Abstract
While the age-related loss in muscle mass partially explains the decline in strength, other yet undefined mechanisms contribute. This study investigates whether changes in myosin-actin stoichiometry and oxidative modification could help explain the decrement in muscle strength with aging. Protein expression and oxidation were evaluated in myosin and actin isolated from the soleus and semimembranosus muscles from young adult, old, and very old Fischer 344 rats. In the soleus muscle, actin and myosin content did not change with aging. In the semimembranosus, actin content was stable, but myosin exhibited decreased content in muscles from very old rats, resulting in a decrease in the myosin-to-actin ratio. 3-Nitrotyrosine and 4-hydroxy-2-nonenal were used as markers of protein oxidative damage. Although myosin and actin are modified with 3-nitrotyrosine and 4-hydroxy-2-nonenal, the extent of chemical modification does not increase with age. The results suggest that the decline in force production with age is not due to the accumulation of these two specific markers of protein oxidation on the myofibrillar proteins. Additionally, age-dependent changes in myofibrillar stoichiometry do not contribute to the decline in force production in the soleus, but may play a role in the semimembranosus with advanced age.
Keywords: 3-nitrotyrosine, 4-hydroxynonenal, skeletal muscle, proteomics
Aging is accompanied by a general decline in muscle strength (3, 36). The decrease in muscle strength is explained, at least in part, by a decrease in muscle mass. However, strength declines more than would be expected from the reduced muscle or fiber size, thus suggesting other mechanisms are involved (3, 4, 35, 37). One possibility is there are defects in the myofibrillar proteins responsible for force generation. This idea is supported by the age-related loss in force measured in permeabilized fibers, where muscle membranes are experimentally removed and force generation is entirely dependent on the interaction of myofibrillar proteins (35, 37, 39).
With aging, a disruption in the interaction of myosin and actin is noted in several studies. Electron paramagnetic resonance spectroscopy was used to measure the percentage of myosin that is in optimal interaction with actin to maximize force generation, referred to as the strong binding structural state, vs. when myosin and actin are weakly bound and thus not producing force. In aged muscle, there was a reduction in the fraction of myosin heads in the strong-binding structural state, such that there are fewer myosin-actin interactions capable of generating force (25). In addition, a significant age-related inhibition of myosin ATPase, critical for generating force, was reported from investigations of isolated proteins (myosin and actin) from young and old animals (32). Thus mechanisms that decrease or interrupt the interaction of myosin and actin are likely to explain the age-related reduction in force-generating capacity.
This study investigates two potential mechanisms that could contribute to the decrease in force-generating interactions of myosin and actin. The first mechanism posits that there is a change in stoichiometry between myosin and actin. Since force generation depends on the interaction of myosin heads with actin molecules, maintaining optimal stoichiometry between myosin and actin is essential. The cellular content of proteins depends on the balance between the two competing processes of protein synthesis and degradation. With aging, there is evidence for decreased myosin heavy chain (MHC) synthesis rates (1, 2, 38, 42) and a loss in the regulation of the proteasome, the main protease responsible for degrading myofibrillar proteins (7, 14). Thus changes in rates of synthesis or degradation could lead to protein-specific declines in either actin or myosin content.
The second mechanism that may play a role in the age-related decline in force generation is oxidative damage to myofibrillar proteins. Past studies showed that, whereas cysteine oxidation was increased in myosin, actin cysteine oxidation was not altered with age (32). These results suggest protein-specific differences in susceptibility to oxidation. The present study examines two additional products of oxidation, 3-nitrotyrosine (3NT) and 4-hydroxy-2-nonenal (HNE). 3NT is the result of tyrosine nitration by the oxidant peroxynitrite, which is generated by the reaction of superoxide and nitric oxide (15). HNE is a reactive aldehyde that originates from the peroxidation of membranes and forms a mixture of adduct types on the side chains of cysteine, lysine, and histidine through a Michael-type nucleophilic addition (6). The protein modifications, HNE (8, 13) and 3NT (41), have been shown to inhibit protein function.
In the present study, we examined actin and myosin isolated from the soleus and semimembranosus muscles to test the hypothesis that age-related changes in the stoichiometry and oxidative modifications are underlying mechanisms for the decline in function. The semimembranosus and soleus are composed predominantly of type II and type I skeletal muscle fibers, respectively. Type II fibers demonstrate an earlier decline in function compared with type I fibers. Thus we hypothesize that 1) myosin and actin will exhibit changes in expression and oxidation with aging; and 2) these changes will occur at an earlier age in the semimembranosus muscle. To test these hypotheses, the expression of actin and myosin and content of HNE and 3NT were compared between young, old, and very old Fischer 344 rats.
METHODS
Animals
Fischer 344 male rats, aged 7–11 mo (young adult), 23–25 mo (old), and 27–33 mo (very old), were obtained from the aging rodent colony of the University of Minnesota that is maintained by the Minneapolis Veterans Administration. The 50% survival rate for the Fischer 344 rat strain is 24 mo (24). All animals were housed in pathogen-free conditions and received food and water ad libitum (12:12-h light-dark daily cycle). The animal care protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Reagents and antibodies
The monoclonal antibodies that recognize myosin-F, myosin-S, and actin were from Novocastra (UK). Polyclonal antibodies that recognize HNE-modified proteins were from Alpha Diagnostics (San Antonio, TX), and those recognizing 3NT modifications were obtained from Cayman Chemical (Ann Arbor, MI). Full-range and low-range prestained molecular weight markers were from Amersham Pharmacia Biotech (Piscataway, NJ). Immobilon-P polyvinylidene difluoride membrane (PVDF, 0.45 µm) for Western immunoblotting was from Millipore (Bedford, MA). The bicinchoninic acid protein assay kit and trifluoroacetic acid (TFA) were from Pierce (Rockford, IL). Alkaline phosphatase-conjugated secondary antibodies (goat anti-rabbit or goat anti-mouse), kaleidoscope prestained molecular weight markers, alkaline phosphatase conjugate substrate kit containing 5-bromo-4-chloro-3′-indolylphosphate-p-toluidine and nitroblue tetrazolium chloride (BCIP-NBT), Silver Stain Plus Kit, Mini Trans-Blot Cell and Transblot SD semidry transfer cell, and all reagents for SDS-PAGE were supplied by Bio-Rad (Hercules, CA). Imidazole, phenylmethylsulfonyl fluoride, tris(2-carboxyethyl)phosphine hydrochloride, tropomyosin, troponin, and myosin light chain were purchased from Sigma (St. Louis, MO). Hi-Pure liquid gelatin for Western immunoblotting was purchased from Norland Products (New Brunswick, NJ).
Tissue preparation
The soleus and semimembranosus muscles were surgically excised from pentobarbital-anesthetized young adult, old, and very old rats. Muscles were trimmed of visible fat and connective tissue and were frozen in liquid nitrogen and stored at −80°C until analysis. Fractionation of muscle samples into extracts 1 and 2 (enriched for cytosolic and myofibrillar proteins, respectively) was performed on ice according to McDonough et al. (27). Briefly, muscle samples (60 mg) were diced with a razor and exposed to 500 µl of subfractionation buffer 1 (20 mM imidazole, 0.25 mM phenylmethylsulfonyl fluoride, pH 7.4) and immediately homogenized with a glass homogenizer (Kontes Duall). Following a 15-min centrifugation at 12,000 g at 4°C, the supernatant was collected, and the remaining pellet was homogenized with 250 µl of buffer 1, and centrifugation was repeated. The supernatants were combined, forming extract 1, and the remaining pellet was then homogenized in 250 µl of subfractionation buffer 2 [0.5% TFA, 1 mM tris(2-carboxyethyl phosphine)hydrochloride] followed by a 15-min centrifugation at 12,000 g. The supernatant was collected, and the remaining pellet was homogenized with 100 µl of buffer 2, and centrifugation was repeated. The combined supernatants comprised extract 2, which was the fraction enriched in myofibrillar proteins. Protein concentration was determined using the bicinchoninic acid protein assay kit, according to the manufacturer’s protocol, with bovine serum albumin as the standard.
Protein separation and identification of oxidative damage by SDS-PAGE and Western blotting
Before immunoblotting, muscle homogenates were electrophoretically separated by SDS-PAGE using a large-format or miniformat (7 or 12% acrylamide) resolving gel with a 3% stacking gel (20). Preliminary experiments showed that the protein loads used in these investigations (2–5 µg) produced an immune signal within the linear range for each antibody. Separation of MHC was accomplished using 7% gels, whereas 12% gels were used to resolve actin. Following electrophoresis, gels were either transferred to PVDF membrane or stained with Coomassie blue or silver, according to manufacturer’s protocols. Specifically, duplicate samples were analyzed via gel electrophoresis (2 gels, identified as 1st and 2nd gel). The proteins on the first gel were silver stained and analyzed for protein content. The first gel was also used for protein identification using mass spectroscopy (see below). The proteins on the second gel were transferred to PVDF and subsequently analyzed via Western blotting for oxidative damage. To document transfer of the proteins to the PVDF membrane, the second gel (after transfer) was stained with Coomassie blue. For miniformat gels, electrophoretic transfer was carried out on a Mini Trans-Blot cell at 110 V for 2–3 h using the following buffer: 2 M glycine, 0.25 M Tris, 8 mM SDS, 20% methanol. Large-format gels were transferred at 800 mA for 45 min using a Transblot SD semidry transfer cell.
Membranes were incubated overnight at 4°C with one of the following primary antibodies at the dilutions indicated: actin (1:1,000), myosin-F (1:1,000), and myosin-S (1:1,000). For determining the extent of protein oxidative modifications, membranes were incubated with primary antibodies that recognize either HNE modifications (1:2,500) or 3NT (1:2,000). Membranes were incubated 1 h at room temperature in either goat anti-rabbit biotin-conjugated (1: 3,000) or goat anti-mouse alkaline phosphatase-conjugated secondary antibody (1:5,000). Those receiving goat anti-rabbit biotin-conjugated secondary were further incubated for 1.5 h in a signal amplification solution containing streptavidin (1:3,000) and biotinyl-alkaline phosphatase (1:3,000) in 1% wash solution. The substrate BCIP-NBT was used to visualize the immunoreaction. Signal from the immunoreaction was imaged using the Fluor-S MultiImaging System (Bio-Rad), and immune reactive bands were quantified by densitometric analysis using Sigma Scan Pro (SPSS, Chicago, IL). All samples were normalized to a standard that was present on each gel. This procedure allowed for comparison of protein samples on multiple blots. Densitometric results are reported as a relative immunoreaction to the reference sample.
Protein identification by mass spectroscopy
To determine protein identification by mass spectroscopy (MS), several steps are taken. First, selected protein bands (and gel matrix) were excised from the silver-stained gel (first gel) and digested overnight at 37°C with trypsin, as described (9, 33). Before protease digestion, the cysteine residues were reduced with DTT and alkylated using iodoacetamide. Second, to separate the peptides from the gel matrix, peptides were extracted by repeated swelling and shrinking of the gel using 25 mM ammonium hydrogen carbonate and acetonitrile 1:1 (vol/vol), followed by 5% formic acid and acetonitrile 1:1 (vol/vol) (9). Third, the extracted peptides were evaporated to near dryness in a Speed Vac and stored at −80°C before mass spectral analysis.
Next, the extracted peptides were prepared for matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS with a Millipore ZipTipC18, according to the manufacturer’s protocol. To crystallize the extracted peptides (samples), 1 µl of 20 mg/ml alpha-cyano-4-hydroxy-trans-cinnamic acid in 0.1% TFA/50% acetonitrile (Sigma-Aldrich, St. Louis, MO) was added to the eluted peptides. The peptides were then spotted onto the MALDI target and dried. Full scans of the peptide mixture from 500–3,500 mass-to-charge ratio (m/z) and tandem mass spectral data of select ions were collected on a QSTAR XL (Applied Biosystems, Foster City, CA) quadrupole TOF MS with an orthogonal MALDI source. The TOF region acceleration voltage was 4 kV, and the injection pulse repetition rate was 6.0 kHz. Laser pulses were generated with a nitrogen laser at 337 nm, ~9 µJ of laser energy using a laser repetition rate of 20 Hz. Mass spectra were the average of ~50–200 laser shots collected in positive mode. External calibration was performed using human angiotensin II (monoisotopic [MH+] m/z 1,046:5,417; Sigma) and ACTH fragment 18–39 (monoisotopic [MH+] m/z 2,465:1,989; Sigma).
Subsequently, the measured peptide m/z were used to search the National Center for Biotechnology Information and Swiss-Prot sequence databases for protein identifications using Mascot (www.matrixscience.com). All searches were performed with a mass tolerance between 50 and 100 ppm. Positive identification required a minimum of three peptide matches and a probability score that indicates high concordance between the masses of experimentally derived peptides with theoretical masses of peptides from the matched protein.
Statistical analysis
Data are presented as means and SE. One-way ANOVA was used to compare protein expression and relative immunoreactive densities between different age groups for each muscle. Differences were judged significant if P ≤ 0.05. Tukey-Kramer multiple-comparison test was performed to determine differences between groups.
RESULTS
Myofibrillar protein expression
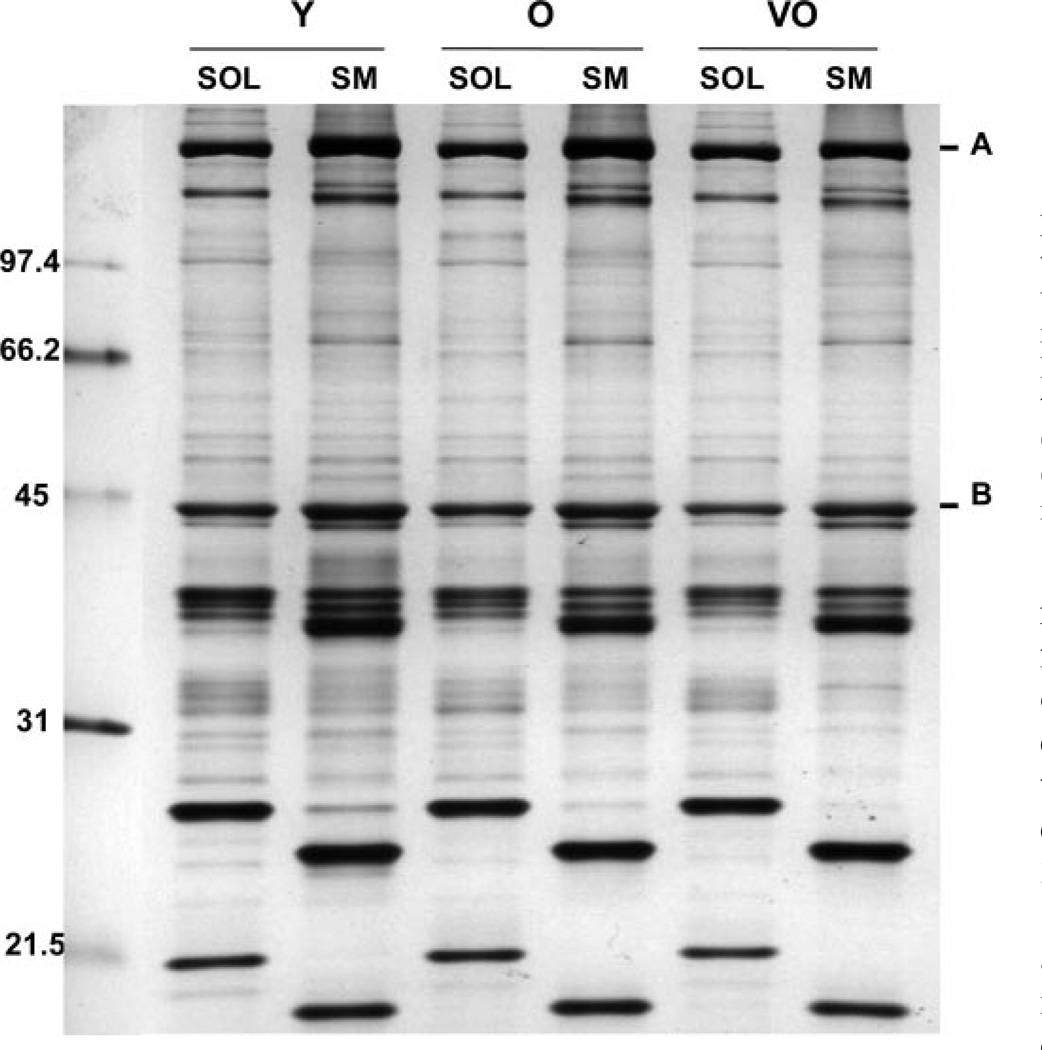
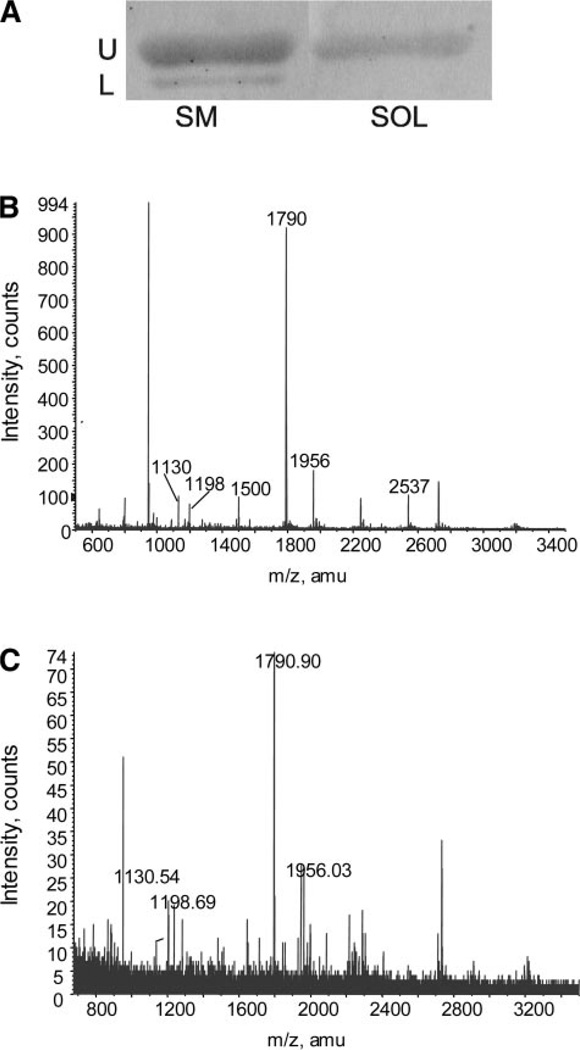
To determine whether myofibrillar proteins are altered with aging, we examined protein expression of the MHC and actin in the myofibrillar fraction of the semimembranosus and soleus muscles. Figure 1 shows a comparison of silver-stained proteins from the myofibrillar fraction isolated from young adult, old, and very old rats. The position of the MHC and actin were determined by Western blotting using antibodies that react with actin (Fig. 2A) and the fast and slow isoforms of MHC (data not shown). In the semimembranosus muscle, the actin antibody reacted with two closely migrating protein bands, with an apparent molecular mass of ~42 kDa. MS confirmed the presence of actin in both bands. Figure 2 shows the mass fingerprints from tryptic digests of the top and bottom bands. The major peaks that matched the m/z values for peptides from actin are indicated. In subsequent investigations of actin in the semimembranosus muscle, both protein bands were analyzed.
Figure 1.
Representative silver-stained gel of the myofibrillar fraction isolated from soleus (SOL) and semimembranosus (SM) muscles in young adult (Y), old (O), and very old (VO) rats. A: myosin heavy chain (MHC). B: actin. Nos. at left are molecular mass markers in kDa.
Figure 2.
Western blot probing for actin and mass spectrometric (MS) analysis of tryptic peptides from actin in the SM muscle. A: antibody reaction to actin (U, upper band; L, lower band). Full scans of a matrix-assisted laser desorption ionization-time-of-flight MS peptide mass fingerprint from upper band (B) and lower band (C). The full scan (B) produced 17 peptides that matched the theoretical mass-to-charge ratio (m/z) values for tryptic peptides from actin (gi 33563240); the 6 major peaks are indicated. MS analysis on the lower band (C) matched 8 peptides to actin; the 4 major peaks are indicated. amu, Atomic mass unit.
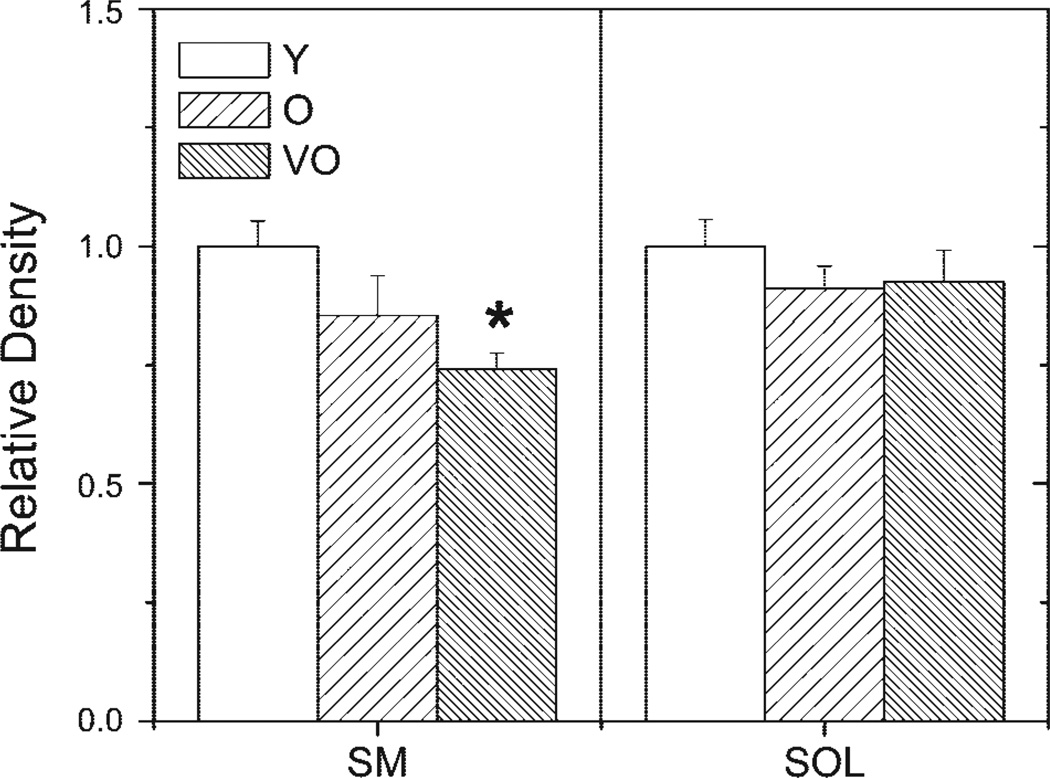
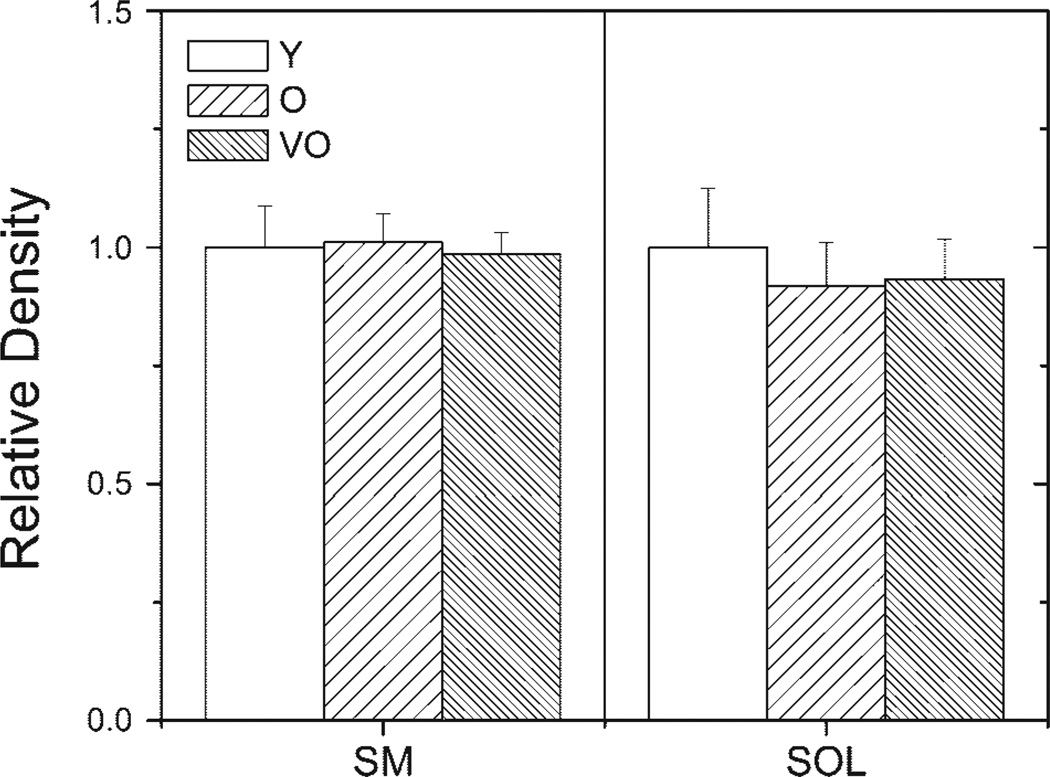
Densitometric analysis of the MHC and actin protein bands on silver-stained gels was used to provide an estimate of the relative content. Equivalent total lane density was the measure for ensuring equal protein loads for each sample. Comparison of protein band density showed no age-dependent difference in either MHC (Fig. 3) or actin (Fig. 4) in the soleus muscle. In the semimembranosus, MHC content was significantly decreased when comparing young adult with very old rats (Fig. 3), but actin content remained constant with aging (Fig. 4).
Figure 3.
Quantification of myosin content in SM and SOL muscles. Graphs show the mean (±SE) density of the MHC protein band on a silver-stained gel for Y, O, or VO rats. N = 6/age group. *P < 0.05 compared with Y rats.
Figure 4.
Quantification of actin content in SM and SOL muscles. Graphs show the mean (±SE) density of the actin protein bands on a silver-stained gel for Y, O, or VO rats. N = 6/age group.
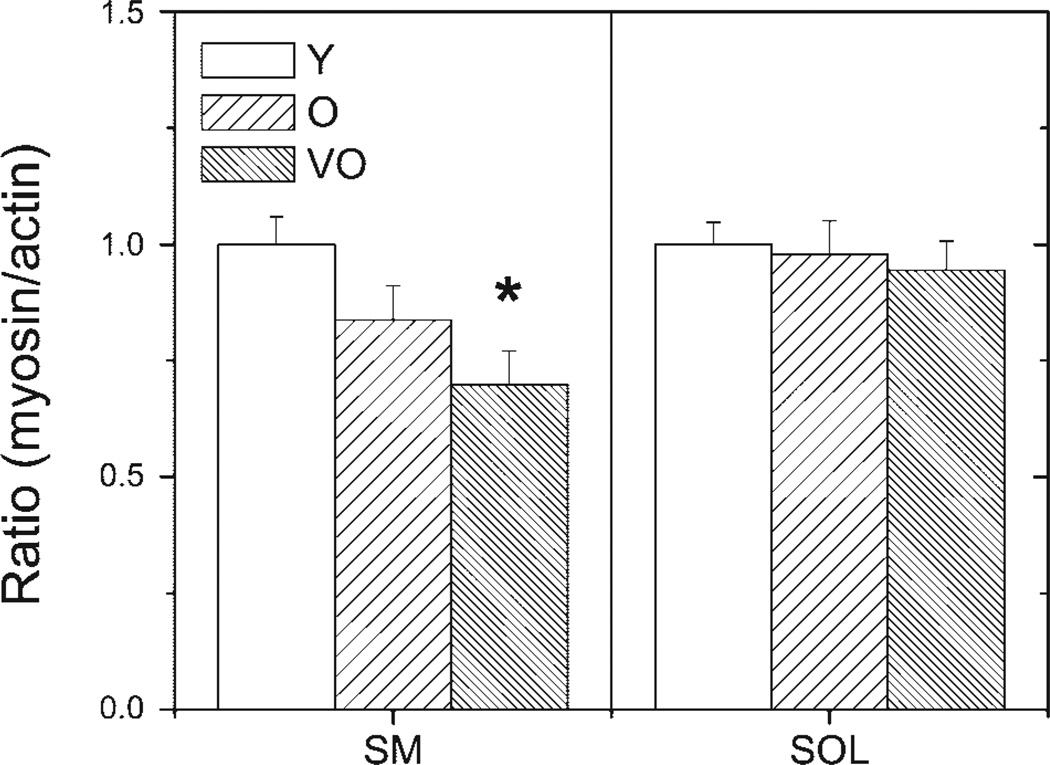
To determine the changes in relative content of myosin and actin, the ratio of relative density was calculated for each muscle type. Figure 5 shows the myosin-to-actin ratio for different ages in each muscle. The myosin-to-actin ratio in the semimembranosus showed an ~30% decline when comparing young adult and very old rats (P = 0.01), but ratios did not differ in the soleus (P = 0.81).
Figure 5.
Ratio of myosin to actin density in SM and SOL muscles. Graphs show the mean ratio (±SE) of the density of MHC to actin for Y, O, and VO rats. *P < 0.05 compared with Y rats.
Protein oxidative modification
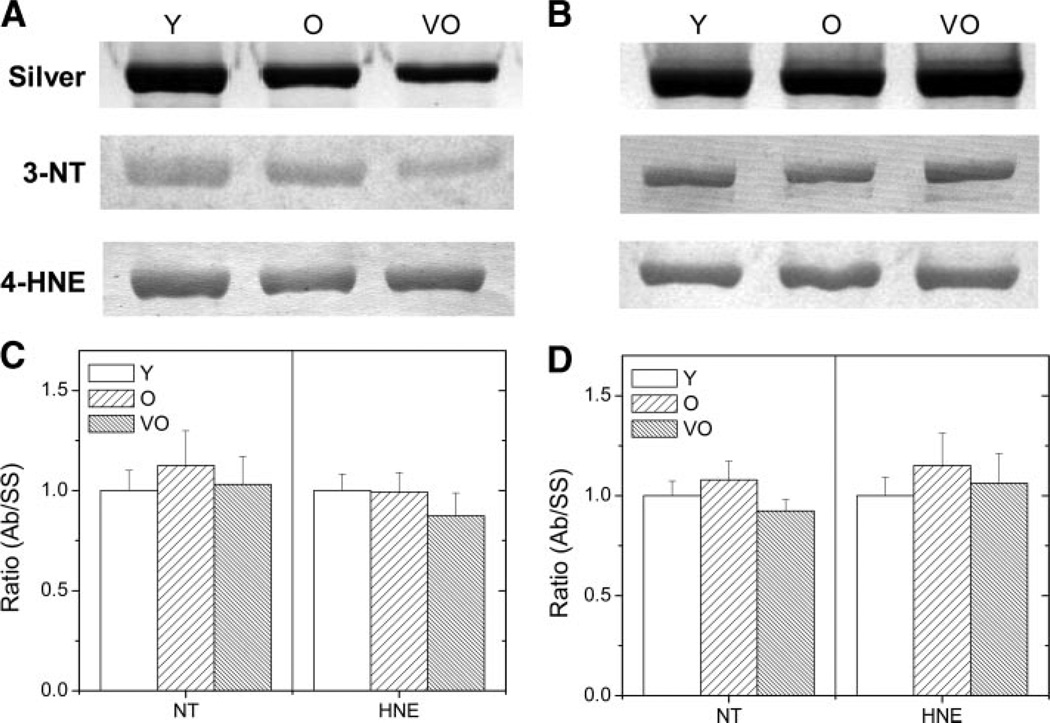
To test the hypothesis that oxidation of myofibrillar proteins could play a role in the decline in force-generating capacity in aged muscle, protein nitration and HNE modification were evaluated in MHC and actin. Western immunoblots of the myofibrillar fraction isolated from the semimembranosus and soleus muscle were probed with antibodies that recognize 3NT and proteins modified by HNE. For MHC, a positive immune reaction to both antibodies was observed for all ages (Fig. 6). Since there is a decrease in total content of MHC in aged semimembranosus, density measures of the immune reactions were normalized to the content of MHC in each sample. Thus the ratio of the density of the immunreaction per MHC density provides an estimate of the extent of oxidative modification. As shown in Fig. 6, there was no age-related difference in MHC modification for both 3NT and HNE.
Figure 6.
Relative content of MHC modified by 3-nitrotyrosine (3NT) or 4-hydroxy-2-nonenal (4-HNE) in SM (A and C) and SOL (B and D) muscles. A and B: representative silver-stained gel (silver) and Western blot (3NT, HNE) of MHC from Y, O, and VO rat muscle. C and D: mean ratios of MHC density on immunoblots per density on silver-stained gels. Data on graphs are normalized to the Y sample. Ab/SS, antibody/silver stained.
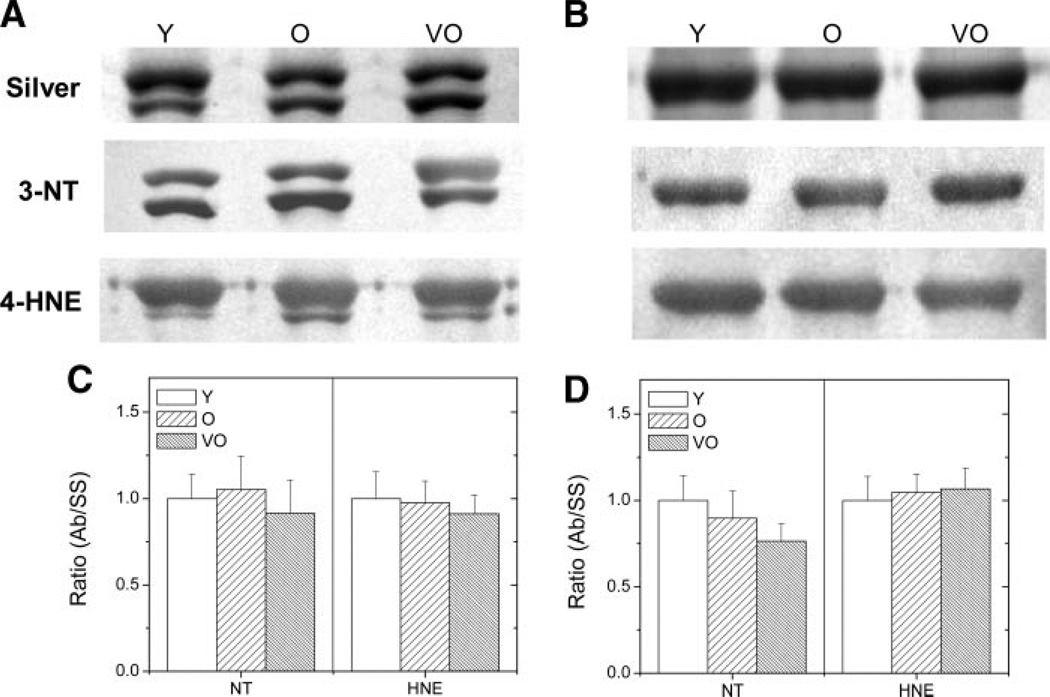
Similar results were also found for actin in the soleus and semimembranosus muscles. While actin is also modified by both 3NT and HNE, there was no age-dependent change in the extent of oxidative modification for this protein (Fig. 7).
Figure 7.
Relative content of actin modified by 3NT or 4-HNE in SM (A and C) and SOL (B and D) muscles. A and B: representative silver-stained gel (silver) and Western blot (3NT, HNE) of actin from Y, O, and VO rat muscle. C and D: mean ratios of actin density on immunoblots per density on silver-stained gels. Data on graphs are normalized to the Y sample.
DISCUSSION
In the present study, we examined actin and myosin isolated from the soleus and semimembranosus muscles to investigate two potential mechanisms that could contribute to the decrease in force-generating interactions of myosin and actin. Three points across the lifespan of the Fischer 344 rat were evaluated. The young adult age group (98% survival) represents an animal with no apparent changes in force-generating capacity of single type I and type II fibers (35–37). In contrast, type I fibers from old animals (50% survival) show an 18% decline in force, whereas the type II fibers exhibit a 30% reduction in force (25, 37). At 25% survival, for very old age, significant declines in force-generating capacity are documented in both fiber types (25, 37). Due to the age-related functional differences between fiber types, the semimembranosus (predominantly type II fibers) and the soleus (predominantly type I fibers) were evaluated.
Optimal stoichiometry of myofibrillar proteins
Force production is dependent on optimal stoichiometry of the myofibrillar proteins, specifically myosin and actin content. Myosin and actin content depends on the balance between protein synthesis and degradation. In the present study, the myosin expression decreases with age in the semimembranosus muscle coupled with no change in actin expression. The preferential reduction in myosin content leads to altered stoichiometry of the myofibrillar proteins. This finding is supported by data showing a significant reduction in muscle protein synthesis rates with age (1, 34, 38, 42). The preferential decrease in myosin expression with age compared with actin expression is consistent with previous studies investigating synthesis rates of the different myofibrillar proteins (1, 2). For example, there is a greater age-related decline in myosin protein synthesis relative to total muscle protein synthesis (1, 11).
In the present study, we observe a selective reduction of myosin expression in the semimembranosus (type II fibers) muscle in contrast to no change in myosin expression in the soleus (type I fibers) muscle. This fiber-type difference is consistent with studies that report a substantial reduction in transcript levels of MHC type II with age compared with MHC type I (2, 34). Thus, over time, this selective reduction of myosin relative to actin in the semimembranosus muscle would result in a change in optimal stoichiometry of the myofibrillar proteins relative to each other. The loss of optimal stoichiometry between these two key proteins critical for force production likely results in decreased force generation in the affected muscle.
Selective myosin protein loss compared with actin is not unprecedented. For example, studies show myosin depletion under the condition of muscle disease and disuse (10, 12). There is significant muscle weakness associated with muscle disease and disuse.
It is important to note that age-related denervation and the process of denervation-reinnervation may play a role in the current muscle-specific findings. For example, during the period between late middle age and old age, substantial structural and functional decreases occur in the number of motor units, the number of innervated fibers, and the ability of the motoneurons to sprout and reinnervate denervated muscle fibers (21). These neural changes occur earlier and to a greater extent in fast-twitch muscles compared with slow-twitch muscles (21). Thus the observed semimembranosus changes in contractile proteins compared with the soleus may be related to innervation.
Protein oxidative modification
Reports of oxidative damage in skeletal muscle with age have produced equivocal results (5, 22,23,26,28–31). Most studies evaluated oxidative damage, i.e., carbonyls, lipid peroxidation, nitration, in muscle samples containing a mixture of fiber types, thus precluding detection of fiber type-specific differences (5, 22, 23). Some of these studies point to an accumulation of oxidative damage in select muscle proteins with age (5, 26, 28). However, the studies did not identify specific protein targets of free radical attack, and therefore direct comparisons between these previous studies and our study is not possible. A few recent studies identified nitrated proteins in cardiac and skeletal muscle from different aged rats (9, 16–19). These studies reported that nitrated proteins are present even in young adult muscle. However, a few specific proteins demonstrated an age-related accumulation of 3-NT (9).
The selective susceptibility of specific proteins to oxidative modification was also demonstrated for the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). In both fast- and slow-twitch muscle, the slow isoform of the Ca2+-ATPase (SERCA2a) was selectively nitrated, even in muscle containing abundant amounts of the fast isoform, SERCA1 (40, 41). Furthermore, MS analysis of tryptic peptides from aged rats identified the sites of nitration at tyrosine residues 294, 295, and 753. Nitration at these specific sites correlated with a significant loss in Ca2+-ATPase function, suggesting the functional importance of one or more of these residues.
Based on the fiber-type-specific reductions in force-generating capacity (30% reduction in force-generating capacity in old rats), we would expect to observe an accumulation of 3NT or HNE on myosin and/or actin from the semimembranosus (50% survival) and in both muscles of the very old rats. However, our data do not show a statistically significant increase in protein oxidation in either protein from the old and very old rats compared with the young adult rats. Thus accumulation of 3NT or HNE in actin and myosin is not the mechanism for the decline in force. However, age-dependent modifications at selective amino acid residues critical for function cannot be ruled out. Additionally, other protein targets that we did not investigate could be modified and affect function.
Summary
To investigate mechanisms that could explain the age-related loss in muscle strength, actin and myosin isolated from the soleus and semimembranosus muscles were examined for changes in protein expression and oxidative modification. We observed a significant decline in myosin protein expression in the semimembranosus muscle that resulted in a change in the optimal stoichiometry between myosin and actin with age. This change in stoichiometry may decrease the number of active cross bridges contributing to force generation, providing a mechanism for muscle weakness. Second, the levels of two markers of oxidative stress, 3NT and HNE, on myosin and actin did not increase with age. This finding suggests that accumulation of oxidative damage to these two key myofibrillar proteins does not occur with age. It should be noted that, with aging, other oxidative modifications might accumulate and/or a site-specific amino acid modification of critical residues on these proteins could adversely affect function and contribute to muscle weakness.
ACKNOWLEDGMENTS
We thank Janice Shoeman and Sheng Zhong for technical assistance.
GRANTS
This work was supported by National Institute on Aging Grants AG-17768 and AG-21626 (L. V. Thompson).
REFERENCES
- 1.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age-underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–439. [PubMed] [Google Scholar]
- 4.Brown M, Hasser EM. Complexity of age-related change in skeletal muscle. J Gerontol A Biol Sci Med Sci. 1996;51:B117–B123. doi: 10.1093/gerona/51a.2.b117. [DOI] [PubMed] [Google Scholar]
- 5.Cakatay U, Telci A, Kayali R, Tekeli F, Akcay T, Sivas A. Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin Biochem. 2003;36:51–55. doi: 10.1016/s0009-9120(02)00407-1. [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Dean RT. Radical-mediated Protein Oxidation. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 7.Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- 8.Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Fugere NA, Ferrington DA, Thompson LV. Protein nitration with aging in the rat semimembranosus and soleus muscles. J Gerontol A Biol Sci Med Sci. 2006;61:806–812. doi: 10.1093/gerona/61.8.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. I. Cellular markers of protein deficits. J Appl Physiol. 2003;95:781–790. doi: 10.1152/japplphysiol.00317.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 12.Haycock JW, Mac Neil S, Mantle D. Differential protein oxidation in Duchenne and Becker muscular dystrophy. Neuroreport. 1998;9:2201–2207. doi: 10.1097/00001756-199807130-00010. [DOI] [PubMed] [Google Scholar]
- 13.Humphries KM, Szweda LI. Selective inactivation of alpha ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with HNE. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 14.Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 16.Kanski J, Alterman MA, Schoneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic Biol Med. 2003;35:1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 17.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kanski J, Hong SJ, Schoneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J Biol Chem. 2005;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- 19.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleaveage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 22.Leeuwenburgh C, Hansen P, Shaish A, Holloszy JO, Heinecke JW. Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R453–R461. doi: 10.1152/ajpregu.1998.274.2.R453. [DOI] [PubMed] [Google Scholar]
- 23.Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- 24.Lipman RCC, Hazzard D, Bronson R. Pathologic characterization of Brown Norway, Brown Norway × Fischer 344, and Fischer 344 × Brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 26.Marzani B, Felzani G, Bellomo RG, Vecchiet J, Marzatico F. Human muscle aging: ROS-mediated alterations in rectus abdominis and vastus lateralis muscles. Exp Gerontol. 2005;40:959–965. doi: 10.1016/j.exger.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 27.McDonough JL, Neverova I, Van Eyk JE. Proteomic analysis of human biopsy samples by single two-dimensional electrophoresis: Coomassie, silver, mass spectrometry, and Western blotting. Proteomics. 2002;2:978–987. doi: 10.1002/1615-9861(200208)2:8<978::AID-PROT978>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 29.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–622. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 30.Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- 31.Pansarasa O, Felzani G, Vecchiet J, Marzatico F. Antioxidant pathways in human aged skeletal muscle: relationship with the distribution of type II fibers. Exp Gerontol. 2002;37:1069–1075. doi: 10.1016/s0531-5565(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 32.Prochniewicz E, Thomas DD, Thompson LV. Age-related decline in actomyosin function. J Gerontol A Biol Sci Med Sci. 2005;60:425–431. doi: 10.1093/gerona/60.4.425. [DOI] [PubMed] [Google Scholar]
- 33.Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mortensen P, Shevchenko A, Boucherie H, Mann M. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Short KR, Vittone JL, Bigelow ML, Proctor DN, Coenen-Schimke JM, Rys P, Nair KS. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99:95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- 35.Thompson LV. Contractile properties and protein isoforms of single skeletal muscle fibers from 12-and 30-mo-old Fischer 344 Brown Norway F1 hybrid rats. Aging (Milano) 1999;11:109–118. [PubMed] [Google Scholar]
- 36.Thompson LV. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002;32:44–57. doi: 10.2519/jospt.2002.32.2.44. [DOI] [PubMed] [Google Scholar]
- 37.Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol. 1999;86:881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- 38.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- 39.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viner RI, Ferrington DA, Huhmer AFR, Bigelow DJ, Schoneich C. Accumulation of nitrotyrosine on the SERCA2a isoform of SR Ca-ATPase of rat skeletal muscle during aging: a peroxynitrite-mediated process? FEBS Lett. 1996;379:286–290. doi: 10.1016/0014-5793(95)01530-2. [DOI] [PubMed] [Google Scholar]
- 41.Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 42.Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol Endocrinol Metab. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]