Abstract
Scope
Sulforaphane (SFN), an isothiocyanate derived from crucifers, has numerous health benefits. SFN bioavailability from dietary sources is a critical determinant of its efficacy in humans. A key factor in SFN absorption is the release of SFN from its glucosinolate precursor, glucoraphanin, by myrosinase. Dietary supplements are used in clinical trials to deliver consistent SFN doses, but myrosinase is often inactivated in available supplements. We evaluated SFN absorption from a myrosinase-treated broccoli sprout extract (BSE) and are the first to report effects of twice daily, oral dosing on SFN exposure in healthy adults.
Methods and results
Subjects consumed fresh broccoli sprouts or the BSE, each providing 200 μmol SFN daily, as a single dose and as two 100-μmol doses taken 12 h apart. Using HPLC-MS/MS, we detected ~3 x higher SFN metabolite levels in plasma and urine of sprout consumers, indicating enhanced SFN absorption from sprouts. Twelve-hour dosing retained higher plasma SFN metabolite levels at later time points than 24-hour dosing. No dose responses were observed for molecular targets of SFN (i.e. heme oxygenase-1, histone deacetylase activity, p21).
Conclusion
We conclude that the dietary form and dosing schedule of SFN may impact SFN absorption and efficacy in human trials.
Keywords: Absorption, Broccoli sprout, Chemoprevention, Excretion, Sulforaphane
1 Introduction
Consuming cruciferous vegetables is associated with many health benefits, including cancer chemoprevention. Sulforaphane (SFN), a major isothiocyanate (ITC) derived from these vegetables, is widely studied for its abilities to prevent cancer onset and progression. SFN facilitates carcinogen metabolism by inducing Phase 2 enzymes and promotes carcinogen excretion by inhibiting Phase 1 enzymes [1–3]. More recently, SFN metabolites were reported to inhibit histone deacetylases (HDAC). HDACs remove acetyl groups from proteins, including histones, which alters gene expression and protein function [4–6]. SFN’s ability to alter epigenetic marks may be important for helping re-express tumor suppressor genes that are often silenced during cancer development. Given these properties, SFN is a promising dietary chemopreventive agent. Here, we focus on how the dietary form and frequency of SFN intake impact SFN absorption. The influence of these factors on specific, putative chemopreventive targets of SFN is also evaluated.
Evidence indicates that SFN absorption is affected by the form consumed. One major determining factor of SFN absorption is the formation of SFN from its glucosinolate precursor, glucoraphanin (GFN). GFN is present in cruciferous vegetables and dietary supplements. The majority of SFN is formed when GFN is hydrolyzed by the plant enzyme, myrosinase, upon plant tissue damage (e.g. chopping, chewing). When plant myrosinase is inactive or absent, a small amount of SFN may still be formed by gut bacteria-derived myrosinase activity [7]. Clarke et al. observed limited SFN absorption in healthy adults after consuming GFN supplements with inactivated myrosinase, which was sevenfold lower than when subjects consumed equivalent levels of GFN from fresh broccoli sprouts containing the active enzyme. These supplements were also less effective than sprouts at lowering HDAC activity [6, 8]. Cramer and Jeffery [9] demonstrated that SFN absorption from a GFN powder devoid of myrosinase activity improved when consumed along with a source of active myrosinase (air-dried broccoli sprouts). These studies clearly demonstrate differences in SFN bioavailability from whole foods and dietary supplements. Since substantial variation in plant glucosinolate content limits the use of whole foods in controlled clinical trials, researchers often employ dietary supplements to deliver consistent SFN doses [10]. Thus, there is a need to identify a suitable supplemental form of SFN. Broccoli sprout extracts (BSE), containing SFN rather than GFN, have been developed and may have enhanced bioavailability compared to GFN supplements lacking active myrosinase. We conducted a human feeding study to evaluate SFN absorption and excretion in individuals consuming fresh broccoli sprouts or a myrosinase-treated BSE containing SFN. Since SFN is mostly excreted within 24 h following consumption, we tested if consuming two doses of SFN 12 h apart (divided dose) could prolong SFN exposure compared to consuming the total amount at a single time point [8,11,12]. Finally, to identify chemopreventive pathways influenced by SFN in humans, we evaluated cancer-relevant putative molecular targets of SFN in subjects before and after SFN consumption. Our observations provide fundamental information for the design of SFN supplementation trials to study the efficacy of dietary SFN in cancer chemoprevention.
2 Materials and methods
2.1 Participants
Twenty healthy adults, 19–50 years, were recruited in Corvallis, Oregon. This study was conducted in the Moore Family Center metabolic kitchen and clinical collection laboratory at Oregon State University (OSU). Exclusion criteria included: smoking, BMI < 18.5 and > 30 kg/m2, vegetarianism, and use of drugs altering lipid metabolism. All participants provided informed consent. Study protocols were approved by the Institutional Review Board at OSU (OSU IRB #4995).
2.2 Dietary interventions
Study design and interventions are depicted in Fig. 1. Subjects were randomized to consume fresh broccoli sprouts or a myrosinase-treated BSE (n = 10), on day 1 of two separate study phases (single and divided dose). In the single-dose phase, subjects (fasting) consumed 200 μmol SFN equivalents from fresh broccoli sprouts or the BSE at 8 AM. In the divided-dose phase (two weeks later), subjects (fasting) consumed half the original dose (100 μmol SFN equivalents) at 8 AM from sprouts or the BSE and the other half (not fasting) 12 h later (Supporting Information Table 1). Sprouts and the BSE were served with bagels, cream cheese, orange juice, milk, or coffee. The same breakfast was provided on days 2 and 3 without sprouts or the BSE. Sprouts were obtained from Sprouters Northwest, Inc. (Kent, WA) and the BSE from Johns Hopkins University (Baltimore, MD). Details of the BSE preparation prior to encapsulation are reported by Egner et al., except supplements used in this study provided 100 μmol SFN per capsule [11]. SFN was determined stable within the BSE stored ≤ 900 days at ≤ 20°C by manufacturers. The BSE was stored in our lab at −20°C ≤ 200 days before consumption and were removed from freezers only moments before being consumed. SFN levels and stability within the BSE were verified in-house, and the “SFN potential” of sprouts was determined to match BSE doses (Section 2.5). Subjects were instructed to avoid consuming foods containing glucosinolates and/or ITCs for 1 week before and throughout each study phase. Subjects attended a prestudy meeting with staff and a registered dietitian regarding study protocol and training for documenting food intake. Self-reported, 3-day diet records collected during each study phase were analyzed using Food Processer® SQL (ESHA, Salem, OR).
Figure 1.
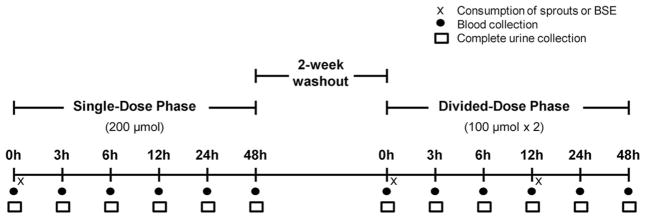
Human feeding study design. Subjects consumed single and divided doses of 200 μmol SFN equivalents from either fresh broccoli sprouts or myrosinase-treated broccoli sprout extract (BSE) supplements (n = 10). Divided doses were consumed 12 h apart within a single day.
2.3 Sample collection
Complete urine collections were obtained following a 12-h overnight fast before SFN consumption and at 3, 6, 12, 24, and 48 h postconsumption. Processing protocols were modified slightly from Janobi et al. [13]. While in subjects’ possession, urine was refrigerated or kept on ice in opaque jugs containing granulated boric acid (~20 mg/mL) to stabilize SFN metabolites. Urine was acidified with TFA to a final concentration of 10% v/v in urine storage tubes (VWR, Radnor, PA) before storing at −80°C. Whole blood (20 mL) was collected by venipuncture into EDTA vacutainers (VWR, Radnor, PA) before SFN consumption and at 3, 6, 12, 24, and 48 h post-consumption. Whole blood was immediately centrifuged 1 min at high speed. Plasma was removed and acidified with TFA to a final concentration of 10% v/v, vortexed, snap-frozen in liquid N2, and stored at −80° C. Remaining whole blood was processed to isolate peripheral blood mononuclear cells (PBMC) as described previously [6]. PBMC protein concentrations were determined using the RC/DC protein assay (Bio-Rad, Hercules, CA). PBMC lysates were frozen at −80°C until analysis of HDAC activity. Phlebotomy was performed in the Moore Family Center clinical collection laboratory by a certified phlebotomist.
2.4 Preparation of standards
R,S-SFN was purchased from LKT Laboratories, Inc. (St. Paul, MN). SFN-glutathione (SFN-GSH), SFN-cysteine (SFN-Cys), and SFN N-acetyl-cysteine (SFN-NAC) were purchased from Toronto Research Chemicals (Canada). Deuterated SFN-NAC (SFN-NAC-D3) and SFN-cysteinylglycine (SFN-CG) were pre-pared in-house as described previously [8].
2.5 Sulforaphane content of sprouts and the broccoli Sprout extract
Sprouts (215 mg) were analyzed for “SFN potential” upon receipt and on nights before consumption. Fresh sprouts were homogenized in 1 mL deionized water and incubated 2 h in the dark at 60°C with 2 mg Sinapis alba thioglucosidase (Sigma-Aldrich, St. Louis, MO). Following incubation, sprouts were filtered using 0.22-μm nylon Spin-X® centrifuge tube filters (VWR, Radnor, PA) (16 000 x g, 5 min, 25°C). The supernatant was diluted 20-fold in 0.1% v/v formic acid (FA) in water. To confirm SFN content of the BSE, 300–400 mg BSE powder was dissolved into 1.5 mL DMSO and diluted to 2 μM in 0.1% v/v FA in water. Sprout and BSE extracts were immediately stored at −80°C until HPLC-MS/MS analysis. We performed quality control experiments to confirm repeatability of extract and analysis procedures, and that SFN within the BSE remained stable over time (data not shown). For HPLC-MS/MS analysis, ten microliters of extracts were injected in duplicate, along with the internal standard, SFN-NAC-D3. HPLC-MS/MS conditions were identical to those in our previous study [14]. The following precursor and product ions were used for detection: SFN (178 > 114), SFN-NAC-D3 (344.1 > 114). Quantitation was based on an 8-point standard curve prepared in 0.1% v/v FA in water. Sprout and BSE doses given to participants were matched to provide equivalent SFN content (Supporting Information Table 1).
2.6 Sulforaphane metabolites in plasma and urine
Plasma was thawed and centrifuged (12 000 x g, 5 min, 4°C) to precipitate proteins. Supernatants were filtered twice through Spin-X® centrifuge tube filters (VWR, Radnor, PA) by centrifugation (12 000 x g, 3.5 min, 4°C). Final filtrates were snap-frozen in liquid N2 and stored at −80°C until HPLC-MS/MS analysis. Urine samples were thawed and centrifuged (400 × g, 5 min, 4°C) to precipitate proteins. Supernatants were filtered twice, as with plasma samples. Final filtrates were diluted 1:2 with 0.1% v/v FA in water, snap-frozen in liquid N2, and stored at −80°C until analysis. Both plasma and urine were analyzed for SFN, SFN-Cys (299 > 114), SFN-GSH (485 > 179), SFN-CG (356 > 114), and SFN-NAC (341.1 > 114). Ten microliters of plasma or urine were injected in duplicate. The same instrumentation and HPLC-MS/MS conditions were used as for sprout and BSE analyses. Quantitation was based on standard curves prepared in 0.1% v/v FA in water. Spike and recovery experiments using an internal standard confirmed consistent and high (>80%) recovery using the documented processing protocols and comparable quantitation of standards prepared in biological matrices versus 0.1% v/v FA in water.
2.7 Urine creatinine
Creatinine was determined using the standard Jaffe reaction method with alkaline picrate [15] and measuring absorbance at 490 nm using a Spectra Max M2 fluorescent plate reader (Molecular Devices, Sunnyvale, CA). Urinary SFN metabolite levels were normalized to urinary creatinine levels where indicated.
2.8 Gene expression
Whole blood was preserved using PAXgene Blood RNA Tubes. Total RNA was isolated using the PAXgene Blood miRNA kit (PreAnalytiX, Hombrechtikon, Switzerland). RNA was reverse transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix for quantitative real-time PCR (qPCR) (Life Technologies, Grand Island, NY). Gene expression was quantified by qPCR using primers specific for p21, heme oxygenase-1 (HO-1), and the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for normalizing gene copy number (Supporting Information Table 2). All qPCR reactions were done using Fast SYBR Green Mastermix on 7900HT Fast Real-Time PCR System (Life Technologies, Grand Island, NY). Gene copies were determined based on standard curves generated from serial dilutions of purified plasmid DNA encoding for genes of interest.
2.9 Protein expression
Plasma HO-1 protein was evaluated in neat, nonacidified plasma obtained during the single-dose phase using an HO-1 ELISA Kit (R&D Systems, Minneapolis, MN) according to manufacturer’s protocol. Optical density was read at 450 nm using a Spectra Max M2 fluorescent plate reader (Molecular Devices, Sunnyvale, CA).
2.10 HDAC activity
HDAC activity was measured in PBMC lysates obtained during the single-dose phase in the Cancer Chemoprevention Program’s Core Laboratory at the Linus Pauling Institute as previously described, with sodium butyrate as the positive control [4]. HDAC substrate and deacetylated standard were custom synthesized (AAPPTec, LLC, Louisville, KY). HDAC activity is expressed relative to lysate protein content.
2.11 Statistical analyses
Initial analyses for SFN metabolite levels included time points where multivariate normality was reasonable (no or few zero responses). Multivariate, repeated measures analyses assessed evidence regarding treatment (sprout or BSE) effect, time effect, and treatment-by-time interaction. When necessary, the repeated measures variance-covariance structure was allowed to vary between treatments (likelihood ratio tests). With strong evidence of time effects or time-by-treatment interactions, treatment effects were evaluated at each time point (unpaired t-tests) and compared between pairs of time points (paired t-tests). With little to no evidence of treatment effects (main or interaction), treatment groups were combined to evaluate changes in responses over time. Use of exact two-sided Wilcoxon rank-sum and exact signed rank tests accommodated time points with nonnormal data (zeroes or nondetectable levels). For molecular responses (HDAC, HO-1, and p21), we analyzed fold change relative to baseline using multivariate, repeated measures analyses including all time points. Two responses (HDAC and HO-1) required additional covariate adjustment for baseline due to significant downward linear trends in fold change with increasing baseline. Whenever residuals exhibited right skew, responses were also analyzed on the log transformed scale with no changes in conclusions. An extreme outlier in the sprouts group for HO-1 protein level led to two different analyses giving similar conclusions: nonparametric analysis (Wilcoxon at each time point) with outlier included and multivariate, repeated measures analysis with outlier excluded. Student’s t-tests were used to compare subject characteristics, reported dietary intakes, and SFN doses between treatment groups (unpaired) and study phases (paired). These analyses were performed using SAS 9.3 (Cary, NC). Pharmacokinetic parameters were analyzed using WinNonLin, version 5.3 (Pharsight Corporation, St. Louis, MO) and compared using paired and unpaired Student’s t-tests. Both compartmental and noncompartmental analyses were performed and returned similar results. Maximum concentration (Cmax) and time at Cmax (Tmax) were determined graphically. Significance for all analyses was determined at α < 0.05.
3 Results
3.1 Subject characteristics
Age, gender, and BMI were similar between sprout and BSE consumers (Supporting Information Table 3). Total intakes of calories and macronutrients were similar between treatment groups and study phases (Supporting Information Table 4). Records indicated compliance with avoiding confounding food items.
3.2 Effects of sulforaphane form and dose
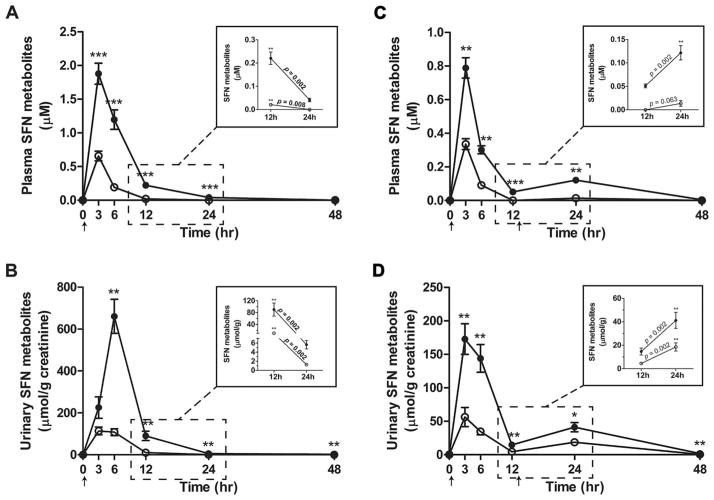
Plasma and urinary levels of total SFN metabolites were ~3–5 times higher in sprout consumers compared to BSE consumers (Fig. 2, Supporting Information Table 5). During both study phases, total SFN metabolites in plasma were significantly higher in sprout consumers at 3, 6, 12, and 24 h after consuming SFN (Figs. 2A and C). In the single-dose phase, urinary levels peaked between 3 and 6 h in sprout consumers and between 0 and 6 h in BSE consumers. In the divided-dose phase, mean urinary levels peaked in both groups between 0 and 3 h following the first 100 μmol SFN dose (Fig. 2D). While SFN absorption from sprouts and the BSE was different, the distribution of SFN metabolites in plasma and urine at Tmax was similar, with the exception that plasma SFN-Cys (p < 0.05) was slightly lower in sprout consumers (Supporting Information Fig. 1A and B, Supporting Information Table 6). SFN-Cys and SFN-CG were the major SFN metabolites detected in plasma at Tmax. After 12 h, the most abundant plasma metabolite was SFN-NAC, followed closely by SFN-CG. In a previous study, we detected SFN-CG as the major plasma metabolite in subjects 12 h after consuming sprouts [8]. Some groups have reported the parent compound, SFN, to be more prevalent in plasma than its metabolites [12, 16]. The reasons for these discrepancies are unclear but may relate to differences in sample preparation, timing of measurements, analytical procedures, or perhaps variation in SFN metabolism among study populations [17, 18]. Prior to analyzing our samples, we conducted multiple spike-recovery experiments to ensure SFN and its metabolites were preserved through sample processing and analysis.
Figure 2.
Sulforaphane (SFN) metabolite levels in plasma and urine following consumption of fresh broccoli sprouts and the broccoli sprout extract (BSE). Total levels (mean ± SEM) of SFN metabolites in plasma (A and C) and urine (B and D) of subjects at time 0 and at 3, 6, 12, 24, and 48 h after consuming broccoli sprouts (closed) or the BSE (open). (A and B) SFN metabolite levels detected following the single dose of 200 μmol SFN equivalents. (C and D) SFN metabolite levels following two 100-μmol SFN doses consumed within a single day. Insets: Close-up of SFN metabolite levels at 12 and 24 h in (A and C) plasma and (B and D) urine. Arrows indicate times of SFN consumption. Free SFN was only detected in four subjects at 3 h following the single dose and comprised < 2% of total SFN metabolites (data not included). *p < 0.05, **p < 0.01, ***p < 0.001.
3.3 Effects of intake frequency
In the divided-dose phase, comparative assessments were performed on plasma concentrations of SFN metabolites 12 h after consuming each dose to evaluate the effects of the twice-daily dosing regimen on achieving steady-state SFN concentrations. In sprout consumers (Fig. 2C inset), plasma concentrations were 2.4-fold higher after consuming the second dose than after the first dose. In BSE consumers, SFN metabolites were only detected after the second dose, and in only 5 of 10 subjects. We also compared 24 h plasma concentrations of SFN metabolites following consumption of the single, 200 μmol dose and the divided dose to evaluate the potential for twice-daily dosing schedules to achieve higher steady-state concentrations than once-daily dosing schedules. In sprout consumers, mean plasma concentrations of total SFN metabolites were significantly higher following consumption of the divided dose compared to the single dose (Fig. 2A inset; 0.12 ± 0.02 μM versus 0.04 ± 0.01 μM, p < 0.001). At the same time points in BSE consumers, SFN metabolites were only detected after consuming the divided dose.
3.4 Chemopreventive targets
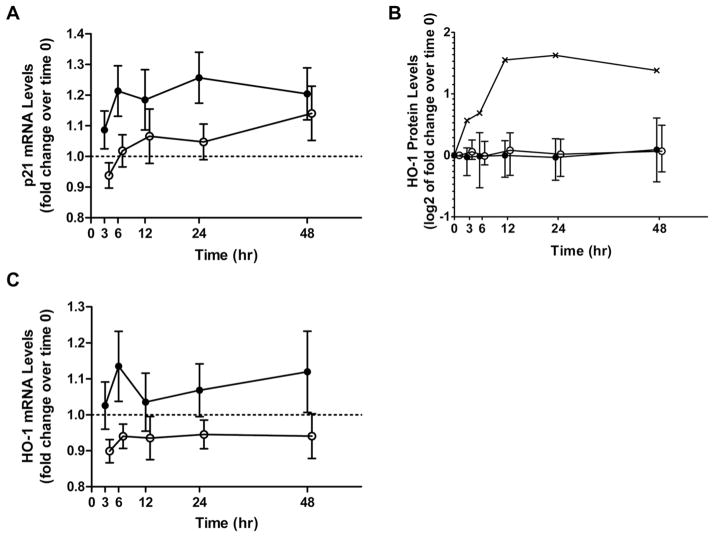
Sprout and BSE consumers had similar fluctuations in PBMC HDAC activity during the study (Fig. 3). Similar to what we have observed previously, HDAC activity decreased transiently, though nonsignificantly (p = 0.097), in most subjects 3 h after consuming sprouts (7 of 9) or the BSE (6 of 10) [6]. Mean HDAC activity increased significantly at the 12-h time point (p < 0.010) (Fig. 3A). One sprout consumer was unable to provide a PBMC sample for analysis at time 0 due to low volume of blood drawn; blood draws at other time points were obtained successfully. For p21 mRNA, the mean fold change from time 0 was greater in sprout consumers than in BSE consumers, but differences between the two groups did not reach statistical significance (p = 0.071; Fig. 4A). In a pooled analysis of sprout and BSE groups, increasing fold changes were not statistically significant (p = 0.185). Sprout and BSE consumers experienced similar changes in plasma HO-1 protein levels with the exception of a single sprout consumer whose response curve was not similar to the other subjects (p = 0.535, excluding outlier; Fig. 4B). Inclusion of the outlier in the analysis did not change statistical conclusions. For HO-1 mRNA, the mean fold change from time 0 was greater in the sprout group than the BSE group, but this difference was not significant after accounting for lower baseline values in the sprout group (p = 0.354; Fig. 4C). In a pooled analysis of sprout and BSE groups, increases in HO-1 expression over time were not significant at the transcript (p = 0.152; Fig. 4B) or protein (p = 0.287; Fig. 4C) level following SFN consumption, though most subjects experienced some increase in HO-1 protein levels during the study (Supporting Information Fig. 2).
Figure 3.
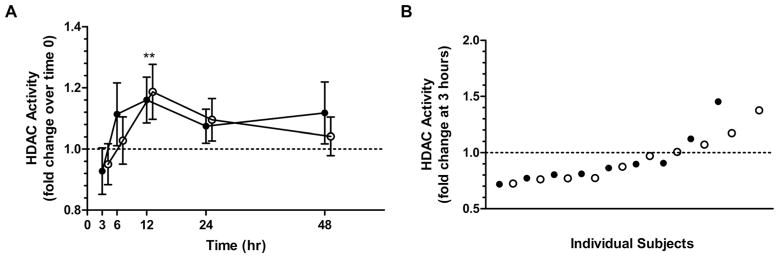
HDAC activity in PBMCs following consumption of broccoli sprouts or the broccoli sprout extract (BSE). (A) Average fold changes (mean ± SEM) in PBMC HDAC activity after consuming a single dose of 200 μmol SFN equivalents from broccoli sprouts (solid, n = 9) or BSEs (open, n = 10). (B) Individual fold changes in HDAC activity from time 0–3 h. Note: Offsets for the BSE group are for visualization purposes only. **p < 0.01. HDAC, histone deacetylase; PBMC, peripheral blood mononuclear cells.
Figure 4.
p21 and HO-1 expression following consumption of broccoli sprouts or the broccoli sprout extract (BSE). Changes in (A) whole blood p21 mRNA levels, (B) plasma HO-1 protein levels, and (C) whole blood HO-1 mRNA after consuming a single dose of 200 μmol SFN equivalents from broccoli sprouts (solid) or BSEs (open). The sprout subject excluded from analyses is shown separately in the figure (-x-). Values represent mean ± SEM (A and B) and geometric mean ± SD (C). Note: Offsets for the BSE group are for visualization purposes only. HO-1, heme oxygenase-1.
4 Discussion
SFN, a phytochemical derived from cruciferous vegetables, has many cancer chemopreventive functions. Details of its molecular mechanisms in humans and its absorption from different dietary sources and forms are emerging. Rich, whole-food SFN sources include cruciferous vegetables, namely broccoli and broccoli sprouts. These foods contain GFN, which is hydrolyzed to yield SFN by the enzyme myrosinase, also found in cruciferous vegetables. Yet, GFN content within vegetables is highly variable, and conversion efficiency of GFN to bioactive SFN is affected by several factors, such as chewing intensity, glutathione-S-transferase (GST) genotype, and presence of gut microbes capable of hydrolyzing GFN when the plant enzyme is inactivated (as occurs with cooking) [10,17,19]. These factors complicate consistent SFN delivery from whole foods in clinical trials, and dietary SFN supplements may be a feasible alternative. Yet, little is known about SFN bioavailability from supplements and how they impact biological processes compared to whole foods. This study is the first to provide evidence for the use of a myrosinase-treated BSE supplement to study cancer chemoprevention strategies involving SFN. We show that SFN absorption and excretion differ in individuals consuming whole foods (broccoli sprouts) and a SFN-containing BSE as well as when a dose is consumed at intervals rather than at a single time. We also describe the impact of these two SFN forms on specific chemopreventive targets.
4.1 Sulforaphane absorption and excretion
Differences in SFN bioavailability among ingested forms of broccoli have largely been attributed to differences in myrosinase activity. Subjects consuming raw broccoli or broccoli sprouts containing intact myrosinase were reported to have relatively high percent recoveries of SFN and SFN metabolites (32–80%). Dietary forms with inactivated myrosinase (e.g. cooked cruciferous vegetables, GFN supplements available over-the-counter) had much lower percent recoveries (10–12%) [8, 20–23]. In a cross-over study (n = 8), Vermeulen et al. [21] observed 11% higher excretion of SFN metabolites following consumption of raw versus cooked broccoli. Also, less SFN was absorbed and excreted from GFN supplements containing inactive myrosinase compared to fresh broccoli sprouts [8]. Supplements containing SFN, such as the BSE used in this study, may deliver higher and more consistent SFN doses than supplements containing GFN and inactive myrosinase. We observed that the myrosinase-treated BSE provided more SFN than previously tested GFN supplements, but SFN absorption was still significantly lower than from fresh broccoli sprouts (Fig. 2A). Validation experiments dismissed inequality of SFN dosing from sprouts and the BSE as an explanation for differences in SFN absorption from the two dietary forms. Our observations suggest that GFN conversion to SFN is not the only factor influencing SFN absorption.
Food matrix and meal composition may have affected SFN absorption from sprouts and the BSE. Whole broccoli sprouts contain nutrients, minerals, and phytochemicals possibly lost during BSE manufacturing that may enhance SFN transport across cell membranes. Compared to the BSE, raw sprouts likely contain more fiber, which can slow gut transit and increase contact time between SFN and absorptive surfaces in the proximal gut. Compounds derived from other ingested foods and beverages could similarly impact SFN absorption. However, all participants consumed similar breakfast meals along with the given dose of sprouts or the BSE. Although composition of subjects’ meals varied slightly, the foods provided did not contain SFN or compounds known to influence SFN absorption or the molecular targets we evaluated. Macronutrient content of subjects’ diets during the study was similar. Despite the many factors possibly influencing SFN absorption, we observed relatively little variability in SFN metabolite levels and percentages within each treatment group. Enzymes involved with SFN absorption and metabolism, such as GSTs, are also involved with absorption and metabolism of other nutrients and xenobiotics and, thus, may be tightly regulated [24]. Variability was further minimized by standardizing breakfast meals and ensuring fasting status prior to morning SFN doses.
Calculated SFN bioavailability from broccoli sprouts exceeded 100% (Supporting Information Table 5), which likely reflects a combination of high SFN bioavailability and interconversion of erucin to SFN in vivo [8]. Interconversion between erucin and SFN may also explain the elevated half-life estimated for SFN absorbed from broccoli sprouts. Glucoerucin levels were not measured in the sprout formulations, and erucin metabolites were not measured in plasma or urine samples, thus their influence on SFN bioavailability and elimination kinetic estimates could not be determined.
This is the first study to report the effects of consuming SFN twice in a single day toward the possibility of achieving higher steady-state SFN levels in humans than consuming an equivalent dose all at once. In the body, SFN is rapidly metabolized, and plasma concentrations of SFN metabolites reach extremely low levels 12–24 h after consuming a single dose [8,11,25,26]. This study demonstrated that 12-h dosing, compared to consuming a single dose, resulted in higher plasma concentrations of SFN metabolites 24 h postconsumption, which was expected based on pharmacokinetic patterns commonly observed with multiple dosing [27]. While the higher concentrations observed at 24 h in the divided-dose phase are likely related to residual SFN metabolites derived from the first divided dose, the increase was not proportional to the amount of residual SFN metabolites expected at the 24-h time point based on previous reports and our observations of SFN kinetics following a single dose [8, 11, 12]. Based on the data, it is unclear what other factors may have contributed to the higher 24-h plasma concentrations observed with the twice-daily dosing schedule. Additional studies are needed to fully understand the impact of consuming multiple daily doses of SFN on SFN absorption and metabolism in humans.
4.2 Chemopreventive targets
SFN is implicated in both genetic and epigenetic mechanisms important for cancer prevention [4, 6, 28]. To identify mechanisms involved with SFN’s ability to prevent carcinogenesis in humans, we evaluated the response of three chemopreventive, mechanistic targets of SFN (i.e. HO-1, HDAC, p21) in healthy adults following consumption of sprouts or the BSE. Despite existing reports that SFN increases expression of Nrf-2 targets, such as HO-1, we did not observe any significant increases in HO-1 expression in whole blood or plasma following sprout or BSE consumption [29–31]. Regardless of the SFN form consumed, subjects experienced a large degree of interindividual variability in HO-1 responses, which could relate to its biological function as an inducible component of cytoprotective mechanisms and prevalence of promoter polymorphism [32, 33]. SFN doses consumed in this study may have been too low or too rapidly metabolized in cells to induce HO-1 to degrees observable in the circulation. Importantly, our observations in whole blood may not represent changes occurring in tissues. Cornblatt et al. [31] observed a tenfold induction of HO-1 in mammary tissue from rats gavaged with 150 μmol SFN over control animals. Additional studies are needed to characterize molecular target responses to consuming SFN from sprouts and the BSE in target organs.
SFN may also prevent cancer through epigenetic mechanisms by functioning as an HDAC inhibitor [34]. Though sprout and BSE subjects consumed similar SFN doses as in previous human studies that have reported decreases in HDAC activity, neither of the two SFN forms produced a significant decrease in HDAC activity. Yet, the trends for decreased activity observed at the 3-h time point in sprout and BSE consumers were similar in magnitude to those reported by Clarke et al. (Fig. 3A) [6]. Larger decreases in HDAC activity may occur in transformed cells or tumors with underlying aberrant HDAC regulation, rather than in nontransformed PBMCs obtained from healthy subjects, as in this study [35, 36]. Higher SFN doses or repeated intake may also result in greater or more sustained decreases in HDAC activity [6]. Increases in HDAC activity observed 12 h after SFN consumption may relate to a homeostatic response to the slight decrease observed 9 h earlier. Rhythmic HDAC expression is not expected as the cause of these increases, as we previously observed no change in HDAC activity over time in healthy adults consuming placebos and avoiding intake of other known dietary HDAC inhibitors (unpublished data). As with HO-1, high interindividual variability was observed in HDAC responses regardless of the SFN form consumed. This variation could relate to biological variation, basal activity levels, or effects on specific HDAC isoforms not influenced by SFN, as we assessed measures of total HDAC activity [37]. Although sprout and BSE consumers did not experience different mean HDAC responses at the doses provided in this study, further evaluation is needed to clarify dose-response relationships between HDAC activity and SFN consumed from sprouts and the BSE as well as tissue-specific effects of SFN on HDAC regulation and downstream targets such as p21.
Despite having lower SFN bioavailability compared to broccoli sprouts, the myrosinase-treated BSE may be an acceptable SFN source for use in clinical trials to study certain chemopreventive mechanisms of SFN. Further, we demonstrated maintenance of plasma SFN levels using a twice-daily dosing schedule, which may be important for increasing or prolonging certain chemopreventive benefits associated with SFN consumption. Together, these data provide important information for using SFN supplementation strategies in clinical studies of cancer chemoprevention.
Supplementary Material
Acknowledgments
This work was supported by the Linus Pauling Institute, Center for Genome Research and Biocomputing Core Facilities, and Mass Spectrometry Lab at Oregon State University, as well as the National Institutes of Health (CA090890, CA122906), National Institute of Environmental Health Sciences (P30 ES000210), and Oregon Agricultural Experimental Station. We gratefully acknowledge technical assistance from Dr. Laura Beaver, Karin Hardin, Jeffery Morre, Jeannie Allen, and Traci Beckman
Abbreviations
- BSE
broccoli sprout extract
- Cys
cysteine
- CG
cysteinylglycine
- FA
formic acid
- GFN
glucoraphanin
- GSH
glutathione
- GST
glutathione-S-transferase
- HDAC
histone deacetylase
- HO-1
heme oxygenase-1
- ITC
isothiocyanate
- NAC
N-acetyl-cysteine
- NAC-D3
deuterated N-acetyl-cysteine
- OSU
Oregon State University
- PBMC
peripheral blood mononuclear cells
- SFN
sulforaphane
Footnotes
The authors have declared no conflict of interest.
Additional supporting information may be found in the online version of this article at the publisher’s web-site
References
- 1.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 2.Petri N, Tannergren C, Holst B, Mellon FA, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 3.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 4.Myzak MC, Karplus PA, Chung FL, Dashwood R. A novel mechanism of chemoprotection by sulforaphane. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 5.Myzak MC, Tong P, Dashwood WM, Dashwood RH, et al. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med. 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke JD, Riedl K, Bella D, Schwartz SJ, et al. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem. 2011;59:10955–10963. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Hullar MA, Beresford SA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr. 2011;106:408–416. doi: 10.1017/S0007114511000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JD, Hsu A, Riedl K, Bella D, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res. 2011;64:456–463. doi: 10.1016/j.phrs.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer. 2011;63:196–201. doi: 10.1080/01635581.2011.523495. [DOI] [PubMed] [Google Scholar]
- 10.Pereira FMV, Rosa E, Fahey JW, Stephenson KK, et al. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agric Food Chem. 2002;50:6239–6244. doi: 10.1021/jf020309x. [DOI] [PubMed] [Google Scholar]
- 11.Egner PA, Chen JG, Wang JB, Wu Y, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4:384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasper AV, Aljanobi A, Smith JA, Bacon JR, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 13.Janobi AAA, Mithen RF, Gasper AV, Shaw PN, et al. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatographytandem electrospray ionisation mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:223–234. doi: 10.1016/j.jchromb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Clarke J, Hsu A, Williams D, Dashwood R, et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2007;28:3171–3179. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urquidi V, Kim J, Chang M, Dai Y, et al. CCL18 in a multiplex urine-based assay for the detection of bladder cancer. PLoS One. 2012;7:e37797. doi: 10.1371/journal.pone.0037797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha S, Hollands W, Teucher B, Needs PA, et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res. 2012;56:1906–1916. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- 17.Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5:603–611. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol. 2007;17:347–353. doi: 10.1016/j.semcancer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzaud G, Young SA, Duncan AJ. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol Biomarkers Prev. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, et al. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J Agric Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen M, Klopping-Ketelaars IWAA, van den Berg R, Vaes WHJ. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–10509. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, et al. Human metabolism and excretion of cancer chemo-protective glucosinolates and isothiocyanate of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 23.Conaway CC, Wang CX, Pittman B, Yang YM, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 24.Josephy PD. Genetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicology. Hum Genomics Proteomics. 2010;2:876940. doi: 10.4061/2010/876940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro TA, Fahey JQ, Wade KL, Stephenson KK, et al. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 27.Brenner GM, Stevens C. Pharmacology: With STUDENT CONSULT Online Access. Elsevier Health Sciences; Philadelphia: 2012. pp. 9–27. [Google Scholar]
- 28.Myzak MC, Dashwood WM, Orner GA, Ho E, et al. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apcmin mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keum YS, Yu S, Chang PPJ, Yuan X, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 30.Prawan A, Keum YS, Khor TO, Yu S, et al. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharm Res. 2008;25:836–844. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 31.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, et al. Preclinical and clinical evaluation of sulforaphane for chemo-prevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 32.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, et al. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2010;87:187–190. doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–1420. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 35.Clarke JD, Hsu A, Yu Z, Dashwood RH, et al. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 37.Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3:344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.