Significance
Self-incompatibility (SI) in plants prevents inbreeding by rejection of pollen from closely related individuals of the same species. Unilateral interspecific incompatibility (UI) blocks cross-hybridization between related species, typically when the pollen donor is self-compatible and the pistil parent is self-incompatible. In this study, we show that ui1.1, a pollen UI factor in tomato, encodes an S-locus F-box protein that is homologous to an SI gene that in Petunia determines pollen specificity. We previously showed that another pollen factor, ui6.1, encodes a Cullin1 protein that functions in both UI and SI. Cullin1 and F-box proteins are components of SCF-type (Skp1, Cullin1, F-box) ubiquitin ligase complexes. The results provide further evidence that pollen rejection in UI involves biochemical mechanisms related to SI.
Keywords: interspecific incompatibility, self-incompatibility, Solanum lycopersicum, Solanum pennellii, S-locus F-box protein
Abstract
Unilateral interspecific incompatibility (UI) is a postpollination, prezygotic reproductive barrier that prevents hybridization between related species when the female parent is self-incompatible (SI) and the male parent is self-compatible (SC). In tomato and related Solanum species, two genes, ui1.1 and ui6.1, are required for pollen compatibility on pistils of SI species or hybrids. We previously showed that ui6.1 encodes a Cullin1 (CUL1) protein. Here we report that ui1.1 encodes an S-locus F-box (SLF) protein. The ui1.1 gene was mapped to a 0.43-cM, 43.2-Mbp interval at the S-locus on chromosome 1, but positional cloning was hampered by low recombination frequency. We hypothesized that ui1.1 encodes an SLF protein(s) that interacts with CUL1 and Skp1 proteins to form an SCF-type (Skp1, Cullin1, F-box) ubiquitin E3 ligase complex. We identified 23 SLF genes in the S. pennellii genome, of which 19 were also represented in cultivated tomato (S. lycopersicum). Data from recombination events, expression analysis, and sequence annotation highlighted 11 S. pennellii genes as candidates. Genetic transformations demonstrated that one of these, SpSLF-23, is sufficient for ui1.1 function. A survey of cultivated and wild tomato species identified SLF-23 orthologs in each of the SI species, but not in the SC species S. lycopersicum, S. cheesmaniae, and S. galapagense, pollen of which lacks ui1.1 function. These results demonstrate that pollen compatibility in UI is mediated by protein degradation through the ubiquitin–proteasome pathway, a mechanism related to that which controls pollen recognition in SI.
Self-incompatibility (SI) in Solanum and other Solanaceae is the S-RNase–based, gametophytic type, in which S-specificity is determined by S-RNases in the pistil (1) and S-locus F-box proteins (SLFs) in pollen (2). F-box proteins, together with Skp1 and Cullin1 proteins, are components of SCF-type (Skp1, Cullin1, F-box) ubiquitin E3 ligases that mark target proteins for degradation by the 26S proteasome (3, 4). The ubiquitin–proteasome pathway controls the pollen compatibility phenotype in SI (5). In the “collaborative non–self-recognition” model (6), the S-locus encodes multiple SLF proteins that together recognize different sets of S-RNases. In a compatible pollination, the SLF/S-RNase interaction leads to protection of pollen tubes against cytotoxic S-RNase, whereas in incompatible pollinations, a failure to recognize self–S-RNase results in pollen tube arrest. In addition, modifier genes, such as those encoding HT-B, NaStEP, and 120-kDa proteins in the pistil, and CUL1 and SSK1 proteins in pollen, are required for SI function but do not confer S-specificity (7–11).
Unilateral incompatibility (UI) is a reproductive barrier related to SI in which pollen from one species or population is rejected on pistils of a related species or population, whereas in the reciprocal crosses, no pollen rejection occurs. Pollen of SC species or populations is almost always rejected on pistils of related SI species or populations, whereas in the reciprocal crosses (SC pollinated by SI), pollen rejection rarely occurs. This unidirectional pattern of pollen rejection is referred to as the “SI × SC rule” (12). Although the mechanisms of pollen recognition and rejection by UI are complex (13), several SI factors, including S-RNase, CUL1, and HT, also function in UI (8, 14, 15).
Cultivated and wild tomatoes provide a powerful model system to study the mechanisms of reproductive barriers in the Solanaceae (16). They display wide variation in mating systems, both between and within species (17). Cultivated tomato, S. lycopersicum, is SC and accepts pollen of its SI or SC wild relatives; conversely, pollen of S. lycopersicum is rejected by pistils of the SI species. Three other red- or orange-fruited species, S. cheesmaniae, S. galapagense, and S. pimpinellifolium, are bilaterally cross-compatible with each other and with S. lycopersicum. There are notable exceptions to SI × SC rule in the tomato clade (18). One is that pollen of all of the red/orange-fruited species (SC) are rejected on pistils of the SC green-fruited species. Another exception is that pollen of some SC biotypes of SI species are compatible on pistils of conspecific SI populations. Therefore, pollen rejection is complex and likely involves more than one mechanism (13). The ability to reject tomato pollen is dominant in interspecific F1 hybrids between cultivated tomato and related wild SI species (i.e., SC × SI hybrids), although pollen tube arrest occurs lower in the style of hybrids, suggesting that expression of the pistil side barrier is weakened (19). Allotriploid hybrids comprised of two genomes from S. lycopersicum (SC) and one genome from S. lycopersicoides (SI) reject tomato pollen tubes lower in the style than corresponding diploid hybrids (19).
We previously reported that two pollen factors from S. pennellii, ui1.1 and ui6.1, are required and sufficient to overcome the UI barrier on allotriploid S. lycopersicum × S. lycopersicoides hybrids (19, 20). These two factors are not sufficient for compatibility on diploid S. lycopersicum × S. lycopersicoides hybrids (19). The ui6.1 locus encodes a pollen specific Cullin1 (CUL1) protein (21) that functions in pollen recognition by UI and SI (8). Pollen lacking ui6.1 are incompatible on pistils expressing active S-RNases, but compatible on pistils expressing a mutant S-RNase lacking RNase activity (8). This observation suggested that ui6.1—and by extension, ui1.1—is required for pollen resistance to S-RNase–based rejection in the pistil. Interestingly, neither ui6.1 nor ui1.1 is required for resistance to S-RNase itself, because tomato pollen is fully compatible on pistils expressing active S-RNase in the absence of a functional HT protein (15, 22). Thus, both SI and UI require multiple pollen and pistil factors. The ui1.1 locus is located at or near the S locus region on the short arm of chromosome 1, suggesting it might encode one or more SLF proteins. The goal of the present research was to isolate ui1.1 from S. pennellii to elucidate the nature of pollen rejection by UI and its relationship to SI.
Results
ui1.1 Maps to the S-Locus.
We previously mapped ui1.1 to a relatively broad (16 cM) genetic interval that spans the S-locus region on chromosome 1 of tomato (20). In the present study, we used recombination data to refine the genetic position of ui1.1, assess the intensity of linkage to the S-locus, and evaluate the prospects for map-based cloning. A backcross-type mapping population was developed from a cross between two plants that were homozygous for the S. pennellii allele at ui6.1, one of which was heterozygous at ui1.1 (i.e., ui1.1+/p-ui6.1p/p × ui1.1+/+-ui6.1p/p, where + represents the S. lycopersicum allele and p the S. pennellii allele). This cross ensured that all pollen express functional ui6.1; because both pollen factors are required for a compatible reaction on the allotriploid tester stock, inferring the genotype of ui1.1 is only possible if pollen express ui6.1. The heterozygous ui1.1/+ plant was used as female parent in the cross because recombination frequency is normally higher in female than in male gametes of tomato (19, 23, 24).
A total of 1,632 individuals were genotyped with two CAPS (cleaved amplified polymorphic sequence) markers, TG184 and C2_At5g18590, spanning the S-locus region. A total of 176 recombinants were recovered, representing a recombination frequency of 10.8%. These flanking markers are separated by ∼17 cM on the reference map of tomato, EXPEN-2000 (solgenomics.net), indicating that recombination was suppressed in our mapping population. Eight additional markers [seven CAPS and one SSR (simple sequence repeat)] situated between TG184 and C2_At5g18590 were used to further genotype the recombinants (Fig. 1B and SI Appendix, Table S1). The markers were also anchored to the tomato reference genome sequence: Tomato WGS Chromosomes version SL2.31 (Fig. 1C). The genetic distance between C2_At1g26940 and C2_At5g18590 was 2.26 cM, which is less than one quarter of the distance on the tomato reference map (Fig. 1A).
Fig. 1.
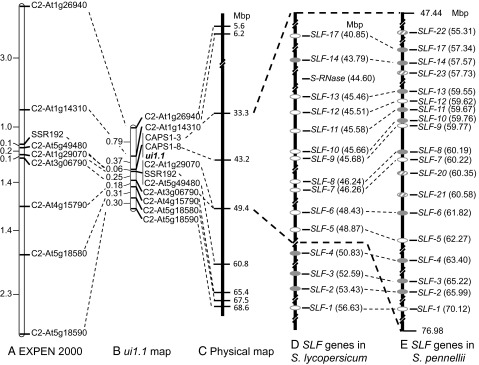
Genetic and physical maps of the ui1.1 region, showing positions of SLF genes in S. lycopersicum and S. pennellii. (A) Genetic map of the S-locus region on chromosome 1, from the EXPEN 2000 interspecific mapping population. (B) Genetic map of the ui1.1 region from a BC mapping population of 1,632 individuals. Recombination suppression reduced the genetic distance of this region to less than 25% of the reference map. (C) Physical map of markers based on the Tomato WGS Chromosomes (ver. SL2.31). (D) Physical map of SLF genes identified in the Tomato WGS Chromosomes. Note: 19 SLF genes were found this region, of which 4 were ruled out as ui1.1 candidates based on the positions of flanking markers. Open ovals, genes with likely loss-of-function mutations; shaded ovals, putative functional genes. (E) SLF genes identified in the S. pennellii genome. Hatched ovals, four S. pennellii SLF genes not present in S. lycopersicum.
Thirty-two recombinants with cross-overs between markers C2_At1g26940 and C2_At5g18580 were tested for pollen compatibility on pistils of the allotriploid tester stock, GH266 (SI Appendix, Table S4). The results indicated that ui1.1 is located between markers C2_At1g14310 and C2_At1g29070, a genetic distance of 0.43 cM on the genetic map and 43.2 Mbp on the physical map (Fig. 1 B and C). The ratio of physical to genetic distance in the ui1.1 region was thus ∼100 Mb/cM, which is >100× higher than the genome-wide average of 750 kb/cM in tomato (25).
In an attempt to refine the map position of ui1.1, we developed additional CAPS markers using publically available genome sequences from the region. Two CAPS markers, CAPS1-3 and CAPS1-8, separated by 10 Mb, cosegregated perfectly with ui1.1 (Fig. 1 B and C). An S-RNase pseudogene was found at position 44.6 Mb in the S. lycopersicum genome, confirming that ui1.1 cosegregates with the S-locus. Because the prospects for map-based cloning of ui1.1 were poor, given the low recombination frequency in this region, we instead pursued a candidate gene approach.
The ui1.1 Region in S. pennellii Includes 23 SLF Genes.
The release of the tomato reference genome sequence (26) provided the opportunity to identify candidate genes underlying ui1.1. We hypothesized that one or more pollen-expressed SLF genes are responsible for ui1.1 function, for the following reasons. First, we showed that ui6.1 encodes CUL1, and both CUL1 and SLF proteins are components of SCF-type ubiquitin E3 ligase complexes. Second, our genetic data established that both ui1.1 and ui6.1 are required to overcome the pistil UI barrier in SC × SI hybrids, which is consistent with the biochemical model in which each gene encodes components of the SCF complex. Third, we showed that CUL1 is required for pollen-side SI function only if pistils express active S-RNase (8). Finally, the pollen determinants of SI specificity in the Solanaceae and related families with the S-RNase–based gametophytic self-incompatibility system are SLF proteins (2, 6).
To test this prediction, we chose a Petunia F-box protein associated with the S1-haplotype (GenBank no. AAS79484) as a query to search the partially released tomato scaffold sequence database and then the fully released Tomato WGS Chromosomes (version SL2.31) (solgenomics.net) using the sequence alignment program tBLASTn. We identified 19 SLF genes (SlSLF-1 to -19) in the S. lycopersicum genome that mapped to the S-locus region on chromosome 1 (SI Appendix). We also searched the S. pennellii accession LA0716 genome sequence (27) using the same Petunia SLF protein to find related proteins. Four additional SLF paralogs (SpSLF-20 to -23) were identified in the S. pennellii genome that were absent from tomato (SI Appendix). All of the genes found in S. lycopersicum were represented by orthologs in S. pennellii. The SLF genes were anchored to the corresponding positions on the Tomato WGS Chromosomes (ver. SL2.31) and the S. pennellii genome sequence (Fig. 1 C and D and SI Appendix, Table S5). Eight loci (SLF-1, -2, -3, -4, -15, 16, -18, and -19) were ruled out for further analysis based on their physical positions on the tomato WGS of chromosome 1 relative to markers C2_At1g14310 and C2_At1g29070 flanking ui1.1. We later discovered a difference in the position of marker C2_At1g29070 in the S. lycopersicum (49.4 Mb) vs. the S. pennellii (76.98 Mb) genome sequences (Fig. 1 D and E); if the S. pennellii sequence is correct, then SpSLF-1, -2, -3, and -4 are located within the marker-delimited ui1.1 region and should not have been ruled out. We identified an S-RNase pseudogene in the S. lycopersicum genome (Fig. 1D and SI Appendix) at position 44.6 Mb. No sequences showing homology to S-RNases were found in the S. pennellii genome, suggesting the SI to SC mutation in accession LA0716 was caused by a deletion of the S-RNase gene.
The Expression of Candidate SLF Genes Is Pollen Specific.
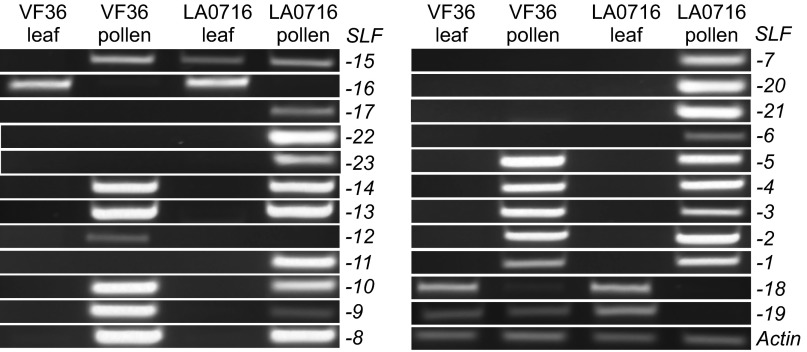
The expression patterns of the 23 ui1.1-linked SLF genes were evaluated in leaves and pollen of tomato cultivar VF36 and S. pennellii LA0716 by RT-PCR (Fig. 2). With the exception of SLF-15, -16, -18, and -19, which are located outside the flanking markers, all genes were abundantly expressed in pollen but not in leaves. Nine SLF paralogs (SLF-1, -2, -3, -4, -5, -8, -10, -13, and -14) were expressed at roughly equal levels in pollen of S. lycopersicum and S. pennellii. Eight genes (SpSLF-6, -7, -11, -17, -20, -21, -22, and -23) were expressed only in pollen of S. pennellii. Four of these, SpSLF-20 to -23, are not represented by orthologs in the S. lycopersicum genome sequence, so no expression in this species was expected. Two paralogs, SLF-9 and -12, were more highly expressed in pollen of S. lycopersicum than in pollen of S. pennellii.
Fig. 2.
Analysis of SLF mRNA levels by RT-PCR. Leaves and pollen of S. lycopersicum cv. VF36 and S. pennellii LA0716 were compared for expression levels of SLF genes. Pollen-specific expression was observed for all genes except four located outside the ui1.1 region. The constitutively expressed Actin gene is included as a control.
F-Box Gene Sequences Are Highly Conserved Across Species.
We annotated the pollen-expressed SLF genes (15 from the S. lycopersicum genome and 19 from S. pennellii) using Genescan gene structure prediction software or manually by querying the NCBI (National Center for Biotechnology Information) protein database. The sequences of 11 SlSLF genes showed evidence of loss of function mutations, whereas the remaining four appeared functional (SI Appendix, Table S5). Five SpSLF genes contained mutations, whereas the other 14 appeared functional. The five SLF loci with mutations in S. pennellii also exhibited mutations in the corresponding S. lycopersicum orthologs.
We studied the sequence divergence between each SLF ortholog in S. pennellii and S. lycopersicum and among the different SLF paralogs within each species. We performed allele sequence alignment between the 10 putative SpSLF genes and the corresponding SlSLF genes, regardless of whether they were functional or mutated in the tomato genome. The four SpSLF genes absent from S. lycopersicum were excluded from this analysis. The results showed that each SLF ortholog is highly conserved between S. lycopersicum and S. pennellii (SI Appendix). Sequence identity ranged from 95% to 99%, despite mutations in six of the SlSLF genes. We also conducted multiple sequence alignment among the 14 putative SpSLF paralogs using Clustalw2. The results indicated that the similarity between paralogs is low, with the percentage of sequence identity ranging from 50.1% to 71.1% at the nucleotide level and from 25.5% to 51.4% at the amino acid level (SI Appendix, Tables S6 and S7). Except for a relatively conserved F-box domain, no other conserved region was found among the SLF proteins (SI Appendix).
SpSLF-23 Is Sufficient for ui1.1 Function.
Of the 23 SpSLF genes identified in S. pennellii, 8 of them were ruled out based on their map location (see above), and 4 (SpSLF-5, -7, -9, and -12) were excluded based on the sequence annotation that showed mutations. The remaining 11 SpSLF genes were used for genetic transformations to test for ui1.1 function. Each gene was introduced by Agrobacterium-mediated transformation into S. lycopersicum IL 6–1, a line homozygous for an S. pennellii introgression which provides functional ui6.1. We obtained two to eight independent transformants for each SpSLF paralog (SI Appendix, Table S8).
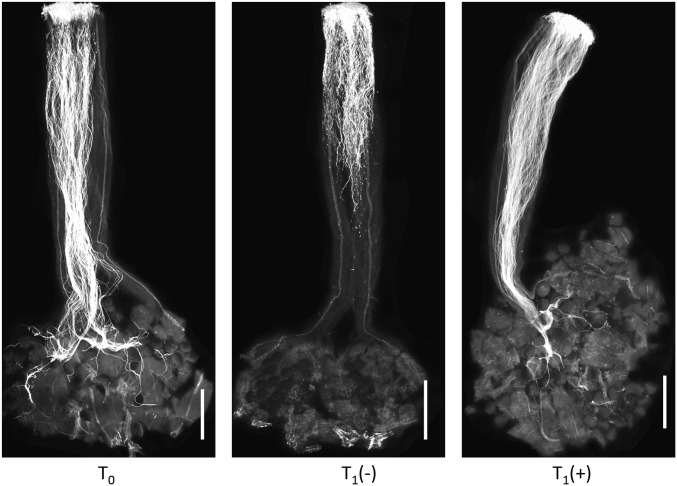
Expression of the transgenes was evaluated by RT-PCR, either in the T0 or T1 generation. Two T0 plants or two T1 progeny arrays representing each SpSLF paralog (one for SpSLF-6) were tested. All but one of the SpSLF transgenes were expressed in anthers of T0 and/or T1 plants; one plant transformed with SpSLF-10 did not show detectable expression (SI Appendix, Fig. S1). The pollen compatibility phenotypes of T0 plants were evaluated on pistils of two independent allotriploid tester lines, GH266 and 10L2411, derived from different accessions of S. lycopersicoides (LA1964 and LA2951, respectively) and containing different S-RNase genes (SI Appendix). Of 11 transformations tested (52 plants total), only transgenic plants expressing SpSLF-23 showed a compatible pollen phenotype (Fig. 3 and SI Appendix, Table S8). All four tested SpSLF-23 transformants were compatible on both allotriploid testers. Transformants for all of the other SpSLF genes elicited only incompatible pollen reactions on the allotriploid tester lines. We further tested for cosegregation between the SpSLF-23 transgene and pollen compatibility phenotypes in the T1 generation. A T1 family derived by self-pollination of a randomly chosen T0 plant segregated 18 transgenic:5 nontransgenic individuals, consistent with the 3:1 ratio expected for a single transgene insertion in the T0 parent [χ2 (3:1) = 0.13ns]. We then tested phenotypes of these T1 plants on pistils of allotriploid tester GH266: all of the transgenic progeny were compatible, and all of the nontransgenic plants were incompatible (Fig. 3). We did not distinguish T1 plants hemizygous or homozygous for the T-DNA; however, both were expected to give an overall compatible reaction on the tester lines because at least 50% of the pollen would be transgenic in either case. These results show that expression of SpSLF-23 in pollen is sufficient to confer ui1.1 function.
Fig. 3.
Compatibility phenotypes of SpSLF-23 transgenic plants on pistils of an allotriploid tester line. T0 transgenic plants showed a compatible phenotype on pistils of allotriploid tester lines, demonstrating that SpSLF-23 is sufficient to confer ui1.1 function in pollen. The compatible phenotype cosegregated with the transgene in the T1 generation. T0, a representative T0 transgenic plant; T1(−), a T1 plant lacking the transgene; T1(+), a T1 plant with the transgene. (Scale bar: 1 mm.) (Full data are in SI Appendix, Table S8.)
SLF-23 Orthologs in Petunia and Other Tomato Species.
We searched the NCBI database using the DNA sequence of SpSLF-23 to find the most closely related SLF genes from petunia. The type-2 SLF genes of petunia showed the highest sequence similarity with the SpSLF-23 gene. We downloaded the DNA and protein sequences of six type-2 SLF genes of petunia and conducted sequence alignment among them. Nucleotide sequence identity between SpSLF-23 and petunia type-2 SLF genes ranged from 77.28% to 89.92%, and the amino acid sequence identity was 64.4–87.5%, suggesting that SpSLF-23 is a type-2 SLF gene (SI Appendix, Tables S9 and S10).
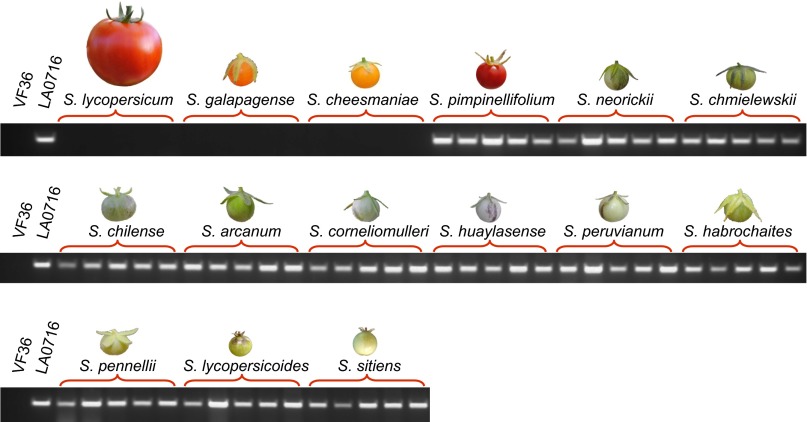
No ortholog of SpSLF-23 was found in the Tomato WGS Chromosomes (ver. SL2.50). PCR tests with other S. lycopersicum genotypes, including IL 6–1 and cv. VF36, also failed to detect this gene. We examined whether SLF-23 orthologs are represented in the genomes of other wild tomato relatives. Five randomly chosen accessions from 15 related Solanum species were genotyped for the SLF-23 gene by genomic PCR with ortholog-specific primers (Fig. 4). Our results show that in addition to S. lycopersicum, this gene is also absent from the genomes of the two yellow- or orange-fruited SC species, S. galapagense and S. cheesmaniae. Several primer pairs were tested, and no amplification was ever observed in these three species. Conversely, an SLF-23 ortholog could be amplified from the closely related red-fruited SC species S. pimpinellifolium, as well as from each of the green-fruited species, which include SC and SI taxa (SI Appendix, Table S11).
Fig. 4.
Survey of cultivated and wild tomato species for the presence or absence of SLF-23 orthologs. Five accessions from each species were tested for the presence of SLF-23 using gene-specific primers (SI Appendix, Table S2). The SC red- or orange-fruited species S. lycopersicum, S. galapagense, and S. cheesmaniae lack SLF-23 orthologs.
Discussion
An S-Locus F-Box Protein Is Sufficient for ui1.1 Function in Pollen.
The major finding of this research is that a pollen-expressed SLF protein underlies ui1.1 function in Solanum. Our results demonstrate that a single SLF transgene, SpSLF-23, is sufficient, in the presence of functional CUL1, to convert the pollen phenotype on allotriploid tester lines from incompatible to compatible. The absence of SLF-23 from the genome of S. lycopersicum explains why pollen of this species are incompatible in assays for ui1.1 function. SLF proteins interact with Skp1 and CUL1 proteins to form SCF ubiquitin E3 ligase complexes and regulate SI by recognition of specific S-RNase forms (5, 10). Thus, the biochemical and genetic data support a model in which pollen rejection by UI, like SI, is controlled by the ubiquitin/proteasome pathway.
Two wild relatives of cultivated tomato, S. cheesmaniae and S. galapagense, both SC, also lack an SLF-23 ortholog, and their pollen is also rejected on pistils of SI species (16, 18). These taxa also lack functional ui6.1 (21). The genome of S. pimpinellifolium does contain an SLF-23 ortholog, yet pollen of this species are nonetheless rejected on pistils of the SI species and the allotriploid tester line. However, because S. pimpinellifolium also lacks functional ui6.1 (21), we cannot draw conclusions about the functionality of ui1.1 or SLF-23 in this species. All of the green-fruited species, which are mostly SI, contain an ortholog of SLF-23. Thus, the presence/absence of this gene correlates relatively well with the SI × SC rule in tomato.
Role of SLF-23 in UI and SI.
Our results show that SLF-23 functions in pollen recognition and rejection during UI in Solanum. SpSLF-23 shows a high degree of amino acid sequence similarity to the Petunia type-2 SLF protein, which has been shown to function in SI by recognizing S9-, S11-, and S19-RNases (6). We have no evidence that SLF-23 is specialized for interspecific pollen rejection: its sequence similarity to type-2 SLF in Petunia suggests it could also function in SI (i.e., in an SI ancestor of SC S. pennellii LA0716), as we previously demonstrated for UI factor CUL1. We show that SpSLF-23 confers pollen resistance to S-RNases in two independent allotriploid tester lines containing different S-RNase genes. The role of SLF-23 in SI is being tested by silencing this gene in S. pennellii to determine whether this affects pollen transmission on SI accessions of this species, as was the case for CUL1 (8).
Role of Other SLF Proteins in S. lycopersicum.
We found at least 23 SLF genes in the genome of S. pennellii and 19 in S. lycopersicum, most of which are pollen specific in their expression. In Petunia inflata, the same set of 17 SLF genes is expressed in pollen of two different S-haplotypes, suggesting that pollen specificity is determined by this combination of genes in each haplotype (28). In the collaborative non–self-recognition model (6), each SLF gene encodes a unique protein capable of recognizing different S-RNase forms. Of the 23 genes identified in S. pennellii by sequence homology, all but 4 are expressed in pollen (the others are located outside the S-locus region). Thus, the number of expressed genes in P. inflata and S. pennellii is similar, consistent with the conservation of S-RNase alleles (shared ancestral polymorhpisms) in divergent Solanaceae species (29).
Another question raised by this study is whether proteins encoded by one of the four nonmutated pollen-expressed SLF genes in S. lycopersicum (SlSLF-2,- 3, -4, or -14) are capable of protecting pollen from specific S-RNases. Diploid F1 hybrids of S. lycopersicum with SI species such as S. peruvianum, S. chilense, S. pennellii, and S. lycopersicoides are self-sterile (30–33). In each of these studies, two to several independent F1 hybrids, presumably with different S-haplotypes, were examined, and none were SC. This trend suggests that the S-haplotype of S. lycopersicum (herein Sc) does not encode active SLF proteins that interact with functional S-RNases from the SI parents. On the pistil side, S. lycopersicum is deficient in S-RNase expression (13) and thus cannot reject pollen with the Sc haplotype. Therefore, if any of the SLF genes expressed in S. lycopersicum pollen (SlSLF-2, -3, -4, and -14) were functional, then at least some F1 interspecific hybrids with SI species are predicted to be partially SC, but this has not been reported. The collaborative non–self-recognition model (6, 34) predicts that each functional SLF protein recognizes one to two different S-RNase alleles; therefore, if the four genes in S. lycopersicum are functional, the expectation is that they should be compatible on four to eight S-haplotypes. However, only 25% of the pollen from the interspecific F1 hybrids would contain both a functional CUL1 gene (from the SI parent) and the Sc allele, including SlSLF genes, from S. lycopersicum. Any other essential pollen SI factors that are nonfunctional in S. lycopersicum would further reduce the expected proportion of self-compatible pollen from these interspecific hybrids. Segregation for multiple pollen loci can be ruled out in allotetraploid SC × SI hybrids, because 100% of diploid pollen should express all necessary pollen factors from the SI parent. Allotetraploid S. lycopersicum × S. chilense and S. lycopersicum × S. lycopersicoides hybrids are also self-sterile (35, 36). These observations imply that the S-haplotype of S. lycopersicum is incapable of overcoming SI through the competitive interaction mechanism in heteroallelic pollen. Thus, the apparent dominance of SI in interspecific SC × SI hybrids suggests the SLF genes expressed in S. lycopersicum pollen are not sufficient to overcome pollen rejection by SI.
SI to SC Transition in S. pennellii LA0716.
We did not detect an S-RNase gene in the genome sequence of S. pennellii LA0716, suggesting this gene was lost, perhaps through a deletion or unequal crossing over event. Previous studies have shown that this SC accession lacks S-RNase mRNA, protein, and activity in the pistil (13, 37). Nonetheless, LA0716 is capable of rejecting pollen of red-fruited species such as S. lycopersicum. Also, a QTL contributing to the strength of this pollen rejection response maps to the same genetic region as the HT-A and HT-B genes, suggesting these genes in LA0716 are probably functional (13). Pollen of LA0716 is fully compatible on pistils of SI accessions of S. pennellii, indicating full pollen function has been retained. These observations suggest that the SI to SC transition in LA0716 was caused by a loss of the S-RNase gene. This loss is likely a recent mutational event, because no other pollen or pistil SC mutations have been detected in this accession.
Kondo et al. (22) failed to amplify S-RNase genes from any of the red-fruited species, which suggested they might be absent from these species. However, as we and others (34) report, the S. lycopersicum genome does contain an S-RNase pseudogene. This gene is predicted to be nonfunctional based on sequence analysis, consistent with the lack of S-RNase activity in styles of S. lycopersicum and the other red-fruited species (13, 22). Thus, SI to SC transitions in both S. pennellii LA0716 and in S. lycopersicum were accompanied or caused by loss of S-RNase expression, whereas loss of the pollen factors ui1.1 and ui6.1 occurred in S. lycopersicum (and other red/orange-fruited species) but not S. pennellii. These observations suggest that mutations in pollen SI factors are relatively late events, preceded in these cases by one or more pistil mutations, and reinforce, rather than initiate, the breakdown of SI.
Materials and Methods
Details about materials and methods are provided in SI Appendix, SI Materials and Methods.
Plant Materials.
To map the ui1.1 locus, we used a backcross-type population derived from S. pennellii LA0716 in which the heterozygote was the female parent (for higher recombination frequency) and in which all progeny expressed functional ui6.1 (required for phenotyping ui1.1). Cultivar VF36 (LA0490) and S. pennellii LA0716 were used for SLF gene expression analysis. Candidate ui1.1 genes were isolated from genomic DNA of LA0716. Tomato introgression line IL 6–1 (LA3500) was used for genetic transformations. Five accessions from cultivated tomato and from each related wild species were surveyed for the presence of SLF-23 orthologs.
DNA Isolation and Genotyping.
DNA was extracted from the mapping population in 96-well microtiter plates as previously described (19). Marker sequences and restriction enzymes used for revealing polymorphism are listed in SI Appendix, Table S1.
Gene Identification, Annotation, and Sequence Alignment.
The tomato whole genome sequence (26) and the S. pennellii genome sequence (27) were searched for SLF coding genes. Candidate genes were annotated using GENSCAN (genes.mit.edu/GENSCAN.html) or manually annotated. Sequence alignments were performed on the Solgenomics website (solgenomics.net). Multiple sequences were aligned using ClustalW2 (www.ebi.ac.uk/Tools/msa/clustalw2/).
Gene Expression Analysis.
Expression of candidate SLF genes and transgenes was assayed by semiquantitative RT-PCR using gene-specific primers and restriction endonucleases, as needed, to distinguish endogenous genes from transgene products (SI Appendix, Table S2).
Gene Isolation and Plant Transformations.
Candidate SLF genes were isolated from genomic DNA by long-distance PCR with Phusion high fidelity DNA polymerase (New England Biolabs) and cloned into a modified pCAMBIA1300 vector. Transgene constructs were inserted by Agrobacterium-mediated transformation into tomato line IL 6–1, which expresses functional ui6.1.
Sequencing S-RNase Genes.
The S-RNase gene sequences of two allotriploid testers (GH266 and 10L2411) were obtained by 3′ rapid amplification of cDNA ends (RACE) as previously described (38).
Supplementary Material
Acknowledgments
We thank the C. M. Rick Tomato Genetics Resource Center staff for supplying seed stocks and Marcus Tamura for preparing style images. Anthony Bolger and Alisdair Fernie kindly provided early access to a draft S. pennellii LA0716 genome sequence database. Bruce McClure and Pat Bedinger provided insightful comments on the manuscript. The project was supported by National Science Foundation Grant MCB 1127059.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423301112/-/DCSupplemental.
References
- 1.McClure BA, et al. Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature. 1989;342(6252):955–957. doi: 10.1038/342955a0. [DOI] [PubMed] [Google Scholar]
- 2.Sijacic P, et al. Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature. 2004;429(6989):302–305. doi: 10.1038/nature02523. [DOI] [PubMed] [Google Scholar]
- 3.Zheng N, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416(6882):703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 4.Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. Plant Cell. 2004;16(12):3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhao Z, Xue Y. Roles of proteolysis in plant self-incompatibility. Annu Rev Plant Biol. 2009;60:21–42. doi: 10.1146/annurev.arplant.043008.092108. [DOI] [PubMed] [Google Scholar]
- 6.Kubo K, et al. Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science. 2010;330(6005):796–799. doi: 10.1126/science.1195243. [DOI] [PubMed] [Google Scholar]
- 7.Hancock CN, Kent L, McClure BA. The stylar 120 kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. Plant J. 2005;43(5):716–723. doi: 10.1111/j.1365-313X.2005.02490.x. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Chetelat RT. The role of a pollen-expressed Cullin1 protein in gametophytic self-incompatibility in Solanum. Genetics. 2014;196(2):439–442. doi: 10.1534/genetics.113.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClure B, Mou B, Canevascini S, Bernatzky R. A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc Natl Acad Sci USA. 1999;96(23):13548–13553. doi: 10.1073/pnas.96.23.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, et al. The Skp1-like protein SSK1 is required for cross-pollen compatibility in S-RNase-based self-incompatibility. Plant J. 2010;62(1):52–63. doi: 10.1111/j.1365-313X.2010.04123.x. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Durán K, et al. NaStEP: A proteinase inhibitor essential to self-incompatibility and a positive regulator of HT-B stability in Nicotiana alata pollen tubes. Plant Physiol. 2013;161(1):97–107. doi: 10.1104/pp.112.198440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis D, Crowe LK. Unilateral interspecific incompatibility in flowering plants. Heredity. 1958;12(2):233–256. [Google Scholar]
- 13.Covey PA, et al. Multiple features that distinguish unilateral incongruity and self-incompatibility in the tomato clade. Plant J. 2010;64(3):367–378. doi: 10.1111/j.1365-313X.2010.04340.x. [DOI] [PubMed] [Google Scholar]
- 14.Murfett J, et al. S RNase and interspecific pollen rejection in the genus Nicotiana: Multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell. 1996;8(6):943–958. doi: 10.1105/tpc.8.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tovar-Méndez A, et al. Restoring pistil-side self-incompatibility factors recapitulates an interspecific reproductive barrier between tomato species. Plant J. 2014;77(5):727–736. doi: 10.1111/tpj.12424. [DOI] [PubMed] [Google Scholar]
- 16.Bedinger PA, et al. Interspecific reproductive barriers in the tomato clade: Opportunities to decipher mechanisms of reproductive isolation. Sex Plant Reprod. 2011;24(3):171–187. doi: 10.1007/s00497-010-0155-7. [DOI] [PubMed] [Google Scholar]
- 17.Rick CM. Evolution of mating systems in cultivated plants. In: Gottlieb LD, Jain SK, editors. Plant Evolutionary Biology. Chapman and Hall; London: 1988. pp. 133–147. [Google Scholar]
- 18.Baek YS, et al. Testing the ‘SI x SC rule’: Pollen-pistil interactions in interspecific crosses between members of the tomato clade (Solanum section Lycopersicon, Solanaceae) Am J Bot. 2014;102(2):1–10. doi: 10.3732/ajb.1400484. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Royer S, Chetelat RT. Fine mapping of ui6.1, a gametophytic factor controlling pollen-side unilateral incompatibility in interspecific solanum hybrids. Genetics. 2010;185(3):1069–1080. doi: 10.1534/genetics.110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chetelat RT, Deverna JW. Expression of unilateral incompatibility in pollen of Lycopersicon pennellii is determined by major loci on chromosomes 1, 6 and 10. Theor Appl Genet. 1991;82(6):704–712. doi: 10.1007/BF00227314. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Chetelat RT. A pollen factor linking inter- and intraspecific pollen rejection in tomato. Science. 2010;330(6012):1827–1830. doi: 10.1126/science.1197908. [DOI] [PubMed] [Google Scholar]
- 22.Kondo K, et al. Insights into the evolution of self-compatibility in Lycopersicon from a study of stylar factors. Plant J. 2002;30(2):143–153. doi: 10.1046/j.1365-313x.2002.01275.x. [DOI] [PubMed] [Google Scholar]
- 23.de Vicente MC, Tanksley SD. Genome-wide reduction in recombination of backcross progeny derived from male versus female gametes in an interspecific cross of tomato. Theor Appl Genet. 1991;83(2):173–178. doi: 10.1007/BF00226248. [DOI] [PubMed] [Google Scholar]
- 24.van Ooijen JW, et al. An RFLP linkage map of Lycopersicon peruvianum. Theor Appl Genet. 1994;89(7-8):1007–1013. doi: 10.1007/BF00224531. [DOI] [PubMed] [Google Scholar]
- 25.Tanksley SD, et al. High density molecular linkage maps of the tomato and potato genomes. Genetics. 1992;132(4):1141–1160. doi: 10.1093/genetics/132.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomato Genome Consortium The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485(7400):635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolger A, et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet. 2014;46(9):1034–1038. doi: 10.1038/ng.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JS, Der JP, dePamphilis CW, Kao TH. Transcriptome analysis reveals the same 17 S-locus F-box genes in two haplotypes of the self-incompatibility locus of Petunia inflata. Plant Cell. 2014;26(7):2873–2888. doi: 10.1105/tpc.114.126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igic B, Bohs L, Kohn JR. Ancient polymorphism reveals unidirectional breeding system shifts. Proc Natl Acad Sci USA. 2006;103(5):1359–1363. doi: 10.1073/pnas.0506283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chetelat RT, Cisneros P, Stamova L, Rick CM. A male-fertile Lycopersicon esculentum x Solanum lycopersicoides hybrid enables direct backcrossing to tomato at the diploid level. Euphytica. 1997;95(1):99–108. [Google Scholar]
- 31.Hardon JJ. Unilateral incompatibility between Solanum pennellii and Lycopersicon esculentum. Genetics. 1967;57(4):795–808. doi: 10.1093/genetics/57.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin FW. The inheritance of self-incompatibility in hybrids of Lycopersicon esculentum Mill. x L. chilense Dun. Genetics. 1961;46(11):1443–1454. doi: 10.1093/genetics/46.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuire DC, Rick CM. Self-incompatibility in species of Lycopersicon Sect. Eriopersicon and hybrids with L. esculentum. Hilgardia. 1954;23(4):101–123. [Google Scholar]
- 34.Kubo K, et al. Gene duplication and genetic exchange drive the evolution of S-RNase-based self-incompatibility in Petunia. Nature Plants. 2015;1(1):1–9. doi: 10.1038/nplants.2014.5. [DOI] [PubMed] [Google Scholar]
- 35.Martin FW. 1960. The genetic determination and action of self-incompatibility in F1, F2 and backcross hybrids of Lycopersicon esculentum x L. chilense. PhD thesis (University of California, Davis, CA)
- 36.Rick CM, De Verna JW, Chetelat RT, Stevens MA. Meiosis in sesquidiploid hybrids of Lycopersicon esculentum and Solanum lycopersicoides. Proc Natl Acad Sci USA. 1986;83(11):3580–3583. doi: 10.1073/pnas.83.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalivendra SC, et al. Developmental onset of reproductive barriers and associated proteome changes in stigma/styles of Solanum pennellii. J Exp Bot. 2013;64(1):265–279. doi: 10.1093/jxb/ers324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igic B, Smith WA, Robertson KA, Schaal BA, Kohn JR. Studies of self-incompatibility in wild tomatoes: I. S-allele diversity in Solanum chilense (Dun.) Reiche [corrected] (Solanaceae) Heredity (Edinb) 2007;99(5):553–561. doi: 10.1038/sj.hdy.6801035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.