Abstract
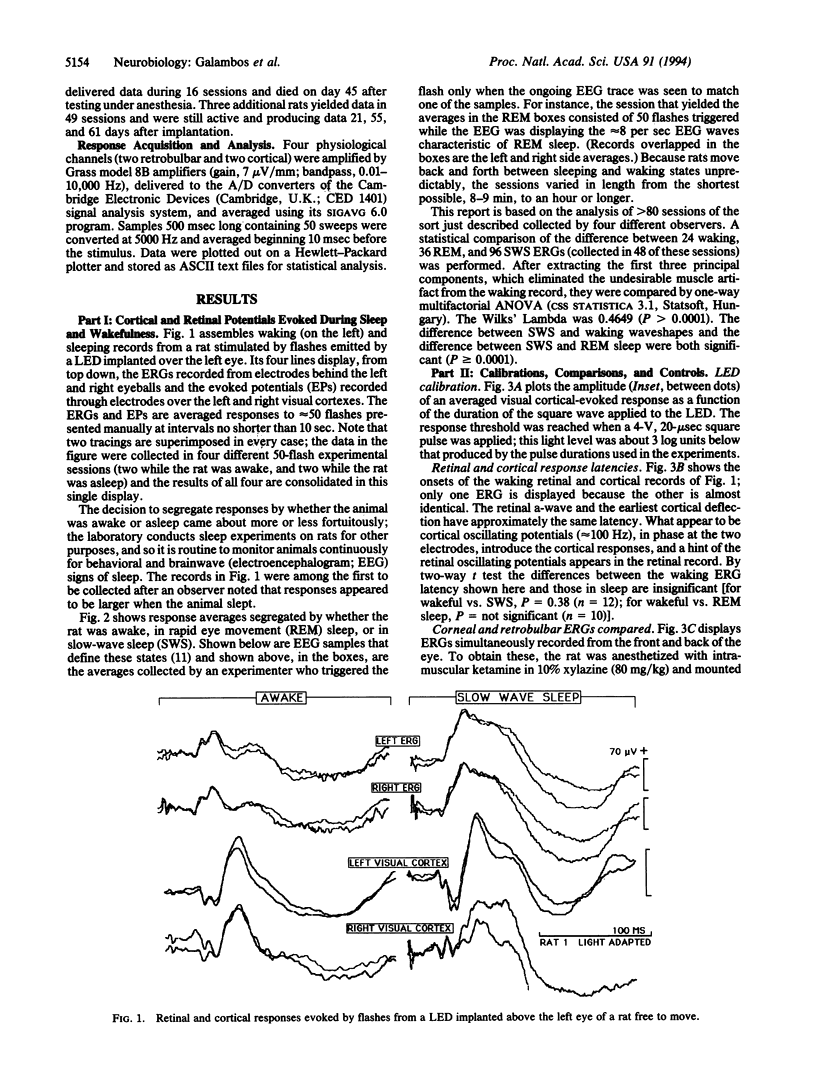
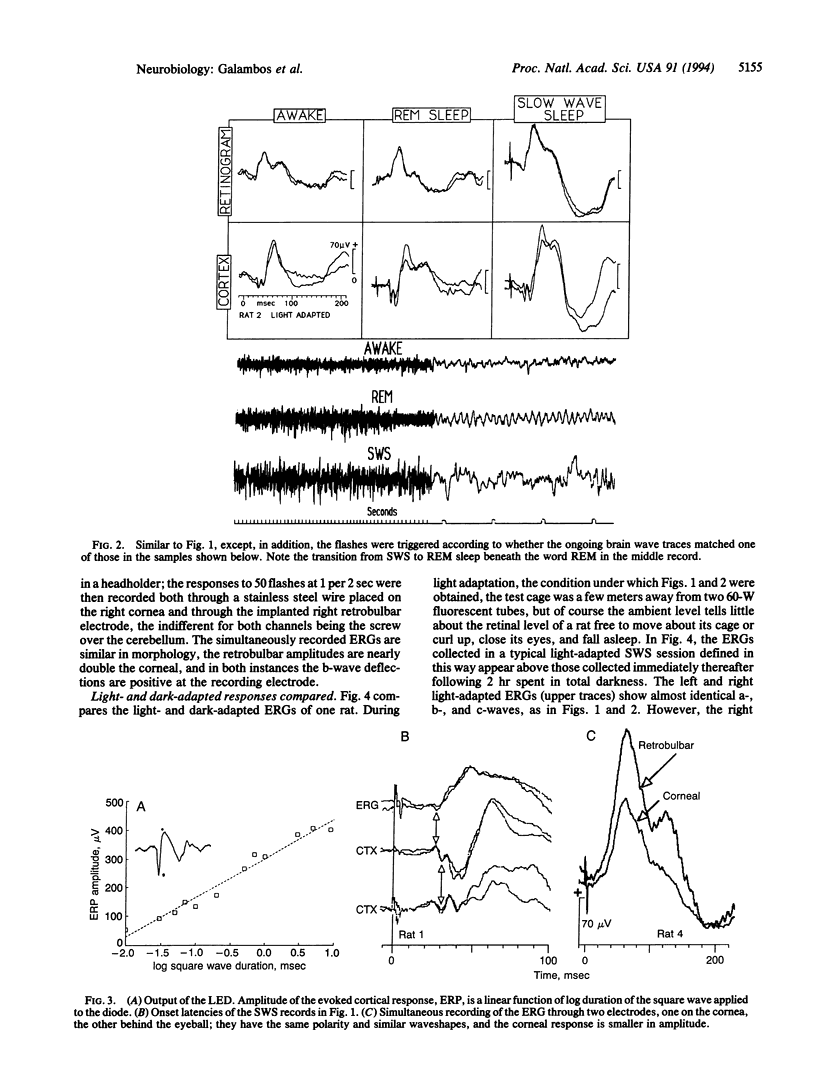
We show here electroretinograms (ERGs) recorded from freely moving rats during sleep and wakefulness. Bilateral ERGs were evoked by flashes delivered through a light-emitting diode implanted under the skin above one eye and recorded through electrodes inside each orbit near the optic nerve. Additional electrodes over each visual cortex monitored the brain waves and collected flash-evoked cortical potentials to compare with the ERGs. Connections to the stimulating and recording instruments through a plug on the head made data collection possible at any time without physically disturbing the animal. The three major findings are (i) the ERG amplitude during slow-wave sleep can be 2 or more times that of the waking response; (ii) the ERG patterns in slow-wave and REM sleep are different; and (iii) the sleep-related ERG changes closely mimic those taking place at the same time in the responses evoked from the visual cortex. We conclude that the mechanisms that alter the visual cortical-evoked responses during sleep operate also and similarly at the retinal level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bons N. Mise en évidence de fibres centrifuges dans le chiasma et les nerfs optiques du rat après lésion d'un noyau suprachiasmatique. C R Seances Soc Biol Fil. 1987;181(3):274–281. [PubMed] [Google Scholar]
- Chun M. H., Han S. H., Chung J. W., Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993 Jun 22;332(4):421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Czepita D. Badania doświadczalne nad rola układu adrenergicznego w kształtowaniu odpowiedzi bioelektrycznej siatkówki i kory wzrokowej. III. Wpływ adrenaliny na ERG i WPW królika. Klin Oczna. 1991 Apr-May;93(4-5):111–113. [PubMed] [Google Scholar]
- GALAMBOS R. A glia-neural theory of brain function. Proc Natl Acad Sci U S A. 1961 Jan 15;47:129–136. doi: 10.1073/pnas.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R. The glia-neuronal interaction: some observations. J Psychiatr Res. 1971 Aug;8(3):219–224. doi: 10.1016/0022-3956(71)90020-3. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. Detection of seven sleep-waking stages in the rat. Neurosci Biobehav Rev. 1992 Spring;16(1):31–38. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- Hoogland P. V., Vanderkrans A., Koole F. D., Groenewegen H. J. A direct projection from the nucleus oculomotorius to the retina in rats. Neurosci Lett. 1985 May 23;56(3):323–328. doi: 10.1016/0304-3940(85)90263-0. [DOI] [PubMed] [Google Scholar]
- Itaya S. K., Itaya P. W. Centrifugal fibers to the rat retina from the medial pretectal area and the periaqueductal grey matter. Brain Res. 1985 Feb 11;326(2):362–365. doi: 10.1016/0006-8993(85)90046-0. [DOI] [PubMed] [Google Scholar]
- JACOBSON J. H., GESTRING G. F. Centrifugal influence on the electroretinogram. Ann N Y Acad Sci. 1959 Nov 12;74(2):362–371. doi: 10.1111/j.1749-6632.1958.tb39557.x. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia J. L., Guerra-Seijas M. J., Gonzalez F., Perez R., Acuña C. Location of neurons projecting to the retina in mammals. Neurosci Res. 1990 Aug;8(4):291–302. doi: 10.1016/0168-0102(90)90035-d. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia J. L. The retinopetal system in the rat. Neurosci Res. 1988 Oct;6(1):88–95. doi: 10.1016/0168-0102(88)90010-7. [DOI] [PubMed] [Google Scholar]
- Larsen J. N., Møller M. The presence of retinopetal fibres in the optic nerve of the Mongolian gerbil (Meriones unguiculatus): a horseradish peroxidase in vitro study. Exp Eye Res. 1987 Dec;45(6):763–768. doi: 10.1016/s0014-4835(87)80093-3. [DOI] [PubMed] [Google Scholar]
- Mangun G. R., Hansen J. C., Hillyard S. A. Electroretinograms reveal no evidence for centrifugal modulation of retinal inputs during selective attention in man. Psychophysiology. 1986 Mar;23(2):156–165. doi: 10.1111/j.1469-8986.1986.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen J. D. Visualization of efferent retinal projections by immunohistochemical identification of cholera toxin subunit B. Brain Res Bull. 1992 Apr;28(4):619–623. doi: 10.1016/0361-9230(92)90112-b. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Inward-rectifying potassium channels in retinal glial (Müller) cells. J Neurosci. 1993 Aug;13(8):3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannita W. G., Balestra V., DiBon G., Hassan K. M., Rosadini G. Placebo effects in standard human neuropharmacological studies: effects of physiological variations of blood glucose and ammonia concentration on the electrophysiology of the visual system. Neuropsychobiology. 1992;25(1):49–60. doi: 10.1159/000118809. [DOI] [PubMed] [Google Scholar]
- Szél A., Röhlich P. Two cone types of rat retina detected by anti-visual pigment antibodies. Exp Eye Res. 1992 Jul;55(1):47–52. doi: 10.1016/0014-4835(92)90090-f. [DOI] [PubMed] [Google Scholar]
- Wässle H., Grünert U., Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993 Jun 22;332(4):407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]


