Abstract
Background
Several meta-analyses confirmed the five most prevalent human papillomavirus (HPV) strains in women with and without cervical neoplastic diseases are HPV16, 18, 31, 52, and 58. HPV16/18 are the predominant oncogenic genotypes, causing approximately 70% of global cervical cancer cases. The vast majority of the women studied in previous analyses were from Europe, North America, Asia, and most recently Latin America and the Caribbean. Despite the high burden of cervical cancer morbidity and mortality in Africa, a robust meta-analysis of HPV genotype prevalence and distribution in African women is lacking.
Methods and Findings
We systematically searched 14 major databases from inception to August 2013 without language restriction, following the Meta-Analysis of Observational Studies in Epidemiology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Seventy-one studies from 23 African countries were identified after screening 1162 citations and data abstracted and study quality appraised from 195 articles. HPV type-specific prevalence and distribution was estimated from 17,273 cases of women with normal cervical cytology; 1019 women with atypical squamous cells of undetermined significance (ASCUS); 1444 women with low-grade squamous intraepithelial lesion (LSIL); 1571 women with high-grade squamous intraepithelial lesion (HSIL); and 4,067 cases of invasive cervical carcinoma (ICC). Overall prevalence of HPV16/18 were 4.4% and 2.8% of women with normal cytology, 12.0% and 4.4% with ASCUS, 14.5% and 10.0% with LSIL, 31.2% and 13.9% with HSIL, and 49.7% and 18.0% with ICC, respectively. Study limitations include the lack of adequate data from Middle and Northern African regions, and variations in the HPV type-specific sensitivity of different genotyping protocols.
Conclusions
To our knowledge, this study is the most comprehensive assessment of the overall prevalence and distribution of HPV genotypes in African women with and without different cervical neoplasias. We have established that HPV16/18 account for 67.7% of ICC cases among African women. Based on our findings, we highly recommend the administration of existing prophylactic vaccines to younger women not infected with HPV16/18 and an increase in HPV screening efforts for high-risk genotypes to prevent cervical cancer.
Review registration: International Prospective Register of Systematic Reviews CRD42013006558.
Introduction
Cervical cancer is the leading cause of mortality and years of life lost due to cancer in Africa [1] and predominantly affects women ages 15–44 years [2]. For decades, 12 highly carcinogenic human papillomavirus (HPV) genotypes have been recognized as the causative agent of cervical cancer [3,4]. Despite having the highest burden of risk factors associated with HPV infection, persistence, and progression to cervical cancer [5], comprehensive data on HPV genotype prevalence and distribution in Africa are lacking. The use of Papanicolaou (Pap) smear screening [6] and HPV prophylactic vaccines [7–9] are effective in preventing cervical cancer in most developed countries. However, their use in African countries remains very limited due to a variety of socio-economic and logistical barriers [10–13].
To date, several global meta-analyses on the distribution of HPV genotypes have confirmed the five most prevalent strains in women with normal cytology [14,15] and cervical neoplastic diseases [16–24] to be HPV16, 18, 31, 52, and 58. HPV16 and 18 were identified as the predominant oncogenic genotypes, causing approximately 70% of global cervical cancer cases [22], with the exception of women infected with HIV, in whom HPV58 is reported to be the second most dominant strain behind HPV16 [20]. The vast majority of the women studied in these investigations, however, were from Europe, North America [14,15,18–24], Asia [16], and most recently Latin America and the Caribbean [17]. Data on African women in these studies are highly variable and incomplete. Thus, the prevalence and distribution of HPV genotypes in Africa among women with normal cervical cytology, neoplastic lesions, and invasive cervical cancer (ICC) is required.
A comprehensive meta-analysis of HPV genotype prevalence and distribution among a large sample size of African women is needed to inform local, national, regional, and global policy to curb the spread of cervical cancer. In May 2013, the Global Alliance for Vaccine and Immunization (GAVI) announced the availability of HPV vaccines (Gardasil and Cervarix) for a reduced price of $4.50 per dose to low-income countries meeting the eligibility criteria [13,25]. With clear indications that the GAVI Alliance is committed to subsidizing HPV vaccines for low-income countries [13,26–29], many African nations are beginning to design effective strategies that address the potential challenges of vaccine delivery and screening for HPV and cervical cancer [13,28,29]. Epidemiological information on the prevalence and distribution of genital HPV infection in Africa is critical for planning for vaccine implementation; evaluating the possible impact of existing prophylactic HPV vaccines; and determining the relevant tools for HPV screening to prevent cervical cancer.
To our knowledge, this systematic review and meta-analysis of HPV genotype prevalence and distribution in Africa is the largest ever performed and includes 25,463 African women. The goal of this study was to establish the overall and type-specific prevalence and distribution of HPV genotypes among women with normal cervical cytology, neoplastic cervical lesions, and cancer in all five World Health Organization (WHO) regions of Africa.
Methods
A systematic review protocol was performed adhering to the guidelines outlined in the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) for systematic reviews [30] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses [31,32] (See S1 Table). The investigators wrote a protocol and registered it with the International Prospective Register of Systematic Reviews (identification number CRD42013006558) in November 2013 [33].
Search Strategy
The search strategy was designed with a librarian (P.A.B.) to identify studies reporting HPV genotypes associated with cervical cancer or neoplasia in women living in Africa by performing a systematic search of literature databases from their earliest dates through August 27, 2013. The search, without any language restrictions, was performed in PubMed/MEDLINE (NCBI), Embase (Elsevier), Web of Science (Thomson), BIOSIS Preview (Thomson), Dissertations and Theses Full Text (ProQuest), Cochrane Central Register of Controlled Trials (Wiley), African Index Medicus (WHO), and POPLINE (K4Health). Exact strategies used to query each of these databases are reported in S1 Appendix Detailed search strategy. We also attempted to identify unpublished studies by examining ClinicalTrials.gov (NIH), International Clinical Trial Registry Platform (WHO), European Union Clinical Trials Register (EMA), and System for Information on Grey Literature in Europe (OpenGrey). The bibliographies of relevant reviews and eligible studies were also examined for additional sources. Databases containing conference proceedings or congress's annals, university theses, and experts were also consulted.
Studies, eligibility criteria, and quality assessment
We used a comprehensive strategy to identify studies that included women living in African countries. Two reviewers (A.J.S. and J.G.O.) read the titles and abstracts to determine each study’s eligibility for full text review if (i) the article and relevant data were accessible, (ii) at least 10 women from an African country were included, (iii) cervical cytology/histology was confirmed by exfoliated cervical cells or fixed/fresh biopsy, (iv) HPV genotype-specific prevalence of at least three HPV genotypes was calculated, and (v) HPV genotype prevalence was calculated separately for each cervical lesion following the Bethesda classification [34,35]. There were no restrictions on the type of HPV assay method used. HPV genotypes were determined by molecular methods [signal amplified hybridization, polymerase chain reaction (PCR), DNA sequencing, type-specific probes, reverse line-blot hybridization, in situ hybridization, southern blot hybridization, or microarray] or by immunological techniques (including ELISA). Articles not meeting these five criteria were excluded. The reference lists of all the articles included in the study were also perused for additional information including non-English articles/abstracts.
Based on the aforementioned pre-defined criteria, two separate reviewers (A.J.S. and R.K.O.) independently reviewed full texts for data abstraction. When further clarification information was required, authors were contacted via email. Discrepancies were resolved by discussion or involvement of a third reviewer (J.G.O.). Two reviewers (A.J.S. and R.K.O.) independently assessed the methodological quality of studies and discrepancies were resolved by consensus [17]. Methodological quality assessment of observational studies was assessed by a checklist of essential items as outlined [24]. A list summarizing all 71 studies included for data abstraction and quality assessment is provided in S2 Table.
Data items
If data or data subsets of the same population were published in multiple articles [36–38], only the publication with the largest sample size was used and the other reports were used as supplementary data. Two studies [39,40] had more detailed data in related doctoral theses, therefore the additional data from the theses were used to supplement the journal articles. Jones et al. [41] compared the results of HPV tests (clinician vs. self-administered), and data from the method considered to be the most sensitive was extracted. Four studies included data from women representing multiple countries [42–45]. Because the studies had clear lesion breakdowns, each of these articles was counted as an independent article in the analysis. Two studies [46,47] reported data from multiple countries without separating individual women studied by country. Though these papers had data broken down by lesion, at the regional level they were grouped into a mixed region category. Okolo et al. [48] independently analyzed for ICC, however, the data on normal cytology was duplicated in Thomas et al. [49]. Thus, the latter publication was used as the source of data for abstraction.
Data extraction
The key information retrieved for analysis included: participant characteristics (population, mean or median age, standard deviation of age), study characteristics (study design, period of data collection, sample size), laboratory location where the samples were processed and genotyped, geographical settings (region according to WHO classification), country, distribution of cases by histological type, tissue source, PCR primers (GP5+/6+, MY09/11, PGMY09/11, SPF10 or individual laboratory designed primers), detailed HPV detection and genotyping methodology, number of HPV positive women, and overall HPV prevalence where given. Data was extracted for both high risk (HR) and low-risk (LR) HPV genotypes [50]. Studies reporting multiple infections with any HPV genotype were also extracted. Specimens negative for beta-globin were not included in the sample size. Cases were classified into five grades of cervical diagnosis by cytology and/or by histology as previously outlined [34,35]. This included women with: 1) normal cervical cytology; 2) atypical squamous cells of undetermined significance (ASCUS); 3) low-grade intraepithelial lesion (LSIL), including cervical intraepithelial neoplasia grade 1 (CIN1); 4) high-grade squamous intraepithelial lesion (HSIL), including CIN2-3; and 5) ICC [including squamous cell carcinoma (SCC) and adeno/adenosquamous carcinoma].
Statistical analysis
Overall HPV prevalence was defined as the proportion of tested individuals that were positive for any HPV infection expressed as a percentage [(the number of HPV positive women/the total number tested)*100]. Similarly, HPV type-specific prevalence by cytology or histology was also calculated for those testing positive for the specific HPV type by cytological category according to the 2001 Bethesda System [34,35]. HPV type-specific prevalence was defined as the proportion of women testing positive for a specific HPV genotype among all HPV positive women tested for that genotype. In HPV type–specific prevalence, only studies that tested for a particular HPV type contributed to the analysis for that type, so sample sizes differed between the type-specific analyses. Multiple HPV infections were separated into constituent types, thus type-specific prevalence represents both single and multiple infections as previously defined [17].
Statistical analysis was conducted as described by Ciapponi et al. [17], using StatsDirect meta-analysis software (Cheshire, United Kingdom) and STATA 13.0 (College Station, TX). Briefly, the sample size (N), number of cases for HPV type (rows), and each cytology/histology classification (column) were calculated by summing the number of all study participants and the number of events in each row/column combination. Raw prevalence was computed by dividing the number of events by sample size, N. Exact 95% confidence interval was computed under binomial. Studies in which the HPV-type was not assessed or was not reported were excluded from the HPV type-specific analyses. Studies in which age was not reported were recorded and analyzed as missing age.
The computed proportions were arcsine square root transformed using the Freeman-Tukey method to stabilize the variance [51]. The meta-analysis was then carried out on the transformed proportions, with the inverse of the variance of the transformed proportion serving as study-specific weight. The arcsine transformations were done to stabilize the variance of simple proportions [17]. We further applied DerSimonian-Laird weights for the random effects model [52] when heterogeneity between studies included in the analysis was found. The I-squared (I2) statistic was used to quantify the heterogeneity between studies.
Additionally, a meta-regression using grouped logistic regression analysis adjusted for age-group was performed to further identify the possible sources of heterogeneity. We then computed lesion-specific age-adjusted prevalence for any HPV and HPV16 for each region. We then used LSMEANS statement with the ILINK options in the SAS LOGISTIC procedure to back transform log-odds into proportions and generate 95% confidence intervals.
Ethical statement
No institutional review board approval was required for this study.
Results
Selection of studies for review
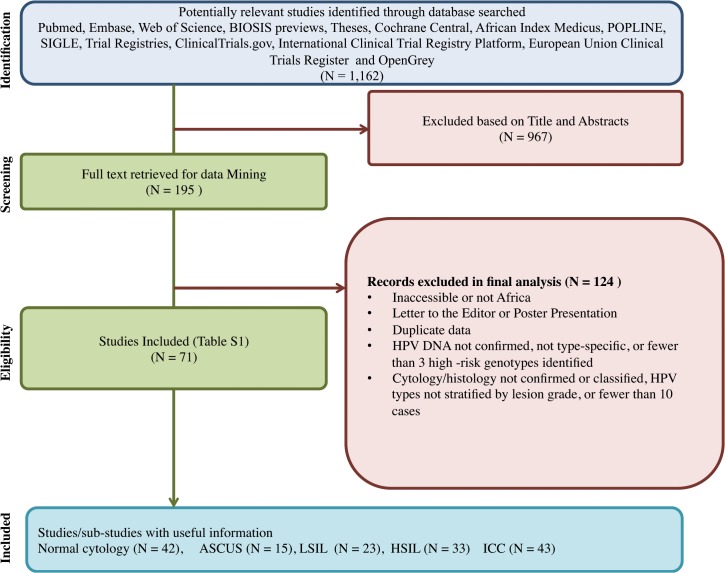
A total of 1162 citations were retrieved and 195 full articles were reviewed. Data was abstracted from 71 epidemiological studies with individual-level data meeting the inclusion criteria. The study flow diagram is shown in Fig 1.
Fig 1. Study flow diagram.
Abbreviation: ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesions; HSIL: High-grade squamous intraepithelial lesions; ICC: Invasive cervical cancer (this includes SCC and ADC).
Study characteristics
Data were collected from studies conducted between 1968 [53] to 2010 [44]. With the exception of most studies from South Africa [41,44,54–68], a study from Morocco [69], Ethiopia and Sudan [42], all HPV genotyping of African participants were performed in laboratories in Europe (France, Spain, Great Britain, Italy, Sweden, the Netherlands, Belgium and Denmark), Japan, or the United States of America (S4 Table). Fifty-six studies reported multiple infections with any HPV type-specific. A total of 30,444 women from 23 African countries were recruited to participate in the various studies included in the systematic review. Beta globin tests were done on 27,915 sample specimens. Finally, HPV DNA testing was done on 25,463 sample specimens from women who had a positive beta globin test result. Their distribution by cytological type and geographical region is given in Table 1.
Table 1. Regional and country specific distribution of studies, study size and prevalence of HPV DNA by cervical disease grade and region.
| African Region (No. studies) | Country [Reference] | Total (studies = 71) | Normal Cytology (studies = 42) | ASCUS (studies = 15) | LSIL (studies = 23) | HSIL (studies = 33) | ICC (studies = 43) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested, N | HPV (+), n | Tested, N | HPV+, n (%) | Tested, N | HPV+, n (%) | Tested, N | HPV+, n(%) | Tested, N | HPV+, n(%) | Tested, N | HPV+, n(%) | ||
| Eastern (26) | Ethiopia [42, 70, 71], Kenya [39, 72–79], Mozambique [36, 80, 81], Rwanda [82], Tanzania [43, 83–85] Ŧ , Uganda [43, 53, 86–88] Ŧ , Zambia [40, 89], Zimbabwe [90–92] | 10246 | 5146 | 6640 | 1907 (29) | 440 | 260 (59) | 605 | 417 (68) | 657 | 563 (85) | 1904 | 1725 (90) |
| Middle (3) | Equatorial Guinea [93], Zaire (DRC) [94] | 30 | 23 | . | . | . | . | 8 | 6 (60) | 19 | 14 (74) | 3 | 3 (100) |
| Northern (6) | Algeria [37, 38, 43] Ŧ , Morocco [69, 95, 96], Sudan [42] | 2510 | 725 | 1865 | 219 (12) | 46 | 4 (7) | 24 | 4 (17) | 8 | 4 (50) | 517 | 473 (90) |
| Southern (19) | Botswana [97, 98], South Africa [41, 44, 54–68] ** | 4983 | 3392 | 3030 | 1505 (50) | 207 | 126 (61) | 521 | 448 (87) | 768 | 645 (85) | 457 | 388 (85) |
| Western (16) | Benin [43, 99] Ŧ , Cote d’Ivoire [100–102], Gambia [103], Ghana [44] **, Guinea [43, 104] Ŧ , Mali [43, 45, 105] * Ŧ , Nigeria [44, 48, 49, 106] **, Senegal [45, 107–109]*, | 7265 | 2878 | 5600 | 1363 (24) | 326 | 85 (26) | 286 | 191 (67) | 102 | 87 (85) | 951 | 845 (89) |
| Mixed studies | Tanzania & South Africa [47], Kenya & South Africa [46], | 429 | 334 | 138 | 58 (42) | . | . | . | . | 17 | 16 (94) | 235 | 225 (96) |
| Overall (71) | . | 25463 | 12498 | 17273 | 5074 (29) | 1019 | 474 (47) | 1444 | 1087 (74) | 1571 | 1371 (85) | 4067 | 3741 (89) |
Abbreviations:
ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesions; HSIL: High-grade squamous intraepithelial lesions; ICC: Invasive cervical cancer included both SCC and ADC.
“Mixed studies” refers to study including women from more than one country without separating individual women to the country where they came from.
*Senegal & Mali counted here as two sub-studies from [45];
** Studies from Ghana, Nigeria, South Africa were from one study [44], but counted here as a sub-study each;
Ŧ All these countries (Algeria, Benin, Guinea, Mali, Uganda, Tanzania) were in one study [43], counted here as stand-alone sub-studies.
Out of 24,643 women tested for any HPV infection, 17,273 had normal cytology; 1,019 were classified as ASCUS; 1,444 were diagnosed with LSIL; 1,571 were HSIL-positive; and 4,067 had ICC. In addition, a total of 346 women were reported as HSIL or higher (either HSIL or ICC), 505 were classified as abnormal without separation into a specific lesion grade, and 257 were of an unspecified low lesion type. The last three categories were expunged from further analysis.
Based on the WHO classification of geographic regions of Africa, Eastern Africa had the highest number of women included in the study (10,246), followed by Western Africa (7,265), Southern Africa (4,983), and Northern Africa (2,510) (Table 1). Middle Africa was the least represented region, including only 30 women from two studies (Equatorial Guinea and Democratic Republic of Congo, formerly Zaire). South Africa (4,962) contributed the largest study size followed by Tanzania (4,398), Senegal (2,360), Nigeria (2,289) and Kenya (2,034). Morocco, Cote d’Ivoire, and Algeria each contributed over 1,000 women to the study. Each of the remaining 15 countries had fewer than 1,000 women included in the study. Two studies [46,47] included 429 women from three different countries without identifying the subjects to their specific country of origin.
Meta-analysis results according to Bethesda Classification
The meta-analysis of HPV type-specific prevalence by lesion types according to the Bethesda classification of women with normal cervical cytology and cervical neoplastic lesions (ASCUS, LSIL, HSIL and ICC) are presented in Table 2. Further sub-group analysis of the 71 studies was performed based on source of tissues, country and Gross National Income (GNI) classification according to the World Bank Table 3and S3 Table. There were no statistically significant differences in all the sub-group analysis for any HPV in women with normal cervix, neoplasia or ICC. Age-adjusted prevalence for any HPV and HPV16 categorized by region and lesion type is provided in Table 4.
Table 2. Prevalence of HPV by Bethesda Classification by cytology.
| HPV types | Normal | ASCUS | LSIL | HSIL | ICC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIGH-RISK | Type | Sample Size, N | # Cases (studies) | Prevalence (%) (95%CI) | Sample Size, N | # Cases (studies) | Prevalence (%) (95%CI) | Sample Size, N | # Cases (studies) | Prevalence (%) (95%CI) | Sample Size, N | # Cases (studies) | Prevalence (%)(95%CI) | Sample Size, N | # Cases (studies) | Prevalence (%) (95%CI) |
| hpv16 | 17273 | 766 (42) | 4.4 (4.1–4.7) | 1019 | 122 (15) | 12 (10.0–14.1) | 1444 | 213 (23) | 14.5 (12.8–16.4) | 1571 | 504 (33) | 31.2 (28.9–33.5) | 4067 | 2078 (43) | 49.7 (48.2–51.2) | |
| hpv18 | 17273 | 491 (42) | 2.8 (2.6–3.1) | 1019 | 45 (15) | 4.4 (3.2–5.9) | 1444 | 146 (23) | 10.0 (8.5–11.6) | 1571 | 224 (33) | 13.9 (12.2–15.6) | 4067 | 751 (43) | 18.0 (16.8–19.2) | |
| hpv31 | 17273 | 329 (42) | 1.9 (1.7–2.1) | 1019 | 27 (15) | 2.6 (1.8–3.8) | 1444 | 67 (23) | 4.6 (3.6–5.8) | 1571 | 133 (33) | 8.2 (6.9–9.7) | 3845 | 104 (38) | 2.7 (2.2–3.3) | |
| hpv33 | 17273 | 273 (42) | 1.6 (1.4–1.8) | 1019 | 24 (15) | 2.4 (1.5–3.5) | 1444 | 73 (23) | 5.0 (3.9–6.2) | 1571 | 167 (33) | 10.3 (8.9–11.9) | 3540 | 167 (32) | 4.7 (4–5.5) | |
| hpv35 | 17105 | 511 (40) | 3.0 (2.7–3.3) | 1019 | 40 (15) | 3.9 (2.8–5.3) | 1444 | 164 (23) | 11.2 (9.6–12.9) | 1571 | 216 (33) | 13.4 (11.7–15.1) | 3619 | 198 (31) | 5.5 (4.8–6.3) | |
| hpv39 | 16128 | 205 (38) | 1.3 (1.1–1.5) | 964 | 28 (13) | 2.9 (1.9–4.2) | 1386 | 50 (20) | 3.6 (2.7–4.7) | 1510 | 55 (31) | 3.4 (2.6–4.4) | 3605 | 39 (36) | 1.1 (0.8–1.5) | |
| hpv45 | 17008 | 339 (40) | 2.0 (1.8–2.2) | 1010 | 37 (14) | 3.7 (2.6–5.0) | 1444 | 76 (22) | 5.2 (4.1–6.5) | 1445 | 104 (31) | 6.4 (5.3–7.7) | 4067 | 408 (42) | 10.0 (9.1–10.9) | |
| hpv51 | 17105 | 363 (40) | 2.1 (1.9–2.3) | 1019 | 31 (15) | 3 (2.1–4.3) | 1444 | 84 (23) | 5.7 (4.6–7.0) | 1571 | 92 (33) | 5.7 (4.6–6.9) | 3619 | 76 (32) | 2.1 (1.7–2.6) | |
| hpv52 | 16128 | 518 (38) | 3.2 (2.9–3.5) | 964 | 66 (13) | 6.8 (5.3–8.6) | 1428 | 148 (21) | 10.4 (8.8–12.1) | 1438 | 191 (30) | 11.8 (10.3–13.5) | 3427 | 146 (30) | 4.3 (3.6–5) | |
| hpv56 | 16128 | 253 (38) | 1.6 (1.4–1.8) | 964 | 22 (13) | 2.3 (1.4–3.4) | 1386 | 89 (20) | 6.4 (5.2–7.8) | 1415 | 72 (29) | 4.5 (3.5–5.6) | 3427 | 33 (30) | 1.0 (0.7–1.3) | |
| hpv58 | 16128 | 421 (38) | 2.6 (2.4–2.9) | 964 | 26 (13) | 2.7 (1.8–3.9) | 1428 | 89 (21) | 6.2 (5.0–7.6) | 1438 | 183 (30) | 11.3 (9.8–13) | 3427 | 83 (30) | 2.4 (1.9–3) | |
| hpv59 | 16128 | 192 (40) | 1.2 (1–1.4) | 964 | 19 (13) | 2 (1.2–3.1) | 1386 | 54 (20) | 3.9 (2.9–5.1) | 1415 | 35 (29) | 2.2 (1.5–3) | 3618 | 27 (36) | 0.7 (0.5–1.1) | |
| LOW-RISK | hpv6 | 17273 | 132 (42) | 0.8 (0.6–0.9) | 1019 | 12 (15) | 1.2 (0.6–2.0) | 1444 | 38 (23) | 2.6 (1.8–3.5) | 1571 | 39 (33) | 2.4 (1.7–3.3) | 4181 | 42 (43) | 1 (0.7–1.4) |
| hpv11 | 13680 | 59 (36) | 0.4 (0.3–0.6) | 964 | 5 (13) | 0.5 (0.2–1.2) | 1230 | 17 (20) | 1.4 (0.8–2.2) | 1336 | 18 (29) | 1.1 (0.7–1.8) | 2919 | 12 (31) | 0.4 (0.2–0.7) | |
| hpv53 | 14664 | 258 (36) | 1.8 (1.6–2.0) | 964 | 28 (13) | 2.9 (1.9–4.2) | 1188 | 85 (19) | 7.2 (5.8–8.8) | 1232 | 92 (28) | 5.6 (4.6–6.9) | 3129 | 19 (28) | 0.6 (0.4–0.9) | |
| hpv54 | 13119 | 99 (31) | 0.8 (0.6–0.9) | 964 | 25 (13) | 2.6 (1.7–3.8) | 981 | 30 (17) | 3.1 (2.1–4.3) | 1085 | 34 (25) | 2.1 (1.5–2.9) | 2233 | 1 (20) | 0 (0–0.2) | |
| hpv66 | 14797 | 201 (36) | 1.4 (1.2–1.6) | 964 | 28 (13) | 2.9 (1.9–4.2) | 1188 | 60 (19) | 5.1 (3.9–6.5) | 1232 | 86 (28) | 5.3 (4.3–6.5) | 3209 | 23 (27) | 0.7 (0.5–1.1) | |
| hpv68 | 15819 | 245 (37) | 1.5 (1.4–1.8) | 964 | 19 (13) | 2 (1.2–3.1) | 1428 | 50 (21) | 3.5 (2.6–4.6) | 1438 | 62 (30) | 3.8 (3.0–4.9) | 2051 | 30 (21) | 1.5 (1–2.1) | |
| hpv70 | 13098 | 108 (30) | 0.8 (0.7–1) | 885 | 9 (12) | 1 (0.5–1.9) | 1002 | 31 (18) | 3.1 (2.1–4.4) | 833 | 22 (22) | 1.4 (0.9–2.1) | 2525 | 5 (27) | 0.2 (0.1–0.5) | |
| hpv82 | 11558 | 20 (27) | 0.2 (0.1–0.3) | 964 | 2 (13) | 0.2 (0–0.7) | 821 | 11 (16) | 1.3 (0.7–2.4) | 1013 | 9 (24) | 0.6 (0.3–1.1) | 1485 | 0 (21) | 0 (0–0.2) | |
| hpvmult* | 15157 | 1399 (37) | 9.2 (8.8–9.7) | 897 | 474 (14) | 20.6 (18–23.4) | 1054 | 475(20) | 44.6 (41.6–47.7) | 1519 | 1371 (29) | 38.5 (36.1–40.9) | 3553 | 545 (33) | 15.3 (14.2–16.6) | |
| anyhpv | 17273 | 5074 (42) | 29.3 (28.6–30.0) | 1019 | 185 (15) | 46.5 (43.4–49.6) | 1444 | 1087(23) | 74.2 (71.9–76.4) | 1571 | 622 (33) | 84.8 (82.9–86.5) | 4067 | 3741 (43) | 89.5 (88.5–90.4) | |
hpvmult*—Multiple HPV genotypes.
Table 3. Overall prevalence of any HPV according to year of publication, primers, and age categories.<.
/Table_Caption>
| ICC | HSIL | LSIL | ASCUS | Normal | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (Studies) | % Prevalence (95% CI) | Cases (Studies) | % Prevalence (95% CI) | Cases (Studies) | % Prevalence (95% CI) | Cases (Studies) | % Prevalence (95% CI) | Cases (Studies) | % Prevalence (95% CI) | ||
| YEAR OF PUBLICATION | 1986–1990 | 13 (1) | 100 (87.6–112.4) | 98 (1) | 67.4 (57.7–77.0) | ||||||
| 1991–1995 | 266 (8) | 85.4 (77.3–93.6) | 74(2) | 70.8 (22.5–119.1) | 6 (1) | 75.0 (44.1–106) | 15 (1) | 19.5 (10.1–28.9) | |||
| 1996–2000 | 181 (3) | 76.4 (62.0–90.8) | 60 (1) | 81.7 (71.2–92.1) | 144 (3) | 72.9 (50.3–95.7) | 2 (1) | 22.2(-6.4–50.8) | 228 (6) | 21.9 (14.8–29.2) | |
| 2001–2005 | 646 (8) | 98.4 (97.2–99.6) | 426 (6) | 88.6 (81.1–95.8) | 72 (3) | 70.2 (39.6–100.9) | 123 (2) | 55.1 (-12.9–123.1) | 818 (8) | 26.5 (18.0–35.0) | |
| 2006–2010 | 1060 (11) | 91.2 (86.1–96.2) | 335 (13) | 93.4 (88.8–98.0) | 389 (10) | 81.6 (71.1–92.1) | 186 (172) | 78.4 (52.3–104.5) | 1672 (15) | 59.1 (46.5–71.7) | |
| 2011–2015 | 1575 (13) | 88.6 (85.2–91.9) | 624 (11) | 88.8 (83.8–93.8) | 476 (8) | 72.8 (60.3–85.3) | 172 (4) | 47.9 (39.4–56.5) | 2341 (13) | 35.9 (23.2–48.7) | |
| Total | 3741 (42) | 90.5 (88.2–92.7) | 1617 (33) | 87.2 (83.4–91.1) | 1087 (23) | 75.8 (68.3–83.4) | 483 (15) | 65.7 (48.0–83.4) | 5074 (43) | 40.2 (33.9–46.5) | |
| PRIMER | (GP)5+/6+ | 1152 (10) | 93.4 (90.3–96.4) | 159 (5) | 80.5 (67.2–93.6) | 193 (3) | 58.4 (45.4–71.4) | 164 (3) | 61.9 (31.5–92.2) | 1054 (12) | 34.7 (22.9–46.7) |
| In-house primer | 385 (8) | 86.3 (77.5–95.1) | 238 (4) | 75.6 (57.5–93.8) | 41 (2) | 70.2 (18.9–121.5) | 2 (1) | 22.2 (-6.4–50.8) | 88 (4) | 24.7 (10.2–39.1) | |
| PGMY&GP | . | . | . | . | . | . | 183 (1) | 40.3 (35.7–44.9) | |||
| PGMY09/11 | 351 (11) | 90.6 (85.2–96.0) | 569 (15) | 92.7 (88.7–96.6) | 523 (11) | 86.9 (79.9–93.9) | 149 (7) | 85.6 (66.1–105.0) | 1773 (13) | 60.3 (44.4–76.2) | |
| SPF | 1772 (13) | 89.8 (85.5–94.2) | 180 (5) | 94.2 (90.4–98.0) | 77 (3) | 79.8 (59.3–100.2) | 70 (1) | 41.4 (33.8–49.1) | 922 (5) | 40.3 (18.9–61.*) | |
| MY09/11 | 81 (2) | 75.6 (38.1–113.2) | 225 (5) | 82.0 (70.4–93.5) | 253 (6) | 66.3 (46.5–86.1) | 89 (3) | 34.2 (3.7–64.6) | 1054 (8) | 24.7 (15.9–33.5) | |
| Total | 3741 (42) | 90.5 (88.2–92.7) | 1379 (33) | 87.2 (83.3–91.1) | 1087 (23) | 75.8 (68.3–83.4) | 474 (15) | 63.5 (45.3–81.6) | 5074 (42) | 40.2 (33.9–46.5) | |
| AGE CATEGORY | 15–24 | . | . | 9 (1) | 100.0 (83.2–116.8) | 10 (1) | 100.0 (84.6–115.4) | 217 (2) | 48.2 (14.7–81.6) | ||
| 25–34 | 599 (11) | 92.5 (86.3–98.7) | 390 (11) | 86.7(78.5–95.0) | 515 (8) | 81.5 (69.7–93.4) | 147 (6) | 80.9 (61.9–99.9) | 886 (11) | 50.5 (37.1–63.8) | |
| 35–44 | 723 (9) | 94.7 (90.9–98.5) | 835 (14) | 91.3 (87.8–94.8) | 529 (13) | 64.8 (52.4–77.2) | 301 (7) | 51.7 (27.0–76.4) | 2579 (20) | 36.1 (26.9–45.2) | |
| 45–54 | 1627 (16) | 91.7 (88.8–94.6) | 12 (2) | 87.1 (67.3–107.0) | 11 (1) | 100.0 (85.8–114.2) | 3 (1) | 6.5 (-1.7–14.8) | 1043 (6) | 31.6 (14.9–48.3) | |
| 55–64 | 480 (5) | 88.1 (82.2–94.0) | . | . | . | . | |||||
| >65 | . | . | . | . | . | . | |||||
| Missing | 312 (3) | 72.6 (52.2–93.1) | 133 (6) | 80.4 (62.9–97.9) | 22 (2) | 91.9 (69.0–114.8) | 23 (1) | 100.0 (92.6–107.4) | 349 (4) | 41.4 (38.0–44.8) | |
| Total | 3741 (44) | 90.5 (88.2–92.7) | 1379 (34) | 87.2 (83.3–91.1) | 1087 (25) | 75.8 (68.3–83.4) | 474 (15) | 63.5 (45.3–81.6) | 5074 (43) | 40.2 (33.9–46.5) | |
Abbreviations: Lesions-ASCUS: Atypical squamous cells of undetermined significance; LSIL: Low-grade squamous intraepithelial lesions; HSIL: High-grade squamous intraepithelial lesions; ICC: Invasive cervical cancer (this included SCC and ADC). CI: Confidence interval; N: Number of cases tested for a given HPV type. Blank boxes means indeterminate.
Table 4. Lesion specific age-adjusted prevalence for any HPV and HPV16 for each region.
| Age | Any HPV | HPV16 | Region | Any HPV | HPV16 | |
|---|---|---|---|---|---|---|
| Normal | <25 | 36.0(32.3–39.9) | 6.8(5.1–9.1) | Eastern | 33.1(31.6–34.5) | 4.5(4.0–5.3) |
| 25-<35 | 47.7(45.4–50.0) | 5.9(5.0–7.1) | Southern | 58.5(56.2–60.7) | 11.2(9.6–13.1) | |
| 35-<45 | 26.8(26.2–27.6) | 4.1(3.7–4.5) | Western | 28.7(27.1–30.8) | 4.2(3.5–5.0) | |
| 45-<55 | 25.3(23.8–26.9) | 3.7(3.2–4.5) | Middle | - | - | |
| = >55’ | - | - | Northern | 16.9(14.9–19.2) | 3.0(2.2–4.2) | |
| ASCUS | <25 | - | - | Eastern | 60.2(55.4–64.8) | 15.6(11.8–19.7) |
| 25-<35 | 65.0(58.6–71.0) | 6.6(4.0–10.7) | Southern | 62.7(55.7–69.2) | 6.3(3.8–10.1) | |
| 35-<45 | 41.2(37.9) | 13.5(11.3–16.1) | Western | 29.6(23.8–36.3) | 1.7(0.9–3.3) | |
| 45-<55 | - | - | Middle | - | - | |
| = >55’ | - | - | Northern | - | - | |
| LSIL | <25 | 100(0–100) | 10.0(2.5–32.4) | Eastern | - | - |
| 25-<35 | 76.0(72.6–79.0) | 16.8(14.2–19.8) | Southern | - | - | |
| 35-<45 | 70.8(67.4–73.9) | 10.8(8.7–13.2) | Western | - | - | |
| 45-<55 | 100(0–100) | 100(0–100) | Middle | - | - | |
| = >55’ | - | - | Northern | - | - | |
| HSIL | <25 | 100.0(0–100) | 5.6(0.7–3.1) | Eastern | - | 27.2(17.3–40.0) |
| 25-<35 | 81.5(77.7–84.7) | 28.6(24.7–32.8) | Southern | - | 28.9(18.2–42.7) | |
| 35-<45 | 80.0(73.1–85.4) | 31.8(29.1–34.7) | Western | - | 26.2(14.5–42.7) | |
| 45-<55 | 85.6(57.2–96.4) | 64.3(37.6–84.3 | Middle | - | - | |
| = >55’ | - | - | Northern | - | - | |
| ICC | <25 | - | - | Eastern | 91.4(89.9–92.9) | - |
| 25-<35 | 91.7(89.4–93.6) | 47.5(43.7–51.3) | Southern | 82.1(77.3–86.0) | - | |
| 35-<45 | 88.2(86.2–90.0) | 47.2(44.3–50.2) | Western | 87.3(84.4–89.7) | - | |
| 45-<55 | 91.1(89.7–92.4) | 52.8(50.4–55.1) | Middle | - | ||
| = >55’ | - | 49.9(45.7–54.1) | Northern | 89.0(85.2–91.9) | - |
'-' Age-group adjusted prevalence cannot be estimated because of sparse data.
Heterogeneity was found to be substantial, ICC: I2 = 88.8%, 43 studies; HSIL I2 = 81.5%, 33 studies; LSIL I2 = 93.3%, 23 studies; ASCUS I2 = 98.5%, 15 studies; and Normal I2 = 99.1%, 42 studies, all p-values <0.001. Random effects age-group adjusted meta-analysis using logistic regression analysis were performed for every HPV genotype.
Prevalence of HPV genotypes in women with normal cervical cytology
Systematic review included 42 studies testing for cervical HPV infection in 17,273 women with normal cytology. This is the highest number of African women with normal cervical cytology ever included in a single HPV prevalence meta-analysis study. Most of the women tested for HPV infections were from Eastern Africa (n = 6,640), followed by Western Africa (n = 5,600), Southern Africa (n = 3,030) and Northern Africa (n = 1,865). A total of 138 individuals were from studies that contained mixed countries (Table 1). HPV16, 52, 35, 18, 58, 51, 45, 31, 53 and 56 were the ten most common genotypes in women with normal cervical cytology in descending order (Table 2).
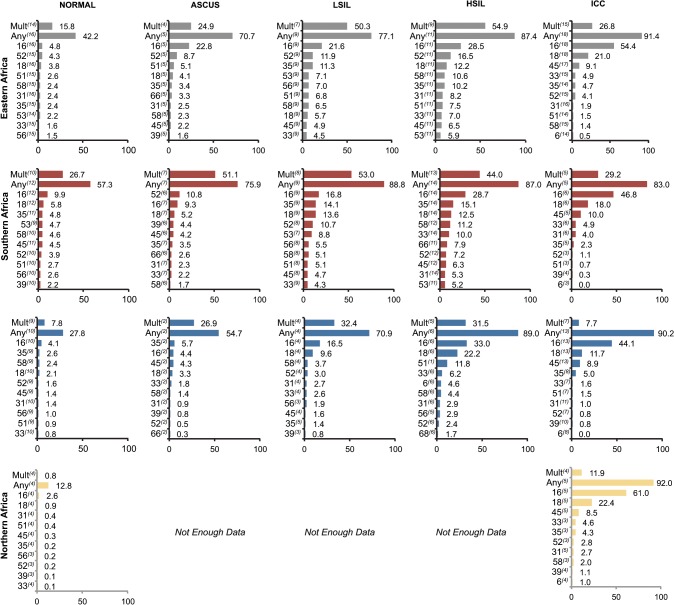
There was wide variation in any HPV infection rates based on region, with Southern Africa (57.3%) having the highest prevalence, followed by Eastern Africa (42.2%), Western Africa (27.8%), and Northern Africa (12.8%) (Fig 2left panel). Type-specific HPV16 and 18 were higher among women with normal cervical cytology from Southern Africa compared to other regions at 9.9% and 5.8% respectively (Fig 2). No study representing women from Middle Africa met the inclusion criteria.
Fig 2. The ten most common HPV genotypes by regions and lesion types according to Bethesda classifications, respectively.
Type specific prevalence is weighted by study size. The superscript numbers in brackets represent the number of studies included for the specific HPV genotype. There was not enough data from the middle African region.
Overall, 39 studies stratified eligible women screened for HPV infection according to age groups. Analysis of these studies revealed that the age group 25–34 years had the highest overall HPV prevalence at 50.5% (95% CI: 37.1–63.8; I2 = 97.2%), closely followed by the age group 15–24 years with 48.2% (95% CI: 14.7–81.6; I2 = 96.6%). Age groups 35–44 and 45–54 years had HPV prevalence of 36.1% (95% CI: 26.9–45.2; I2 = 99.3%) and 31.6% (95% CI: 14.9–48.3; I2 = 99.2%), respectively, all p-values <0.001 (Table 3). Further lesion-specific age-adjusted prevalence for any HPV and HPV16 for each region is provided in Table 4.
Prevalence of HPV genotypes in Women with ASCUS and LSIL
To date, only one meta-analysis of HPV type-specific prevalence among women from Africa with ASCUS and LSIL exists [21]. However, in that study there were less than 300 women included in each lesion type. Our meta-analysis included fifteen studies testing for HPV infection in 1,019 women diagnosed with ASCUS. Most of the women tested for HPV infections were from Eastern Africa (n = 440), followed by women from Western Africa (n = 326), Southern Africa (n = 207) and Northern Africa (n = 46). Of the women tested for an HPV infection in Middle Africa, none were diagnosed with ASCUS (Table 1).
HPV16, 52, 18, 35, 45, 51, 66, 53, 39 and 58 were the ten most common genotypes in women with ASCUS (Table 2). The prevalence of type-specific HPV and distribution in women diagnosed with ASCUS for each geographic region is presented in Fig 2, in the second panel from the left.
For LSIL, data from a total of 1,444 women from 23 studies were abstracted. A majority of the patients were from Eastern (n = 605), followed by Southern (n = 521), Western (n = 286, Northern (n = 24) and Middle (n = 8) (Table 1) Africa regions. HPV16, 35, 52, 18, 53, 56, 58, 51, 45 and 66 were the top ten most common genotypes in women with LSIL (Table 2). HPV type specific prevalence and distribution by region for LSIL is summarized in (Fig 2, in the third panel from the left).
Prevalence of HPV genotypes in high-grade intraepithelial lesions
Guan et al. [21] attempted to analyze African HPV genotype prevalence in high-grade lesions, where only 185 out of 245 patient samples tested positive for HPV. In our systematic review, 33 studies comprising a total of 1,571 HSIL cases were included in the analysis. A majority of HSIL cases came from Southern (n = 768), followed by Eastern (n = 563), Western (n = 102), and Middle (n = 19) regions of Africa. Only 8 and 17 cases were from the Northern region and mixed region, respectively.
HPV16, 18, 35, 52, 58, 33, 31, 53, 45 and 66 were the ten most frequently detected genotypes in HSIL cases (Table 2). HPV type-specific prevalence and distribution by region for HSIL is summarized in (Fig 2). Western Africa reported the highest prevalence of HPV16 and 18 at 33% and 22.2% respectively for HSIL. In Eastern and Southern regions, HPV16 prevalence was tied at 28%, followed by HPV 52 (16.5%) and HPV 35 (15.1%), respectively.
Overall, 30 studies stratified eligible women screened for HPV infection by age. Analysis of these studies revealed that the age group 45–54 years had the highest overall prevalence of any HPV for HSIL at 87.1% (95% CI: (67.3–107.0; I2 = 0.0%) as shown in Table 3.
Prevalence of HPV genotypes in cervical cancer
A total of 4,067 cases from 43 studies were included in the ICC systematic review analysis (Table 1). A majority of the ICC cases were from the Eastern region (n = 1,905), followed by Western (n = 951), Northern (n = 517), Southern (n = 457) regions, and only three patients were from the Middle Africa region. Two hundred and thirty-five patients were from mixed regions.
HPV16, 18, 45, 35, 33, 52, 31, 58, 51 and 68 were the top ten most frequently detected genotypes among ICC cases (Table 2). While HPV16, 18, 35, and 52 were the four most common types in normal cytology through HSIL lesions; in ICC tissues HPV45 replaced HPV52 as the third most common genotype (Table 2). Together HPV16, 18, and 45 accounted for 77.7% of all ICC cases in African women.
HPV type-specific prevalence and distribution by region is presented in Fig 2. The prevalence of HPV16, 18, and 45 in ICC cases were 49.7% (95% CI: 48.2–51.2; I2 = 88.6%), 18.0% (95% CI: 16.8–19.2; I2 = 78.2%) and 10.0% (95% CI: 9.1–10.9; I2 = 85.7%), respectively. HPV16 and 18 varied by region; in descending order: Northern (61%, and 22.4%), Eastern (54.4% and 21.0%), Southern (46.8% and 18%), and Western (44.1% and 11.7%) regions of Africa.
Discussion
Comprehensive data on the geographic distribution of specific HPV genotypes in women with all grades of cervical diagnosis are crucial for estimating the baseline burden of disease, the future impact of HPV vaccines on cervical cancer prevention, and the identification of optimal HPV screening tools. To the best of our knowledge, our report is the largest meta-analysis to date of HPV genotype distribution and prevalence in women from Africa by region, lesion type, age group, publication year, and PCR primers for genotyping. Seventy-one studies matched the inclusion criteria and were abstracted for systematic review and meta-analysis. Each of the five sub-regions of African countries (n = 23) was fairly represented with the exception of the Middle region, where only two studies were included.
Overall, the prevalence of any HPV infection in Africa was higher than other world regions; our meta-analysis determined the prevalence rates in women with normal cervical cytology (29.3%), ASCUS (46.5%), LSIL (74.2%), HSIL (84.8%) and ICC (89.5%) [21]. Our study revealed that by pooling several studies examining HPV infection among women with normal cervical cytology, Southern Africa has the highest of any HPV infections at 57.3%, followed by Eastern Africa (42.2%), Western Africa (27.8%) and Northern Africa (12.8%). The prevalence of any HPV infection increased among women by cervical disease grade in all regions, except Southern Africa. However, the inclusion in this analysis of various genotyping techniques with varying sensitivities might impact the accuracy of HPV specific genotype prevalence.
Nevertheless, the four most common genotypes, HPV16, 18, 52 and 35, were identical from normal cervical cytology through HSIL. In ICC cases, however, HPV45 replaced HPV52 as the third most common genotype. The high prevalence rate of HPV45 in Africa was previously reported among ICC cases [18,19,22,110,111]. Further analysis of women diagnosed with ICC by region confirmed that despite the differences in prevalence of HPV16, 18 and 45, these three genotypes remained the most common in all regions for ICC. HPV33 was the fourth most common in all regions except in Western Africa, where it is HPV35. These findings suggest a higher prevalence of HPV45 in ICC and HPV52 in HSIL than previously found [23]. The prevalence of HPV16 and 18 were much greater in ICC cases than in HSIL cases (49.7% vs. 31.2% and 18.0% vs. 13.9%, respectively). The combined prevalence of HPV16 and 18 in cases of ICC is 67.7%, in agreement with the ~70% global estimate found by other meta-analyses [19,21–23].
While there has been a substantial push toward mass vaccination with either Gardasil or Cervarix [112], until now there was insufficient data to demonstrate the high prevalence of HPV16/18 in cases of ICC in all sub-regions of the African continent. Data from the present study indicate that vaccines currently available could prevent nearly 70% of cervical cancer cases in all sub-regions of Africa. Moreover, a polyvalent vaccine in Phase III clinical trials known to protect against HPV16, 18, 31, 33, 35, 45, 51, 52, and 58 could prevent nearly all cases of cervical cancer in African women. Unfortunately, because most African countries are currently unprepared to implement HPV vaccination, even the availability of this nonavalent vaccine would leave an entire generation unprotected in Africa [113]. These barriers include the long duration of time it would take for the vaccine to be ready for mass distribution, the negotiation of its cost, and the placement of infrastructure to deliver the vaccine to adolescent girls at risk of developing cervical cancer [13,114]. Thus, other cancer prevention tools such as cervical screening through visual inspection with acetic acid and/or Lugol’s iodine, or detection of high-risk HPV genotypes may help reduce the high burden of cervical cancer morbidity and mortality in the region.
Using the stringent inclusion and exclusion criteria outlined in our meta-analysis, six studies from Northern Africa qualified for data abstraction for women with normal cervical cytology (n = 1,865) and ICC (n = 517). Notably, 83.4% of ICC cases were associated with either HPV16 (61%) or 18 (22.4%) in the sub-region, the highest number ever reported from any part of the globe. However, the prevalence of any HPV infection among women with normal cytology was low (12.8%) compared to other regions which ranged between 27.8–57.3%. The prevalence of HPV16 and 18 is 2.9% and 0.9%, respectively. Since most of the women screened for cervical cancers are adults over 18 years of age, low prevalence of HPV16 and 18 in the population suggests that there is a wider window available for delivering HPV vaccine, particularly if it is combined with HPV DNA screening among older women. A recent review on the burden of HPV infections and related diseases in the extended Middle East and Northern Africa reported very low prevalence of HPV infection in women with normal cervical cytology, similar to current findings [115]. However, our study revealed a higher prevalence of HPV16/18 among women diagnosed with ICC than previously reported [115]. The high prevalence of HPV16/18 in ICC in Northern Africa requires urgent attention such as encouraging the use of culturally acceptable tools to increase cervical cancer screenings and HPV DNA testing among the population so as to have a complete picture of the prevalence of HPV genotypes by all stages of cervical cancer grade. Due to limited data, our study was unable to yield conclusive HPV prevalence in women from Northern Africa diagnosed with ASCUS, LSIL and HSIL cervical lesions, due to limited data. Of great concern to policy makers in Northern Africa region is that very few interventions to create awareness about cervical cancer, implementation of cervical cancer screening programs, HPV DNA testing, or use of prophylactic vaccines to prevent HPV infection have been reported.
A majority of the population in Northern Africa share similar cultural and religious traditions and known to have more conservative views towards sexual behavior than countries in sub-Saharan Africa. If indeed future studies in Northern Africa clearly indicate that a majority of women in the region with normal cervical cytology have lower risk of any HPV infection even at advanced ages (18–26 years), then it will be important to increase the age of vaccination for Northern African girls up from the current WHO recommended 9–13 years to a much older age [116]. The legal adult age is between 16–18 years in most countries, which could serve as a more appropriate vaccination age for the region.
The increasing availability of additional tools to prevent and fight cervical cancer provides an opportunity for all nations to implement cervical cancer control programs that are both cost-effective and easier to deliver or to improve existing programs by saving additional lives and reducing cost [117]. The GAVI Alliance, in an effort to close the gap in access to life-saving vaccines [28], recently announced a deal with pharmaceutical industries to offer the HPV vaccine at US $4.50 per dose [28,29] to developing countries meeting GAVI’s eligibility criteria [13]. To date, 19 sub-Saharan African countries have been approved to pilot HPV vaccine demonstration projects, however, despite the high prevalence of HPV16/18 in ICC cases in Northern Africa, not a single country from the region has been approved for HPV vaccine support [29].
The implementation of one lifetime screening at age 35 with either visual inspection with acetic acid or HPV DNA testing would reduce the risk of developing cervical cancer by 25% and cost less than $500 per person per year of life saved [11]. However, the lack of laboratory infrastructure for HPV genotyping in nearly the entire African continent, with the exception of South Africa, Morocco and Ethiopia as shown in our study, should be a major concern to all who are working to fight cervical cancer in Africa. In our systematic review, the majority of the HPV DNA genotyping (for the studies eligible for data abstraction) was performed outside of Africa in the USA, Japan or Europe. HPV DNA testing is currently the most sensitive and reproducible cervical screening test [118], therefore, there is an urgent need to build the infrastructure in Africa to facilitate efficient cervical screening.
The major contribution of our study to the current body of literature is the assessment of 25,463 women from all African regions of whom 46.8% were HPV infected. A further stratification of specific HPV genotype prevalence by disease severity will help identify appropriate tools for HPV genotyping and assess the future impact of HPV vaccines. However, the applicability of this data throughout Africa is limited, since 95% of the studies were obtained from Eastern, Western and Southern African regions, partially attributable to the lack of well-designed studies in the remainder of Africa, especially Middle and Northern Africa. Indeed, quality studies meeting stringent meta-analysis criteria for inclusion were missing from 30 African countries, thus future cervical cancer prevention policies should consider building laboratory infrastructure in these countries.
The current WHO recommendations on cervical cancer screening are based on age [119], however, only a few studies stratified the population studied by age. A majority of studies reported mean or median age, which is not helpful in defining the prevalence of HPV infection by age-group to determine an appropriate age to target for HPV DNA testing or cervical cancer screening. This consideration should be factored in the design of future studies in the continent.
Some of the limitations to our study include: (i) the inclusion of cross-sectional studies and their inherent risk of bias; (ii) inclusion of studies that over sampled HIV-seropositive patients in the analysis; and (iii) there may be lack of representativeness of HPV type specific prevalence due to variation in different genotyping methods used and tissue sources. Finally, while we made every effort to identify and include in our meta-analysis all eligible studies, it is possible that we missed some.
Although previous research has established the relationship between HIV and HPV infection, the inter-relationship is not fully understood [63,102,120]. Of the estimated 35.3 million individuals worldwide living with HIV/AIDS in 2012, approximately 70% are living in Africa [121]. Women make up 60% of the HIV-infected population in Africa [121], and nearly 40% of HIV seropositive individuals without cervical abnormalities test positive for HPV infection [110]. Studies are underway in our group to specifically estimate the prevalence of HPV genotypes among women living with HIV in Africa.
Supporting Information
(DOC)
(PDF)
(DOC)
(DOCX)
(DOCX)
(PDF)
Acknowledgments
This study was not funded, mainly done on the kitchen table. Authors have no conflict of interest to declare. We are grateful to Dr. Melanie Trombly for her critical review of the manuscript and language editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sitas F, Parkin M, Chirenje Z, Stein L, Mqoqi N, Wabinga H (2006) Cancer In: Dean T. Jamison, Richard G. Feachem, Malegapuru W. Makgoba, Eduard R. Bos, Florence K. Baingana et al. , editors. Diseases and mortality in Sub-Saharan Africa: World Bank. [Google Scholar]
- 2. Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. International journal of cancer 118: 3030–3044. [DOI] [PubMed] [Google Scholar]
- 3. Burd EM (2003) Human papillomavirus and cervical cancer. Clinical microbiology reviews 16: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munoz N, Bosch FX, De Sanjose S, Herrero R, Castellsague X, Shah KV, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England Journal of Medicine 348: 518–527. [DOI] [PubMed] [Google Scholar]
- 5. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA: a cancer journal for clinicians 61: 69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 6. Gakidou E, Nordhagen S, Obermeyer Z (2008) Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS medicine 5: e132 10.1371/journal.pmed.0050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK (2014) Early Impact of Human Papillomavirus Vaccination on Cervical Neoplasia—Nationwide Follow-up of Young Danish Women. Journal of the National Cancer Institute 106: djt460 10.1093/jnci/djt460 [DOI] [PubMed] [Google Scholar]
- 8. Mesher D, Soldan K, Howell-Jones R, Panwar K, Manyenga P, Jit M, et al. (2013) Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine 32: 26–32. 10.1016/j.vaccine.2013.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. (2012) Fall in human papillomavirus prevalence following a national vaccination program. Journal of Infectious Diseases 206: 1645–1651. 10.1093/infdis/jis590 [DOI] [PubMed] [Google Scholar]
- 10. Agosti JM, Goldie SJ (2007) Introducing HPV vaccine in developing countries,Äîkey challenges and issues. New England Journal of Medicine 356: 1908–1910. [DOI] [PubMed] [Google Scholar]
- 11. Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mah√© C, et al. (2005) Cost-effectiveness of cervical-cancer screening in five developing countries. New England Journal of Medicine 353: 2158–2168. [DOI] [PubMed] [Google Scholar]
- 12. Ogembo JG, Manga S, Nulah K, Foglabenchi LH, Perlman S, Wamai RG, et al. (2014) Achieving High Uptake of Human Papillomavirus Vaccine in Cameroon: Lessons learned in overcoming challenges. Vaccine 32: 4399–4403. 10.1016/j.vaccine.2014.06.064 [DOI] [PubMed] [Google Scholar]
- 13. Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG (2014) Knowledge and Awareness of HPV Vaccine and Acceptability to Vaccinate in Sub-Saharan Africa: A Systematic Review. PLoS One 9: e90912 10.1371/journal.pone.0090912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, de Sanjosé S (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. Journal of Infectious Diseases 202: 1789–1799. 10.1086/657321 [DOI] [PubMed] [Google Scholar]
- 15. de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Munoz N, et al. (2007) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet infectious diseases 7: 453–459. [DOI] [PubMed] [Google Scholar]
- 16. Bhatla N, Lal N, Bao YP, Ng T, Qiao YL (2008) A meta-analysis of human papillomavirus type-distribution in women from South Asia: Implications for vaccination. Vaccine 26: 2811–2817. 10.1016/j.vaccine.2008.03.047 [DOI] [PubMed] [Google Scholar]
- 17. Ciapponi A, Bardach A, Glujovsky D, Gibbons L, Picconi MA (2011) Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: Systematic Review and Meta-analysis. PloS one 6: e25493 10.1371/journal.pone.0025493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clifford G, Smith J, Aguado T, Franceschi S (2003) Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. British Journal of Cancer 89: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clifford G, Smith J, Plummer M, Munoz N, Franceschi S (2003) Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. British Journal of Cancer 88: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clifford GM, Gonçalves MAG, Franceschi S (2006) Human papillomavirus types among women infected with HIV: a meta-analysis. Aids 20: 2337 [DOI] [PubMed] [Google Scholar]
- 21. Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. (2012) Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 131: 2349–2359. 10.1002/ijc.27485 [DOI] [PubMed] [Google Scholar]
- 22. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM (2011) Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 128: 927–935. 10.1002/ijc.25396 [DOI] [PubMed] [Google Scholar]
- 23. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. International journal of cancer 121: 621–632. [DOI] [PubMed] [Google Scholar]
- 24. Tricco A, Ng C, Gilca V, Anonychuk A (2011) Canadian oncogenic human papillomavirus cervical infection prevalence: Systematic review and meta-analysis. BMC infectious diseases 11: 235 10.1186/1471-2334-11-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Youngblood R (2013) GAVI injects new life into HPV vaccine rollout. Lancet 381: 1688 10.1016/S0140-6736(13)61059-4 [DOI] [PubMed] [Google Scholar]
- 26. Campos NG, Kim JJ, Castle PE, Ortendahl JD, O'Shea M, Diaz M, et al. (2012) Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. International Journal of Cancer 130: 2672–2684. 10.1002/ijc.26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Louie KS, De Sanjose S, Mayaud P (2009) Epidemiology and prevention of human papillomavirus and cervical cancer in sub Saharan Africa: a comprehensive review. Tropical Medicine & International Health 14: 1287–1302. [DOI] [PubMed] [Google Scholar]
- 28.GAVI (2011) GAVI welcomes lower prices for life-saving vaccines.[press release], June 6, 2011.
- 29.GAVI (2014) 206,000 more girls to benefit from HPV vaccine with GAVI Alliance support
- 30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. (2000) Meta-analysis of observational studies in epidemiology. JAMA: the journal of the American Medical Association 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogembo JG, Bain PA, Seymour A, Park HS (2013) Human papillomavirus genotype prevalence among African women with normal cervical cytologies and lesions: a systematic review and meta-analysis. PROSPERO International prospective register of systematic reviews.
- 34. Solomon CG, Schiffman M, Solomon D (2013) Cervical-Cancer Screening with Human Papillomavirus and Cytologic Cotesting. New England Journal of Medicine 369: 2324–2331. 10.1056/NEJMcp1210379 [DOI] [PubMed] [Google Scholar]
- 35. Solomon N, Nayar R (2004) The Bethesda System for reporting cervical cytology: definitions, criteria, and explanatory notes: Springer. [Google Scholar]
- 36. Castellsagué X, Klaustermeier J, Carrilho C, Albero G, Sacarlal J, Quint W, et al. (2008) Vaccine-related HPV genotypes in women with and without cervical cancer in Mozambique: Burden and potential for prevention. International Journal of Cancer 122: 1901–1904. [DOI] [PubMed] [Google Scholar]
- 37. Hammouda D, Clifford GM, Pallardy S, Ayyach G, Chékiri A, Boudrich A, et al. (2011) Human papillomavirus infection in a population-based sample of women in Algiers, Algeria. International journal of cancer 128: 2224–2229. 10.1002/ijc.25539 [DOI] [PubMed] [Google Scholar]
- 38. Hammouda D, Munoz N, Herrero R, Arslan A, Bouhadef A, Oublil M, et al. (2005) Cervical carcinoma in Algiers, Algeria: human papillomavirus and lifestyle risk factors. International journal of cancer 113: 483–489. [DOI] [PubMed] [Google Scholar]
- 39. Rogo K (1990) Human papillomavirus and human immunodeficiency virus infection in relation to cervical cancer: Studies and observations of basic clinical and epidemiological aspects of cancer of the cervix with special reference to Kenya, Africa: Umea University. [Google Scholar]
- 40. Sahasrabuddhe V, Mwanahamuntu M, Vermund S, Huh W, Lyon M, Stringer J, et al. (2007) Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. British Journal of Cancer 96: 1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jones HE, Allan BR, van de Wijgert JHHM, Altini L, Taylor SM, de Kock A, et al. (2007) Agreement between self-and clinician-collected specimen results for detection and typing of high-risk human papillomavirus in specimens from women in Gugulethu, South Africa. Journal of clinical microbiology 45: 1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abate E, Aseffa A, El-Tayeb M, El-Hassan I, Yamuah L, Mihret W, et al. (2013) Genotyping of human papillomavirus in paraffin embedded cervical tissue samples from women in Ethiopia and the Sudan. J Med Virol 85: 282–287. 10.1002/jmv.23437 [DOI] [PubMed] [Google Scholar]
- 43. Bosch FX, Manos MM, MuN" d N, Sherman M, Jansen AM, Peto J, et al. (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. Journal of the National Cancer Institute 87: 796–802. [DOI] [PubMed] [Google Scholar]
- 44. Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. (2014) Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. International journal of cancer 134: 1389–1398. 10.1002/ijc.28425 [DOI] [PubMed] [Google Scholar]
- 45.Ndiaye C, Alemany L, Ndiaye N, Kamate B, Diop Y, Odida M, et al. (2012) Human papillomavirus distribution in invasive cervical carcinoma in sub-Saharan Africa: could HIV explain the differences? Trop Med Int Health. [DOI] [PubMed]
- 46.De Vuyst H, Ndirangu G, Moodley M, Tenet V, Estambale B, Meijer CJLM, et al. (2011) Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa. International journal of cancer. [DOI] [PubMed]
- 47. Dols JA, Reid G, Brown JM, Tempelman H, Bontekoe TR, Quint WG, et al. (2012) HPV Type Distribution and Cervical Cytology among HIV-Positive Tanzanian and South African Women. ISRN Obstet Gynecol 2012: 514146 10.5402/2012/514146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okolo C, Franceschi S, Adewole I, Thomas JO, Follen M, Snijders PJF, et al. (2010) Human papillomavirus infection in women with and without cervical cancer in Ibadan, Nigeria. Infectious Agents and Cancer 5: 24 10.1186/1750-9378-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas J, Herrero R, Omigbodun A, Ojemakinde K, Ajayi I, Fawole A, et al. (2004) Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. British Journal of Cancer 90: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arbyn M, Tommasino M, Depuydt C, Dillner J (2014) Are 20 human papillomavirus types causing cervical cancer? J Pathol 234: 431–435. 10.1002/path.4424 [DOI] [PubMed] [Google Scholar]
- 51. Stuart A, Ord JK (1994) Kendall’s advanced theory of statistics Vol. I Distribution theory. Arnold, London. [Google Scholar]
- 52. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 53. Odida M, de Sanjosé S, Quint W, Bosch XF, Klaustermeier J, Weiderpass E (2008) Human Papillomavirus type distribution in invasive cervical cancer in Uganda. Bmc Infectious Diseases 8: 85 10.1186/1471-2334-8-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allan B, Marais DJ, Hoffman M, Shapiro S, Williamson AL (2008) Cervical human papillomavirus (HPV) infection in South African women: implications for HPV screening and vaccine strategies. Journal of clinical microbiology 46: 740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cooper K, Herrington C, Graham A, Evans M, McGee J (1991) In situ evidence for HPV 16, 18, 33 integration in cervical squamous cell cancer in Britain and South Africa. Journal of clinical pathology 44: 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Denny L, Boa R, Williamson AL, Allan B, Hardie D, Stan R, et al. (2008) Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstetrics & Gynecology 111: 1380. [DOI] [PubMed] [Google Scholar]
- 57. Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, Sanne IM, et al. (2010) Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes and Control 21: 433–443. 10.1007/s10552-009-9475-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Firnhaber C, Zungu K, Levin S, Michelow P, Montaner LJ, McPhail P, et al. (2009) Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus-infected women in Johannesburg, South Africa. Acta cytologica 53: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kay P, Soeters R, Nevin J, Denny L, Dehaeck C, Williamson AL (2003) High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. Journal of medical virology 71: 265–273. [DOI] [PubMed] [Google Scholar]
- 60. Marais DJ, Passmore JAS, Denny L, Sampson C, Allan BR, Williamson AL (2008) Cervical and oral human papillomavirus types in HIV-1 positive and negative women with cervical disease in South Africa. Journal of medical virology 80: 953–959. 10.1002/jmv.21166 [DOI] [PubMed] [Google Scholar]
- 61. Marais DJ, Rose RC, Lane C, Kay P, Nevin J, Denny L, et al. (2000) Seroresponses to human papillomavirus types 16, 18, 31, 33, and 45 virus-like particles in South African women with cervical cancer and cervical intraepithelial neoplasia. Journal of medical virology 60: 403–410. [DOI] [PubMed] [Google Scholar]
- 62. McDonald AC, Denny L, Wang C, Tsai WY, Wright TC Jr., Kuhn L (2012) Distribution of high-risk human papillomavirus genotypes among HIV-negative women with and without cervical intraepithelial neoplasia in South Africa. PLoS One 7: e44332 10.1371/journal.pone.0044332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moodley J, Constant D, Hoffman M, Salimo A, Allan B (2009) Human papillomavirus prevalence, viral load and pre-cancerous lesions of the cervix in women initiating highly active antiretroviral therapy in South Africa: a cross-sectional study. BMC cancer 9: 275 10.1186/1471-2407-9-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramesar JE, Dehaeck CMC, Soeters R, Williamson A (1996) Human papillomavirus in normal cervical smears from Cape Town. South African Medical Journal-Cape Town-Medical Association of South Africa- 86: 1402–1405. [PubMed] [Google Scholar]
- 65. Richter KL, Van Rensburg EJ, Van Heerden WFP, Boy SC (2008) Human papilloma virus types in the oral and cervical mucosa of HIV-positive South African women prior to antiretroviral therapy. Journal of oral pathology & medicine 37: 555–559. [DOI] [PubMed] [Google Scholar]
- 66. Said H, Ahmed K, Burnett R, Allan B, Williamson AL, Hoosen AA (2009) HPV genotypes in women with squamous intraepithelial lesions and normal cervixes participating in a community-based microbicide study in Pretoria, South Africa. Journal of Clinical Virology 44: 318–321. 10.1016/j.jcv.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 67. Williamson AL, Brink NS, Dehaeck C, Ovens S, Soeters R, Rybicki EP (1994) Typing of human papillomaviruses in cervical carcinoma biopsies from Cape Town. Journal of medical virology 43: 231–237. [DOI] [PubMed] [Google Scholar]
- 68. Williamson AL, Dehaeck C, Soeters R (1989) Typing of human papillomaviruses in cervical intraepithelial neoplasia grade 3 biopsies from Cape Town. Journal of medical virology 28: 146–149. [DOI] [PubMed] [Google Scholar]
- 69. Mzibri ME, Mhand RA, Benider A, Benchekroun N, Benchekroun M, Ennaji M (2009) Molecular detection and genotyping of human papillomavirus in cervical carcinoma biopsies in an area of high incidence of cancer from Moroccan women. Journal of medical virology 81: 678–684. 10.1002/jmv.21279 [DOI] [PubMed] [Google Scholar]
- 70. Bekele A, Baay M, Mekonnen Z, Suleman S, Chatterjee S (2010) Human papillomavirus type distribution among women with cervical pathology- study over 4 years at Jimma Hospital, southwest Ethiopia. Tropical Medicine & International Health 15: 890–893. [DOI] [PubMed] [Google Scholar]
- 71. Fanta B (2005) The distribution of Human Papilloma Virus infection in women with cervical histological abnormalities from an area with high incidence of cervical cancer. Ethiopian medical journal 43: 151 [PubMed] [Google Scholar]
- 72. Czegledy J, Rogo K, Evander M, Wadell G (1992) High-risk human papillomavirus types in cytologically normal cervical scrapes from Kenya. Medical microbiology and immunology 180: 321–326. [DOI] [PubMed] [Google Scholar]
- 73. De Vuyst H, Lillo F, Broutet N, Smith JS (2008) HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. European Journal of Cancer Prevention 17: 545 10.1097/CEJ.0b013e3282f75ea1 [DOI] [PubMed] [Google Scholar]
- 74. De Vuyst H, Mugo NR, Chung MH, McKenzie KP, Nyongesa-Malava E, Tenet V, et al. (2012) Prevalence and determinants of human papillomavirus infection and cervical lesions in HIV-positive women in Kenya. Br J Cancer 107: 1624–1630. 10.1038/bjc.2012.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Vuyst H, Ndirangu G, Moodley M, Tenet V, Estambale B, Meijer CJLM, et al. (2012) Human papillomavirus prevalence in invasive cervical carcinoma by HIV Status. Infectious Agents and Cancer 7. [DOI] [PubMed] [Google Scholar]
- 76. De Vuyst H, Parisi MR, Karani A, Mandaliya K, Muchiri L, Vaccarella S, et al. (2010) The prevalence of human papillomavirus infection in Mombasa, Kenya. Cancer Causes and Control 21: 2309–2313. 10.1007/s10552-010-9645-z [DOI] [PubMed] [Google Scholar]
- 77. De Vuyst H, Steyaert S, Van Renterghem L, Claeys P, Muchiri L, Sitati S, et al. (2003) Distribution of human papillomavirus in a family planning population in Nairobi, Kenya. Sexually transmitted diseases 30: 137 [DOI] [PubMed] [Google Scholar]
- 78. Maranga IO, Hampson L, Oliver AW, He X, Gichangi P, Rana F, et al. (2013) HIV Infection Alters the Spectrum of HPV Subtypes Found in Cervical Smears and Carcinomas from Kenyan Women. Open Virol J 7: 19–27. 10.2174/1874357901307010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rahman M, Sasagawa T, Yamada R, Kingoro A, Ichimura H, Makinoda S (2011) High prevalence of intermediate‐risk human papillomavirus infection in uterine cervices of kenyan women infected with human immunodeficiency virus. J Med Virol 83: 1988–1996. 10.1002/jmv.22203 [DOI] [PubMed] [Google Scholar]
- 80. Naucler P, da Costa FM, da Costa JL, Ljungberg O, Bugalho A, Dillner J (2011) Human papillomavirus type-specific risk of cervical cancer in a population with high human immunodeficiency virus prevalence: case‚Äìcontrol study. Journal of general virology 92: 2784–2791. 10.1099/vir.0.034298-0 [DOI] [PubMed] [Google Scholar]
- 81. Naucler P, Da Costa FM, Ljungberg O, Bugalho A, Dillner J (2004) Human papillomavirus genotypes in cervical cancers in Mozambique. Journal of general virology 85: 2189–2190. [DOI] [PubMed] [Google Scholar]
- 82. Singh DK, Anastos K, Hoover DR, Burk RD, Shi Q, Ngendahayo L, et al. (2009) Human Papillomavirus Infection and Cervical Cytology in HIV-Infected and HIV-Uninfected Rwandan Women. Journal of Infectious Diseases 199: 1851–1861. 10.1086/599123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, et al. (2012) Human Papillomavirus Prevalence and Type Distribution in 3603 HIV-Positive and HIV-Negative Women in the General Population of Tanzania: The PROTECT Study. Sexually Transmitted Diseases 39: 201–208. 10.1097/OLQ.0b013e31823b50ad [DOI] [PubMed] [Google Scholar]
- 84. Mayaud P, Weiss HA, Lacey CJ, Gill DK, Mabey DC (2003) Genital human papillomavirus genotypes in northwestern Tanzania. J Clin Microbiol 41: 4451–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vidal AC, Murphy SK, Hernandez BY, Vasquez B, Bartlett JA, Oneko O, et al. (2011) Distribution of HPV genotypes in cervical intraepithelial lesions and cervical cancer in Tanzanian women. Infectious Agents and Cancer 6: 1–8. 10.1186/1750-9378-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Blossom D, Beigi R, Farrell J, Mackay W, Qadadri B, Brown D, et al. (2007) Human papillomavirus genotypes associated with cervical cytologic abnormalities and HIV infection in Ugandan women. J Med Virol 79: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Odida M, de Sanjose S, Sandin S, Quiros B, Alemany L, Lloveras B, et al. (2010) Comparison of human papillomavirus detection between freshly frozen tissue and paraffin embedded tissue of invasive cervical cancer. Infect Agent Cancer 5: 15 10.1186/1750-9378-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Odida M, Sandin S, Mirembe F, Kleter B, Quint W, Weiderpass E (2011) HPV types, HIV and invasive cervical carcinoma risk in Kampala, Uganda: a case-control study. Infectious Agents and Cancer 6: 8 10.1186/1750-9378-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ng'andwe C, Lowe JJ, Richards PJ, Hause L, Wood C, Angeletti PC (2007) The distribution of sexually-transmitted Human Papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC infectious diseases 7: 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gravitt PE, Kamath AM, Gaffikin L, Chirenje ZM, Womack S, Shah KV (2002) Human papillomavirus genotype prevalence in high-grade squamous intraepithelial lesions and colposcopically normal women from Zimbabwe. International journal of cancer 100: 729–732. [DOI] [PubMed] [Google Scholar]
- 91. Stanczuk GA, Kay P, Allan B, Chirara M, Tswana SA, Bergstrom S, et al. (2003) Detection of human papillomavirus in urine and cervical swabs from patients with invasive cervical cancer. Journal of medical virology 71: 110–114. [DOI] [PubMed] [Google Scholar]
- 92. Stanczuk GA, Kay P, Sibanda E, Allan B, Chirara M, Tswana SA, et al. (2003) Typing of human papillomavirus in Zimbabwean patients with invasive cancer of the uterine cervix. Acta obstetricia et gynecologica Scandinavica 82: 762–766. [DOI] [PubMed] [Google Scholar]
- 93. Garcia-Espinosa B, Nieto-Bona MP, Rueda S, Silva-Senchez LF, Piernas-Morales MC, Carro-Campos P, et al. (2009) Genotype distribution of cervical human papillomavirus DNA in women with cervical lesions in Bioko, Equatorial Guinea. Diagnostic pathology 4: 31 10.1186/1746-1596-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Icenogle JP, Laga M, Miller D, Manoka AT, Tucker RA, Reeves WC (1992) Genotypes and Sequence Variants of Human Papillomavirus DNAs from Human Immunodeficiency Virus Type I-Infected Women with Cervical Intraepithelial Neoplasia. Journal of Infectious Diseases 166: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 95. Alhamany Z, El Mzibri M, Kharbach A, Malihy A, Abouqal R, Jaddi H, et al. (2010) Prevalence of human papillomavirus genotype among Moroccan women during a local screening program. The Journal of Infection in Developing Countries 4: 732–739. [DOI] [PubMed] [Google Scholar]
- 96. Chaouki N, Bosch FX, Muñoz N, Meijer CJ, El Gueddari B, El Ghazi A, et al. (1998) The viral origin of cervical cancer in Rabat, Morocco. International journal of cancer 75: 546–554. [DOI] [PubMed] [Google Scholar]
- 97. MacLeod IJ, O'Donnell B, Moyo S, Lockman S, Shapiro RL, Kayembe M, et al. (2011) Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV‚Äêinfected women in Botswana. Journal of medical virology 83: 1689–1695. 10.1002/jmv.22178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ramogola-Masire D, de Klerk R, Monare B, Ratshaa B, Friedman HM, Zetola NM (2012) Cervical Cancer Prevention in HIV-Infected Women Using the "See and Treat" Approach in Botswana. Jaids-Journal of Acquired Immune Deficiency Syndromes 59: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Piras F, Piga M, De Montis A, Zannou ARF, Minerba L, Perra MT, et al. (2011) Prevalence of human papillomavirus infection in women in Benin, West Africa. Virology Journal 8: 514 10.1186/1743-422X-8-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Adjorlolo-Johnson G, Unger E, Boni-Ouattara E, Touré-Coulibaly K, Maurice C, Vernon S, et al. (2010) Assessing the relationship between HIV infection and cervical cancer in Côte d'Ivoire A case-control study. BMC infectious diseases 10: 242 10.1186/1471-2334-10-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jaquet A, Horo A, Charbonneau V, Ekouevi DK, Roncin L, Toure B, et al. (2012) Cervical human papillomavirus and HIV infection in women of child-bearing age in Abidjan, Cote d'Ivoire, 2010. Br J Cancer 107: 556–563. 10.1038/bjc.2012.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. La Ruche G, You B, Mensah-Ado I, Bergeron C, Montcho C, Ramon R, et al. (1998) Human papillomavirus and human immunodeficiency virus infections: relation with cervical dysplasia-neoplasia in African women. International journal of cancer 76: 480–486. [DOI] [PubMed] [Google Scholar]
- 103. Wall S, Scherf C, Morison L, Hart K, West B, Ekpo G, et al. (2005) Cervical human papillomavirus infection and squamous intraepithelial lesions in rural Gambia, West Africa: viral sequence analysis and epidemiology. British Journal of Cancer 93: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Keita N, Clifford G, Koulibaly M, Douno K, Kabba I, Haba M, et al. (2009) HPV infection in women with and without cervical cancer in Conakry, Guinea. British Journal of Cancer 101: 202–208. 10.1038/sj.bjc.6605140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bayo S, Bosch FX, De Sanjosé S, Munoz N, Combita AL, Coursaget P, et al. (2002) Risk factors of invasive cervical cancer in Mali. International journal of epidemiology 31: 202–209. [DOI] [PubMed] [Google Scholar]
- 106. Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Eklund C, Reilly M, et al. (2012) The age-specific prevalence of human papillomavirus and risk of cytologic abnormalities in rural Nigeria: Implications for screen-and-treat strategies. International Journal of Cancer 130: 2111–2117. 10.1002/ijc.26211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Astori G, Beltrame A, Pipan C, Raphenon G, Botta G (1999) PCR-RFLP-detected human papilloma virus infection in a group of Senegalese women attending an STD clinic and identification of a new HPV-68 subtype. Intervirology 42: 221–227. [DOI] [PubMed] [Google Scholar]
- 108. Chabaud M, Le Cann P, Mayelo V, Leboulleux D, Diallo A, Enogat N, et al. (1996) Detection by PCR of human papillomavirus genotypes in cervical lesions of Senegalese women. Journal of medical virology 49: 259–263. [DOI] [PubMed] [Google Scholar]
- 109. Xi LF, Touré P, Critchlow CW, Hawes SE, Dembele B, Sow PS, et al. (2003) Prevalence of specific types of human papillomavirus and cervical squamous intraepithelial lesions in consecutive, previously unscreened, West-African women over 35 years of age. International journal of cancer 103: 803–809. [DOI] [PubMed] [Google Scholar]
- 110. Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL (2006) HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 24: S26–S34. [DOI] [PubMed] [Google Scholar]
- 111. De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, Banura C, et al. (2013) The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine 31: F32–F46. 10.1016/j.vaccine.2012.07.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Broutet N, Huntington D, Cutts F, Hall P (2006) Preparing for the introduction of HPV vaccines: policy and programme guidance for countries Switzerland: World Health Organization; 21. [Google Scholar]
- 113. Peres J (2011) For cancers caused by HPV, two vaccines were just the beginning. Journal of the National Cancer Institute 103: 360–362. 10.1093/jnci/djr053 [DOI] [PubMed] [Google Scholar]
- 114. Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G, Denny LA (2013) Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine 31 Suppl 5: F47–52. 10.1016/j.vaccine.2012.06.066 [DOI] [PubMed] [Google Scholar]
- 115. Vaccarella S, Bruni L, Seoud M (2013) Burden of human papillomavirus infections and related diseases in the extended Middle East and North Africa region. Vaccine 31 Suppl 6: G32–44. 10.1016/j.vaccine.2012.06.098 [DOI] [PubMed] [Google Scholar]
- 116. WHO (2013) Comprehensive cervical cancer prevention and control: A healthier future for girls and women WHO, Geneva. [Google Scholar]
- 117. Denny LA, Sankaranarayanan R, De Vuyst H, Kim JJ, Adefuye PO, Alemany L, et al. (2013) Recommendations for cervical cancer prevention in sub-saharan Africa. Vaccine 31 Suppl 5: F73–74. 10.1016/j.vaccine.2012.11.077 [DOI] [PubMed] [Google Scholar]
- 118. Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC Jr. (2010) Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst 102: 1557–1567. 10.1093/jnci/djq342 [DOI] [PubMed] [Google Scholar]
- 119. WHO (2013) Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention: supplemental material: GRADE evidence-to-recommendation tables and evidence profiles for each recommendation Geneva: WHO. [Google Scholar]
- 120. Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J (2012) Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 30 Suppl 5: F168–174. 10.1016/j.vaccine.2012.06.045 [DOI] [PubMed] [Google Scholar]
- 121. UNAIDS (2013) Global Report 2013: UNAIDS Report on the Global AIDS Epidemic. Geneva: UNAIDS. ISBN 978-92-9253-032-7 ISBN 978-92-9253-032-7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOC)
(DOCX)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.