Abstract
Volvocine algae are a group of chlorophytes that together comprise a unique model for evolutionary and developmental biology. The species Chlamydomonas reinhardtii and Volvox carteri represent extremes in morphological diversity within the Volvocine clade. Chlamydomonas is unicellular and reflects the ancestral state of the group, while Volvox is multicellular and has evolved numerous innovations including germ-soma differentiation, sexual dimorphism, and complex morphogenetic patterning. The Chlamydomonas genome sequence has shed light on several areas of eukaryotic cell biology, metabolism and evolution, while the Volvox genome sequence has enabled a comparison with Chlamydomonas that reveals some of the underlying changes that enabled its transition to multicellularity, but also underscores the subtlety of this transition. Many of the tools and resources are in place to further develop Volvocine algae as a model for evolutionary genomics.
1. Introduction
Ever since van Leeuwenhoek first described Volvox he had collected from a roadside ditch (van Leeuwenhoek, 1700), this elegant organism and its cousins, the Volvocine algae, have fascinated biologists. Just over 300 years after their first published description Volvocine algae entered the genomic era with genome sequencing of the unicellular species Chlamydomonas reinhardtii (Merchant et al., 2007) followed by that of Volvox carteri (Prochnik et al., 2010). These two species are at the forefront of research in diverse areas including evolution, motility, photosynthesis, and development before their genomes were completed. It has now become possible to add an arsenal of -omics tools to speed progress and answer new questions. Genomics research with Chlamydomonas has shed light on several areas of biology by helping define conserved genes related to both plant and animal biology. The addition of the Volvox genome makes possible comparisons within Volvocine algae aimed at deciphering the genetic changes that underlie a major transition in biological complexity represented by differences between unicellular Chlamydomonas and multicellular Volvox. These comparisons have revealed how remarkably similar these two species are at the genome level, and have prompted a reconsideration of how much change is required to drive a major evolutionary transition. In this chapter we introduce the Volvocine algae and highlight key areas of genomics research to which they have contributed.
2. The Biology of the Volvocales
The Volvocales (or Volvocine algae) are a sub-group of chlorophytes (green algae) comprising dozens of species in seven major genera (Fig. 1). The two best-known species are unicellular Chlamydomonas reinhardtii, and multicellular Volvox carteri (Fig. 2). These two species represent two extremes in a range of developmental complexity observed within the Volvocales (Fig. 2). Each of the major genera is defined by its level of organization such as colony size and morphology, cell number, cell differentiation, and the degree of germ-soma specialization (Fig. 2). A brief introduction to the biology, taxonomy, ecology, and culture resources for Volvocine algae follows.
Figure 1. The phylogenetic relationship of the Volvocales to other algae, plants and eukaryotes.
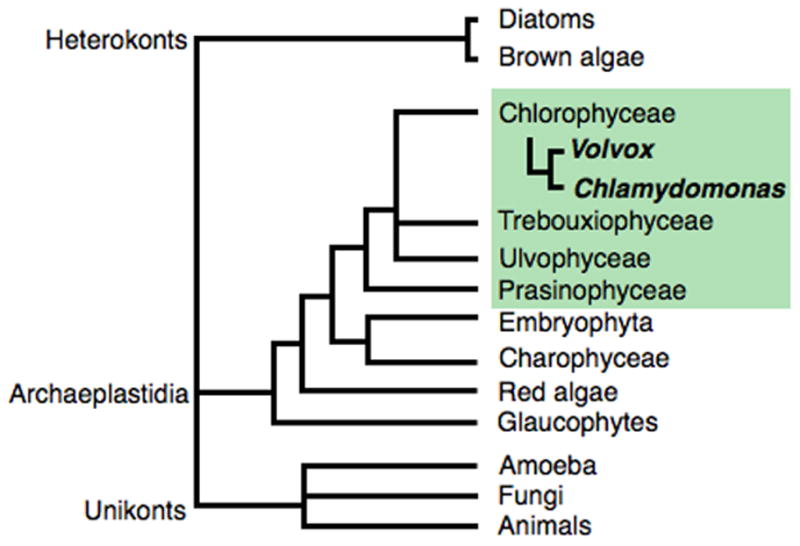
The Chlorophyceae, with Chlamydomonas and Volvox indicated, are members Chlorophyta, or green algae, one of the five major groups of Archaeoplastidia. The unrooted cladogram is adapted from (Baldauf, 2003).
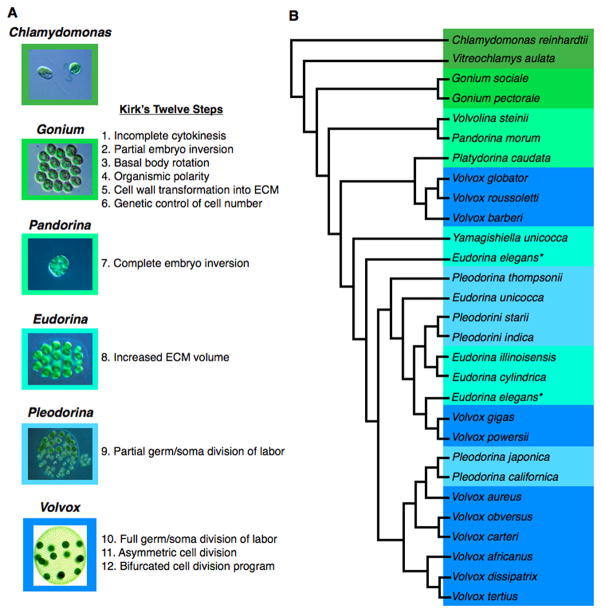
Figure 2. The morphology and phylogeny of the Volvocales.
(A) Micrographs of Volvocine species and their relationship to each of David Kirk’s twelve steps of multicellular evolution (Kirk, 2005). (B) Phylogeny of selected Volvocales adapted from (Nozaki, 2003; Kirk, 2005). Species are color-coded based on morphology as indicated in panel (A). Asterisks indicate two isolates of Eudorina elegans that subsequently were reclassified as distinct species (Nozaki, 2003; Yamada et al., 2008).
2a. Chlamydomonas is a prototypical Volvocine alga
Chlamydomonas reinhardtii has been used as a model for many decades to investigate diverse areas of biology (Harris, 2001; 2001; Grossman et al., 2003; 2003; Harris, 2004; Peers and Niyogi, 2008; Stern et al., 2009; 2009). Molecular genetic tools and resources have been developed for Chlamydomonas including nuclear and organellar transformation, insertional mutagenesis, RNAi-mediated silencing, microarrays, EST libraries, BAC libraries, cDNA libraries, and numerous strains and mutants (see Tables 1 and 2). The following section provides a brief overview of Chlamydomonas.
Table 1.
Volvocales Culture Collections
| Culture Collection | Species available | Media | Other | Accepts New Cultures? | Web Site |
|---|---|---|---|---|---|
| Chlamydomonas collection | Chlamydomonas reinhardtii, some other chlamydomonadaceae | Recipes and for sale | Plasmids cDNA libraries, Genomic libraries | Yes, all chlamydomonadaceae | http://chlamycollection.org/ |
| UTEX | Some Chlamydomonas, many Volvocales, other Chlorophytes and protists | Recipes | Genomic DNA for PCR analysis | Yes, with prior approval | http://web.biosci.utexas.edu/utex/ |
| SAG | Many Volvocales, many other Chlorophytes and Protists | References | Yes, with prior approval | http://epsag.uni-goettingen.de/cgi-bin/epsag/website/cgi/show_page.cgi?kuerzel=start | |
| CCAP | Many Volvocales, many other Chlorophytes and Protists | Recipes and for sale | Yes, also accepts private collections | http://www.ccap.ac.uk/index.htm | |
| NIES | A wide array of algal strains, extensive collection of the Volvocales | Recipes | Yes, with prior approval | http://mcc.nies.go.jp/ |
Table 2.
Genomic Resources for Chlamydomonas and Volvox
| Resource | Description | Reference(s) | URL |
|---|---|---|---|
| General Resources | |||
| Chlamy.org | General landing page for all information about Chlamydomonas | http://www.chlamy.org | |
| Chlamy Collection | Strain and plasmid stock center and general information about Chlamydomonas | http://www.chlamycollection.org | |
| Sequences | |||
| Phytozome | Latest assembly and annotations of the Chlamydomonas and Volvox genomes and comparison tools. | http://www.phytozome.net | |
| JGI Genome Browser for Chlamydomonas | Version 4 of the Chlamydomonas genome including use annotations. All future assemblies will be deposited on Phytozome | http://genome.jgi-psf.org/Chlre4/Chlre4.home.html | |
| Chlamydomonas reinhardtii minus mating type locus | GenBank Accession GU814015 | Ferris, Olson et al. 2010 | http://www.ncbi.nlm.nih.gov/nuccore/GU814015 |
| Chlamydomonas reinhardtii plus mating type locus | GenBank Accession GU814014 | Ferris, Olson et al. 2010 | http://www.ncbi.nlm.nih.gov/nuccore/GU814014 |
| Chlamydomonas reinhardtii chloroplast genome | GenBank Accession GU814014 | Maul et al. 2002 | http://www.chlamy.org/chloro/default.html |
| Chlamydomonas reinhardtii mitochondrial genome | GenBank Accession FJ423446 | Ma et al. 1992 | http://www.ncbi.nlm.nih.gov/nuccore/NC_001638 |
| Chlamydomonas incerta EST database | GenBank Accessions GI106792973-GI106798104 | Popescu et al. 1996 | http://megasun.bch.umontreal.ca/pepdb/pep.html |
| JGI Genome Browser for Volvox | Version 1 of the Volvox carteri genome including user annotations. All future versions of the genome will be deposited on Phytozome | http://genome.jgi.doe.gov/Volca1/Volca1.info.html | |
| Volvox carteri Female mating type locus | GenBank Accession GU784915 | Ferris, Olson et al. 2010 | http://www.ncbi.nlm.nih.gov/nuccore/GU784915 |
| Volvox carteri Male mating type locus | GenBank Accession GU784916 | Ferris, Olson et al. 2010 | http://www.ncbi.nlm.nih.gov/nuccore/GU784916 |
| Volvox carteri chloroplast genome | GenBank Accession GU084820.1 | Smith and Lee 2009 | http://www.ncbi.nlm.nih.gov/nuccore/GU084820.1 |
| Volvox carteri mitochondrial genome | GenBank Accession EU760701 | Smith and Lee 2009 | http://www.ncbi.nlm.nih.gov/nuccore/EU760701 |
| Libraries | |||
| Chlamydomonas reinhardtii genomic BAC library | http://www.genome.clemson.edu | ||
| Volvox carteri Female genomic BAC libraries | http://www.genome.arizona.edu/ | ||
| Volvox cateri Male genomic BAC libraries | Ferris, Olson et al. 2010 | http://www.genome.clemson.edu/ | |
| Chlamydomonas EST database | Asamizu et al. 2004 | http://est.kazusa.or.jp/en/plant/chlamy/EST/ | |
| Chlamydomonas 454 read database | 2 Gb of 454 sequencing of Chlamydomonas cDNAs from various growth conditions | http://genomes.mcdb.ucla.edu/Cre454/ | |
| Functional Annotation and Expression Tools | |||
| Algal Functional Annotation Tool | Comprehensive algal genomics, annotation and expression tool | Lopez et al. 2011 | http://pathways.mcdb.ucla.edu/algal/index.html |
| GreenGenie2 | Gene predictions for version 4 of the Chlamydomonas genome | Kwan et al 2009 | http://stormo.wustl.edu/GreenGenie2/ |
| ChlamyCyc | Chlamydomonas centric, integrated annotation tool that integrates with other related plant databases | http://chlamyto.mpimp-golm.mpg.de/chlamycyc/index.jsp | |
| Plant Genome Database | Plant genome annotation comparison tool that includes Chlamydomonas reinhardtii | http://www.plantgdb.org/search/misc/ | |
| PLAZA | Tools for comparative plant genomics | Van Bel et al. 2012 | http://bioinformatics.psb.ugent.be/plaza/ |
| Proteomic Databases | |||
| Chlamydomonas flagellar proteome | http://labs.umassmed.edu/chlamyfp/protector_login.php | ||
| Plant Transcription Factor Databases | |||
| Plant Transcription Factor Database | Includes both Chlamydomonas reinhardtii and Volvox carteri. Version 2.0 | Zhang et al. 2010 | http://planttfdb.cbi.edu.cn/ |
| Plant Transcription Factor Database | Includes Chlamydomonas reinhardtii. Version 3.0 | Pérez-Rodríguez et al. 200 | http://plntfdb.bio.uni-potsdam.de/v3.0/ |
| Microarrays | |||
| Chlamydomonas reinhardtii microarray | Agilent Chlamydomonas reinhardtii microarray, number 024664 | Toepel et al. 2011 | https://earray.chem.agilent.com/earray/ |
| Chlamydomonas reinhardtii UNIGENE cDNA array | cDNA array based on the first Chlamydomonas UNIGENE set superseded by the Agilent arrays | Jain et al. 2007 | |
| Chlamydomonas reinhardtii oligo array | Oligonucleotide array based on the first Chlamydomonas EST set superseded by the Agilent arrays | Eberhard et al. 2006 | |
| Chlamydomonas sRNA Database | |||
| SiLoDb sRNA Locus Database | Database of sRNA locations in the Chlamydomonas genome | http://silodb.cmp.uea.ac.uk/ | |
2a.i Chlamydomonas taxonomy
Chlamydomonas reinhardtii belongs to the chlorophycean family Chlamydomonaceae (Fig. 1). The genus Chlamydomonas encompasses over 600 species, but is now recognized as being polyphyletic and the genus will need substantial revision (Lemieux et al., 1985; Buchheim et al., 1997; Morita et al., 1999; Pröschold et al., 2001; Hoham et al., 2002; Stern et al., 2009). Molecular phylogenies place Chlamydomonas reinhardtii and a few other Chlamydomonas species as the closest unicellular relatives of colonial Volvocine algae that are together part of a coherent, monophyletic grouping (Fig. 2) (Nozaki and Itoh, 1994; Nozaki et al., 1995; Coleman, 1999; Nozaki et al., 2000; 2002; Nakada et al., 2010) with an estimated divergence time of ~200 MY (Herron et al., 2009). We refer here to Volvocine algae as the group containing colonial Volvocales plus their closest unicellular relatives including Chlamydomonas reinhardtii (Fig. 2).
2a.ii Chlamydomonas cell structure
Each Chlamydomonas cell is 5–10 μM in diameter and is shaped like a pear or ovoid (Fig. 3). Surrounding the plasma membrane is a cell wall composed of distinct layers of crosslinked hydroxyproline-rich glycoproteins (Woessner and Goodenough, 1994; Stern et al., 2009). A pair of basal bodies at the anterior end of the cell nucleates two flagella that provide motility and chemosensory functions. The basal bodies also nucleate and organize the microtubule cytoskeleton within the cell body (Dutcher, 2003; Pearson and Winey, 2009) (Fig. 3). The flagella and basal bodies are structurally and functionally homologous to cilia and basal bodies of animal cells and Chlamydomonas serves as a model for investigating these organelles (Dutcher, 2003; Pedersen and Rosenbaum, 2008). The nucleus is positioned below the basal bodies, while the posterior of each cell is occupied by a single large cup-shaped chloroplast that encompasses over half of the total cell volume (Fig. 3). Chlamydomonas is of particular interest for evolutionary genomics because it has attributes of land plants (chloroplast, photosynthetic metabolism) and of animals, lower plants and protists (flagella and basal bodies) (Harris, 2001; Grossman et al., 2003; Merchant et al., 2007). Indeed, as described below, the unique biology of this duality is reflected in its protein coding gene repertoire and has been exploited to identify new candidate genes that are involved in plant- and animal-specific processes.
Figure 3. Cell structure of Chlamydomonas reinhardtii.
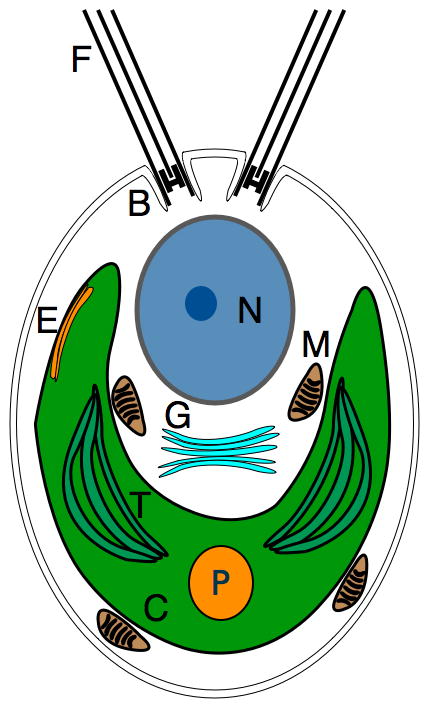
Schematic representation of the subcellular components of a typical Chlamydomonas cell; flagella (F), basal body (B), nucleus (N), eye spot (E), golgi apparatus (G), mitochondria (M), chloroplast (C), thylakoids (T), and the pyrenoid (P).
2a.iii Chlamydomonas vegetative and sexual cycles
Chlamydomonas cells are haploid and have two mating types, plus and minus, that are specified by a mating locus (MT) (Ferris et al., 2002; Goodenough et al., 2007; Ferris et al., 2010). Under nutrient replete conditions Chlamydomonas cells proliferate mitotically using a modified cell cycle termed multiple fission (also called palintomy) that is shared by most other Volvocine algae and many other chlorophytes (Kirk, 1995; 2005; Graham et al., 2009). This mode of reproduction involves a long growth period followed by a rapid series of S phases and mitoses to produce 2n daughters (2, 4, 8, 16 …) where n is typically 1, 2, 3 or 4 in Chlamydomonas (Fig. 4). In a diurnal environment the cell cycle becomes synchronized with growth occurring during the day and division occurring at night (Harris, 2001). In Chlamydomonas the division number (n) is variable but in colonial Volvocine algae it is more stereotyped with variability of usually just one cycle within a species.
Figure 4. The multiple fission cell cycle and sexual cycles of Chlamydomonas and Gonium.
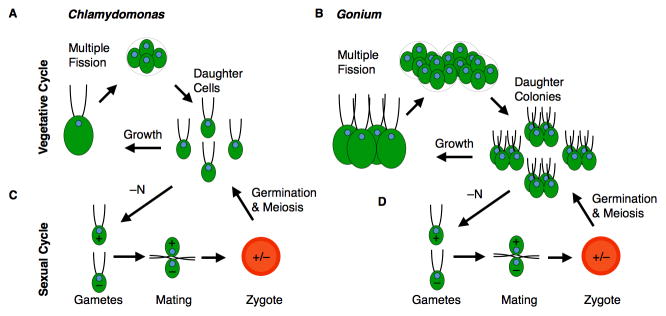
During vegetative growth (A), Chlamydomonas cells may grow many fold size with the extent of growth somewhat indeterminate. Cells then divide multiple times to produce uniform-sized daughters. Two rounds of cell division are shown in this panel, with division numbers ranging from one to four in a typical culture. Gonium colonies (B) follow a similar growth and division pattern as Chlamydomonas, but the daughter cells remain attached to each other through cytoplasmic bridges and ECM (see Fig. 6). The Chlamydomonas sexual cycle (C) is induced by lack of nitrogen (-N) that causes cells to differentiate into gametes. Gametes from each mating type (plus and minus) are similar in size. Flagellar adhesion between gametes of opposite mating type precedes cell fusion to form a diploid zygote. Upon germination four meiotic progeny are produced that can reenter the vegetative cycle. In Gonium –N also triggers gametogenesis that involves colony dissolution into unicellular gametes. Post-meiotic Gonium progeny are single cells that produce a new vegetative colony after their first passage through the cell cycle.
When starved of nitrogen, vegetative Chlamydomonas cells differentiate into mating-competent gametes. While gametes are morphologically similar to vegetative cells, they express a genetic program that allows them to recognize cells of the opposite mating type and to fuse into a zygote that differentiates to become a dormant diploid zygospore. Upon return to light and nutrients the spores undergo meiosis and germinate to yield four haploid progeny that reenter the vegetative reproductive cycle (Goodenough et al., 1995; 2007; Stern et al., 2009) (Fig. 4).
2a.iv Chlamydomonas Metabolism
Chlamydomonas cells can grow phototrophically on simple mineral nutrient media, but can also grow heterotrophically using external acetate as a carbon source for respiration. Its ability to grow heterotrophically has made Chlamydomonas an important model for photosynthesis research since photosynthetically inactive mutants are viable when supplied with acetate (Harris, 2001; Grossman et al., 2003; Dent et al., 2005). Moreover, Chlamydomonas also has evolved anaerobic metabolic pathways that are of significant interest for understanding biological hydrogen (H2) production (Rupprecht, 2009; Kruse and Hankamer, 2010; Costa and de Morais, 2011). Pathways for key nutrients and metabolites such as nitrogen, sulfur, phosphorous, iron, copper, starch lipids and others have been investigated extensively (Harris, 2001; Grossman et al., 2003; Stern et al., 2009).
2b. Colonial Volvocine algae show convergent evolution of form and function
2b.i Overview of colonial Volvocine forms
The colonial Volvocine algae come in a diverse forms that can be organized based on colony size, cell type specialization and other morphological attributes. These attributes were traditionally used to group the Volvocine algae into genera and are a useful means of describing different types; but as described below and in Fig. 2, the morphological classification scheme is misleading with respect to phylogenetic relationships within the Volvocales where multiple instances of convergent evolution have occurred.
The simplest colonial genera is Gonium. Morphologically, Gonium looks like a group of 4, 8 or 16 Chlamydomonas cells that are stuck together. Indeed, each Gonium colony arises from a single cell that undergoes multiple fission and whose daughters remain attached after division (Hoops et al., 2006) (Fig. 4). Gonium colonies also have polarity with cells arranged in a slightly curved sheet with flagella on the apical surface that corresponds to the convex side of the colony Fig. 2. This configuration is achieved through a post-mitotic contortion termed inversion that bends the cell sheet outward so that the flagella (that originally faced inward after division) end up on the outside face of the colony. Polarity is also evident in the orientation of flagella in 16-cell Gonium colonies where the inner four cells have their flagella arranged as those in Chlamydomonas (with opposed beat directions), while the outer twelve cells have rotated basal bodies that allow the two flagella to beat with a more parallel stroke that is characteristic of all the larger colonial genera (Fig. 6). Partial inversion in Gonium is functionally similar to the process of full inversion in the remaining spheroidal genera that involves a complete turning inside-out of the post-mitotic colony (Kirk, 2005).
Figure 6. The mating-type loci of Chlamydomonas and Volvox.
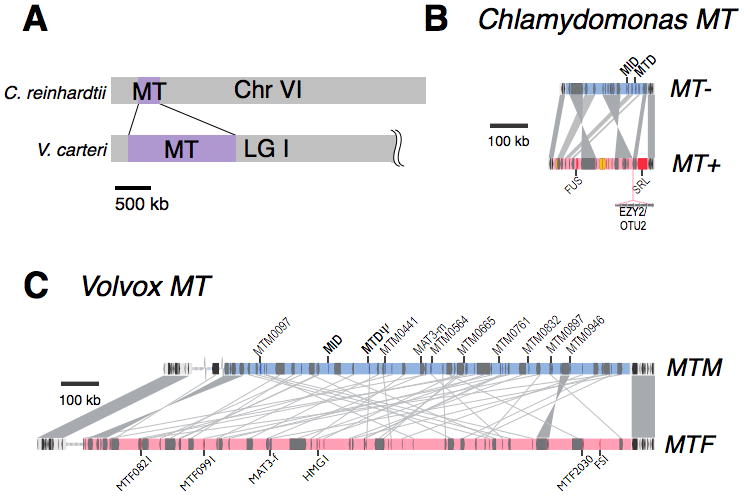
(A) The mating-type locus for both species is near the telomere of a syntenic chromosome (chromosome 6 in Chlamydomonas, linkage group I of Volvox). (B) MT+ and MT− mating haplotypes of Chlamydomonas. Rearrangements between the two haplotypes are indicated by gray shading. Locations of the sex determining genes MID and MTD1 and the sex limited gene gamete fusion gene FUS1 are shown. Also indicated are the EZY2/OTU2 regions that may be important for uniparental chloroplast inheritance (Ferris et al., 2002; Goodenough et al., 2007). (C) The Volvox male and female mating-type loci are about six times larger than Chlamydomonas MT and contain very little syntenic gene order between haplotypes. Several novel genes are shown as well as genes encoding homologs MID, MTD, and the retinoblastoma tumor suppressor homolog, MAT3. Figure adapted from (Ferris et al., 2010; Umen, 2011).
The remaining genera include Pandorina that contain 8, 16 or 32 similar-sized cells in a compact ball with flagella pointing outward (Fig. 2). Eudorina colonies have 16, 32 or 64 cells but are much larger than Pandorina due to the secretion of an extensive glycoprotein-rich extracellular matrix in which cells are embedded. Pleodorina colonies are larger still (32, 64 or 128 cells) and show partial cell type specialization with some small somatic cells on the anterior of the spheroid that never grow or divide, but only provide motility. The remaining cells are flagellated and motile, but continue to grow. Eventually these cells withdraw their flagella and divide into new colonies.
Volvox is the most morphologically complex Volvocine in terms of size and cell-type specialization. Each spherical colony is up to 0.5 mm in diameter and contains 2000–4000 small sterile somatic cells around the outside embedded in an extensive network of ECM (Fig. 6). The somatic cells provide motility for the colony and secrete ECM, but do not grow or reproduce. Within each colony are large reproductive cells termed gonidia that grow and undergo a morphogenesis program that is described in more detail below for the model species Volvox carteri (see section 6a, below).
Colonial Volvocine algae have sexual cycles similar to that of Chlamydomonas with the smaller colonial forms producing gametes of equal size like Chlamydomonas. Larger colonial genera such as Eudorina, Pleodorina and Volvox are anisogamous or oogamous with males producing packets of small sperm and females producing large eggs (Fig. 5) (Nozaki et al., 2000).
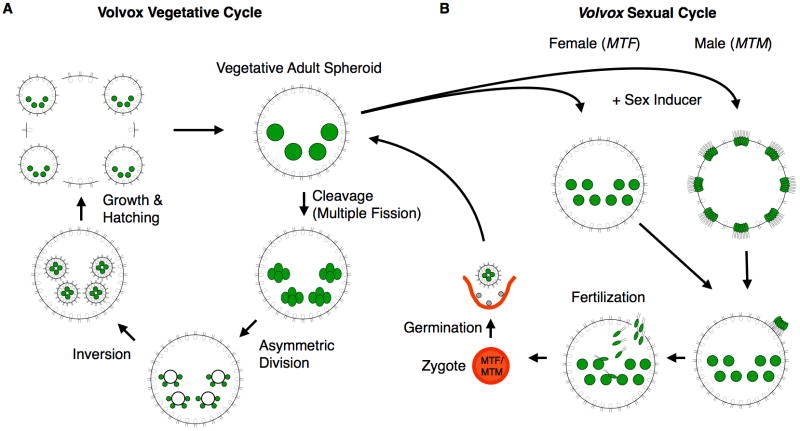
Fig. 5. Vegetative and sexual developmental in Volvox.
(A) The Volvox vegetative life cycle for males and females is identical and begins with a mature adult (upper right) whose gonidia (large green cells) undergo cleavage to begin embryogenesis. During the sixth cleavage cycle asymmetric division occurs and leads to production of 16 large anterior cells that are destined to form the germ cells in the next generation. After a total of 12 cleavage cycles the ~2000-celled embryo undergoes inversion to place the gonidial precursors inside the spheroid and the flagella of the somatic cells pointing outside in their final adult configuration. After a period of growth the daughter spheroids hatch and continue to grow and mature into adults that can restart the vegetative cycle. (B) Sexual development begins when immature gonidia are exposed to sex-inducer protein. Their subsequent embryogenesis is then altered to produce egg-bearing females or sperm-packet-bearing males (Hallmann et al., 1998; Ferris et al., 2010). Females contain 32–48 eggs and ~2000 somatic cells, while males contain 128 somatic cells and 128 packets of 64–128 sperm. Sperm packets are released whereupon they swim as a single unit until they encounter a sexual female. Upon interacting with a sexual female the sperm packet dissolves into individual cells that enter the female ECM through a fertilization pore and swim until an individual sperm encounters and fuses with an egg to form a diploid zygote. When a Volvox zygospore germinates, only one of the four meiotic products survives and differentiates into a new vegetative spheroid.
2b.ii Phylogenetic relationships among Volvocine algae
Morphological criteria that led to grouping the various Volvocine species into the genera described above also suggested a simple, step-wise progression in developmental complexity termed the Volvocine lineage hypothesis (Larson et al., 1992; Kirk, 1998). In its simplest form this hypothesis predicts a nested phylogenetic tree with each new genera arising once from a less complex ancestral form. Molecular systematics reveals a more complex history in this group with multiple independent transitions between levels of organization. For example, colonials with morphology similar to Eudorina (Fig. 2) have arisen independently at least three times to create the genera now termed Eudorina, Volvulina and Yamagishiella (Nozaki et al., 2000; Herron et al., 2009). Likewise, Volvox-like colonial forms have also evolved at least three times independently with distinct developmental patterning mechanisms that give rise to germ-soma differentiation (Desnitski, 1995). Even more problematic for the Volvocine lineage hypothesis are trait losses such as the absence of expanded ECM in some subgroups of Eudorina that are morphologically closer to Pandorina than to other members of their clade (Fig. 2). While complicating the task of classification, convergent evolution within the Volvocine algae makes them appealing as a model system because it allows comparative studies of how similar developmental innovations arose independently within a well-defined clade.
2c. Ecology of the Volvocine algae
Volvocine algae are freshwater species that are distributed world-wide. Long distance dispersion is possible due to the production of dormant sexual zygospores that can survive for years in desiccated environments and are highly resistant to stress. Wind, attachment to animals or ingestion by animals provides a means for the spores to travel and allows for maintenance of genetic continuity between geographically distant populations. Chlamydomonas is often isolated from soil samples though it can also be found in ponds and temporary bodies of water. The methods for isolating Chlamydomonas and other algae from soils favor germinating zygospores, so it is not clear whether significant populations of vegetative Chlamydomonas are present in soils where they are collected. In contrast, colonial Volvocine algae are typically isolated as vegetative forms from transient puddles, rice paddies and warm ponds. The presence of vegetative colonial Volvocine algae in temporary bodies of water implies the presence of zygospores in the soil prior to inundation as vegetative forms do not survive desiccation. Collection method biases notwithstanding, there are several potential advantages to a colonial lifestyle in an open water environment. The first is increased size. Colonies can be resistant to grazing predators that eat unicells but which have an upper limit to the size of prey they can ingest (Bell, 1985; Kirk, 1998; Bonner, 2000). The second potential advantage of being colonial is increased efficiency in resource utilization. In general such efficiency is thought to be of greatest benefit for multicellular species in environments that fluctuate from highly nutrient-rich to nutrient poor. Under these conditions a multicellular organism can collect and store nutrients allowing it to outcompete unicellular species whose growth is more tightly coupled to immediately available nutrients (Kirk, 1998). A third potential benefit of multicellularity is increased mobility. For example, the combined effort of 4000 somatic flagella beating on the surface of a Volvox spheroid supports motility at rates of ~500–900 μm s−1 (Solari et al., 2006; 2008; Ueki et al., 2010) while the two flagella on a Chlamydomonas cell allow movement at a rate of ~100–200 μm s−1 (Ojakian and Katz, 1973). The faster speed of a Volvox spheroid would allow it to travel much greater distances through a stratified water column in search of mineral nutrients. Moreover, the second advantage (efficiency of resource utilization) may be enhanced by the third advantage (motility) in the action of flagellar beating that disrupts the diffusion boundary layer around each spheroid and thereby increases the efficiency of nutrient uptake (Koufopanou, 1994; Short et al., 2006).
2d. Culture resources for Volvocine algae
Chlamydomonas and other Volvocine algae are maintained and distributed from any of several culture collections. Some of the major sources of Volvocine culture stocks are summarized in Table 1. The Chlamydomonas Stock Center has wild-type strains, including inbred isogenic reference strains, many published and unpublished mutants, and several independent wild isolates. In addition to cultures, the Chlamydomonas Stock Center also distributes cDNA libraries and plasmids. The UTEX algal collection at the University of Texas Austin has a good representation of Volvocine algal strains. UTEX also maintains a comprehensive database of freshwater algal media recipes and protocols. Additional collections of Volvocine algae are SAG at the University of Gottingen in Germany, CCAP in UK, and NIES in Japan. Currently, two web-based resources provide such information for Volvocine algae and other groups. Protist Images (http://protist.i.hosei.ac.jp/Protist_menuE.html) has a large collection of photos and taxonomy of protists, including Volvcine algae. Algaebase (http://www.algaebase.org/) includes a comprehensive taxonomy for many Volvocine species.
3. Chlamydomonas and Volvox Genome structure and content
The genome sequence of Chlamydomonas reinhardtii was published in 2007 (Merchant et al., 2007), and that of Volvox carteri in 2010 (Prochnik et al., 2010). These two sequences have provided new insights into the biology of Volvocine algae and have enabled a host of additional “omics” and systems-level approaches (Gonzalez-Ballester, 2005; Eberhard et al., 2006; Jamers et al., 2009; Rolland et al., 2009; Schmidt et al., 2010; Castruita et al., 2011). The majority of genomics research on Volvocine algae has been done with Chlamydomonas; however, the genome of Volvox has already provided new perspectives on the evolution of multicellularity and of Volvocine sexual cycles (Ferris et al., 2010; Prochnik et al., 2010), and we can expect the area of comparative Volvocine algal genomics to be one of increasing activity and interest in the near future. In this review we focus first on Chlamydomonas genomics and the insights it has provided on the evolution of a remarkably complex and sophisticated unicellular eukaryote. We then focus on the emerging area of comparative genomics of Chlamydomonas, Volvox and soon-to-be sequenced species of Volvocine algae that will be of increasing future interest.
3a. Chlamydomonas Nuclear Genome Structure
The Chlamydomonas reinhardtii nuclear genome sequence is ~117 MB distributed on 17 chromosomes. The nucleotide composition is GC biased (64% GC) as is 3rd position codon usage (Merchant et al., 2007). Protein coding gene density is relatively high with 16.7% exonic sequence and 12.5% total repeats. Chlamydomonas chromosomes have genetically mappable centromeres that are not well-characterized, but likely contain repeats (Preuss and Mets, 2002; Merchant et al., 2007). Telomeric repeats are around 300–350 bp and are composed of the sequence (TTTTAGGG)n (Petracek et al., 1990).
3a.i. The Chlamydomonas Mating-type Locus
Chlamydomonas has two mating types, plus and minus, that are governed by a mating locus with haplotypes designated MT+ and MT−. Although it segregates as a single Mendelian trait, MT is a large multigenic region of around 200–300 kb (Fig. 6). Sequence rearrangements (inversions and transpositions) between MT+ and MT− suppress recombination in this region and keep the genes within and around MT in linkage disequilibrium. This rearranged configuration keeps sex-related genes together, but also keeps over a dozen housekeeping genes trapped within a non-recombining region (Umen, 2011). Thus, the MT locus has acquired some properties of a haploid sex chromosome and its expansion in Volvox is one of the most notable differences between the two algal genomes (Ferris et al., 2010). The comparative genomics of the Chlamydomonas and Volvox mating loci are discussed in more detail below.
3a.ii Protein coding genes
Various strategies have been employed for protein coding gene predictions that are homology based or use empirical data. One of the most successful gene prediction programs for Chlamydomonas is AUGUSTUS that combines evidence-based and de novo gene model predictions (Stanke and Morgenstern, 2005; Stanke et al., 2006; 2008). The de novo gene prediction program GreenGenie2 has been trained on a large set of known Chlamydomonas cDNA sequences from which it has generated high-quality gene models (Kwan et al., 2009) that are available pre-formatted for processing with Cufflinks (Trapnell et al., 2010), a popular tool for transcript assembly and differential gene expression analysis.
3a.iii Genes for RNAs
Chlamydomonas encodes 259 tRNAs, over half of which are present in clusters that arose through serial gene duplication (Merchant et al., 2007). Compared to other eukaryotes Chlamydomonas tRNAs are relatively intron rich, with 60% intron-containing tRNA genes, and contain an unusually large number that encode a 3′ terminal CCA sequence that is usually added post-transcriptionally in eukaryotes (Merchant et al., 2007).
Cytosolic ribosomal RNA (rRNA) encoding gene clusters (18S, 5.8S, 28S) are found at two locations: one end of Chromosome 14, and at one end of Chromosome 8. A single cytosolic 5S rRNA cluster is located on chromosome 8 about 200 kb from 18S/5.8S/28S cluster with several dozen predicted protein coding genes in between the two loci (J. Umen, unpublished observation). The highly repetitive nature of these clusters makes their exact size difficult to determine.
The nuclear genome encodes 322 snoRNAs that are involved in rRNA processing and base modifications. Chlamydomonas snoRNAs are notable for being clustered and for the high proportion that are encoded within spliceosomal introns of protein coding genes (Merchant et al., 2007; Chen et al., 2008).
Chlamydomonas contains a complex repertoire of small 22–24 nt RNAs (sRNAs), some of which appear to be microRNAs (miRNAs) (Molnár et al., 2007; Zhao et al., 2007). The Chlamydomonas genome also encodes key proteins involved in sRNA biogenesis and function including Argonaute proteins and Dicer nuclease, though it does not appear to encode any RNA-dependent RNA polymerases that are potentially involved in generation or amplification of double stranded RNAs that act as sRNA precursors (Schroda, 2006; Casas-Mollano et al., 2008; Cerutti et al., 2011). As discussed below, RNAi-based gene silencing has proven to be a powerful reverse genetics tool in Volvocine algae.
3b. Chlamydomonas Organellar Genomes
The Chlamydomonas chloroplast genome is typical for those in the green algal/land plant lineage. It is a 200 kb circle with two large inverted repeats that contain rRNA genes and the psbA gene. In total the chloroplast encodes around 99 genes including protein coding genes, tRNAs and rRNAs (Maul et al., 2002; Grossman et al., 2003). The chloroplast DNA (cpDNA) is present in around 80 copies per cell and is packaged into nucleoids containing around 10 copies each. Chloroplast DNA replication occurs continuously through the cell cycle and is not synchronized with the nuclear division cycle, while chloroplast division is tightly coordinated with cytokinesis (Chiang and Sueoka, 1967; Johnson and Porter, 1968; Turmel et al., 1980; Adams et al., 2008). Though typical in size and protein coding capacity for a chloroplast genome, the Chlamydomonas cpDNA is relatively rich in non-conserved short intergenic repeats that may contribute to its high rate of rearrangement (Maul et al., 2002; Odom et al., 2008).
The Chlamydomonas mitochondrial (mt) genome is a 15.8 kb linear molecule with inverted repeats at each end (Vahrenholz et al., 1993). The mt genome encodes 8 proteins, mitochondrial rRNAs, and three tRNAs (Grant and Chiang, 1980; Gray and Boer, 1988; Michaelis et al., 1990). The Chlamydomonas mt genome is notable in being linear in structure and significantly smaller than the circular mt genomes found in other algae and most plants (Grant and Chiang, 1980; Boer et al., 1985; Stern et al., 2009). Other notable features of the mt genome are fragmentation of its rRNA genes that are separately transcribed as pieces that assemble non-covalently into intact mt ribosomes (Boer and Gray, 1988; Denovan-Wright et al., 1996) and are rapidly evolving with respect to other mt genes cytosolic rRNAs (Popescu and Lee, 2007).
The Chlamydomonas organellar genomes are inherited uniparentally through postzygotic mechanisms that are still not well understood. In meiotic progeny cpDNA is inherited from the MT+ parent while mitohondrial DNA is inherited from the MT− parent (Boynton et al., 1988). cpDNA inheritance involves targeted destruction of MT− derived cpDNA in early zygotes (Nishimura et al., 1998; Kuroiwa, 2010), and may also involve differential replication in germinating zygotes mediated by cpDNA methylation (Umen, 2001). Loss of female mtDNA appears to occur during meiosis as zygotes germinate (Aoyama et al., 2006).
3c. The Volvox Nuclear Genome
Previous genetic mapping defined 19 linkage groups for Volvox that probably correspond to chromosomes (Kirk, 1998), though exact chromosome number remains to be established. The ~131 Mbp Volvox nuclear genome sequence assembly (version 2) contains 434 scaffolds, 100 of which are >50 kbp and contain over 98% of the sequence. Updated assemblies and annotations are available on the Phytozome web site (Table 2) (Goodstein et al., 2011). The extra ~14 Mbp of sequence in Volvox compared with Chlamydomonas is composed of repeats that are interspersed within and between genes (Prochnik et al., 2010). A second difference between the two algae is that the Volvox genome is ~56% GC while Chlamydomonas is ~64% GC. However, with respect to coding capacity Volvox is highly similar to Chlamydomonas with ~15,000 predicted protein coding genes (Prochnik et al., 2010). A more detailed comparison of gene content between the two species is in sections 5 and 6 below.
3d. The Volvox Organellar Genomes
Compared with Chlamydomonas and with most other green algae, the Volvox carteri organellar genomes are atypically large and filled with non-coding sequences, most of which are palindromic repeats (Smith and Lee, 2009). The cp genome is 420 kb and the mt genome is 30 kb. The cp genome is the largest and most repeat-rich within the green lineage (Chlorophytes and Streptophytes), while the Volvox mt genome is also repeat rich with few genes. Other than their expanded repeat content, the organellar protein coding genes of Volvox are very similar to those of Chlamydomonas (Smith and Lee, 2009). The increased non-coding content of all three Volvox genomes compared with Chlamydomonas (nuclear, cp and mt) is consistent with less efficient natural selection on a smaller, and possibly fragmented population of Volvox carteri (Smith and Lee, 2010).
3d.i Organelle DNA inheritance in Volvox
Like Chlamydomonas, the Volvox organellar genomes are inherited uniparentally, but unlike Chlamydomonas, both derive from the maternal parent (Adams et al., 1990). The presence of the putative mating type specification gene MID in males of colonial Volvocine algae makes them formally equivalent to the MT− mating type of Chlamydomonas that also has a MID gene (Ferris and Goodenough, 1997; Nozaki et al., 2006; Hamaji et al., 2008; Ferris et al., 2010). Therefore, with respect to mating type there has been at least one switch in mtDNA inheritance patterns in the lineage. In Gonium pectorale organellar DNA inheritance was found to follow a similar pattern to that of Chlamydomonas with cpDNA inherited from the MT+ parent and mtDNA from the MT− parent (Hamaji et al., 2008; Setohigashi et al., 2011). These findings are consistent with the Chlamydomonas/Gonium organellar inheritance patterns being ancestral, but more data from other Volvocine species will be required to confirm this idea.
Moreover, even though cpDNA is inherited maternally in all three Volvocine algal species described above, Volvox female MT is missing EZY1, EZY2 and other MT+ specific genes that are thought to be associated with uniparental cpDNA inheritance in Chlamydomonas (Fig. 6) (Armbrust et al., 1995; Ferris et al., 2002; Goodenough et al., 2007). The absence of these genes in Volvox suggests that the underlying mechanism that specifies uniparental cpDNA inheritance in the two species may differ as well. A default hypothesis for uniparental organelle DNA inheritance in oogamous species such as Volvox is by a passive mechanism based on differential contributions of organellar DNA from eggs versus sperm (as opposed to active elimination that occurs in Chlamydomonas). Nonetheless, additional active mechanisms to ensure uniparental organelle DNA inheritance may also operate in Volvox as they do in Chlamydomonas and other eukaryotic taxa (Shitara et al., 2000; Zouros, 2000; Rawi et al., 2011; Sato and Sato, 2011; DeLuca and O’Farrell, 2012).
3d.ii The Volvox Mating Locus
Volvox and other large colonials (Pleodorina, Eudorina) transitioned from an isogamous ancestral mating system to an anisogamous or oogamous one with eggs and sperm. Volvox sexual development occurs via a modified embryogenesis program that differs between males and females and is controlled by a mating locus with two haplotypes, MTF (female) and MTM (male).
There are striking similarities and differences between the mating loci of Chlamydomonas and Volvox. They both are relatively large regions with rearrangements that suppress recombination (Fig. 6). Somewhat surprisingly, the location of MT in both species is in the same region of a syntenic chromosome (chromosome 6 in Chlamydomonas reinhardtii, scaffold 1 in Volvox carteri) indicating that MT has not moved during the ~200 Mya since the Chlamydomonas and Volvox lineages last shared a common ancestor (Fig. 7A) (Ferris et al., 2010). Unlike Chlamydomonas MT, Volvox MT is repeat-rich with low gene density compared with the rest of the genome, making it similar in most respects to classical sex chromosomes (Ferris et al., 2010). However, unlike diploid heteromorphic sex chromosomes, haploid sex chromosomes are not sheltered from gene loss and are less likely to lose essential genes (Bull, 1978). At >1Mbp the Volvox MT region is about five times larger than Chlamydomonas MT and unlike the case for MT+/MT−, MTF and MTM contain almost no residual synteny (identical gene order) between the 70+ shared genes that they have in common (Fig. 6C).
Figure 7. Comparison of the ECM structure of Chlamydomonas, Gonium and Volvox.
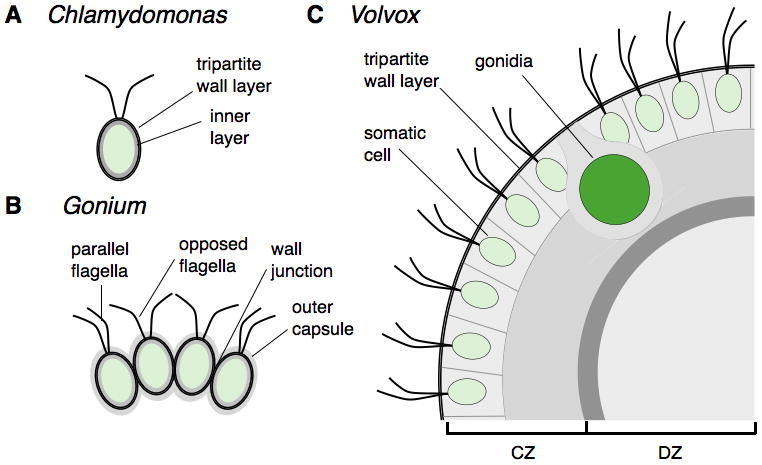
The Chlamydomonas cell wall (A) is composed of an inner layer and an outer tripartite layer that is conserved with other Volvocales (indicated as a black layer around the cell). Individual Gonium cells (B) have an identical tripartite layer and the entire colony is surrounded by an additional outer capsule layer of ECM. Cells are held together by specialized attachments at their wall junctions. (C) Volvox cells are completely embedded in an expanded and compartmentalized ECM with a conserved tripartite boundary layer surrounding the entire colony (instead of individual cells). Inside the tripartite layer of the somatic cells are the cellular zones of ECM and the deep zone of the interior.
3e. Additional Volvocine algal genomes
Chlamydomonas and Volvox represent two extremes of organismal complexity in the Volvocales. Many questions about evolution in the Volvocine algae can only be answered by sequencing additional genomes of intermediate species. Projects to do so are underway and include genomes of Gonium pectorale and Pleodorina starrii (B. Olson, H. Nozaki, P. Durrand, R. Michod, personal communication).
4. Molecular genetics and genomics tools for Volvocine algae
Genomics resources for Chlamydomonas and Volvox are steadily growing and include informatics sites that provide access to browsers and computational tools as well as repositories for strains and cDNA or genomic libraries. These resources are described in Tables 1 and 2.
4a. The Chlamydomonas molecular genetics toolkit
4a.i Transformation
All three genomes (nuclear, chloroplast, mitochondrial) of Chlamydomonas are transformable (Kindle et al., 1989; Kindle, 1990). Transformed nuclear genes insert randomly, a property that can be exploited for making tagged mutants (Dent et al., 2005; Galván et al., 2007). Transformation of the nuclear genome by electroporation with either a circular or linearized plasmid is a routine procedure typically resulting in hundreds to thousands of independent transformants (Keller, 1995; Shimogawara et al., 1998). Chlamydomonas can also be transformed by vortexing gametolysin-treated cells with glass beads or silicon carbide whiskers (Kindle, 1990; Dunahay et al., 1997). Chloroplast and mitochondrial transformation occurs by homologous recombination. It is now possible to transform not just one or a few genes into the Chlamydomonas chloroplast, but to re-engineer the entire chloroplast genome ex-vivo and then reintroduce it to obtain gene replacements and modifications at multiple loci (O’Neill et al., 2012).
4a.ii. Promoters for nuclear transgene expression
Unlike the case in higher plants, heterologous promoters have so far not been successful in Chlamydomonas. A handful of endogenous promoters have been successfully used to drive transgene expression (Neupert et al., 2012). These include an engineered hybrid promoter from the HSP70A and RBCS2 genes that also contains the first intron of RBSC2 (Schroda et al., 2000; 2002; Lodha et al., 2008), The HSP70a and HSP70b promoters alone (Schroda et al., 2000), and the PSAD promoter that is derived from an intronless gene and well-suited to drive expression of cDNAs (Fischer and Rochaix, 2001).
4a.iii. Regulated Promoters
Several regulated promoters have been described that utilize environmentally responsive transcriptional responses to drive transgene expression. These include a copper-responsive promoter from the cytochrome b6 gene CYC6 that can be induced using Ni2+ (Quinn et al., 2003; Ferrante et al., 2008), the nitrate inducible/ammonium repressible promoter from the nitrate reductase gene NIT1, and the low-CO2-inducible promoter from the carbonic anhydrase gene CA1 (Ohresser et al., 1997; Villand et al., 1997). The activities of these promoters are coupled to environmental conditions, a property that might limit their utility. Synthetic or heterologous regulated promoters would be useful for expanding the molecular genetic and genomic toolkits of both Chlamydomonas and Volvox.
4a.iv. Selectable markers in Chlamydomonas
Two classes of selectable markers have been utilized for nuclear transformation in Chlamydomonas; endogenous genes that complement auxotrophies, and dominant antibiotic resistance makers (Neupert et al., 2012). Complementable mutations include nit1 (nitrate requiring) (Kindle et al., 1989), arg7 (arginine requiring) (Debuchy et al., 1989), nic7 (nicotinamide requiring) (Ferris, 1995), and thi10 (thiamine requiring) (Ferris, 1995). Dominant antibiotic resistance markers that have been engineered for Chlamydomonas include ble (zeocin resistance) (Stevens et al., 1996), aphVIII (paromomycin resistance) (Sizova et al., 2001), aph7″ (hygromycin resistance) (Berthold et al., 2002), aadA (spectinomycin resistance) (Cerutti et al., 1997), cry1-1 (emetine resistance) (Nelson et al., 1994), and ppx1 (protoporphyrinogen oxidase mutant conferring resistance to the herbicide S-23142) (Randolph-Anderson et al., 1993).
Chloroplast transformants are typically selected by either complementation of an atpB mutation to restore phototrophic growth (Boynton et al., 1988) or with a dominant spectinomycin/kanamycin resistance gene (aadA) (Purton, 2007; Ramesh et al., 2011). Mitochondrial transformants are selected by complementation of the respiration deficient mutant dum1 (Boynton and Gillham, 1996; Yamasaki et al., 2005; Hu et al., 2011).
4a.v. Reporter genes
Green fluorescent protein (GFP) has been codon optimized for use in Chlamydomonas (Fuhrmann et al., 1999) and has been expressed transgenically from the nucleus, chloroplast and mitochondrial genomes (Fuhrmann et al., 1999; Purton, 2007; Hu et al., 2011; Ramesh et al., 2011). Live cell imaging with GFP in algae is a challenge due to high background fluorescence from chlorophyll and other auto-fluorescent compounds, but can be effective in cases where the GFP is well expressed or concentrated in a specific subcellular location (Fuhrmann et al., 1999; Yoshihara et al., 2008; Diener, 2009; Hayashi and Shinozaki, 2011; Neupert et al., 2012). Luciferase reporters have also been developed for Chlamydomonas (Fuhrmann et al., 2004; Mayfield and Schultz, 2004; Shao and Bock, 2008), and adapted for various purposes including extracellular secretion (Eichler-Stahlberg et al., 2009) and for monitoring circadian gene expression (Minko et al., 1999; Matsuo et al., 2008) (Mayfield and Schultz, 2004).
4a.vi. RNAi and antisense technology in Chlamydomonas
RNA interference (RNAi) is an effective tool for gene silencing in Chlamydomonas that has all the essential components of the RNAi machinery (see section 4a). Two strategies that have proven successful for generating targeted gene knockdowns are double strand hairpin-forming constructs (Rohr et al., 2004) and artificial microRNAs (Molnar et al., 2009; Zhao et al., 2009) that can also be coupled to regulated promoters (Schmollinger et al., 2010).
4b. Forward genetics and functional genomics in Chlamydomonas
Positional cloning of Chlamydomonas mutants can be accomplished using PCR mapping makers (Kathir et al., 2003; Rymarquis et al., 2005), that are available from the Chlamydomonas Resource Center (http://www.chlamycollection.org). Next-generation re-sequencing-based strategies for mutant gene identification are also now feasible and should allow more rapid identification of point mutants (Dutcher et al., 2012).
Insertional mutagenesis is a powerful tool for isolating tagged mutants in Chlamydomonas (Galván et al., 2007), but its potential is even greater when combined with high-throughput methods for identifying and cataloging insertion sites. This potential is gradually being realized. One approach for doing so involves pooling sets of mutant DNA and screening for insertions in genes of interest using PCR with a gene-specific and insert-specific pair of primers. When optimized this method has a very high success rate (Pootakham et al., 2010; González-Ballester et al., 2011). A variant approach involves screening insertion mutants that have been enriched for specific phenotypes such as photoprotection or nitrogen utilization, and then identifying mutants en masse by sequencing the insertion border in all of the identified strains (Dent et al., 2005; Gonzalez-Ballester, 2005). A next-generation approach to insertional mutant screening using bar-coded sequencing of selected pools may be on the horizon. A drawback of using insertional lines in a haploid strain is that essential genes will be highly underrepresented. A diploid insertion library is a feasible way around this problem, but has yet to be generated as a reverse genetics resource.
4c. The Volvox toolkit
4c.i Volvox transformation
The Volvox nuclear genome can be transformed by particle bombardment using the nitA gene (encoding nitrate reductase) to complement a nitA− strain (Schiedlmeier et al., 1994; Gruber et al., 1996). Dominant antibiotic resistance markers including aphVIII (conferring paromomycin resistance) and ble (conferring zeocin resistance) have also been adapted for use in Volvox (Hallmann and Rappel, 1999; Jakobiak et al., 2004). An sex pheromone inducible promoter system has also been developed for regulated gene expression (Hallmann and Sumper, 1994). Some Volvox transformation vectors have also been used successfully to transform another Volvocine alga, Gonium pectorale (Lerche and Hallmann, 2009). GFP fusion proteins expressed in Volvox have been used to localize proteins within the ECM (Ender et al., 2002; Ishida, 2007) and in nuclei (Pappas and Miller, 2009). Gene expression can be knocked down in Volvox using antisense constructs as demonstrated for GlsA (Cheng et al., 2003) and a photoreceptor, Volvox rhodopsin (Ebnet, 1999).
4c.ii Forward genetics by transposon tagging
it is challenging to create and maintain thousands of insertion tagged mutants in Volvox due to its relatively low transformation efficiency and the difficulty of maintaining so many transformed lines (Kirk, 2000). An alternative strategy for forward genetics is transposon tagging mutagenesis using the cold-inducible transposons Idaten (Ueki and Nishii, 2008) or Jordan (Miller et al., 1993). This method relies on endogenous transposons that can occasionally excise to yield revertants. While successful, the method is relatively time consuming and inefficient. The development of artificial transposons would be a useful advance.
Classical genetics has been used successfully in Volvox to identify developmental mutants, but was done in a pre-genome era with few molecular markers (Huskey et al., 1979). The decreasing costs of resequencing may reopen this route for identifying important developmental regulators in Volvox.
5. The Chlamydomonas genome as a window into plant and animal evolution
In this section we highlight areas where genomic information from Chlamydomonas has shed light on eukaryotic cell biology and the evolution of the green plant lineage. While every species is in some ways specialized, Chlamydomonas is particularly interesting in that it does not have a reduced genome size as do marine picoalgae such as Ostreococcus and Micromonas (Derelle et al., 2006; Misumi et al., 2007; Palenik et al., 2007; Worden et al., 2009), and it has retained features that were likely present in the last common eukaryotic ancestor such as flagella and basal bodies. In addition, Chlamydomonas is part of the Archeaplastidia or green lineage that includes a diverse group of green algae and land plants (Fig 1A), all of which are descended from a unicellular eukaryote that underwent a primary endosymbiotic event of engulfing a cyanobacterium that subsequently evolved into the chloroplast. The tractability of Chlamydomonas as an experimental model makes it highly attractive for genomic studies that can then lead to experimentally testable hypotheses about gene function. Some examples of genomics-based discoveries in Chlamydomonas are described below.
5a. Cell motility and the flagella
Long before its genome was sequenced, it was recognized that the flagella and basal bodies of Chlamydomonas are structurally and functionally homologous to those in other eukaryotes including animals (Preble et al., 1999; Silflow and Lefebvre, 2001). Indeed, a key process for flagellar biogenesis that was discovered in Chlamydomonas, intraflagellar transport (IFT) (Kozminski et al., 1993), is now widely recognized for its role in human ciliary signaling and a variety of genetic diseases (Sharma et al., 2008; Hildebrandt et al., 2011). Chlamydomonas is also a valuable resource for identifying new basal body and flagellar genes. Part of its utility for this approach stems from it having one of the best annotated sets of flagellar and basal body protein coding genes, many of which were validated by empirical proteomics (Table 2) (Keller et al., 2005; Pazour et al., 2005; Merchant et al., 2007).
Comparative approaches have been successful at identifying gene sets enriched for those encoding flagella and basal body proteins. This approach is based on the fact that higher plants, fungi and slime molds have lost flagella and basal bodies whereas most animals and unicellular eukaryotes have retained these organelles. By focusing on protein families where ciliated species have at least one member, but where non-ciliated species do not, new cilia or basal body proteins were identified (Avidor-Reiss et al., 2004; Li et al., 2004). These studies identified known proteins, but also new ones such as BBS5 whose human homolog is encoded by a gene associated with the genetic disease Bardet-Biedl syndrome (Li et al., 2004). A Chlamydomonas-centric comparative genomics approach was also used to subclassify flagellar genes by identifying those that are common to organisms with different cilia subtypes. Using a similar comparative approach as above, a more comprehensive analysis of flagella genes was undertaken. Starting with a set of proteins that are present in flagellated/ciliated organisms but not in those without flagella, a more refined subset of classifications were made. For example, the nematode C. elegans has non-motile sensory cilia, and proteins found in Chlamydomonas and other species with motile cilia but not in C. elegans were provisionally designated as MOT genes. Similar functional comparisons were made with the moss Physcomitrella patens and diatom Thalassiosira pseudonana, each of which have structurally modified motile cilia that are missing or thought to be missing structural components found in the Chlamydomonas flagella that has a standard 9+2 microtubule doublet structure with inner and outer dynein arms and radial spokes (Merchant et al., 2007).
5b. Green Genes
Genomic analyses in Chlamydomonas have aided progress in understanding photosynthetic metabolism and cell biology in the green lineage. A green lineage specific tool based on the predicted Chlamydomonas proteome was used to identify gene families that are specific to photosynthetic species. Indeed, 83% of the Green Cut proteins from Chlamydomonas whose functions were already known were chloroplast localized (Merchant et al., 2007). However, the Green Cut set also includes proteins that evolved specifically in the green lineage but may not be directly related to photosynthesis. These include proteins with predicted functions in signaling and nuclear transcription factors that may be specialized within the green lineage. Since they were first identified, a number of Green Cut protein family members were characterized and functionally verified (Grossman et al., 2003; Karpowicz et al., 2011) and this process will likely accelerate in the next few years as functional genomic resources such as insertional library screening are applied to Chlamydomonas and other green organisms.
Large-scale approaches such as Green Cut have inherent limitations that are complemented by more directed studies. For example some chloroplast import machinery proteins were missed by Green Cut, probably due to sequence divergence or incomplete gene models, but could be identified in directed searches based on screening for Arabidopsis Tic (translocon in the inner chloroplast envelope) and Toc (translocon in the outer chloroplast envelope) homologs (Kalanon and McFadden, 2008). Homologs of all but two Arabidopsis Tic and Toc proteins were identified in Chlamydomonas, and the two missing proteins (Tic62 and Toc64) are also missing from one or more other algal species. Comparison of the chloroplast transit peptide receptor complex proteins CrToc34 and CrToc159 indicated differences in amino acid composition that may reflect differences in composition of transit peptides between Chlamydomonas and higher plants (Franzén et al., 1990; Patron and Waller, 2007; Kalanon and McFadden, 2008).
5c. Selenoproteins
One interesting surprise in the Chlamydomonas genome was the presence of selenocysteine-containing proteins that are absent from land plants and fungi, but present in animals (Novoselov et al., 2002). Selenosysteine (Sec) is inserted into translating polypeptides by a modified tRNA-Sec that recognizes the codon UGA (normally a translation terminator) whose insertion is specified by a stem-loop forming sequence element (termed SECIS) in the 3′ UTR of the message containing the tRNA-Sec codon (Novoselov et al., 2002). Chlamydomonas was found to encode a single tRNA-Sec gene (RAO et al., 2003) as well as the enzymes and factors needed to produce and insert tRNA-Sec into elongating polypeptide chains (Grossman et al., 2007). Twelve putative selenoproteins have been identified in Chlamydomonas, five of which are involved in redox biochemistry (Grossman et al., 2007). Five other selenoproteins have no known function, but do have orthologs in mammals. This finding makes Chlamydomonas a potential new model for investigating selenoprotein-related biology.
5d. Carbon concentrating mechanism
Inorganic carbon (Ci) is often a limiting nutrient for aquatic photosynthetic microbes such as Chlamydomonas. Unlike terrestrial environments with relatively stable atmospheric CO2 concentrations, aquatic Ci exists in gaseous (CO2) and ionic (HCO3, CO32−) forms that are not always in equilibrium with atmospheric CO2 and whose ratios fluctuate depending on pH. Aquatic algae have evolved carbon concentrating mechanisms (CCMs) to help overcome this limitation CCM proteins are part of an energy mediated transport system to move Ci from outside the cell and concentrate it in the chloroplast where it serves as the substrate for RuBisCo in photosynthetic dark reactions. CCM proteins include carbonic anhydrases (CAs) that interconvert CO2 and HCO32−, and transporters that move HCO3 or CO2 across membranes. While forward genetic screens have identified many CCM proteins (Spalding, 2008; Wang et al., 2011), genome-wide homology searches have revealed additional candidate genes that may play a role in the CCM or related processes. These include a large repertoire of twelve CAs that localize to different subcellular compartments or the periplasmic space. They also include additional paralogs of LCI proteins (LCIA, LCIB, LCIC, LCID, LCIE), that are thought to be involved in CO2 transport or uptake (Wang and Spalding, 2006; Grossman et al., 2007) and which have homologs in other algae such as Ostreococcus. A second observation from the Chlamydomonas genome sequence is that several CCM genes cluster in a single 75 kb region of chromosome 4 (Merchant et al., 2007). These include CCP1, CCP2, LCID, LCIE and two carbonic anhydrase paralogs, CAH1 and CAH2. Whether such clustering is connected to gene regulation remains to be determined.
EST and genome sequencing identified a medically relevant protein family, Rh, as another possible connection to CO2 physiology. Rh is a red blood cell antigen and integral plasma membrane protein that is part of an ammonium transporter superfamily (PFAM 00909). The discovery of Rh proteins in non-metazoans such as Chlamydomonas was unexpected (Huang and Liu, 2001), as is their overall distribution in a few select non-metzoan taxa that include green algae (Chlamydomonas, Coccymyxa, Chlorella), oomycetes (Phytophthora), slime molds (Dictyostelium, Polysphondylium), Choanoflagellates (Monosiga, Salpingoeca), Incertae sedis (Capsaspora) and Heteroloboseans (Naeglaria). Whether Rh transports NH4/NH3+ or CO2/HCO3 is not fully resolved, however the Chlamydomonas Rh paralogs are linked to high CO2 acclimation possibly by acting as a bidirectional CO2 gas exchange channel (Soupene et al., 2004; 2004).
5e. Vitamin biosynthesis
Many algae require one or more of vitamins B1 (thiamine), B7 (biotin) and B12 (cobalamin) for growth (Croft et al., 2006). Vitamin auxotrophies are distributed across algal taxa in a manner that suggests multiple independent losses of genes or enzymes requiring the co-factors. Chlamydomonas does not require any vitamin supplements. It has biosynthetic pathways for thiamine and biotin, and its single cobalamin-dependent enzyme, the methionine synthase METH, is redundant with a cobalamin-independent enzyme, METE (Croft et al., 2006). Interestingly, Volvox carteri requires B12 supplementation for growth suggesting that it has lost its cobalamin-independent methionine synthase gene, METE (or that it has acquired another essential enzyme that is cobalamin dependent). Indeed, the sequence of Volvox METE has multiple frame-shift mutations and a premature stop indicating that it has become a pseudogene, thus explaining the dependence of Volvox on B12 (Helliwell et al., 2011). Moreover, METE loss has occurred at least one other time in the Volvocine lineage. The 16-cell colonial species Gonium pectorale METE was also found to be a pseudogene whose loss appears to have occurred independently from that in V. carteri (Helliwell et al., 2011), though some strains of Gonium pectorale are B12 independent suggesting a very recent loss of METE in some lines (Stein, 1966). The loss of METE implies that Volvox carteri and some Gonium pectorale strains acquire B12 environmentally and raise the question of whether they do so in specific association with other microbes.
Volvox carteri has orthologs of the thiamine and biotin biosynthetic enzymes identified in Chlamydomonas (Croft et al., 2006) (J. Umen, unpublished observation), but their expression and function have not been carefully examined. The question of whether Volvox carteri truly requires biotin and thiamine supplementation merits reexamination in light of this observation.
5f. Cell cycle
The non-canonical multiple fission cell cycle of Chlamydomonas might be expected to require innovation in cell cycle control machinery compared to organisms that divide by binary fission. In addition, multiple-fission is likely to have been an important factor in facilitating the evolution of colonialism in Volvocine algae (see section 6e). A genomic survey of cell cycle regulatory proteins in Chlamydomonas revealed a typical repertoire of eukaryotic cell division control proteins that are similar to those in land plants, though with far less gene duplication than is seen in plants (Bisova et al., 2005). A similar situation was observed for another alga, the Prasinophyte Ostreococcus taurii (Robbens et al., 2005). A few notable observations arose from these studies. First, both algae encode homologs of the plant-lineage-specific cyclin dependent kinase (CDK) CDKB that is expressed during cell division (Bisova et al., 2005; Corellou, 2005). This finding broadens the possible roles for CDKB and suggests an ancestral function that encompasses both algae and land plants. A notable feature of Chlamydomonas cell cycle genes is the presence of novel cyclin dependent kinases, CDKG1 and CDKG2, that are not found in species outside of Volvocine algae and may be important for the multiple fission cycle found in the Volvocales. Genomic level analysis also confirmed the presence of key components of the retinoblastoma (RB) tumor suppressor pathway that are conserved in the green lineage and in animals, but have been lost from fungi (Xie et al., 1996; Umen and Goodenough, 2001; Bisova et al., 2005; Robbens et al., 2005; Grafi et al., n.d.). Interestingly, the only cell cycle gene family in Chlamydomonas that underwent expansion is the D-type cyclins which are presumed to activate CDKs that regulate RB-related proteins through phosphorylation. Chlamydomonas has four D-type cyclins that are diverged from each other enough to suggest an ancient duplication event. This idea is supported by the finding that Volvox has orthologs for each of the four D-type cyclin subfamilies that are found in Chlamydomonas plus additional paralogous duplications whose potential significance is discussed in section 6e (Prochnik et al., 2010). This finding indicates that a D-cyclin family expansion occurred early in the Volvocine lineage and has been maintained in two independent branches suggesting an important role for each of the D-type cyclin sub-types in Volvocine algal cell cycle control.
5g. Scavenger receptors
Scavenger receptor and cysteine rich domain (SRCR) proteins are found in metazoans where they have diverse roles in the immune system (e.g. pathogen recognition by macrophages) and elsewhere such the sea urchin sperm flagellar receptor for the egg peptide speract (Cardullo et al., 1994; Resnick et al., 1994). SRCR proteins contain a ~100 amino acid repeat module with conserved cysteine pairs that form disulfide bridges (Hohenester et al., 1999). SRCR proteins are abundant in metazoans, but are absent from higher plants, and most algae. Remarkably, Chlamydomonas encodes 35 SRCR domain containing proteins, many of which were described previously (Wheeler et al., 2008), and few of which have appeared in a more updated genome assembly from Phytozome (Goodstein et al., 2011). Volvox has at least 21 SRCR domain-containing proteins, though its genome annotation is not as comprehensive as that of Chlamydomonas, so this family awaits a true interspecies comparison in Volvocine algae. SRCR domains are often repeated multiple times in a protein with a range of 1–11 copies in Chlamydomonas SRCR proteins. A notable feature of several of the Chlamydomonas SRCR proteins is their enormous size, with the largest containing 8671 amino acids and encoded by a gene that spans almost 60 kb. Several of the Chlamydomonas SRCR proteins also contain lectin domains that bind to carbohydrates and are widespread in eukaryotes (Wheeler et al., 2008). The presence of these large metazoan-like adhesion molecules or receptors in Chlamydomonas and Volvox raises many interesting questions about their function(s) and provides a new avenue for investigating their evolutionary origins.
6. The Volvox genome as a window into multicellular evolution
6a. Volvox development
Volvox carteri has a complex developmental program that involves conserved processes that are shared with Chlamydomonas and other features with no obvious analogs in Chlamydomonas (Fig. 4 and 5). Insights into the evolution of Volvox have come from genomics and from isolation of developmental mutants (Kirk, 1998; Nishii and Miller, 2010; Prochnik et al., 2010). Below we outline key stages of Volvox development and discuss what genetic and genomic approaches have revealed about the origins of multicellularity in Volvocine algae. More comprehensive reviews of Volvox carteri development are also available (Kirk, 1998; 2005).
Each Volvox carteri vegetative spheroid is composed of ~2000 sterile somatic cells and sixteen large reproductive cells called gonidia, all of which are embedded within a complex compartmentalized ECM that occupies 99% of the spheroid volume. Somatic cells do not normally grow or reproduce but provide motility for the spheroid and secrete extracellular matrix. They may also help concentrate nutrients in the interior to support growth of reproductive cells (Koufopanou, 1994). The vegetative gonidial cells undergo embryogenesis involving successive cleavage divisions that follows a modified program of multiple fission in which incomplete cytokinesis leaves post-mitotic cells attached through a network of cytoplasmic bridges. At cycle 6 (32-->64 cell stage transition) 16 cells on the anterior of the embryo divide asymmetrically to produce a large and a small cell while the remaining cells continue to divide a total of twelve times. The 16 large cells from the asymmetric division divide one or two more times asymmetrically, and then withdraw from cleavage. By the end of embryonic division these 16 large cells will end up differentiating into the next generation of germ cells, while the small cells become somatic. Interestingly, germ-soma differentiation is controlled by cell size rather than by partitioning of specific cell fate determinants into either of the two cell types (Kirk et al., 1993). At the end of cleavage the embryo is inside out with respect to its final configuration with gonidial cells on the outside of the spheroid and flagella of somatic cells pointing inwards. Through the remarkable process of inversion the embryo turns itself right-side out into its adult configuration with gonidial precursor cells on the interior of the spheroid and the flagella of the somatic cells facing the exterior (Viamontes and Kirk, 1977; Viamontes et al., 1979; Kirk and Nishii, 2001; Hallmann, 2006a). Juvenile spheroids remain inside their mother where they continue to grow for another day or two before hatching out to repeat the vegetative growth cycle.
In addition to its vegetative asexual reproductive cycle Volvox carteri has a sexual development cycle (Fig. 5) that is under control of its mating locus (Fig. 6). While vegetative males and females of Volvox carteri are indistinguishable, the male and female sexual forms are different from each other and from vegetative Volvox. Sexual differentiation in Volvox is triggered by a glycoprotein sex-inducer produced by males that causes modified embryogenesis programs in males and females (Fig. 5) (Kochert, 1968; Tschochner et al., 1987; Mages et al., 1988). Females treated with sex inducer produce sexual offspring with 32–48 egg cells instead of vegetative gonidia, while males treated with sex inducer produce sexual offspring containing a 1:1 ratio of 128 somatic cells and 128 sperm packets, each containing 64–128 sperm. Sperm packets travel as a unit and upon encountering a sexual female, break apart and tunnel inside the ECM where they find and fertilize eggs to produce a diploid zygote spore. Upon germination the spore undergoes meiosis and produces 3 polar bodies plus one new viable haploid progeny spheroid. While not described in Kirk’s twelve steps (see below), innovations in the Volvox sexual cycle have been recently summarized (Ferris et al., 2010; Umen, 2011).
6b. Kirk’s twelve steps of multicellular evolution in the Volvocales
David Kirk proposed a twelve-step model of multicellular evolution in the Volvocales (Kirk, 1998; 1999; 1999; 2005) that posited the minimum changes or innovations required for a unicellular Chlamydomonas-like ancestor to evolve into a multicellular Volvox carteri-like spheroid (Fig. 2A). When broken into discrete steps it becomes clear that half of the innovations occur in the first transition to form the simplest colonial genus exemplified by species such as Gonium (Kirk, 1998; 1999; 1999; 2005; Herron and Michod, 2008; Herron et al., 2009; Nishii and Miller, 2010). This finding implies that the most difficult transition is evolving the minimal functional colonial form, a finding that is partly supported by the monophyly of colonial Volvocines as a whole (i.e., only one original instance of colonialism) compared to polyphyly of several genera within the Volvocine algae (i.e. more than one instance of a transition to a more complex form) (Fig. 1).
Evolving Gonium-like colonies from Chlamydomonas-like unicells requires a minimum of six changes: 1. incomplete cytokinesis to maintain linkage between daughter cells (i.e. cytoplasmic bridges), 2. partial inversion to reorient post-mitotic daughters within the new colony. 3. basal body rotation by ninety degrees in peripheral cells to align flagella, (Kirk, 1998; 2005). 4. establishment of organismal polarity as evidenced by the difference in basal body and flagellar orientations of central versus peripheral cells in the colony, 5. modification of the cell wall to keep daughters attached 6. genetic control of cell number. After the evolution of a colonial morphology typified by Gonium, the Volvocales show a significant increase is colony size/cell number. The spherical genera starting with Pandorina undergo full inversion corresponding to Kirk’s step 7. Eudorina is representative of step 8--expansion of the extracellular matrix (ECM). Pleodorina represents Kirk’s step 9--partial division of labor between germ and somatic cells. Finally, Volvox carteri represents Kirk’s steps 11 and 12–asymmetric cell divisions and a bifurcated cell division program. Notably, these last two steps are found in only one subgroup of Volvox species from the section Merrillosphaera. Other, species of Volvox develop in a different manner through progressive binary divisions that represent an alternative evolutionary solution to colony growth (Desnitski, 1995; Kirk, 1998; Coleman, 2012).
6c. Comparing the content of the Chlamydomonas and Volvox genomes
It has long been assumed that an extensive new genetic toolkit would be required for multicellular evolution (King, 2004; Ruiz-Trillo et al., 2007; Rokas, 2008a; 2008b). Previous genomic comparisons reinforced this idea (Putnam et al., 2007; Srivastava et al., 2010), though in no case had two closely related species with such divergent morphology, such as Chlamydomonas and Volvox, been compared (Prochnik et al., 2010). On a global level the genomes of these two algae are remarkably similar, including extensive regions of synteny (Ferris et al., 2010; Prochnik et al., 2010). The total protein coding gene count for Chlamydomonas is ~14,516 (Merchant et al., 2007) while that for the first version of the Volvox genome was ~14,520 genes (Prochnik et al., 2010). Protein domain content is also similar between the two species: Chlamydomonas has 2,354 PFAM domains while Volvox has 2,431 PFAM domains that are largely overlapping (Prochnik et al., 2010). Of the ~15,000 genes found in Chlamydomonas and Volvox, ~64% can be assigned into protein families that are shared with other eukaryotes (Prochnik et al., 2010). Of these, 80% show a 1:1 orthology between the two algae. In summary, with a few exceptions described below, large-scale comparisons of Chlamydomonas and Volvox reveal very few differences that can be tied to the substantial differences in biological organization that distinguish them.
The overall similarity between the two genomes is mirrored by results of forward genetics that identified key developmental regulators in Volvox. Mutants that affect somatic cell specification (regA) (Kirk et al., 1999), asymmetric cell division (glsA) (Miller and Kirk, 1999), and inversion (invA, invB, invC) (Nishii et al., 2003; Ueki and Nishii, 2008; 2009) have all been identified and cloned. Remarkably, four of the genes underlying these processes that are unique to Volvox have orthologs in Chlamydomonas (glsA, invA, invB, invC), and the fifth, regA, is part of a complex gene family that has members in both species. Moreover, two of the Volvox mutants, invA and glsA, can be complemented by their Chlamydomonas orthologs (Cheng et al., 2003; Nishii et al., 2003; Duncan et al., 2007; Ueki and Nishii, 2008; 2009). Thus, it appears that many ancestral functions carried out by proteins in Chlamydomonas could be modified or coopted to perform developmental functions in Volvox, suggesting that the genetic toolkit required for multicellularity may have been largely present in ancestral unicells (King, 2004; Prochnik et al., 2010).
26% (1,835) of the 15,000 genes in Chlamydomonas and Volvox are only found in these algae, and are thus Volvocine specific. This latter group of Volvocine algal protein families is of special interest since they show asymmetric expansion/contraction patterns between Chlamydomonas and Volvox. These Volvocine algal protein families tend to be larger in Volvox than in Chlamydomonas (Prochnik et al., 2010). Moreover, this asymmetric distribution of expansions/contractions does not extend to protein families that are shared with species outside Volvocine algae. This latter group of more ancient proteins shows similar degrees of loss/gain between the Volvox and Chlamydomonas lineages (Prochnik et al., 2010).
What might be responsible for the preferential expansion/retention of Volvocine algal specific protein families in Volvox? While answers to this question are speculative, it is striking that among these protein families are two whose members are components of the Volvox ECM (see below), which is itself a massive elaboration of the ancestral cell wall of its Chlamydomonas-like ancestor. Extrapolating further, one can speculate that the entire group of Volvocine-algal-specific proteins might be highly evolvable. By definition they have been around long enough to remain established in both algal lineages, but are still young enough to be amenable to evolutionary experimentation and elaboration. Unfortunately, the functions of most Volvocine-algal-specific proteins are unknown since they are restricted to this lineage, but they may be a rich source of evolutionary plasticity and novelty that merits further examination. Supporting this idea are recent findings indicating that the origins of many metazoan proteins associated with multicellular functions (e.g. cell adhesion) extend back to their last unicellular ancestor that resembled a choanoflagellate, and are also clade-specific proteins that are not found outside of holozoa (e.g. integrins, cadherins, tyrosine kinases) (King et al., 2008; Abedin and King, 2010; 2010).
6c.i Gene family expansions in Chlamydomonas
Notable gene family expansions in Volvox relative to Chlamydomonas are discussed below, but there are a few instances where Chlamydomonas has more gene family members than Volvox (Prochnik et al., 2010). Chlamydomonas has ~35 histone gene clusters while Volvox has less than half this number (Prochnik et al., 2010). The Chlamydomonas histone content is also exceptionally high compared with plants and other algae. Increased gene copy number is a possible means of increasing the rate of gene product synthesis which might be important for multiple fission where rapid successive divisions occur, but it is not clear why Chlamydomonas would need more histone clusters than Volvox since their cell division rates during mitosis are similar (Harper and John, 1986; Kirk, 1998). Proteins with ankyrin repeats were reported to be highly enriched in Chlamydomonas versus Volvox (~40 versus 12) but a reevaluation of protein domain content using more sensitive criteria in the new version of the Volvox genome (Goodstein et al., 2011) shows that Chlamydomonas has 146 ankyrin repeat proteins versus 80 in Volvox (J. Umen, unpublished observation). This is still a biased ratio, but no longer appears exceptional and may be a result of genetic drift.
6c.i. Cell cycle modifications - key steps in Kirk’s twelve steps
Four of Kirk’s twelve steps of multicellular evolution in the Volvocales are closely or directly related to cell cycle modifications: incomplete cytokinesis (1), genetic modulation of cell number (6), Asymmetric cell division (11), Bifurcated cell division program (12) (Kirk, 2005). In addition, sexual development in Volvox entails further cycle modifications that include altered timing of embryonic asymmetric cell division in males and females, and a set of post-embryonic germ cell divisions to produce sperm packets in males (Ferris et al., 2010; Umen, 2011). As described above Volvox utilizes a Chlamydomonas-like multiple fission cell cycle for embryonic and post-embryonic divisions. Unlike the case in Chlamydomonas where division number is flexible, the number of divisions in Volvox and other colonial Volvocine algae is more stereotyped so that cell number per colony stays relatively constant and is always a power of 2 (e.g. 4, 8, 16, 32 …). The triggering of asymmetric cell division at a specific embryonic cell cycle number and the subsequent bifurcated division program for germ cell and somatic cell precursors in Volvox is not understood, but may be coupled in some manner to cell size, as is the final process of germ-soma differentiation (Kirk et al., 1993; Kirk, 2005).
Forward genetics in Chlamydomonas identified the retinoblastoma (RB) tumor suppressor pathway as a central regulator of size control during multiple fission (Umen and Goodenough, 2001; Fang et al., 2006). It might be expected that this pathway is modified or elaborated in Volvox and perhaps other Volvocine species to accommodate alterations in colony size and/or developmental timing. While the cell division cycle protein coding genes in Volvox are overall very similar to those in Chlamydomonas, there are two intriguing differences uncovered by genome sequencing that suggest possible modifications in Volvox. First, an expansion of the D1 cyclin subfamily was found in Volvox that has single orthologs of the other Chlamydomonas cyclins (D2, D3, D4) but four cyclin D1 paralogs (Prochnik et al., 2010). D type cyclins are the activator subunits of cyclin dependent kinases that have retinoblastoma tumor suppressor related proteins (RBRs) as their substrates and key targets for controlling cell cycle progression. The elaboration of D1 cyclins in Volvox may enable more fine-tuned developmental control over multiple fission than in Chlamydomonas, an idea that merits further investigation.
A second change in the Volvox RB pathway compared with Chlamydomonas is the incorporation of the RBR homolog MAT3 into the Volvox mating locus and subsequent divergence of the male and female alleles (vcMAT3f and vcMAT3m) (Fig. 6) (Ferris et al., 2010). The large divergence between MAT3f and MAT3m as well as their sex-regulated alternative splicing patterns suggests that this protein has acquired a role in controlling developmental patterning differences that distinguish male and female sexual forms of Volvox.
6c.ii. Asymmetric cell division - Kirk’s 11th step
Chlamydomonas does not divide asymmetrically, so it might be expected that Volvox would have evolved one or more proteins that facilitate this function, perhaps through modifications to its cytoskeletal protein repertoire. On the contrary, no major differences in cytoskeletal proteins were detected between the two species (Prochnik et al., 2010). Moreover, the single identified mutant that affects asymmetric division in Volvox, glsA, has a Chlamydomonas ortholog that complements its phenotype (Miller and Kirk, 1999; Cheng et al., 2003). glsA encodes a DnaJ-domain protein that interacts with heat shock protein HSP70 to promote asymmetric cell division in an unknown manner (Cheng et al., 2005; 2006). The investigation of GAR1, the Chlamydomonas glsA ortholog, may shed light on the ancestral role of this protein in Chlamydomonas cell division.
6c.iii. Inversion - Kirk’s 7th step
Like the case for asymmetric cell division, there is no obvious correlate of inversion in Chlamydomonas and no candidate cytoskeletal genes that emerged from genomic comparisons as candidate mediators of this process. Forward genetic screens have identified three genes required for Volvox inversion– invA, invB and invC--that encode a kinesin, a sugar transporter, and a glycosyl transferase respectively (Nishii et al., 2003; Ueki and Nishii, 2008; 2009). InvA is thought to interact with the microtubule cytoskeleton at cytoplasmic bridge attachment sites to effect cell-shape changes required for inversion (Nishii et al., 2003). InvB and InvC are required for expansion of the vesicle that surrounds the inverting embryo and which physically restricts inversion in the invB and invC mutants. Each of the above proteins has a highly similar ortholog in Chlamydomonas indicating that the pathways they control have cognate processes in Chlamydomonas from which they were coopted. The invB and invC functions might have ancestral roles in cell wall expansion that must occur in Chlamydomonas to accommodate cell growth. The potential role of the Chlamydomonas invA ortholog, IAR1, is harder to imagine but suggests the existence of cytoskeletal processes that have not yet been characterized.
6c.iv. Evolution of an elaborated ECM
Cell-cell adhesion is a critical aspect of multicellularity (Bonner, 2000; Abedin and King, 2010). In the Volvocales adhesion is accomplished by ECM proteins that are related to cell wall proteins from Chlamydomonas (Kirk et al., 1986; Adair et al., 1987; Hallmann, 2006b). Indeed, the importance of cell-cell adhesion and ECM modifications in colonial Volvocine algae is reflected in two of Kirk’s twelve steps of multicellular evolution that are changes to the ECM (Kirk, 2005). Step 5 is modification of the cell wall to maintain adhesion between post-mitotic daughters, while step 8 is expansion of the ECM that allows for spheroid growth in the absence of cell growth and division.
The Chlamydomonas cell wall is composed of three major layers of hydroxyproline rich glycoproteins that can be divided into seven sub-layers (Goodenough and Heuser, 1985). The outermost of these layers can be used to nucleate the assembly of Volvox cell walls demonstrating the evolutionary conservation of Volvocine cell wall structural components (Adair et al., 1987; Adair, 1988). The expanded ECM in Volvox is organized and compartmentalized as revealed by various structural and localization studies (Fig. 7) (Kirk et al., 1986; Ertl et al., 1989; Godl et al., 1995; Hallmann and Kirk, 2000; Hallmann, 2006b).
Paralleling the expansion of its ECM, the Volvox genome codes a much larger number of ECM-related proteins than does Chlamydomonas. Two families of ECM proteins whose members are known constituents of Volvox ECM underwent notable expansion in the Volvox lineage. These are a family of hydroxyproline-rich glycoproteins called pherophorins and a family of metalloproteases called VMPs (Sumper and Hallmann, 1997; Hallmann, 2002; 2007; Prochnik et al., 2010). Chlamydomonas has 27 predicted pheophorins versus 45 in Volvox, and 8 VMPs versus 42 in Volvox.
ECM proteins in Volvox also play a role in the sexual cycle. The Volvox sex inducer is a pherophorin-related protein (Mages et al., 1988), and expression of several pherophorin genes is up-regulated during sexual differentiation (Hallmann, 2006b; Prochnik et al., 2010). Likewise, three related Volvox ECM metalloproteinases are also sexually regulated (Hallmann, 2006b; Prochnik et al., 2010). The remarkable organizational properties of the Volvox ECM and the potential roles of ECM proteins in signaling and nutrient storage/transport are all exciting topics for future investigation.
6c.v. Terminal differentiation of somatic cells and the origins of VARL Genes
Somatic cell fate in Volvox is controlled by regA, whose protein product is a nuclear localized putative transcriptional repressor (Tam et al., 1991; Kirk et al., 1999; Meissner et al., 1999; Duncan et al., 2006; 2007). RegA belongs to a family of proteins in Volvocine algae called VARL (Volvocine Algae RegA Like) that all share a putative DNA binding domain related to the SAND domain found in some transcriptional repressors (Duncan et al., 2006; 2007). Unlike the previously described glsA, invA, invB and invC genes, Volvox regA has no clear ortholog in Chlamydomonas. Instead, both algae have multiple VARL paralogs that arose from a complicated pattern of duplications and/or losses in each of the two species (Duncan et al., 2006; 2007). regA is part of a four gene paralogous duplication that is unique to Volvox. Interestingly, it is the only one of the four paralogs that has been identified as a developmental mutant (Kirk et al., 1999). In Chlamydomonas the closest VARL protein to Volvox regA is called RLS1 whose expression has been linked to stress and environmental responses (Nedelcu and Michod, 2006; Nedelcu, 2009), but whose function is not known. These data again point to the unicellular origins of proteins associated with multicellular development in the Volvocine algae.
6c.vi Chlamydomonas and Volvox microRNAs are not homologous
Both Chlamydomonas and Volvox have RNAi machinery (Cerutti et al., 2011) and it might be expected that they contain homologous microRNA genes as do many higher plants and animals (Berezikov, 2011; Cuperus et al., 2011). On the contrary, when Chlamydomonas microRNAs were compared to those predicted from Volvox, there was very little overlap (Zhao et al., 2007), though there are caveats to this conclusion. The comparison was based on empirical data for small RNAs and predicted microRNAs in Chlamydomonas from studies that captured only a fraction of possible stages in the life cycle. An empirical evaluation of Volvox miRNAs and small RNA targets will be required to know if any underlying homology exists in the small RNA networks of the two algae. Equally important will be an improved understanding of how miRNAs and other small regulatory RNAs contribute to the developmental programs of Volvocine algae.
6c.vi. The Volvox mating locus and evolution of sexual dimorphism
As discussed above, the sexual cycles of Chlamydomonas and Volvox are under the control of a mating locus (MT) whose location is similar in the two species, but whose size, content, and evolutionary dynamics are very different (Ferris et al., 2010; Umen, 2011). The structure of the MT region from Volvox and Chlamydomonas is described in Section 3. Besides its relative expansion and loss of residual synteny between shared genes (those with an allele in both mating types), the divergence rates of Volvox mating locus genes are up to two orders of magnitude higher than those in Chlamydomonas (Ferris et al., 2010). Whereas shared genes in Chlamydomonas MT retain high nucleotide identity (typically 99% in coding regions, and >90% in introns), shared genes are in Volvox have coding region identities as low as 40–50% and typically have no similarity in their intron sequences. This large divergence is the result of completely blocked recombination that can be traced back through speciation events in sister species of Volvox (Ferris et al., 2010). Paradoxically, these differences with Chlamydomonas MT makes Volvox MT look older even though it is part of a presumably younger and more recently-derived lineage (Charlesworth and Charlesworth, 2010; Umen, 2011). One hypothesis that needs testing is whether cryptic recombination might occur in Chlamydomonas MT that acts to maintain homogeneity between shared genes in the two MT haplotypes (Umen, 2011). The effect of long-term isolation of female and male alleles of Volvox mating locus genes is divergence in both primary sequence and expression patterns, with a remarkable number of genes having acquired sex-regulated expression including female or male biases, and up- or down-regulation during sexual differentiation (Ferris et al., 2010). Thus, even shared genes in the Volvox mating locus have diverged enough to acquire male or female specific functions.
Another remarkable feature of Volvox MT is the presence of numerous male-limited and female-limited genes that do not have an allelic counterpart in the opposite mating type (Fig. 6). Of fifteen such genes (five female-specific, ten male-specific) only two, VcMID and VcMTD, have any similarity to Chlamydomonas genes, and only one, encoding an HMG-box protein, shows similarity to genes in any other species (Ferris et al., 2010). The number of “new” genes in the Volvox mating locus stands in contrast to the paucity of Volvox-specific autosomal genes (Prochnik et al., 2010) (and see below). This finding implies that the Volvox mating locus has evolved into a hotbed of evolutionary experimentation (Ferris et al., 2010), an idea that might be confirmed by obtaining sequences from mating loci of related Volvocine species and additional wild isolates of Volvox carteri..
VcMID and VcMTD are in male MT and have homologs CrMId and CrMTD in the Chlamydomonas MT− locus. MId and MTD homologs have been found in MT− strains of Gonium pectorale (Hamaji et al., 2008; 2008), and MId has also been found in males of Pleodorina starrii (Nozaki et al., 2006). Mid proteins are related to RWP-RK family putative transcription factors, while MTD proteins are Volvocine-algal-specific. In Chlamydomonas MID is a key sex determining gene whose presence or absence dictates differentiation as minus or plus gametes (Ferris and Goodenough, 1997). MTD appears to play an auxiliary role in augmenting the expression of MID (Lin and Goodenough, 2007). Functional studies of MID have not been reported outside of Chlamydomonas, but two observations suggest that sexual differentiation in Volvox will involve additional factors besides VcMId and VcMTD. First, VcMId mRNA is expressed constitutively in both vegetative and sexually induced males, and is not controlled by sex inducer or by nitrogen starvation as is the case for Mid orthologs in Chlamydomonas, Gonium and Pleodorina (Ferris and Goodenough, 1997; Nozaki et al., 2006; Lin and Goodenough, 2007; Hamaji et al., 2008). Second, VcMTD appears to be a pseudogene in Volvox with no start codon anywhere near the 5′ end of its mRNA (Ferris et al., 2010). These observations suggest that other genes in MT or elsewhere contribute to sexual differentiation in Volvox.
7. Future Perspectives
Genomics research with Volvocine algae is still far from reaching its potential. While the unexpectedly similar genome sequences of Chlamydomonas and Volvox have not led to a simple list of genes that underlie each major trait difference that distinguishes them, they have provided a framework for more in-depth studies. Genomics has also refocused investigations into the origin of complexity towards the unicellular species Chlamydomonas that is remarkably sophisticated and appears to possess most of the genetic toolkit that was required for Volvocine algae to become multicellular. Critical for understanding how Volvocine algae and their genomes evolve will be sequencing additional species. Such sequencing will allow important genomic attributes such as gene gains and losses to be polarized in order to determine the direction of change within the group. Given the complex evolutionary history within Volvocine algae (Fig. 2) additional genomes will provide insights into how convergent traits evolved in different subgroups. As genomes from closely related Volvocine algal subgroups are compared the key genetic changes that distinguish them may come into focus. The decreasing cost of sequencing will soon make it possible to do population genomics on Volvocine algae that will begin to shed light on how population structure and natural variation relate to long-term evolutionary change and speciation. Low cost genome sequencing is an enabling technology for large-scale forward genetics and functional genomics that are of increasing importance for Chlamydomonas and will soon be so for Volvox and other species. Sequencing is also revolutionizing the ability to rapidly create new model systems from previously unstudied species. Efforts to develop additional Volvocine algal models such as Gonium pectorale (whose genome is currently being sequenced) will greatly expand the utility of Volvocine algae as a premier model for evolution and development.
Contributor Information
James G. Umen, Donald Danforth Plant Science Center, 975 North Warson Rd., St. Louis, MO 63132 USA
Bradley J.S.C. Olson, Molecular Cellular and Developmental Biology, Ecological Genomics Institute, Division of Biology, Kansas State University, Manhattan, KS 66506 USA
References
- Abedin M, King N. Diverse evolutionary paths to cell adhesion. Trends Cell Biol. 2010;20:734–742. doi: 10.1016/j.tcb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair W. Organization and in vitro assembly of the Chlamydomonas reinhardtii cell wall. Self-assembling architecture 1988 [Google Scholar]
- Adair WS, Steinmetz SA, Mattson DM, Goodenough UW, Heuser JE. Nucleated assembly of Chlamydomonas and Volvox cell walls. J Cell Biol. 1987;105:2373–2382. doi: 10.1083/jcb.105.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CR, Stamer KA, KMJ, McNally JG, Kirk MM, Kirk DL. Patterns of organellar and nuclear inheritance among progeny of two geographically isolated strains of Volvox carteri. Current Genetics. 1990;18:141–153. doi: 10.1007/BF00312602. [DOI] [PubMed] [Google Scholar]
- Adams S, Maple J, Møller SG. Functional conservation of the MIN plastid division homologues of Chlamydomonas reinhardtii. Planta. 2008;227:1199–1211. doi: 10.1007/s00425-008-0692-6. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Hagiwara Y, Misumi O, Kuroiwa T, Nakamura S. Complete elimination of maternal mitochondrial DNA during meiosis resulting in the paternal inheritance of the mitochondrial genome in Chlamydomonas species. Protoplasma. 2006;228:231–242. doi: 10.1007/s00709-006-0155-5. [DOI] [PubMed] [Google Scholar]
- Armbrust EV, Ibrahim A, Goodenough UW. A mating type-linked mutation that disrupts the uniparental inheritance of chloroplast DNA also disrupts cell-size control in Chlamydomonas. Mol Biol Cell. 1995;6:1807–1818. doi: 10.1091/mbc.6.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Baldauf S. The deep roots of eukaryotes. Science. 2003;300:1703. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- Bell G. The Origin and evolution of sex. In: Halvorson HO, Monroy A, editors. The Origin and Evolution of Sex. Alan R. Liss Inc; 1985. pp. 221–256. [Google Scholar]
- Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- Berthold P, Schmitt R, Mages W. An engineered Streptomyces hygroscopicus aph 7″ gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii. Protist. 2002;153:401–412. doi: 10.1078/14344610260450136. [DOI] [PubMed] [Google Scholar]
- Bisova K, Krylov DM, Umen JG. Genome-wide annotation and expression profiling of cell cycle regulatory genes in Chlamydomonas reinhardtii. 2005;137:475–491. doi: 10.1104/pp.104.054155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer PH, Bonen L, Lee RW, Gray MW. Genes for respiratory chain proteins and ribosomal RNAs are present on a 16-kilobase-pair DNA species from Chlamydomonas reinhardtii mitochondria. Proc Natl Acad Sci USA. 1985;82:3340–3344. doi: 10.1073/pnas.82.10.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer PH, Gray MW. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988:55. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed]
- Bonner J. First Signals, The Evolution of Multicellular Development. Princeton University Press; Princeton, NJ: 2000. [Google Scholar]
- Boynton J, Gillham N, Harris E, Hosler J, Johnson A, Jones A, Randolph-Anderson B, Robertson D, Klein T, Shark K, et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Boynton JE, Gillham NW. Genetics and transformation of mitochondria in the green alga Chlamydomonas. Methods in enzymology. 1996;264:279–296. doi: 10.1016/s0076-6879(96)64027-0. [DOI] [PubMed] [Google Scholar]
- Buchheim MA, Buchheim JA, Chapman RL. Phylogenyof The VLE-14 Chlamyomonas (Chlorophyceae) Group: A Study Of 18S rRNA Gene Sequences. Journal of Phycology. 1997;33:1024–1030. [Google Scholar]
- Bull J. Sex Chromosomes in Haploid Dioecy: A Unique Contrast To Muller’s Theory For Diploid Dioecy. Am Nat. 1978;112:245–250. [Google Scholar]
- Cardullo RA, Herrick SB, Peterson MJ, Dangott LJ. Speract receptors are localized on sea urchin sperm flagella using a fluorescent peptide analog. Dev Biol. 1994;162:600–607. doi: 10.1006/dbio.1994.1113. [DOI] [PubMed] [Google Scholar]
- Casas-Mollano JA, Rohr J, Kim EJ, Balassa E, van Dijk K, Cerutti H. Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing. Genetics. 2008;179:69–81. doi: 10.1534/genetics.107.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castruita M, Casero D, Karpowicz SJ, Kropat J, Vieler A, Hsieh SI, Yan W, Cokus S, Loo JA, Benning C, Pellegrini M, Merchant SS. Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. THE PLANT CELL ONLINE. 2011;23:1273–1292. doi: 10.1105/tpc.111.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti H, Johnson AM, Gillham NW, Boynton JE. Epigenetic silencing of a foreign gene in nuclear transformants of Chlamydomonas. Plant Cell. 1997;9:925–945. doi: 10.1105/tpc.9.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti H, Ma X, Msanne J, Repas T. RNA-mediated silencing in Algae: biological roles and tools for analysis of gene function. Eukaryotic Cell. 2011;10:1164–1172. doi: 10.1128/EC.05106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Evolutionary biology: the origins of two sexes. Curr Biol. 2010;20:R519–21. doi: 10.1016/j.cub.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chen CJ, Vallon O, Huang ZP, Zhou H, Qu LH. Genomewide analysis of box C/D and box H/ACA snoRNAs in Chlamydomonas reinhardtii reveals an extensive organization into intronic gene clusters. Genetics. 2008;179:21–30. doi: 10.1534/genetics.107.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Fowler R, Tam LW, Edwards L, Miller SM. The role of GlsA in the evolution of asymmetric cell division in the green alga Volvox carteri. Dev Genes Evol. 2003;213:328–335. doi: 10.1007/s00427-003-0332-x. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Hallmann A, Edwards L, Miller SM. Characterization of a heat-shock-inducible hsp70 gene of the green alga Volvox carteri. Gene. 2006;371:112–120. doi: 10.1016/j.gene.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Pappas V, Hallmann A, Miller SM. Hsp70A and GlsA interact as partner chaperones to regulate asymmetric division in Volvox. Dev Biol. 2005;286:537–548. doi: 10.1016/j.ydbio.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Chiang KS, Sueoka N. Replication of chloroplast DNA in Chlamydomonas reinhardi during vegetative cell cycle: its mode and regulation. Proc Natl Acad Sci USA. 1967;57:1506. doi: 10.1073/pnas.57.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman A. A Comparative Analysis Of The Volvocaceae (Chlorophyta) Journal of Phycology. 2012 doi: 10.1111/j.1529-8817.2012.01168.x. [DOI] [PubMed] [Google Scholar]
- Coleman AW. Phylogenetic analysis of “Volvocacae” for comparative genetic studies. Proc Natl Acad Sci USA. 1999;96:13892–13897. doi: 10.1073/pnas.96.24.13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corellou F. Atypical Regulation of a Green Lineage-Specific B-Type Cyclin-Dependent Kinase. Plant Physiol. 2005;138:1627–1636. doi: 10.1104/pp.105.059626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JAV, de Morais MG. The role of biochemical engineering in the production of biofuels from microalgae. Bioresource Technology. 2011;102:2–9. doi: 10.1016/j.biortech.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Croft MT, Warren MJ, Smith AG. Algae Need Their Vitamins. Eukaryotic Cell. 2006;5:1175–1183. doi: 10.1128/EC.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus JT, Fahlgren N, Carrington JC. Evolution and Functional Diversification of MIRNA Genes. THE PLANT CELL ONLINE. 2011;23:431–442. doi: 10.1105/tpc.110.082784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuchy R, Purton S, Rochaix JD. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. The EMBO Journal. 1989;8:2803. doi: 10.1002/j.1460-2075.1989.tb08426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca SZ, O’Farrell PH. Barriers to male transmission of mitochondrial DNA in sperm development. Dev Cell. 2012;22:660–668. doi: 10.1016/j.devcel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright EM, Sankoff D, Spencer DF, Lee RW. Evolution of fragmented mitochondrial ribosomal RNA genes in Chlamydomonas. J Mol Evol. 1996;42:382–391. doi: 10.1007/BF02498632. [DOI] [PubMed] [Google Scholar]
- Dent RM, Haglund CM, Chin BL, Kobayashi MC, Niyogi KK. Functional genomics of eukaryotic photosynthesis using insertional mutagenesis of Chlamydomonas reinhardtii. Plant Physiol. 2005;137:545–556. doi: 10.1104/pp.104.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derelle E, Ferraz C, Rombauts S, Rouze P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynié S, Cooke R. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA. 2006;103:11647–11652. doi: 10.1073/pnas.0604795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnitski A. A Review On The Evolution Of Development In Volvox-Morphological And Physiological Aspects. European journal of protistology. 1995;31:241–247. [Google Scholar]
- Diener D. Methods in Cell Biology, Methods in Cell Biology0091679X. Elsevier; 2009. [Google Scholar]
- Dunahay TG, Adler SA, Jarvik JW. Transformation of microalgae using silicon carbide whiskers. Methods Mol Biol. 1997;62:503–509. doi: 10.1385/0-89603-480-1:503. [DOI] [PubMed] [Google Scholar]
- Duncan L, Nishii I, Harryman A, Buckley S, Howard A, Friedman NR, Miller SM. The VARL Gene Family and the Evolutionary Origins of the Master Cell-Type Regulatory Gene, regA, in Volvox carteri. J Mol Evol. 2007;65:1–11. doi: 10.1007/s00239-006-0225-5. [DOI] [PubMed] [Google Scholar]
- Duncan L, Nishii I, Howard A, Kirk D, Miller SM. Orthologs and paralogs of regA, a master cell-type regulatory gene in Volvox carteri. Current Genetics. 2006;50:61–72. doi: 10.1007/s00294-006-0071-4. [DOI] [PubMed] [Google Scholar]
- Dutcher SK. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic. 2003;4:443–451. doi: 10.1034/j.1600-0854.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- Dutcher SK, Li L, Lin H, Meyer L, Giddings TH, Jr, Kwan AL, Lewis BL. Whole-Genome Sequencing to Identify Mutants and Polymorphisms in Chlamydomonas reinhardtii. G3. 2012;2:15–22. doi: 10.1534/g3.111.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard S, Jain M, Im CS, Pollock S, Shrager J, Lin Y, Peek AS, Grossman AR. Generation of an oligonucleotide array for analysis of gene expression in Chlamydomonas reinhardtii. Current Genetics. 2006;49:106–124. doi: 10.1007/s00294-005-0041-2. [DOI] [PubMed] [Google Scholar]
- Ebnet E. Volvoxrhodopsin, a Light-Regulated Sensory Photoreceptor of the Spheroidal Green Alga Volvox carteri. Plant Cell. 1999;11:1473–1484. doi: 10.1105/tpc.11.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler-Stahlberg A, Weisheit W, Ruecker O, Heitzer M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta. 2009;229:873–883. doi: 10.1007/s00425-008-0879-x. [DOI] [PubMed] [Google Scholar]
- Ender F, Godl K, Wenzl S, Sumper M. Evidence for autocatalytic cross-linking of hydroxyproline-rich glycoproteins during extracellular matrix assembly in Volvox. Plant Cell. 2002;14:1147–1160. doi: 10.1105/tpc.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H, Mengele R, Wenzl S, Engel J, Sumper M. The extracellular matrix of Volvox carteri: molecular structure of the cellular compartment. J Cell Biol. 1989;109:3493–3501. doi: 10.1083/jcb.109.6.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SC, de los Reyes C, Umen JG. Cell size checkpoint control by the retinoblastoma tumor suppressor pathway. PLoS Genet. 2006;2:e167. doi: 10.1371/journal.pgen.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante P, Catalanotti C, Bonente G, Giuliano G. An optimized, chemically regulated gene expression system for Chlamydomonas. PLoS ONE. 2008;3:e3200. doi: 10.1371/journal.pone.0003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris P, Goodenough U. Mating Type in Chlamydomonas Is Specified by mid, the Minus-Dominance Gene. Genetics. 1997;146:859–869. doi: 10.1093/genetics/146.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ. Localization of the nic-7, ac-29 and thi-10 genes within the mating-type locus of Chlamydomonas reinhardtii. Genetics. 1995;141:543–549. doi: 10.1093/genetics/141.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ, Armbrust EV, Goodenough UW. Genetic structure of the mating-type locus of Chlamydomonas reinhardtii. Genetics. 2002;160:181–200. doi: 10.1093/genetics/160.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris PJ, Olson B, de Hoff PL, Douglass S, Casero D, Prochnik SE, Geng S, Rai R, Grimwood J, Schmutz J, Nishii I, Hamaji T, Nozaki H, Pellegrini M, Umen JG. Evolution of an expanded sex-determining locus in Volvox. Science. 2010;328:351–354. doi: 10.1126/science.1186222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Rochaix JD. The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol Gen Genomics. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- Franzén LG, Rochaix JD, von Heijne G. Chloroplast transit peptides from the green alga Chlamydomonas reinhardtii share features with both mitochondrial and higher plant chloroplast presequences. FEBS Lett. 1990;260:165–168. doi: 10.1016/0014-5793(90)80094-y. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Hausherr A, Ferbitz L, Schödl T, Heitzer M, Hegemann P. Monitoring dynamic expression of nuclear genes in Chlamydomonas reinhardtii by using a synthetic luciferase reporter gene. Plant Mol Biol. 2004;55:869–881. doi: 10.1007/s11103-004-2150-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Oertel W, Hegemann P. A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J. 1999;19:353–361. doi: 10.1046/j.1365-313x.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- Galván A, González-Ballester D, Fernández E. Insertional mutagenesis as a tool to study genes/functions in Chlamydomonas. Adv Exp Med Biol. 2007;616:77–89. doi: 10.1007/978-0-387-75532-8_7. [DOI] [PubMed] [Google Scholar]
- Godl K, Hallmann A, Rappel A, Sumper M. Pherophorins: a family of extracellular matrix glycoproteins from Volvox structurally related to the sex-inducing pheromone. Planta. 1995:196. [PubMed] [Google Scholar]
- Gonzalez-Ballester D. Functional Genomics of the Regulation of the Nitrate Assimilation Pathway in Chlamydomonas. Plant Physiol. 2005;137:522–533. doi: 10.1104/pp.104.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ballester D, Pootakham W, Mus F, Yang W, Catalanotti C, Magneschi L, de Montaigu A, Higuera JJ, Prior M, Galván A, Fernández E, Grossman AR. Reverse genetics in Chlamydomonas: a platform for isolating insertional mutants. Plant Methods. 2011;7:24. doi: 10.1186/1746-4811-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U, Lin H, Lee JH. Sex determination in Chlamydomonas. Semin Cell Dev Biol. 2007;18:350–361. doi: 10.1016/j.semcdb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Goodenough UW, Armbrust EV, Campbell AM, Ferris PJ. Molecular Genetics of Sexuality in Chlamydomonas. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:21–44. [Google Scholar]
- Goodenough UW, Heuser JE. The Chlamydomonas cell wall and its constituent glycoproteins analyzed by the quick-freeze, deep-etch technique. J Cell Biol. 1985;101:1550–1568. doi: 10.1083/jcb.101.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2011;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafi G, Burnett RJ, Helentjaris T, Larkins BA, DeCaprio JA, Sellers WR, Kaelin WG. A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication. n.d doi: 10.1073/pnas.93.17.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LE, Graham J, Graham JM, Wilcox LW. Algae. Benjamin-Cummings Pub Co; 2009. [Google Scholar]
- Grant D, Chiang KS. Physical mapping and characterization of Chlamydomonas mitochondrial DNA molecules: Their unique ends, sequence homogeneity, and conservation. Plasmid. 1980;4:82–96. doi: 10.1016/0147-619x(80)90085-2. [DOI] [PubMed] [Google Scholar]
- Gray MW, Boer PH. Organization and expression of algal (Chlamydomonas reinhardtii) mitochondrial DNA. Philos Trans R Soc Lond, B, Biol Sci. 1988;319:135–147. doi: 10.1098/rstb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Grossman A, Harris E, Hauser C, Lefebvre P, Martinez D, Rokhsar D, Shrager J, Silflow C, Stern D, Vallon O. Chlamydomonas reinhardtii at the Crossroads of Genomics. Eukaryotic Cell. 2003;2:1137. doi: 10.1128/EC.2.6.1137-1150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AR, Croft M, Gladyshev VN, Merchant SS, Posewitz MC, Prochnik S, Spalding MH. Novel metabolism in Chlamydomonas through the lens of genomics. Curr Opin Plant Biol. 2007;10:190–198. doi: 10.1016/j.pbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Gruber H, Kirzinger SH, Schmitt R. Expression of the Volvox gene encoding nitrate reductase: mutation-dependent activation of cryptic splice sites and intron-enhanced gene expression from a cDNA. Plant Mol Biol. 1996;31:1–12. doi: 10.1007/BF00020601. [DOI] [PubMed] [Google Scholar]
- Hallmann A. Extracellular matrix and sex-inducing pheromone in Volvox. Int Rev Cytol. 2002;227:131–182. doi: 10.1016/s0074-7696(03)01009-x. [DOI] [PubMed] [Google Scholar]
- Hallmann A. Morphogenesis in the family Volvocaceae: different tactics for turning an embryo right-side out. Protist. 2006a;157:445–461. doi: 10.1016/j.protis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Hallmann A. The pherophorins: common, versatile building blocks in the evolution of extracellular matrix architecture in Volvocales. Plant J. 2006b;45:292–307. doi: 10.1111/j.1365-313X.2005.02627.x. [DOI] [PubMed] [Google Scholar]
- Hallmann A. A small cysteine-rich extracellular protein, VCRP, is inducible by the sex-inducer of Volvox carteri and by wounding. Planta. 2007;226:719–727. doi: 10.1007/s00425-007-0519-x. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Godl K, Wenzl S, Sumper M. The highly efficient sex-inducing pheromone system of Volvox. Trends Microbiol. 1998;6:185–189. doi: 10.1016/s0966-842x(98)01234-7. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Kirk DL. The developmentally regulated ECM glycoprotein ISG plays an essential role in organizing the ECM and orienting the cells of Volvox. J Cell Sci. 2000;113(Pt 24):4605–4617. doi: 10.1242/jcs.113.24.4605. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Rappel A. Genetic engineering of the multicellular green alga Volvox: a modified and multiplied bacterial antibiotic resistance gene as a dominant selectable marker. Plant J. 1999;17:99–109. doi: 10.1046/j.1365-313x.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Sumper M. Reporter genes and highly regulated promoters as tools for transformation experiments in Volvox carteri. Proc Natl Acad Sci USA. 1994;91:11562–11566. doi: 10.1073/pnas.91.24.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaji T, Ferris PJ, Coleman AW, Waffenschmidt S, Takahashi F, Nishii I, Nozaki H. Identification of the minus-dominance gene ortholog in the mating-type locus of Gonium pectorale. Genetics. 2008;178:283–294. doi: 10.1534/genetics.107.078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, John P. Coordination of division events in the Chlamydomonas cell cycle. Protoplasma. 1986:131. [Google Scholar]
- Harris EH. Chlamydomonas As A Model Organism. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:363–406. doi: 10.1146/annurev.arplant.52.1.363. [DOI] [PubMed] [Google Scholar]
- Harris EH. Introduction to Chlamydomonas, The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers; Dordrecht: 2004. [Google Scholar]
- Hayashi Y, Shinozaki A. Visualization of microbodies in Chlamydomonas reinhardtii. J Plant Res. 2011 doi: 10.1007/s10265-011-0469-z. [DOI] [PubMed] [Google Scholar]
- Helliwell KE, Wheeler GL, Leptos KC, Goldstein RE, Smith AG. Insights into the Evolution of Vitamin B12 Auxotrophy from Sequenced Algal Genomes. Mol Biol Evol. 2011;28:2921–2933. doi: 10.1093/molbev/msr124. [DOI] [PubMed] [Google Scholar]
- Herron MD, Hackett JD, Aylward FO, Michod RE. Triassic origin and early radiation of multicellular volvocine algae. Proceedings of the National Academy of Sciences. 2009;106:3254–3258. doi: 10.1073/pnas.0811205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron MD, Michod RE. Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye. Evolution. 2008;62:436–451. doi: 10.1111/j.1558-5646.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoham RW, Bonome TA, Martin CW, Leebens Mack JH. A Combined 18S rDNA and rbcL Phylogenetic Analysis Of Chloromonas And Chlamydomonas (Chlorophyceae, Volvocales) Emphasizing Snow And Other Cold-Temperature Habitats. Journal of Phycology. 2002;38:1051–1064. [Google Scholar]
- Hohenester E, Sasaki T, Timpl R. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat Struct Biol. 1999;6:228–232. doi: 10.1038/6669. [DOI] [PubMed] [Google Scholar]
- Hoops H, Nishii I, Kirk D. Cytoplasmic Bridges in Volvox and Its Relatives. In: Baluška F, Volkmann D, Barlow P, editors. Cell-Cell Channels. Landes Bioscience; 2006. [Google Scholar]
- Hu Z, Zhao Z, Wu Z, Fan Z, Chen J, Wu J, Li J. Successful expression of heterologous egfp gene in the mitochondria of a photosynthetic eukaryote Chlamydomonas reinhardtii. Mitochondrion. 2011 doi: 10.1016/j.mito.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Huang CH, Liu PZ. New Insights into the Rh Superfamily of Genes and Proteins in Erythroid Cells and Nonerythroid Tissues. Blood Cells, Molecules, and Diseases. 2001;27:90–101. doi: 10.1006/bcmd.2000.0355. [DOI] [PubMed] [Google Scholar]
- Huskey R, Griffin B, Cecil P, Callahan A. A Preliminary Genetic Investigation of Volvox Carteri. Genetics. 1979;91:229–244. doi: 10.1093/genetics/91.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K. Sexual pheromone induces diffusion of the pheromone-homologous polypeptide in the extracellular matrix of Volvox carteri. Eukaryotic Cell. 2007;6:2157–2162. doi: 10.1128/EC.00304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobiak T, Mages W, Scharf B, Babinger P, Stark K, Schmitt R. The bacterial paromomycin resistance gene, aphH, as a dominant selectable marker in Volvox carteri. Protist. 2004;155:381–393. doi: 10.1078/1434461042650343. [DOI] [PubMed] [Google Scholar]
- Jamers A, Blust R, De Coen W. Omics in algae: paving the way for a systems biological understanding of algal stress phenomena? Aquat Toxicol. 2009;92:114–121. doi: 10.1016/j.aquatox.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Johnson UG, Porter KR. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol. 1968;38:403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanon M, McFadden GI. The chloroplast protein translocation complexes of Chlamydomonas reinhardtii: a bioinformatic comparison of Toc and Tic components in plants, green algae and red algae. Genetics. 2008;179:95–112. doi: 10.1534/genetics.107.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz SJ, Prochnik SE, Grossman AR, Merchant SS. The GreenCut2 Resource, a Phylogenomically Derived Inventory of Proteins Specific to the Plant Lineage. J Biol Chem. 2011;286:21427–21439. doi: 10.1074/jbc.M111.233734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P, LaVoie M, Brazelton WJ, Haas NA, Lefebvre PA, Silflow CD. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryotic Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LC, Romijn EP, ZAMORA I, Yates JR, Marshall WF. Proteomic Analysis of Isolated Chlamydomonas Centrioles Reveals Orthologs of Ciliary-Disease Genes. Curr Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Keller LR. Electroporation of DNA into the unicellular green alga Chlamydomonas reinhardtii. Methods Mol Biol. 1995;55:73–79. doi: 10.1385/0-89603-328-7:73. [DOI] [PubMed] [Google Scholar]
- Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL, Schnell RA, Fernández E, Lefebvre PA. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol. 1989;109:2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N. The unicellular ancestry of animal development. Dev Cell. 2004;7:313–325. doi: 10.1016/j.devcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JGI, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. Asymmetric division, cell size and germ-soma specification in Volvox. Seminars in Developmental Biology. 1995;6:369–379. [Google Scholar]
- Kirk D. Volvox: molecular-genetic origins of multicellularity and cellular differentiation. Cambridge University Press; 1998. [Google Scholar]
- Kirk DL. Evolution of multicellularity in the volvocine algae. Curr Opin Plant Biol. 1999;2:496–501. doi: 10.1016/s1369-5266(99)00019-9. [DOI] [PubMed] [Google Scholar]
- Kirk DL. Volvox as a Model System for Studying the Ontogeny and Phylogeny of Multicellularity and Cellular Differentiation. Journal of Plant Growth Regulation. 2000;19:265–274. [Google Scholar]
- Kirk DL. A twelve-step program for evolving multicellularity and a division of labor. Bioessays. 2005;27:299–310. doi: 10.1002/bies.20197. [DOI] [PubMed] [Google Scholar]
- Kirk DL, Birchem R, King N. The extracellular matrix of Volvox: a comparative study and proposed system of nomenclature. J Cell Sci. 1986;80:207–231. doi: 10.1242/jcs.80.1.207. [DOI] [PubMed] [Google Scholar]
- Kirk DL, Nishii I. Volvox carteri as a model for studying the genetic and cytological control of morphogenesis. Development, Growth & Differentiation. 2001;43:621–631. doi: 10.1046/j.1440-169x.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Kirk MM, Ransick A, McRae SE, Kirk DL. The relationship between cell size and cell fate in Volvox carteri. J Cell Biol. 1993;123:191–208. doi: 10.1083/jcb.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk MM, Stark K, Miller SM, Müller W, Taillon BE, Gruber H, Schmitt R, Kirk DL. regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein. Development. 1999;126:639–647. doi: 10.1242/dev.126.4.639. [DOI] [PubMed] [Google Scholar]
- Kochert G. Differentiation of reproductive cells in Volvox carteri. J Protozool. 1968;15:438–452. doi: 10.1111/j.1550-7408.1968.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Koufopanou V. The evolution of soma in the Volvocales. American Naturalist. 1994:907–931. [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse O, Hankamer B. Microalgal hydrogen production. Current Opinion in Biotechnology. 2010;21:238–243. doi: 10.1016/j.copbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T. The Replication, Differentiation, and Inheritance of Plastids with Enlphasis on the Concept of Organelle Nuclei. Int Rev Cytol. 2010;128:1–33. [Google Scholar]
- Kwan AL, Li L, Kulp DC, Dutcher SK, Stormo GD. Improving gene-finding in Chlamydomonas reinhardtii:GreenGenie2. BMC Genomics. 2009;10:210. doi: 10.1186/1471-2164-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson A, Kirk MM, Kirk DL. Molecular phylogeny of the volvocine flagellates. Mol Biol Evol. 1992;9:85–105. doi: 10.1093/oxfordjournals.molbev.a040710. [DOI] [PubMed] [Google Scholar]
- Lemieux B, Turmel M, Lemieux C. Chloroplast DNA variation in Chlamydomonas and its potential application to the systematics of this genus. BioSystems. 1985;18:293–298. doi: 10.1016/0303-2647(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Lerche K, Hallmann A. Stable nuclear transformation of Gonium pectorale. BMC Biotechnol. 2009;9:64. doi: 10.1186/1472-6750-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC. Comparative Genomics Identifies a Flagellar and Basal Body Proteome that Includes the BBS5 Human Disease Gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Lin H, Goodenough UW. Gametogenesis in the Chlamydomonas reinhardtii minus mating type is controlled by two genes, MID and MTD1. Genetics. 2007;176:913–925. doi: 10.1534/genetics.106.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodha M, Schulz-Raffelt M, Schroda M. A new assay for promoter analysis in Chlamydomonas reveals roles for heat shock elements and the TATA box in HSP70A promoter-mediated activation of transgene expression. Eukaryotic Cell. 2008;7:172–176. doi: 10.1128/EC.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mages HW, Tschochner H, Sumper M. The sexual inducer of Volvox carteri. Primary structure deduced from cDNA sequence. FEBS Lett. 1988;234:407–410. doi: 10.1016/0014-5793(88)80126-1. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Okamoto K, Onai K, Niwa Y, Shimogawara K, Ishiura M. A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes Dev. 2008;22:918–930. doi: 10.1101/gad.1650408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul JE, Lilly JW, Cui L, Depamphilis CW, Miller W, Harris EH, Stern DB. The Chlamydomonas reinhardtii plastid chromosome: islands of genes in a sea of repeats. Plant Cell. 2002;14:2659–2679. doi: 10.1105/tpc.006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield SP, Schultz J. Development of a luciferase reporter gene, luxCt, for Chlamydomonas reinhardtii chloroplast - Mayfield - 2003 - The Plant Journal - Wiley Online Library. Plant J. 2004;37:449–458. doi: 10.1046/j.1365-313x.2003.01965.x. [DOI] [PubMed] [Google Scholar]
- Meissner M, Stark K, Cresnar B, Kirk DL, Schmitt R. Volvox germline-specific genes that are putative targets of RegA repression encode chloroplast proteins. Current Genetics. 1999;36:363–370. doi: 10.1007/s002940050511. [DOI] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Maréchal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernández E, Fukuzawa H, González-Ballester D, González-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riaño-Pachón DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan J, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang P, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo Y, Martínez D, Ngau WCA, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou K, Grigoriev IV, Rokhsar DS, Grossman AR. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis G, Vahrenholz C, Pratje E. Mitochondrial DNA of Chlamydomonas reinhardtii: The gene for apocytochrome b and the complete functional map of the 15.8 kb DNA. Mol Gen Genomics. 1990:223. doi: 10.1007/BF00265056. [DOI] [PubMed] [Google Scholar]
- Miller SM, Kirk DL. glsA, a Volvox gene required for asymmetric division and germ cell specification, encodes a chaperone-like protein. Development. 1999;126:649–658. doi: 10.1242/dev.126.4.649. [DOI] [PubMed] [Google Scholar]
- Miller SM, Schmitt R, Kirk DL. Jordan, an active Volvox transposable element similar to higher plant transposons. Plant Cell. 1993;5:1125–1138. doi: 10.1105/tpc.5.9.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko I, Holloway SP, Nikaido S, Carter M, Odom OW, Johnson CH, Herrin DL. Renilla luciferase as a vital reporter for chloroplast gene expression in Chlamydomonas. Mol Gen Genet. 1999;262:421–425. doi: 10.1007/s004380051101. [DOI] [PubMed] [Google Scholar]
- Misumi O, Yoshida Y, Nishida K, Fujiwara T, Sakajiri T, Hirooka S, Nishimura Y, Kuroiwa T. Genome analysis and its significance in four unicellular algae, Cyanidioshyzon merolae, Ostreococcus tauri, Chlamydomonas reinhardtii, and Thalassiosira pseudonana. J Plant Res. 2007;121:3–17. doi: 10.1007/s10265-007-0133-9. [DOI] [PubMed] [Google Scholar]
- Molnar A, Bassett A, Thuenemann E, Schwach F, Karkare S, Ossowski S, Weigel D, Baulcombe D. Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J. 2009 doi: 10.1111/j.1365-313X.2008.03767.x. [DOI] [PubMed] [Google Scholar]
- Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature. 2007;447:1126–1129. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- Morita E, Abe T, Tsuzuki M, Fujiwara S, Sato N, Hirata A, Sonoike K, Nozaki H. Role of pyrenoids in the CO2-concentrating mechanism: comparative morphology, physiology and molecular phylogenetic analysis of closely related strains of Chlamydomonas and Chloromonas (Volvocales) Planta. 1999;208:365–372. [Google Scholar]
- Nakada T, Nozaki H, Tomita M. Another origin of coloniality in volvocaleans: the phylogenetic position of Pyrobotrys arnoldi (Spondylomoraceae, Volvocales) J Eukaryot Microbiol. 2010;57:379–382. doi: 10.1111/j.1550-7408.2010.00488.x. [DOI] [PubMed] [Google Scholar]
- Nedelcu AM. Environmentally induced responses co-opted for reproductive altruism. Biol Lett. 2009;5:805–808. doi: 10.1098/rsbl.2009.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcu AM, Michod RE. The evolutionary origin of an altruistic gene. Mol Biol Evol. 2006;23:1460–1464. doi: 10.1093/molbev/msl016. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Savereide PB, Lefebvre PA. The CRY1 gene in Chlamydomonas reinhardtii: structure and use as a dominant selectable marker for nuclear transformation. Mol Cell Biol. 1994;14:4011–4019. doi: 10.1128/mcb.14.6.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert J, Shao N, Lu Y, Bock R. Genetic transformation of the model green alga Chlamydomonas reinhardtii. Methods Mol Biol. 2012;847:35–47. doi: 10.1007/978-1-61779-558-9_4. [DOI] [PubMed] [Google Scholar]
- Nishii I, Miller SM. Volvox: simple steps to developmental complexity? Curr Opin Plant Biol. 2010;13:646–653. doi: 10.1016/j.pbi.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Nishii I, Ogihara S, Kirk DL. A kinesin, invA, plays an essential role in volvox morphogenesis. Cell. 2003;113:743–753. doi: 10.1016/s0092-8674(03)00431-8. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Higashiyama T, Suzuki L, Misumi O, Kuroiwa T. The biparental transmission of the mitochondrial genome in Chlamydomonas reinhardtii visualized in living cells. Eur J Cell Biol. 1998;77:124–133. doi: 10.1016/S0171-9335(98)80080-0. [DOI] [PubMed] [Google Scholar]
- Novoselov SV, Rao M, Onoshko NV, Zhi H, Kryukov GV, Xiang Y, Weeks DP, Hatfield DL, Gladyshev VN. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. The EMBO Journal. 2002;21:3681–3693. doi: 10.1093/emboj/cdf372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki H. Origin and evolution of the genera Pleodorina and Volvox (Volvocales) Biologia. 2003:425–431. [Google Scholar]
- Nozaki H, Itoh M. Phylogenetic Relationships Within The Colonial Volvocales (Chlorophyta) Inferred From Cladistic Analysis Based On Morphological Data. Journal of Phycology. 1994;30:353–365. [Google Scholar]
- Nozaki H, Itoh M, Sano R, Uchida H, Watanabe M, Kuroiwa T. Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data. Journal of Phycology. 1995;31:970–978. [Google Scholar]
- Nozaki H, Misawa K, Kajita T, Kato M, Nohara S, Watanabe MM. Origin and evolution of the colonial volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Mol Phylogenet Evol. 2000;17:256–268. doi: 10.1006/mpev.2000.0831. [DOI] [PubMed] [Google Scholar]
- Nozaki H, Mori T, Misumi O, Matsunaga S, Kuroiwa T. Males evolved from the dominant isogametic mating type. Curr Biol. 2006;16:R1018–20. doi: 10.1016/j.cub.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Nozaki H, Takahara M, Nakazawa A, Kita Y, Yamada T, Takano H, Kawano S, Kato M. Evolution of rbcL group IA introns and intron open reading frames within the colonial Volvocales (Chlorophyceae) Mol Phylogenet Evol. 2002;23:326–338. doi: 10.1016/s1055-7903(02)00030-1. [DOI] [PubMed] [Google Scholar]
- O’Neill BM, Mikkelson KL, Gutierrez NM, Cunningham JL, Wolff KL, Szyjka SJ, Yohn CB, Redding KE, Mendez MJ. An exogenous chloroplast genome for complex sequence manipulation in algae. Nucleic Acids Res. 2012;40:2782–2792. doi: 10.1093/nar/gkr1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom OW, Baek KH, Dani RN, Herrin DL. Chlamydomonas chloroplasts can use short dispersed repeats and multiple pathways to repair a double-strand break in the genome. Plant J. 2008;53:842–853. doi: 10.1111/j.1365-313X.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- Ohresser M, Matagne RXF, Loppes R. Expression of the arylsulphatase reporter gene under the control of the nit1 promoter in Chlamydomonas reinhardtii. Current Genetics. 1997;31:264–271. doi: 10.1007/s002940050204. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Katz DF. A simple technique for the measurement of swimming speed of Chlamydomonas. Exp Cell Res. 1973;81:487–491. doi: 10.1016/0014-4827(73)90540-5. [DOI] [PubMed] [Google Scholar]
- Palenik B, Grimwood J, Aerts A, Rouze P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci USA. 2007;104:7705. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas V, Miller SM. Functional analysis of the Volvox carteri asymmetric division protein GlsA. Mech Dev. 2009;126:842–851. doi: 10.1016/j.mod.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Patron NJ, Waller RF. Transit peptide diversity and divergence: a global analysis of plastid targeting signals. Bioessays. 2007;29:1048–1058. doi: 10.1002/bies.20638. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Winey M. Basal body assembly in ciliates: the power of numbers. Traffic. 2009;10:461–471. doi: 10.1111/j.1600-0854.2009.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Chapter Two Intraflagellar Transport (IFT):: Role in Ciliary Assembly, Resorption and Signalling. Current Topics in Developmental Biology. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Peers G, Niyogi KK. Pond scum genomics: the genomes of Chlamydomonas and Ostreococcus. Plant Cell. 2008;20:502–507. doi: 10.1105/tpc.107.056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracek ME, Lefebvre PA, Silflow CD, Berman SD. Chlamydomonas telomere sequences are A+T-rich but contain three consecutive G-C base pairs. Proc Natl Acad Sci USA. 1990;87:8222. doi: 10.1073/pnas.87.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pootakham W, González-Ballester D, Grossman AR. Identification and regulation of plasma membrane sulfate transporters in Chlamydomonas. Plant Physiol. 2010;153:1653–1668. doi: 10.1104/pp.110.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu CE, Lee RW. Mitochondrial Genome Sequence Evolution in Chlamydomonas. Genetics. 2007;175:819–826. doi: 10.1534/genetics.106.063156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble AM, Giddings TH, Jr, Dutcher SK. Basal bodies and centrioles: Their function and structure. Current Topics in Developmental Biology. 1999;49:207–233. doi: 10.1016/s0070-2153(99)49010-6. [DOI] [PubMed] [Google Scholar]
- Preuss D, Mets L. Summaries of National Science Foundation-Sponsored Arabidopsis 2010 Projects and National Science Foundation-Sponsored Plant Genome Projects That Are Generating Arabidopsis Resources for the Community. Plant Physiol. 2002;129:421–422. [Google Scholar]
- Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, Hellsten U, Chapman J, Simakov O, Rensing SA, Terry A, Pangilinan J, Kapitonov V, Jurka J, Salamov A, Shapiro H, Schmutz J, Grimwood J, Lindquist E, Lucas S, Grigoriev IV, Schmitt R, Kirk D, Rokhsar DS. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science. 2010;329:223–226. doi: 10.1126/science.1188800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pröschold T, Marin B, Schlösser UG, Melkonian M. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist. 2001;152:265–300. doi: 10.1078/1434-4610-00068. [DOI] [PubMed] [Google Scholar]
- Purton S. Tools and techniques for chloroplast transformation of Chlamydomonas. Adv Exp Med Biol. 2007;616:34–45. doi: 10.1007/978-0-387-75532-8_4. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Kropat J, Merchant S. Copper Response Element and Crr1-Dependent Ni2+-Responsive Promoter for Induced, Reversible Gene Expression in Chlamydomonas reinhardtii. Eukaryotic Cell. 2003;2:995–1002. doi: 10.1128/EC.2.5.995-1002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V, Bingham S, Webber A. A simple method for chloroplast transformation in Chlamydomonas reinhardtii. Methods in Molecular Biology. 2011;684:313–320. doi: 10.1007/978-1-60761-925-3_23. [DOI] [PubMed] [Google Scholar]
- Randolph-Anderson BL, Boynton JE, Gillham NW, Harris EH, Johnson AM, Dorthu M-P, Matagne RF. Further characterization of the respiratory deficient dum-1 mutation of Chlamydomonas reinhardtii and its use as a recipient for mitochondrial transformation. Mol Gen Genomics. 1993;236–236:235–244. doi: 10.1007/BF00277118. [DOI] [PubMed] [Google Scholar]
- RAO M, CARLSON BA, Novoselov SV, Weeks DP, Gladyshev VN, HATFIELD DL. Chlamydomonas reinhardtii selenocysteine tRNA[Ser]Sec. RNA. 2003;9:923–930. doi: 10.1261/rna.5510503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Robbens S, Khadaroo B, Camasses A, Derelle E, Ferraz C, Inzé D, van de Peer Y, Moreau H. Genome-wide analysis of core cell cycle genes in the unicellular green alga Ostreococcus tauri. Mol Biol Evol. 2005;22:589–597. doi: 10.1093/molbev/msi044. [DOI] [PubMed] [Google Scholar]
- Rohr J, Sarkar N, Balenger S, Jeong BR, Cerutti H. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 2004;40:611–621. doi: 10.1111/j.1365-313X.2004.02227.x. [DOI] [PubMed] [Google Scholar]
- Rokas A. The molecular origins of multicellular transitions. Curr Opin Genet Dev. 2008a;18:472–478. doi: 10.1016/j.gde.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Rokas A. The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu Rev Genet. 2008b;42:235–251. doi: 10.1146/annurev.genet.42.110807.091513. [DOI] [PubMed] [Google Scholar]
- Rolland N, Atteia A, Decottignies P, Garin J, Hippler M, Kreimer G, Lemaire SD, Mittag M, Wagner V. Chlamydomonas proteomics. Curr Opin Microbiol. 2009;12:285–291. doi: 10.1016/j.mib.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Ruiz-Trillo I, Burger G, Holland PWH, King N, Lang BF, Roger AJ, Gray MW. The origins of multicellularity: a multi-taxon genome initiative. Trends Genet. 2007;23:113–118. doi: 10.1016/j.tig.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rupprecht J. From systems biology to fuel—Chlamydomonas reinhardtii as a model for a systems biology approach to improve biohydrogen production. Journal of Biotechnology. 2009;142:10–20. doi: 10.1016/j.jbiotec.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Rymarquis LA, Handley JM, Thomas M, Stern DB. Beyond complementation. Map-based cloning in Chlamydomonas reinhardtii. Plant Physiol. 2005;137:557–566. doi: 10.1104/pp.104.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- Schiedlmeier B, Schmitt R, Müller W, Kirk MM, Gruber H, Mages W, Kirk DL. Nuclear transformation of Volvox carteri. Proc Natl Acad Sci USA. 1994;91:5080–5084. doi: 10.1073/pnas.91.11.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Lin-Schmidt X, Chamberlin A, Salehi-Ashtiani K, Papin JA. Metabolic systems analysis to advance algal biotechnology. Biotechnol J. 2010;5:660–670. doi: 10.1002/biot.201000129. [DOI] [PubMed] [Google Scholar]
- Schmollinger S, Strenkert D, Schroda M. An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Current Genetics. 2010;56:383–389. doi: 10.1007/s00294-010-0304-4. [DOI] [PubMed] [Google Scholar]
- Schroda M. RNA silencing in Chlamydomonas: mechanisms and tools. Current Genetics. 2006;49:69–84. doi: 10.1007/s00294-005-0042-1. [DOI] [PubMed] [Google Scholar]
- Schroda M, Beck CF, Vallon O. Sequence elements within an HSP70 promoter counteract transcriptional transgene silencing in Chlamydomonas. Plant J. 2002;31:445–455. doi: 10.1046/j.1365-313x.2002.01371.x. [DOI] [PubMed] [Google Scholar]
- Schroda M, Blöcker D, Beck CF. The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J. 2000;21:121–131. doi: 10.1046/j.1365-313x.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- Setohigashi Y, Hamaji T, Hayama M, Matsuzaki R, Nozaki H. Uniparental Inheritance of Chloroplast DNA Is Strict in the Isogamous Volvocalean Gonium. PLoS ONE. 2011;6:e19545. doi: 10.1371/journal.pone.0019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao N, Bock R. A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Current Genetics. 2008;53:381–388. doi: 10.1007/s00294-008-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Current Topics in Developmental Biology. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- Shimogawara K, Fujiwara S, Grossman A, Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara H, Kaneda H, Sato A, Inoue K, Ogura A, Yonekawa H, Hayashi JI. Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics. 2000;156:1277–1284. doi: 10.1093/genetics/156.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MB, Solari CA, Ganguly S, Powers TR, Kessler JO, Goldstein RE. Flows driven by flagella of multicellular organisms enhance long-range molecular transport. Proc Natl Acad Sci USA. 2006;103:8315–8319. doi: 10.1073/pnas.0600566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow CD, Lefebvre PA. Assembly and Motility of Eukaryotic Cilia and Flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 2001;127:1500–1507. [PMC free article] [PubMed] [Google Scholar]
- Sizova I, Fuhrmann M, Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Smith DR, Lee RW. The mitochondrial and plastid genomes of Volvox carteri: bloated molecules rich in repetitive DNA. BMC Genomics. 2009;10:132. doi: 10.1186/1471-2164-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Lee RW. Low Nucleotide Diversity for the Expanded Organelle and Nuclear Genomes of Volvox carteri Supports the Mutational-Hazard Hypothesis. Mol Biol Evol. 2010;27:2244–2256. doi: 10.1093/molbev/msq110. [DOI] [PubMed] [Google Scholar]
- Solari CA, Kessler JO, Michod RE. A hydrodynamics approach to the evolution of multicellularity: flagellar motility and germ-soma differentiation in volvocalean green algae. Am Nat. 2006;167:537–554. doi: 10.1086/501031. [DOI] [PubMed] [Google Scholar]
- Solari CA, Michod RE, Goldstein RE. Volvox Barberi, The Fastest Swimmer Of The Volvocales (Chlorophyceae) Journal of Phycology. 2008;44:1395–1398. doi: 10.1111/j.1529-8817.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci USA. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MH. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. Journal of Experimental Botany. 2008;59:1463–1473. doi: 10.1093/jxb/erm128. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier MEA, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–644. doi: 10.1093/bioinformatics/btn013. [DOI] [PubMed] [Google Scholar]
- Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33:W465–7. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Schöffmann O, Morgenstern B, Waack S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics. 2006;7:62. doi: 10.1186/1471-2105-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. Growth And Mating Of Gonium Pectorale (Volvocales) In Defined Media. Journal of Phycology. 1966;2:23–28. doi: 10.1111/j.1529-8817.1966.tb04587.x. [DOI] [PubMed] [Google Scholar]
- Stern DB, Witman G, Harris EH. The Chlamydomonas Sourcebook. 2. Academic Press; 2009. [Google Scholar]
- Stevens DR, Purton S, Rochaix JD. The bacterial phleomycin resistance geneble as a dominant selectable marker inChlamydomonas. Mol Gen Genomics. 1996;251:23–30. doi: 10.1007/BF02174340. [DOI] [PubMed] [Google Scholar]
- Sumper M, Hallmann A. Biochemistry of the extracellular matrix of Volvox. Int Rev Cytol. 1997;180:51–85. doi: 10.1016/s0074-7696(08)61770-2. [DOI] [PubMed] [Google Scholar]
- Tam L, Stamer KA, Kirk DL. Early and late gene expression programs in developing somatic cells of Volvox carteri*1. Dev Biol. 1991;145:67–76. doi: 10.1016/0012-1606(91)90213-m. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschochner H, Lottspeich F, Sumper M. The sexual inducer of Volvox carteri: purification, chemical characterization and identification of its gene. EMBO J. 1987;6:2203–2207. doi: 10.1002/j.1460-2075.1987.tb02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M, Lemieux C, Lee RW. Net synthesis of chloroplast DNA throughout the synchronized vegetative cell-cycle of Chlamydomonas. Current Genetics. 1980;2:229–232. doi: 10.1007/BF00435691. [DOI] [PubMed] [Google Scholar]
- Ueki N, Matsunaga S, Inouye I, Hallmann A. How 5000 independent rowers coordinate their strokes in order to row into the sunlight: phototaxis in the multicellular green alga Volvox. BMC Biol. 2010;8:103. doi: 10.1186/1741-7007-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki N, Nishii I. Idaten Is a New Cold-Inducible Transposon of Volvox carteri That Can Be Used for Tagging Developmentally Important Genes. Genetics. 2008;180:1343–1353. doi: 10.1534/genetics.108.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki N, Nishii I. Controlled enlargement of the glycoprotein vesicle surrounding a volvox embryo requires the InvB nucleotide-sugar transporter and is required for normal morphogenesis. Plant Cell. 2009;21:1166–1181. doi: 10.1105/tpc.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen J, Goodenough U. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev. 2001;15:1652. doi: 10.1101/gad.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen JG. Chloroplast DNA methylation and inheritance in Chlamydomonas. Genes Dev. 2001;15:2585–2597. doi: 10.1101/gad.906701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen JG. Evolution of sex and mating loci: An expanded view from Volvocine algae. Curr Opin Microbiol. 2011;14:634–641. doi: 10.1016/j.mib.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrenholz C, Riemen G, Pratje E, Dujon B, Michaelis G. Mitochondrial DNA of Chlamydomonas reinhardtii: the structure of the ends of the linear 15.8-kb genome suggests mechanisms for DNA replication. Current Genetics. 1993;24:241–247. doi: 10.1007/BF00351798. [DOI] [PubMed] [Google Scholar]
- van Leeuwenhoek A. Part of a Letter from Mr Antony van Leeuwenhoek, concerning the Worms in Sheeps Livers, Gnats, and Animalcula in the Excrements of Frogs. Philosophical Transactions. 1700;22:509–518. [Google Scholar]
- Viamontes GI, Fochtmann LJ, Kirk DL. Morphogenesis in Volvox: analysis of critical variables. Cell. 1979;17:537–550. doi: 10.1016/0092-8674(79)90262-9. [DOI] [PubMed] [Google Scholar]
- Viamontes GI, Kirk DL. Cell shape changes and the mechanism of inversion in Volvox. J Cell Biol. 1977;75:719–730. doi: 10.1083/jcb.75.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villand P, Eriksson M, Samuelsson G. Carbon dioxide and light regulation of promoters controlling the expression of mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Biochem J. 1997;327 (Pt 1):51–57. doi: 10.1042/bj3270051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Duanmu D, Spalding MH. Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: inorganic carbon transport and CO2 recapture. Photosyn Res. 2011;109:115–122. doi: 10.1007/s11120-011-9643-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spalding MH. An inorganic carbon transport system responsible for acclimation specific to air levels of CO2 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2006;103:10110–10115. doi: 10.1073/pnas.0603402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Miranda-Saavedra D, Barton GJ. Genome Analysis of the Unicellular Green Alga Chlamydomonas reinhardtii Indicates an Ancient Evolutionary Origin for Key Pattern Recognition and Cell-Signaling Protein Families. Genetics. 2008;179:193–197. doi: 10.1534/genetics.107.085936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J, Goodenough U. Volvocine cell walls and their constituent glycoproteins: an evolutionary perspective. Protoplasma. 1994;181:245–258. [Google Scholar]
- Worden AZ, Lee JH, Mock T, Rouze P, Simmons MP, Aerts AL, Allen AE, Cuvelier ML, Derelle E, Everett MV, Foulon E, Grimwood J, Gundlach H, Henrissat B, Napoli C, McDonald SM, Parker MS, Rombauts S, Salamov A, Von Dassow P, Badger JH, Coutinho PM, Demir E, Dubchak I, Gentemann C, Eikrem W, Gready JE, John U, Lanier W, Lindquist EA, Lucas S, Mayer KFX, Moreau H, Not F, Otillar R, Panaud O, Pangilinan J, Paulsen I, Piegu B, Poliakov A, Robbens S, Schmutz J, Toulza E, Wyss T, Zelensky A, Zhou K, Armbrust EV, Bhattacharya D, Goodenough UW, van de Peer Y, Grigoriev IV. Green Evolution and Dynamic Adaptations Revealed by Genomes of the Marine Picoeukaryotes Micromonas. Science. 2009;324:268–272. doi: 10.1126/science.1167222. [DOI] [PubMed] [Google Scholar]
- Xie Q, Sanz-Burgos AP, Hannon GJ, Gutiérrez C. Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 1996;15:4900–4908. [PMC free article] [PubMed] [Google Scholar]
- Yamada TK, Miyaji K, Nozaki H. A taxonomic study of Eudorina unicocca (Volvocaceae, Chlorophyceae) and related species, based on morphology and molecular phylogeny. European Journal of Phycology. 2008;43:317–326. [Google Scholar]
- Yamasaki T, Kurokawa S, Watanabe KI, Ikuta K, Ohama T. Shared molecular characteristics of successfully transformed mitochondrial genomes in Chlamydomonas reinhardtii. Plant Mol Biol. 2005;58:515–527. doi: 10.1007/s11103-005-7081-3. [DOI] [PubMed] [Google Scholar]
- Yoshihara C, Inoue K, Schichnes D, Ruzin S, Inwood W, Kustu S. An Rh1-GFP Fusion Protein Is in the Cytoplasmic Membrane of a White Mutant Strain of Chlamydomonas reinhardtii. Mol Plant. 2008;1:1007–1020. doi: 10.1093/mp/ssn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, Qi Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007;21:1190–1203. doi: 10.1101/gad.1543507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Wang W, Bai X, Qi Y. Gene silencing by artificial microRNAs in Chlamydomonas. The Plant Journal. 2009;58:157–164. doi: 10.1111/j.1365-313X.2008.03758.x. [DOI] [PubMed] [Google Scholar]
- Zouros E. The exceptional mitochondrial DNA system of the mussel family Mytilidae. Genes & genetic systems. 2000;75:313–318. doi: 10.1266/ggs.75.313. [DOI] [PubMed] [Google Scholar]