Abstract
Adipose tissue derived stem cells (ASCs) are mesenchymal stem cells which can be obtained from different adipose tissue sources within the body. It is an abundant cell pool, which is easy accessible and the cells can be obtained in large numbers, cultivated and expanded in vitro and prepared for tissue engineering approaches, especially for skeletal tissue repair. In the recent years this cell population has attracted a great amount of attention among researchers in human as well as in veterinary medicine. In the meantime ASCs have been well characterized and their use in regenerative medicine is very well established. This review focuses on the characterization of ASCs for their use for tissue engineering approaches especially in veterinary medicine and also highlights a selection of clinical trials on the basis of ASCs as the relevant cell source.
Keywords: Fat derived stem cells, regenerative medicine, orthopedic diseases, horse, dog
Introduction
Mesenchymal stem cells are one type of adult stem cells which were originally identified as multi-potent cells isolated from the bone marrow [1,2]. Later on Caplan and co-workers used the term mesenchymal stem cells (MSCs) [3]. They described them as clonal, plastic adherent, non-hematopoetic cells, which are the source for mesodermal cell lineages such as osteoblasts, chondroblasts and adipocytes [1]. Additionally, mesenchymal stem cells also have an endodermal and a neuroectodermal differentiation potential, which has so far only been shown in vitro [4-6]. After their discovery MSCs have been isolated from nearly all tissues of the body, for example bone marrow and adipose tissue [7], umbilical cord blood [8], synovia [9], periost [10], skeletal muscle [11], lung [12], teeth [13], skin [14] blood [15] and bone [16].
Similar to other stem cells MSCs are present in the different tissues throughout life. However, their number is correlated inversely with the health status as well as with age and tissue origin [17]. MSCs play a very important role in all mature tissues, thus it is assumed that they form a kind of pool of cells for tissue repair, which can be mobilized rather quickly during injury and disease [18].
These cells are able to synthesize a broad variety of growth factors and cytokines, which may influence cells in their proximate vicinity [19]. Thus, bioactive factors secreted by MSCs may have an autocrine or paracrine effect on the local environment [20].
Adipose tissue derived stem cells
Adult adipose tissue is the source of fibroblast-like cells capable of multipotential differentiation in a number of species [21-24]. These cells were first identified as MSCs in human adipose tissue in 2001 [5,7]. During the following years the establishment of this tissue as a cell source for MSCs suitable for tissue engineering and regenerative medicine was continuously promoted. They could be isolated from lipoaspirate and after isolation they exhibited an osteogenic, adipogenic, myogenic and chondrogenic differentiation potential in vitro. As a variety of names were used to describe the plastic adherent cell population, in the year 2004 the “Fat Applied Technology Society” reached a consensus to adopt the term adipose derived stem cells ASC [25]. There are several types of adipose tissue as cell source for ASCs e.g. subcutaneous or abdominal fat tissue, with subcutaneous as the clinically most relevant source. Because white adipose tissue is the most abundant adipose tissue in the organism, ASCs are preferentially isolated from this tissue and they can be isolated without any major clinical risk.
The initial method to isolate cells from the adipose tissue was developed by Rodbell in the 1960s [26] using rat fat pads. According to this protocol the tissue was minced and afterwards it undergoes enzymatic digestions with collagenase type II. Later on after centrifugation, the resulting cell pellet was called the stroma vascular fraction (SVF). ASCs are obtained by plastic adherence from the whole SVF, which also includes endothelial cells, pericytes, smooth muscle cells and blood cells [27]. Subsequently, this procedure has been modified for the isolation of human adipose tissue specimens [7].
Isolation and culture of ASCs
Similar to the isolation of ASCs from human tissue the isolation and characterization of ASCs from other species has been carried out just a few years later. Most of the species used served as translational models for human diseases. Thus, it has been shown, that ASCs can be isolated from the adipose tissue of the mouse [28], rat [29], rabbit [30] and pig [31]. The isolation of ASCs for the actual application in veterinary regenerative medicine has been carried out in the horse [32] and in the dog [33]. The ASCs isolation method was based on the technique originally reported for human ASC isolation [7,34,35]. Independent of the species the adipose tissue is collected either by needle biopsy or by liposuction aspiration.
While in the horse for the isolation of ASCs almost exclusively subcutaneous tissue is used which is preferentially collected from the region above the dorsal gluteal muscles [32] in canines adipose tissue is additionally collected from the abdominal cavity as well as from inguinal fat depots [33]. It could however been shown that there are individual factors as well as location dependent influences on the cell yield in the SVF [36].
After collection, the adipose tissue is washed in an equal volume of phosphate buffered saline (PBS) supplemented with 1% Penicillin/Streptomycin. For transport reasons it can also be stored in PBS for a limited time. For cell isolation the adipose tissue is minced and then undergoes enzymatic digestions using collagenase type I dissolved in BSA. The adipose tissue within the enzymatic solution is placed in a water-bath at 37°C for 10 minutes and afterwards in an incubator for about 30 minutes. The digested fat tissue is centrifuged for 5 minutes at 260 g. Afterwards the pellet is squashed through a 70 µm nylon cell strainer filter mesh and then pipetted into a 50 ml Falcon tube. The cells are washed in PBS at 300 g for 5 minutes and resuspended in 1 ml DMEM and 10% FCS. Finally, the cells are transferred to a culture dish at a density of 250.000 cells per cm2 (Figure 1). After 24 h the cultures are washed with PBS to remove the nonadherent cells. During the expansion period, the medium is replaced three times per week. When the cells reach 80% of confluence, they are detached from the culture dish using accutase (PAA, Germany), then are washed with DMEM, counted and plated again. For cryopreservation after passage 1, the cells are detached and suspended in cryopreservation medium containing 30% FCS and 10% dimethylsulfoxide [37,38]. They are not negatively affected by the cryopreservation procedure so that long term storage is guaranteed (Figure 2) [38-41].
Figure 1.
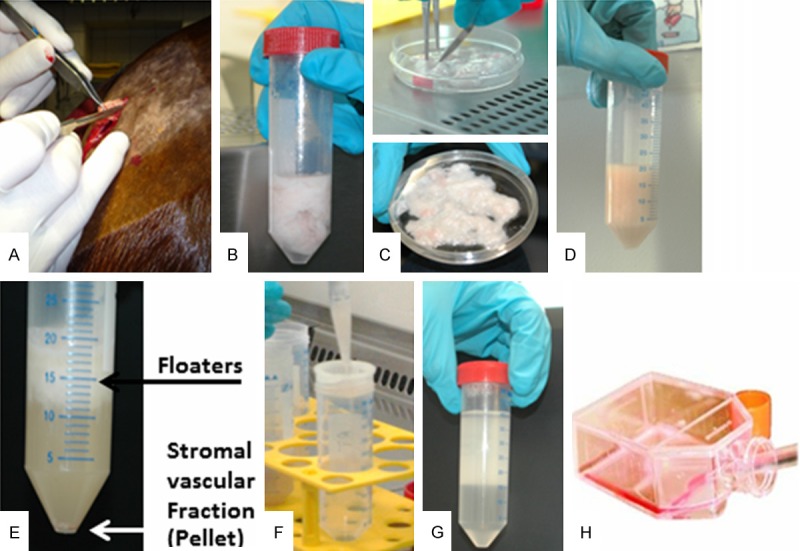
Isolation and preparation of equine ASCs in the cell culture lab. ASCs are isolated and prepared according the following procedure: A. Surgical resection of adipose tissue from the region of the horse’s tail. B. Adipose tissue dissolved in PBS in a Falcon tube. C. Mechanical disintegration of fat in the cell culture dish. D. Enzymatic digestion using collagenase I. E. After centrifugation the floating fat tissue is separated from the stromal vascular fraction at the bottom of the tube. F. After resuspension of the pellet in buffer (PBS) the suspension is filtered through a 70 µm nylon cell strainer and centrifuged again. G. The resulting pellet after the second centrifugation step. H. After resuspension in the standard medium the cells are plated in cell culture flasks or petri dishes.
Figure 2.
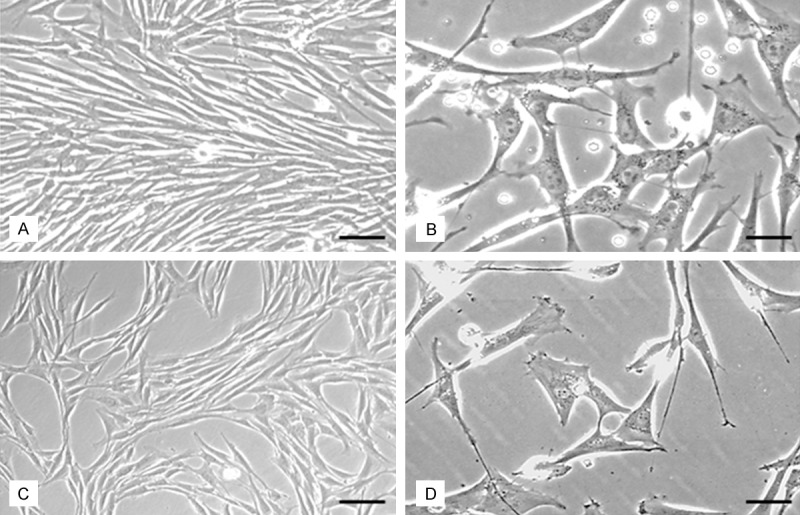
Morphologic characterization of eqine ASCs. Morphological characterization is carried out in passage 3 before and after cryopreservation. (A) Phase contrast micrograph of ASCs before cryopreservation. (B) Higher magnification of (A). (C) Phase contrast micrograph of ASCs after cryopreservation and thawing, note the cell morphologies are not altered compared to (A). (D) Higher magnification of (C) no significant alteration of cell shape can be detected compared to non-cryopreserved cells. Scale bar in (A and C) = 50 µm, in (B and D) = 20 µm.
Characterization of ASCs
Similar to ASCs derived from human tissue, ASCs isolated from species relevant in veterinary medicine are characterized by their plastic adherence and by stem cell specific marker expression. These are first of all the stem cell specific markers associated with pluripotency such as Oct-4, Nanog and SOX2 in canine ASCs [33,38] and also Oct4 and Nanog in equine ASCs [42], for review see [43]. According to the minimal standards set up by the international society for cellular therapy (ISCT) to characterize mesenchymal stem cells by the expression of certain cell surface markers, equine adipose derived stem cells have been shown to be additionally immunopositive for CD29, CD90, CD105 and CD73 and negative for the hematopoietic markers CD45, CD34, CD14, CD11b, CD79 alpha or CD19 as detected by immunohistochemistry but also quantified by flow cytometry [42], for review see [43]. Immunophenotyping in canine ASCs using the surface markers as defined by the ISCT the majority of cells reveals the expression of CD44, CD29 and CD90 [40], [38] while other markers, including CD14, CD34, CD44 and CD117 were consistently absent [40]. It has to be emphasized however, that according to the antibodies used expression rates may be either higher or lower or even absent at all.
Pluripotency of equine and canine ASCs is proven by their differentiation capacity into multiple lineages under culturing with specific conditions from which also their use for some of the clinical applications may be deduced (Figure 3). For ASCs of both species (canine and equine) the adipogenic, osteogenic and chondrogenic differentiation can be shown using specific histological staining procedures (Figure 4) [32,33,38,42] and additionally by investigating the expression of the adipogenic, osteogenic and chondrogenic marker genes amongst others RUNx2, Aggrecan and PPARγ2 [33,38,40,42,44,45]. In order to evaluate the potency of equine ASCs for an application in tendon regeneration additionally the tenogenic differentiation of ASCs can be demonstrated using the combination of growth and differentiation factors as well as tensile strain and by carrying out a histological and PCR analysis for the tenogenic marker genes COMP and Scleraxis [46].
Figure 3.
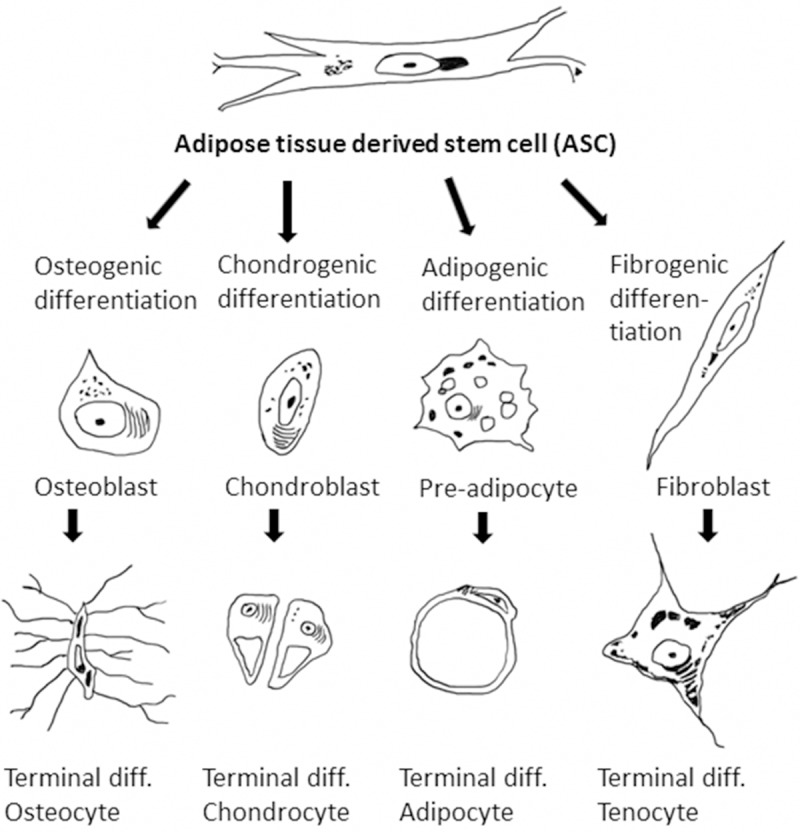
Differentiation lineages of ASCs. Schematic drawing of the most important mesenchymal differentiation lineages of adipose tissue derived stem cells. The most common and most important differentiation lineages are the osteogenic, chondrogenic and the adipogenic lineage. Additionally, in some reports also the tenogenic differentiation potential is mentioned.
Figure 4.
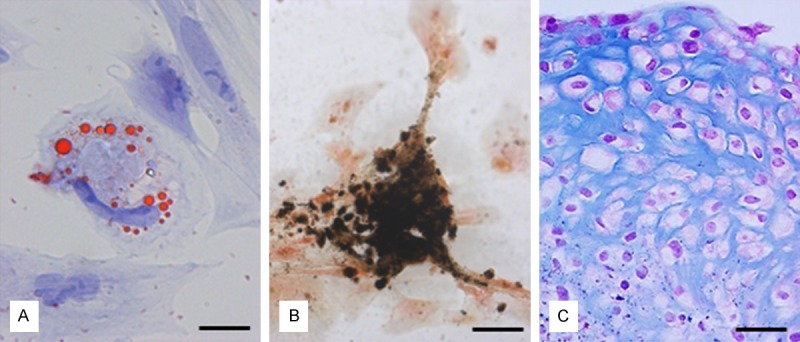
Histological staining procedures for demonstrating ASCs differentiation. Differentiation of canine ASCs into the three classical differentiation lineages after cultivation of cells in the appropriate differentiation media can be demonstrated using the characteristic staining procedures (A) red oil O for the adipogenic differentiation, (B) van Kossa for the osteogenic differentiation and (C) Alcian blue for the chondrogenic differentiation. Scale bar in (A) = 12 µm, in (B) = 20 µm and in (C) = 25 µm.
Behavior of ASCs under special culture conditions
In order to cultivate ASCs under conditions with the aim to improve their therapeutic potential there are several attempts to cultivate, expand and differentiate ASCs under special culture conditions.
In many tissues and organs the oxygen tension is well below 21% with a mean tissue level of 3% O2 [47]. Thus, the physiologic environment where MSCs reside is rather hypoxic and this is in contrast to culture conditions usually applied in in vitro cell and tissue culture. As the oxygen tension has an important role in the outcome of in vitro differentiation, there are several reports elucidating the culture of cells under various oxygen tensions. For equine ASCs some contradictory results were published in the literature. On one hand it could be shown that while the proliferation rate of hypoxic ASCs was lower than in normoxic cells, because of a significantly higher presence of dead cells, the immunophenotype of both BMSCs and ASCs was maintained under both oxygen conditions. So it can be deduced that hypoxia attenuates the proliferative capacity of equine MSCs but keeps them in a more undifferentiated state as embryonic markers are upregulated [48]. Besides an inhibited cell proliferation under hypoxia there are also hints for an altered differentiation potential in equine ASCs. While the adipogenic and chondrogenic differentiation was improved under hypoxia, the osteogenic and tenogenic differentiation was more effective in 21% O2 [45,46]. These findings are in line with observations in canine MSCs in which an inhibitory effect of hypoxia on both cell proliferation and differentiation could be observed [49]. For all these effects two aspects have to be taken into account: first of all the culture conditions in various studies such as passage number, media, serum batch etc. may be different and secondly also interindividual differences in stem cell behavior have to be considered.
It is also worthwhile considering the effects of the extracorporal shock wave (ESWT) on ASCs as in equine veterinary medicine ESWT is used to optimize healing conditions of bone, tendon as well as of cartilage. Moreover, a shock wave therapy is very often combined with a stem cell injection. One major effect of ESWT treatment of ASCs is the increase in the proliferation rate with a slightly improved differentiation potential. Especially the increased proliferation rate might give a hint for a possible endogenous stem cell activation within the treated tissue after ESWT application [50]. A similar approach was carried out by looking at the effect of a magnetic field on ASC behavior. This was actually done as devices that generate a static magnetic field are used in veterinary orthopedics especially for pain treatment termed magnetotherapy. Based on this fact and the observation that magnetic fields activate microvesicles in stem cells, canine and equine ASCs have been exposed to a magnetic field produced by neodymium magnets. Interestingly there were differences between canine and equine ASCs under these conditions. While equine ASCs maintained a high proliferation rate and showed an increase of secretory microvesicles, canine ASCs reacted with a decreased proliferative activity and revealed a poor secretory activity [51].
Comparison of bone marrow and adipose tissue derived stem cells
For regenerative therapies in veterinary medicine the use of mesenchymal stem cells is increasingly discussed and actually applied. Consequently, there is the need for a comparing the potency of bone marrow derived and adipose tissue derived stem cells as adipose derived stem cells are much easier to harvest. Such direct comparisons in regard of cell proliferation, stem cell marker expression and lineage specific differentiation potential have been carried out for equine as well as for canine mesenchymal stem cells of both tissues of origin. For equine MSCs it has been shown that the proliferation rate of ASCs is slightly lower than that of BMSCs, while stem marker expression is similar in both cell populations. In regard of the differentiation potential for all major differentiation lineages there is no marked difference between both cell types [44]. However, high interindividual differences were observed in the proliferation capacity of both cell types, particularly in higher passages. For canine MSCs the situation is slightly different. While cells of both groups display a remarkable resemblance in morphology and expression of MSC-specific markers, they showed considerable differences in the proliferation rate and differentiation into the chondrogenic and osteogenic direction. Population doubling occurred significantly faster in ASCs compared to BMSCs [49]. However, the differentiation into the chondrogenic and osteogenic lineage was much weaker compared to BMSCs [38], while the adipogenic differentiation potential has been shown to be similar in both cell populations [52].
Therapeutic application of ASCs in equine veterinary medicine
Since mesenchymal stem cells have the potency to differentiate into cell types of the musculoskeletal tissue, they are promising candidates for tissue engineering and regenerative medicine in the equine patient. Cartilage lesions and cartilage degeneration, bone fractures and especially tendon lesions are extremely common in equine athletes. The lesions are known for their long recovery times and long non-productive periods have to be taken into account [53]. The application of MSCs in tissue engineering raise hopes for a faster healing and moreover, for complete regeneration after musculoskeletal injuries [54]. There have been several reports about the use of MSCs from different sources for regenerative therapies in horses and so far the clinical outcome after using these alternative sources seems to be comparable with BMSCs. However, although ASCs have a similar differentiation potential as their bone marrow counterparts and their isolation bears some significant advantages like their easy recovery, in veterinary medicine their disadvantages such as higher donor site morbidity [55] is very often pronounced. In contrast, in human medicine a number of clinical approaches using ASC can be found through searches and on clinical trial web sites and thus, ASCs have prominent implications in tissue regeneration due to their high cell yield in adipose tissue, the ability to differentiate into multiple lineages and the secretion of various cytokines and their immunomodulatory effects [56,57]. Therefore, it is also worthwhile looking at regenerative approaches exclusively using ASCs in veterinary medicine. The application of ASCs has mainly been carried out in equine tendon regeneration [58]. A cell fraction called “adipose-derived mononuclear cells”, which contains in addition to ASCs also fibroblasts and other nucleated cells induced improved fiber linearity, uniformity and crimp pattern and a recovery of the tendon architecture after implantation in an experimentally induced tendon lesion using collagenase [59]. In a further clinical approach 16 horses were treated with allogenic ASCs grown in the presence of autologous platelet lysate. After the recovery period 14 horses returned into working and remained active over the two year follow up period [60]. Similar results could also be shown in the course of an experimentally induced tendinitis using a collagenase gel, which was directly injected into the superficial digital flexor tendon of eight equines. Ultrasound assessment over 16 weeks and histologic investigation revealed that the injection of ASCs suspended in platelet concentrate (PC) prevented the progression of the lesion and resulted in a greater organization and decreased inflammation [61]. Furthermore, neovascularization was assessed in a standardized surgical model in superficial flexor digital tendons of nine horses 22 weeks after stem cell treatment [62]. The safety and efficacy of a therapy based on the use of allogeneic adipose tissue-derived mesenchymal stem cells (ASCs) in combination with platelet rich plasma (PRP) were evaluated in 19 horses affected by acute or subacute overstrain superficial digital flexor tendonitis (SDFT). It could be shown that the application of allogeneic ASCs neither raised clinical sign of acute or chronic adverse tissue reactions, nor the formation of abnormal tissue in the long-term. Therefore, the combination of allogeneic ASCs and PRP can be considered as a safe and effective strategy for the treatment of SDF tendonitis in the horse [63].
Besides the use of ASCs in tendon regeneration there is also a report about the long-term beneficial influence of ASCs in horses suffering from bone spavin in comparison to routine steroid injections [64]. So far there are no reports about an application of equine ASCs in cartilage regeneration.
Therapeutic application of ASCs in small animal veterinary medicine
The therapeutic application of ASCs in small animals is mainly restricted to canines and the clinical use in dogs seems to be rather versatile due to the differentiation potential of the cells as well as to the secretion of immunomodulatory factors. Based on the use of the dog as a model for human medicine, there are a number of studies dealing with regenerative processes, which may have additionally a significant impact also for veterinary medicine. While publications about the use of ASCs for the treatment of central or peripheral nervous system lesions [65,66] and for the treatment of cardiomyopathies [67] are of barely scientific interest for human medicine, descriptions about the use of ASCs in orthopedic diseases have a much stronger association with applications in veterinary practice. The majority of studies focus on the use of ASCs in dogs with chronic osteoarthritis (OA). Thus, dogs suffering from OA of the hip or humeroradial joints treated with autologous ASCs within the whole SVF, had significantly improved scores for lameness, pain and range motion compared to control animals [68,69]. In these cases the ASCs were not isolated from the SVF, so that a heterogenous mixture of cells was injected into the lesioned sites. A similar approach was carried out in dogs with OA in humeroradial joints using isolated and well characterized ASCs having a significant potential for the treatment of lameness [70]. In a force platform analysis of dogs treated with ASCs by direct injection into the hip joint by analyzing the vertical force and the vertical impulse revealed an objectively improved limb function after exclusive ASC treatment [71]. However, it is questionable whether the observed beneficial effects exerted by ASCs are based on the secreted cytokines or on their differentiation capacity and thus on a direct impact on the regenerating cartilage. This question can only be elucidated if labelled stem cells for injection are used. However, these kind of experiments have only been carried out using BMSCs in ovine and caprine OA animal models [72] in the course of histologic analysis carried out after euthanasia.
The second major field of application for ASCs in small animal veterinary medicine is bone defects, which may occur in fractures associated with a massive loss of bone mass after trauma as well as after surgical resection of bone tumors or bone cysts. In case when spontaneous bone healing is hampered, usually autologous bone grafts or spongiosa are used to fill large bony defects. Alternatively to actual bone grafts isolated and expanded MSCs from bone marrow or adipose tissue in combination with scaffold materials can be used in order to achieve an osteogenic differentiation as well as an osteoinductive effect [73]. Interestingly, most approaches using BMSCs to treat bone defects in canines have been carried out in an experimental setting by creating an artificial critical size defects. However, as for the isolation of autologous BMSCs an invasive surgical operation has to be applied, the isolation of ASCs represents a far less invasive procedure. Li et al. could demonstrate that the osteogenic and osteoinductive potential of BMSCs is much better than that of ASCs in an experimentally induced CSD in the ulnar bone [74]. In order to improve the osteoinductive characteristics, ASCs were pre-differentiated and additionally transduced with the gene coding for BMP-2, which is an osteogenic growth factor responsible for an improved osteogenic differentiation and therefore a better fracture healing. In a clinical study the effect of cells from the autologous SVF and that of allogenic ASCs was evaluated in dogs with hip dysplasia after injection of cells into acupuncture points. After injection all animals showed an improved clinical condition and a reduced lameness. However, obviously positive results were more pronounced in the dogs treated with autologous SVF cells. Anyway, an important therapeutic alternative for the therapy of hip dysplasia is demonstrated [75].
Further studies on the use of ASCs in bone regeneration in dogs have been carried out on a more experimental level using the animals as models for bone defects in human medicine. Thus, new bone formation could be detected in mandibular bone [76] or in experimentally induced long bone defects using ASCs and the greater omentum as a scaffold material [77]. Furthermore, allogenic ASCs in combination with a coral scaffold have been shown to improve bone regeneration in bilateral cranial critical size defects using Beagle dogs [78]. Additionally, bone regeneration could also be demonstrated using ASCs incorporated into platelet rich plasma scaffolds transplanted into experimentally created tibia bone defects of six beagle dogs [79].
Legal aspects for the use of stem cells in veterinary medicine
Within the European Union the use of stem cells in human medicine is regulated by the European Medicines Agency, which follows research into the use of stem cells in medicines and is responsible for marketing-authorization applications for medicines containing stem cells. However, regardless the fact that medical products for the veterinary field are also regulated and registered by the European Medicines Agency, for the use and application of stem cells of different sources including ASCs in veterinary regenerative medicine there are currently no regulations applicable. This situation is similar in the United States, where the American Veterinary Medical Association monitors legal and regulatory issues for the veterinary practice. The use of medicinal products however is regulated by the US Food and Drug administration (FDA) [80], which is also responsible for the regulations on the use of stem cells in humans. According to the situation in the EU, in the US regulations regarding the use of stem cells in veterinary medicine have not been installed yet, although in the meantime there is a widespread commercialization for cell based regenerative therapies [81], especially promoting the use of ASCs.
Conclusions
Out of adult mesenchymal stem cells ASCs have gained a high significance in experimental and clinical veterinary medicine. They can be characterized by their easy access, their good proliferative and differentiation properties. Furthermore, they secrete a variety of cytokines and growth factors and are outstanding because of their immunomodulatory effects. Because of the latter characteristic there is an increase in studies promoting the use of allogenic cells for therapeutic usage. The number of clinical studies using ASCs for therapeutic applications in horses as well as in dogs is continuously rising, although they are in high competition with studies using BMSCs or even the unpurified SVF. Nevertheless, the preparation and clinical use of ASCs in veterinary practice is increasingly commercialized. Further experimental studies, also investigating the clinical effect of pre-differentiated cells or the possibility of a genetic modification of ASCs to promote the differentiation properties and to improve the clinical outcome have to be carried out in future. This should be done with regard to safety issues in order to guarantee the beneficial effects of this promising cell source also of allogenic origin for tissue engineering in veterinary medicine.
Acknowledgements
The authors wish to thank Manuela Heimann for technical assistance and for critically reading the manuscript.
Disclosure of conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- 1.Friedenstein A. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M, Mackay A, Beck S, Jaiswal R, Douglas R, Mosca J, Moorman M, Simonetti D, Craig S, Marshak D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;5411:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Caplan A. Mesenchymal stem cells. J Orthop Res. 1991;5:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith A, Nishikawa SI. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;7:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Wenisch S, Trinkaus K, Hild A, Hose D, Heiss C, Alt V, Klisch C, Meissl H, Schnettler R. Immunochemical, ultrastructural and electrophysiological investigations of bone-derived stem cells in the course of neuronal differentiation. Bone. 2006;6:911–921. doi: 10.1016/j.bone.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Arnhold S, Klein H, Klinz FJ, Absenger Y, Schmidt A, Schinköthe T, Brixius K, Kozlowski J, Desai B, Bloch W, Addicks K. Human bone marrow stroma cells display certain neural characteristics and integrate in the subventricular compartment after injection into the liquor system. Eur J Cell Biol. 2006;6:551–565. doi: 10.1016/j.ejcb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, Benhaim P, Lorenz H, Hedrick M. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;2:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Erices A, Conget P, Minguell J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;1:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 9.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;8:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto T, Sperling J, Sanyal A, Fitzsimmons J, Reinholz G, Conover C, O’Driscoll S. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartil. 2003;1:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 11.Jankowski R, Deasy B, Huard J. Muscle-derived stem cells. Gene Ther. 2002;10:642–647. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 12.Noort WA, Kruisselbrink AB, in’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;8:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 13.Miura M, Gronthos S, Zhao M, Lu B, Fisher L, Robey P, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;10:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young H, Steele T, Bray R, Hudson J, Floyd J, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas P, Black A. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;1:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 15.Zvaifler N, Marinova-Mutafchieva L, Adams G, Edwards C, Moss J, Burger J, Maini R. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;6:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenisch S, Trinkaus K, Hild A, Hose D, Herde K, Heiss C, Kilian O, Alt V, Schnettler R. Human reaming debris: a source of multipotent stem cells. Bone. 2005;1:74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Majors A, Boehm C, Nitto H, Midura R, Muschler G. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;4:546–557. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 18.Barry F, Murphy J. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;4:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Haynesworth S, Baber M, Caplan A. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;3:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Caplan A, Dennis J. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;5:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 21.Hollenberg C, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969;11:2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dardick I, Poznanski W, Waheed I, Setterfield G. Ultrastructural observations on differentiating human preadipocytes cultured in vitro. Tissue Cell. 1976;3:561–571. doi: 10.1016/0040-8166(76)90013-6. [DOI] [PubMed] [Google Scholar]
- 23.Poznanski W, Waheed I, Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab Invest. 1973;5:570–576. [PubMed] [Google Scholar]
- 24.Dixon-Shanies D, Rudick J, Knittle J. Observatons on the growth and metabolic functions of cultured cells derived from human adipose tissue. Proc Soc Exp Biol Med. 1975;2:541–545. doi: 10.3181/00379727-149-38846. [DOI] [PubMed] [Google Scholar]
- 25.Bunnell B, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;2:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966;1:130–139. [PubMed] [Google Scholar]
- 27.Rodbell M, Jones A. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966;1:140–142. [PubMed] [Google Scholar]
- 28.Safford K, Hicok K, Safford S, Halvorsen YD, Wilkison W, Gimble J, Rice H. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;2:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 29.Tholpady S, Katz A, Ogle R. Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat Rec A Discov Mol Cell Evol Biol. 2003;1:398–402. doi: 10.1002/ar.a.10039. [DOI] [PubMed] [Google Scholar]
- 30.Peptan I, Hong L, Mao J. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast Reconstr Surg. 2006;5:1462–1470. doi: 10.1097/01.prs.0000206319.80719.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu CQ, Zhang GH, Zhang LJ, Yang GS. Osteogenic and adipogenic potential of porcine adipose mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2007;2:95–100. doi: 10.1007/s11626-006-9008-y. [DOI] [PubMed] [Google Scholar]
- 32.Vidal M, Kilroy G, Lopez M, Johnson J, Moore R, Gimble J. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;7:613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 33.Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;6:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 34.Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;1:92–102. doi: 10.1089/scd.2005.14.92. [DOI] [PubMed] [Google Scholar]
- 35.Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;1:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 36.Astor D, Hoelzler M, Harman R, Bastian R. Patient factors influencing the concentration of stromal vascular fraction (SVF) for adipose-derived stromal cell (ASC) therapy in dogs. Can J Vet Res. 2013;3:177–182. [PMC free article] [PubMed] [Google Scholar]
- 37.Raabe O, Reich C, Wenisch S, Hild A, Burg-Roderfeld M, Siebert HC, Arnhold S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem Cell Biol. 2010;6:545–554. doi: 10.1007/s00418-010-0760-4. [DOI] [PubMed] [Google Scholar]
- 38.Reich C, Raabe O, Wenisch S, Bridger P, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells--a comparative study. Vet Res Commun. 2012;2:139–148. doi: 10.1007/s11259-012-9523-0. [DOI] [PubMed] [Google Scholar]
- 39.Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;2:401–410. doi: 10.1097/01.prs.0000298322.70032.bc. [DOI] [PubMed] [Google Scholar]
- 40.Vieira N, Brandalise V, Zucconi E, Secco M, Strauss B, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;3:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 41.Mambelli LI, Santos EJ, Frazão PJ, Chaparro MB, Kerkis A, Zoppa AL, Kerkis I. Characterization of equine adipose tissue-derived progenitor cells before and after cryopreservation. Tissue Eng Part C Methods. 2009;15:87–94. doi: 10.1089/ten.tec.2008.0186. [DOI] [PubMed] [Google Scholar]
- 42.Raabe O, Shell K, Würtz A, Reich C, Wenisch S, Arnhold S. Further insights into the characterization of equine adipose tissue-derived mesenchymal stem cells. Vet Res Commun. 2011;6:355–365. doi: 10.1007/s11259-011-9480-z. [DOI] [PubMed] [Google Scholar]
- 43.Burk J, Badylak S, Kelly J, Brehm W. Equine cellular therapy--from stall to bench to bedside? Cytometry A. 2013;1:103–113. doi: 10.1002/cyto.a.22216. [DOI] [PubMed] [Google Scholar]
- 44.Ranera B, Lyahyai J, Romero A, Vázquez F, Remacha A, Bernal M, Zaragoza P, Rodellar C, Martín-Burriel I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 2011;144:147–154. doi: 10.1016/j.vetimm.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Shell K, Raabe O, Freitag C, Ohrndorf A, Christ HJ, Wenisch S, Arnhold S. Comparison of Equine Adipose Tissue-Derived Stem Cell Behavior and Differentiation Potential Under the Influence of 3% and 21% Oxygen Tension. Journal of Equine Veterinary Science. 2013;2:74–82. [Google Scholar]
- 46.Raabe O, Shell K, Fietz D, Freitag C, Ohrndorf A, Christ H, Wenisch S, Arnhold S. Tenogenic differentiation of equine adipose-tissue-derived stem cells under the influence of tensile strain, growth differentiation factors and various oxygen tensions. Cell Tissue Res. 2013;3:509–521. doi: 10.1007/s00441-013-1574-1. [DOI] [PubMed] [Google Scholar]
- 47.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 48.Ranera B, Remacha A, Álvarez-Arguedas S, Romero A, Vázquez F, Zaragoza P, Martín-Burriel I, Rodellar C. Effect of hypoxia on equine mesenchymal stem cells derived from bone marrow and adipose tissue. BMC Vet Res. 2012;8:142. doi: 10.1186/1746-6148-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung DJ, Hayashi K, Toupadakis C, Wong A, Yellowley C. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012;1:66–75. doi: 10.1016/j.rvsc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Raabe O, Shell K, Goessl A, Crispens C, Delhasse Y, Eva A, Scheiner-Bobis G, Wenisch S, Arnhold S. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. Am J Stem Cells. 2013;1:62–73. [PMC free article] [PubMed] [Google Scholar]
- 51.Marędziak M, Marycz K, Smieszek A, Lewandowski D, Toker N. The influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissue. In Vitro Cell Dev Biol Anim. 2014;6:562–571. doi: 10.1007/s11626-013-9730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey C, Reid S, Hodgson D, Rose R. Impact of injuries and disease on a cohort of two- and three-year-old thoroughbreds in training. Vet Rec. 1999;17:487–493. doi: 10.1136/vr.145.17.487. [DOI] [PubMed] [Google Scholar]
- 54.Koch T, Berg L, Betts D. Current and future regenerative medicine-principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can Vet J. 2009;2:155–165. [PMC free article] [PubMed] [Google Scholar]
- 55.Richardson L, Dudhia J, Clegg P, Smith R. Stem cells in veterinary medicine--attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007;9:409–416. doi: 10.1016/j.tibtech.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Nauta A, Fibbe W. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;10:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 57.Tsuji W, Rubin J, Marra K. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;3:312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed S, Leahy E. Growth and Development Symposium: Stem cell therapy in equine tendon injury. J Anim Sci. 2013;1:59–65. doi: 10.2527/jas.2012-5736. [DOI] [PubMed] [Google Scholar]
- 59.Nixon A, Dahlgren L, Haupt J, Yeager A, Ward D. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;7:928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- 60.Del Bue M, Riccò S, Ramoni R, Conti V, Gnudi G, Grolli S. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: their association in vitro and in vivo. Vet Res Commun. 2008;32(Suppl 1):S51–5. doi: 10.1007/s11259-008-9093-3. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho Ade M, Badial PR, Álvarez LE, Yamada AL, Borges AS, Deffune E, Hussni CA, Garcia Alves AL. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. Stem Cell Res Ther. 2013;4:85. doi: 10.1186/scrt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conze P, van Schie HT, van Weeren R, Staszyk C, Conrad S, Skutella T, Hopster K, Rohn K, Stadler P, Geburek F. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen Med. 2014;6:743–757. doi: 10.2217/rme.14.55. [DOI] [PubMed] [Google Scholar]
- 63.Ricco S, Renzi S, Del Bue M, Conti V, Merli E, Ramoni R, Lucarelli E, Gnudi G, Ferrari M, Grolli S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol. 2013;1(Suppl):61–68. doi: 10.1177/03946320130260s108. [DOI] [PubMed] [Google Scholar]
- 64.Nicpoń J, Marycz K, Grzesiak J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol J Vet Sci. 2013;4:753–754. doi: 10.2478/pjvs-2013-0107. [DOI] [PubMed] [Google Scholar]
- 65.Ryu H, Lim J, Byeon Y, Park J, Seo M, Lee Y, Kim W, Kang K, Kweon O. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;4:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoreishian M, Rezaei M, Beni B, Javanmard S, Attar B, Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J Oral Maxillofac Surg. 2013;3:577–587. doi: 10.1016/j.joms.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 67.Pogue B, Estrada A, Sosa-Samper I, Maisenbacher H, Lamb K, Mincey B, Erger K, Conlon T. Stem-cell therapy for dilated cardiomyopathy: a pilot study evaluating retrograde coronary venous delivery. J Small Anim Pract. 2013;7:361–366. doi: 10.1111/jsap.12098. [DOI] [PubMed] [Google Scholar]
- 68.Black L, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich D, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;4:272–284. [PubMed] [Google Scholar]
- 69.Black L, Gaynor J, Adams C, Dhupa S, Sams A, Taylor R, Harman S, Gingerich D, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;3:192–200. [PubMed] [Google Scholar]
- 70.Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;2:189–194. doi: 10.1042/CBI20110304. [DOI] [PubMed] [Google Scholar]
- 71.Vilar J, Batista M, Morales M, Santana A, Cuervo B, Rubio M, Cugat R, Sopena J, Carrillo J. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 2014;10:143. doi: 10.1186/1746-6148-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy J, Fink D, Hunziker E, Barry F. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;12:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 73.Harasen G. Stimulating bone growth in the small animal patient: Grafts and beyond! Can Vet J. 2011;2:199–200. [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007;356:836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- 75.Marx C, Silveira MD, Selbach I, da Silva AS, Braga LM, Camassola M, Nardi NB. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014;2014:391274. doi: 10.1155/2014/391274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haghighat A, Akhavan A, Hashemi-Beni B, Deihimi P, Yadegari A, Heidari F. Adipose derived stem cells for treatment of mandibular bone defects: An autologous study in dogs. Dent Res J (Isfahan) 2011;8(Suppl 1):S51–7. [PMC free article] [PubMed] [Google Scholar]
- 77.Bigham-Sadegh A, Mirshokraei P, Karimi I, Oryan A, Aparviz A, Shafiei-Sarvestani Z. Effects of adipose tissue stem cell concurrent with greater omentum on experimental long-bone healing in dog. Connect Tissue Res. 2012;4:334–342. doi: 10.3109/03008207.2012.660585. [DOI] [PubMed] [Google Scholar]
- 78.Liu G, Zhang Y, Liu B, Sun J, Li W, Cui L. Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials. 2013;11:2655–2664. doi: 10.1016/j.biomaterials.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Cruz AC, Caon T, Menin Á, Granato R, Boabaid F, Simões CM. Adipose-derived stem cells incorporated into platelet-rich plasma improved bone regeneration and maturation in vivo. Dent Traumatol. 2014;31:42–8. doi: 10.1111/edt.12134. [DOI] [PubMed] [Google Scholar]
- 80.Nobert K. The regulation of veterinary regenerative medicine and the potential impact of such regulation on clinicians and firms commercializing these treatments. Vet Clin North Am Equine Pract. 2011;2:383–391. doi: 10.1016/j.cveq.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Volk S, Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. 2013;3:382–394. doi: 10.1111/wrr.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]


