Significance
Increasing evidence indicates that Mars might have been habitable early in its history, and that it might harbor liquid water at present in the form of brines associated with features known as recurrent slope lineae (RSL). However, even if brines do occur, it is unclear what substrates any relict microbes could metabolize. Results presented here show that carbon monoxide, which is abundant in Mars’ atmosphere, could be used at local scales under conditions that occur at RSL, including moderate temperatures, low pressure, high CO2, low oxygen concentrations, and extreme water potentials. Halophilic CO-oxidizing Proteobacteria, and recently discovered extremely halophilic CO-oxidizing Euryarchaeota described in this study, represent ideal models for understanding the capacity of Mars’ atmosphere to support microbial communities.
Keywords: extreme halophiles, Euryarchaeota, carbon monoxide, Mars regolith
Abstract
Carbon monoxide occurs at relatively high concentrations (≥800 parts per million) in Mars’ atmosphere, where it represents a potentially significant energy source that could fuel metabolism by a localized putative surface or near-surface microbiota. However, the plausibility of CO oxidation under conditions relevant for Mars in its past or at present has not been evaluated. Results from diverse terrestrial brines and saline soils provide the first documentation, to our knowledge, of active CO uptake at water potentials (−41 MPa to −117 MPa) that might occur in putative brines at recurrent slope lineae (RSL) on Mars. Results from two extremely halophilic isolates complement the field observations. Halorubrum str. BV1, isolated from the Bonneville Salt Flats, Utah (to our knowledge, the first documented extremely halophilic CO-oxidizing member of the Euryarchaeota), consumed CO in a salt-saturated medium with a water potential of −39.6 MPa; activity was reduced by only 28% relative to activity at its optimum water potential of −11 MPa. A proteobacterial isolate from hypersaline Mono Lake, California, Alkalilimnicola ehrlichii MLHE-1, also oxidized CO at low water potentials (−19 MPa), at temperatures within ranges reported for RSL, and under oxic, suboxic (0.2% oxygen), and anoxic conditions (oxygen-free with nitrate). MLHE-1 was unaffected by magnesium perchlorate or low atmospheric pressure (10 mbar). These results collectively establish the potential for microbial CO oxidation under conditions that might obtain at local scales (e.g., RSL) on contemporary Mars and at larger spatial scales earlier in Mars’ history.
The search for past or extant microbial life remains a focal point for astrobiological research on Mars and elsewhere (1–8). Orbiting and rover-based explorations have emphasized historical and contemporary distributions of liquid water as central elements of theories about when, where, and under what conditions life might have existed or continues to exist on Mars. Geological, geochemical, and geomorphological observations have provided definitive evidence for large, ancient fluvial systems that might have been conducive to life (8–13), but the timing, temperature, and composition of surface water have been controversial (14–17). Although unequivocal evidence for contemporary liquid water reservoirs has not yet been obtained, experimental evidence suggests plausible conditions under which brines might form (18), and observations from the Mars Reconnaissance Orbiter suggest that recurring slope lineae (RSL) at latitudes between 32°S and 48°S might develop in association with seasonally moderate conditions during late spring-summer (19–21). McEwen et al. (19) have proposed that these features can be best explained by near-surface liquid water brines that form between temperatures of about −10 °C and 25 °C during late spring-summer.
However, even if liquid water was at one time or is now sufficient to support microbial life, energy sources that could sustain metabolism in near-surface regolith have not been identified. Although the 1976 Viking lander results appeared to exclude surface organic matter (e.g., 22, 23), recent evidence suggests that low organic matter levels might indeed occur in some deposits (e.g., 12). Even so, it is uncertain whether this material exists in a form or concentrations suitable for microbial use.
The Martian atmosphere has largely been ignored as a potential energy source, because it is dominated by CO2 (24, 25). Ironically, UV photolysis of CO2 forms carbon monoxide (CO), a potential bacterial substrate that occurs at relatively high concentrations: about 800 ppm on average, with significantly higher levels for some sites and times (26, 27). In addition, molecular oxygen (O2), which can serve as a biological CO oxidant, occurs at about 1,450 ppm (25). In contrast, Earth’s atmosphere contains only about 0.3 ppm CO, a comparatively low concentration that nevertheless supports the activity of numerous soil microbes (28).
One of the few studies that have considered CO as an energy source for putative Mars microbes used estimates of atmospheric CO concentrations and production rates to model uptake by a hypothetical global regolith sink (27). Based on their results, Weiss et al. (27) argued that CO uptake, if it occurs, must take place at very low rates, and that the atmosphere is a marginal energy source. Although this might be true at a global scale, substantial activity could occur at more local scales, such as those of RSL. However, CO utilization has not been previously documented for terrestrial brines or soils experiencing water potential extremes that would characterize putative RSL brines, nor has CO oxidation been demonstrated for extreme halophiles, which have been posited as models for life on Mars (29–32).
Results presented here establish the plausibility of atmospheric CO as an energy source for hypothetical RSL brine microbes. We observed that intact saline soil cores with surface water potentials of about −41 MPa consumed atmospheric CO, and that surface soil (0–2 cm) from this site oxidized exogenous CO, as did saline soils, sediments, and crusts from three other systems with water potentials consistent with brines that might exist on Mars (33, 34). These systems included salinized volcanic ash and sand from Hawai’i Island (water potentials from about −29 to −117 MPa), mixed gypsum and halite deposits from solar salterns in the Atacama Desert, Chile (water potentials of about −8 to −12 MPa), and salt-encrusted sediments from the Bonneville Salt Flats, Utah (BSF; water potentials of about −42 MPa).
We also observed CO uptake using a medium with 5.3 M NaCl and a water potential of −39 MPa with Halorubrum str. BV1 (from BSF), the first extremely halophilic euryarchaeote isolated, to our knowledge, as a CO oxidizer. In addition, we documented CO uptake by two other extremely halophilic euryarchaeotes that possess form I CO dehydrogenase genes: Natronorubrum bangense and N. sulfidifaciens. Finally, we demonstrated that a gammaproteobacterial halophile, Alkalilimnicola ehrlichii MLHE-1, oxidized CO at −19 MPa, at atmospheric pressures (10 mbar) similar to those on Mars, and under suboxic and anoxic conditions with nitrate present. A. ehrlichii MLHE-1 also tolerated perchlorate concentrations comparable to those that occur in Mars’ regolith.
Results and Discussion
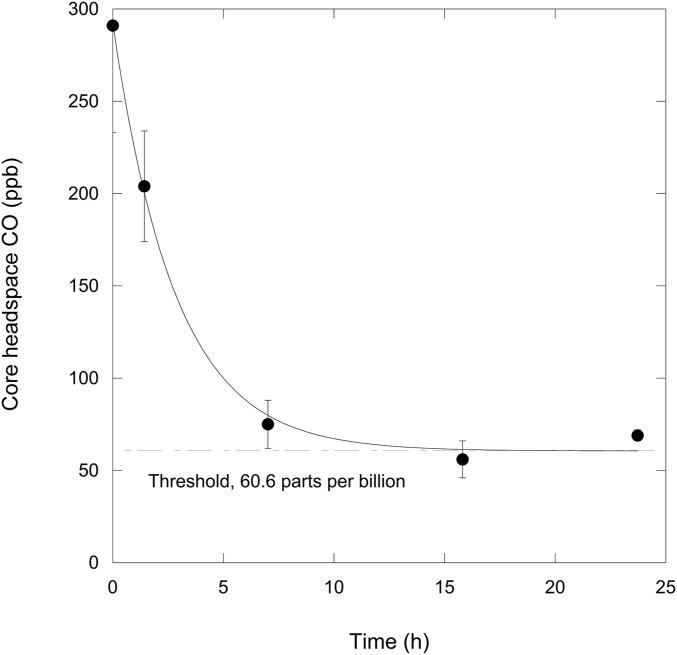
Atmospheric CO (about 290 parts per billion; ppb) was consumed at rates of 0.40 ± 0.16 mg CO⋅m−2⋅d−1 by intact saline soil cores from a Wendover, Utah (WEND), site with surface water potentials (0- to 2-cm-depth interval) of −41 MPa (Fig. 1). The threshold for uptake, 61 ppb, was well below ambient CO concentrations, and more than four orders of magnitude lower than levels in Mars’ atmosphere. WEND CO uptake rates were somewhat less than values reported for Maine forest soils and Hawaiian volcanic deposits (35, 36), but activity by the latter was substantially inhibited at water potentials below −0.1 to −1 MPa (37). Thus, results from WEND provide the first evidence, to our knowledge, for active atmospheric CO uptake under conditions of extreme water limitation.
Fig. 1.
Headspace CO concentrations (ppb; means ± 1 SE, n = 3) as a function of time for intact cores from Wendover, Utah, saline soil (July 2014); soil surface water potentials were approximately −41 MPa. Initial CO concentrations represented ambient atmospheric CO; the dashed line indicates the apparent CO uptake threshold.
Active CO uptake at −41 MPa was also confirmed using WEND soil from the 0- to 2-cm-depth interval incubated with exogenous CO (100 ppm). Apparent maximum CO uptake rates by these soils (0.30 ± 0.04 nmol g−1⋅h−1) were similar to rates for soil from the 8- to 10-cm interval (0.24 ± 0.03 nmol g−1⋅h−1) with water potentials of −6.3 MPa. This indicated that CO-oxidizing bacterial communities in the saline surface soils were adapted to high salt and low water potential. The fact that these soils have subsequently yielded a CO-oxidizing extremely halophilic isolate provided further support for the presence of bacterial communities adapted to salt stress.
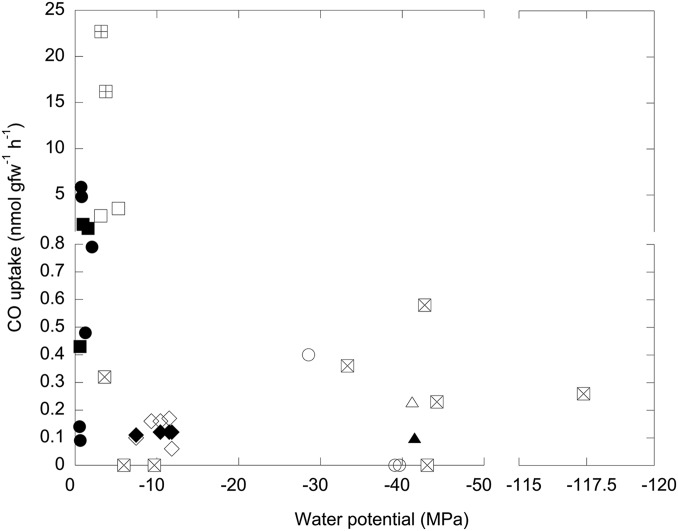
Results of similar assays for several additional sites indicated that CO oxidation at low water potentials was widespread among diverse saline systems (Fig. 2 and Table S1). CO uptake by BSF saline crusts and sediments and Hawaiian volcanic ash and cinders (Holei Sea Arch, HSA; MacKenzie State Park, MSP) occurred over a range of water potentials from about −3.6 to −117 MPa. Rates varied across this range, but with no obvious relationship to water potential (Fig. 2 and Table S1). CO was oxidized at −28.5 MPa at a saline MSP site [0.40 nmol gfw (g fresh weight)−1⋅h−1], whereas no activity was detected for two adjacent samples with water potentials of −39.1 to −39.7 MPa. In contrast, CO uptake was readily observed for four of five HSA sites with water potentials ranging from −33.3 to −117.4 MPa; notably, two of three HSA samples with water potentials >−10 MPa showed no uptake, whereas activity at the HSA site with the highest water potential (0.32 nmol g−1⋅h−1, −3.6 MPa) was comparable to that for the site with the lowest water potential (0.26 nmol g−1⋅h−1, −117.4 MPa). Active CO uptake was also observed for crystalline sediment from two Atacama Desert solar salterns (ASL), Salar de Llamara and Laguna Cejar, at −7.5 to −11.8 MPa (Fig. 2 and Table S1). ASL rates (0.06–0.17 nmol g−1⋅h−1) were somewhat lower than those for HSA and MSP; however, ASL CO uptake potential was stable for 9 mo without exogenous CO (Fig. 2 and Table S1), suggesting that ASL CO oxidizers tolerated both low water potential and starvation substrate regimes. Activity for brine-saturated BSF sediment and salt crusts was comparable to that of the ASL samples, but at water potentials of approximately −41 MPa (Fig. 2 and Table S1). Collectively, these results documented a previously unsuspected capacity of extreme halophiles to oxidize CO in geographically dispersed hypersaline systems with ecologically distinct characteristics (e.g., saline soils versus saltern sediments).
Fig. 2.
CO uptake rates (nmol gfw−1⋅h−1) for individual samples versus sample water potential (MPa). ⊞, ○, and □ represent MacKenzie State Park samples from sites 1, 2, and 3 in April 2014, respectively; ● and ■ represent MacKenzie State Park samples from sites 2 and 3 in July 2014, respectively; ⊠ represents HSA samples from July 2014; ◇ and ◆ represent Atacama samples from June 2013 and April 2014, respectively; △ and ▲ represent Wendover, Utah, soil (0–2 cm) and Bonneville Salt Flats sediment, respectively.
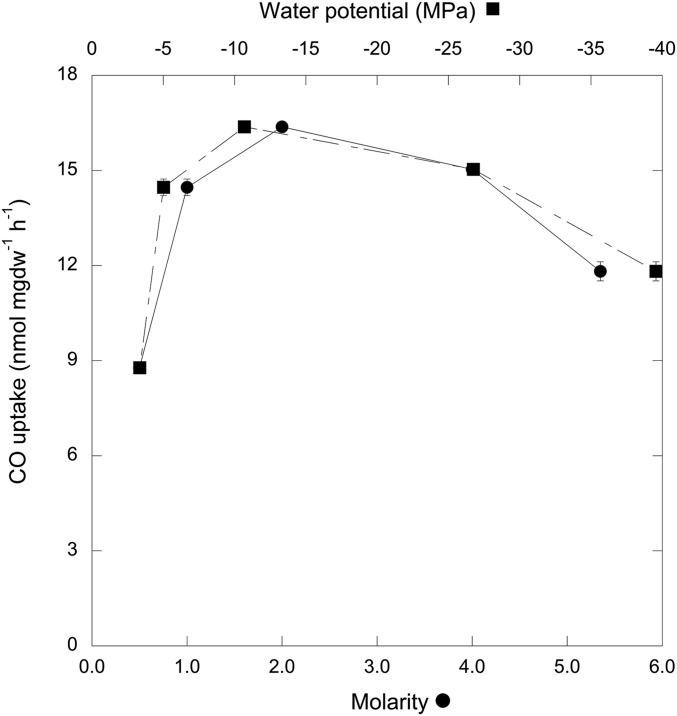
CO uptake by BSF samples at −41 MPa was consistent with results from the first documented extremely halophilic CO-oxidizing euryarchaeote, Halorubrum str. BV1, which was isolated from BSF sediment (Fig. S1). Halorubrum str. BV1 oxidized CO maximally at about −10.7 MPa, but activity was reduced by only 28% at −39.6 MPa (5.3 M NaCl; Fig. 3), which suggested that CO uptake was well-adapted for water stress and the high salt regimes that typify BSF. Additional assays have also revealed CO oxidation by the extremely halophilic euryarchaeotes N. bangense and N. sulfidifaciens, both isolated from Chinese salt lakes (38, 39). Collectively, these results suggested that novel extremely halophilic CO oxidizers derived from diverse terrestrial systems might prove suitable as models for metabolism and survival on Mars or elsewhere.
Fig. 3.
CO uptake rates [nmol (mg cell dry weight)−1⋅h−1; means ± 1 SE, n = 3) for Halorubrum sp. BV1 incubated at varied NaCl concentrations (molar, ●) and water potentials (MPa, ■).
To assess the responses of halophilic CO oxidation to conditions relevant for Mars, washed cells of the gammaproteobacterial halophile A. ehrlichii MLHE-1 (40) were incubated on basalt cinders. A. ehrlichii MLHE-1 oxidized CO at 6 °C (Table S2), which was within the range of RSL temperatures reported by McEwen et al. (19). However, significant CO uptake likely occurs at even lower temperatures in other halophiles, because Octadecabacter antarcticus 307, a marine psychrophile with form I genes for CO oxidation, grows at 4 °C or lower in brines with at least twice the salinity of full-strength seawater (41). Extreme halophiles have also been isolated from and are active in subzero Antarctic and Arctic brines (42–48). In addition, atmospheric CO uptake has been documented for Maine forest soils incubated at 0 °C (35), which indicates that cold-tolerant CO-oxidizing populations might be widely distributed, and provides important insights about the behavior of extraterrestrial systems, such as the RSL, that experience large seasonal shifts in temperature.
Although high salt concentrations and low water potential partially inhibited A. ehrlichii MLHE-1, no adverse effect on CO uptake was observed for 50 mM magnesium perchlorate (Table 1), a concentration that can be anticipated for regolith with volumetric water contents of 10% and total perchlorate levels by weight of 0.1%. Perchlorate has been previously reported at somewhat higher levels for two locations on Mars’ surface (49–51), but higher concentrations might not prove to be a serious constraint, because Oren et al. (32) have reported tolerance of up to 0.4 M sodium perchlorate by several extreme halophiles. Indeed, in addition to tolerating perchlorate, some CO oxidizers might be able to use it as a respiratory electron acceptor. Perchlorate reduction using organic substrates has been reported for several extremely halophilic euryarchaeotes (32, 52), but A. ehrlichii MLHE-1 did not oxidize CO with perchlorate (Fig. S2), nor has perchlorate-linked CO oxidation been documented yet for other CO oxidizers. Nonetheless, perchlorate-coupled CO oxidation is thermodynamically favorable (ΔGo −1,042 kJ⋅mol−1), and an analogous process, perchlorate-dependent hydrogen oxidation, has been used for perchlorate bioremediation (53). These observations suggest that Mars’ regolith could support communities of novel CO-oxidizing perchlorate reducers when or if conditions are favorable for the development of near-surface brines.
Table 1.
CO uptake observed for A. ehrlichii MLHE-1 incubated with varied electron acceptors (suboxic, 0.2% O2; anoxic, +5 mM nitrate) or magnesium perchlorate (50 mM) or magnesium sulfate (500 mM)
| Uptake units | Treatments | ||
| Oxic | Suboxic | Anoxic | |
| Rate, nmol−1·mgdw−1·h−1 | 1.61 ± 0.39 | 1.49 ± 0.26 | 0.23 ± 0.03 |
| Control | Mg(ClO4)2 | MgSO4 | |
| Rate, nmol−1·mgdw−1·h−1 | 0.49 ± 0.07 | 0.52 ± 0.05 | 0.63 ± 0.09 |
| Control | Vacuum control | 10 mbar | |
| Rate constant, h−1 | 0.91 ± 0.04 | 1.25 ± 0.31 | 0.67 ± 0.16 |
Uptake rate constants (h−1 ± 1 SE, n = 3) were observed for ambient and low-pressure incubations. Rates for varied electron acceptor and magnesium salt availability [nmol (mg cell dry weight)−1⋅h−1 ± 1 SE, n = 3] were determined for cells incubated with initial headspace CO concentrations of 8 ppm and were based on first-order uptake rate constants. Rate constants for varied pressure incubations were obtained for treatments initiated at 6 ppm CO and 1 atm pressure (controls), for treatments with 6 ppm CO and 1 atm pressure reestablished after reducing pressure to 10 mbar (vacuum controls), and for treatments with 600 ppm CO at 10 mbar pressure. mgdw, mg cell dry weight.
CO oxidation need not depend on perchlorate, however. Molecular oxygen occurs in the Martian atmosphere at concentrations sufficient to support aerobic activity [about 1,450 ppm (25)]. When incubated with 2,000-ppm headspace oxygen concentrations in the absence of nitrate, A. ehrlichii MLHE-1 consumed CO nearly as rapidly (92.4%) and completely as it did in the presence of 21% oxygen (Table 1 and Fig. S3); the small difference between fully oxic and suboxic was not significantly different (ANOVA, P = 0.59, Bonferroni post hoc test).
Nitrate might also serve as an electron acceptor for CO oxidation. Although the availability of nitrate has not yet been established, evidence from Martian meteorites suggests that it could be distributed as widely as perchlorate (54). Previous studies have shown that both denitrification and dissimilatory nitrate reduction can be coupled to CO oxidation (55) and, in this study, A. ehrlichii MLHE-1, a nitrate respirer, oxidized CO anaerobically in the presence of 5 mM nitrate, albeit at rates significantly slower (14.3–15.4%) than under oxic or suboxic conditions (Table 1 and Fig. S3).
Although temperature and water availability might change spatially and temporally at Mars’ surface, atmospheric pressure is consistently low and potentially constrains metabolic activity. Hypobaria inhibits growth of several Bacillus species, Deinococcus radiodurans R1, and Escherichia coli K12 (56, 57). Nonetheless, hypobaria did not affect A. ehrlichii MLHE-1 CO uptake over a range from 10 to 1,020 mbar, because first-order CO uptake rate constants were equivalent regardless of incubation pressure (Table 1). Low-pressure tolerances have also been observed for growth of Carnobacterium isolates, Psychrobacter cryohalolentis K5, Serratia liquefaciens ATCC 27592, and a Vibrio isolate (58). These results show collectively that bacterial responses to hypobaria vary, but they also suggest that hypobaria need not constrain atmosphere–regolith CO exchanges.
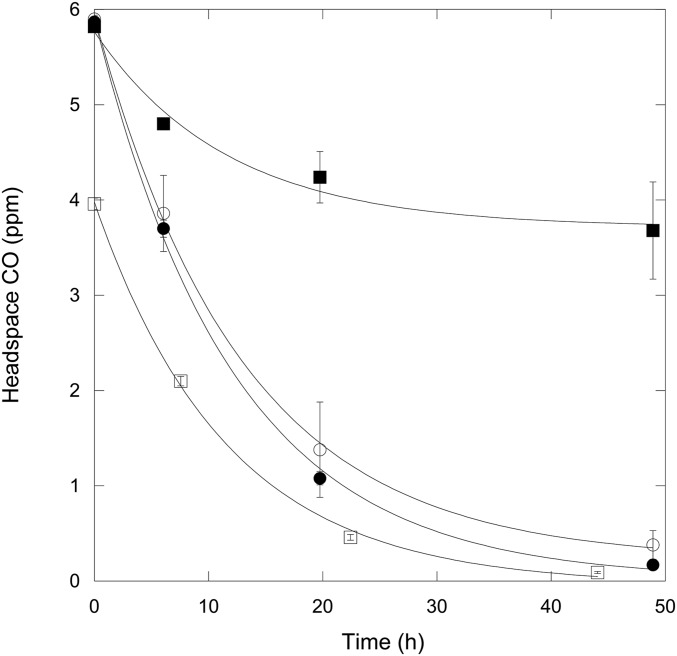
Although hypobaria might adversely affect some bacteria, it could also reduce potentially inhibitory effects of high CO2 (Mars atmosphere contains 96% CO2). Elevated CO2 concentrations have long been known to inhibit bacterial food spoilage and inhibit growth of several isolates (59–64). Consistent with these observations, CO uptake by A. ehrlichii MLHE-1 was partially inhibited during incubation in an atmosphere with 95% CO2/4.8% N2/0.2% O2 at 1,020 mbar relative to activity in an atmosphere with 99.8% N2/0.2% O2 (Fig. 4). However, no inhibition was observed when cells were incubated with 9.5% CO2/89.5% N2/1% O2 (Fig. 4). Because previous analyses have shown that oxygen concentrations from 0.2% to 21% did not affect A. ehrlichii MLHE-1 CO uptake (Table 1), the lack of inhibition by 9.5% CO2 can be attributed to the lower CO2 concentration. This is significant, because CO2 concentrations in a near-surface Mars RSL brine in equilibrium with a CO2 partial pressure (pCO2) of 0.96 at 7 mbar would be more than 10-fold lower than in similar brines equilibrated with a pCO2 of 0.095 at 1,020 mbar. Thus, despite its high CO2 partial pressure, the low total pressure for Mars might preclude significant CO2 inhibition of bacterial activity. A similar conclusion has been drawn from growth analyses of two facultative anaerobes, E. coli ATCC 35218 and S. liquefaciens ATCC 27592, and an obligately anaerobic Carnobacterium spp. (65, 66).
Fig. 4.
Headspace CO concentrations (ppm; means ± 1 SE, n = 3) as a function of time for A. ehrlichii MLHE-1 incubated on basalt cinders with the following treatments: ○ and ● represent ambient atmosphere and 99.8% N2/0.2% O2, respectively; ■ represents 95% CO2/4.8% N2/0.2% O2; and □ represents 9.5% CO2/89.5% N2/1% O2.
Collectively, these results establish the plausibility of CO as a substrate that can contribute to the energy needs of microbes in extraterrestrial environments with conditions that permit formation of stable brines. Such conditions might exist on Mars at RSL, and on the moons Enceladus and Europa (67, 68). If extremely halophilic CO oxidizers exist on contemporary Mars, they could use multiple electron acceptors, including molecular oxygen. Any such microbes might represent relicts from earlier periods in Mars’ history when atmospheric CO concentrations could have been much higher than at present (69, 70). The plausibility of microbial CO oxidation on Mars also suggests that extremely halophilic CO oxidizers as well as other CO-oxidizing microbes might be exploited in efforts to manipulate Mars’ atmosphere and to establish multifunctional microbial communities in the regolith or in engineered environments.
Of course, it must be noted that the persistence of extant microbial populations, if any exist, or populations transplanted in the future, depends on factors other than energy substrates. For example, the availability for biosynthesis of major and trace nutrients in the regolith (e.g., phosphorus, sulfur, molybdenum, etc.) could prove an important constraint. Results from Meslin et al. (71) indicate that Mars soil elemental compositions are comparable to terrestrial soils, including volcanic deposits known to support assemblages of CO oxidizers (36), but additional effort is certainly needed to establish the limits of regolith as a growth medium.
Materials and Methods
Sites and Sampling.
Samples were collected from two sites on the southeastern coast of Hawai’i Island during April and July 2014. MacKenzie State Park sites were located in the vicinity of 19.439389 x 155.862472 and 19.355878 x 155.86222; Holei Sea Arch sites were located in the vicinity of 19.389361 x 155.249028. All sites were near the edge of a lava escarpment at the sea margin. Sea spray from waves breaking at the escarpment base created small pools of seawater on top of the escarpment; water potentials in the pools varied from less than seawater due to rainfall input up to those of salt-saturated brines due to evaporation. Sea spray also moistened weathered basalt, cinders, and ash on top of the escarpment; water potentials for this material also varied due to rainfall and evaporation. Sediment from the bottom of small shallow pools was collected using a sterile spatula and transferred to sterile Whirl-Pak sample bags for subsequent ex situ analyses of water potential and CO uptake rates at a nearby field facility. Weathered basalt, cinder, and ash deposits were collected and analyzed similarly; these samples included depths of 2 cm or less. Samples from Laguna Cejar and Salar de Llamara, Chile were collected by B. Bebout (NASA Ames Research Center, Moffett Field, CA) and C. Kelley (University of Missouri, Columbia, MO) at approximately −23.062444 x 68.215278 and −21.266667 x 69.616667, respectively. These sites have been described previously (72). Samples of saline sediments were collected aseptically during May 2013, and then transferred to 50-mL conical disposable centrifuge tubes that were capped and shipped to Louisiana State University for water potential and initial CO uptake analysis in June 2013. Samples from the Bonneville Salt Flats were collected in July 2013 and again in July 2014. The July 2013 samples were collected aseptically from a small salt-crusted pool (40.737722 x 113.858694; BSF-1) and transferred to Whirl-Pak bags for transport to Louisiana State University for analysis. July 2014 samples were collected similarly, but were assayed within a few hours of collection at a field facility. Sites included the original July 2013 site plus an additional site (40.774583 x 113.858917; BSF-2). A third set of samples was collected from a saline soil (WEND) supporting sparse vegetation south of the Bonneville Salt Flats (40.515472 x 114.044917). Saline soil was collected from the 0- to 2-cm interval and transferred to Whirl-Pak and Ziplock food storage bags. A set of intact triplicate cores was also collected at WEND using 7.5-cm (inner diameter) × 30-cm-long aluminum tubes; the tubes were sealed and transported with minimal disturbance to a nearby field facility for analysis of atmospheric CO uptake.
CO Uptake Assays.
Subsamples (5–10 gfw) from the upper two 2 cm of BSF-1, BSF-2, HSA, and MSP and from the 0- to 2-cm and 8- to 10-cm intervals of WEND were transferred to either 135-cm3 serum bottles or 110-cm3 glass jars (HSA and MSP) that were sealed with neoprene rubber stoppers; samples from ASL were processed similarly, except that 75-cm3 serum bottles were used. CO was added to all bottles at a final concentration of about 100 ppm, and headspaces were sampled at intervals for assays using a Peak Laboratories reduced gas detector operated according to the manufacturer’s instructions. CO was separated using a 1-m stainless steel column (3.2-mm outer diameter × 1.8-mm inner diameter) packed with Molecular Sieve 5A (Sigma-Aldrich) and operated at 122 °C with a carrier gas of air at a flow of about 35 cm3⋅min−1. All samples were incubated at ambient temperature (about 20–23 °C). For atmospheric uptake assays, the upper ends of WEND core tubes were sealed with plastic caps containing a septum, thus entraining ambient air (36). Atmospheric CO uptake was monitored by sampling at intervals, and assaying CO was as described above.
Water Potential.
Water potential was measured using a Decagon Instruments WP4T dew point hygrometer standardized with −2.2 MPa (0.5 M KCl) and −190 MPa (0.25 aw LiCl; aw = water activity) standards obtained from the manufacturer. Subsamples from all sites were transferred to disposable sample cups and analyzed within several hours of collection following the manufacturer’s protocols as described by Weber and King (37).
Isolate Assays.
The responses of CO uptake by Halorubrum strain BSF-1 to varied salt concentrations and water potentials were determined by growing the isolate in CM1 medium to an absorbance of 0.8 at 600 nm. Five equal volumes of medium were centrifuged at 10,000 × g for 10 min at 4 °C to harvest cells. Cell pellets were resuspended in one of five modified versions of the CM1 medium containing 0.5, 1.0, 2.0, 4.0, or 5.3 M NaCl and 2.5 mM sodium pyruvate as a maintenance substrate. Cells were centrifuged a second time (10,000 × g, 10 min), and then resuspended in the appropriate modified CM1 medium. Triplicate aliquots for each sodium chloride treatment were incubated in 160-cm3 serum bottles at 40 °C with shaking at 100 rpm and initial headspace CO concentrations of 100 ppm. CO uptake was assayed as described above for other samples.
Several members of the genus Natronorubrum were obtained from either the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) or the Japan Collection of Microorganisms (JCM): N. aibiense (JCM-13488), N. bangense (JCM-10635), N. sulfidifaciens (JCM-14089), N. texcoconense (JCM-17497), and N. tibetense (DSM-13204). Isolates were grown in DSMZ Medium 371. Cells were harvested by centrifugation as above, and then resuspended in 5 mL Medium 371 with 0.05% yeast extract and no added carbon source; cells were incubated with 100 ppm CO in sealed 60-cm3 serum bottles with uptake assays conducted as described previously. Genome sequences for N. bangense, N. sulfidifaciens, and N. tibetense (available from Integrated Microbial Genomes) contained the canonical form I genes for CO dehydrogenase (27); however, CO uptake by these isolates has not been previously reported.
Assays with the extremely halophilic proteobacterium A. ehrlichii MLHE-1 were conducting using a strain obtained from the DSMZ (DSM-17681). A. ehrlichii MLHE-1 was grown in Artificial Mono Lake medium [AML; (73)] supplemented with 25 mM pyruvate as a carbon and energy source and 0.05% yeast extract (YE). NaCl concentrations were varied from 0.5 to 3.1 M. For all assays, cells in AML were grown to an absorbance of about 1.4 at 600 nm, and then harvested by centrifugation (10 min at 4 °C and 10,000 × g) and resuspended in AML with 0.05% YE. One-milliliter volumes of resuspended cells were pipetted onto 10-g dry weight masses of washed, organic-free (fired at 550 °C, 3-h minimum) basalt cinders in 110-cm3 glass jars, the contents of which were gently but thoroughly mixed to homogenize the cells and cinders. After sealing with neoprene rubber stoppers, CO was added to jar headspaces for uptake assays as described above.
The effect of oxygen availability on CO uptake was assessed using triplicate sets of neoprene-stoppered 110-cm3 jars with headspaces at ambient pressure and temperature and headspace compositions of ambient air, 99.8% N2 plus 0.2% O2, or 100% oxygen-free nitrogen. Anoxic treatments included incubations with or without 5 mM sodium nitrate. Nitrate supports dissimilatory nitrate reduction by A. ehrlichii MLHE-1 (40). The effect of perchlorate was assessed at ambient temperature using ambient air headspaces and treatments (in triplicate) with no added perchlorate or 50 or 500 mM magnesium perchlorate; A. ehrlichii MLHE-1 was also incubated with perchlorate anaerobically, and in anoxic treatments with no added electron acceptors.
The effect of subambient pressure on CO uptake was determined by incubating at ambient temperature four triplicate sets of neoprene-stoppered 110-cm3 jars with ambient air headspace pressures of 10 mbar and initial CO concentrations of about 600 ppm. Sealed jars were incubated in an aluminum vacuum chamber maintained at 10 mbar. At intervals, the chamber was returned to ambient pressure, and one set of triplicates was removed for CO assays as described above after raising the jar headspaces to ambient pressure using CO-free air. The vacuum chamber was resealed and the remaining jars were incubated at 10 mbar for up to 34 h. Results were compared with two treatments. One set of triplicate jars was incubated with ambient pressure and air with 6 ppm CO; the headspace pressures of a second set of triplicate jars were reduced to 10 mbar and then returned to ambient, at which point a final concentration of 6 ppm CO was added.
The effects of CO2 on CO uptake were determined with two separate assays. One used triplicate 110-cm3 jars with A. ehrlichii MLHE-1 incubated at ambient temperature on basalt cinders as described above for each of three headspace treatments: ambient air, 95% CO2/4.8% N2/0.2% O2, or 99.8% N2/0.2% O2. The second assay was conducted similarly, but with the exception that CO uptake from an ambient air atmosphere was compared with uptake from an atmosphere of 9.5% CO2/89.5% N2/1% O2. CO was added at final concentrations of 4–6 ppm for both assays, and initial CO uptake rates were estimated using a nonlinear curve-fitting algorithm (35).
Supplementary Material
Acknowledgments
G.M.K. gratefully acknowledges the contributions of S. McDuff, C. Judd, M. Myers, and S. Neupane to the isolation and initial characterization of Halorubrum sp. BV1 and several additional extremely halophilic CO oxidizers. S. McDuff and M. Myers also provided support during field excursions to the Bonneville Salt Flats, and C. Judd provided support for routine laboratory operations. Drs. B. Bebout (NASA Ames Research Center) and C. Kelley (University of Missouri) generously shared samples collected from solar salterns in the Atacama Desert. Partial funding support was provided by awards from the National Science Foundation (DEB-1146444) and NASA (NNH10ZNE003C).
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The Halorubrum sp. BV1 16S rRNA gene sequence reported in this paper has been deposited in GenBank (accession no. KP334116).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424989112/-/DCSupplemental.
References
- 1.Kargel J. Europa’s crust and ocean: Origin, composition, and the prospects for life. Icarus. 2000;148(1):226–265. [Google Scholar]
- 2.McKay CP, Porco CC, Altheide T, Davis WL, Kral TA. The possible origin and persistence of life on Enceladus and detection of biomarkers in the plume. Astrobiology. 2008;8(5):909–919. doi: 10.1089/ast.2008.0265. [DOI] [PubMed] [Google Scholar]
- 3.Brack A, et al. Origin and evolution of life on terrestrial planets. Astrobiology. 2010;10(1):69–76. doi: 10.1089/ast.2009.0374. [DOI] [PubMed] [Google Scholar]
- 4.Cockell CS. Vacant habitats in the Universe. Trends Ecol Evol. 2011;26(2):73–80. doi: 10.1016/j.tree.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 5.McKay CP, et al. The Icebreaker Life mission to Mars: A search for bimolecular evidence for life. Astrobiology. 2013;13:335–353. doi: 10.1089/ast.2012.0878. [DOI] [PubMed] [Google Scholar]
- 6.Michalski JR, et al. Groundwater activity on Mars and implications for a deep biosphere. Nat Geosci. 2013;6:133–138. [Google Scholar]
- 7.Nixon SL, Cousins CR, Cockell C. Plausible microbial metabolisms on Mars. Astron Geophys. 2013;54(1):1.13–1.16. [Google Scholar]
- 8.Arvidson RE, et al. Ancient aqueous environments at Endeavour crater, Mars. Science. 2014;343(6169):1248097. doi: 10.1126/science.1248097. [DOI] [PubMed] [Google Scholar]
- 9.Wray JJ, et al. Columbus crater and other possible groundwater-fed paleolakes of Terra Sirenum, Mars. J Geophys Res. 2011;116(E1):E01001. [Google Scholar]
- 10.Grotzinger JP, et al. A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science. 2014;343(6169):1242777. doi: 10.1126/science.1242777. [DOI] [PubMed] [Google Scholar]
- 11.McLennan SM, et al. MSL Science Team Elemental geochemistry of sedimentary rocks at Yellowknife Bay, Gale Crater, Mars. Science. 2014;343(6169):1244734. doi: 10.1126/science.1244734. [DOI] [PubMed] [Google Scholar]
- 12.Ming DW, et al. Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science. 2014;343(6169):1245267. doi: 10.1126/science.1245267. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa H, et al. Evolution of water reservoirs on Mars: Constraints from hydrogen isotopes in martian meteorites. Earth Planet Sci Lett. 2014;394:179–185. [Google Scholar]
- 14.Gaidos E, Marion G. Geological and geochemical legacy of a cold early Mars. J Geophys Res. 2003;108(E6):5055. [Google Scholar]
- 15.McEwen AS, et al. A closer look at water-related geologic activity on Mars. Science. 2007;317(5845):1706–1709. doi: 10.1126/science.1143987. [DOI] [PubMed] [Google Scholar]
- 16.Fairén AG. A cold and wet Mars. Icarus. 2010;208(1):165–175. [Google Scholar]
- 17.Wordsworth R, et al. Global modelling of the early martian climate under a denser CO2 atmosphere: Water cycle and ice evolution. Icarus. 2013;222(1):1–19. [Google Scholar]
- 18.Fischer E, Martínez GM, Elliott HM, Rennó NO. Experimental evidence for the formation of liquid saline water on Mars. Geophys Res Lett. 2014;41(13):4456–4462. doi: 10.1002/grl.51829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen AS, et al. Seasonal flows on warm Martian slopes. Science. 2011;333(6043):740–743. doi: 10.1126/science.1204816. [DOI] [PubMed] [Google Scholar]
- 20.Ojha L, et al. Spectral constraints on the formation mechanism of recurring slope lineae. Geophys Res Lett. 2013;40(21):5621–5626. [Google Scholar]
- 21.Ojha L, et al. HiRISE observations of recurring slope lineae (RSL) during southern summer on Mars. Icarus. 2014;231:365–376. [Google Scholar]
- 22.Navarro-González R, Vargas E, de la Rosa J, Raga AC, McKay CP. Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res. 2010;115(E12):E12010. [Google Scholar]
- 23.Moores JE, Schuerger AC. UV degradation of accreted organics on Mars: IDP longevity, surface reservoir of organics, and relevance to the detection of methane in the atmosphere. J Geophys Res. 2012;117(E8):E08008. [Google Scholar]
- 24.Atreya SK, Mahaffy PR, Wong A-S. Methane and related trace species on Mars: Origin, loss, implications for life, and habitability. Planet Space Sci. 2007;55(3):358–369. [Google Scholar]
- 25.Mahaffy PR, et al. MSL Science Team Abundance and isotopic composition of gases in the martian atmosphere from the Curiosity rover. Science. 2013;341(6143):263–266. doi: 10.1126/science.1237966. [DOI] [PubMed] [Google Scholar]
- 26.Sindoni G, Formisano V, Geminale A. Observations of water vapour and carbon monoxide in the Martian atmosphere with the SWC of PFS/MEX. Planet Space Sci. 2011;59(2):149–162. [Google Scholar]
- 27.Weiss BP, Yung YL, Nealson KH. Atmospheric energy for subsurface life on Mars? Proc Natl Acad Sci USA. 2000;97(4):1395–1399. doi: 10.1073/pnas.030538097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King GM, Weber CF. Distribution, diversity and ecology of aerobic CO-oxidizing bacteria. Nat Rev Microbiol. 2007;5(2):107–118. doi: 10.1038/nrmicro1595. [DOI] [PubMed] [Google Scholar]
- 29.Litchfield CD. Survival strategies for microorganisms in hypersaline environments and their relevance to life on early Mars. Meteorit Planet Sci. 1998;33(4):813–819. doi: 10.1111/j.1945-5100.1998.tb01688.x. [DOI] [PubMed] [Google Scholar]
- 30.Leuko S, Rothschild LJ, Burns BP. Halophilic archaea and the search for extinct and extant life on Mars. J Cosmol. 2010;5:940–950. [Google Scholar]
- 31.Bryanskaya AV, Berezhnoy AA, Rozanov AS, Peltek SE, Pavlov AK. Adaptive capabilities of microorganisms of salt lakes of the Altai Region under conditions of early Mars. Paleontol J. 2013;47(9):1089–1092. [Google Scholar]
- 32.Oren A, Elevi Bardavid R, Mana L. Perchlorate and halophilic prokaryotes: Implications for possible halophilic life on Mars. Extremophiles. 2014;18(1):75–80. doi: 10.1007/s00792-013-0594-9. [DOI] [PubMed] [Google Scholar]
- 33.Davila AF, et al. Hygroscopic salts and the potential for life on Mars. Astrobiology. 2010;10(6):617–628. doi: 10.1089/ast.2009.0421. [DOI] [PubMed] [Google Scholar]
- 34.Tosca NJ, McLennan SM, Lamb MP, Grotzinger JP. Physicochemical properties of concentrated Martian surface waters. J Geophys Res. 2011;116(E5):E05004. [Google Scholar]
- 35.King GM. Attributes of atmospheric carbon monoxide oxidation by Maine forest soils. Appl Environ Microbiol. 1999;65(12):5257–5264. doi: 10.1128/aem.65.12.5257-5264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King GM. Contributions of atmospheric CO and hydrogen uptake to microbial dynamics on recent Hawaiian volcanic deposits. Appl Environ Microbiol. 2003;69(7):4067–4075. doi: 10.1128/AEM.69.7.4067-4075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber CF, King GM. Water stress impacts on bacterial carbon monoxide oxidation on recent volcanic deposits. ISME J. 2009;3(12):1325–1334. doi: 10.1038/ismej.2009.70. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Zhou P, Tian X. Characterization of two novel haloalkaliphilic archaea Natronorubrum bangense gen. nov., sp. nov. and Natronorubrum tibetense gen. nov., sp. nov. Int J Syst Bacteriol. 1999;49(Pt 1):261–266. doi: 10.1099/00207713-49-1-261. [DOI] [PubMed] [Google Scholar]
- 39.Cui H-L, et al. Natronorubrum sulfidifaciens sp. nov., an extremely haloalkaliphilic archaeon isolated from Aiding salt lake in Xin-Jiang, China. Int J Syst Evol Microbiol. 2007;57(Pt 4):738–740. doi: 10.1099/ijs.0.64651-0. [DOI] [PubMed] [Google Scholar]
- 40.Hoeft SE, et al. Alkalilimnicola ehrlichii sp. nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int J Syst Evol Microbiol. 2007;57(Pt 3):504–512. doi: 10.1099/ijs.0.64576-0. [DOI] [PubMed] [Google Scholar]
- 41.Gosink JJ, Herwig RP, Staley JT. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst Appl Microbiol. 1997;20(3):356–365. [Google Scholar]
- 42.Perreault NN, Andersen DT, Pollard WH, Greer CW, Whyte LG. Characterization of the prokaryotic diversity in cold saline perennial springs of the Canadian high Arctic. Appl Environ Microbiol. 2007;73(5):1532–1543. doi: 10.1128/AEM.01729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondino LJ, Asao M, Madigan MT. Cold-active halophilic bacteria from the ice-sealed Lake Vida, Antarctica. Arch Microbiol. 2009;191(10):785–790. doi: 10.1007/s00203-009-0503-x. [DOI] [PubMed] [Google Scholar]
- 44.Niederberger TD, et al. Microbial characterization of a subzero, hypersaline methane seep in the Canadian High Arctic. ISME J. 2010;4(10):1326–1339. doi: 10.1038/ismej.2010.57. [DOI] [PubMed] [Google Scholar]
- 45.Murray AE, et al. Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. Proc Natl Acad Sci USA. 2012;109(50):20626–20631. doi: 10.1073/pnas.1208607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dassarma S, Capes MD, Karan R, Dassarma P. Amino acid substitutions in cold-adapted proteins from Halorubrum lacusprofundi, an extremely halophilic microbe from Antarctica. PLoS ONE. 2013;8(3):e58587. doi: 10.1371/journal.pone.0058587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeMaere MZ, et al. High level of intergenera gene exchange shapes the evolution of haloarchaea in an isolated Antarctic lake. Proc Natl Acad Sci USA. 2013;110(42):16939–16944. doi: 10.1073/pnas.1307090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickson JL, Head JW, Levy JS, Marchant DR. Don Juan Pond, Antarctica: Near-surface CaCl(2)-brine feeding Earth’s most saline lake and implications for Mars. Sci Rep. 2013;3:1166. doi: 10.1038/srep01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht MH, et al. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science. 2009;325(5936):64–67. doi: 10.1126/science.1172466. [DOI] [PubMed] [Google Scholar]
- 50.Catling DC, et al. Atmospheric origins of perchlorate on Mars and in the Atacama. J Geophys Res. 2010;115:E00E11. [Google Scholar]
- 51.Davila AF, Willson D, Coates JD, McKay CP. Perchlorate on Mars: A chemical hazard and a resource for humans. Int J Astrobiol. 2013;12(4):321–325. [Google Scholar]
- 52.Okeke BC, Giblin T, Frankenberger WT., II Reduction of perchlorate and nitrate by salt tolerant bacteria. Environ Pollut. 2002;118(3):357–363. doi: 10.1016/s0269-7491(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 53.Ahn CH, et al. Bacterial biofilm-community selection during autohydrogenotrophic reduction of nitrate and perchlorate in ion-exchange brine. Appl Microbiol Biotechnol. 2009;81(6):1169–1177. doi: 10.1007/s00253-008-1797-3. [DOI] [PubMed] [Google Scholar]
- 54.Kounaves S, Carrier BL, O’Neil GD, Stroble ST, Claire MW. Evidence of martian perchlorate, chlorate, and nitrate in Mars 23 meteorite EETA79001: Implications for oxidants and organics. Icarus. 2014;229:206–213. [Google Scholar]
- 55.King GM. Nitrate-dependent anaerobic carbon monoxide oxidation by aerobic CO-oxidizing bacteria. FEMS Microbiol Ecol. 2006;56(1):1–7. doi: 10.1111/j.1574-6941.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 56.Schuerger A, Nicholson W. Interactive effects of hypobaria, low temperature, and CO2 atmospheres inhibit the growth of mesophilic Bacillus spp. under simulated martian conditions. Icarus. 2006;185(1):143–152. [Google Scholar]
- 57.Nicholson WL, et al. Exploring the low-pressure growth limit: Evolution of Bacillus subtilis in the laboratory to enhanced growth at 5 kilopascals. Appl Environ Microbiol. 2010;76(22):7559–7565. doi: 10.1128/AEM.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuerger AC, Ulrich R, Berry BJ, Nicholson WL. Growth of Serratia liquefaciens under 7 mbar, 0°C, and CO2-enriched anoxic atmospheres. Astrobiology. 2013;13(2):115–131. doi: 10.1089/ast.2011.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill CO, Tan KH. Effect of carbon dioxide on growth of Pseudomonas fluorescens. Appl Environ Microbiol. 1979;38(2):237–240. doi: 10.1128/aem.38.2.237-240.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon NM, Kell DB. The inhibition by CO2 of the growth and metabolism of micro-organisms. J Appl Bacteriol. 1989;67(2):109–136. doi: 10.1111/j.1365-2672.1989.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 61.Koizumi H, et al. Effect of carbon dioxide concentration on microbial respiration in soil. Ecol Res. 1991;6(3):227–232. [Google Scholar]
- 62.Eyles MJ, Moir CJ, Davey JA. The effects of modified atmospheres on the growth of psychrotrophic pseudomonads on a surface in a model system. Int J Food Microbiol. 1993;20(2):97–107. doi: 10.1016/0168-1605(93)90097-z. [DOI] [PubMed] [Google Scholar]
- 63.Martin JD, Werner BG, Hotchkiss JH. Effects of carbon dioxide on bacterial growth parameters in milk as measured by conductivity. J Dairy Sci. 2003;86(6):1932–1940. doi: 10.3168/jds.S0022-0302(03)73780-1. [DOI] [PubMed] [Google Scholar]
- 64.Leisner JJ, Laursen BG, Prévost H, Drider D, Dalgaard P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol Rev. 2007;31(5):592–613. doi: 10.1111/j.1574-6976.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berry BJ, Jenkins DG, Schuerger AC. Effects of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl Environ Microbiol. 2010;76(8):2377–2386. doi: 10.1128/AEM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholson WL, Krivushin K, Gilichinsky D, Schuerger AC. Growth of Carnobacterium spp. from permafrost under low pressure, temperature, and anoxic atmosphere has implications for Earth microbes on Mars. Proc Natl Acad Sci USA. 2013;110(2):666–671. doi: 10.1073/pnas.1209793110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waite JH, Jr, et al. Cassini ion and neutral mass spectrometer: Enceladus plume composition and structure. Science. 2006;311(5766):1419–1422. doi: 10.1126/science.1121290. [DOI] [PubMed] [Google Scholar]
- 68.Zolotov MY. An oceanic composition on early and today’s Enceladus. Geophys Res Lett. 2007;34(23):L23203. [Google Scholar]
- 69.Zahnle K, Haberle RM, Catling DC, Kasting JF. Photochemical instability of the ancient Martian atmosphere. J Geophys Res. 2008;113(E11):E11004. [Google Scholar]
- 70.Ramirez RM, et al. Warming early Mars with CO2 and H2. Nat Geosci. 2013;7:59–63. [Google Scholar]
- 71.Meslin P-Y, et al. MSL Science Team Soil diversity and hydration as observed by ChemCam at Gale Crater, Mars. Science. 2013;341(6153):1238670. doi: 10.1126/science.1238670. [DOI] [PubMed] [Google Scholar]
- 72.Kelley CA, Nicholson BE, Beaudoin CS, Detweiler AM, Bebout BM. Trimethylamine and organic matter additions reverse substrate limitation effects on the δ13C values of methane produced in hypersaline microbial mats. Appl Environ Microbiol. September 19, 2014 doi: 10.1128/AEM.02641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blum JS, et al. Desulfohalophilus alkaliarsenatis gen. nov., sp. nov., an extremely halophilic sulfate- and arsenate-respiring bacterium from Searles Lake, California. Extremophiles. 2012;16(5):727–742. doi: 10.1007/s00792-012-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.