Abstract
Infectious triggers are associated with the induction of transient antiphospholipid antibodies. One therefore wonders if microbes that permanently colonize us play a role in the pathogenesis of antiphospholipid syndrome (APS). The microbiota represents the collection of all microorganisms colonizing humans and is necessary for normal host physiology. The microbiota, however, is a constant stress on the immune system, which is tasked with recognizing and eliminating pathogenic microbes while tolerating commensal populations. A growing body of literature supports a critical role for the commensal-immune axis in the development of autoimmunity against colonized barriers (e.g., gut or skin) and sterile organs (e.g., pancreas or joints). Whether these interactions affect the development and sustainment of autoreactive CD4+ T cells and pathogenic autoantibodies in APS is unknown. This review provides an overview of the current understanding of the commensal-immune axis in autoimmunity with a focus on the potential relevance to APS. Additionally, we discuss emerging findings supporting the involvement of the gut microbiota in a spontaneous model of APS, the (NZW×BXSB)F1 hybrid, and formalize hypotheses to explain how interactions between the immune system and the microbiota may influence human APS etiopathogenesis.
Keywords: Commensal, Microbiome, Cross-reactivity, β2-Glycoprotein I, Gut barrier, Antiphospholipid syndrome, Antiphospholipid antibodies, Molecular mimicry, NZW, BXSB, Th17, Tfh, Rheumatic fever, Guillain-Barré syndrome, LPS, Segmented filamentous bacteria, SFB, Leaky gut
Introduction
The gut microbiota or the collection of all gut commensals, a previously “forgotten organ,” lives in a symbiotic relationship with its host and influences a wide range of physiological processes including nutrient availability, metabolism, behavior, and immune system development and homeostasis [1, 2]. Commensal bacteria within an individual are estimated to outnumber human cells ten to one; on average, the human gut is colonized by ~160 bacterial species, and more than 1000 species in total can be found across the human population [3, 4]. Compositional or functional disturbances in the microbiota (so-called dysbiosis) are linked to a variety of chronic metabolic and inflammatory diseases including obesity, cardiovascular disease, and cancer [2]. In addition, immune system development and homeostasis, both at barrier sites (gut, skin, lung) and systemically, are greatly influenced by the microbial community composition [5, 6]. Intriguingly, commensal bacteria, particularly those colonizing the gut, exert profound effects on a number of experimental autoimmune models [7, 8]. Our laboratory focuses on understanding the role of the gut microbiota on the induction and maintenance of autoreactive T cells and autoantibodies that mediate systemic autoimmunity, in particular antiphospholipid syndrome (APS) and lupus.
Antiphospholipid syndrome is a prototypical autoimmune disease mediated by T cell-dependent antiphospholipid antibodies (aPLs) that interfere with coagulation and result in thrombosis and miscarriages. The general mechanism leading to thrombotic events is thought to involve a local procoagulant state at sites where autoantigen-bound antibodies trigger the activation of endothelial cells, platelets, and monocytes. These processes manifest as both venous and arterial thromboses and obstetric complications leading to significant morbidity and mortality in APS patients [9, 10]. In the appropriate clinical scenarios, patients are tested for the presence of lupus anticoagulant (LA), anti-β2-glycoprotein I (anti-β2GPI), and anti-cardiolipin (aCL) antibodies. These aPLs can fluctuate over time and be present in healthy individuals without a history of thrombosis or pregnancy complications [11, 12]. Persistently “triple positive” (LA+, anti-β2GPI+, and aCL+) asymptomatic carriers of aPLs, however, have a markedly increased risk of thrombosis [13]. Transient aPLs can be induced by a variety of infectious agents but are generally not considered pathogenic. Clinical criteria for a diagnosis of APS thus require consecutive positive LA tests or detection of high titers of IgM or IgG antibodies against β2GPI or CL separated by a minimum of 12 weeks [11, 14]. Additional isotypes and targets of aPLs are currently under investigation but are not considered standard APS diagnostic criteria. These include anti-β2GPI of the IgA isotype and aPLs against prothrombin, phosphatidylserine/ prothrombin, and phosphatidylethanolamine [15].
While a variety of autoantigens are identified in APS, β2GPI is the most commonly detected in patients. Lupus anticoagulant correlates strongest with thrombotic events when antibodies against β2GPI are present, providing further evidence for its importance in APS [16]. β2GPI is a common serum protein with pleiotropic functions including anticoagulant properties, scavenging lipopolysaccharide (LPS), mediating apoptotic cell clearance, and limiting oxidative stress during apoptosis [17–20, 21••]. β2GPI contains five domains and is found in a closed spherical confirmation in the blood; the positively charged cysteine residues in domain V bind to negatively charged surfaces such as CL or other negatively charged phospholipids, which leads to a conformational change that exposes a cryptic epitope in domain I [22] (Fig. 1). This epitope, with the core sequence RGGMR, is a major target of thrombogenic autoantibodies against β2GPI in humans [23, 24]. Other pathogenic antibody targets of β2GPI have also been described such as human IgA anti-β2GPI binding to domain Vor the TLRVYK sequence within domain III in mice [25–27].
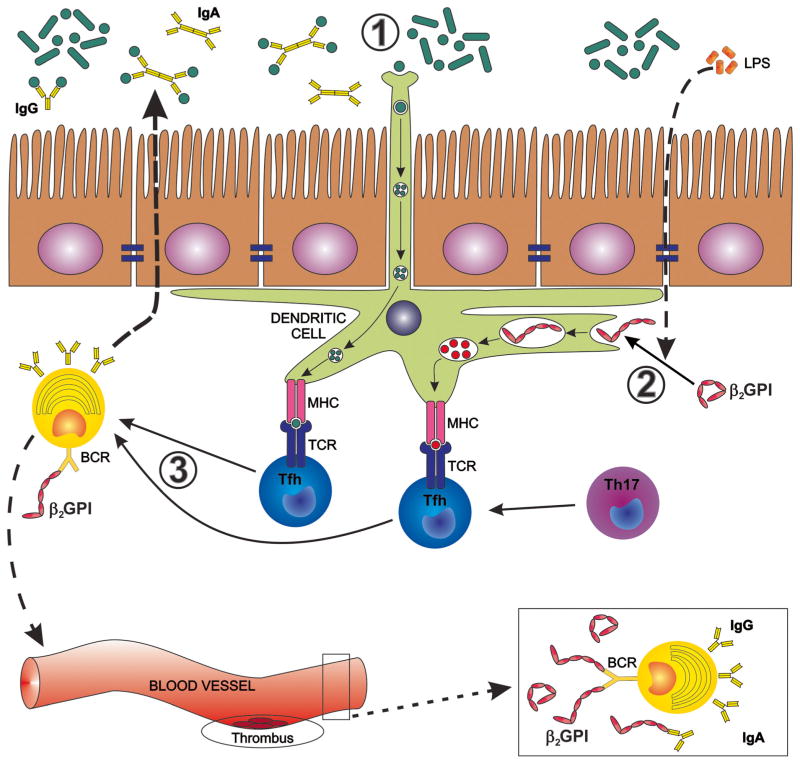
Fig. 1.
Proposed influence of the gut microbiota on induction and maintenance of anti-β2-glycoprotein I antibodies (anti-β2GPIs). In genetically predisposed individuals, the gut microbiota may drive the induction of anti-β2GPI antibodies by several mechanisms that can occur separately or in combination. 1 Cross-reactive gut commensal antigens are recognized by mucosal dendritic cells (DCs) sampling the intestinal lumen or by phagocytosis after barrier disruption or apoptosis of intestinal epithelial cells (not shown). 2 Commensal-derived LPS, phospholipids, and oxidative stress lead to a conformational change of β2GPI that exposes cryptic epitopes in domains I and V of β2GPI. DCs and other antigen-presenting cells take up unfolded β2GPI bound to LPS or phospholipids via receptors that bind these complexes, e.g., Toll-like receptors. 3 DCs present cross-reactive commensal antigens, cryptic β2GPI antigens, or both in an HLA class II-restricted manner to cognate CD4+ helper T cells in secondary lymphoid organs. CD4+ helper Tcell subsets assist antigen-specific B cells via CD40 ligand and other co-stimulatory receptors (not shown). These B cells then secrete IgA/IgG in the mucosal lumen and in the systemic circulation, respectively. The helper T cells leading to IgA/IgG production are follicular helper Tcells (Tfh) or, as shown in the gut, also ex-Th17 cells that convert to Tfh-like cells [62•]. A “second hit” in the vasculature then leads to thrombotic events as detailed in the main text [41].
The major autoepitopes in APS are characterized, but the exact mechanism that contributes to the development of anti-β2GPI antibodies is unknown. The production of autoantibodies against β2GPI and the pathogenesis of a spontaneous APS murine model were shown to be Tcell dependent [28–30]. While the T cell epitopes in humans are not exhaustively defined, Kuwana and colleagues demonstrated that CD4+ T cells from HLA-DRB4*0103 (DR53) APS patients recognized an HLA-restricted dominant epitope in domain V of β2GPI with the amino acid sequence KVSFFCKNKEKKCSY [31–33]. Binding of β2GPI to LPS or phospholipids that are exposed on the surface of apoptotic cells changes its conformation from a closed, circular state to an open, hook-like structure (Fig. 1), which is thought to contribute to antigenicity by exposing cryptic epitopes as mentioned above. The context in which this conformational change occurs could be important for the production of autoantibodies. In addition, the anatomical localization of β2GPI in the body is another variable. For instance, β2GPI coats in the open conformation the endothelial cells of the uterus and the trophoblast during pregnancy, which is likely why miscarriages and pre-eclampsia are such a frequent complication in APS [34].
In general, aPLs develop not only in isolation but also in various rheumatic diseases, suggesting a general mechanism of systemic autoimmunity with broad clinical implications [35]. It is notable that repetitive injections of β2GPI together with LPS into non-autoimmune murine strains induce not only antiphospholipid antibodies but also multiple lupus-specific autoantibodies [36]. The temporal sequence of autoantibody induction is similar to the sequence detected in humans years before the onset of systemic lupus [37]. These findings suggest that an initial autoimmune response against β2GPI might interfere with non-inflammatory processing of apoptotic material generating epitope spreading. These processes are likely important for the development of systemic autoimmunity in those patients that are serologically positive for anti-β2GPI antibodies. Interestingly, deletion of the β2GPI gene in an APS-prone model aggravates lupus nephritis, which supports this notion [38•]. Therefore, elucidating the initiating factors of APS will lead to a better understanding of systemic autoimmunity in general.
Despite a growing understanding of APS pathophysiology, the etiology remains unknown. Several mechanisms are proposed to explain the etiopathogenesis of APS including impaired clearance of apoptotic material, activation of innate pattern recognition receptors (the Toll-like receptor hypothesis) and molecular mimicry with pathogens [39, 40]. Once aPLs are generated and sustained, it is thought that a “second hit” is needed for thrombus formation [41•]. As with any complex autoimmune disease, a genetic predisposition forms the basis for disease susceptibility but is not sufficient to trigger autoimmunity [42–46]. Taken together, a genetically predisposed individual produces pathogenic autoantibodies that trigger thrombosis after a second hit that damages the endothelium or potentiates thrombus formation.
One potential piece in the “APS puzzle” could be the contribution of commensal bacteria to the development and maintenance of autoreactive CD4+ T cells and autoantibodies. This hypothesis is supported by early data from our laboratory using (NZW×BXSB)F1 mice, a spontaneous APS animal model. In the following sections, we describe recent advances in the understanding of commensals as mediators of autoimmunity and hypothesize how commensals may play a role in the development of β2GPI-mediated APS through antigen-dependent and antigen-independent mechanisms.
Commensals as Mediators of Autoimmunity
The microbiota affects the adaptive immune system in both antigen-independent and antigen-specific ways. Antigen-independent mechanisms, such as the production of symbiotic metabolites or molecules like indoles, polysaccharide A, and short-chain fatty acids can induce helper T cell subsets [47, 48, 49•, 50]. In addition to these broader effects, it is now well established that commensals play a role in shaping the antigen-specific repertoire of the mucosal and systemic immune system, respectively. Colonic regulatory T cells (Tregs) recognize specific gut commensal antigens [51, 52]. In addition, segmented filamentous bacteria (SFB) induce SFB-specific intestinal Th17 cells [53, 54]. Particularly interesting is that commensal-specific Tregs can switch from a tolerogenic phenotype to a pro-inflammatory effector phenotype upon breakdown of intestinal homeostasis. Using a T cell receptor (TCR) transgenic model, Hand et al. elegantly demonstrated that commensal-specific T cells, normally tolerant to commensal clostridial bacteria, lost tolerance and gained effector qualities. During acute infection with Toxoplasma gondii, this leads to the subsequent development of long-lived memory T cells [55••]. It is therefore the context in which commensals are recognized by the adaptive immune system that determines if a regulatory or pathogenic effector response is mounted.
Importantly, the microbiota not only influences T cell phenotypes and functions but also both T-dependent and T-independent antibody production [56]. Antibody cloning and expression experiments support that human IgA+ and IgG+ plasmablasts in the gut recognize not only enteric pathogens but also commensal bacteria [57]. Even B cell development was recently shown to occur in the gut lamina propria of mice [58•]. Furthermore, healthy human serum contains antibodies specific to a variety of gut bacteria [59]. Taken together, commensal-specific T and B cell responses occur in healthy hosts and can elicit systemic antibody production. These responses could form the basis for the development of autoreactive clones in a genetically predisposed individual.
The gut microbiota also exerts profound effects on systemic adaptive immune responses not directed against commensal antigens. This is illustrated by murine studies that involve depleting the gut microbiota with antibiotics, affecting antiviral adaptive immune responses such as those against influenza [60, 61].
In summary, the gut microbiota modulates adaptive immune responses and is also recognized by the adaptive immune system, specifically by commensal-specific CD4+ helper T subsets and B cells that lead to production of local and systemic IgG and IgA responses. Helper T cell-dependent antibody production is generally mediated by follicular helper T cells (Tfh). In the gut, however, Th17 can acquire a Tfh phenotype to induce high-affinity IgA responses [62•]. Th17 cells are implicated in the pathogenesis of various human autoimmune diseases and are expanded by several commensals (e.g., SFB and Alcaligenes) [5, 63]. Interestingly, SFB’s effect on autoimmunity is model dependent, being pathogenic in animal models of rheumatory arthritis and multiple sclerosis [64, 65] but protective in the nonobese diabetic mouse model of type 1 diabetes [66, 67]. It is tempting to speculate that the various effects on autoimmunity are due to antigen-specific recognition of Th17 cells and that a switch to Tfh in the gut is involved in autoantibody-mediated autoimmune diseases, in particular, in settings of a breached gut barrier that lead to long-lived memory responses to commensals as discussed above [55••]. Bystander activation, epitope spread, and molecular mimicry, processes described for infectious agents, are possible mechanisms of how commensals could trigger autoimmune responses [68]. Bystander activation and epitope spreading likely require more tissue damage than most innocuous gut commensals can invoke, but cross-reactivity with self-antigens could occur in a genetically susceptible individual that mounts physiologic adaptive immune responses to contain the microbiota.
Molecular Mimicry, APS, and β2GPI
A very intriguing, yet incomplete, hypothesis to explain the development of aPLs is the role of microbes through antigen-dependent effects, particularly molecular mimicry, besidesantigen-independent effects such as breaks in tolerance driven by inflammation. A long-standing hypothesis in the development of autoimmunity is that of molecular mimicry, which refers to the generation of cross-reactive T and B cells that recognize antigens from microbial pathogens but cross-react to autoantigens [69, 70]. Two classic examples of human autoimmune diseases that are thought to originate from cross-reactivity with protein or carbohydrate structures from pathogens are rheumatic fever and Guillain-Barré syndrome (GBS), respectively [71, 72]. In both of these syndromes, the immune pathology that characterizes the disease correlates with the presence of infectious agents, and these infectious agents have epitopes that share linear and structural similarities to the self-antigens targeted by the adaptive immune system.
In rheumatic heart disease, cross-reactive T cell clones isolated from human valvular tissues recognized streptococcal, myocardial, and valvular peptides [73]. Intralesional T cell clones recognized similar antigens as those in the peripheral blood and are, interestingly, HLA-DR53 associated, which is the same MHC class II restriction that predominates in APS [29, 74]. Clinically, there are several similarities between rheumatic fever and APS (most notably the cardiac and neurologic features), which is intriguing given that streptococcal proteins can also bind to or cross-react with β2GPI [75–77].
In GBS, lipooligosaccharides from Campylobacter jejuni are thought to mimic human gangliosides on peripheral nerves. Cross-reactivity was linked to GBS mechanistically both by studying T cell clones and by confirming molecular mimicry in in vivo models [73, 78]. In both GBS and rheumatic fever, the autoimmune response is transient and dependent on the infectious trigger even though long-term (or even permanent) sequelae can occur secondary to damage caused by the autoimmune pathology. We speculate that chronic autoimmune diseases are in part sustained by cross-reactive microbiota as opposed to acute infections with pathogens that are eventually cleared by the host.
Considering the high degree of human gut microbial diversity, colonization, and commensal antigenic load [3, 4], it is not surprising that commensal bacteria would statistically share significant peptide sequence homology with autoantigens. Additionally, given the degeneracy of the TCR, antigen-specific TCRs also recognize numerous cross-reactive antigens, including autoantigens [79, 80, 81••]. Pathogen-reactive memory T cells and antibodies can be found in both unexposed individuals and patients, which cross-react with commensals, inferring a possible evolutionary advantage allowing a more rapid response to pathogenic microbes [82•, 83••].
Molecular mimicry has been implicated in APS. Transient anti-β2GPI antibody production was induced experimentally in vivo by injection of pathogen proteins or peptides [26, 76]. Molecular mimicry has been best studied using antibodies that target the TLRVYK sequence in domain III [26, 25]. Notably, the Shoenfeld group showed that mice immunized with proteins from Haemophilus influenzae, Neisseria gonorrhoeae, or tetanus toxoid, which all share sequence homology to the core region TLRVYK. These immunizations led to the production of antibodies that recognized cardiolipin, β2GPI, and the TLRVYK sequence. Naïve mice infused with these antibodies developed significant thrombocytopenia, prolonged activated partial thromboplastin time, and pregnancy loss similar to mice treated with pathogenic anti-β2GPI. These studies showed for the first time that cross-reactivity offered a potential explanation for the induction of pathogenic aPLs. Pathogen-induced antibodies, however, are generally not considered thrombogenic in humans [84], although many micro-organisms are linked to the induction of aPLs [85]. This might be due to the fact that they are transient and potentially require a second hit to cause thrombosis [41]. In either case, their contribution to thrombosis is likely important in some patients. We propose that commensal bacteria can act as a source of persistent cross-reactive antigen in APS and other chronic autoimmune syndromes. In a similar vein, transient aPLs could be caused by cross-reactive pathogens in human subjects, whereas chronic autoimmunity in APS might be mediated by cross-reactive gut commensals (Fig. 1).
Supporting this theory, we have identified potential cross-reactive peptides using six to eight amino acid-overlapping sequences of the dominant epitopes in APS. Interestingly, some bacteria contain highly homologous sequences to both the major B and T cell epitopes. One of them, the gram-positive anaerobic commensal Roseburia intestinalis, is particularly abundant in the human gut and stimulatory to lymphocytes from APS patients compared to controls (unpublished observations). It remains unknown whether this effect is due to cross-reactivity, but preliminary studies in murine models support a general role for the gut microbiota in APS pathogenesis, which we will discuss in the next section.
Proof of Principle in Animal Studies
A classic model for APS is the (NZW×BXSB)F1 hybrid. These mice develop not only lupus-like systemic autoimmunity but also high titers of β2GPI antibodies and APS manifestations including acute myocardial infarctions and immune thrombocytopenia [86]. Interestingly, mortality is markedly reduced by dietary restriction [87]. Because dietary changes not only affect the host but also profoundly alter the gut microbiome [88–92], we have tested a role for the microbiota in this APS model independently from the mimicry hypothesis [8]. Of note, a fermented milk product that contains a probiotic bacterial strain was shown to slightly alter aPLs in non-autoimmune animals, suggesting indirectly that gut microbes could modulate also pathogenic autoantibodies and APS [93]. Indeed, depletion of the gut microbiota with broad-spectrum antibiotics in young adult APS-prone (NZW×BXSB)F1 animals (after maturation of the immune system) markedly prevented myocardial infarctions and other thrombotic events that otherwise led to death in this model (unpublished observations, [94•]). Specifically, depletion of gut microbiota increased survival and suppressed serum anti-β2GPI IgG antibody titers. Importantly, mice treated with vancomycin or ampicillin alone are similarly protected as with broad-spectrum antibiotics (unpublished observations). These emerging data suggest that gram-positive bacteria within the gut microbiome play a role in driving APS in this model.
Commensal bacteria or their products mediate differentiation and homeostasis of gut-resident and systemic CD4+ T cells in mice. Remarkably, both Th17 and Treg populations can be induced by certain commensals as discussed above. Distinct from these phenotypes are Tfh cells characterized as CD4+CXCR5+ICOShighPD-1high in the lymphoid organs and peripheral blood. Tfh cells are specialized to help B cells undergo isotype switching and affinity maturation in germinal centers [95]. Importantly, besides their prominent role in autoimmunity, follicular helper T cells also appear to interact with the microbiota and are essential for the maintenance of mucosal barriers [96, 97]. In agreement with these results, we found that the frequency of splenic Tfh cells is reduced in antibiotic-treated mice relative to control-treated, APS-prone mice (unpublished observations). Exactly how specific commensals mediate the spontaneous APS phenotype in (NZW×BXSB)F1 mice needs to be studied in more depth, but our work supports that there is a previously underappreciated role for gut commensals in the pathogenesis of APS.
Future Directions: from Mice to Humans
Despite promising links between gut bacteria and the development of anti-β2GPI-mediated pathology in animal models, a link between the gut microbiota and development of human APS remains to be elucidated. Going forward, it will be important to determine if APS patients are affected by gut or other microbiota and to identify the causative commensal bacteria (so-called pathobionts). 16S ribosomal RNA and metagenomic sequencing, culture of specific strains of human commensals, and transfer of candidates into gnotobiotic animals are sophisticated strategies to test interactions between candidate commensals and the host [98, 99]. Such approaches have already led to the identification of human gut commensals with immune modulatory capacities in gnotobiotic models [100•]. Longitudinal studies of patient microbiomes using next-generation 16S rRNA sequencing, combined with advances in detecting human antigen-specific T and B cells, will allow researchers to link the human host immune system with recognition of, or response to, human commensals of the same host [101, 102]. As laid out above, one testable hypothesis is that commensal bacteria can lead to the induction of autoreactive lymphocytes through cross-reactivity in a genetically predisposed individual. Supporting this hypothesis is the recent data showing that murine T cell hybridomas reactive to the lupus and Sjögren’s syndrome autoantigen Ro60 cross-react with synthetic peptides and recombinant protein from commensals [103•]. Advances in cloning of human CD4+ T cells from the peripheral blood without skewing phenotypes should enable the exploration of commensal-specific CD4+ T cells, their function, and their cross-reactive potential with autoantigens [101]. These approaches, however, would only address one side of the “commensal-immune equation.” Studying the effects of the microbiota on the innate immune system and autoantigens will be equally important to understand the overall contribution of commensals to the pathogenesis of APS. Proinflammatory strains of the gut microbiota, acute infections with pathogens, or other “gut trauma” (e.g., critical illness) could mediate local innate inflammation at the gut barrier, promoting a “leaky gut” and formation of commensal-specific memory T cells as pointed out above [55••, 104, 105]. In this context, it is noteworthy that oxidative stress, LPS, and phospholipids that open the conformation of β2GPI could be derived from gut commensals and further promote autoreactivity by uncovering cryptic epitopes, whereas cross-reactivity with commensals could account for induction of β2GPI-directed responses (Fig. 1). Specifically, molecular mimicry with the microbiota could activate anti-β2GPI-specific T cells and lead to generation of pathogenic anti-β2GPI antibodies that subsequently bind to cryptic epitopes revealed by microbial products (Fig. 1). These two processes are not dependent on each other and could occur separately, i.e., the anti-β2GPI might be formed months or years before an inflammatory trigger uncovers sufficient amounts of the cryptic epitopes within β2GPI. This phenomenon might also account for the frequent association of various infections with catastrophic APS [106].
Conclusion
Interactions between the microbiota and the immune system have a profound impact on the pathogenesis of several autoimmune disease models. In healthy individuals, the microbiota sustains a state of tolerance by promoting an anti-inflammatory environment. When this homeostasis is disrupted, a skewing towards pro-inflammatory interactions occurs, which can have local and systemic effects on the immune system, including breaches of the mucosal barriers and generation of commensal-specific memory T cells. Given the known links between transient aPLs and pathogenic microbes, it is plausible that commensal bacteria may promote breaks in tolerance and the induction of persistent aPLs in genetically predisposed individuals. Indeed, preliminary results from our laboratory suggest a role for gram-positive commensal bacteria in the development of murine APS. Understanding these interactions on a molecular level in animal models, healthy human subjects, and patients could lead to new ways to diagnose, prevent, and treat patients suffering from APS and related auto-immune disorders. Most importantly, it might also give us a deeper insight into the etiopathogenesis of this fascinating disease.
Acknowledgments
We would like to thank all members of the Kriegel lab for constructive discussions on this topic and Andrew Yu and Dr. Amar Manvar for critical review of the manuscript. This work was supported by grants from the National Institutes of Health (NIH) (K08 AI095318), the Yale Rheumatic Diseases Research Core (NIH P30 AR053495), the Women’s Health Research at Yale, the O’Brien Center at Yale (NIH P30DK079310), the Arthritis National Research Foundation (all to M.A.K.), and the Yale Interdisciplinary Immunology Training Program (NIH T32AI07019) (to W.E.R.).
Footnotes
Conflict of Interest William E. Ruff, Silvio M. Vieira, and Martin A. Kriegel declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
William E. Ruff, Email: william.ruff@yale.edu, Department of Immunobiology, Yale University School of Medicine, 300 George St, Suite 353G, New Haven, CT 06511, USA
Silvio M. Vieira, Email: silvio.vieria@yale.edu, Department of Immunobiology, Yale University School of Medicine, 300 George St, Suite 353G, New Haven, CT 06511, USA
Martin A. Kriegel, Email: martin.kriegel@yale.edu, Department of Immunobiology, Yale University School of Medicine, 300 George St, Suite 353G, New Haven, CT 06511, USA. Section of Rheumatology, Department of Medicine, Yale University School of Medicine, New Haven, CT, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–95. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathis D, Benoist C. Microbiota and autoimmune disease: the hosted self. Cell Host Microbe. 2011;10(4):297–301. doi: 10.1016/j.chom.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Vieira SM, Pagovich OE, Kriegel MA. Diet, microbiota and autoimmune diseases. Lupus. 2014;23(6):518–26. doi: 10.1177/0961203313501401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498–509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 10.Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramon E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Annals of the Rheumatic Diseases. 2014 doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 11.Erkan D, Derksen WJM, Kaplan V, Sammaritano L, Pierangeli SS, Roubey R, et al. Real world experience with antiphospholipid antibody tests: how stable are results over time? Ann Rheum Dis. 2005;64(9):1321–5. doi: 10.1136/ard.2004.031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev. 2010;9(5):A299–304. doi: 10.1016/j.autrev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118(17):4714–8. doi: 10.1182/blood-2011-03-340232. [DOI] [PubMed] [Google Scholar]
- 14.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 15.Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmunity Reviews. 2014 doi: 10.1016/j.autrev.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 16.De Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104(12):3598–602. doi: 10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 17.Nimpf J, Wurm H, Kostner GM. Interaction of beta 2-glycoprotein-I with human blood platelets: influence upon the ADP-induced aggregation. Thromb Haemost. 1985;54(2):397–401. [PubMed] [Google Scholar]
- 18.Miyakis S, Giannakopoulos B, Krilis SA. Beta 2 glycoprotein I—function in health and disease. Thromb Res. 2004;114(5–6):335–46. doi: 10.1016/j.thromres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Maiti SN, Balasubramanian K, Ramoth JA, Schroit AJ. Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J Biol Chem. 2008;283(7):3761–6. doi: 10.1074/jbc.M704990200. [DOI] [PubMed] [Google Scholar]
- 20.Ioannou Y, Zhang JY, Passam FH, Rahgozar S, Qi JC, Giannakopoulos B, et al. Naturally occurring free thiols within beta 2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood. 2010;116(11):1961–70. doi: 10.1182/blood-2009-04-215335. [DOI] [PubMed] [Google Scholar]
- 21••.Agar C, de Groot PG, Morgelin M, Monk SD, van Os G, Levels JH, et al. beta(2)-glycoprotein I: a novel component of innate immunity. Blood. 2011;117(25):6939–47. doi: 10.1182/blood-2010-12-325951. This study demonstrates that LPS binds to domain V of β2GPI, changing its conformation, which prompts clearance of the complex by macrophages. This paper also shows that β2GPI is inversely correlated with temperature rise and inflammatory markers in healthy subjects injected with LPS. [DOI] [PubMed] [Google Scholar]
- 22.de Groot PG, Meijers JC. Beta(2)-glycoprotein I: evolution, structure and function. J Thromb Haemost. 2011;9(7):1275–84. doi: 10.1111/j.1538-7836.2011.04327.x. [DOI] [PubMed] [Google Scholar]
- 23.de Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of beta 2-glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105(4):1540–5. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou Y, Pericleous C, Giles I, Latchman DS, Isenberg DA, Rahman A. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human beta(2)-glycoprotein I: mutation studies including residues R39 to R43. Arthritis Rheum. 2007;56(1):280–90. doi: 10.1002/art.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank M, Shoenfeld Y, Cabilly S, Heldman Y, Fridkin M, Katchalski-Katzir E. Prevention of experimental antiphospholipid syndrome and endothelial cell activation by synthetic peptides. Proc Natl Acad Sci U S A. 1999;96(9):5164–8. doi: 10.1073/pnas.96.9.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109(6):797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy V, Willis R, Romay-Penabad Z, Ruiz-Limon P, Martinez-Martinez LA, Jatwani S, et al. Value of isolated IgA anti-β2-glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum. 2013;65(12):3186–93. doi: 10.1002/art.38131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi Y, Inaba M, Sugihara A, Koshiji M, Sugiura K, Amoh Y, et al. Effects of administration of monoclonal antibodies (anti-CD4 or anti-CD8) on the development of autoimmune diseases in (NZW × BXSB)F1 mice. Immunobiology. 1998;198(4):451–64. doi: 10.1016/s0171-2985(98)80052-1. [DOI] [PubMed] [Google Scholar]
- 29.Hattori N, Kuwana M, Kaburaki J, Mimori T, Ikeda Y, Kawakami Y. T cells that are autoreactive to beta2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 2000;43(1):65–75. doi: 10.1002/1529-0131(200001)43:1<65::AID-ANR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Arai T, Kaburaki J, Ikeda Y, Kawakami Y, Kuwana M. Restricted T-cell receptor beta-chain usage by T cells autoreactive to beta(2)-glycoprotein I in patients with antiphospholipid syndrome. Blood. 2002;99(7):2499–504. doi: 10.1182/blood.v99.7.2499. [DOI] [PubMed] [Google Scholar]
- 31.Arai T, Yoshida K, Kaburaki J, Inoko H, Ikeda Y, Kawakami Y, et al. Autoreactive CD4+ T-cell clones to beta2-glycoprotein I in patients with antiphospholipid syndrome: preferential recognition of the major phospholipid-binding site. Blood. 2001;98(6):1889–96. doi: 10.1182/blood.v98.6.1889. [DOI] [PubMed] [Google Scholar]
- 32.Kuwana M, Matsuura E, Kobayashi K, Okazaki Y, Kaburaki J, Ikeda Y, et al. Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood. 2005;105(4):1552–7. doi: 10.1182/blood-2004-08-3145. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi Y, Seta N, Kaburaki J, Kobayashi K, Matsuura E, Kuwana M. Excessive exposure to anionic surfaces maintains autoantibody response to beta(2)-glycoprotein I in patients with antiphospholipid syndrome. Blood. 2007;110(13):4312–8. doi: 10.1182/blood-2007-07-100008. [DOI] [PubMed] [Google Scholar]
- 34.Agostinis C, Biffi S, Garrovo C, Durigutto P, Lorenzon A, Bek A, et al. In vivo distribution of β2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118(15):4231–8. doi: 10.1182/blood-2011-01-333617. [DOI] [PubMed] [Google Scholar]
- 35.Rand JH. The antiphospholipid syndrome. Annu Rev Med. 2003;54:409–24. doi: 10.1146/annurev.med.54.101601.152412. [DOI] [PubMed] [Google Scholar]
- 36.Levine JS, Subang R, Nasr SH, Fournier S, Lajoie G, Wither J, et al. Immunization with an apoptotic cell-binding protein recapitulates the nephritis and sequential autoantibody emergence of systemic lupus erythematosus. J Immunol. 2006;177(9):6504–16. doi: 10.4049/jimmunol.177.9.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 38•.Giannakopoulos B, Mirarabshahi P, Qi M, Weatherall C, Qi JC, Tanaka K, et al. Deletion of the antiphospholipid syndrome autoantigen β2-glycoprotein I potentiates the lupus autoimmune phenotype in a Toll-like receptor 7-mediated murine model. Arthritis Rheumatol. 2014;66(8):2270–80. doi: 10.1002/art.38646. This study demonstrates both the protective and pleiotropic role of β2GPI in animal models susceptible to lupus. In addition to inducing thrombosis, autoantibodies against β2GPI may interfere with clearance pathways, which could induce antinuclear systemic autoimmunity. [DOI] [PubMed] [Google Scholar]
- 39.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109(2):422–30. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Tapias P, Blank M, Anaya JM, Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24(4):389–93. doi: 10.1097/BOR.0b013e32835448b8. [DOI] [PubMed] [Google Scholar]
- 41•.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–44. doi: 10.1056/NEJMra1112830. Detailed summary of the current understanding of APS pathogenesis. [DOI] [PubMed] [Google Scholar]
- 42.Sebastiani GD, Galeazzi M, Morozzi G, Marcolongo R. The immunogenetics of the antiphospholipid syndrome, anticardiolipin antibodies, and lupus anticoagulant. Semin Arthritis Rheum. 1996;25(6):414–20. doi: 10.1016/s0049-0172(96)80006-0. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez ML, Katsumata K, Atsumi T, Romero FI, Bertolaccini ML, Funke A, et al. Association of HLA-DM polymorphism with the production of antiphospholipid antibodies. Ann Rheum Dis. 2004;63(12):1645–8. doi: 10.1136/ard.2003.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sestak A, O’Neil KM. Familial lupus and antiphospholipid syndrome. Lupus. 2007;16(8):556–63. doi: 10.1177/0961203307078071. [DOI] [PubMed] [Google Scholar]
- 45.De Angelis V, Scurati S, Raschi E, Liutkus A, Belot A, Borghi MO, et al. Pro-inflammatory genotype as a risk factor for aPL-associated thrombosis: report of a family with multiple anti-phospholipid positive members. J Autoimmun. 2009;32(1):60–3. doi: 10.1016/j.jaut.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Kamboh MI, Wang X, Kao AH, Barmada MM, Clarke A, Ramsey-Goldman R, et al. Genome-wide association study of antiphospholipid antibodies. Autoimmune Dis. 2013;2013:761046. doi: 10.1155/2013/761046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 49•.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. This study demonstrates that short-chain fatty acids, independent of specific bacteria, exert a protective effect against colitis by inducing FOXP3+ CD4+ T cells. The production of short-chain fatty acids promotes intestinal homeostasis and health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, de Roos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, et al. Thymus-derived regulatory Tcells contribute to tolerance to commensal microbiota. Nature. 2013;497(7448):258–62. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40(4):594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510(7503):152–6. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Sci. 2012;337(6101):1553–6. doi: 10.1126/science.1220961. This study is the first to demonstrate that gut insults with either pathogens or chemical irritation can lead to commensal-specific memory T cells in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–73. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 57.Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121(5):1946–55. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–5. doi: 10.1038/nature12496. This study is the first to demonstrate commensal influence on B cell lineage development outside of the bone marrow. The development of B cells in the lamina propria was a novel finding which implicates that commensals can influence the mature B cell repertoire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas A, Zimmermann K, Graw F, Slack E, Rusert P, Ledergerber B, et al. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut. 2011;60(11):1506–19. doi: 10.1136/gut.2010.224774. [DOI] [PubMed] [Google Scholar]
- 60.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–9. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–70. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of Tcell-dependent IgA responses. Nat Immunol. 2013;14(4):372–9. doi: 10.1038/ni.2552. This study demonstrated that Th17 cells convert to follicular helper T cells in the gut, which are necessary for the production of T cell dependent antigen-specific IgA responses in the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunisawa J, Kiyono H. Alcaligenes is commensal bacteria habituating in the gut-associated lymphoid tissue for the regulation of intestinal IgA responses. Front Immunol. 2012;3:65. doi: 10.3389/fimmu.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108 (Suppl 1):4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108(28):11548–53. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–12. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9(4):246–58. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341(27):2068–74. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 70.Oldstone MB. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon Antib Immunodiagn Immunother. 2014;33(3):158–65. doi: 10.1089/mab.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ang CW, Jacobs BC, Laman JD. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25(2):61–6. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24(4):408–16. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fae KC, da Silva DD, Oshiro SE, Tanaka AC, Pomerantzeff PM, Douay C, et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J Immunol. 2006;176(9):5662–70. doi: 10.4049/jimmunol.176.9.5662. [DOI] [PubMed] [Google Scholar]
- 74.Guilherme L, Oshiro SE, Fae KC, Cunha-Neto E, Renesto G, Goldberg AC, et al. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect Immun. 2001;69(9):5345–51. doi: 10.1128/IAI.69.9.5345-5351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blank M, Krause I, Magrini L, Spina G, Kalil J, Jacobsen S, et al. Overlapping humoral autoimmunity links rheumatic fever and the antiphospholipid syndrome. Rheumatol. 2006;45(7):833–41. doi: 10.1093/rheumatology/kel118. [DOI] [PubMed] [Google Scholar]
- 76.Gharavi AE, Pierangeli SS, Espinola RG, Liu X, Colden-Stanfield M, Harris EN. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46(2):545–52. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- 77.van Os GM, Meijers JC, Agar C, Seron MV, Marquart JA, Akesson P, et al. Induction of anti-beta2-glycoprotein I autoanti-bodies in mice by protein H of Streptococcus pyogenes. J Thromb Haemost. 2011;9(12):2447–56. doi: 10.1111/j.1538-7836.2011.04532.x. [DOI] [PubMed] [Google Scholar]
- 78.Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, et al. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci U S A. 2004;101(31):11404–9. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ausubel LJ, Kwan CK, Sette A, Kuchroo V, Hafler DA. Complementary mutations in an antigenic peptide allow for crossreactivity of autoreactive T-cell clones. Proc Natl Acad Sci U S A. 1996;93(26):15317–22. doi: 10.1073/pnas.93.26.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2012;287(2):1168–77. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157(5):1073–87. doi: 10.1016/j.cell.2014.03.047. Extensive in vitro study demonstrating that cross-reactivity occurs if key residues are conserved within a target sequence. This suggest that this phenomenon might be more widespread than previously thought but difficult to detect with conventional homology searches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Trama AM, Moody MA, Alam SM, Jaeger FH, Lockwood B, Parks R, et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe. 2014;16(2):215–26. doi: 10.1016/j.chom.2014.07.003. Commensal cross-reactive antibodies against HIV gp41 envelope protein develop in both patients and healthy donors, supporting commensal antigens that influence B cell reactivity. Additionally, this study showed that an E. coli intracellular protein RNA polymerase can give rise to antibodies that are cross-reactive to gp41. This supports a role for commensal antigens, both extracellular and intra-cellular, in the development of the B cell repertoire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83••.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38(2):373–83. doi: 10.1016/j.immuni.2012.10.021. This study demonstrates that human influenza- and HIV-specific CD4+ T cells are present in unexposed individuals due to cross-reactivity with environmental and commensal antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62(5):388–93. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amin NM. Antiphospholipid syndromes in infectious diseases. Hematol/Oncol Clin N Am. 2008;22(1):131–43. doi: 10.1016/j.hoc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Hang LM, Izui S, Dixon FJ. (NZW × BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981;154(1):216–21. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mizutani H, Engelman RW, Kinjoh K, Kurata Y, Ikehara S, Matsuzawa Y, et al. Calorie restriction prevents the occlusive coronary vascular disease of autoimmune (NZW × BXSB)F1 mice. Proc Natl Acad Sci U S A. 1994;91(10):4402–6. doi: 10.1073/pnas.91.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science (New York, NY) 2011;333(6038):101–4. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Sci. 2011;332(6032):970–4. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. doi: 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amital H, Gilburd B, Shoenfeld Y. Probiotic supplementation with Lactobacillus casei (Actimel) induces a Th1 response in an animal model of antiphospholipid syndrome. Ann N Y Acad Sci. 2007;1110:661–9. doi: 10.1196/annals.1423.069. [DOI] [PubMed] [Google Scholar]
- 94•.Vieira SM, Yu A, Pagovich OE, Tiniakou E, Sterpka J, Kriegel MA. Depletion of the gut microbiota prevents β2-glycoprotein I antibody production and mortality in a model of antiphospholipid syndrome. Arthritis Rheum. 2013 Oct 1;:S1–S1331. An abstract describing the first in vivo evidence that the gut microbiota is involved in the pathogenesis of APS based on antibiotic depletion studies. [Google Scholar]
- 95.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8(6):337–47. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kato LM, Kawamoto S, Maruya M, Fagarasan S. Gut TFH and IgA: key players for regulation of bacterial communities and immune homeostasis. Immunol Cell Biol. 2014;92(1):49–56. doi: 10.1038/icb.2013.54. [DOI] [PubMed] [Google Scholar]
- 97.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Sci. 2012;336(6080):485–9. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 98.Dantas G, Sommer MO, Degnan PH, Goodman AL. Experimental approaches for defining functional roles of microbes in the human gut. Annu Rev Microbiol. 2013;67:459–75. doi: 10.1146/annurev-micro-092412-155642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahern PP, Faith JJ, Gordon JI. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40(6):815–23. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100•.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6(220):220ra11. doi: 10.1126/scitranslmed.3008051. Important work demonstrating novel tools to parse the effects of specific gut bacteria on host physiology including regulatory T cell numbers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engen SA, Valen Rukke H, Becattini S, Jarrossay D, Blix IJ, Petersen FC, et al. The oral commensal streptococcus mitis shows a mixed memory Th cell signature that is similar to and cross-reactive with Streptococcus pneumoniae. PLoS ONE. 2014;9(8):e104306. doi: 10.1371/journal.pone.0104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206(7):1525–34. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103•.Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjögren’s syndrome antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014;152(1–2):1–9. doi: 10.1016/j.clim.2014.02.004. Using murine T cell hybridomas, this study demonstrates that several commensal peptides and one commensal recombinant protein can cross-react with the lupus and Sjögren’s syndrome autoantigen Ro60 that is also one of the most frequent antigen specificities of antinuclear antibodies (ANAs) in healthy subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–84. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–73. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asherson RA, Cervera R, Piette JC, Shoenfeld Y, Espinosa G, Petri MA, et al. Catastrophic antiphospholipid syndrome: clues to the pathogenesis from a series of 80 patients. Medicine. 2001;80(6):355–77. doi: 10.1097/00005792-200111000-00002. [DOI] [PubMed] [Google Scholar]