Abstract
Mupirocin decolonization of nasal Staphylococcus aureus prior to surgery decreases surgical-site infections; however, treatment requires 5 days, compliance is low, and resistance occurs. In 2010, 3M Company introduced povidone-iodine (PVP-I)-based skin and nasal antiseptic (Skin and Nasal Prep [SNP]). SNP has rapid, broad-spectrum antimicrobial activity. We tested SNP's efficacy using full-thickness tissue (porcine mucosal [PM] and human skin) explant models and human subjects. Prior to or following infection with methicillin-resistant Staphylococcus aureus (MRSA) (mupirocin sensitive and resistant), explants were treated with Betadine ophthalmic preparation (Bet), SNP, or mupirocin (Bactroban nasal ointment [BN]) or left untreated. One hour posttreatment, explants were washed with phosphate-buffered saline (PBS) plus 2% mucin. One, 6, or 12 h later, bacteria were recovered and enumerated. Alternatively, following baseline sampling, human subjects applied two consecutive applications of SNP or saline to their anterior nares. One, 6, and 12 h after application of the preparation (postprep), nasal swabs were obtained, and S. aureus was enumerated. We observed that treatment of infected PM or human skin explants with SNP resulted in >2.0 log10 CFU reduction in MRSA, regardless of mupirocin sensitivity, which was significantly different from the values for BN- and Bet-treated explants and untreated controls 1 h, 6 h, and 12 h after being washed with PBS plus mucin. Swabbing the anterior nares of human subjects with SNP significantly reduced resident S. aureus compared to saline 1, 6, and 12 h postprep. Finally, pretreatment of PM explants with SNP, followed by a mucin rinse prior to infection, completely prevented MRSA infection. We conclude that SNP may be an attractive alternative for reducing the bioburden of anterior nares prior to surgery.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as one of the most important pathogens in nosocomial or hospital-acquired infections (HAI) (1). Surgical-site infections (SSIs), a class of HAI, are defined by the National Healthcare Safety Network (NHSN) as those infections that occur up to 30 days postsurgery or up to 90 days after surgical implantation of a medical device and remain a significant clinical problem despite advances made in reducing the risk of SSIs (2). The frequency of SSI can be as high as 20%, depending on the type of surgery (3). S. aureus is the leading cause of SSIs, accounting for 30% of all SSIs with almost half (49.2%) of those caused by MRSA (4). Preventing a single case of MRSA SSI can reduce hospital costs by as much as $42,300 and reduce the length of stay by 50% (median, 2 weeks) (5–7).
S. aureus colonizes the anterior nares, skin, and mucosal surfaces of approximately 30% of the population (8–10). Nasal colonization with S. aureus is a well-known risk factor for acquisition of SSIs (11–13). Decolonization of the anterior nares is one strategy for reducing risk of SSIs. Intranasally applied mupirocin has been the therapy of choice since the 1980s (14). The most efficacious regimen for S. aureus eradication from the anterior nares is twice-daily applications of mupirocin ointment for 5 days for a total of 10 doses (15, 16). In a prospective study, Yano et al. saw a reduction in the rate of S. aureus SSIs in patients decolonized with mupirocin prior to upper gastrointestinal surgery compared to the control group (0.71% versus 11.7% [P < 0.001]) (17). Others have observed >60% reduction in SSIs in a cohort of cardiothoracic patients treated with mupirocin prior to surgery (18, 19). Furthermore, there was a significant cost savings, considering that the average cost of mupirocin treatment was $12.47 compared to $10,428 ± $9,125 for a superficial sternal infection or $81,018 ± $41,567 for a deep sternal infection (18). Finally, in a review by T. Perl, although not statistically significant, a subset of surgical patients decolonized with mupirocin experienced approximately 50% fewer SSIs than those who did not receive treatment (20).
In contrast to these studies, double-blind, randomized, placebo-controlled clinical trials have failed to demonstrate a significant impact of nasal decolonization by mupirocin on SSI. For example, intranasal mupirocin administered to S. aureus carriers did not reduce the rates of overall cardiac surgical SSIs caused by this organism (21). In 2002, Perl et al. failed to demonstrate a significant difference in the rate of SSI patients treated prophylactically with mupirocin versus those treated with a placebo (22).
Treatment failure has been associated with increased mortality (23). Patient noncompliance may contribute to treatment failure (24). However, there is growing evidence that treatment failure may also be due to acquired antibiotic resistance (25–27). The prevalence rates of high-level mupirocin resistance (Mupr) increased from 1.6% of MRSA strains during the 5-year period of 1995 to 1999 to 7% for 2000 to 2004 (28). The increasing prevalence of Mupr has important implications for institutions where decolonization is the standard of care. While there are no standardized guidelines for screening or decolonization, most clinicians attempt to decolonize patients who are at risk with a combination of chlorhexidine gluconate applied to the skin and intranasal mupirocin (29). The use of mupirocin enhances selective pressure, decreasing its effectiveness as a decolonization strategy (30). This has prompted the evaluation of alternative strategies for reducing the risk of MRSA SSIs.
In early 2010, 3M Company launched 3M skin and nasal antiseptic (Skin and Nasal Prep [SNP]), an alternative to topical antibacterial therapy with mupirocin, as a topical patient preoperative antiseptic prep for the reduction of microbial bioburden on the skin and in the anterior nares. The preparation is based on povidone-iodine (PVP-I), which has broad-spectrum antibacterial activity, as well as activity against fungi, protozoa, viruses, and some bacterial spores (31, 32). PVP-I has rapid in vitro activity (bactericidal within 15 to 20 s), and the duration of the effect on skin has been reported to be 12 to 14 h due to a phenomenon called back-diffusion (33, 34). In contrast to the use of antibiotics, there is minimal potential for the development of resistance to PVP-I due to multiple cellular targets (35, 36). Finally, excipients in the 3M SNP formulation protect PVP-I from inactivation by organic compounds such as blood or mucin and increase mucoadhesion.
Recently, Phillips et al. conducted a prospective, open-label, randomized clinical study to evaluate 3M SNP as an alternative to intranasal mupirocin because of lack of patient compliance and increasing mupirocin resistance (37). They demonstrated that 3M SNP resulted in significantly fewer S. aureus SSIs than mupirocin. Therefore, we conducted an analysis of 3M SNP and mupirocin and determined their ability to reduce MRSA burden on mucosal tissues and human skin. The aims of this study were threefold: (i) to demonstrate that 3M Skin and Nasal Prep is efficacious at reducing microbial bioburden and in prevention of methicillin-resistant S. aureus infections in an ex vivo model, (ii) to show efficacy against Mupr MRSA in a novel ex vivo human skin MRSA infection model, and (iii) to validate reduction in the anterior nares S. aureus bioburden of human subjects.
MATERIALS AND METHODS
Bacterial growth and explant inoculation.
Methicillin-resistant S. aureus (MRSA) strain Xen30 was purchased from Caliper Life Sciences (Hopkinton, MA). Mupirocin-resistant clinical MRSA isolates were received from the Minnesota Department of Health's repository. Mupirocin susceptibility was evaluated using Etest strips (bioMérieux, Durham, NC) according to the manufacturer's instructions. Strips were interpreted according to literature and opinion, as they were for research use only. Bacterial strains described in these studies are maintained in our laboratories as frozen glycerol stocks. Prior to experimentation, tryptic soy agar containing 5% sheep blood (TSB) (Becton Dickinson, Franklin Lakes, NJ) is inoculated from frozen stocks. On the afternoon prior to initiation of the experiment, Todd-Hewitt broth (BD Biosciences, San Jose, CA) is inoculated with colonies from fresh TSB plates. Stationary-phase (overnight) cultures are washed in RPMI 1640 medium (Invitrogen, Carlsbad, CA) and resuspended to a concentration of approximately 5 × 108 CFU/ml. Two-microliter portions of this suspension are used to inoculate explants on the mucosa or stratum corneum surface (1 × 106 CFU/explants). Explants are returned to 37°C and 7% CO2 and incubated for 1 to 24 h.
Explants of healthy porcine vaginal mucosa (5-mm diameter; full-thickness squamous epithelium) or human skin (5-mm diameter; full thickness) were infected with methicillin-resistant S. aureus. Prior to or following infection, explants were treated with Betadine solution (ophthalmic preparation) (Betadine Ophthalmic) (Alcon, Fort Worth, TX), 3M skin and nasal antiseptic (Skin and Nasal Prep [SNP]) (St. Paul, MN), or mupirocin ointment (Bactroban nasal ointment [Bactroban Nasal]; Glaxo Smith Kline, Research Triangle Park, NC) or left untreated (controls). Bacteria were enumerated by transferring explants to 2× Dey-Engley (DE) broth (Becton Dickinson, Franklin Lakes, NJ) for neutralization, vortex mixing, and then plating onto TSB plates neat or serially diluted in phosphate-buffered saline (PBS; Sigma, St. Louis, MO).
Ex vivo porcine vaginal mucosal (PVM) culture.
In a previous publication, we describe a novel model for determining the efficacy of antimicrobial agents (38). Briefly, specimens of healthy porcine vaginal mucosa are excised from animals at slaughter in the University of Minnesota Andrew Boss Laboratory of Meat Science. The tissue is a by-product of the slaughter of animals for human consumption and therefore Institutional Animal Care and Use Committee (IACUC) exempt. Specimens are then transported to the laboratory in RPMI 1640 medium supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA) on ice. Tissue was utilized within 3 h of excision. Tissue explants of uniform size were obtained from the porcine vagina using a 5-mm biopsy punch. Excess muscle tissue was trimmed away with a scalpel. The explants are washed in serum and antibiotic-free medium 3 times and then placed mucosal side up on a 0.4-μm cell culture insert (BD Bioscience, San Jose, CA) in 6-well plates containing fresh serum and antibiotic-free RPMI 1640 medium. The mucosal surface was continually exposed to air.
Procurement culture of human skin explants.
Healthy human skin (deidentified) is procured by the National Disease Research Interchange (NDRI) and is exempt from Institutional Review Board review. Healthy skin specimens are excised from cadavers and transported to the laboratory in HypoThermosol (BioLife Solutions, Bothell, WA), a cryopreservation medium. Tissue is utilized within 24 h of excision. Decolonization of normal flora is achieved by drying the surface of the specimen and swabbing the area with ChloraPrep (CareFusion, San Diego, CA) twice. Explants of uniform size are then obtained from the specimen using a 5-mm biopsy punch. Tissue explants are washed in RPMI 1640 medium containing 2% (vol/vol) normal human serum. The explants are then placed on a polyethylene terephthalate (PET) track-etched 0.4-μm cell culture insert (BD Biosciences, San Jose, CA) in 6-well plates containing fresh RPMI 1640 medium plus 2% normal human serum (Invitrogen, Carlsbad, CA) and incubated at 37°C and 7% CO2. An air interface is maintained with the stratum corneum.
Application of test formulations or comparators.
The model was developed to be a semihigh-throughput screen of full-strength formulations in the form of liquids, pastes, gels, foams, or dressings. One hundred microliters of Betadine ophthalmic preparation, 3M Skin and Nasal Prep, or mupirocin (Bactroban Nasal) ointment is applied topically to each explant, and the explants were incubated for 1 to 24 h at 37°C and 7% CO2.
Mucin wash.
To mimic mucociliary clearance, 1 h following application of treatments to the explants, 1 ml of 2% (wt/vol) mucin (Sigma, St. Louis, MO) in PBS was pipetted into each well containing an explant. The plate was swirled gently to wash the explants, and the suspension was aspirated from each well. The explants were then returned to culture for the indicated time periods.
Bacterial (CFU) enumeration.
Bacteria are enumerated from infected explants by vortex mixing (medium-high setting, 4 min) in 250 μl sterile antimicrobial neutralizing DE broth at twice the manufacturer's recommended concentration. Suspensions are serially diluted in PBS (or plated neat) and spread on TSB plates using a spiral plater (Biotek, Microbiology International).
Reduction of normal nasal flora in human subjects.
Baseline samples (n = 70) were taken from healthy human subjects prior to application of the preparation or saline. Only subjects with baseline levels of >5 × 103 CFU/swab were included in this efficacy study. Depending on the sampling time, 7 to 18 subjects applied 3M Skin and Nasal Prep or 0.9% saline control to their nostrils for 30 s each, followed by an immediate repeat application, for a total application time of 1 min per naris (3M study EM-05-011100). Samples for quantitative cultures were obtained from the anterior nares using a standardized swabbing procedure. Briefly, one dry, sterile rayon swab was used to sample both the right and left nostrils. For each nostril, the rayon swab was inserted carefully into the anterior, apex portion of the nostril and rotated 2 times with slight pressure. This swabbing procedure was used to collect study day baseline levels and 1, 6, and 12 h after application of the preparation (postprep) nasal samples. Following collection, the swab sample was immediately immersed into a tube containing 1 ml of a neutralizer solution (NS) described previously (39). The sample tube was capped tightly and vortex mixed for ∼1 min and then serially diluted in phosphate-buffered saline. Duplicate 0.1-ml aliquots were spread on HardyCHROM Staph aureus (CSA; Hardy Diagnostics, Santa Maria, CA) and Trypticase soy agar with 5% sheep blood (SBA). All samples were plated within 20 min of collection. After 20 to 28 h of aerobic incubation at 35 to 37°C, CSA plates were evaluated for differentially selective growth (smooth, deep pink to fuchsia colonies), and SBA plates were evaluated for total growth using a Quebec colony counter (Reichert Technologies, Depew, NY). Data presented are mean ± standard deviation (SD) log10 reductions of S. aureus from the baseline level.
Statistical analysis.
Each ex vivo experiment was repeated a minimum of three times. Data presented are means ± standard errors of the means (SEMs) for three replicate samples. Analysis of variance (ANOVA) followed by Bonferroni's posttest were performed using the GraphPad PRISM software (GraphPad Software, Inc., La Jolla, CA). The human subject study was performed once, and data presented are mean ± SD log10 reduction from baseline. Student's t test was used to evaluate significant differences at each time point.
RESULTS
Efficacy of 3M SNP, Betadine Ophthalmic, and Bactroban Nasal in treatment of MRSA-infected ex vivo porcine vaginal mucosa.
We tested the antimicrobial activity of two povidone-iodine-based preparations (5%, wt/vol) and 2% mupirocin (Bactroban Nasal [BN]) using a modified version (Fig. 1a) of a previously developed ex vivo full-thickness tissue model of MRSA infection (38). Ex vivo porcine vaginal mucosal (PVM) explants were infected with MRSA (strain Xen30) for 2 h and then treated with antimicrobials for 1 h. Treated and untreated control explants were washed in PBS plus 2% (wt/vol) porcine mucin to mimic mucociliary clearance. CFU were enumerated from neutralized explants following 1, 6, or 12 h (to mimic short, average, and long surgeries) of incubation at 37°C. One hour after the explants were washed (postwash), MRSA bacterial densities of infected PVM explants treated with 3M SNP or Betadine Ophthalmic were significantly lower than that of untreated controls (bacterial densities [log10 CFU/explant] of 1.09 ± 0.57, 2.51 ± 0.20, and 5.30 ± 0.06, respectively) (Fig. 1b). Treatment with BN had no effect on CFU recovered at this time point (log10 5.14 ± 0.09 CFU/explant). There was bacterial growth in untreated control explants and Betadine Ophthalmic-treated explants 6 h postwash (6.86 ± 0.01 and 5.12 ± 0.48 log10 CFU/explant), whereas the bacterial burden from BN-treated explants remained static and not different from that of the untreated control (log10 5.07 ± 0.06 CFU/explant). 3M SNP exerted a significant persistent effect at this time point, further reducing bacterial densities (log10 0.43 ± 0.43 CFU/explant).
FIG 1.
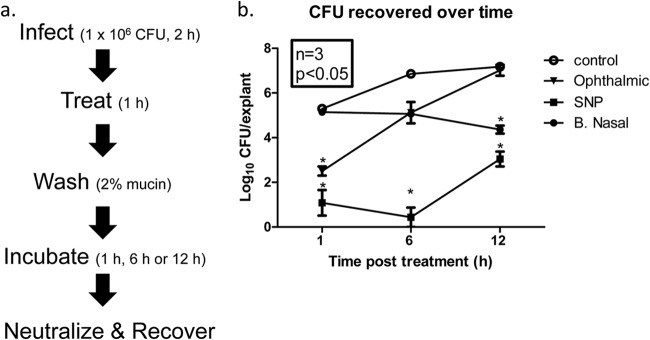
Efficacy of PVP-I formulations and Bactroban Nasal against MRSA infection in an ex vivo PVM model. (a) Schematic of experimental design. (b) Explants of normal PVM were infected with S. aureus Xen30, treated, washed with sterile PBS containing 2% mucin and then incubated as shown in panel a. The explants were treated with Betadine Ophthalmic, 3M SNP, or Bactroban Nasal (B. Nasal) or left untreated as a control. Following incubation for 1, 6, or 12 h, explants were transferred to a neutralization buffer containing sodium thiosulfate and vortex mixed to release surviving bacteria. Serial dilutions were made in sterile PBS and plated onto tryptic soy agar supplemented with sheep blood, using a spiral plater. The number of viable bacterial cells is expressed as log10 CFU/explants recovered over time. Values are means ± SEMs (error bars). Values that are statistically significantly different (P < 0.05) from the value for the untreated control are indicated by an asterisk.
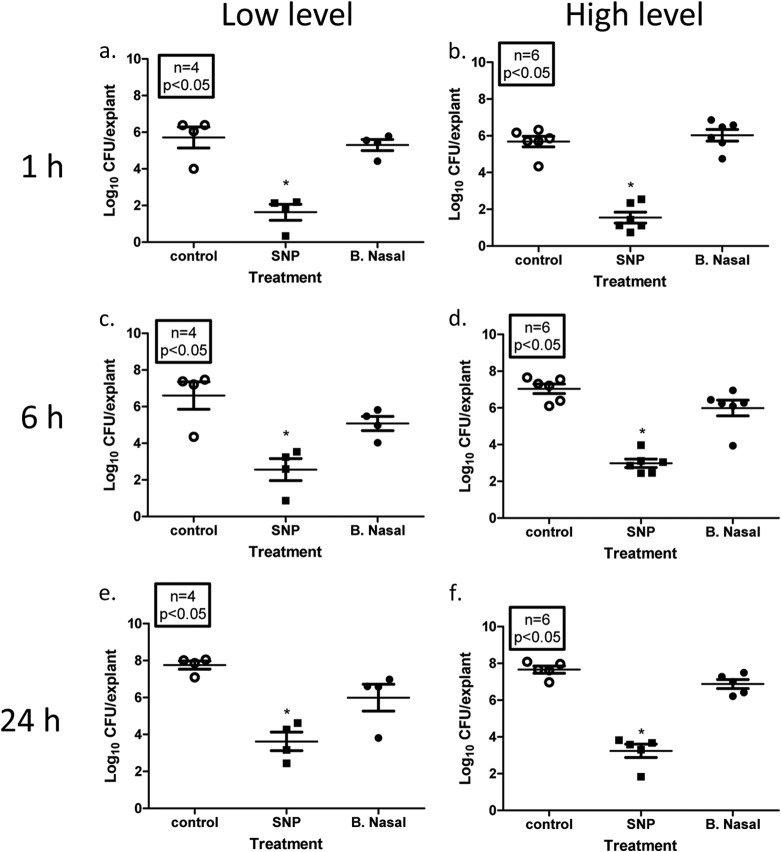
Efficacy of 3M SNP and Bactroban Nasal against mupirocin-resistant MRSA infections of ex vivo porcine vaginal mucosa.
We next evaluated the antimicrobial effect of 3M SNP compared to Bactroban Nasal on 10 Mupr MRSA isolates (both high-level resistance [HLR] and low-level resistance [LLR]) (see Table 1 for mupirocin sensitivity data) using the PVM infection model described above. As expected, 1 h following 2% mucin wash, 3M SNP-treated explants had significantly less LLR MRSA bacteria on infected PVM explants than untreated controls and BN-treated explants (1.63 ± 0.44 versus 5.30 ± 0.30 and 5.71 ± 0.57, respectively [log10 CFU/explant] [Fig. 2a]). Although some regrowth was observed at 6 h (Fig. 2c) and 24 h (Fig. 2e), 3M SNP-treated explants were associated with significantly lower bacterial densities (2.56 ± 1.60 and 3.62 ± 0.50, respectively) than untreated controls (6.60 ± 0.75 and 7.76 ± 0.22, respectively) or BN-treated explants (5.08 ± 0.39 and 5.99 ± CFU/explant) (all values are shown in log10 CFU/explant). BN appeared to be bacteriostatic, but it had no significant effect on LLR MRSA CFU recovered at any time point compared to the control. Similar results were observed with the HLR MRSA isolates as well (Fig. 2b, d, and f). At all three time points examined, HLR MRSA-infected explants treated with 3M SNP had significantly lower bacterial densities than untreated or BN-treated explants (at 1 h, 1.55 ± 0.29 versus 5.68 ± 0.29 or 6.03 ± 0.32, respectively; at 6 h, 2.98 ± 0.23 versus 7.04 ± 0.26 or 5.99 ± 0.43, respectively; at 24 h, 3.24 ± 0.36 versus 7.66 ± 0.19 or 6.88 ± 0.24, respectively) (all values are shown in log10 CFU/explant). Although not significantly different from the control, some growth was observed in the HLR MRSA isolates at 24 h compared to 1 h.
TABLE 1.
Mupirocin susceptibilities of clinical MRSA isolates by Etest
| MRSA isolate | Mupirocin susceptibility (μg/ml) by Etest | Mupirocin resistancea |
|---|---|---|
| 146 | 8 | LLR |
| 823 | 12 | LLR |
| 815 | 8 | LLR |
| 748 | 12 | LLR |
| 103 | ≥1,024 | HLR |
| 920 | ≥1,024 | HLR |
| 559 | ≥1,024 | HLR |
| 476 | ≥1,024 | HLR |
| 993 | ≥1,024 | HLR |
| 329 | ≥1,024 | HLR |
LLR, low-level mupirocin resistance; HLR, high-level mupirocin resistance.
FIG 2.
Efficacy of 3M SNP or Bactroban Nasal against low- and high-level mupirocin-resistant MRSA isolates. Explants of normal PVM were infected with ∼1 × 106 CFU low-level Mupr MRSA isolates (a, c, and e) or high-level Mupr MRSA isolates (b, d, and f) for 2 h. Infected explants were then treated with SNP or Bactroban Nasal (B. Nasal) or left untreated (control) for 1 h, followed by washing with sterile PBS supplemented with 2% mucin. Washed explants were then returned to the incubator for 1 h (a and b), 6 h (c and d), or 24 h (e and f). Following incubation, explants were transferred to 2× DE broth and vortex mixed to release surviving bacteria. Serial dilutions were made in sterile PBS and plated onto tryptic soy agar supplemented with sheep blood, using a spiral plater. The number of viable bacterial cells is expressed as log10 CFU/explants. Each symbol represents the value for an individual explant, and the mean (horizontal bar) ± SEM (error bar) for each group are shown. Mean values that are significantly different (P < 0.05) from the mean value for the untreated control group are indicated by an asterisk.
Efficacy of 3M SNP, Betadine Ophthalmic, and Bactroban Nasal in the treatment of MRSA-infected ex vivo human skin.
We adapted our MRSA PVM infection model to fresh, full-thickness human skin which is more representative of the tissue type in the anterior nares and therefore more translational. Explants of normal human skin were prepared as described above and infected with MRSA (strain Xen30) for 2 h (Fig. 3a). Similar to the above-described experiment on PVM, explants were treated with 3M SNP, Betadine Ophthalmic, or BN for 1 h and then washed with PBS containing 2% (wt/vol) mucin. Explants were incubated for 1, 6, or 12 h prior to neutralization and bacterial recovery. One hour postwash, the MRSA burden of infected PVM explants treated with 3M SNP or Betadine Ophthalmic was significantly reduced compared to that of untreated controls (0.33 ± 0.33, 2.09 ± 0.60, and 4.06 ± 0.19, respectively [log10 CFU/explant]; Fig. 3b). Further, CFU recovered from 3M SNP-treated explants were significantly lower than the Betadine Ophthalmic-treated explants. Treatment with BN had no effect on CFU recovered at this time point (log10 4.50 ± 0.08 CFU/explant). Six hours postwash, there was evidence of growth in the untreated controls (log10 5.50 ± 0.40 CFU/explant) which continued through the 12-h time point (log10 6.57 ± 0.35 CFU/explant). Again, 3M SNP was significantly more effective at reducing MRSA burden than Betadine Ophthalmic or BN (0 CFU recovered versus 1.72 ± 0.0.40 or 4.20 ± 0.24, respectively [log10 CFU/explant]). BN treatment appeared bacteriostatic at 6 h postwash; however, this was not statistically different from control. By 12 h postwash, the difference between untreated controls and BN treatment was significant (6.57 ± 0.35 versus 3.92 ± 0.11, respectively [log10 CFU/explant]). Zero CFU were recovered from the 3M SNP-treated explants 12 h postwash, which was significantly lower than BN-treated and untreated controls. At this time point, no statistically significant difference between CFU recovered from the 3M SNP-treated explants and the Betadine Ophthalmic-treated explants was observed; however, numerically, 3M SNP treatment was more effective at eradicating MRSA (0 versus log10 0.63 ± 0.34 CFU/explant).
FIG 3.
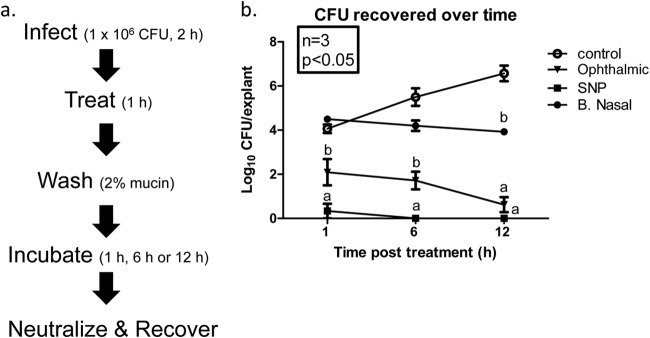
Efficacy of PVP-I formulations and Bactroban Nasal against MRSA infection in an ex vivo, full-thickness, fresh human skin model. (a) Schematic of experimental design. (b) Explants of normal human skin were infected with S. aureus Xen30, treated, washed with sterile PBS containing 2% mucin, then incubated as shown in panel a. The explants were treated with Betadine Ophthalmic, 3M SNP, or Bactroban Nasal (B. Nasal) or left untreated as a control. Following incubation for 1, 6, or 12 h, explants were transferred to 2× DE broth and vortex mixed to release surviving bacteria. Serial dilutions were made in sterile PBS and plated onto tryptic soy agar supplemented with sheep blood, using a spiral plater. The number of viable bacterial cells is expressed as log10 CFU/explants recovered over time. Values are means ± SEMs (error bars). Values with different letters are significantly different (P < 0.05).
Efficacy of 3M SNP on normal flora in the anterior nares of human subjects.
Having demonstrated that 3M SNP significantly reduced MRSA in two ex vivo full-thickness models, we next determined whether it could reduce normal flora of human anterior nares. Baseline samples (n = 70) were taken by swabbing the anterior nares. The mean baseline level of S. aureus in subjects included in this study was log10 4.77 ± 0.62 CFU. The anterior nares of subjects were then sampled at 1, 6, or 12 h following application of SNP (n = 13 to 18) or saline control (n = 7 to 9). At all three time points, the S. aureus log10 reduction from the baseline level in 3M SNP-treated subjects was significantly greater than that observed in the saline control subjects (2.3 ± 1.68 versus 0.86 ± 0.73 at 1 h, 2.79 ± 1.52 versus 0.76 ± 0.58 at 6 h and 2.37 ± 1.77 versus 0.6 ± 0.9 at 12 h [all values are log10 CFU]) (Fig. 4).
FIG 4.
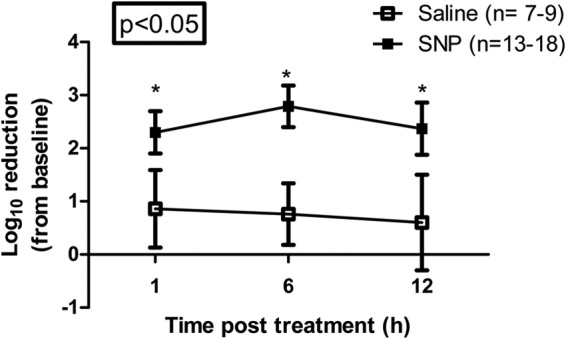
3M SNP reduces normal flora of human anterior nares. Thirteen to 18 human subjects (3M study EM-05-011100) applied 3M Skin and Nasal Prep (3M SNP) and seven to nine human subjects applied 0.9% saline control to their nostrils for 30 s each, followed by an immediate repeat application, for a total application time of 1 min per naris. Postprep samples were taken via swabbing the nares at 1 h, 6 h, and 12 h. Baseline samples were taken prior to the application of prep or saline. The mean baseline level of S. aureus in subjects included in this study was log10 4.77 ± 0.62 CFU. The numbers of viable bacterial cells are expressed as log10 reduction from baseline level over time. Values are means ± SEMs (error bars). Mean values that are significantly different (P < 0.05) from the mean value for the untreated saline control group are indicated by an asterisk.
3M SNP prevents MRSA infection of PVM explants.
Since the intended outcome of 3M SNP use is to prevent SSIs, we next determined whether treatment could prevent PVM explants from becoming infected with MRSA. We slightly modified the PVM infection model described above, as depicted in Fig. 5a. Explants were treated for 5 min with 3M SNP, Betadine Ophthalmic, or mupirocin or left untreated, incubated for 15 min, washed with PBS containing 2% mucin (wt/vol), infected with MRSA (Xen30), incubated for 1 h, and then neutralized, and CFU were recovered. The number of CFU recovered from untreated control explants was log10 4.19 ± 0.12 CFU/explant (Fig. 5b). Treatment with either 5% povidone-iodine-based product resulted in a reduction in the ability of MRSA to infect explants, with 3M SNP being far superior to the Betadine ophthalmic formulation (log10 0.00 ± 0.00 versus 2.34 ± 0.12 CFU/explant, respectively). CFU recovered from 2% mupirocin-treated explants was equivalent to that recovered from controls (log10 4.53 ± 0.05 CFU/explant).
FIG 5.
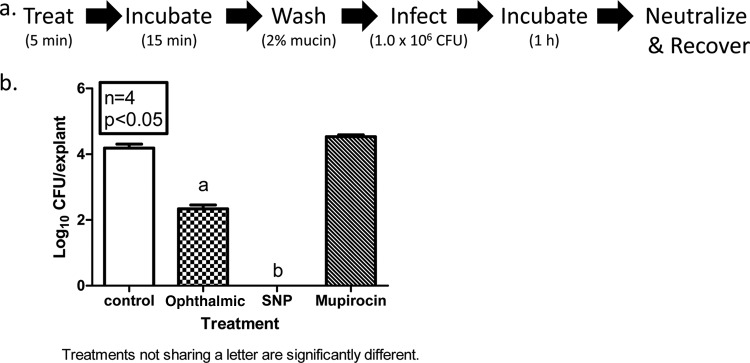
3M SNP prevents MRSA infection in an ex vivo model. (a) Schematic of experimental design. (b) Explants of normal PVM were treated with PVP-I formulations or mupirocin ointment (2%) for 5 min at room temperature (RT), followed by 15-min incubation at 37°C. Explants were then washed with sterile PBS containing 2% mucin. After the wash, explants were infected with ∼1 × 106 CFU of MRSA Xen30 and incubated at 37°C for 1 h. Explants were transferred to 2× DE broth and vortex mixed to release surviving bacteria. Serial dilutions were made in sterile PBS and plated onto tryptic soy agar supplemented with sheep blood, using a spiral plater. The number of viable bacterial cells is expressed as log10 CFU/explants. Values are means plus SEMs (error bars).
DISCUSSION
There are numerous commercially manufactured PVP-I-containing preoperative skin preparations that meet FDA requirements, including the recently developed 3M SNP. To our knowledge, this is the first study in translational, full-thickness tissue models, which directly compares PVP-I formulations and topical application of 2% mupirocin ointment (BN). Using an ex vivo porcine vaginal mucosal (PVM) infection model, we demonstrated that treatment with 3M SNP or Betadine Ophthalmic was bactericidal against MRSA within 2 h of application. This is consistent with the reported rapid activity of PVP-I-based antimicrobials in the ophthalmic surgical setting (40), which, in turn, is associated with a decreased risk of postoperative infections (41). In contrast, no change in the MRSA burden of explants treated with BN was observed out to 14 h postapplication (12 h after the mucin wash), which is consistent with its known slow mode of action (42). While we recognize that the mupirocin decolonization strategy includes twice-daily application for 5 days intranasally prior to surgery, there is also a risk of patient noncompliance. Therefore, our studies are intended to mimic mupirocin efficacy in a noncompliant patient being decolonized prior to surgery.
PVP-I kinetics are well understood, and it is well-known that iodine is the active antimicrobial component. For example, Schenck et al. showed that povidone complexes with hydrogen triiodide through hydrogen bonding with the proton (43). Triiodide is in equilibrium with iodine (I2) and iodide (I−) shown by the reaction I3− = I2 + I−. There are other excipients in 3M SNP which buffer the composition and protect the iodine from reduction to inactive iodide, which happens rapidly at increased pH (above 4.5) and reaction with organic matter.
Treatment with 3M SNP resulted in sustained bactericidal activity for up to 8 h postapplication (6 h after the mucin wash), which was a significant improvement over the Betadine Ophthalmic-treated explants where >5.0 log10 unit growth was observed. We believe that the sustained activity is a result of increased adhesion to the net negatively charged mucus on the tissue surface. This mucoadherence results from the presence of a proprietary cationic polymer in the formulation (44, 45). We acknowledge that recolonization after 8 h is a possibility and that is the subject of ongoing studies.
Recently, universal decolonization was shown to be more effective than targeted decolonization in the prevention of MRSA intensive care unit (ICU) infections (46). This, along with the increased use of mupirocin for prevention of recurrent skin and soft tissue MRSA infections (47–49), and growing interest in perioperative eradication of MRSA for the prevention of SSIs has important implications for the spread of mupirocin resistance (50). In 2002, Deshpande et al. (51) performed an extensive study of mupirocin resistance and noted a marked increase globally compared to an earlier study. Widespread use of mupirocin is commonly associated with increased incidence of resistance (25, 52–54). The short, defined course (5 days) of mupirocin prescribed in clinical trials of efficacy does not appear to select for mupirocin resistance in one study (22). In contrast, Watanabe et al. reported the development of low-level mupirocin resistance after intranasal treatment to reduce nasal carriage (55). This same research group also presented a case study in 2001 which described the development of mupirocin resistance in the pharynx of a patient during the course of intranasal application (56). Mupirocin resistance has been associated with decolonization failure, which correlates with increased SSIs (57–59). We evaluated 10 Mupr MRSA clinical isolates, both HLR and LLR, in our PVM infection treatment model. We saw that both HLR and LLR MRSA isolates were sensitive to 3M SNP. This is not surprising due to the differences in mechanism of action by the two agents. Mupirocin reversibly binds to the isoleucyl tRNA synthetase, resulting in inhibition of protein synthesis (60). Resistance is mediated by the acquisition of a plasmid-encoded mupA gene (HLR) (61) or a mutation in the native ileS gene (LLR) (62). In contrast, iodine has many cellular targets, including fatty acids, nucleotides and the free-sulfur amino acids cysteine and methionine in proteins (63). This makes the development of resistance unlikely, and in fact, has not been reported (35).
We previously developed a semihigh-throughput ex vivo PVM infection model for determining efficacy and toxicity of antimicrobial agents that is translational in nature (38). Its use as a substrate for bacterial attachment and nutrition provides an alternative, cost-effective method that more closely mimics in vivo conditions than classic in vitro studies. The indications for use of 3M SNP for nasal bacterial reduction is application to the anterior nares, which more closely resembles skin in terms of cell type and microflora (64). The ex vivo PVM explant infection model does not entirely mimic the host factors or microbial components found in skin; therefore, we extended the principles of the PVM infection model to a model that uses fresh human skin as the substrate. Using this ex vivo human skin MRSA infection model, we observed results similar to those obtained in the ex vivo PVM model. This not only confirms that the PVM infection model is a valuable tool for the preclinical evaluation of topical biocides for infection prevention or treatment but also suggests that 3M SNP could reduce skin or anterior nares flora.
3M SNP rapidly achieved a significant reduction in the resident S. aureus from the anterior nares of human test subjects, demonstrating clinical translation of the PVM and ex vivo human skin models. This is consistent with a recent report of a prospective, open-label, randomized clinical trial where bacterial reduction with 3M SNP resulted in significantly fewer S. aureus SSIs than in mupirocin-treated patients (twice daily for 5 days) undergoing primary or revision arthroplasty or spinal fusion (37).
In conclusion, given the issues with medication compliance and evolving mupirocin resistance and the importance of reducing the risk of S. aureus SSIs, the benefits of 3M SNP should be considered. They include rapid efficacy, broad-spectrum activity against multiple opportunistic pathogens, lack of development of antimicrobial resistance, and ease of use. 3M SNP provides just-in-time, health care provider directly observed preventive application and may be an attractive alternative for reducing the bioburden of anterior nares prior to surgery.
ACKNOWLEDGMENT
This work was supported by a grant from 3M Company.
REFERENCES
- 1.Sader HS, Streit JM, Fritsche TR, Jones RN. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002-2004). Clin Microbiol Infect 12:844–852. doi: 10.1111/j.1469-0691.2006.01550.x. [DOI] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. 1999. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–280. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 3.Owens CD, Stoessel K. 2008. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 70(Suppl 2):3–10. doi: 10.1016/S0195-6701(08)60017-1. [DOI] [PubMed] [Google Scholar]
- 4.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ, Kaye KS, Chen LF, Schmader KE, Choi Y, Sloane R, Sexton DJ. 2009. Clinical and financial outcomes due to methicillin resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS One 4:e8305. doi: 10.1371/journal.pone.0008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. 2002. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 23:183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 7.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. 2013. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 8.Ringberg H, Petersson AC, Walder M, Johansson PJH. 2006. The throat: an important site for MRSA colonization. Scand J Infect Dis 38:888–893. doi: 10.1080/00365540600740546. [DOI] [PubMed] [Google Scholar]
- 9.Rimland D, Roberson B. 1986. Gastrointestinal carriage of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 24:137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigi R, Hanrahan J. 2007. Staphylococcus aureus and MRSA colonization rates among gravidas admitted to labor and delivery: a pilot study. Infect Dis Obstet Gynecol 2007:70876. doi: 10.1155/2007/70876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluytmans JA, Mouton JW, Ijzerman EP, Vandenbroucke-Grauls CM, Maat AW, Wagenvoort JH, Verbrugh HA. 1995. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis 171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 12.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. 2000. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol 21:319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 13.Levy PY, Ollivier M, Drancourt M, Raoult D, Argenson JN. 2013. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthop Traumatol Surg Res 99:645–651. doi: 10.1016/j.otsr.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.van Rijen M, Bonten M, Wenzel R, Kluytmans J. 2008. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev 2008:CD006216. doi: 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta MS, Hacek DM, Kufner BA, Price C, Peterson LR. 2013. Dose-ranging study to assess the application of intranasal 2% mupirocin calcium ointment to eradicate Staphylococcus aureus nasal colonization. Surg Infect (Larchmt) 14:69–72. doi: 10.1089/sur.2012.086. [DOI] [PubMed] [Google Scholar]
- 16.Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. 2009. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis 48:922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- 17.Yano M, Doki Y, Inoue M, Tsujinaka T, Shiozaki H, Monden M. 2000. Preoperative intranasal mupirocin ointment significantly reduces postoperative infection with Staphylococcus aureus in patients undergoing upper gastrointestinal surgery. Surg Today 30:16–21. doi: 10.1007/PL00010040. [DOI] [PubMed] [Google Scholar]
- 18.Cimochowski GE, Harostock MD, Brown R, Bernardi M, Alonzo N, Coyle K. 2001. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg 71:1572–1579. doi: 10.1016/S0003-4975(01)02519-X. [DOI] [PubMed] [Google Scholar]
- 19.Kluytmans JA, Mouton JW, VandenBergh MF, Manders MJ, Maat AP, Wagenvoort JH, Michel MF, Verbrugh HA. 1996. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol 17:780–785. doi: 10.1086/647236. [DOI] [PubMed] [Google Scholar]
- 20.Perl TM. 2003. Prevention of Staphylococcus aureus infections among surgical patients: beyond traditional perioperative prophylaxis. Surgery 134:S10–S17. doi: 10.1016/S0039-6060(03)00391-X. [DOI] [PubMed] [Google Scholar]
- 21.Konvalinka A, Errett L, Fong IW. 2006. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect 64:162–168. doi: 10.1016/j.jhin.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 23.Schmid H, Romanos A, Schiffl H, Lederer SR. 2013. Persistent nasal methicillin-resistant Staphylococcus aureus carriage in hemodialysis outpatients: a predictor of worse outcome. BMC Nephrol 14:93. doi: 10.1186/1471-2369-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caffrey AR, Woodmansee SB, Crandall N, Tibert C, Fielding C, Mikolich DJ, Vezeridis MP, LaPlante KL. 2011. Low adherence to outpatient preoperative methicillin-resistant Staphylococcus aureus decolonization therapy. Infect Control Hosp Epidemiol 32:930–932. doi: 10.1086/661787. [DOI] [PubMed] [Google Scholar]
- 25.Vasquez JE, Walker ES, Franzus BW, Overbay BK, Reagan DR, Sarubbi FA. 2000. The epidemiology of mupirocin resistance among methicillin-resistant Staphylococcus aureus at a Veterans' Affairs hospital. Infect Control Hosp Epidemiol 21:459–464. doi: 10.1086/501788. [DOI] [PubMed] [Google Scholar]
- 26.Mongkolrattanothai K, Mankin P, Raju V, Gray B. 2008. Surveillance for mupirocin resistance among methicillin-resistant Staphylococcus aureus clinical isolates. Infect Control Hosp Epidemiol 29:993–994. doi: 10.1086/590536. [DOI] [PubMed] [Google Scholar]
- 27.Jones JC, Rogers TJ, Brookmeyer P, Dunne WM Jr, Storch GA, Coopersmith CM, Fraser VJ, Warren DK. 2007. Mupirocin resistance in patients colonized with methicillin-resistant Staphylococcus aureus in a surgical intensive care unit. Clin Infect Dis 45:541–547. doi: 10.1086/520663. [DOI] [PubMed] [Google Scholar]
- 28.Simor AE, Stuart TL, Louie L, Watt C, Ofner-Agostini M, Gravel D, Mulvey M, Loeb M, McGeer A, Bryce E, Matlow A. 2007. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian hospitals. Antimicrob Agents Chemother 51:3880–3886. doi: 10.1128/AAC.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson DJ, Podgorny K, Berrios-Torres SI, Bratzler DW, Dellinger EP, Greene L, Nyquist AC, Saiman L, Yokoe DS, Maragakis LL, Kaye KS. 2014. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35:605–627. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel JB, Gorwitz RJ, Jernigan JA. 2009. Mupirocin resistance. Clin Infect Dis 49:935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 31.Gershenfeld L. 1962. Povidone-iodine as a sporocide. Am J Pharm Sci Support Public Health 134:78–81. [PubMed] [Google Scholar]
- 32.Russell AD. 1990. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev 3:99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLure AR, Gordon J. 1992. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect 21:291–299. doi: 10.1016/0195-6701(92)90139-D. [DOI] [PubMed] [Google Scholar]
- 34.Gottardi W. 1995. The uptake and release of molecular iodine by the skin: chemical and bactericidal evidence of residual effects caused by povidone-iodine preparations. J Hosp Infect 29:9–18. doi: 10.1016/0195-6701(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 35.Houang ET, Gilmore OJ, Reid C, Shaw EJ. 1976. Absence of bacterial resistance to povidone iodine. J Clin Pathol 29:752–755. doi: 10.1136/jcp.29.8.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanker Klossner B, Widmer HR, Frey F. 1997. Nondevelopment of resistance by bacteria during hospital use of povidone-iodine. Dermatology 195(Suppl 2):10–13. doi: 10.1159/000246024. [DOI] [PubMed] [Google Scholar]
- 37.Phillips M, Rosenberg A, Shopsin B, Cuff G, Skeete F, Foti A, Kraemer K, Inglima K, Press R, Bosco J. 2014. Preventing surgical site infections: a randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infect Control Hosp Epidemiol 35:826–832. doi: 10.1086/676872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson MJ, Scholz MT, Parks PJ, Peterson ML. 2013. Ex vivo porcine vaginal mucosal model of infection for determining effectiveness and toxicity of antiseptics. J Appl Microbiol 115:679–688. doi: 10.1111/jam.12277. [DOI] [PubMed] [Google Scholar]
- 39.Anderson MJ, Horn ME, Lin YC, Parks PJ, Peterson ML. 2010. Efficacy of concurrent application of chlorhexidine gluconate and povidone iodine against six nosocomial pathogens. Am J Infect Control 38:826–831. doi: 10.1016/j.ajic.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Halachmi-Eyal O, Lang Y, Keness Y, Miron D. 2009. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg 35:2109–2114. doi: 10.1016/j.jcrs.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 41.Wu PC, Li M, Chang SJ, Teng MC, Yow SG, Shin SJ, Kuo HK. 2006. Risk of endophthalmitis after cataract surgery using different protocols for povidone-iodine preoperative disinfection. J Ocul Pharmacol Ther 22:54–61. doi: 10.1089/jop.2006.22.54. [DOI] [PubMed] [Google Scholar]
- 42.Casewell MW, Hill RL. 1985. In-vitro activity of mupirocin (‘pseudomonic acid') against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 15:523–531. doi: 10.1093/jac/15.5.523. [DOI] [PubMed] [Google Scholar]
- 43.Schenck HU, Simak P, Haedicke E. 1979. Structure of polyvinylpyrrolidone-iodine (povidone-iodine). J Pharm Sci 68:1505–1509. doi: 10.1002/jps.2600681211. [DOI] [PubMed] [Google Scholar]
- 44.Bogataj M, Vovk T, Kerec M, Dimnik A, Grabnar I, Mrhar A. 2003. The correlation between zeta potential and mucoadhesion strength on pig vesical mucosa. Biol Pharm Bull 26:743–746. doi: 10.1248/bpb.26.743. [DOI] [PubMed] [Google Scholar]
- 45.Ko S, Roh YH, Choo JH, Jang SH, Han SH, Jang HG. 2010. Effect of cationic polymer treatment on adhesion of iron oxide to eyelashes. Int J Cosmet Sci 32:314. doi: 10.1111/j.1468-2494.2010.00580_3.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R. 2013. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West SK, Plantenga MS, Strausbaugh LJ. 2007. Use of decolonization to prevent staphylococcal infections in various healthcare settings: results of an Emerging Infections Network survey. Infect Control Hosp Epidemiol 28:1111–1113. doi: 10.1086/519930. [DOI] [PubMed] [Google Scholar]
- 48.Creech CB, Beekmann SE, Chen Y, Polgreen PM. 2008. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 27:270–272. doi: 10.1097/INF.0b013e31815c9068. [DOI] [PubMed] [Google Scholar]
- 49.Szumowski JD, Cohen DE, Kanaya F, Mayer KH. 2007. Treatment and outcomes of infections by methicillin-resistant Staphylococcus aureus at an ambulatory clinic. Antimicrob Agents Chemother 51:423–428. doi: 10.1128/AAC.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C, Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. 2007. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg 83:1569–1576. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 51.Deshpande LM, Fix AM, Pfaller MA, Jones RN. 2002. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn Microbiol Infect Dis 42:283–290. doi: 10.1016/S0732-8893(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 52.Upton A, Lang S, Heffernan H. 2003. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother 51:613–617. doi: 10.1093/jac/dkg127. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Fontan M, Rosales M, Rodriguez-Carmona A, Falcon TG, Valdes F. 2002. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 39:337–341. doi: 10.1053/ajkd.2002.30553. [DOI] [PubMed] [Google Scholar]
- 54.Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, Jassal V, Oreopoulos D. 2001. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 21:554–559. [PubMed] [Google Scholar]
- 55.Watanabe H, Masaki H, Asoh N, Watanabe K, Oishi K, Furumoto A, Kobayashi S, Sato A, Nagatake T. 2001. Emergence and spread of low-level mupirocin resistance in methicillin-resistant Staphylococcus aureus isolated from a community hospital in Japan. J Hosp Infect 47:294–300. doi: 10.1053/jhin.2000.0931. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Masaki H, Asoh N, Watanabe K, Oishi K, Kobayashi S, Sato A, Sugita R, Nagatake T. 2001. Low concentrations of mupirocin in the pharynx following intranasal application may contribute to mupirocin resistance in methicillin-resistant Staphylococcus aureus. J Clin Microbiol 39:3775–3777. doi: 10.1128/JCM.39.10.3775-3777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker ES, Vasquez JE, Dula R, Bullock H, Sarubbi FA. 2003. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infect Control Hosp Epidemiol 24:342–346. doi: 10.1086/502218. [DOI] [PubMed] [Google Scholar]
- 58.Simor AE, Phillips E, McGeer A, Konvalinka A, Loeb M, Devlin HR, Kiss A. 2007. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 44:178–185. doi: 10.1086/510392. [DOI] [PubMed] [Google Scholar]
- 59.Harbarth S, Liassine N, Dharan S, Herrault P, Auckenthaler R, Pittet D. 2000. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 31:1380–1385. doi: 10.1086/317484. [DOI] [PubMed] [Google Scholar]
- 60.Parenti MA, Hatfield SM, Leyden JJ. 1987. Mupirocin: a topical antibiotic with a unique structure and mechanism of action. Clin Pharm 6:761–770. [PubMed] [Google Scholar]
- 61.Hodgson JE, Curnock SP, Dyke KG, Morris R, Sylvester DR, Gross MS. 1994. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob Agents Chemother 38:1205–1208. doi: 10.1128/AAC.38.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonio M, McFerran N, Pallen MJ. 2002. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 46:438–442. doi: 10.1128/AAC.46.2.438-442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Block SS. (ed). 1991. Disinfection, sterilization, and preservation. Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 64.Wilson M, McNab R, Henderson B (ed). 2002. Bacterial disease mechanisms. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]