Significance
Chronic obstructive pulmonary disease (COPD) is the fourth-leading cause of death worldwide. Roflumilast has been approved for COPD. However, its efficacy has been hampered by the development of tolerance, and the underlying mechanism remains unknown but may be attributed to phosphodiesterase (PDE) 4B up-regulation. Here we found that PDE4B2 is synergistically up-regulated by inflammatory stimuli (e.g. bacteria) and cAMP-elevating agents (e.g. roflumilast) via cross-talk between protein kinase A catalytic subunit β and NF-κB p65 in a cAMP-dependent manner. Up-regulated PDE4B2 contributes to the induction of certain key chemokines in both enzymatic activity-dependent and activity-independent manners. Our findings may explain, at least in part, the development of tolerance and help develop new therapeutics to improve efficacy of roflumilast.
Keywords: PDE4B, Roflumilast, nontypeable Haemophilus influenzae, PKA-Cβ, p65
Abstract
Phosphodiesterase 4B (PDE4B) plays a key role in regulating inflammation. Roflumilast, a phosphodiesterase (PDE)4-selective inhibitor, has recently been approved for treating severe chronic obstructive pulmonary disease (COPD) patients with exacerbation. However, there is also clinical evidence suggesting the development of tachyphylaxis or tolerance on repeated dosing of roflumilast and the possible contribution of PDE4B up-regulation, which could be counterproductive for suppressing inflammation. Thus, understanding how PDE4B is up-regulated in the context of the complex pathogenesis and medications of COPD may help improve the efficacy and possibly ameliorate the tolerance of roflumilast. Here we show that roflumilast synergizes with nontypeable Haemophilus influenzae (NTHi), a major bacterial cause of COPD exacerbation, to up-regulate PDE4B2 expression in human airway epithelial cells in vitro and in vivo. Up-regulated PDE4B2 contributes to the induction of certain important chemokines in both enzymatic activity-dependent and activity-independent manners. We also found that protein kinase A catalytic subunit β (PKA-Cβ) and nuclear factor-κB (NF-κB) p65 subunit were required for the synergistic induction of PDE4B2. PKA-Cβ phosphorylates p65 in a cAMP-dependent manner. Moreover, Ser276 of p65 is critical for mediating the PKA-Cβ–induced p65 phosphorylation and the synergistic induction of PDE4B2. Collectively, our data unveil a previously unidentified mechanism underlying synergistic up-regulation of PDE4B2 via a cross-talk between PKA-Cβ and p65 and may help develop new therapeutic strategies to improve the efficacy of PDE4 inhibitor.
Cyclic adenosine monophosphate (cAMP), an important second messenger, plays a pivotal role in regulating inflammatory and immune response (1–5). The intracellular concentration of cAMP depends largely on the activity of phosphodiesterases (PDEs) that catalyze its breakdown. To date 11 PDE families (PDE1–11) have been identified, many of which have several different isoforms and transcriptional/splice variants with distinct properties (6–8). PDE4 family, the primary cAMP-specific enzyme, comprises four genes (PDE4A–D), each with multiple variants that encode more than 20 different protein products (8). The therapeutic potential of PDE4 inhibition in airway inflammatory diseases such as chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis (CF) has long been explored (9–12). Recently, roflumilast has been approved as the first PDE4 inhibitor for oral, once-daily treatment of severe COPD with symptoms of chronic bronchitis and a history of exacerbations (12–14). However, its wide clinical use has been limited by the severe dose-limiting side effects such as emesis and nausea (12, 15).
PDE4B has been shown to play a critical role in mediating inflammatory response (16, 17). Gene deletion studies in mice have established that expression of PDE4B, but not PDE4A or PDE4D, is crucial for lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α) production and allergen-induced airway hyperresponsiveness in leukocytes (18–20). Previous studies, including ours, have shown that cAMP elevators, LPS and nontypeable Haemophilus influenzae (NTHi), a major bacterial cause of COPD exacerbation (21), induce PDE4B expression in various cell types, including leukocytes and epithelial cells (16–18, 22–27). In particular, cAMP elevators induce PDE4B as a negative-feedback mechanism for controlling cAMP signaling. In contrast, inflammatory stimuli up-regulate PDE4B as a counterregulatory mechanism for antagonizing the antiinflammatory action of cAMP signaling. The airway epithelium is an essential barrier that responds to environmental stimuli and has a vital role as an immune regulator through the secretion of cytokines, chemokines, growth factors, antimicrobial peptides, and the recruitment of leukocytes (28). Up-regulation of PDE4B expression in airway epithelial cells may contribute significantly to the inflammatory response in the pathogenesis of COPD. Interestingly, there is also clinical evidence suggesting the development of tachyphylaxis or tolerance on repeated dosing of roflumilast and the possible contribution of PDE4B up-regulation, which could be counterproductive for suppressing inflammation (29–32). Thus, understanding how PDE4B is up-regulated in the context of the complex pathogenesis and medications of COPD may help improve the efficacy and possibly ameliorate the tolerance of roflumilast.
On the basis that expression of PDE4 isoforms is induced by cAMP elevators including PDE4 inhibitors (18, 23–26, 33) and PDE4B is induced by NTHi (27), we sought to determine whether roflumilast synergizes with NTHi to induce PDE4B expression in the context of the complex pathogenesis and medications of COPD. Here we found that roflumilast synergized with NTHi to up-regulate PDE4B2 expression in human airway epithelial cells in vitro and in mouse lungs in vivo. Up-regulated PDE4B2 contributes to the induction of certain important chemokines in both enzymatic activity-dependent and activity-independent manners. Protein kinase A catalytic subunit β (PKA-Cβ) and nuclear factor-κB (NF-κB) p65 subunit were required for the synergistic induction of PDE4B2. PKA-Cβ phosphorylates p65 in a cAMP-dependent manner. Thus, our study provides new insights into the synergistic regulation of PDE4B2 via cross-talk between PKA-Cβ and p65 and may help develop new therapeutic strategies to improve the efficacy of PDE4 inhibitor in patients with COPD exacerbation.
Results
Roflumilast Synergizes with NTHi to Up-Regulate PDE4B2 Expression in Vitro and in Vivo.
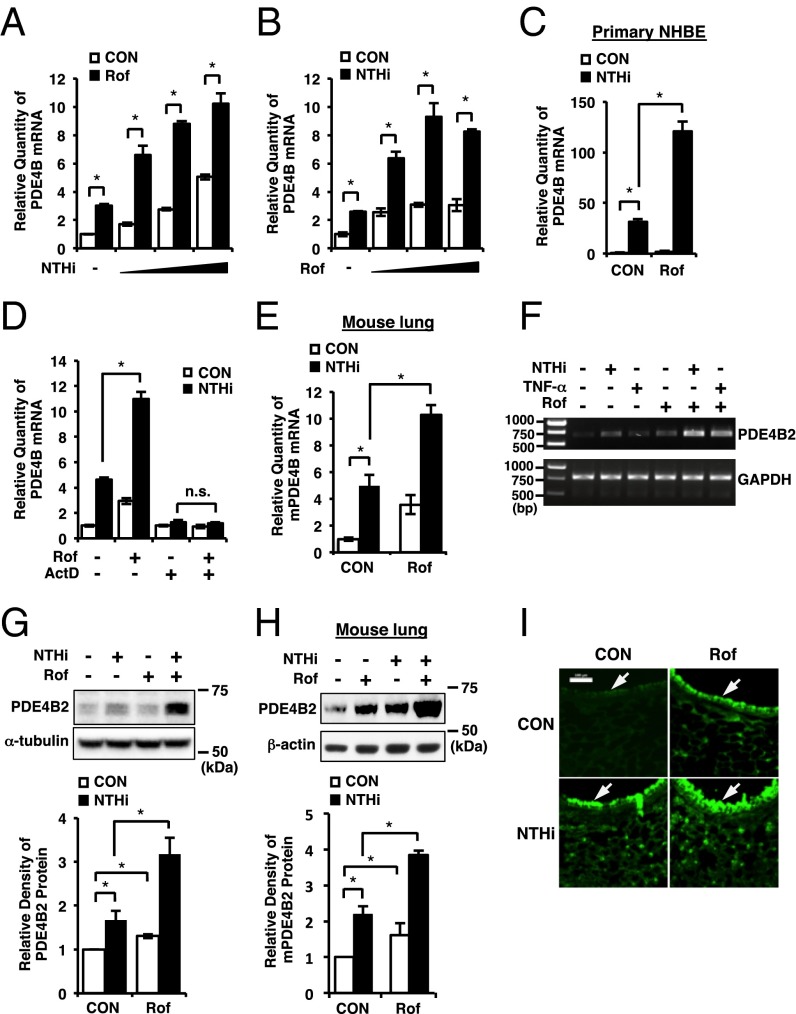
Because the expression of PDE4 isoforms is induced by PDE4 inhibitors (24, 26, 33) and PDE4B is also induced by NTHi (27), we sought to determine whether roflumilast synergizes with NTHi to induce PDE4B expression in human airway epithelial cells. As shown in Fig. 1 A and B, roflumilast synergized with NTHi to up-regulate PDE4B expression at the mRNA level in human bronchial epithelial BEAS-2B cells in a dose-dependent manner as assessed by quantitative PCR (Q-PCR) analysis. A similar result was also confirmed in primary normal human bronchial epithelial (NHBE) cells (Fig. 1C). We also analyzed the expressions of other PDE4 family members under the same condition. As shown in Fig. S1 A and B, PDE4A and 4C were not up-regulated by NTHi or roflumilast. PDE4D was up-regulated by NTHi or roflumilast, but no significant synergistic effect was observed, suggesting that PDE4B is specifically regulated by NTHi and roflumilast in a synergistic manner. To further determine whether the synergistic induction of PDE4B occurs at the transcriptional level, we treated BEAS-2B cells with actinomycin D (ActD), a transcriptional inhibitor. ActD completely abrogated the PDE4B induction by NTHi and roflumilast, suggesting that the synergistic induction of PDE4B occurs at the transcriptional level (Fig. 1D). We next determined whether roflumilast synergizes with NTHi to up-regulate PDE4B expression in vivo. Consistent with the in vitro results, roflumilast synergistically enhanced NTHi-induced PDE4B expression at mRNA level in mouse lungs (Fig. 1E).
Fig. 1.
Roflumilast synergizes with NTHi to up-regulate PDE4B2 expression in vitro and in vivo. (A) BEAS-2B cells were pretreated with roflumilast (Rof) (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi (MOI of 25, 50, and 100), and PDE4B mRNA expression was analyzed. (B) BEAS-2B cells were pretreated with Rof (0.01, 0.1, and 1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (C) Primary NHBE cells were pretreated with Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (D) BEAS-2B cells were pretreated with ActD (5 ng/mL) and Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. PDE4B mRNA expression was normalized with each control of ActD-treated or nontreated. (E) Mice were inoculated with Rof (5 mg/kg i.p.) for 2 h, followed by intratracheal inoculation with NTHi (5 × 107 cfu per lung). After 5 h, PDE4B mRNA expression in lung tissues was analyzed. (F) BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi or TNF-α (10 ng/mL), and PDE4B2 mRNA expression was analyzed. (G) BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 3-h stimulation with NTHi, and PDE4B2 protein expression was analyzed. (H and I) Mice were inoculated with Rof and NTHi as described in E. After 5 h, PDE4B2 protein expression in lung tissues was analyzed (H) and lung tissues were stained against PDE4B (I). (Magnification: 200×.) (Scale bar: 100 μm.) The relative density of PDE4B2 protein was normalized with α-tubulin (G) or β-actin (H). Data in A–E, G, and H are mean ± SD (n = 3); *P < 0.05; n.s. = P > 0.05. Data are representative of three or more independent experiments. CON, control; n.s., nonsignificant.
We also performed semiquantitative RT-PCR analysis to determine which PDE4B isoforms are up-regulated by NTHi and roflumilast. The human PDE4B gene encodes a number of distinct isoforms, so-called long forms PDE4B1 and PDE4B3, short form PDE4B2, and supershort form PDE4B5 (34–38). We were unable to examine another long form, PDE4B4, because its cDNA has been cloned only in rat and this isoform appears not to be encoded by human genome (39). As shown in Fig. 1F and Fig. S1C, the expression of PDE4B2 was synergistically up-regulated by roflumilast and NTHi or TNF-α. The amplified PCR bands for PDE4B1, PDE4B3, and PDE4B5 were not detected even after 40 cycles of amplification in BEAS-2B cells (Fig. S1C).
The synergistic induction of PDE4B2 expression was also verified at the protein level in vitro and in vivo (Fig. 1 G–I). Western blot analyses in airway epithelial cell extracts and mouse lung tissue extracts revealed that NTHi and roflumilast induced an increase in the ∼70-kDa PDE4B isoform, which comigrates with overexpressed human PDE4B2 protein (Fig. 1 G and H and Fig. S2 A and B) (34, 40–42). Immunofluorescent staining showed that high intensity of PDE4B immunofluorescence signals was largely detected in bronchial epithelium of mouse lung tissues (Fig. 1I, arrows). Consistently, the PDE4B enzyme activity was also increased due to the up-regulation of PDE4B2 protein expression caused by roflumilast and NTHi (Fig. S1D). Together, our data suggest that roflumilast synergizes with NTHi to specifically up-regulate PDE4B2 expression at both mRNA and protein levels in vitro and in vivo.
PDE4B2 Is Required for NTHi-Induced Expression of Proinflammatory Mediators.
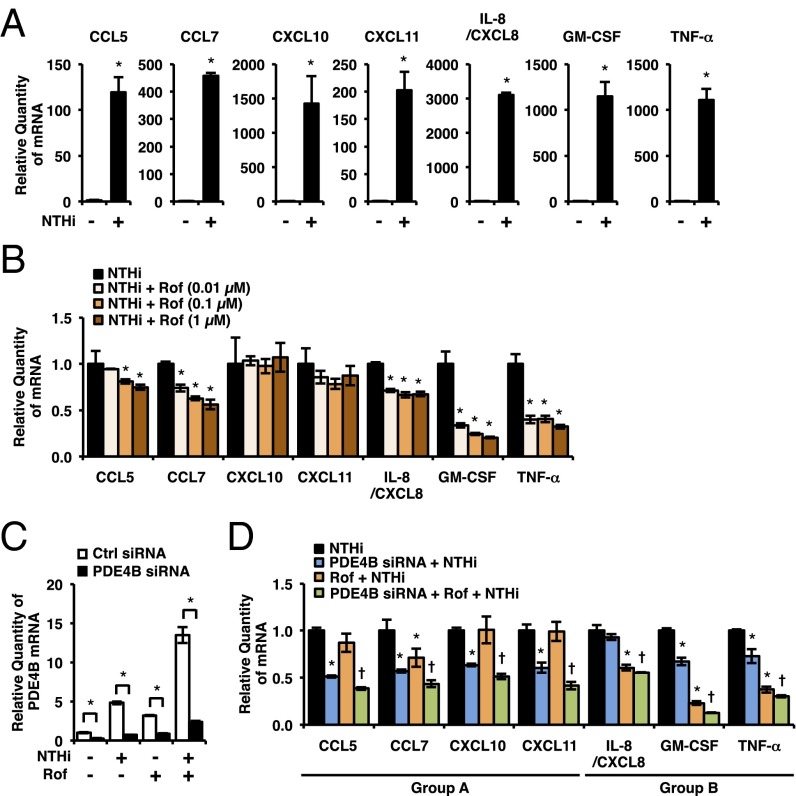
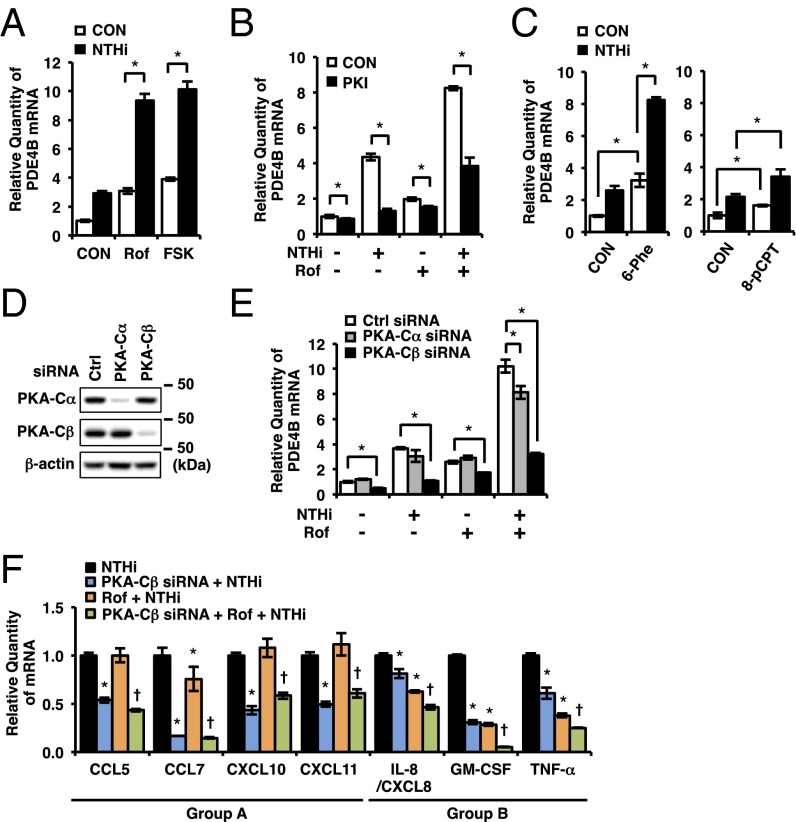
Next, we sought to determine the role of PDE4B2 expression in NTHi-induced inflammatory response in human airway epithelial cells. Proinflammatory mediators including cytokines and chemokines play critical roles in the recruitment and activation of leukocytes from the circulation to the lung in airway inflammatory diseases (43–45). Airway epithelial cells are the important sources of proinflammatory mediators induced by bacterial pathogens (28). Thus, we first determined whether NTHi induces the expression of a number of key proinflammatory mediators that have been shown to be critical in the pathogenesis of COPD. As shown in Fig. 2A, NTHi significantly up-regulated the expression of CC chemokine ligand 5 (CCL5), CCL7, CXC chemokine ligand 10 (CXCL10), CXCL11, interleukin-8 (IL-8/CXCL8), granulocyte-macrophage colony-stimulating factor (GM-CSF), and TNF-α in BEAS-2B cells. Interestingly, roflumilast inhibited NTHi-induced expression of these proinflammatory mediators to various extents (Fig. 2B). Roflumilast markedly inhibited the induction of TNF-α and GM-CSF, but modestly suppressed the induction of CCL5, CCL7, and IL-8. In contrast, it exhibited almost no inhibitory effect on CXCL10 and CXCL11 induction even at higher concentration. These interesting results have led us to postulate that the low or limited efficacy of roflumilast in suppressing these proinflammatory mediators may be attributed to the up-regulated expression of PDE4B2 by roflumilast in the presence of NTHi. We thus determined the contribution of PDE4B2 by assessing the effects of PDE4B2 depletion by using PDE4B siRNA on induction of these proinflammatory mediators by NTHi. As expected, PDE4B siRNA markedly depleted PDE4B2 expression in BEAS-2B cells (Fig. 2C and Fig. S2C). PDE4B2 depletion further significantly inhibited the induction of CCL5, CCL7, CXCL10, and CXCL11 (group A) that were modestly or minimally inhibited by roflumilast alone (Fig. 2D). In contrast, PDE4B2 depletion only minimally affected the induction of IL-8, GM-CSF, and TNF-α (group B) that were modestly or markedly inhibited by roflumilast alone (Fig. 2D and Table S1).
Fig. 2.
PDE4B is required for NTHi-induced expression of proinflammatory mediators. (A) BEAS-2B cells were stimulated with NTHi for 5 h, and mRNA expressions were analyzed. *P < 0.05 vs. CON. (B) BEAS-2B cells were pretreated with Rof for 1 h followed by 5-h stimulation with NTHi, and mRNA expressions were analyzed. Each expression was normalized with NTHi treated. *P < 0.05 vs. NTHi. (C) After 72-h transfection of siRNA, BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. *P < 0.05. (D) After 72-h transfection of siRNA, BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 5-h stimulation with NTHi, and mRNA expressions were analyzed. Each expression was normalized with NTHi treated. *P < 0.05 vs. NTHi; †P < 0.05 vs. Rof + NTHi. Data are mean ± SD (n = 3). Data are representative of three or more independent experiments.
PDE4D was also up-regulated by NTHi and roflumilast (Fig. S1 A and B), and previous studies have suggested that PDE4D has different and nonredundant role from PDE4B (46, 47). We thus also evaluated the effect of PDE4D depletion on NTHi-induced expression of proinflammatory mediators (Fig. S3 A and B). The induction of chemokines in group A was attenuated by PDE4D depletion, but to a much lesser extent compared with PDE4B depletion. The induction of chemokines and cytokines in group B was not affected or even slightly enhanced by PDE4D depletion (Fig. S3B). Collectively, these results suggest that different proinflammatory mediators are differentially regulated by PDE4B and PDE4D, and the expression of PDE4B plays a more crucial role in NTHi-induced expression of CCL5, CCL7, CXCL10, and CXCL11 in bronchial epithelial cells.
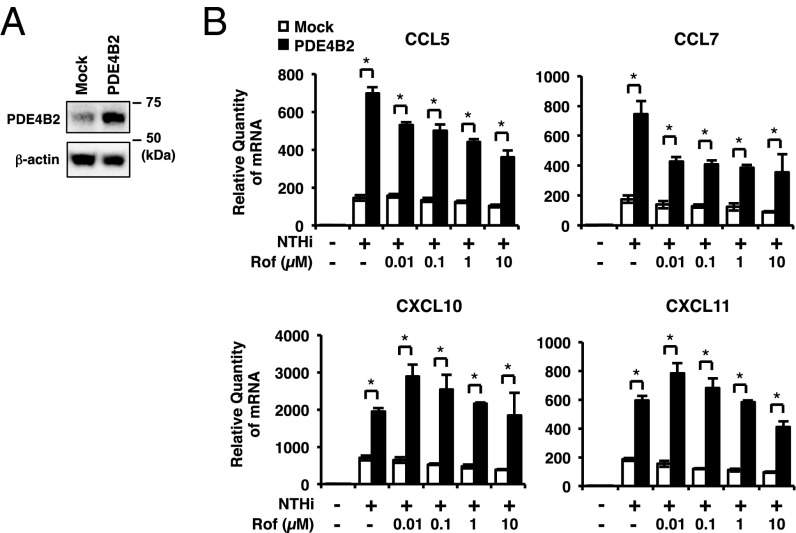
To further confirm the role of PDE4B2 up-regulation in NTHi-induced expression of chemokines, we developed the cells stably overexpressing wild-type PDE4B2 (PDE4B2-stable cells). The PDE4B2 expression in PDE4B2-stable cells was higher compared with mock cells (Fig. 3A and Figs. S2D and S4A). As shown in Fig. 3B, NTHi-induced expression of CCL5, CCL7, CXCL10, and CXCL11 was significantly enhanced in PDE4B2-stable cells compared with mock cells. Interestingly, roflumilast was unable to fully inhibit the NTHi-induced expression of these chemokines in PDE4B2-stable cells even at the highest concentration tested (10 µM). Of note, the NTHi-induced expression of CXCL10 and CXCL11 was even slightly enhanced by lower dose (<1 µM) of roflumilast in PDE4B2-stable cells (Fig. 3B). In line with the results from PDE4B depletion (Fig. 2D), PDE4B2 overexpression did not markedly increase the expression of IL-8, GM-CSF, and TNF-α induced by NTHi in the presence of roflumilast (Fig. S4 B and C). Together, these results suggest that synergistically up-regulated PDE4B2 by NTHi and roflumilast may contribute, at least in part, to the decreased efficacy of roflumilast in suppressing CCL5, CCL7, CXCL10, and CXCL11 induction in bronchial epithelial cells.
Fig. 3.
Increased PDE4B2 enhances NTHi-induced expression of chemokines. (A) PDE4B protein expression in mock and PDE4B2-stable cells. (B) Mock or PDE4B2-stable cells were pretreated with Rof for 1 h followed by 5 h stimulation with NTHi, and mRNA expressions were analyzed. Data in B are mean ± SD (n = 3); *P < 0.05. Data are representative of three or more independent experiments.
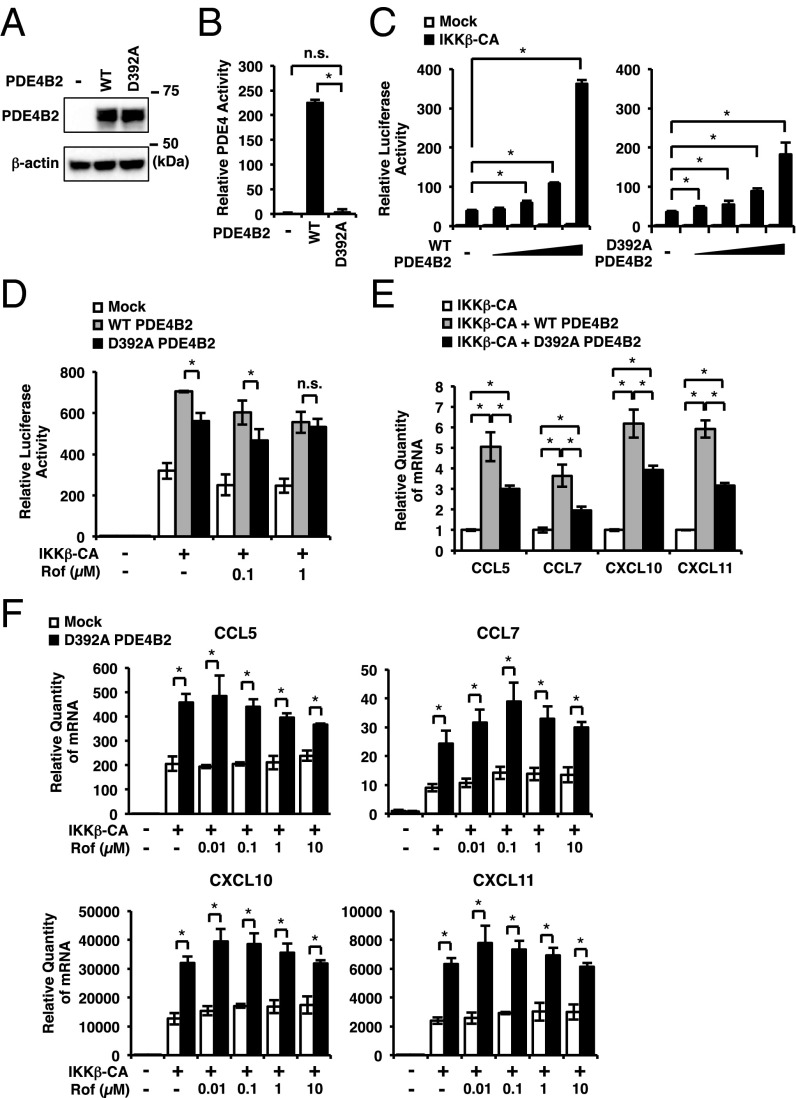
It has been shown that the enzymatic activity of PDE4 is critical for regulating inflammatory response (8, 10–12). However, our data revealed that the up-regulated PDE4B2 leads to the induction of certain proinflammatory mediators in a manner that cannot be overcome by high doses of roflumilast (up to 10 μM), a very high level of this drug because the IC50 has been estimated at less than 1 nM (8). These very interesting but rather unexpected results thus led us to hypothesize that PDE4B2 may regulate the expression of these chemokines at least in part independently of its well-known enzymatic activity, e.g., acting as an adaptor protein. To test this hypothesis, we compared the effects of expressing wild-type PDE4B2 (PDE4B2-WT) and catalytically inactive form of PDE4B2 (PDE4B2-D392A) (48, 49) on NF-κB-dependent promoter activity and the chemokine induction. Both PDE4B2-WT and PDE4B2-D392A were equally expressed in the transfected BEAS-2B cells, and PDE4 activity was significantly lower in the cells transfected with PDE4B2-D392A compared with the cells transfected with PDE4B2-WT (Fig. 4 A and B and Fig. S2E). We found that PDE4B2-WT markedly enhanced constitutively active form of inhibitor of NF-κB (IκB) kinase β (IKKβ-CA)–induced NF-κB promoter activity in a dose-dependent manner and also enhanced IKKβ-CA–induced group A-chemokine expression (Fig. 4 C–E). Interestingly, PDE4B2-D392A also enhanced IKKβ-CA–induced NF-κB promoter activity and chemokine expression, although to a lesser extent compared with PDE4B2-WT (Fig. 4 C–E). Moreover, roflumilast (up to 10 µM) was unable to fully inhibit the IKKβ-CA–induced NF-κB promoter activity and group A-chemokine expression in the cells transfected with PDE4B2-D392A (Fig. 4 D and F). Together, these results suggest PDE4B2 enhances inflammatory response in both PDE enzymatic activity-dependent and activity-independent manners, which may contribute to the tolerance to roflumilast.
Fig. 4.
Effects of PDE4B2-WT and PDE4B2-D392A on NF-κB promoter activity and chemokine expression. (A and B) BEAS-2B cells were transfected with mock, PDE4B2-WT or PDE4B2-D392A. After 48 h, PDE4B protein expression (A) and PDE4 enzymatic activity (B) were analyzed. (C and D) BEAS-2B cells were transfected with NF-κB luciferase plasmid together with mock, IKKβ-CA, PDE4B2-WT, or PDE4B2-D392A. After 24 h, NF-κB activity was measured (C), or after 40 h, cells were treated with Rof for 5 h and NF-κB activity was measured (D). (E) BEAS-2B cells were transfected with IKKβ-CA, mock, PDE4B2-WT, and PDE4B2-D392A. After 40 h, mRNA expressions were analyzed. Each expression was normalized with IKKβ-CA transfected. (F) BEAS-2B cells were transfected with mock, IKKβ-CA, and PDE4B2-D392A. After 40 h, cells were treated with Rof for 5 h and mRNA expressions were analyzed. Data in B–F are mean ± SD (n = 3); *P < 0.05; n.s. = P > 0.05. Data are representative of three or more independent experiments. n.s., nonsignificant.
PKA-Cβ but Not PKA-Cα Is Required for Synergistic Induction of PDE4B2.
To investigate the mechanism underlying the synergistic induction of PDE4B2, we first examined whether cAMP, which is increased by roflumilast, is involved in the synergistic induction of PDE4B2. As shown in Fig. 5A, forskolin (FSK), a potent cAMP elevator, synergized with NTHi to up-regulate PDE4B2 expression, suggesting that cAMP is involved in the synergistic induction of PDE4B2 in BEAS-2B cells. Thus, we further investigated the involvement of two ubiquitously expressed intracellular cAMP effectors, PKA and exchange protein directly activated by cAMP (Epac). A specific PKA inhibitor (PKI) significantly suppressed the synergistic induction of PDE4B2 by NTHi and roflumilast (Fig. 5B). Consistent with this result, a PKA-selective activator 6-Phe-cAMP, which does not activate Epac, synergistically enhanced NTHi-induced PDE4B2 expression (Fig. 5C, Left). In contrast, an Epac-selective activator 8-pCPT-2′-O-Me-cAMP did not markedly synergize with NTHi to induce PDE4B2 expression (Fig. 5C, Right). These results suggest that cAMP-dependent activation of PKA but not Epac is required for the synergistic induction of PDE4B2 in bronchial epithelial cells.
Fig. 5.
PKA-Cβ but not PKA-Cα is required for synergistic induction of PDE4B. (A) BEAS-2B cells were pretreated with Rof (0.1 µM) or FSK (1 μM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (B) BEAS-2B cells were pretreated with Rof (0.1 µM) and PKI (60 μM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (C) BEAS-2B cells were pretreated with 6-Phe-cAMP (6-Phe, 0.5 mM) or 8-pCPT-2′-O-Me-cAMP (8-pCPT, 0.5 mM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (D) Protein expression was analyzed after 48-h transfection of siRNA in BEAS-2B cells. (E) After 48 h transfection of siRNA, BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. Data in A–C and E are mean ± SD (n = 3); *P < 0.05. (F) After 48-h transfection of siRNA, BEAS-2B cells were pretreated with Rof (0.1 µM) for 1 h followed by 5-h stimulation with NTHi, and mRNA expressions were analyzed. Each target expression was normalized with NTHi treated. Data are mean ± SD (n = 3); *P < 0.05 vs. NTHi; †P < 0.05 vs. Rof + NTHi. Data are representative of three or more independent experiments. CON, control; FSK, forskolin.
PKA is a tetrameric enzyme consisting of two catalytic (C) and two regulatory (R) subunits. Binding of cAMP to R subunits results in relief of R subunit inhibition of the C subunits, which then phosphorylate a variety of protein substrates (50–54). Three C subunit isoforms, PKA-Cα, PKA-Cβ, and PKA-Cγ, have been identified in human, although PKA-Cγ is testis specific (55). We next determined which PKA isoform is involved in the synergistic induction of PDE4B2 by depleting PKA-Cα and PKA-Cβ using specific siRNAs. Western blot analysis revealed that the protein expression of PKA-Cα and PKA-Cβ was efficiently and selectively decreased in siRNA-transfected cells, respectively (Fig. 5D). Interestingly, the synergistic induction of PDE4B2 by NTHi and roflumilast was significantly attenuated by PKA-Cβ depletion, whereas only a slight suppression was observed in PKA-Cα–depleted cells (Fig. 5E). Next, we determined the effect of PKA-Cβ depletion on the up-regulation of proinflammatory mediators induced by NTHi in the presence of roflumilast. As shown in Fig. 5F, the inhibitory effect of PKA-Cβ depletion on the expression of proinflammatory mediators is highly consistent with that of PDE4B2 depletion, except that PKA-Cβ depletion attenuated CCL7 and GM-CSF up-regulation to a greater extent compared with PDE4B2 depletion. These results suggest that PKA-Cβ acts as a key positive regulator in the synergistic up-regulation of PDE4B2 and proinflammatory mediators induced by NTHi and roflumilast. It should be noted that some of the NTHi-induced proinflammatory mediators were even increased by PKA-Cα depletion (Fig. S3C), which is line with the antiinflammatory effects of PKA-Cα reported in other cell types (1).
Because it has been shown that cAMP response element (CRE) plays a particularly important role in up-regulation of PDE4B2 expression in rat neurons (25), we examined the involvement of CRE-binding protein (CREB) and activating transcription factor 1 (ATF1), two ubiquitously expressed PKA-dependent transcription factors (56), on the synergistic induction of PDE4B2 by NTHi and roflumilast in BEAS-2B cells. Interestingly, depletion of either CREB or ATF1 decreased the synergistic induction of PDE4B2 but to a much lesser extent compared with PKA-Cβ depletion (Fig. S5). These results suggest that other downstream molecules of PKA-Cβ may play a more important role in the synergistic induction of PDE4B2 in bronchial epithelial cells. Nonetheless, our data suggest that PKA-Cβ but not PKA-Cα is crucial for the synergistic induction of PDE4B2 by NTHi and roflumilast.
IKKβ-p65 but Not c-Rel Is Required for Synergistic Induction of PDE4B2.
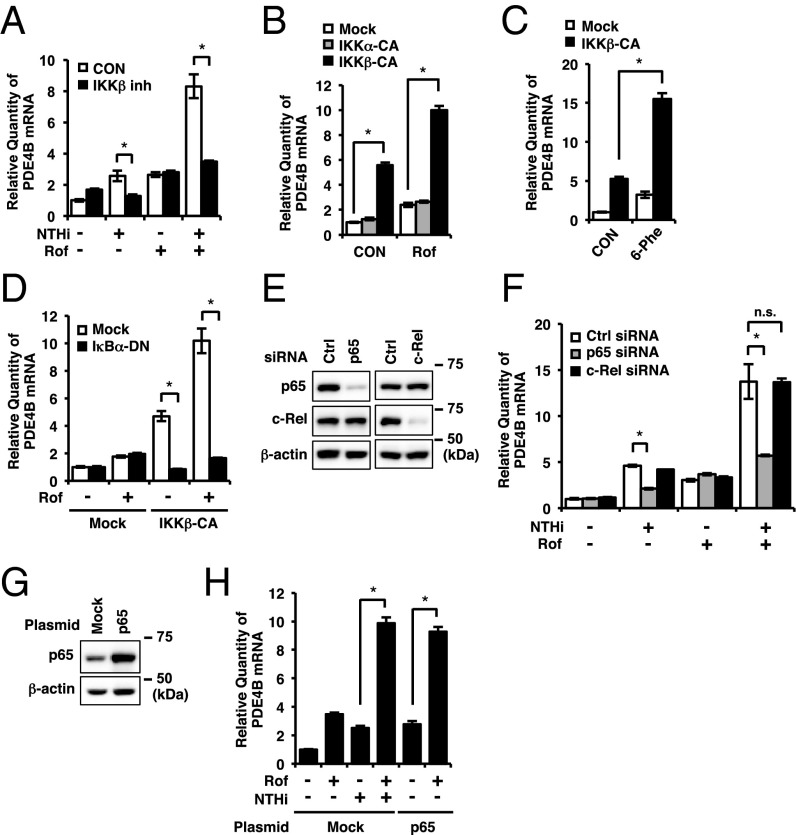
Because NTHi is known as a potent activator of IKKβ (also known as IKK2), leading to the activation of NF-κB–dependent inflammatory response (57–59), we examined the requirement of IKKβ-NF-κB signaling in the synergistic induction of PDE4B2. We first evaluated the role of IKKβ. As shown in Fig. 6A, IKKβ inhibitor significantly inhibited the synergistic induction of PDE4B2 by NTHi and roflumilast, but did not affect the induction of PDE4B2 by roflumilast alone. To further determine whether the activation of IKKβ synergizes with roflumilast to induce the synergistic up-regulation of PDE4B2, BEAS-2B cells were transfected with the constitutively active form of IKKα (IKKα-CA) and IKKβ (IKKβ-CA). As shown in Fig. 6 B and C, PDE4B2 expression was synergistically enhanced by roflumilast or a PKA activator 6-Phe-cAMP in IKKβ-CA– but not IKKα-CA–transfected cells. In addition, we also demonstrated that the expression of a dominant-negative mutant of IκBα (IκBα-DN), the downstream molecule of IKKβ, completely blocked IKKβ-CA–induced PDE4B2 expression and the synergistic induction of PDE4B2 by roflumilast in IKKβ-CA–transfected cells (Fig. 6D). These results suggest that IKKβ-IκBα signaling pathway is required for the synergistic induction of PDE4B2.
Fig. 6.
IKKβ-p65 but not c-Rel is required for synergistic induction of PDE4B. (A) BEAS-2B cells were pretreated with Rof (0.1 µM) and IKKβ inhibitor (1 μM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed. (B and C) BEAS-2B cells were transfected with mock, IKKα-CA, or IKKβ-CA. After 40 h, cells were treated with Rof (0.1 µM) or 6-Phe-cAMP (6-Phe, 0.5 mM) for 1.5 h and PDE4B mRNA expression was analyzed. (D) BEAS-2B cells were transfected with mock, IKKβ-CA, and IκBα-DN. After 40 h, cells were treated with Rof (0.1 µM) for 1.5 h and PDE4B mRNA expression was analyzed. (E–H) BEAS-2B cells were transfected with siRNA (E and F) or plasmid (G and H). After 48 h, the protein expressions were analyzed (E and G), or cells were pretreated with Rof (0.1 µM) for 1 h followed by 1.5-h stimulation with NTHi, and PDE4B mRNA expression was analyzed (F and H). Data in A–D, F, and H are mean ± SD (n = 3); *P < 0.05; n.s. = P > 0.05. Data are representative of three or more independent experiments. CON, control; n.s., nonsignificant.
IκBα prevents the activation and nuclear translocation of NF-κB complexes including p65 and c-Rel, which have been known to be activated by PKA-Cα and PKA-Cβ, respectively (4, 60–62). Thus, we first investigated whether NTHi induces nuclear translocation of p65 and c-Rel. As shown in Fig. S6A, both p65 and c-Rel were translocated to the nucleus within 60 min after the NTHi treatment in BEAS-2B cells. These results thus led us to further determine the requirement of p65 and c-Rel for the synergistic induction of PDE4B2 by using siRNA to selectively deplete p65 or c-Rel (Fig. 6E). We found that depletion of p65, but not c-Rel, significantly inhibited the synergistic induction of PDE4B2 by both NTHi and roflumilast or PDE4B2 induction by NTHi alone but not by roflumilast alone (Fig. 6F), thereby indicating an important role of p65 in NTHi-induced PDE4B2 expression. We further determined whether overexpression of p65 synergizes with roflumilast to induce PDE4B2. As shown in Fig. 6 G and H, roflumilast indeed synergistically enhanced PDE4B2 expression in p65-transfeceted cells. Collectively, our data demonstrate that IKKβ-IκBα-p65 signaling pathway is required for the synergistic induction of PDE4B2 in bronchial epithelial cells.
PKA-Cβ Phosphorylates p65.
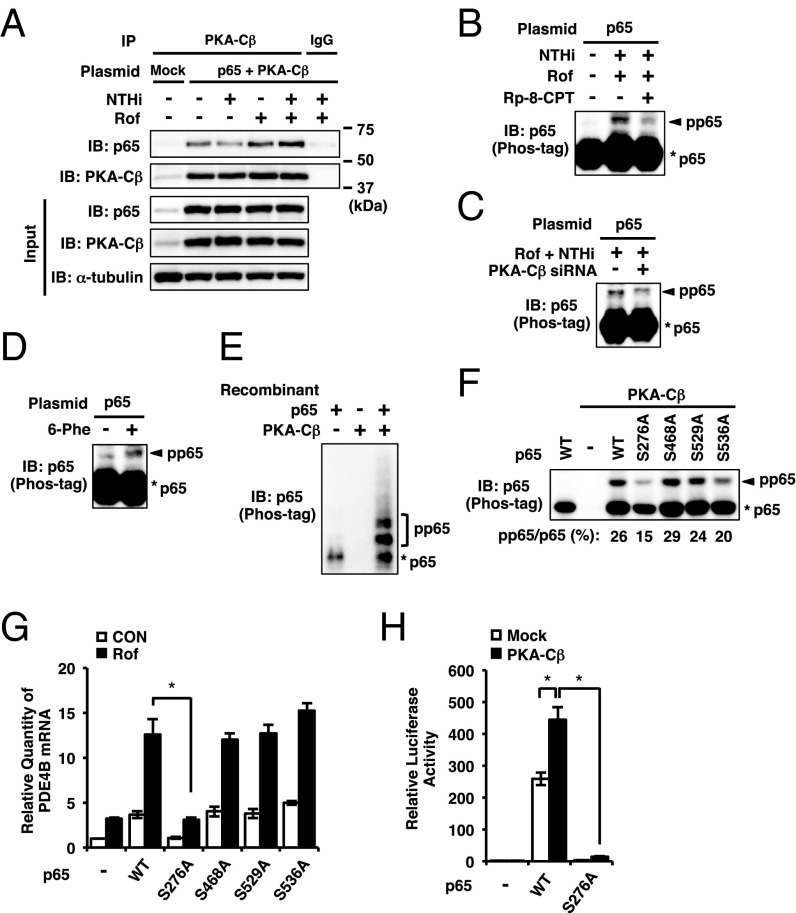
To further determine how PKA-Cβ interacts with p65 and synergizes with each other to up-regulate PDE4B2 expression, we first investigated whether p65 is physically associated with PKA-Cβ by performing coimmunoprecipitation experiments. As shown in Fig. 7A, p65 and PKA-Cβ were physically associated with each other in BEAS-2B cells transfected with both p65 and PKA-Cβ. Moreover, their interaction was enhanced by cotreatment of roflumilast with NTHi (Fig. 7A). We found that roflumilast did not affect the nuclear expression level of p65 or PKA-Cβ in BEAS-2B cells (Fig. S6 A–D). Thus, we next examined whether PKA-Cβ affects p65 phosphorylation. Phosphorylation at multiple residues of p65 has been shown to regulate various functions of p65, such as DNA binding and transcriptional activities (63, 64). However, the role of PKA-Cβ in regulating p65 phosphorylation remains unknown. To evaluate p65 phosphorylation, we performed phosphate-affinity (Phos-tag) SDS-PAGE, a phosphate-binding tag-based method that has been developed to specifically decrease the migration speed of phosphorylated proteins so that the phosphorylated protein can be separated from nonphosphorylated protein (Fig. S7A) (65). As shown in Fig. 7B, cotreatment of roflumilast with NTHi induced phosphorylation of p65, which was inhibited by Rp-8-CPT-cAMPS, a specific inhibitor of cAMP-dependent PKA activation. PKA-Cβ depletion or H89 treatment exhibited similar inhibitory effects (Fig. 7C and Figs. S6E and S7B). Consistent with these results, a cAMP-dependent PKA-selective activator 6-Phe-cAMP also induced this phosphorylation (Fig. 7D). Of note, NTHi alone did not induce the similar phosphorylation in BEAS-2B cells (Fig. S7B), suggesting that PKA-Cβ activation by cAMP is required for p65 phosphorylation. We also found that the overexpression of PKA-Cβ1, the major subtype of Cβ in BEAS-2B cells (Fig. S6F), induced the phosphorylation of p65, which was inhibited by H89, but not by a p38 inhibitor (SB203580) or an ERK inhibitor (PD98059) (Fig. S7C). These results suggest that PKA-Cβ1 directly phosphorylates p65, independently of activation of p38, ERK, or their downstream kinase MSK1 (mitogen- and stress-activated kinase 1) that has been shown to phosphorylate p65 (66–68). To further confirm whether PKA-Cβ directly phosphorylates p65, we performed in vitro kinase assay by using recombinant p65 and PKA-Cβ proteins. As shown in Fig. 7E and Fig. S7D, PKA-Cβ directly phosphorylates p65.
Fig. 7.
PKA-Cβ phosphorylates p65. (A) BEAS-2B cells were transfected with mock or p65 together with PKA-Cβ. After 48 h, cells were pretreated with Rof (0.1 µM) for 1 h followed by 1-h stimulation with NTHi. PKA-Cβ in whole-cell extracts was pulled down with anti-PKA-Cβ antibody and immunoblotted against p65 and PKA-Cβ. (B–D and F) BEAS-2B cells were transfected with plasmid or siRNA for 48 h, and p65 phosphorylation in nuclear extracts was analyzed by Phos-tag SDS-PAGE. Rp-8-CPT-cAMPS (Rp-8-CPT, 0.1 mM) was pretreated for 0.5 h before the Rof treatment (B). Rof (0.1 µM) was pretreated for 1 h followed by 1-h stimulation with NTHi (B and C). Cells were treated with 6-Phe-cAMP (6-Phe, 0.5 mM) for 2 h (D). (E) Recombinant p65 and PKA-Cβ proteins were mixed and incubated at 37 °C for 0.5 h and analyzed by Phos-tag SDS-PAGE. (G) After 48-h transfection of plasmid, BEAS-2B cells were treated with Rof (0.1 µM) for 2.5 h, and PDE4B mRNA expression was analyzed. (H) BEAS-2B cells were transfected with NF-κB luciferase plasmid together with mock, p65, S276A p65, and PKA-Cβ. After 24 h, NF-κB activity was measured. Data in G and H are mean ± SD (n = 3); *P < 0.05. Data are representative of three or more independent experiments. CON, control; pp65, phosphorylated p65.
Searching for PKA consensus phosphorylation sequence (RRXS/T) revealed that the serine 276 residue (Ser276) is a potential PKA phosphorylation site (69), which is in line with the previous studies showing that PKA-Cα phosphorylates p65 at Ser276 residue (61, 62). To determine whether p65 is also phosphorylated by PKA-Cβ at Ser276, BEAS-2B cells were transfected with the phosphorylation-deficient mutant (S276A) of p65 and analyzed by Phos-tag SDS-PAGE. The p65 constructs with mutation of other serine residues (S468A, S529A, and S536A) known to be phosphorylated by other kinases were also analyzed. The intensity ratio of PKA-Cβ–induced phosphorylation was decreased in S276A- and S536A-transfected cells compared with the wild-type p65-transfected cells (Fig. 7F and Fig. S6G). To further determine the functional involvement of p65 phosphorylation at these residues in PDE4B2 induction, we examined PDE4B2 expression in the cells transfected with these different p65 phosphorylation site mutants. As shown in Fig. 7G, roflumilast and NTHi synergistically enhanced PDE4B2 expression in the cells transfected with p65 mutants S468A, S529A, and S536A, but not S276A. We next determined the effect of Ser276 phosphorylation by PKA-Cβ on the transcriptional activity of p65 by performing the NF-κB promoter activity analysis. Cotransfection with PKA-Cβ1 significantly enhanced the p65-induced NF-κB promoter activity, which was abrogated by coexpressing S276A mutant of p65 (Fig. 7H). Together, these results suggest that roflumilast and NTHi increase the physical interaction of PKA-Cβ with p65, which, in turn, leads to the phosphorylation of p65 at Ser276 and subsequent up-regulation of p65-dependent transcriptional activity.
Discussion
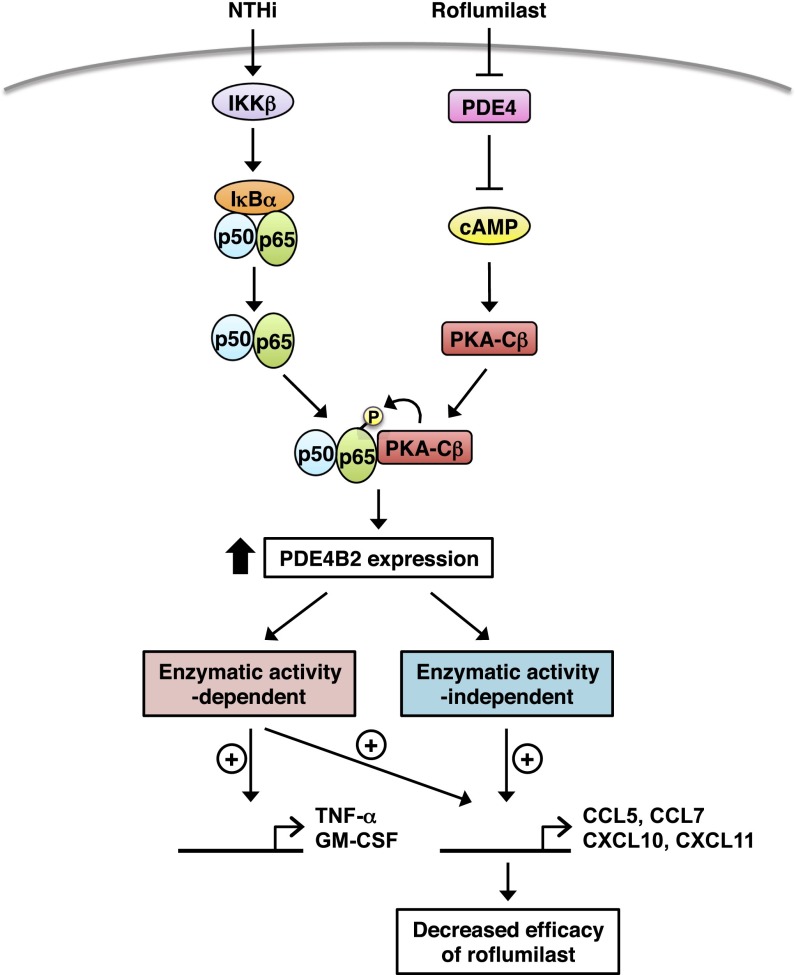
PDE4B has been shown to be up-regulated by various inflammatory stimuli, which plays a critical role in mediating inflammatory response (16–18, 22, 70–72). We recently also found that PDE4B is induced by bacterium NTHi (27). It is also well known that the expression level of PDE4 isoforms is induced by cAMP elevators including PDE4 inhibitor itself (18, 23–26, 33), which is believed to be important in negative feedback regulation of cAMP signaling. However, it remains unclear how PDE4 is regulated in the presence of both bacterial pathogen and cAMP elevators. In this study, we showed for the first time to our knowledge that roflumilast (a clinically approved PDE4 inhibitor for COPD exacerbation) synergizes with NTHi (a major bacterial cause of COPD exacerbation) to induce PDE4B2 expression in the context of the complex pathogenesis and medications of COPD, via a cross-talk between cAMP/PKA-Cβ and p65 (Fig. 8). The synergistic induction of PDE4B2 was also observed in the presence of other inflammatory stimuli, such as TNF-α and IL-1β (Fig. S1E), and cAMP elevators. PDE4B2 expression plays a critical role in NTHi-induced expression of chemokines CCL5, CCL7, CXCL10, and CXCL11, which have previously been shown to be crucial in the pathogenesis of COPD exacerbation. These chemokines are increased in induced sputum, bronchoalveolar lavage (BAL) fluid, or peripheral airways in patients with COPD (73–77) and play important roles in the recruitment of macrophages, CD8+ T cells, B cells, eosinophils, and neutrophils into the airway lumen (43–45, 78, 79).
Fig. 8.
Schematic representation of PDE4B2 induction by NTHi and roflumilast via p65 and PKA-Cβ.
The second major finding of this study is that PDE4B2 regulates certain chemokine expression in both enzymatic activity-dependent and activity-independent manner. This enzymatic activity-independent function may lead to the reduced efficacy of roflumilast in suppressing inflammation under certain clinical conditions due to the synergistically up-regulated PDE4B2. For example, we observed that the effect of roflumilast in suppressing NTHi-induced CCL5, CCL7, CXCL10, and CXCL11 (named group A-chemokines) is less than its effect in suppressing some other proinflammatory mediators such as GM-CSF and TNF-α in bronchial epithelial cells. In addition, roflumilast became more efficacious in suppressing group A-chemokines in PDE4B-depleted, but not in PDE4D-depleted, cells. Consistent with these results, roflumilast (even at 10 µM) was unable to fully inhibit the NTHi-induced expression of group A-chemokines in the stable cell line overexpressing PDE4B2. Moreover, both PDE4B2-WT and PDE4B2-D392A (the mutant with no enzymatic activity) markedly enhanced the NF-κB promoter activity and group A-chemokine expression, although PDE4B2-D392A enhanced NF-κB activation and chemokine expression to a lesser extent compared with PDE4B2-WT. It should also be noted that roflumilast (up to 10 µM) was unable to fully inhibit the IKKβ-CA–induced NF-κB promoter activity and group A-chemokine expression in the cells overexpressing PDE4B2-WT or PDE4B2-D392A. Together, these results demonstrate that up-regulated PDE4B2 may contribute to the up-regulation of these chemokines in both enzymatic activity-dependent and activity-independent manners, thereby providing the evidence for the first time to our knowledge that PDE4B2 may act as a nonenzymatic adaptor protein in regulating inflammatory response. Future studies are warranted to further elucidate the underlying mechanism.
The third major finding in the present study is that PKA-Cβ, but not PKA-Cα, is specifically required for mediating the synergistic induction of PDE4B2 and the resultant inflammatory response in human airway epithelial cells. Previously, it has been shown that PKA-Cα activates NF-κB signaling via phosphorylating p65 at Ser276 in a cAMP-independent manner (61). PKA-Cα has been shown to be maintained in an inactive state through association with IκB complex. Signals that lead to IκB degradation result in PKA-Cα activation and subsequent phosphorylation of p65 at Ser276. This previous study suggests that the cAMP-independent PKA activation mechanism is involved in NF-κB activation and inflammation in response to inflammatory stimuli such as LPS, mitogens, cytokines, and viruses. In the current study, we reported a distinct mechanism by which cAMP synergizes with NF-κB signaling to up-regulate PDE4B2 through PKA-Cβ, but not PKA-Cα, mediated phosphorylation of p65 at Ser276 in the contexts of the presence of both bacterial pathogen and cAMP-elevating agents. Future study is required to further determine whether PKA-Cα and PKA-Cβ specifically mediate the cAMP-independent and cAMP-dependent regulation of p65 phosphorylation, respectively.
In addition to the distinct roles of PKA-Cα and PKA-Cβ in regulating PDE4B2 expression, PKA-Cα and PKA-Cβ also appear to differentially modulate the expression of proinflammatory mediators. For example, we found that some of the NTHi-induced proinflammatory mediators were increased upon PKA-Cα knockdown (Fig. S3C), which is line with the antiinflammatory role of PKA-Cα reported in other cell types (1). The role of PKA-Cβ in mediating inflammatory response remains largely unclear. In this study, we found that the expression of proinflammatory mediators was reduced by PKA-Cβ knockdown (Fig. 5F), indicating the proinflammatory role of PKA-Cβ. These data suggest that PKA-Cβ–selective inhibition may represent a promising strategy to suppress proinflammatory mediators without compromising the antiinflammatory effects mediated by PKA-Cα. Given the distinct roles of PKA-Cα and PKA-Cβ in regulating PDE4B2 and proinflammatory mediators, it is likely that PKA may be involved in regulating the physiological and pathological responses in an isoform-specific manner (54). Thus, special attention needs to be paid to the role of various isoforms of PKA for the studies aimed to determine the involvement of PKA.
The role and underlying mechanisms of PKA in regulating NF-κB signaling appears to be rather complex. In some model systems, the activation of PKA inhibits NF-κB nuclear translocation. For example, TNF-α–mediated nuclear translocation of p65 is enhanced by PKA depletion using siRNA in HeLa cells (80). Forskolin impairs the nuclear translocation of p65 in Jurkat T lymphocytes (81). There are also NF-κB nuclear translocation-independent mechanisms. For example, in human monocytes and endothelial cells, cAMP inhibits NF-κB–mediated transcription without preventing the nuclear translocation of NF-κB complex. Instead, cAMP/PKA inhibits NF-κB–dependent transcriptional activity by phosphorylating CREB, which competes with p65 for limiting amounts of NF-κB coactivator CREB-binding protein (CBP) (82). It has also been shown that the inhibitory action of the cAMP/PKA pathway on the transcriptional activity of NF-κB in Jurkat T lymphocytes is exerted through directly or indirectly modifying the C-terminal transactivation domain of p65, which is independent of CREB and p65 phosphorylation at Ser276 (83). All above-mentioned studies provide evidence for the inhibitory effect of cAMP/PKA on NF-κB transcriptional activity. However, there is also evidence that PKA is able to activate NF-κB signaling, in which phosphorylation of p65 at Ser276 appears to be critical. For example, phosphorylation of NF-κB at Ser276 by PKA stimulates NF-κB transcriptional activity by promoting the interaction of NF-κB with the coactivator CBP/p300 (62). NF-κB p65 phosphorylation at Ser276 by PKA activates NF-κB and contributes to the malignant phenotype of head and neck cancer (84). It has been also shown that PKA-Cα activates NF-κB signaling via phosphorylating p65 at Ser276 in an IKKβ-dependent but cAMP-independent manner (61). In the present study, we show that PKA-Cβ also phosphorylates p65 at Ser276 but in a cAMP-dependent manner, which is critical for the synergistic induction of PDE4B2 by NTHi and roflumilast in bronchial epithelial cells. Taken together, these lines of experimental evidence suggest that cAMP and/or PKA can modulate NF-κB activation/inactivation via various mechanisms, which might depend on cell types and sources of cAMP and PKA as well as the NF-κB subunits present in different signaling complexes. In addition, the expression levels of A kinase-interacting protein 1 (AKIP1) in various cell types or conditions also appear to be important for the effect of PKA on NF-κB activity. It has been shown that in cells with low levels of AKIP1, PKA-activating agents inhibit NF-κB transcriptional activity. In contrast, in cells with high levels of AKIP1, the PKA activation increases p65 phosphorylation at Ser276 and synergizes with NF-κB activation (85).
In summary, our studies provide insights not only into the molecular mechanism underlying the synergistic induction of PDE4B2 expression in the context of the complex pathogenesis and medications of COPD but also into the signaling cross-talk between PKA-Cβ and p65 via a cAMP-dependent manner. Our results also provide the evidence for the first time to our knowledge that PDE4B2 may act, at least in part, as a nonenzymatic adaptor protein in regulating inflammatory response. Combinational administration of roflumilast with PKA-Cβ–selective inhibitor may help attenuate the unwanted up-regulation of PDE4B2, thereby representing a promising therapeutic strategy to improve the efficacy, decrease the effective dose, and possibly ameliorate the tolerance of PDE4 inhibitors in patients with COPD exacerbation.
Materials and Methods
Reagents and Antibodies.
Actinomycin D and protease inhibitor mixture (PIC) were purchased from Sigma-Aldrich. Myristoylated PKA inhibitor, and IKKβ inhibitor IV were purchased from EMD Millipore. Roflumilast was purchased from Santa Cruz Biotechnology. N6-Phenyl-cAMP (6-Phe-cAMP), 8-pCPT-2′-O-Me-cAMP, and Rp-8-CPT-cAMPS were purchased from BioLog. Forskolin was purchased from Enzo Life Sciences. Phos-tag Acrylamide was purchased from Wako Chemicals USA. Recombinant p65 protein was purchased from Active Motif. Recombinant PKA-Cβ protein was purchased from R&D Systems. Antibodies for PKA-Cβ (sc-904), p65 (sc-8008), β-actin (sc-8432), α-Tubulin (sc-69969), PDE4B (sc-25812), and TFIIB (sc-225) were purchased from Santa Cruz Biotechnology, and PKA-Cα (4782) and c-Rel (4727) were purchased from Cell Signaling.
Bacterial Strains and Culture Condition.
Clinical isolates of NTHi strain 12 was used in this study (86). Bacteria were grown on chocolate agar plate at 37 °C in an atmosphere of 5% (vol/vol) CO2 overnight, and subsequently inoculated in brain heart infusion (BHI) broth supplemented with 3.5 μg/mL NAD and 10 μg/mL hemoglobin (BD Biosciences). After overnight incubation, bacteria were subcultured into fresh broth and the log phase bacteria, monitored by measurement of optical density (OD) value, washed, and suspended in DMEM for in vitro cell experiments and in isotonic saline for in vivo animal experiments. For the all in vitro experiments except the dose-dependent experiment, NTHi was treated at multiplicity of infection (MOI) of 50.
Cell Culture.
All media described below were supplemented with 10% (vol/vol) FBS (Sigma-Aldrich). Human bronchial epithelial BEAS-2B cells were maintained in RPMI medium 1640 (Life Technologies). Human primary bronchial epithelial NHBE (Lonza) cells were maintained in bronchial epithelial growth media (BEGM) supplemented with BEGM SingleQuots (87). BEAS-2B cells stably expressing human PDE4B2 (PDE4B2-stable cells) were obtained by plasmid transfection following Geneticin selection (300 µg/mL). Cells were cultured in a humidified atmosphere of 5% (vol/vol) CO2 at 37 °C.
Real-Time Quantitative and Semiquantitative RT-PCR Analyses.
Total RNA was isolated with TRIzol reagent (Life Technologies) by following the manufacturer’s instruction. For the reverse transcription reaction, TaqMan reverse transcription reagents (Life Technologies) were used as described previously. For quantitative RT-PCR analysis, PCR amplifications were performed by using SYBR Green Universal Master Mix (Life Technologies). In brief, reactions were performed in triplicate containing 2× Universal Master Mix, 1 μL of template cDNA, 500 nM primers in a final volume of 12.5 μL, and they were analyzed in a 96-well optical reaction plate (USA Scientific). Reactions were amplified and quantified by using a StepOnePlus Real-Time PCR System and the manufacturer’s corresponding software (StepOnePlus Software v2.3; Life Technologies). The relative quantities of mRNAs were obtained by using the comparative Ct method and were normalized by using human cyclophilin or mouse glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as an endogenous control. For semiquantitative RT-PCR analysis, PCR amplifications were performed with PrimeSTAR Max polymerase (Takara) by following the manufacturer’s instruction. The primer sequences used are listed in Table S2.
Plasmids, Transfections, and Luciferase Assay.
The expression plasmids, constitutively active forms of IKKα (IKKα-CA, S176E/S180E) and IKKβ (IKKβ-CA, S177E/S181E) and a dominant negative form of IκBα (IκBα-DN, S32A/S36A) were described (57, 86). Luciferase reporter construct of NF-κB (pGL4.32) was purchased from Promega. Human PDE4B2, p65 (RelA), and PKA-Cβ1/2 cDNA sequences were generated and inserted into the BamHI and HindIII sites of the pcDNA3.1/mycHis(-) vector. Mutant p65 and PDE4B2-D392A were generated by using PrimeSTAR Max (Takara). Empty vector was used as a control and was also added where necessary to ensure an equivalent amount of input DNA. All transient transfections were carried out in triplicate by using TransIT-LT1 reagent (Mirus) following the manufacturer’s instruction.
siRNA-Mediated Knockdown.
Human validated siRNA oligos were obtained from GE Healthcare (Negative Control, D001810-10; PDE4B, L007648-01; PKA-Cα, M004649-01; PKA-Cβ, M004650-00; p65, L003533-00; c-Rel, L004768-00). Cells were transfected with 50 nM siRNA by using DharmaFECT-4 (Thermo Scientific) and collected or treated 48 h later. For the cotransfection of siRNA with DNA, cells were transfected with 10 nM siRNA by using Lipofectamine 3000 (Life Technologies).
Subcellular Fractionation.
Cells were washed twice and corrected with ice cold PBS and centrifuge at 3,000 × g for 5 min. The cells were suspended with buffer A (10 mM Hepes at pH 7.4, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, supplemented with 1 mM Na3VO4, and PIC) and incubated on ice for 10 min (88). The cells were lysed by adding Nonidet P-40 (0.5%) and vortex for 15 s. Cells were centrifuged at 3,000 × g for 5 min and the supernatants (Cytosol fraction) were removed. Precipitates were resuspended in buffer B (20 mM Hepes at pH 7.5, 5 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, supplemented with 1 mM Na3VO4 and PIC) and incubated on ice for 10 min. The cells were vortexed and centrifuged at 16,000 × g for 15 min, and the supernatants were recovered (nuclear fraction).
Western Blot.
Whole-cell extracts and mouse lung tissue extracts were recovered with the lysis buffer (50 mM Tris⋅HCl at pH 7.4, 1% Nonidet P-40, 0.25% deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, supplemented with 1 mM Na3VO4 and PIC). For PDE4B protein, cell extracts were recovered with buffer A as described above. Cell or tissue extracts were separated on 8% (wt/vol) SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences). The membrane was blocked with a solution of TBS containing 0.1% Tween 20 (TBS-T) and 5% (wt/vol) nonfat dry milk. The membrane was then incubated in a 1:1,000–1:2,000 dilution of a primary antibody in 5% (wt/vol) BSA-TBS-T. After three washes with TBS-T, the membrane was incubated with 1:5,000 dilution of the corresponding secondary antibody in 2.5% (wt/vol) nonfat dry milk-TBS-T. Respective proteins were visualized by using Amersham ECL Prime Regent (GE Healthcare Life Sciences).
Immunoprecipitation.
Cell extracts were incubated with 1 µg of primary antibodies for overnight at 4 °C, followed by 2-h incubation with protein G PLUS-agarose beads (Santa Cruz Biotechnology). Immunoprecipitates were then suspended in a sample buffer, separated on 8% (wt/vol) SDS-PAGE gels, transferred to PVDF membrane, and detected by immunoblot analysis as described above.
PDE4 Activity.
PDE4 activity in whole-cell extracts from the cells transfected with PDE4B2 constructs were measured by using cyclic nucleotide PDE assay kit (Enzo Life Sciences) following the instructions. PDE4 activity was estimated from the difference between total and roflumilast-resistant PDE activity.
Phos-tag SDS-PAGE.
Recombinant p65 protein or nuclear extracts recovered without EDTA/EGTA were separated on 6% (wt/vol) SDS-PAGE gels containing 50 µM Mn2+-Phos-tag Acrylamide and transferred to PVDF membrane according to the manufacture’s instructions (65).
In Vitro Kinase Assay.
Recombinant p65 protein (70 ng) and recombinant PKA-Cβ (50 ng) were mixed in kinase assay buffer (20 mM Hepes at pH 7.5, 1 M MgCl2, 1 mM DTT, 10 mM ATP) and incubated at 30 °C for 0.5 h. Reaction was stopped by adding 4× SDS sample buffer [0.24 M Tris⋅HCl at pH 6.8, 40% (vol/vol) glycerol, 8% (wt/vol) SDS, 20% (vol/vol) 2-mercaptoehanol, 0.04% bromophenol blue].
Mice and Animal Experiments.
For NTHi-induced inflammation in C57BL/6J mice (7 wk old), anesthetized mice were intratracheally inoculated with NTHi at a concentration of 5 × 107 cfu per mouse and saline was inoculated as control. The inoculated mice were then killed 5 h after NTHi inoculation. For inhibition study, mice were pretreated with roflumilast intraperitoneally 2 h before NTHi inoculation. All animal experiments were approved by the Institutional Animal Care and Use Committee at Georgia State University.
Immunofluorescent Staining.
Formalin-fixed paraffin-embedded mouse lung tissue was sectioned (4 μm) and PDE4B protein was detected by using rabbit anti-PDE4B and FITC-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology). Stained sections were then imaged, and images were recorded under light-microscopy and fluorescence-microscopy systems (AxioVert 40 CFL, AxioCam MRC, and AxioVision LE Image system; Carl Zeiss).
Statistical Analysis.
All experiments were repeated at least three times with consistent results. Data were shown as mean ± SD. Statistical analysis was assessed by unpaired two-tailed Student’s t test and P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DC005843, DC004562, and GM107529 (to J.-D.L.), HL111291 and HL088400 (to C.Y.). J.-D.L. is Georgia Research Alliance Eminent Scholar in Inflammation and Immunity.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418716112/-/DCSupplemental.
References
- 1.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271(34):20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: A tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40(7):651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertz AL, et al. Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA. 2009;106(51):21978–21983. doi: 10.1073/pnas.0911684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlo S, et al. Cyclic AMP: A selective modulator of NF-kappaB action. Cell Mol Life Sci. 2011;68(23):3823–3841. doi: 10.1007/s00018-011-0757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldenburger A, et al. Anti-inflammatory role of the cAMP effectors Epac and PKA: Implications in chronic obstructive pulmonary disease. PLoS ONE. 2012;7(2):e31574. doi: 10.1371/journal.pone.0031574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 7.Conti M, Mika D, Richter W. Cyclic AMP compartments and signaling specificity: Role of cyclic nucleotide phosphodiesterases. J Gen Physiol. 2014;143(1):29–38. doi: 10.1085/jgp.201311083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58(3):488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard E, et al. Anchored PDE4 regulates chloride conductance in wild-type and DeltaF508-CFTR human airway epithelia. FASEB J. 2014;28(2):791–801. doi: 10.1096/fj.13-240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365(9454):167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson JA, Souness J, Webber S, Pollock K, Raeburn D. Anti-inflammatory effects of the novel phosphodiesterase IV inhibitor RP 73401. Int Arch Allergy Immunol. 1995;107(1-3):425–426. doi: 10.1159/000237066. [DOI] [PubMed] [Google Scholar]
- 12.Michalski JM, Golden G, Ikari J, Rennard SI. PDE4: A novel target in the treatment of chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2012;91(1):134–142. doi: 10.1038/clpt.2011.266. [DOI] [PubMed] [Google Scholar]
- 13.Price D, Chisholm A, Ryan D, Crockett A, Jones R. The use of roflumilast in COPD: A primary care perspective. Prim Care Respir J. 2010;19(4):342–351. doi: 10.4104/pcrj.2010.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashkin DP. Roflumilast: The new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014;15(1):85–96. doi: 10.1517/14656566.2013.837159. [DOI] [PubMed] [Google Scholar]
- 15.Robichaud A, et al. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002;135(1):113–118. doi: 10.1038/sj.bjp.0704457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: Relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G718–G724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gobejishvili L, et al. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. J Pharmacol Exp Ther. 2011;337(2):433–443. doi: 10.1124/jpet.110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci USA. 2002;99(11):7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175(3):1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- 20.Jin SL, et al. Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2010;126(6):1252–1259.e12. doi: 10.1016/j.jaci.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moghaddam SJ, et al. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38(6):629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D, Wu P, Egan RW, Billah MM, Wang P. Phosphodiesterase 4B gene transcription is activated by lipopolysaccharide and inhibited by interleukin-10 in human monocytes. Mol Pharmacol. 1999;55(1):50–57. doi: 10.1124/mol.55.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Méhats C, et al. Selective up-regulation of phosphodiesterase-4 cyclic adenosine 3′,5′-monophosphate (cAMP)-specific phosphodiesterase variants by elevated cAMP content in human myometrial cells in culture. Endocrinology. 1999;140(7):3228–3237. doi: 10.1210/endo.140.7.6847. [DOI] [PubMed] [Google Scholar]
- 24.Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154(1):82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Sa C, Tolbert LM, Conti M, Duman RS. Regulation of cAMP-specific phosphodiesterases type 4B and 4D (PDE4) splice variants by cAMP signaling in primary cortical neurons. J Neurochem. 2002;81(4):745–757. doi: 10.1046/j.1471-4159.2002.00878.x. [DOI] [PubMed] [Google Scholar]
- 26.Dlaboga D, Hajjhussein H, O’Donnell JM. Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: Comparison with rolipram. Brain Res. 2006;1096(1):104–112. doi: 10.1016/j.brainres.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu K, et al. Inhibition of PDE4B suppresses inflammation by increasing expression of the deubiquitinase CYLD. Nat Commun. 2013;4:1684. doi: 10.1038/ncomms2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallstrand TS, et al. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151(1):1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109(3):366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Rabe KF, et al. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 31.Calverley PM, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, Tan L, Atkinson G, Ward J. UK-500,001 Global Study Team A controlled trial of 6-weeks’ treatment with a novel inhaled phosphodiesterase type-4 inhibitor in COPD. Eur Respir J. 2009;33(5):1039–1044. doi: 10.1183/09031936.00068908. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154(1):99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Huston E, et al. Molecular cloning and transient expression in COS7 cells of a novel human PDE4B cAMP-specific phosphodiesterase, HSPDE4B3. Biochem J. 1997;328(Pt 2):549–558. doi: 10.1042/bj3280549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung YF, et al. PDE4B5, a novel, super-short, brain-specific cAMP phosphodiesterase-4 variant whose isoform-specifying N-terminal region is identical to that of cAMP phosphodiesterase-4D6 (PDE4D6) J Pharmacol Exp Ther. 2007;322(2):600–609. doi: 10.1124/jpet.107.122218. [DOI] [PubMed] [Google Scholar]
- 36.Bolger G, et al. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13(10):6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colicelli J, et al. Isolation and characterization of a mammalian gene encoding a high-affinity cAMP phosphodiesterase. Proc Natl Acad Sci USA. 1989;86(10):3599–3603. doi: 10.1073/pnas.86.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15(14):1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd M, et al. Molecular cloning and subcellular distribution of the novel PDE4B4 cAMP-specific phosphodiesterase isoform. Biochem J. 2003;370(Pt 2):429–438. doi: 10.1042/BJ20021082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquette A, André J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat Struct Mol Biol. 2011;18(5):584–591. doi: 10.1038/nsmb.2022. [DOI] [PubMed] [Google Scholar]
- 41.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310(5751):1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 42.Yougbare I, et al. NCS 613, a potent and specific PDE4 inhibitor, displays anti-inflammatory effects on human lung tissues. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L441–L450. doi: 10.1152/ajplung.00407.2010. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2006;27(10):546–553. doi: 10.1016/j.tips.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1(2):95–104. [PubMed] [Google Scholar]
- 45.Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2007;119(5):1065–1071. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- 46.Ariga M, et al. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173(12):7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- 47.Blackman BE, et al. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem. 2011;286(14):12590–12601. doi: 10.1074/jbc.M110.203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mongillo M, et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95(1):67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 49.Xu RX, et al. Atomic structure of PDE4: Insights into phosphodiesterase mechanism and specificity. Science. 2000;288(5472):1822–1825. doi: 10.1126/science.288.5472.1822. [DOI] [PubMed] [Google Scholar]
- 50.Krebs EG, Beavo JA. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 51.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 52.Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- 53.Gamm DM, Baude EJ, Uhler MD. The major catalytic subunit isoforms of cAMP-dependent protein kinase have distinct biochemical properties in vitro and in vivo. J Biol Chem. 1996;271(26):15736–15742. doi: 10.1074/jbc.271.26.15736. [DOI] [PubMed] [Google Scholar]
- 54.Padmanabhan A, Li X, Bieberich CJ. Protein kinase A regulates MYC protein through transcriptional and post-translational mechanisms in a catalytic subunit isoform-specific manner. J Biol Chem. 2013;288(20):14158–14169. doi: 10.1074/jbc.M112.432377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beebe SJ, et al. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis—representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990;4(3):465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- 56.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 57.Shuto T, et al. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci USA. 2001;98(15):8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen R, et al. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324(3):1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 60.Yu SH, Chiang WC, Shih HM, Wu KJ. Stimulation of c-Rel transcriptional activity by PKA catalytic subunit beta. J Mol Med (Berl) 2004;82(9):621–628. doi: 10.1007/s00109-004-0559-7. [DOI] [PubMed] [Google Scholar]
- 61.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89(3):413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 62.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1(5):661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 63.Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22(9):1282–1290. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-κB addiction and its role in cancer: ‘One size does not fit all’. Oncogene. 2011;30(14):1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5(4):749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: Comradeship and hostility. Cell Signal. 2008;20(9):1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Joo JH, Jetten AM. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. J Biol Chem. 2008;283(24):16391–16399. doi: 10.1074/jbc.M800945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS ONE. 2009;4(2):e4393. doi: 10.1371/journal.pone.0004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Songyang Z, et al. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4(11):973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 70.Cohen P, et al. Monitoring cellular responses to Listeria monocytogenes with oligonucleotide arrays. J Biol Chem. 2000;275(15):11181–11190. doi: 10.1074/jbc.275.15.11181. [DOI] [PubMed] [Google Scholar]
- 71.Borysiewicz E, Fil D, Dlaboga D, O’Donnell JM, Konat GW. Phosphodiesterase 4B2 gene is an effector of Toll-like receptor signaling in astrocytes. Metab Brain Dis. 2009;24(3):481–491. doi: 10.1007/s11011-009-9150-9. [DOI] [PubMed] [Google Scholar]
- 72.Christiansen SH, Selige J, Dunkern T, Rassov A, Leist M. Combined anti-inflammatory effects of β2-adrenergic agonists and PDE4 inhibitors on astrocytes by upregulation of intracellular cAMP. Neurochem Int. 2011;59(6):837–846. doi: 10.1016/j.neuint.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Saetta M, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165(10):1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 74.Fujimoto K, et al. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25(4):640–646. doi: 10.1183/09031936.05.00047504. [DOI] [PubMed] [Google Scholar]
- 75.Costa C, et al. CXCR3 and CCR5 chemokines in induced sputum from patients with COPD. Chest. 2008;133(1):26–33. doi: 10.1378/chest.07-0393. [DOI] [PubMed] [Google Scholar]
- 76.Hacievliyagil SS, Gunen H, Mutlu LC, Karabulut AB, Temel I. Association between cytokines in induced sputum and severity of chronic obstructive pulmonary disease. Respir Med. 2006;100(5):846–854. doi: 10.1016/j.rmed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 77.Frankenberger M, et al. Chemokine expression by small sputum macrophages in COPD. Mol Med. 2011;17(7-8):762–770. doi: 10.2119/molmed.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michalec L, et al. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168(2):846–852. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 79.Gross NJ. Novel antiinflammatory therapies for COPD. Chest. 2012;142(5):1300–1307. doi: 10.1378/chest.11-2766. [DOI] [PubMed] [Google Scholar]
- 80.King CC, Sastri M, Chang P, Pennypacker J, Taylor SS. The rate of NF-κB nuclear translocation is regulated by PKA and A kinase interacting protein 1. PLoS ONE. 2011;6(4):e18713. doi: 10.1371/journal.pone.0018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neumann M, et al. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995;14(9):1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–5456. [PubMed] [Google Scholar]
- 83.Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem. 2002;269(18):4559–4565. doi: 10.1046/j.1432-1033.2002.03157.x. [DOI] [PubMed] [Google Scholar]
- 84.Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C. Nuclear NF-kappaB p65 phosphorylation at serine 276 by protein kinase A contributes to the malignant phenotype of head and neck cancer. Clin Cancer Res. 2009;15(19):5974–5984. doi: 10.1158/1078-0432.CCR-09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao N, Hibi Y, Cueno M, Asamitsu K, Okamoto T. A-kinase-interacting protein 1 (AKIP1) acts as a molecular determinant of PKA in NF-kappaB signaling. J Biol Chem. 2010;285(36):28097–28104. doi: 10.1074/jbc.M110.116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishinaga H, et al. TGF-beta induces p65 acetylation to enhance bacteria-induced NF-kappaB activation. EMBO J. 2007;26(4):1150–1162. doi: 10.1038/sj.emboj.7601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jono H, et al. Transforming growth factor-beta -Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription. J Biol Chem. 2002;277(47):45547–45557. doi: 10.1074/jbc.M206883200. [DOI] [PubMed] [Google Scholar]
- 88.Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17(15):6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.