Abstract
Adult-born hippocampal neurons are important for cognitive plasticity in rodents. There is evidence for hippocampal neurogenesis in adult humans, although whether its extent is sufficient to have functional significance has been questioned. We have assessed the generation of hippocampal cells in humans by measuring the concentration of nuclear bomb test-derived 14C in genomic DNA and we present an integrated model of the cell turnover dynamics. We found that a large subpopulation of hippocampal neurons, constituting one third of the neurons, is subject to exchange. In adult humans, 700 new neurons are added per day, corresponding to an annual turnover of 1.75% of the neurons within the renewing fraction, with a modest decline during aging. We conclude that neurons are generated throughout adulthood and that the rates are comparable in middle aged humans and mice, suggesting that adult hippocampal neurogenesis may contribute to human brain function.
New neurons integrate throughout life in the hippocampus and olfactory bulb of most mammals. The newborn neurons have enhanced synaptic plasticity for a limited time after their differentiation (Ge et al., 2007; Schmidt-Hieber et al., 2004), which is critical for their role in mediating pattern separation in memory formation and cognition in rodents (Clelland et al., 2009; Nakashiba et al., 2012; Sahay et al., 2011). It has been long debated whether adult neurogenesis decreased during primate evolution and if there is sufficient generation of neurons in adult humans to contribute to brain function (Kempermann, 2012; Rakic, 1985). A seminal study by Eriksson, Gage and colleagues provided the only direct evidence to date for adult neurogenesis in humans (Eriksson et al., 1998), although it did not enable assessing the number of new neurons generated or the dynamics of this process.
To estimate the extent of adult neurogenesis in humans, recent studies have quantified the number of cells expressing the neuronal precursor (neuroblast) marker doublecortin in the subventricular zone, which gives rise to olfactory bulb neurons, and in the dentate gyrus of the hippocampus (Knoth et al., 2010; Sanai et al., 2011; Wang et al., 2011). Very similar dynamics have been revealed in these two regions, which contain a large number of neuroblasts shortly after birth that then decreases sharply during the first postnatal year and then declines more moderately through childhood and adult life (Göritz and Frisén, 2012; Knoth et al., 2010; Sanai et al., 2011; Wang et al., 2011). The decrease in neuroblast numbers in the subventricular zone and their migratory path suggested that there is negligible, if any, adult olfactory bulb neurogenesis in humans (Arellano and Rakic, 2011; Sanai et al., 2011; Wang et al., 2011). Retrospective birth dating established that olfactory bulb neurons are as old as the individual, and if there is any addition of neurons in the adult human olfactory bulb, less than 1% of the neurons are exchanged over a century (Bergmann et al., 2012). It appears unlikely that adult olfactory bulb neurogenesis has any functional significance in humans. The similar decline in neuroblast numbers in the subventricular zone and the hippocampus poses the question of whether there is postnatal hippocampal neurogenesis in humans to an extent that may have an impact on brain function.
Analysis of the number of neuronal progenitor cells gives an indirect indication of the possible extent of neurogenesis. However, it does not provide information on whether the neuroblasts differentiate and integrate as mature neurons. This is evident from the studies of the subventricular zone and olfactory bulb, where the generation of neuroblasts does not result in detectable integration of new neurons in the olfactory bulb (Bergmann et al., 2012). The strategies used to study the generation of mature neurons in experimental animals are not readily applicable to humans. To be able to study cell turnover dynamics in humans, we have developed a strategy to retrospectively birth date cells (Spalding et al., 2005a). This strategy takes advantage of the elevated atmospheric 14C levels caused by above ground nuclear bomb testing 1955–63 during the Cold War (De Vries, 1958; Nydal and Lovseth, 1965). After the International Test Ban Treaty in 1963, the atmospheric levels have declined due to uptake by the biotope and diffusion from the atmosphere (Levin and Kromer, 2004; Levin et al., 2010). 14C in the atmosphere reacts with oxygen to form CO2, which is taken up by plants in photosynthesis. When we eat plants, or animals that live off plants, we take up 14C, making atmospheric 14C levels mirrored in the human body at all times (Harkness, 1972; Libby et al., 1964; Spalding et al., 2005b). When a cell goes through mitosis and duplicates its chromosomes, it integrates 14C in the synthesized genomic DNA with a concentration corresponding to that in the atmosphere at the time, creating a date mark in the DNA (Spalding et al., 2005a). The cumulative nature of 14C integration, makes the method especially suited for establishing the kinetics of slowly turning over cell populations. The accuracy of individual datings is approximately ±1.5 years (Spalding et al., 2005b), but higher accuracy is reached by integrating data from many independent measurements.
We have retrospectively birth dated hippocampal cells and provide an integrated model for adult hippocampal neurogenesis in humans. We report that there is substantial neurogenesis in the human hippocampus throughout life, to an extent comparable to that in the middle aged mouse, supporting that adult hippocampal neurogenesis may contribute to human brain function.
Results
Retrospective birth dating of cells from the human hippocampus
Cell nuclei were isolated by gradient centrifugation from dissected human postmortem hippocampi. The nuclei were incubated with antibodies against the neuron specific nuclear epitope NeuN, and neuronal and non-neuronal nuclei were isolated by flow cytometry (Fig. 1 and Fig. S1) (Bergmann et al., 2012; Bhardwaj et al., 2006; Spalding et al., 2005a). The 14C concentration in genomic DNA from hippocampal neurons (n=55) and non-neuronal cells (n=65) was measured by accelerator mass spectrometry in subjects between 19 and 92 years of age (14C data is given in Table S1).
Figure 1. Isolation of neuronal and non-neuronal nuclei from the human hippocampus.

Cell nuclei were isolated from the human postmortem hippocampus and incubated with an isotype control antibody (A) or with an antibody against the neuron-specific epitope (NeuN) (B), and the neuronal and non-neural populations were isolated by flow cytometry. The sorting gate for neuronal nuclei is indicated.
Standard accelerator mass spectrometry analysis requires samples corresponding to about 1 mg of carbon. The total amount of carbon in genomic DNA samples from hippocampal cell populations, after cell sorting and purifications steps is typically in the range 10–20 μg, necessitating a different approach. Consequently, a new experimental method had to be developed, including a new sample preparation setup and laboratory procedure to address various critical issues including reliability and accuracy (Salehpour et al., 2013).
To infer the cell turnover dynamics in the adult hippocampus, several mathematical models, or scenarios, with increasing detail were fitted to the 14C data. All scenarios were based on a birth-and-death process, by which cells can die or be added to a cell population. A scenario defines a set of rules for how cells are born, die or renew; i.e. it sets whether there should be more, less or equal birth and death, which cells will die preferentially or renew, etcetera. For each of these scenarios, a set of parameters quantifies the extent of renewal. The mathematical model tracks the chronological age of each cell and the age of the person with a variable n(t, α), with the cell density (units in cells per year) of age α in a person aged t. The evolution of the cell density is given by a biological transport equation, which move cells along age as time progresses, with a loss term accounting for cell death: ∂n(t, α)/∂t + ∂n(t, α)/dα = γ(t, α)n(t, α). An initial condition describing the cell population at birth and a boundary condition describing how new cells are added are supplemented to the transport equation to solve the problem fully (equations are given in the Extended Experimental Procedures). Solving the problem allows the prediction of the 14C level for a sample, by integrating the solution n(t, α) along the atmospheric 14C curve between the birth and death of the individual. By comparing the model prediction to all neuronal or non-neuronal cell data, best parameter sets for each scenario was found. The best scenarios were selected based on Akaike Information Criterion (AIC), i.e. their goodness-of-fit and their level of detail. For Scenario A (constant turnover) and Scenario 2POP (constant turnover in a fraction of cells), individual turnover rates could also be estimated.
Turnover of non-neuronal cells in the adult human hippocampus
We first assessed the turnover dynamics of non-neuronal (NeuN-) cells in the human hippocampus. The 14C concentration in genomic DNA corresponded to time points after the birth of the individuals (Figure 2A, B), establishing turnover of non-neuronal cells in the human hippocampus. Mathematical modeling of 14C data allowed a detailed analysis of the dynamics of cell turnover (Bergmann et al., 2009; Bergmann et al., 2012; Spalding et al., 2008). By fitting the models to the data, we can infer how much cell renewal is needed to reproduce the observed 14C levels and whether the renewal is restricted to a subpopulation (see Fig. S2 and the extended experimental procedures). The best model, based on AIC, was Scenario 2POP, in which a fraction of the population is renewing and the other is not. In this scenario, cells within the renewing fraction are set to turn over at a constant rate throughout life. This scenario indicated that a large proportion of the non-neuronal cells (51%, CI [22%, 88%]) is continuously exchanged. The median turnover rate within the subpopulation of non-neuronal cells undergoing exchange is 3.5%/year (Figure 2C, Table S2). Individual turnover estimates suggest that there is a decline in the turnover of non-neuronal cells during aging (r=−0.35, p=0.04). The average age of non-neuronal cells within the renewing fraction at different ages of an individual is shown in Figure 2D.
Figure 2. Turnover dynamics of non-neuronal cells.
(A) Schematic illustration of the representation of the measured 14C concentration in genomic DNA. The black line indicates the 14C concentration in the atmosphere at different time points in the last century. Individually measured 14C concentrations in genomic DNA of human hippocampal cells are plotted at the time of the subject's birth (vertical lines), before (green dot) or after the 14C bomb spike (orange dot). 14C concentrations above the bomb curve (subjects born before the bomb peak) and data points below the bomb curve (subjects born after the nuclear tests) indicate cellular turnover. (B) The 14C concentrations of genomic DNA from non-neuronal cells demonstrate post-natal cell turnover in subjects born before and after the bomb spike. (C) Individual turnover rates for non-neuronal cells computed based on individual data fitting. Individual turnover rate calculations are sensitive to deviations in measured 14C and values <0.001 or >1.5 were excluded from the plot, but the full data is given in Table S1. (D) Non-neuronal average cell age estimates of cells within the renewing fraction are depicted (red curve). The dashed line represents a no-cell-turnover scenario.
Hippocampal neurogenesis in adult humans
We next analyzed the 14C concentration in neuronal genomic DNA. One can draw several conclusions regarding hippocampal neurogenesis from the raw data (Figure 3). First, the 14C concentration in genomic DNA of hippocampal neurons corresponds to the concentration in the atmosphere after the birth of the individual, confirming postnatal generation of hippocampal neurons in humans (Eriksson et al., 1998). This finding is in contrast to cortical and olfactory bulb neurons, which are not exchanged postnatally to a detectable degree in humans, with 14C levels corresponding to the time around the birth of the individual (Bergmann et al., 2012; Bhardwaj et al., 2006; Spalding et al., 2005a). Second, the oldest studied subjects had higher 14C concentrations in neuronal DNA than were present in the atmosphere before 1955 (Figure 3). This finding establishes that there has been DNA synthesis after 1955, indicating hippocampal neurogenesis at least into the fifth decade of life (the oldest individual was 42 years old in 1955). Third, the rather uniformly elevated levels of 14C in individuals born before the onset of the nuclear bomb tests indicate that there can be no dramatic decline in hippocampal neurogenesis with age; if there was a substantial decrease in neurogenesis during aging, individuals born longer before the rise in atmospheric 14C would have incorporated less of the elevated 14C levels present after 1955. Fourth, individuals born before the onset of nuclear bomb tests have lower 14C levels in hippocampal neuron DNA than at any time after 1955, establishing that, although some neurons are generated postnatally, the hippocampus is heterogeneous and a large subset of hippocampal neurons is not exchanged postnatally. Thus, it is evident from the raw data that there is substantial generation of hippocampal neurons in humans, restricted to a subpopulation, without any dramatic decline during adulthood.
Figure 3. Hippocampal neurogenesis in adult humans.
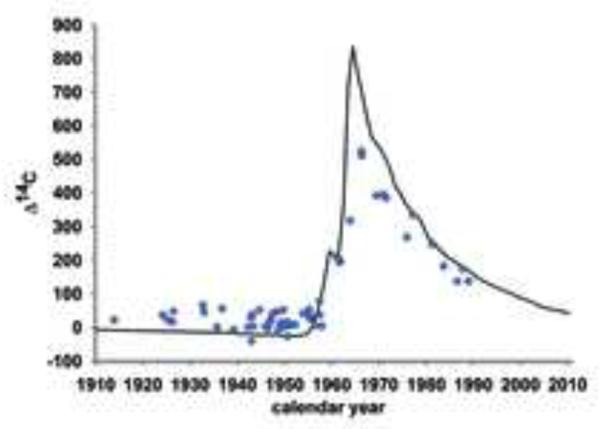
14C concentrations in hippocampal neuron genomic DNA correspond to a time after the date of birth of the individual, demonstrating neurogenesis throughout life.
A large proportion of hippocampal neurons is subject to turnover
Adult hippocampal neurogenesis in mammals is restricted to the dentate gyrus (Kempermann, 2012). With the current sensitivity of accelerator mass spectrometry, it is not possible to separately carbon date neurons from subdivisions of the human hippocampus. However, neuroblasts and BrdU-labeled neurons have only been demonstrated in the dentate gyrus in adult humans (Eriksson et al., 1998; Knoth et al., 2010), indicating that neuronal turnover is restricted to the dentate gyrus also in humans. With the term turnover of neurons, it is not implied that individual neurons that are lost are replaced by new neurons taking over their function, but that there is an exchange of neurons at the population level. It was evident from the raw data (Figure 3) that not all hippocampal neurons are exchanged postnataly in humans. Models that allowed two compartments, one continuously turning over population and one non-renewing, fitted the data much better than any other model (see the supplemental material). Scenario 2POP indicates that the size of the cycling neuronal population constitutes 35% (CI [12%, 63%]) of hippocampal neurons (Figure 4A), corresponding to slightly less than the proportion of hippocampal neurons that constitutes the dentate gyrus in humans (see further below). This finding indicates that the vast majority of dentate gyrus neurons are subject to exchange in humans, differing from the situation in the mouse, in which approximately 10% of the dentate gyrus neurons are subject to exchange (Imayoshi et al., 2008; Santos et al., 2007). The proportion of hippocampal neurons that are exchanged has not been addressed in other species.
Figure 4. Subpopulation dynamics of hippocampal neurons and non-neurons.
(A) Hill function indicates that the fraction of neurons being exchanged is homogenous and confers to one mode of exchange. (B) In line with a non-neuronal population comprised of several cell types, the Hill function indicates that the nonneuronal cells form a heterogeneous group, with some subpopulations having high turnover rates and some very low. The z-axis indicates different possible solutions compatible with the data. Only solutions with a good fit are shown, with those with the highest probability indicated in red and lower probability in blue.
It is possible that cells in the hippocampus form a heterogeneous population in terms of renewal. A scenario with a continuum of turnover rates was used to assess the heterogeneity of the neuronal and non-neuronal cell populations (Scenario XPOP). The modeling indicates that the neuronal subpopulation that is turning over in the hippocampus is rather homogeneous and confers to one mode of exchange (Figure 4A). The non-neuronal cells form a heterogeneous group of cells, consisting mainly of astrocytes, microglia and oligodendrocyte-lineage cells, but also containing several smaller populations of, for example, leukocytes and blood vessela-ssociated endothelial and perivascular cells. In line with this, models that allowed subpopulations to have different turnover dynamics fitted the non-neuronal data best. The non-neuronal cells appear more heterogeneous than the neurons, with some having high turnover rates and some very low (Figure 4B).
The rate of neuronal turnover in the human hippocampus
As the majority of hippocampal neurons are not exchanged, the average age of hippocampal neurons increases with the age of the individual, which may give the false impression that the turnover rate decreases sharply during aging. However, when taking into account that neurogenesis is restricted to a subpopulation, individual estimates of turnover rates indicate a more modest decline in turnover with aging within this population (Fig. 5A, Fig. S3, Table S3, r=−0.31, p=0.03, Scenario 2POP). The median turnover rate of neurons within the renewing subpopulation is 1.75%/year during adulthood, corresponding to approximately 700 new neurons/day or 0.004% of the dentate gyrus neurons/day in the human hippocampus. The turnover rate of hippocampal neurons is not significantly different between men and women (P=0.41, ANOVA). The average age of neurons within the renewing fraction at different ages of an individual is shown in Figure 5B.
Figure 5. Neuronal turnover dynamics in the human hippocampus.
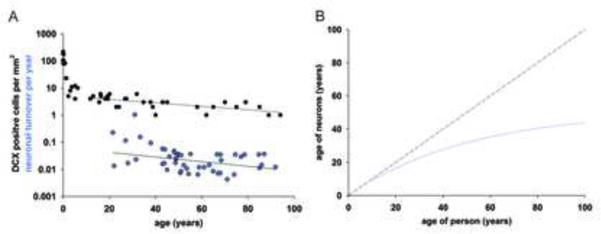
(A) Individual turnover rates for neuronal cells within the renewing fraction were computed based on individual data fitting. The number of doublecortin (DCX)-positive cells per mm2 in the dentate gyrus (data from Knoth et al, 2010) shows a similar modest decline during adult ages as the computed neuronal turnover rates. Straight lines depict linear regression curves, with the regression line for DCX cell counts being calculated for individuals 10 years and older. Individual turnover rate calculations are sensitive to deviations in measured 14C and values <0.001 or >1.5 were excluded from the plot, but the full data is given in Table S1. (B) The average age of the neurons within the renewing fraction (blue curve). The dashed line represents the no-cell-turnover scenario.
Comparing the turnover rates between the full neuronal and non-neuronal hippocampal populations reveals a significantly higher turnover rate within the non-neuronal compartment (p<2e-5, Wilcoxon signed rank test, Scenario A). This finding is largely explained by a larger subset of cells turning over within the non-neuronal population than within the neuronal population, and when comparing the turnover rates specifically within the respective subpopulations that are subject to cellular exchange, there was no significant difference in turnover rates between the neuronal and non-neuronal populations (p=0.054, Wilcoxon signed rank test, Scenario 2POP). However, as non-neuronal cells are more abundant than neurons in the human hippocampus (Fig. 1A), a larger number of non-neuronal cells in absolute numbers is generated.
There was no correlation between the neuronal and non-neuronal turnover rates within individuals older than 50 years (r=−0.14, p=0.58, Scenario 2POP), suggesting that the generation of these different cell types is regulated independently, as in the mouse (Steiner et al., 2004). However there was a correlation in young individuals (<50 years, r=−0.62, p=0.003). The inter-individual variation in the turnover rate of neurons and non-neuronal cells in the hippocampus is similar, with a median absolute deviation of 0.0226 and 0.0158 per year, respectively. The inter-individual variation may appear largest in the younger subjects, but this is a consequence of the shallow slope of the atmospheric 14C levels in recent times, which provides less resolution and therefore introduces higher variability.
An integrated model of neuronal dynamics in the human hippocampus
The determination of the fraction of neurons that is subject to exchange in the human hippocampus and their turnover rate makes it possible to infer the age of the full complement of neurons in individuals of different ages. The hippocampus is a mosaic of neurons of different ages, with a large fraction of cells remaining from development and with neurons generated at different times throughout life. Stereological quantifications have revealed a decrease in the number of hippocampal neurons during aging in humans, with the dentate gyrus being least affected (Fig. S4). A relative increase in the proportion of neurons in the renewing fraction with age fits the 14C data well.
The most detailed model, Scenario 2POPEd, provides a global picture of the dynamics of neuronal turnover. Non-renewing neurons die without being replaced, resulting in a slow decrease during life. Within the renewing neuron population, young cells die faster, leading to a neuron age distribution with less middle-aged cells than would be expected if all neurons were as likely to be replaced. One observation from the modeling is that adult-born neurons are preferentially lost and do not survive as long as the neurons generated during development. The half-life of a neuron in the renewing fraction is 7.1 years, or 10 times shorter than in the non-renewing fraction. Although it is known that adult-born neurons integrate long-term in rodents, whether they last for the remainder of the animal's life has not been studied, although the available data are compatible with a preferential loss of adult-born neurons (Imayoshi et al., 2008; Kempermann et al., 2003; Ninkovic et al., 2007). The integrated model of the dynamics of hippocampal neuron numbers and exchange in humans is shown in Figure 6.
Figure 6. An integrated model of the number and age of neurons in the human hippocampus.
Schematic illustration of the number of neurons in the dentate gyrus (above the white line) and the other subdivisions of the hippocampus (below the white line), and the age of neurons within the dentate gyrus at different ages. The total number of neurons declines with age in the hippocampus, with the dentate gyrus being relatively spared. The dentate gyrus is composed of a declining fraction of cells generated during development (black), which is gradually replaced by postnatally generated cells. For a given age of the person, postnatally generated cells are in different shades of gray, indicating decade intervals, with the lightest gray being cells generated during the last decade, one shade darker being cells generated 10–20 years ago, and so on. This way, at age 15, among postnatally generated cells, only cells generated 0–10 years ago and 10–20 years ago are present. Read vertically, for a fixed age of the person, the cell age distribution goes from oldest cells (black) to the youngest ones (light gray). Read horizontally, the fraction of adult-born cells (non-black) increases with age. The model is based on Scenario 2POPEd. The figure was generated using parameters: initial fraction of renewing neurons: 0.31, death rate of the non-renewing neurons: 0.0035/year, death rate of newborn neurons: 0.11/year, cell age at which the death rate has reduced by half: 19 years. The parameter set was selected among the 3% best out of 3×105 parameter sets explored using a Markov Chain Monte Carlo algorithm, and consistent with Scenario 2POP.
Discussion
Newborn neurons in the adult hippocampus have distinct features for a limited period after their differentiation that give them a key role in pattern separation and cognitive adaptability in rodents. We have birth dated hippocampal cells to assess whether adult neurogenesis occurs to a significant extent in adult humans, and provide a detailed view of the cell turnover dynamics. There is substantial neurogenesis throughout life in the human hippocampus, with only a modest decline during aging. There is a preferential loss of adult-born neurons and a larger proportion of hippocampal neurons is subject to exchange in humans compared to the mouse. Nonneuronal cells have more heterogeneous turnover dynamics than hippocampal neurons.
It is important to consider whether DNA repair may contribute to 14C integration in hippocampal cells. DNA damage and repair are largely restricted to proliferating cells and are believed to be several orders of magnitude lower in postmitotic cells than is detectable by 14C dating (Spalding et al., 2005a). DNA repair during cell proliferation will not affect the assessment of cell generation, as 14C integrates in DNA at a concentration corresponding to that in the atmosphere during mitosis. We have not found any measurable 14C integration in the DNA of cortical, cerebellar or olfactory bulb neurons over many decades in humans (Bergmann et al., 2012; Bhardwaj et al., 2006; Spalding et al., 2005a). Not even neurons surviving at the perimeter of an ischemic cortical stroke, a situation where there is substantial DNA damage and repair, incorporate sufficient 14C to be detected (our unpublished data). The dynamics of 14C integration in the DNA of hippocampal neurons does not appear to be compatible with any pattern of DNA repair previously described; a large fraction of hippocampal neurons (35%) would have to exchange their entire genome by DNA repair during the lifetime of an individual, whereas there would be no detectable DNA repair in the remaining hippocampal neurons. In contrast, the number of neuroblasts reported in the adult human dentate gyrus (Knoth et al., 2010) is sufficient to give rise to the number of new neurons indicated by the 14C analysis and the decline in neurogenesis closely parallels the decrease in the number of neuroblasts (Figure 5A). Thus, the 14C concentration in genomic DNA of hippocampal neurons is likely to accurately reflect neurogenesis.
Retrospective birth dating reveals that what appears as small numbers of neuroblasts present in adulthood (Knoth et al., 2010) give rise to a substantial number of new neurons over time in the hippocampus. It is interesting in this context that the similar density of neuroblasts in the subventricular zone to that in the hippocampal dentate gyrus does not result in any detectable addition of new neurons to the olfactory bulb (Bergmann et al., 2012; Göritz and Frisén, 2012; Knoth et al., 2010; Sanai et al., 2011; Wang et al., 2011). The lack of olfactory bulb neurogenesis thus appears to be a consequence of an absence of migration and/or integration of new neurons in the olfactory bulb, rather than a lack of generation of neuroblasts.
There are some distinct differences in the pattern of adult hippocampal neurogenesis in humans compared to rodents, in which this process has been most extensively characterized. First, a much larger proportion of hippocampal neurons are subject to exchange in humans. In mice, 10% of the neurons in the dentate gyrus are added in adulthood and subject to exchange (Imayoshi et al., 2008; Ninkovic et al., 2007). In humans, approximately one third of the hippocampal neurons turn over, corresponding to the vast majority of the dentate gyrus neurons. Second, although hippocampal neurogenesis declines with age in both rodents and humans, the relative decline during adulthood appears smaller in humans compared to mice. Comparisons of the kinetics of the age-dependent decline in hippocampal neurogenesis between different species have revealed a similar chronology, rather than correlating to developmental milestones (Amrein et al., 2011). In line with this, the most dramatic decrease in the number of neuroblasts in the dentate gyrus occurs during the first postnatal months in both mice and humans (Ben Abdallah et al., 2010; Knoth et al., 2010). An effect of this is that young adult mice are still in the most steeply declining phase of neurogenesis, making the relative decrease in neurogenesis during adult life being much larger in mice than humans. Whereas there is an approximate ten-fold decrease in neurogenesis from 2 to 9 months of age in mice (Ben Abdallah et al., 2010), there is an approximate four-fold decline during the entire adult lifespan in humans (Fig. 5A). Third, the impact of adult neurogenesis on the total number of neurons in the dentate gyrus differs between rodents and humans. Hippocampal neurogenesis in mice and rats is additive and results in a net increase in the number of dentate gyrus neurons with age (Bayer, 1985; Imayoshi et al., 2008; Kempermann et al., 2003; Ninkovic et al., 2007). This is not the case in humans, where there is a net loss of dentate gyrus neurons during adult life. Although the decrease in neuronal numbers is less pronounced in the dentate gyrus than other subdivisions of the human hippocampus, the generation of new neurons does not keep up with the neuronal loss (Fig. 6). Computational models have indicated that addition of new neurons to the circuitry, together with loss of older redundant cells and enhanced synaptic plasticity can maximize the effect of the new neurons, whereas an isolated exchange of neurons would have less influence (Appleby et al., 2011). The adult generation of neurons serves to uphold a pool of neurons with specific functional properties, rather than replacing individual neurons that are lost. The continuous generation of new neurons in the adult human hippocampus may therefore have an additive role functionally in the circuitry, although more neurons are lost than generated.
Can the number of new neurons generated in the adult human hippocampus have functional significance? An indication of this may be gained by comparing the extent of adult neurogenesis in humans with that in other species, in particular the mouse, in which most experiments on the function of adult hippocampal neurogenesis have been carried out. It is difficult to make direct comparisons as there are several factors influencing the potential impact of newborn neurons that may vary between species, for example the total number of cells in the circuitry and for how long newborn neurons have distinct features. The best measure of adult neurogenesis when comparing across species may be the relative proportion of newborn to old neurons (Kempermann, 2012). We conclude that 0.004% of the dentate gyrus neurons are exchanged daily in adult humans, which can be compared to 0.03–0.06%/day in 2-month-old mice and 0.004–0.02%/day in 5- to 16-year-old macaque monkeys (Jabès et al., 2010; Kempermann et al., 1997; Kornack and Rakic, 1999). Hippocampal neurogenesis has been estimated to decrease approximately 10-fold between 2 months and 9 months of age in the mouse (Ben Abdallah et al., 2010), indicating that the rate of neurogenesis in adult humans may correspond to that of a 9-month-old mouse. Together with the extended period of immature features of the adult-born neurons in non-human primates (Kohler et al., 2011), and potentially humans, the relative proportion of adult born neurons with unique functions in the human hippocampus may not be smaller than that in a middle-aged mouse. Thus, the extent of neurogenesis in the adult human hippocampus may be sufficient to convey similar functions as in the mouse, in which adult neurogenesis is important for cognitive adaptability.
Adult-born hippocampal neurons have enhanced synaptic plasticity for a period of time after their differentiation (Ge et al., 2007; Schmidt-Hieber et al., 2004). This, together with the dentate gyrus being a bottleneck in the network, allows a small proportion of neurons to have a substantial influence on the circuitry and hippocampal function. The new neurons are required for efficient pattern separation, the ability to distinguish and store similar experiences as distinct memories, whereas the old granule cells are necessary for pattern completion, which serves to associate similar memories to each other (Clelland et al., 2009; Nakashiba et al., 2012; Sahay et al., 2011). Failing pattern separation may result in generalization, a common feature in anxiety and depression in humans (Kheirbek et al., 2012). There are a number of indications that implicate reduced neurogenesis in psychiatric disease, but it has been difficult to explore whether there is a link in humans (Eisch and Petrik, 2012). We find considerable interindividual variation in this study, and assessment of hippocampal neurogenesis together with reconstruction of medical histories may reveal whether reduced neurogenesis is associated with psychiatric disease in humans.
Experimental procedures
Tissue Collection
Tissues were procured between 2000 and 2012 from autopsy cases following informed consent from the individual or next-of-kin, from the Department of Forensic Medicine in Stockholm, Sweden, Department of Neurology, Miller School of Medicine Brain Endowment Bank in Miami, FL (USA) and the Department of Pathology, University of Debrecen, Hungary. Ethical permission for this study was granted by the Regional Ethical Committee in Stockholm (No 02-418, 2005/185, 2006/1029-31/2, 2006/189-31, 2010-313/31-3), Institutional Review Board of the University of Miami Miller School of Medicine (FWA00002247; IRB00005622) and the local institutional review board of the medical faculty in Debrecen, Hungary. Whole hippocampi were dissected and analyzed. Brain tissue was frozen and stored at −80°C until further analysis.
Nuclei Isolation
Tissue samples were thawed and Dounce homogenized in 10 ml lysis buffer (0.32 M sucrose, 5 mM CaCl2, 3 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-HCl [pH 8.0], 0.1% Triton X-100, 1 mM DTT). Homogenized samples were suspended in 20 ml of sucrose solution (1.7 M sucrose, 3 mM magnesium acetate, 1 mM DTT, 10 mM Tris-HCl [pH 8.0]), layered onto a cushion of 10 ml sucrose solution, and centrifuged at 36,500 g for 2.4 hr at 4°C. The isolated nuclei were re-suspended in Nuclei storage buffer (NSB) (10 mM Tris, pH=7.2; 2 mM MgCl2; 70 mM KCl and 15% sucrose) for consecutive immunostaining and flow cytometry analysis.
FACS sorting and analysis
Isolated nuclei were stained with mouse NeuN (A-60) (Millipore, 1:1000). NeuN (A-60) antibody was directly conjugated to Alexa 647 (Invitrogen Antibody Labeling Kit Alexa 647). Flow cytometry analyses and sorting were performed using a BD FACS Diva or Beckman Coulter MoFlo XDP high-speed sorter. FACS correction for sort purity is detailed in the extended experimental procedures. FACS gating strategy for sorts is shown in Figure 1 and Figure S1.
Correction for FACS impurities: In case the sorting purity was less than 100%, FACS impurities were corrected for by solving the following equation system for: Δ14Cnon–neurons_corrected and Δ14Cneurons_corrected (Yimpurity_non–neurons and Ximpurity–neurons are given in percent). Corrected values are shown in table S1.
| (I) |
| (II) |
DNA purification
All experiments were carried out in a clean room (ISO8) to prevent any carbon contamination of the samples. All glassware was pre-baked at 450°C for 4 hours. DNA isolation was performed according to a modified protocol from Miller et al (Miller et al., 1988). Five-hundred μl DNA lysis buffer (100 mM Tris pH=8.0; 200 mM NaCl; 1% SDS; 5 mM EDTA) and 6 μl Proteinase K (20 mg/ml) were added to the collected nuclei and incubated overnight at 65°C. RNAse cocktail was added (Ambion) and incubated at 65°C for 1 hour. Half of the existing volume of 5 M NaCl solution was added and agitated for 15 seconds. The solution was spun down at 13,000 rpm for 3 min. The supernatant containing the DNA was transferred to a 12-ml glass vial. Three times the volume of absolute ethanol was added and the glass vial was inverted several times to precipitate the DNA. The DNA precipitate was washed three times in DNA washing solution (70% Ethanol (v/v) and 0.1 M NaCl) and transferred to 500 μl DNase/RNAase free water (Gibco/Invitrogen). The DNA was quantified and DNA purity verified by UV spectroscopy (NanoDrop).
Accelerator Mass Spectrometry
All AMS analyses were performed blind to age and origin of the sample. Purified DNA samples suspended in water were lyophilized to dryness. To convert the DNA sample into graphite, excess CuO was added to each dry sample and the tubes were evacuated and sealed with a high temperature torch. Tubes were placed in a furnace set at 900°C for 3.5 h to combust all carbon to CO2. The evolved CO2 was purified, trapped and reduced to graphite in the presence of iron catalyst in individual reactors at 550 °C for 6 hour. Graphite targets were measured independently at the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory (Fallon et al., 2007) and the Department of Physics and Astronomy, Ion Physics, Uppsala University (Salehpour et al., 2013). Large CO2 samples (>100 μg) were split and δ13C was measured by stable isotope ratio mass spectrometry, which established the δ13C correction to −22.3 ± 0.5‰ (1 SD), which was applied for all samples. Corrections for background contamination introduced during sample preparation were made as described (Brown and Southon, 1997; Hua et al., 2004; Santos et al., 2007). The measurement error was determined for each sample and ranged between ±4‰ and 12‰ (1 SD) Δ14C for the large sample and small samples (10μgC) respectively. All 14C data are reported as decay-corrected Δ14C or Fraction Modern.
Statistics and Mathematical Modeling
Statistical methodologies used in this paper are the following: the median, which is robust in presence of outliers, is chosen as location parameter. For comparison of two matched samples we use Wilcoxon signed rank test, which test the median difference between the pairs to be zero. As median is used as location parameter we decide to measure the deviation in terms of absolute deviation from median. The link between two quantitative variables is assessed using correlation measure and test, while the influence of a qualitative variable on a quantitative one is measured using ANOVA.
Mathematical modeling was based on birth death processes and renewal equations representing different scenarios essentially as described (Bergmann et al., 2009) and as outlined in detail in the Extended Experimental Procedures. These models were integrated along the atmospheric 14C curve to yield a prediction of the DNA 14C concentration. Nonlinear least-square and Monte Carlo Markov Chain algorithms were used to estimate the best global parameters for each scenario, for the neuronal and the non-neuronal cell samples. The Akaike Information Criterion was used to compare the different scenarios. Individual turnover rates were estimated from Scenarios A and 2POP, for all the samples. Scenario A provided a direct turnover rate estimate for each sample. However, for Scenario 2POP, the fraction of renewing cells had to be fixed, in order to uniquely determine the turnover rates. The fraction was set to 0.35 for the neurons and 0.55 for the non-neuronal cells.
Supplementary Material
Research Highlights.
Nuclear bomb test-derived 14C in human hippocampal neurons reveal adult neurogenesis
One third of hippocampal neurons are subject to exchange
The annual turnover rate is 1.75% within the renewing fraction in adult humans
The extent of adult neurogenesis is comparable in middle aged humans and mice
Acknowledgments
We thank Marcelo Toro, Sarantis Giatrellis, Albert Busch, Endre Kiss and Haythem H.M. Ismail for flow cytometry, Karl Håkansson for AMS sample preparation, Dr. Attila Rácz for tissue procurement and Drs. Fred Gage, Gerd Kempermann and Lutz Slomianka for valuable discussions. The staff at the Swedish National Board of Forensic Medicine is acknowledged for cooperation in tissue donation. This study was supported by the Swedish Research Council, Tobias Stiftelsen, Hjärnfonden, SSF, NARSAD, AFA Försäkringar, Knut och Alice Wallenbergs Stiftelse, NIDA DA031429, the ERC, NIH/NCRR 5P41RR013461, NIH/NIGMS 8P41GM103483 and the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institute (ALF 20080508). This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. H.B.H. was funded by a grant from the German Research Foundation (DFG: Hu 1961/1-1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amrein I, Isler K, Lipp HP. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci. 2011;34:978–987. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Appleby PA, Kempermann G, Wiskott L. The role of additive neurogenesis and synaptic plasticity in a hippocampal memory model with grid-cell like input. PLoS computational biology. 2011;7:e1001063. doi: 10.1371/journal.pcbi.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Rakic P. Neuroscience: Gone with the wean. Nature. 2011;478:333–334. doi: 10.1038/478333a. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neuron production in the hippocampus and olfactory bulb of the adult rat brain: addition or replacement? Ann N Y Acad Sci. 1985;457:163–172. doi: 10.1111/j.1749-6632.1985.tb20804.x. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiology of aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Southon JR. Corrections for contamination background in AMS 14C measurements. Nucl Instrum Methods Phys Res Sect B. 1997;123:208–213. [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries H. Atomic bomb effect: variation of radiocarbon in plants, shells, and snails in the past 4 years. Science. 1958;128:250–251. doi: 10.1126/science.128.3318.250. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fallon SJ, Guilderson TP, Brown TA. CAMS/LLNL ion source efficiency revisited. Nucl Instrum Meth B. 2007;259:106–110. [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göritz C, Frisén J. Neural stem cells and neurogenesis in the adult. Cell Stem Cell. 2012;10:657–659. doi: 10.1016/j.stem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Harkness DD. Further investigations of the transfer of bomb 14 C to man. Nature. 1972;240:302–303. doi: 10.1038/240302a0. [DOI] [PubMed] [Google Scholar]
- Hua Q, Zoppi U, Williams A, Smith A. Small-mass AMS radiocarbon analysis at ANTARES. Nucl Instr and Meth. 2004;B223–B224:284–292. [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New neurons for `survival of the fittest'. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci U S A. 2011;108:10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I, Kromer B. The tropospheric 14CO2 level in mid latitudes of the northern hemisphere (1959–2003) Radiocarbon. 2004;46:1261–1272. [Google Scholar]
- Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, Steele LP, Wagenbach D, Weller R, Worthy DE. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO2. Tellus. 2010;62:26–46. [Google Scholar]
- Libby WF, Berger R, Mead JF, Alexander GV, Ross JF. Replacement Rates for Human Tissue from Atmospheric Radiocarbon. Science. 1964;146:1170–1172. doi: 10.1126/science.146.3648.1170. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nydal R, Lovseth K. Distribution of radiocarbon from nuclear tests. Nature. 1965;206:1029–1031. doi: 10.1038/2061029a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour M, Hakansson K, Possnert G. Accelerator mass spectrometry of ultra-small samples with applications in the biosciences. Nucl Instrum Meth B. 2013;294:97–103. [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GM, Southon JR, Griffin S, Beaupre SR, Druffel ERM. Ultra small-mass AMS C-14 sample preparation and analyses at KCCAMS/UCI Facility. Nucl Instrum Meth B. 2007;259:293–302. [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Spalding K, Bhardwaj RD, Buchholz B, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005a;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Buchholz BA, Bergman L-E, Druid H, Frisén J. Age written in teeth by nuclear tests. Nature. 2005b;437:333–334. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu Y-Y, Zhao C-H, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Research. 2011;21:1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





