the limiting membrane of multivesicular bodies (MVBs). These intraluminal vesicles (ILVs) are released from cells when the MVBs fuse with the PM (20). Sorting of proteins into ILVs of signalling endosomes may terminate signalling by shielding catalytic domains from the cytoplasm (21). How LMP1 traffics towards MVBs, what sorting requirements determine incorporation into ILVs, and what the effect is on its TRAF clustering are not fully understood.
In this report, we studied the role of palmitoylation as a likely membrane anchor that targets LMP1 to tetraspanin (CD63)-enriched exosomes (22). We demonstrate that LMP1–TRAF2 complexes localize at and signal from endosomal membranes, but only LMP1 is sorted into exosomes. We discuss our findings in the context of “signalling endosomes” as a platform for signal initiation and termination (2,23), and how this may affect the properties of exosomes.
Materials and methods
Cell lines and culture conditions
HEK293 cells are derived from human embryonic kidney cells grown in tissue culture. HeLa-CIITA cells are HeLa cells stably transduced with CIITA, a key regulator of the MHC class II promoter (24), and selected for HLA-DR expression. Both HEK293 and HeLa-CIITA were cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza) containing 10% foetal bovine serum (FBS; Perbio science HyClone), 100 U/ml penicillin G, 100 µg/ml streptomycin sulphate, and 2 mM glutamine. RN, an EBV-transformed human B-cell line (HLA-DR15, a kind gift from W. Stoorvogel), was cultured as previous described by Pegtel et al. in 2010 (25). The BJAB–LMP1 cell line, a cell line expressing LMP1 under control of an inducible promoter, and its LMP1-negative counterpart (BJAB-tTA, kind gifts from M. Rowe) were cultured in RPMI-1640 (Lonza) containing 10% FBS (Perbio science HyClone), 100 U/ml penicillin, 100 µg/ml streptomycin sulphate, and 2 mM glutamine. For induction of LMP1, expression cells were washed 5 times with phosphate buffered saline (PBS) and cultured in the absence of tetracycline.
Plasmids and transfections
Transfections were performed using Lipofectamine 2000 reagent (Invitrogen), typically with 500 ng plasmid unless noted otherwise. Cells were seeded at a density of 40,000 cell/well in a 24-well plate and transfected the following day using Lipofectamine 2000 (Invitrogen). For transfections in a T75 flask, typically 10 µg plasmid was used with 30 µl Lipofectamine 2000 (Invitrogen) (24).
Plasmids pGK2-LMP1 wild type (wt), pSG5-LMP1-DM, pSG5-LMP1wt, and pCDNA3-LMP1wt were kind gifts from Ellen Cahir-McFarland and Rajiv Khanna. pCDNA3-LMP1ΔTM1-2 was described before (26). pGK2-LMP1-C78A was constructed by targeted mutagenesis of pGK2-LMP1wt, using the QuikChange Lightning Site-Directed Mutagenesis Kit protocol (Agilent, Santa Clara, CA, USA) and 5′-TTCAGAAGAGACCTTCTCGCTCCACTTGGAGCCCTTTG-3′ as primer. Plasmid-enhanced green florescence protein (pEGFP)-Rab5 and pEGFP-Rab7 were kind gifts of Jolanda Smit. FU-CRW vectors containing wild-type Src, Src (Y529F), Src (S3C/S6C), Src (Y529F/S3C/S6C), wild-type Fyn, Fyn (Y528F), Fyn (C3S/C6S), or Fyn (Y528F/C3S/C6S) were described before (27).
Antibodies and reagents
Mouse-anti-LMP1 OT21C is a noncommercial monoclonal antibody that reacts with a conformational epitope mapping at residues 290–318, described previously (27). Mouse monoclonal antibody against CD63 was purchased from BD Biosciences (clone H5C6). Polyclonal antibody against CD63 (NKI-C3) (28) was kindly provided by Dr Jacques Neefjes (NKI, Amsterdam, the Netherlands). Rabbit polyclonal antibody against TRAF2 and mouse monoclonal antibodies against heat shock protein 70 (HSP70) and β-actin were purchased from Santa Cruz. Rabbit polyclonal antibodies against Src and Fyn were purchased from Cell Signaling. The secondary antibodies swine-anti-rabbit FITC, swine-anti-rabbit HRP, and rabbit-anti-mouse HRP were purchased from DAKO, and goat-anti-mouse Alexa594 and goat-anti-rabbit Alexa594 were from Molecular Probes. Poly-L-lysine was obtained from Sigma. For Western blotting, cells or exosomes were lysed in a 1% sodium dodecyl sulphate (SDS) buffer, and equal amounts of protein were loaded onto an SDS/PAGE (poly-acrylamide gel electrophoresis) gel. All gels were run under reducing conditions. 2-bromopalmitate (2BP) was purchased from Sigma.
Confocal laser scanning microscopy
For immune-fluorescence and Confocal laser scanning microscopy (CLSM) analysis, HEK293 or HeLa-CIITA cells were seeded on 10 mm poly-L-lysine-coated cover slips and transfected the following day. Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde [20 min room temperature (RT)]. For lysosome (co-) staining, lysotracker Red (Invitrogen) was added 60 min before fixation to the medium at 1 µM concentration and incubated at 37°C. After fixation, cells were permeabilized with 0.1% Triton x-100 in PBS for 10 min at 4°C, then blocked with 10% foetal calf serum (FCS)/PBS (30 min RT). After blocking, the first antibody was diluted in 0.1% bovine serum albumin (BSA)/PBS and incubated for 30 min at RT. Cells were washed 3 times with PBS before incubation with the secondary antibody diluted in 0.1% BSA/PBS and incubated for 30 min at RT. Finally, cells were washed 3 times with PBS before the coverslips were embedded in Vectashield reagent (Vector Laboratories Inc., Burlingame, CA, USA) and sealed with nail polish. Slides were imaged with a Leica DMRB microscope (Leica, Cambridge, UK). All confocal images were obtained through sequential scanning with a pinhole of 1 AE. Fluorophores were excited using 488 nm (FITC) and 561 nm (Alexa594) laserlines. ImageJ software was used to process the images.
NFκB reporter assays
Dual luciferase reporter assays were normalized for transfection efficiency by co-transfecting a Gaussian luciferase expression plasmid and dividing Firefly luciferase by Gaussian luciferase activity at 20–24 hours after transfection of cells. Luciferase activities in the presence of the plasmid of interest were plotted relative to a luciferase reporter construct (p3X-kB-L) (29) in the presence of a control vector, typically at 500 ng plasmid unless otherwise noted, or LMP1-wt was set at 100%. Luciferase assays were performed according to the manufacturer's protocol (Promega).
Exosome isolation
Exosomes were purified from the cultured media (consisting of DMEM with 5% exosome-free serum, spun overnight at 70,000 g) by differential centrifugations at 500 g (2×10 min), 2,000 g (2×25 min), and 10,000 g (2×30 min), which removed cellular debris, and centrifugation at 70,000 g (60 min) pelleted exosomes. The exosome pellet was washed once in a large volume of PBS followed by a centrifugation step of 70,000 g for 1 hour, and re-suspended in sample buffer for Western blot analysis (24).
Soft-agar transformation assay
HEK293 cells were transfected as described above with pGK2-LMP1wt or pGK2LMP1-C78A or pEGFP-N1. After 24 hours, a soft-agar plate (in a 6-well plate) was prepared with a bottom layer of 0.6% agarose (Sea Plague, Lonza) in DMEM; on the top, cells were diluted in a concentration of 1×105 cells/ml in 2.4% agarose–DMEM solution. After 1.5 weeks, the top medium was refreshed, and after 3 weeks pictures were made and colonies counted.
Nano-LC separation
Peptides were separated by an Ultimate 3,000 nano-LC system (Dionex LC-Packings, Amsterdam, the Netherlands) equipped with a 20 cm×75 µm ID fused silica column custom packed with 3 µm 120 Å ReproSil Pur C18 aqua (Dr Maisch GMBH, Ammerbuch-Entringen, Germany). After injection, peptides were trapped on a 1 cm×100 µm ID precolumn packed with 5 µm ReproSil Pur C18 aqua. Peptides were separated in 60-min gradients at 300 nl/min (8–32% acetonitrile in 0.05% formic acid).
Mass spectrometry
Intact peptide MS spectra and MS/MS spectra were acquired on a label-free quantitation Fourier transform (LTQ-FT) hybrid mass spectrometer (Thermo Fisher, Bremen, Germany), as described in detail in Albrethsen et al. (30) and Piersma et al. (31). Intact masses were measured at 50,000× resolution in the ion cyclotron resonance cell. The top 5 most intense signals (charge state 2+ and higher) were subjected to collision-induced dissociation (CID) in the linear ion trap. Dynamic exclusion was applied with a repeat count of 1 and an exclusion time of 30 seconds.
Protein identification
MS/MS spectra were searched against the Uniprot human reference proteome (release September 2012) using MaxQuant 1.3.0.5 (32). Enzyme specificity was set to trypsin, and up to 2 missed cleavages were allowed. Peptide precursor ions were searched with a maximum mass deviation of 6 ppm, and fragment ions with a maximum mass deviation of 0.5 Da. Peptide and protein identifications were filtered at a false discovery rate of 1% using the decoy database strategy. The minimal peptide length was 7 amino acids. Proteins that could not be differentiated based on MS/MS spectra alone were grouped to protein groups (default MaxQuant settings).
Statistical analysis
We performed statistical analysis (Student's t-test for significance) using GraphPad Prism version 6.0 (GraphPad software). Shown are representative experiments except when indicated otherwise. Asterisks indicate significance: **p < 0.01, **p < 0.001. Experiments are replicated in independent experiments.
Results
LMP1 traffics directly to (late) endosomes for downstream signalling
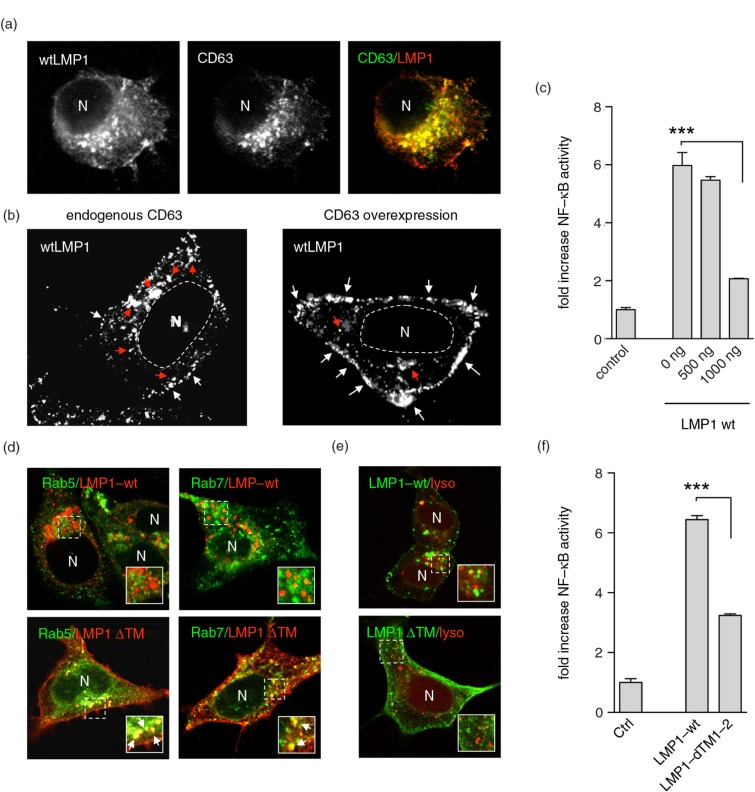
LMP1 strongly associates with endosomes marked by the presence of CD63 (Fig. 1a), and this association with CD63 is important for LMP1 subcellular trafficking, exosomal sorting, and signalling (6). To study how LMP1 trafficking in the endosomal pathway is coupled to downstream signalling, LMP1 was co-transfected with increasing amounts of a CD63-expressing plasmid. We observed increased levels of LMP1 in the PM compared to wtLMP1 (Fig. 1b). Because the signalling activity of certain RTK receptors critically depends on accumulation at endosomal membranes (2), we examined signalling of mislocalized LMP1 using dual NFκB–luciferase reporter assays. Accumulation of LMP1 in the PM due to CD63 overexpression reduced NFκB signalling up to 3-fold depending on the dose (Fig. 1c). Next, we analysed a mutant form of LMP1, LMP1ΔTM1-2, that lacks the first 2 transmembrane domains important for membrane aggregation (13,14). Fluorescent confocal analysis (CLSM) showed that the LMP1ΔTM1-2 mutant accumulated at the PM, phenotypically mimicking LMP1 localization under CD63 overexpressing conditions (Fig. 1d). LMP1ΔTM1-2 is endocytosed from the PM, as shown by co-localization with Rab5-GFP and Rab7-GFP, which are indicative of early and late endosomes, respectively (33,34). In contrast, wtLMP1 does not accumulate at the PM and does not co-localize with either of these Rab-GFP proteins (Fig. 1d). Prior studies showed that exogenously expressed LMP1 and endogenous LMP1 expressed in naturally EBV-infected B lymphoblasts associate with the late-endosomal protein CD63 and escape lysosomal degradation through release via exosomes (6,7,35). To investigate whether LMP1ΔTM1-2, which is similar to wtLMP1, escapes degradation by lysosomes, we performed co-localization experiments with LysotrackerTM. Neither wtLMP1 nor LMP1ΔTM1-2 showed extensive co-localization with Lysotracker in HEK293 cells (Fig. 1e), suggesting that LMP1ΔTM1-2 escapes lysosomal degradation by incorporation into exosomes or is shed directly from the PM (22,36). Finally, to study whether the altered distribution of LMP1ΔTM1-2 has an effect on NFκB activation, we performed luciferase reporter assays. Indeed, the LMP1ΔTM1-2 mutant showed roughly a 2-fold decreased signalling activity in HEK293 cells compared to wtLMP1 (Figure 1f).
Fig. 1.
LMP1 accumulates at and signals from CD63+ endosomes. (a) Immunofluorescent labelling of wtLMP1 (red) and CD63 (green) in HEK293 cells. (b) Immunofluorescent labelling of wtLMP1 in HEK293 cells with endogenous CD63 levels or overexpression of CD63. White and red arrowheads indicate LMP1 localized at the plasma membrane or endosomal membranes, respectively, and N indicates nucleus. (c) Reporter assay for effect of CD63 on LMP1-wt NFκB activity. Cell lysates of HEK293 cells transfected for 24 hours with wtLMP1 (LMP1-WT) and increasing amounts of CD63 plasmid or empty vector (control), together with an NFκB–reporter construct. Error bars represent s.d.; shown is one representative experiment; n=3. (d) Immunofluorescent labelling of LMP1-wt or LMP1ΔTM1-2 (LMP1 ΔTM) (both in red) in HEK293 cells co-transfected with Rab5- or Rab7-GFP (both in green). N indicates nucleus. (e) Immunofluorescent labelling of Lysotracker (red) in LMP1-wt or LMP1 ΔTM (green) transfected HEK293 cells. N indicates nucleus. (f) Reporter assay for LMP1-wt or LMP1-ΔTM1-2 NFκB activity. Cell lysates of HEK293 cells transfected with wtLMP1 (LMP1-WT), LMP1-ΔTM1-2 (LMP1-ΔTM1-2), or empty vector (control), together with an NFκB–reporter construct. Error bars represent s.d.; shown is one representative experiment; n=3.
Altogether, these results suggest that LMP1 by default does not traffic to the PM but directly traffics to late endosomes while escaping degradation by lysosomes through exosomal release. Endosomal membranes thus represent the main site for LMP1–NFκB activation.
LMP1 specifically clusters TRAF2 via its CTAR domains but is not sorted in exosomes
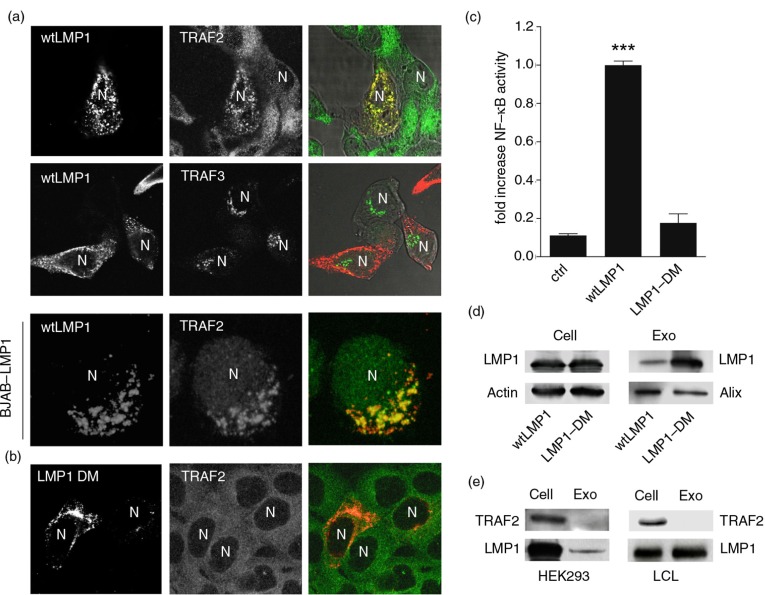
Because a significant pool of LMP1 escapes endolysosomal degradation, we sought to decipher how LMP1 signalling is controlled as NFκB overstimulation is associated with inflammation, pro-tumourigenicity, and cytotoxicity (37,38). To this end, we first studied which TRAFs are recruited by LMP1 as these are the critical mediators of NFκB activation (16,17). CLSM analysis in HEK293 showed strong co-localization between LMP1 and endogenous TRAF2 but not with endogenous TRAF3 (Fig. 2a). The recruitment of TRAF2 was also examined in EBV-negative B cells that can be induced to express LMP1 (BJAB-LMP1), where we confirmed that LMP1 clusters endogenous TRAF2 (Fig. 2a, lower panel). Thus, LMP1 specifically recruits TRAF2 in epithelial and B cells, consistent with siRNA knockdown studies in human lymphoma and LCL cells (18).
Fig. 2.
LMP1–TRAF2 association at endosomal membranes controls signalling and sorting. (a) Immunofluorescent labelling of endogenous TRAF2 and TRAF3 (all in green) in wtLMP1 (red) transfected HEK293 cells. Lower panel: Immunofluorescent labelling on EBV-negative BJAB cells carrying a tetracycline-inducible (TET-Off) LMP1 expression construct, induced for 24 hours (LMP1 in red) and labelled for endogenous TRAF2 (green). N indicates nucleus. (b) Immunofluorescent labelling of endogenous TRAF2 (green) in HEK293 cells transfected with LMP1 constructs with point mutations in (LMP1 DM) the suspected TRAF-binding sites (red). N indicates nucleus. (c) Reporter assay for LMP1-wt or LMP1-DM NFκB activity. Cell lysates of HEK293 cells transfected for 24 hours with wtLMP1, LMP1-DM, or empty vector (control), together with an NFκB–reporter construct. Error bars represent s.d.; shown is one representative experiment; n=3. (d) Western blotting analysis on TRAF2 and LMP1 protein levels in EBV-infected LCL cell and exosome lysates (left) and in wtLMP1-transfected HEK293 cell and exosome lysates (right). (e) Western blotting analysis on LMP1 protein levels in wtLMP1– or LMP1-DM-transfected HEK293 cell and exosome lysates.
To explore whether the CTAR domains are involved in the TRAF2 association with LMP1, we performed CLSM experiments on HEK293 cells expressing a LMP1 mutant (LMP1-DM) with point mutations in the suspected TRAF-binding domains. As expected from prior biochemical experiments (39), mutations in these domains abolished TRAF2 co-localization with LMP1 in intact cells (Fig. 2b). We confirmed with dual NFκB–luciferase reporter assays that mutations of these domains were functional in that they diminished LMP1-mediated NFκB activation (Fig. 2c).
LMP1 incorporation into exosomes contributes to the regulation of its NFκB signalling activity (6). To investigate whether the CTAR domains of LMP1 are critical for late-endosomal sorting, we collected exosomes from the supernatant of HEK293 cells transfected with wtLMP1 or LMP1-DM. Surprisingly, LMP1-DM was strongly enriched in the exosomal fraction as compared to wtLMP1 (Fig. 2d), suggesting that dissociation of TRAF2 is a prerequisite for the internalization of LMP1 into MVBs. To verify whether TRAF2 is co-sorted into exosomes, we purified exosomes from EBV-transformed B cells and HEK293 cells transfected with wtLMP1, as described previously (24,25,40). Western blotting showed that whereas LMP1 is present in both cell lysates and exosomes, as expected, TRAF2 is not detectable in exosomes (Fig. 2e), indicating that TRAF2 dissociates from LMP1 at endosomal membranes before sorting into exosomes.
Thus, LMP1 selectively recruits TRAF2 via its CTAR domains for downstream signalling at endosomal membranes. At this location, LMP1–TRAF2 signalling complexes dissociate before LMP1 is incorporated into MVBs, restricting NFκB activation and explaining release via exosomes.
Mutation of LMP1's active palmitoylation site diminishes its exosomal sorting and transformation capacity
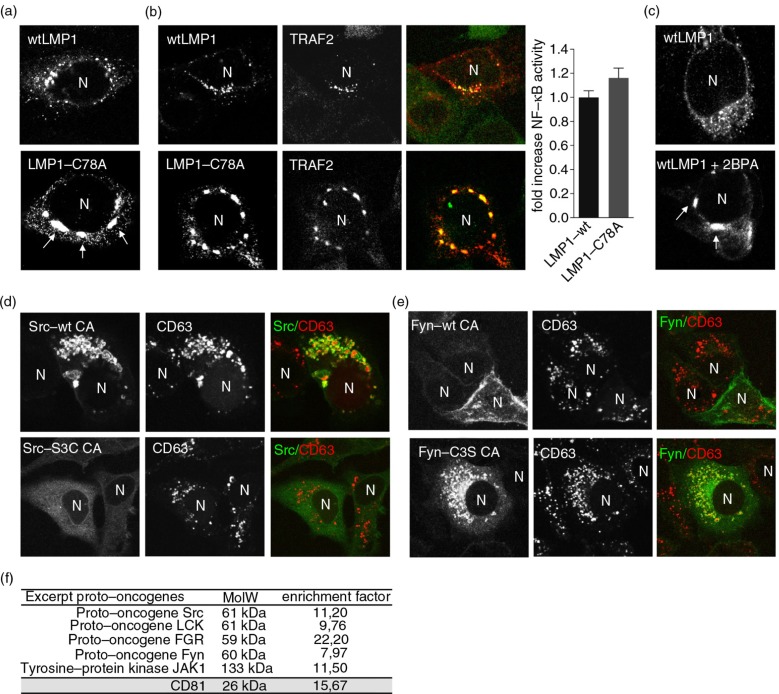
To establish a functional role for palmitoylation in the targeting of oncoproteins to late endosomes, we generated an EBV-LMP1 mutant (LMP1-C78A) in which cysteine 78, a confirmed LMP1 palmitoylation site, is changed into an alanine (41). We transfected HEK293 cells with wtLMP1 or LMP1-C78A, and visualized both LMP1 and CD63 with fluorescent antibodies. Mutation of the only active palmitoylation site of LMP1 resulted in a pronounced peri-nuclear accumulation, although co-localization with CD63 was retained (Fig. 3a). Next, we determined whether LMP1-C78A still recruits TRAF2 in HEK293 cells. Indeed, LMP1-C78A was seemingly equally efficient in recruiting TRAF2 when compared to wtLMP1, suggesting the mutant may still activate NFκB (Fig. 3b). Somewhat surprisingly, dual NFκB–luciferase reporter assays revealed that LMP1-C78A activated NFκB at comparable levels as wtLMP1 (Fig. 3b). Moreover, we found a comparable localization defect using a chemical inhibitor for palmitoylation, 2-bromopalmitate (2BPA) (Fig. 3c).
Fig. 3.
Palmitoylation controls subcellular trafficking of LMP1 but not TRAF2 association. (a) Immunofluorescent co-labelling of transfected wtLMP1 or LMP1-C78A (red) in HEK293 cells. N indicates nucleus. (b) Immunofluorescent labelling of endogenous TRAF2 (green) in HEK293 cells transfected with wtLMP1 or LMP1-C78A (red). To the right, reporter assay for LMP1-wt or LMP1-C78A NFκB activity. Cell lysates of HEK293 cells transfected for 24 hours with wtLMP1 or LMP1-C78A, together with an NFκB–reporter construct. Error bars represent s.e.m.; n>3. N indicates nucleus. (c) Immunofluorescent labelling of transfected wtLMP1 in 2BPA and control treated HEK293 cells. N indicates nucleus. (d, e) Immunofluorescent labelling of constitutive active Src-wt CA, Src-S3C CA, Fyn-wt CA, or Fyn-C3S CA (all in green) transfected HeLa-CIITA cells, co-labelled for CD63 (red). N indicates nucleus. (f) Table showing the enrichment of proto-oncogenes in exosomes versus cell lysates from 6 different B-cell lines referenced to the total number of peptides identified in each analysis.
To investigate whether observations made on the influence of palmitoylation on LMP1 trafficking could be extended to cellular oncoproteins, we studied SFK members. Non-palmitoylated, constitutively active (CA) SFKs are more oncogenic than their palmitoylated counterparts (26). To determine if endosomal localization is related to the oncogenicity of SFKs, we performed CSLM analysis. For this, we used CA c-Src and CA Fyn C3S (loss of palmitoylation mutant), and their less oncogenic counterparts CA c-Src S3C (gain of palmitoylation) and CA Fyn constructs, transfected in HeLa-CIITA cells that were co-labelled for CD63. The strongly oncogenic CA c-Src showed pronounced endosomal association in ring-like structures. In contrast, its less oncogenic counterpart CA c-Src S3C showed a predominantly cytoplasmic localization (Fig. 3d). Notably, these endosomal ring-like structures are reminiscent of “swollen” endosomes observed in activated Rab5 (or Rab5-Q79L) endosomes in v-Src expressing cells (42,43). Comparable results were found for CA Fyn C3S (Fig. 3e). SFKs are suggested to localize at the PM or at Rab7+ (late) endosomal and lysosomal structures (44,45). To study the effect of palmitoylation on trafficking, we wished to define the endosomal CD63+ structures associated with the oncogenic Src and Fyn variants in the absence of palmitoylation. To this end, we performed CSLM analysis on HeLa-CIITA cells transfected with CA c-Src, CA Fyn-C3S, and Rab5-GFP. The endosomal-associated ring structures of CA forms of c-Src and Fyn-C3S were decorated with early-endosomal Rab5 (Supplementary Fig. 2a and b), suggesting that PM-localized Src/Fyn are internalized and retained at Rab5/CD63+ early-endosomal structures where signalling is sustained.
Overall, these studies confirm that palmitoylation is a critical modification that supports trafficking of both cytoplasmic proteins (Fyn and Src) and the integral membrane protein (LMP1) to signalling endosomes.
Recent studies suggest a link between oncogene trafficking to and signalling from endosomal membranes (6,46). If signalling endosomes produce exosomes, as suspected, then the proteome of exosomes may provide clues regarding the composition of endosomal signalling platforms. To explore this possibility, we performed label-free quantitative proteomics (LTQ-FTMS) on highly purified endosome-derived exosomes that are derived from various B-cell lines, including LMP1-positive and -negative tumour cells. We identified 2,100 proteins, including numerous well-known proto-oncogenes and exosomal marker proteins. Consistent with our hypothesis, among the proteins identified were SFK family members, including Src, Fyn, Lck, Fgr, and tyrosine-protein kinase JAK1 (Fig. 3f). These results are consistent with endosomal membranes having a role as signalling platforms. Of the proteins listed in Fig. 3f, Fyn, Lck, Fgr, and JAK1 are naturally occurring signalling proteins with an active palmitoylation site (26,47,48), consistent with palmitoylation being a regulatory mechanism for targeting certain signalling proteins to late endosomes.
Palmitoylation controls LMP1 transformation capacity
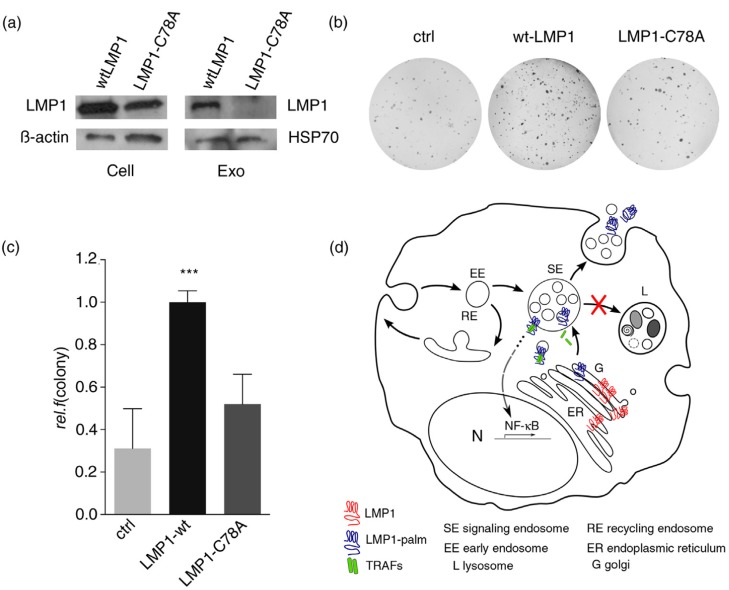
The proteomic data we obtained (Fig. 3f) revealed the presence of palmitoylated (proto-)oncogenes in exosomes, suggesting that palmitoylation may control signal termination of signalling molecules at endosomal membranes. To study whether palmitoylation of LMP1 is required for sorting into exosomes, we performed Western blotting analysis on cell lysates and corresponding exosomes purified from supernatant of HEK293 cells transfected with wtLMP1 or LMP1-C78A. The results show that LMP1-C78A is precluded from sorting into exosomes, whereas the levels of HSP70, a common exosomal marker, were not affected (Fig. 4a).
Fig. 4.
Palmitoylation controls LMP1 transformation capacity. (a) Western blotting analysis on LMP1 protein levels in wtLMP1 or LMP1-C78A transfected HEK293 cell and exosome lysates; β-actin and HSP70 as loading controls. (b, c) Soft-agar assay for anchorage-independent growth of HEK293 cells transfected with wtLMP1, LMP1-C78A, or GFP control (ctrl) construct. Error bars represent s.e.m.; n>3. (d) Hypothetical model showing that newly synthesized LMP1 (blue) assembles with CD63 in the ER; traffics to the Golgi (G), where it is palmitoylated (red); and buds off in CD63-positive transport vesicles that form or assemble at limiting membranes of signalling endosomes (SEs). Whereas palmitoylation targets LMP1 to SE membranes, non-palmitoylated LMP1 is retained in the ER–Golgi region. Wild-type LMP1 recruits TRAF2, and the activated signalling complexes accumulate at endosomal membranes activating NFκB. Upon LMP1 sorting into the intra-luminal vesicles, LMP1 is then secreted via exosomes while TRAF2 dissociates and is presumably left behind in the cytosol.
The subcellular localization of mutated signalling proteins can elicit strong oncogenic capacity (2). To investigate if the altered distribution of LMP1-C78A affects oncogenicity, we performed transformation assays of HEK293 cells upon transfection with wtLMP1 and LMP1-C78A. Surprisingly, the wtLMP1-transfected cells formed many more colonies compared to LMP1-C78A, indicating that despite its TRAF2 clustering and NFκB activation potency, the trafficking mutant of LMP1 has reduced transformation efficiency (Fig. 4b and c).
Thus, the post-translational modification palmitoylation on cysteine 78 controls the downstream signalling and oncogenicity of LMP1 by targeting it into the (late-)endosomal pathway. Overall, our studies indicate that palmitoylation is a critical modification that supports endosomal membrane trafficking of cytoplasmic and integral membrane signalling proteins required for signal termination. Disturbances in palmitoylation disrupt proto-oncoprotein trafficking to and from endosomal membranes, causing overstimulation and transformation.
Discussion
Kaposi sarcoma–associated virus (KSHV) and EBV are associated with human cancers. EBV-infected B cells and lymphoma cells may transfer specific viral and cellular components via exosomes influencing the tumour microenvironment (7,39,49,50). Exosomes produced by EBV-infected nasopharyngeal carcinoma cells contain high levels of the viral oncogene LMP1 and viral microRNAs (miRNAs) that activate critical signalling pathways in recipient cells (34,51). Here, we provide evidence that palmitoylation-dependent trafficking of LMP1–TRAF2 complexes to late endosomes supports oncogenic signalling, which is restricted by disengagement from the signalling adapter TRAF2 at this location. We propose that a process of fine-tuning downstream signalling at these subcellular sites explains the selective sorting and release of LMP1 via exosomes with possible consequences for the tumour microenvironment.
A long-held paradigm in receptor signalling was that surface receptors are, upon internalization, either degraded or recycled back to the PM. Once inside, they were thought to be functionally inactive. Nonetheless, it emerged that receptor signalling may also occur from endosomes. Previously, we have shown that the CA, intracellular, viral oncoprotein EBV-LMP1 associates with the tetraspanin CD63 at endosomal membranes and is rapidly released via CD63-enriched exosomes (6). Here, we questioned what molecular requirements have a role in this seemingly highly efficient targeting and sorting process. Prior studies using trafficking of specific fusion proteins suggested that PM anchors target oligomeric, cytoplasmic proteins to exosomes and microvesicles (22). Our study extends this knowledge to naturally palmitoylated oncoproteins, including EBV-LMP1. LMP1 harbours 3 potential palmitoylation sites (C78, C84, and C116), of which only C78 is palmitoylated as shown by [3H]palmitate labelling (41). We found that a single point mutation in C78 hindered LMP1 exit from the endoplasmic reticulum (ER)–Golgi region, reduced sorting, released via exosomes, and remarkably also impaired transformation capacity yet left NFκB activation unaltered. This is consistent with previous findings suggesting that LMP1 (C78) palmitoylation does not affect raft association nor is required for NFκB and c-Jun N-terminal kinase activation (41). One question remains: whether palmitoylation could actually promote LMP1 anchoring in late-endosomal membranes, as not all LMP1-C78A is retained peri-nuclearly (Fig. 3a). We cannot rule out the active involvement of palmitoylation in small membrane domain formation, which has been shown to favour ILV formation through inward budding of limiting membranes (52). In light of the membrane juxtapositioning of the cysteine residue in this integral membrane protein LMP1, specific targeting to tetraspanin-enriched microdomains containing CD63 seems plausible (22).
We wished to address whether ILV incorporation of LMP1 is a mechanism for attenuating downstream signalling. LMP1 downstream signalling activation depends on the recruitment TRAFs at its cytoplasmic CTAR domains (13–15). A siRNA inhibition study of endogenous TRAFs demonstrated that TRAF2 is critically involved in the growth stimulatory properties of LMP1 in various human lymphoma cell lines and LCLs (18). IP studies in multiple cell types showed binding of LMP1 to TRAF1, 2, 3, 5, and 6 (16,17), although such studies provide no information on subcellular compartmentalization (53). We found in intact LMP1-expressing lymphoblasts and HEK293 cells that LMP1 recruits TRAF2, but not TRAF3 or TRAF6 (data not shown), at endosomal membranes. Strikingly, Western blotting analysis revealed that TRAF2 was absent from exosomal lysates. Because LMP1 that localizes at the PM due to CD63 overexpression recruits TRAF2 (Supplementary Fig. 1), we argue that LMP1 is actively sorted into ILVs of MVBs as a primary source of LMP1-containing EVs. Passive sorting would likely result in TRAF2 being detectable in EV. Thus, TRAF2 is likely to dissociate from active LMP1 signalling complexes before incorporation into ILVs. Despite many lines of evidence suggesting that LMP1 is sorted into bona fide MVBs, we cannot rule out the possibility that a proportion of LMP1 molecules is directly released from the PM. Nevertheless, our findings are consistent with prior observations coupling LMP1 release via exosomes to control downstream signalling (25). Notably, LMP1 mutated in its TRAF2 association domain (LMP1-DM) is secreted much more efficiently compared to wtLMP1. It is thus tempting to speculate that CTAR-associated TRAFs somehow restrict LMP1 sorting into ILVs of signalling endosomes. Possibly, the sorting–budding machinery that drives LMP1 into ILVs actively dissociates TRAFs or is controlled by ubiquitination–deubiquitination cycles (54,55). An alternative explanation is that LMP1–TRAF2 complexes are sorted into lysosomes, whereas unbound LMP1 is passively sorted into ILVs. Although we cannot formally exclude this possibility, it should be noted that low levels of LMP1 are already sorted into exosomes (6), making it less plausible that TRAF availability per se is decisive for the fate of LMP1, but rather indicates dynamic TRAF2 association and dissociation kinetics. Moreover, there are many more LMP1 signalling adapters that control downstream NFκB activation. It seems likely that additional TRAF and interacting molecules also have a role in LMP1 sorting (14,15,18,56).
Our current results establish that LMP1 activates NFκB from signalling endosomes for multiple reasons. Firstly, mutation of the active palmitoylation site of LMP1 results in an early block in trafficking and shows lower transformation capacity. Secondly, forced routing of LMP1 to the PM by either deletion of the first 2 TM regions or overexpression of CD63 impairs the LMP1-mediated NFκB activation, whereas TRAF2 recruitment seems unaffected (Supplementary Fig. 1). Thirdly, deletion of its first 2 TM regions redirects LMP1 into a Rab5–Rab7 endocytic pathway also utilized by EGFR (57), suggesting that LMP1, by default, does not traffic via this PM-shuttling pathway but goes directly to endosomal membranes. Thus, PM trafficking, as seen for cellular RTKs and SFKs, is not the default pathway for LMP1 and only moderately contributes to NFκB activation. We further found that constitutively activated tyrosine kinases c-Src and Fyn accumulate at CD63 + /Rab5+ early-endosomal membranes consistent with endocytosis from the PM. These mutants have increased signalling and transformation capacity when palmitoylation sites are absent, causing mistargeting to endosomes (26). We speculate that targeting of SFK–receptor complexes to lysosomes and/or the internalization into ILVs of MVBs is hampered in the oncogenic forms, explaining prolonged signalling and enhanced oncogenicity of these mutants. Only recently, it became clear that cycles of (de-)palmitoylation are required for the proper functioning of specific proteins localized at the PM, as substitution of palmitoylation by an irreversible membrane anchor showed perturbed localization of proteins (58). This, combined with our findings on oncoproteins, suggests that palmitoylation might play an important role in the cellular transformation process. Indeed, palmitoylation has previously been linked to prostate (26) and gastric cancer (59), raising possibilities for the modification of palmitoylation status of proteins for therapeutic intervention (60).
The findings presented here are consistent with the notion that subcellular trafficking of LMP1 towards late endosomes affects downstream signalling and oncogenesis. Possibly, the sorting of oncogenic receptors into exosomes prevents overstimulation, which would explain why tumour cells would benefit from oncoprotein clearance via exosomes and explain systemic physiological effects (3,61). From studies on Wnt-signalling, we know that at least a subset of signalling endosomes in healthy cells are bona fide MVBs that can escape fusion with lysosomes and fuse with the PM instead to secrete exosomes (46,62). Endosome-based signalling responses are increasingly being recognized as a widespread phenomenon extending beyond the RTK and SFK superfamilies. Indeed, recent evidence showed that signalling from internalized receptors has a distinct physiological outcome (63). New fundamental discoveries in this area are expected to have consequences for exosome-mediated cell–cell communication physiology as well. Our study provides a rationale for investigating the content of highly purified endosome-derived exosomes as a read-out for ongoing intracellular signalling processes. This may hold clinical relevance as exosomes originating from certain signalling endosomes could promote tumour progression (3,64).
Supplementary Material
Conflict of interest and funding
This work was funded by the Dutch Cancer Society grants KWF-3775 (JMM and DMP) and KWF-5510 (DMP).
References
- 1.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 2.Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011;13:827–37. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 3.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4."/>Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 5.Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008;18:322–9. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-kappaB activation. EMBO J. 2011;30:2115–29. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meckes DG, Jr., Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010;107:20370–5. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 9.Thorley-Lawson DA, Gross A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 10.Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA. 1998;95:11963–8. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hömig-Hölzel C, Hojer C, Rastelli J, Casola S, Strobl LJ, Müller W, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–29. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–18. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 13.Yasui T, Luftig M, Soni V, Kieff E. Latent infection membrane protein transmembrane FWLY is critical for intermolecular interaction, raft localization. and signaling. Proc Natl Acad Sci USA. 2004;101:278–83. doi: 10.1073/pnas.2237224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soni V, Yasui T, Cahir-McFarland E, Kieff E. LMP1 transmembrane domain 1 and 2 (TM1-2) FWLY mediates intermolecular interactions with TM3-6 to activate NF-kappa. B J Virol. 2006;80:10787–93. doi: 10.1128/JVI.01214-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Sugden B. A membrane leucine heptad contributes to trafficking, signaling, and transformation by latent membrane protein 1. J Virol. 2007;81:9121–30. doi: 10.1128/JVI.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. J Virol. 1997;71:4649–56. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arcipowski KM, Stunz LL, Graham JP, Kraus ZJ, Vanden Bush TJ, Bishop GA. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1) J Biol Chem. 2011;286:9948–55. doi: 10.1074/jbc.M110.185983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guasparri I, Bubman D, Cesarman E. EBV LMP2A affects LMP1-mediated NF-kappaB signaling and survival of lymphoma cells by regulating TRAF2 expression. Blood. 2008;111:3813–20. doi: 10.1182/blood-2007-03-080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masui H, Wells A, Lazar CS, Rosenfeld MG, Gill GN. Enhanced tumorigenesis of NR6 cells which express non-down-regulating epidermal growth factor receptors. Cancer Res. 1991;51:6170–5. [PubMed] [Google Scholar]
- 20.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Eden ER, White IJ, Futter CE. Down-regulation of epidermal growth factor receptor signalling within multivesicular bodies. Biochem Soc Trans. 2009;37:173–7. doi: 10.1042/BST0370173. [DOI] [PubMed] [Google Scholar]
- 22.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286:14383–95. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandal MV, Zandi R, Pedersen MW, Willumsen BM, van Deurs B, Poulsen HS. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis. 2007;28:1408–17. doi: 10.1093/carcin/bgm058. [DOI] [PubMed] [Google Scholar]
- 24.Verweij FJ, van Eijndhoven MAJ, Middeldorp J, Pegtel DM. Analysis of viral microRNA exchange via exosomes in vitro and in vivo. Methods Mol Biol. 2013;1024:53–68. doi: 10.1007/978-1-62703-453-1_5. [DOI] [PubMed] [Google Scholar]
- 25.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij FJ, Middeldorp JM, Pegtel DM. Intracellular signaling controlled by the endosomal-exosomal pathway. Commun Integr Biol. 2012;5:88–93. doi: 10.4161/cib.18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Smith DA, Memarzadeh S, Lowell CA, Cooper JA, Witte ON. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci USA. 2011;108:6579–84. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demetrick DJ, Herlyn D, Tretiak M, Creasey D, Clevers H, Donoso LA, et al. ME491 melanoma-associated glycoprotein family: antigenic identity of ME491, NKI/C-3, neuroglandular antigen (NGA), and CD63 proteins. J Natl Cancer Inst. 1992;84:422–9. doi: 10.1093/jnci/84.6.422. [DOI] [PubMed] [Google Scholar]
- 29.Luftig M, Prinarakis E, Yasui T, Tsichritzis T, Cahir-McFarland E, Inoue J, et al. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc Natl Acad Sci USA. 2003;100:15595–600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albrethsen J, Knol JC, Piersma SR, Pham TV, de Wit M, Mongera S, et al. Subnuclear proteomics in colorectal cancer: identification of proteins enriched in the nuclear matrix fraction and regulation in adenoma to carcinoma progression. Mol Cell Proteomics. 2010;9:988–1005. doi: 10.1074/mcp.M900546-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piersma SR, Fiedler U, Span S, Lingnau A, Pham TV, Hoffmann S, et al. Workflow comparison for label-free, quantitative secretome proteomics for cancer biomarker discovery: method evaluation, differential analysis, and verification in serum. J Proteome Res. 2010;9:1913–22. doi: 10.1021/pr901072h. [DOI] [PubMed] [Google Scholar]
- 32.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–72. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 33.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21:1289–300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–22. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–85. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 38.Le Clorennec C, Ouk T-S, Youlyouz-Marfak I, Panteix S, Martin C-C, Rastelli J, et al. Molecular basis of cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV latency III B cells: LMP1 induces type II ligand-independent autoactivation of CD95/Fas with caspase 8-mediated apoptosis. J Virol. 2008;82:6721–33. doi: 10.1128/JVI.02250-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devergne O, Hatzivassiliou E, Izumi KM, Kaye KM, Kleijnen MF, Kieff E, et al. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol Cell Biol. 1996;16:7098–108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dukers DF, Meij P, Vervoort MB, Vos W, Scheper RJ, Meijer CJ, et al. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165:663–70. doi: 10.4049/jimmunol.165.2.663. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi M, Izumi KM, Kieff E. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc Natl Acad Sci USA. 2001;98:4675–80. doi: 10.1073/pnas.081075298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts R, Barbieri M, Pryse K, Chua M, Morisaki J, Stahl P. Endosome fusion in living cells overexpressing GFP-rab5. J Cell Sci. 1999;112:3667–75. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- 44.Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–75. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- 45.Kasahara K, Nakayama Y, Kihara A, Matsuda D, Ikeda K, Kuga T, et al. Rapid trafficking of c-Src, a non-palmitoylated Src-family kinase, between the plasma membrane and late endosomes/lysosomes. Exp Cell Res. 2007;313:2651–66. doi: 10.1016/j.yexcr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–91. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijlmakers M-J, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 48.Ren W, Jhala US, Du K. Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte. 2013;2:17–28. doi: 10.4161/adip.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol. 2008;18:388–96. doi: 10.1016/j.semcancer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Pegtel DM. Oncogenic herpesviruses sending mixed signals. Proc Natl Acad Sci USA. 2013;110:12503–4. doi: 10.1073/pnas.1310928110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meckes DG, Jr., Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci USA. 2013;110(31) doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 53.Williamson R, Thompson AJ, Abu M, Hye A, Usardi A, Lynham S, et al. Isolation of detergent resistant microdomains from cultured neurons: detergent dependent alterations in protein composition. BMC Neurosci. 2010;11:120. doi: 10.1186/1471-2202-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown KD, Hostager BS, Bishop GA. Regulation of TRAF2 signaling by self-induced degradation. J Biol Chem. 2002;277:19433–8. doi: 10.1074/jbc.M111522200. [DOI] [PubMed] [Google Scholar]
- 55.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106:17615–22. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu HP, Wu CC, Chang YS. PRA1 promotes the intracellular trafficking and NF-kappaB signaling of EBV latent membrane protein 1. EMBO J. 2006;25:4120–30. doi: 10.1038/sj.emboj.7601282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceresa BP. Regulation of EGFR endocytic trafficking by rab proteins. Histol Histopathol. 2006;21:987–93. doi: 10.14670/HH-21.987. [DOI] [PubMed] [Google Scholar]
- 58.Jia L, Chisari M, Maktabi MH, Sobieski C, Zhou H, Konopko AM, et al. A mechanism regulating G protein-coupled receptor signaling that requires cycles of protein palmitoylation and depalmitoylation. J Biol Chem. 2014;289:6249–57. doi: 10.1074/jbc.M113.531475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mo M-L, Li M-R, Chen Z, Liu X-W, Sheng Q, Zhou H-M. Inhibition of the Wnt palmitoyltransferase porcupine suppresses cell growth and downregulates the Wnt/β-catenin pathway in gastric cancer. Oncol Lett. 2013;5:1719–23. doi: 10.3892/ol.2013.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linder ME, Jennings BC. Mechanism and function of DHHC S-acyltransferases. Biochem Soc Trans. 2013;41:29–34. doi: 10.1042/BST20120328. [DOI] [PubMed] [Google Scholar]
- 61.Ménard L, Parker PJ, Kermorgant S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat Commun. 2014;5:3907. doi: 10.1038/ncomms4907. [DOI] [PubMed] [Google Scholar]
- 62.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–48. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–8. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–56. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.