Abstract
Background and Purpose
Diffusion-weighted imaging (DWI) lesion volume is associated with poor outcome after thrombolysis, and it is unclear whether endovascular therapies are beneficial for large DWI lesion. Our aim was to assess the impact of pretreatment DWI lesion volume on outcomes after endovascular therapy, with a special emphasis on patients with complete recanalization.
Methods
We analyzed data collected between April 2007 and November 2011 in a prospective clinical registry. All acute ischemic stroke patients with complete occlusion of internal carotid artery or middle cerebral artery treated by endovascular therapy were included. DWI lesion volumes were measured by the RAPID software. Favorable outcome was defined by modified Rankin Scale of 0 to 2 at 90 days.
Results
A total of 139 acute ischemic stroke patients were included. Median DWI lesion volume was 14 cc (interquartile range, 5–43) after a median onset time to imaging of 110 minutes (interquartile range, 77–178). Higher volume was associated with less favorable outcome (adjusted odds ratio, 0.55; 95% confidence interval, 0.31–0.96). A complete recanalization was achieved in 65 (47%) patients after a median onset time of 238 minutes (interquartile range, 206–285). After adjustment for volume, complete recanalization was associated with more favorable outcome (adjusted odds ratio, 6.32; 95% confidence interval, 2.90–13.78). After stratification of volume by tertiles, complete recanalization was similarly associated with favorable outcome in the upper 2 tertiles (P<0.005).
Conclusions
Our results emphasize the importance of initial DWI volume and recanalization on clinical outcome after endovascular treatment. Large DWI lesions may still benefit from recanalization in selected patients.
Keywords: acute stroke syndromes, diffusion-weighted imaging, endovascular reperfusion, thrombolysis
The recanalization of the occluded artery is the major goal of the treatment of acute ischemic stroke (AIS).1 The ability to restore cerebral blood flow within the shortest delay by intravenous, intra-arterial, combined intravenous/intra-arterial thrombolysis, and, more recently, mechanical thrombectomy, has been consistently associated with an increased rate of good clinical recovery.2–8 Besides recanalization, the extension of early ischemic signs on an acute head computed tomography (CT), assessed by the Alberta Stroke Program Early CT Score, has been associated with a worse outcome in patients treated by intravenous recombinant tissue–type plasminogen activator (rt-PA) or intra-arterial prourokinase.9,10 Compared with CT scan, diffusion-weighted imaging (DWI) is the method of choice to estimate the extension of the acute ischemic lesion within the first hours after stroke onset.11,12 Several studies have demonstrated that the extent of DWI lesion volume is associated with poor outcome of AIS.13–16 The mismatch between diffusion and perfusion is the preferred method to assess ischemic penumbra in clinical practice. The mismatch between DWI lesion volume and the National Institutes of Health Stroke Scale (NIHSS) or the site of vessel occlusion has been proposed as surrogate to select patients for an acute reperfusion therapy.17–19 In 2009, 1 study showed that none of the 6 patients with a DWI lesion >70 cc benefited from endovascular recanalization.20 In this context, we investigated the relationship between acute DWI lesion volume outlined by the RAPID software and clinical outcome according to recanalization rate in a cohort of acute ischemic stroke patients treated by endovascular treatment.
Methods
Bichat Stroke Program
Patients were identified from a prospective clinical registry of patients with AIS treated between April 2007 and November 2011 at Bichat University Hospital. Detailed material and methods have been previously reported.21 All patients underwent a magnetic resonance angiography (MRA) or CT angiography before treatment to document arterial occlusion. Patients eligible for intravenous treatment with a documented vessel occlusion received a combined intravenous/ intra-arterial dose of 0.9 mg/kg (intravenous, 0.6 mg/kg; intra-arterial, 0.3 mg/kg) in case of documented arterial occlusion; in the absence of recanalization after intravenous/intra-arterial rt-PA administration, additional mechanical endovascular therapy was performed using either the snare (eV3) or the Solitaire (Covidien) devices.5 Patients with a documented arterial occlusion who were not eligible for intravenous rt-PA were treated by intra-arterial rt-PA (dose 0.5 mg/kg), followed by adjunctive mechanical endovascular therapy if the occlusion persisted. In patients with a contraindication to rt-PA, a direct mechanical endovascular therapy approach was considered.21
Patient Consent and Protocol Approval
Informed consent was obtained from the patient or surrogate, and the research protocol was approved by the Ethics Committee from Ambroise Pare Hospital.
Sample Selection
Patients with a complete occlusion of internal carotid artery or middle cerebral artery (M1 and M2 segments) treated by endovascular therapy and evaluated by a pretreatment diffusion-weighted (DWI) imaging were enrolled in this substudy.
Data Collection
Data were prospectively collected using a structured questionnaire. The severity of the ischemic stroke was assessed using the NIHSS score at admission and 1, 3, and 24 hours after initiation of treatment. Time from symptom onset to cerebral imaging (MRI), to initiation of therapy (rt-PA administration by intravenous or intra-arterial if contraindicated or local thrombectomy device deployment if rt-PA is contraindicated) was also recorded. Arterial status of the occluded artery was monitored with conventional angiography during the intra-arterial approach, and time to recanalization was noted. Recanalization was measured using the thrombolysis in myocardial infarction (TIMI) score22 by 2 staff members (E.M. and M.M.). Successful complete recanalization was defined as a complete restoration of blood flow (TIMI 3). All patients had a CT or MRI scan 24 hours after treatment onset to assess hemorrhagic complications. The modified Rankin Scale at 3 months was assessed during face-to-face interviews or via telephone calls by a senior vascular neurologist (E.M. or M.M.) certified for modified Rankin Scale scoring.
DWI Lesion Measurement
Diffusion maps were reprocessed by the RAPID software.15 DWI masks and maps were compared and independently reviewed by 2 stroke neurologists (M.I. and J.-M.O.) blinded from clinical data and recanalization rates. When needed, DWI lesion masks were manually corrected as previously described in the pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial-Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution database and Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 study.23,24
Clinical Outcome Definitions
The primary study outcome was the percentage of patients who achieved a favorable outcome at 90 days, defined by a modified Rankin Scale score of 0 to 2. Secondary outcomes included 90-day mortality, hemorrhagic transformation, and symptomatic intracerebral hemorrhage, as described.25
Statistical Analysis
Data are presented as mean (SD) or median (interquartile range [IQR]) for continuous variables and percentage (count) for categorical variables. DWI lesions were divided into tertiles to describe the association with baseline characteristics and outcome. Comparisons between tertiles were made using the Cochran–Mantel–Haenszel trend test for categorical variables and ANOVA (nonparametric for skewed baseline data) with linear contrast. Similar results were obtained when DWI lesion volume was analyzed as continuous variable (using the Spearman rank correlation for continuous baseline data and Mann–Whitney U test for categorical variables). For each clinical outcome, we calculated the odds ratios (ORs) for the upper tertiles of DWI lesion volume relative to the lowest; because a gradual change in ORs was found, we also computed the ORs per 1 SD increase of DWI lesion volume (after logtransformation of volume+1 to reduce the skewness). Logistic regression analyses were used to adjust for potential confounding factors selected on the basis of their significance with DWI lesion volume in univariate analyses (P<0.20). Because a strong correlation was found between DWI lesion volume and admission NIHSS score, multivariate analyses were performed with and without the inclusion of admission NIHSS score. Finally, to further evaluate the impact of DWI lesion volume on the success of reperfusion therapy, we compared clinical outcomes between the TIMI grades in each DWI lesion volume tertile using the χ2 test (or Fisher exact test when the expected cell frequency was <5). Further comparisons were done in subgroups defined by previously published DWI selection criteria (the MRA–DWI mismatch19; the clinical–DWI mismatch18; and very large infarction >70 cc).20 We did not attempt to test heterogeneity in the DWI lesion subgroups because of the small sample size in some strata. Statistical testing was done at the 2-tailed α level of 0.05. Data were analyzed using the Statistical Analysis Software package, release 9.3 (SAS Institute, Cary, NC).
Results
Over a 4.5-year study period, 210 consecutive patients with an AIS complicating an acute internal carotid artery or middle cerebral artery occlusion were treated by endovascular therapy. Among them, 157 patients (75%) underwent an MRI before recanalization therapy. Of these, 18 patients with no or poor quality DWI were excluded, resulting in a study sample size of 139 patients with a measured DWI lesion volume (Figure I in the online-only Data Supplement).
DWI Lesion Volume, Baselines, and Clinical Outcomes
The median delay from symptom onset to MRI was 110 minutes (IQR, 77–178), and the median DWI lesion volume was 14 cc (IQR, 5–43). Five (4%) patients had no DWI lesion. Increasing DWI lesion volumes were correlated with admission NIHSS score (r=0.42; P<0.001) and not with MRI delay (Spearman correlation coefficient, r=0.14; P=0.10). Table 1 shows baseline characteristics of the study sample. Large DWI lesion volume was more frequently associated with internal carotid artery occlusion. Patients with large DWI lesion were more frequently treated by direct endovascular treatment and tended to be younger.
Table 1.
Baseline Characteristics According to the Tertile of DWI Lesion Volume
| DWI Lesion Volume, cc | |||||
|---|---|---|---|---|---|
| Overall (n=139) | <8 (n=46) | 8–32 (n=47) | >32 (n=46) | P for Trend | |
| DWI volume, cc, median (IQR) | 14 (5–43) | 3 (1–5) | 14 (10–20) | 60 (43–104) | NA |
| Age, y, mean (SD) | 69.8 (17.6) | 76.2 (13.4) | 67.1 (17.8) | 66.2 (19.6) | 0.006 |
| Men | 65 (46.8) | 21 (45.6) | 26 (55.3) | 18 (39.1) | 0.53 |
| Medical history | |||||
| Hypertension | 69 (50.4) | 26 (56.5) | 19 (40.4) | 25 (54.4) | 0.84 |
| Diabetes mellitus | 17 (12.2) | 6 (13.0) | 4 (8.5) | 7 (15.2) | 0.75 |
| Hypercholesterolemia | 35 (25.6) | 13 (28.9) | 10 (21.3) | 12 (26.7) | 0.81 |
| Current or former smoker | 49 (35.3) | 19 (41.3) | 15 (31.9) | 15 (32.6) | 0.38 |
| Antithrombotic medications | 53 (38.1) | 20 (43.5) | 15 (31.9) | 18 (39.1) | 0.67 |
| Clinical measure | |||||
| Blood glucose, mg/dL, median (IQR) | 117 (101–146) | 122 (110–155) | 112 (95–133) | 124 (101–162) | 0.83 |
| SBP, mm Hg, mean (SD) | 148 (23) | 152 (20) | 146 (24) | 146 (23) | 0.27 |
| DBP, mm Hg, mean (SD) | 79 (14) | 80 (15) | 79 (13) | 80 (13) | 0.92 |
| NIHSS score, median (IQR) | 16 (10–21) | 12 (9–17) | 16 (10–22) | 18 (15–22) | <0.001 |
| Cardioembolism | 81 (58.3) | 27 (58.7) | 30 (63.8) | 24 (52.2) | 0.53 |
| ICA occlusion (isolated or tandem with MCA) | 41 (29.5) | 12 (26.1) | 10 (21.3) | 19 (41.3) | 0.11 |
| Time to MRI, min, median (IQR) | 110 (77–178) | 95 (73–135) | 120 (80–210) | 121 (85–190) | 0.11 |
| AIS treatment | |||||
| Combined IV/IA | 97 (69.8) | 40 (87.0) | 30 (63.8) | 27 (58.7) | 0.003 |
| IA alone | 42 (30.2) | 6 (13.0) | 17 (36.2) | 19 (41.3) | |
| Time to AIS treatment, min, median (IQR) | |||||
| Combined IV/IA | 132 (110–155) | 135 (102–157) | 128 (110–155) | 133 (110–154) | 0.82 |
| IA alone | 314 (260–350) | 260 (195–320) | 322 (309–370) | 300 (220–346) | 0.28 |
Data are presented as no. (%) unless otherwise indicated.
AIS indicates acute ischemic stroke; BP, blood pressure; DBP, diastolic BP; DWI, diffusion-weighted imaging; IA, intra-arterial; ICA, internal carotid artery; IQR, interquartile range; IV, intravenous; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; MCA, middle cerebral artery; and SBP, systolic BP.
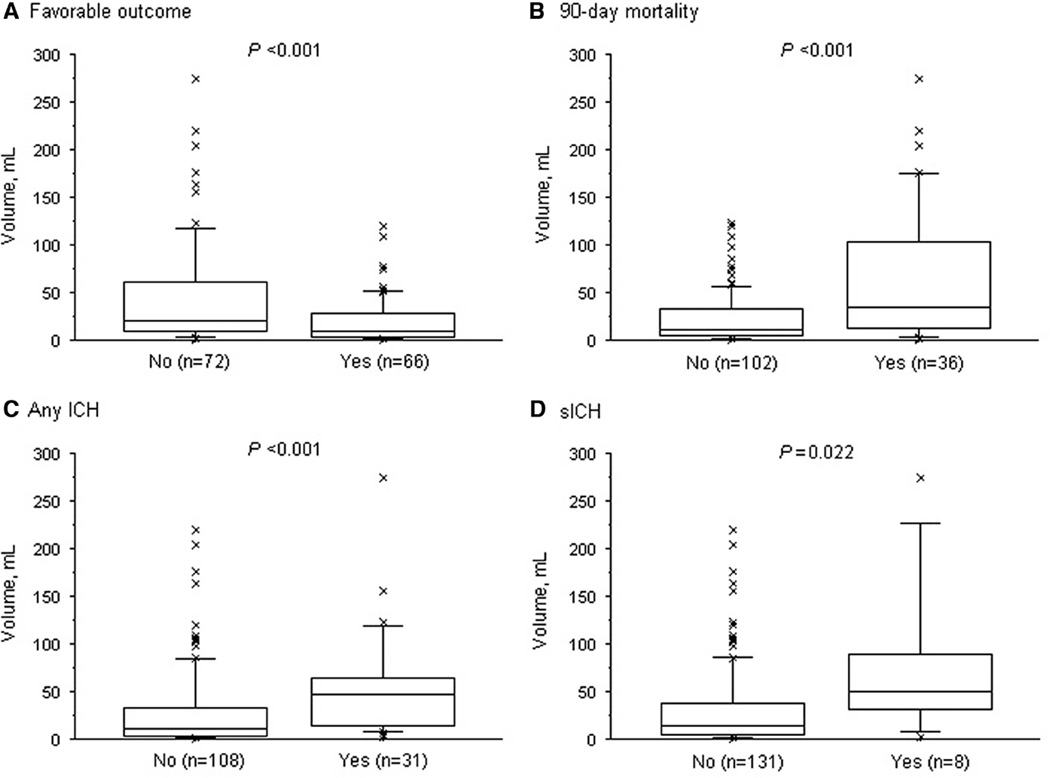
Compared with patients who did not experience clinical recovery, a favorable outcome was associated with a smaller DWI lesion volume (median, 9 versus 21 cc; P<0.001). Conversely, patients who died at 90 days and those who experienced a hemorrhagic transformation after reperfusion therapy had a larger DWI lesion volume (Figure 1). After categorization of DWI lesion volume into tertiles, the rate of favorable outcome decreased gradually with increasing tertiles, whereas the 90-day mortality and hemorrhagic complication rates increased. These relationships between increasing DWI lesion volume and outcome remained significant in multivariate analysis, including potential confounding factor (Table 2).
Figure 1.
Distribution of diffusion-weighted imaging (DWI) lesion volume according to favorable outcome (A), 90-day mortality (B), any intracerebral hemorrhage (ICH) (C), and symptomatic ICH (D). Boxes show the 25th, 50th, and 75th, and whiskers show the 5th and 95th percentiles. P values were calculated using the Mann–Whitney U test. Favorable outcome was defined as 90-day modified Rankin Scale ≤2 and symptomatic intracerebral hemorrhage (sICH) as European Co-operative Acute Stroke Study-II criteria.
Table 2.
Impact of DWI Lesion Volume on Clinical Outcomes
| DWI Lesion Volume, cc | ||||
|---|---|---|---|---|
| <8.0 (n=46) | 8.0–31.7 (n=47) | >31.7 (n=46) | P for Trend or OR per SD* | |
| Favorable outcome | ||||
| n, % | 29 (64.4) | 23 (48.9) | 14 (30.4) | 0.001 |
| Crude OR (95% CI) | 1.00 (ref) | 0.53 (0.23–1.22) | 0.24 (0.10–0.58) | 0.49 (0.33–0.72) |
| Model 1–adjusted OR (95% CI) | 1.00 (ref) | 0.32 (0.11–0.90) | 0.14 (0.04–0.44) | 0.38 (0.23–0.63) |
| Model 2–adjusted OR (95% CI) | 1.00 (ref) | 0.53 (0.17–1.66) | 0.34 (0.10–1.17) | 0.55 (0.31–0.96) |
| 90-day mortality | ||||
| n, % | 7 (15.6) | 10 (21.3) | 19 (41.3) | 0.005 |
| Crude OR (95% CI) | 1.00 (ref) | 1.47 (0.51–4.26) | 3.82 (1.41–10.36) | 2.30 (1.46–3.62) |
| Model 1–adjusted OR (95% CI) | 1.00 (ref) | 1.90 (0.53–6.82) | 6.29 (1.76–22.46) | 2.76 (1.61–4.75) |
| Model 2–adjusted OR (95% CI) | 1.00 (ref) | 1.49 (0.39–5.75) | 4.65 (1.20–17.94) | 2.60 (1.45–4.66) |
| Intracerebral hemorrhage | ||||
| n, % | 3 (6.5) | 10 (21.3) | 18 (39.1) | <0.001 |
| Crude OR (95% CI) | 1.00 (ref) | 3.87 (0.99–15.14) | 9.21 (2.48–34.21) | 2.39 (1.47–3.89) |
| Model 1–adjusted OR (95% CI) | 1.00 (ref) | 4.42 (1.08–18.04) | 11.71 (2.90–47.31) | 2.54 (1.51–4.27) |
| Model 2–adjusted OR (95% CI) | 1.00 (ref) | 2.98 (0.69–12.84) | 6.57 (1.50–28.75) | 1.97 (1.11–3.47) |
Model 1: logistic regression analysis adjusted for age, time from symptom onset to MRI, prior IV rt-PA (ie, combined IV/IA approach), and ICA occlusion. Model 2: logistic regression analysis adjusted for age, time from symptom onset to MRI, prior IV rt-PA, ICA occlusion, and admission NIHSS score.
CI indicates confidence interval; DWI, diffusion-weighted imaging; IA, intra-arterial; ICA, internal carotid artery; IV, intravenous; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; ref, reference; and rt-PA, recombinant tissue-type plasminogen activator.
OR computed per 1 SD increased in log (volume+1). Favorable outcome was defined as 90-day modified Rankin Scale ≤2.
DWI Lesion Volume and Impact of Recanalization
Sixty-five (47%) patients experienced a complete recanalization (TIMI 3) within a median delay from symptom onset of 238 minutes (IQR, 206–285), and 39 (28%) a partial recanalization (TIMI 2) within a median delay of 269 minutes (IQR, 219–350). Patients who experienced a complete recanalization tended to have a smaller median DWI lesion volume (10 cc; IQR, 3–31) than patients with partial recanalization (21 cc; IQR, 6–59) or than patients with no recanalization (19 cc; IQR, 9–46; P for between-group comparison=0.07).
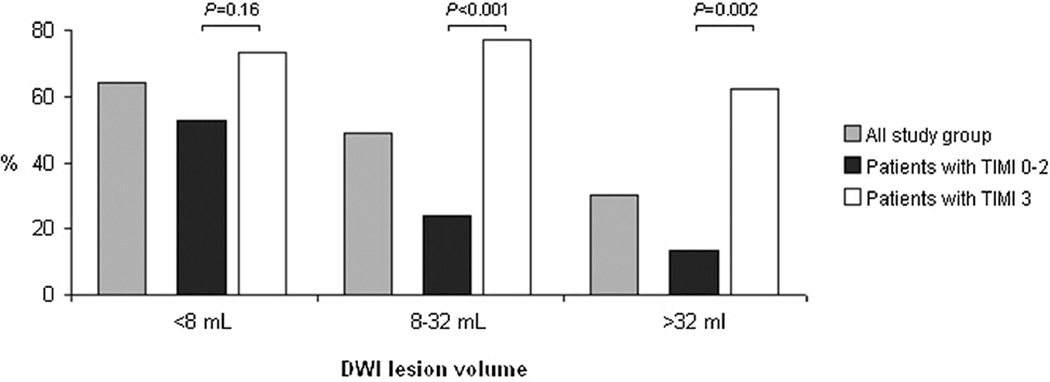
Overall recanalization was associated with an increased rate of favorable outcome, especially when complete recanalization was achieved (Table I in the online-only Data Supplement). After adjustment for DWI lesion volume and compared with patient with no recanalization, the OR of favorable outcome was 2.96 (95% confidence interval, 0.95–9.25) for partial recanalization and 11.85 (95% confidence interval, 4.06–34.57) for complete recanalization. When patients with no or partial recanalization were combined together, the OR of favorable outcome after complete recanalization was 6.32 (95% confidence interval, 2.90–13.78). After stratification on DWI lesion volume tertiles, a complete recanalization remained associated with an increased rate of favorable outcome in the upper 2 tertiles (Figure 2); crude ORs (95% confidence interval) of favorable outcome for complete recanalization was 2.44 (0.70–8.52) in the first tertile, 10.77 (2.78–41.75) in the second tertile, and 10.83 (2.51–46.66) in the last tertile. A complete recanalization was also associated with a favorable outcome in patients who did and in those who did not have DWI–MRA or a clinical–DWI mismatch (Table 3). Among the 19 patients with a large DWI lesion volume (>70 cc), a complete recanalization tended to be associated with an increased rate of clinical recovery (43% versus 8%; P=0.12). The largest DWI lesion volume associated with a favorable outcome was 20 cc for patients with no recanalization, 76 cc for patients with partial recanalization, and 121 cc for patients with complete recanalization. Recanalization was also associated with a lower rate of mortality (Table II in the online-only Data Supplement) and with a lower rate of hemorrhagic transformation (Table III in the online-only Data Supplement). As for favorable outcome, the impact was stronger when complete recanalization was achieved. Regarding the DWI lesion volume subgroups, complete recanalization was associated with a lower rate in the upper 2 tertiles of DWI lesion volume and among patients with a DWI lesion volume >70 cc (Table 3). The rate of hemorrhagic transformation was essentially lower among recanalizers in the subgroup of patients with a DWI lesion volume ≤70 cc (Table 3).
Figure 2.
Functional outcome rates by tertiles of diffusion-weighted imaging (DWI) lesion volume, overall and according to successful complete recanalization. TIMI indicates thrombolysis in myocardial infarction.
Table 3.
Impact of Complete Recanalization on Favorable Outcome, 90-day Mortality, Hemorrhagic Transformation, in Overall and According to DWI Lesion Volume Subgroups
| Favorable Outcome | 90-Day Mortality | Hemorrhagic Transformation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TIMI 0–2 | TIMI 3 | P Value | TIMI 0–2 | TIMI 3 | P Value | TIMI 0–2 | TIMI 3 | P Value | |
| Overall (n=139) | 20 (27.0) | 46 (71.9) | <0.001 | 29 (39.2) | 7 (10.9) | <0.001 | 23 (31.1) | 8 (12.3) | 0.008 |
| DWI lesion volume, tertiles | |||||||||
| <8 cc (n=46) | 10 (52.6) | 19 (73.1) | 0.16 | 3 (15.8) | 4 (15.4) | 1.00 | 3 (15.8) | 0 (0.0) | 0.06 |
| 8–32 cc (n=47) | 6 (24.0) | 17 (77.3) | <0.001 | 10 (40.0) | 0 (0.0) | <0.001 | 6 (24.0) | 4 (18.2) | 0.73 |
| >32 cc (n=46) | 4 (13.3) | 10 (62.5) | 0.002 | 16 (53.3) | 3 (18.8) | 0.023 | 14 (46.7) | 4 (25.0) | 0.15 |
| MRA–DWI mismatch status | |||||||||
| Yes (n=82)* | 15 (37.5) | 29 (70.7) | 0.003 | 10 (25.0) | 4 (9.8) | 0.08 | 7 (17.5) | 4 (9.5) | 0.29 |
| No (n=57) | 5 (14.7) | 17 (73.9) | <0.001 | 19 (55.9) | 3 (13.0) | 0.002 | 16 (47.1) | 4 (17.4) | 0.021 |
| NIHSS–DWI mismatch criteria | |||||||||
| NIHSS ≥8 and volume ≤25 cc (n=70) | 13 (36.1) | 22 (66.7) | 0.011 | 9 (25.0) | 3 (9.1) | 0.11 | 8 (22.2) | 4 (11.8) | 0.25 |
| NIHSS ≥8 and volume >25 cc (n=51) | 4 (12.5) | 13 (68.4) | <0.001 | 18 (56.3) | 3 (15.8) | 0.005 | 15 (46.9) | 4 (21.1) | 0.07 |
| NIHSS <8 (n=18) | 3 (50.0) | 11 (91.7) | 0.083 | 2 (33.3) | 1 (8.3) | 0.25 | 0 (0.0) | 0 (0.0) | NA |
| Very large DWI lesion group | |||||||||
| Volume ≤70 cc (n=120) | 19 (30.7) | 43 (75.4) | <0.001 | 19 (30.7) | 5 (8.8) | 0.003 | 20 (32.3) | 5 (8.6) | 0.001 |
| Volume >70 cc (n=19) | 1 (8.3) | 3 (42.9) | 0.12 | 10 (83.3) | 2 (28.6) | 0.045 | 3 (25.0) | 3 (42.9) | 0.62 |
Data are no. (%) of patients with outcomes unless otherwise indicated. Comparisons in favorable outcome between TIMI flow grades were done using χ2 or Fisher exact test.
DEFUSE indicates Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution; DWI, diffusion-weighted imaging; ICA, internal carotid artery; MCA, middle cerebral artery; MRA, magnetic resonance angiography; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; and TIMI, thrombolysis in myocardial infarction.
DEFUSE model (volume <25 cc for ICA or MCA-M1 occlusion and volume <15 cc for MCA-M2 occlusion).
Discussion
The results presented above demonstrate that the size of acute ischemic lesion outlined by DWI was strongly related to clinical outcome in patients undergoing endovascular treatment. Compared with partial and nonrecanalizers, patients with large DWI lesions (>32 cc) did experience an increased rate (>60%) of clinical recovery after a complete recanalization.
Concurring with previous studies,26,27 the first part of our results confirms that baseline DWI lesion volume is a strong independent predictor of dependency, death, and hemorrhagic transformation after endovascular treatment. In 1999, the investigators of the Prolyse in Acute Cerebral Thromboembolism II trial used NIHSS to stratify the severity of acute ischemic stroke and demonstrated that intra-arterial prourokinase was beneficial in the subgroup of patients with moderately severe AIS defined by NIHSS 10 to 20 but not in patients with a mild (NIHSS 0–10) or a very severe AIS (NIHSS >20).8 The second part of our study supports the same type of relationship using DWI lesion volume, a criteria highly correlated with NIHSS. In the present study, successful recanalization was associated with a higher rate of clinical recovery in patients with no clinical–DWI or MRA–DWI mismatch who did not seem to benefit from early recanalization in previous studies.18,19 However, Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 1 and 2 substudies suggested that several patients with no clinical–DWI or MRA–DWI mismatch did have a target DWI–perfusion-weighted imaging mismatch and may, therefore, be improved by a complete reperfusion.28,29 In addition, a complete recanalization was associated with an increased odd of clinical recovery and a decreased mortality rate in subgroups of patients with DWI volumes associated with poor outcome in nonrecanalizers or partial recanalizers. Finally, some patients with a DWI lesion volume >70 cc did experience a good outcome after recanalization, conversely to what has been previously published.20 These original findings suggest that the occurrence of a complete recanalization significantly alters the outcome despite a large DWI lesion.
Although MRI and femoral puncture were obtained 4.6 and 6.2 hours in the target mismatch patient in Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2, MRI and recanalization were achieved, respectively, 2 and 4 hours after symptom onset in the present study.23 In addition, the median delay from onset to recanalization measured in the study by Yoo et al20 that reported no benefit in patients with a DWI lesion >70 cc was 7 hours. Therefore, we hypothesize that the delay to recanalization might have played a role in the results observed. Nonetheless, 63% of the patients with a DWI lesion volume >70 cc died after endovascular treatment. In conclusion, regarding the conflicting results and the few published cases, additional studies are needed at this early timepoint to define which patients with a very large ischemic lesion on DWI may benefit from endovascular treatment. In the meantime, a cautious case by case selection is strongly recommended.
However, there was no significant relationship between a complete recanalization and an increased rate of good outcome in patients with a small DWI lesion (<8 cc). In this subgroup, the vast majority (85%) of the patients did experience a partial or complete recanalization. Patients with both TIMI 2 or 3 recanalization rate had high rate (>70%) of clinical recovery, whereas only 28% of the nonrecanalizers (TIMI 0–1) experienced a good outcome (P=0.069). We assume that the absence of statistical difference may be because of a lack of power and should not preclude treating those patients.
Our study has several limitations. As reported in the results, patients with the largest ischemic lesion were younger than the patients with small DWI lesion volume. This selection bias is a consequence of previous analyses that demonstrated with others that intra-arterial treatment was not beneficial in octogenarians.30,31 The second shortcoming of the study is related to its small sample size. As a consequence, some strata defined by DWI lesion volume and TIMI flow grades were too small to detect some differences and perform multivariable analyses as the relationship with delay to MRI or type of treatment. Our results remain to be confirmed in larger multicenter trials. Third, as demonstrated by previous studies, DWI/perfusion-weighted imaging MRI criteria, such as target mismatch or malignant profile, outperform DWI-based criteria for the selection of patients to recanalization therapy.19,29 Hence, the lack of perfusion imaging in our study has to be considered as a limitation.
In conclusion, our results emphasize the importance of initial DWI volume and recanalization on clinical outcome after endovascular treatment. Because of the potential beneficial effect of complete recanalization on patients with large DWI volume, our findings suggest that endovascular reperfusion treatment may be considered cautiously on a case-by-case basis. Future studies comparing medical with endovascular treatment and using the last development of multimodal imaging may help clarify the selection criteria for acute endovascular treatment.
Supplementary Material
Acknowledgments
Sources of Funding
Funding for this study was provided in part by SOS-ATTAQUE CEREBRALE.
Dr Mosimann received a scholarship grant from Center Hospitalier Universitaire Vaudois and University of Lausanne during the research period.
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.113.000911/-/DC1.
Disclosures
Dr Albers has equity interest in iSchemaView and has worked as a consultant for Covidien and Stryker. Dr Bammer has equity interest in iSchemaView.
References
- 1.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke. 2000;31:610–614. doi: 10.1161/01.str.31.3.610. [DOI] [PubMed] [Google Scholar]
- 3.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial Doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31:1812–1816. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 4.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, et al. RECANALISE Investigators. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 8.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. J Am Med Assoc. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 9.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 10.Hill MD, Rowley HA, Adler F, Eliasziw M, Furlan A, Higashida RT, et al. PROACT-II Investigators. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke. 2003;34:1925–1931. doi: 10.1161/01.STR.0000082483.37127.D0. [DOI] [PubMed] [Google Scholar]
- 11.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 12.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996;16:53–59. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke. 2000;31:2597–2602. doi: 10.1161/01.str.31.11.2597. [DOI] [PubMed] [Google Scholar]
- 15.Oppenheim C, Samson Y, Manaï R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31:2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- 16.Baird AE, Dambrosia J, Janket S, Eichbaum Q, Chaves C, Silver B, et al. A three-item scale for the early prediction of stroke recovery. Lancet. 2001;357:2095–2099. doi: 10.1016/s0140-6736(00)05183-7. [DOI] [PubMed] [Google Scholar]
- 17.Donnan GA, Baron JC, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8:261–269. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- 18.Dávalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- 19.Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, et al. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39:2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, González RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouchaud A, Mazighi M, Labreuche J, Meseguer E, Serfaty JM, Laissy JP, et al. Outcomes of mechanical endovascular therapy for acute ischemic stroke: a clinical registry study and systematic review. Stroke. 2011;42:1289–1294. doi: 10.1161/STROKEAHA.110.599399. [DOI] [PubMed] [Google Scholar]
- 22.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. DEFUSE 2 study investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 26.Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezène Y, Philippeau F, et al. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 2004;225:3–9. doi: 10.1016/j.jns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, et al. Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra NK, Albers GW, Marks MP, Liggins J, Hamilton S, Straka M, et al. Patient selection for endovascular stroke therapy: can MRA substitute for PWI? Stroke. 2013;44:A179. Abstract. [Google Scholar]
- 29.Lansberg MG, Thijs VN, Hamilton S, Schlaug G, Bammer R, Kemp S, et al. DEFUSE Investigators. Evaluation of the clinical-diffusion and perfusion-diffusion mismatch models in DEFUSE. Stroke. 2007;38:1826–1830. doi: 10.1161/STROKEAHA.106.480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazighi M, Labreuche J, Meseguer E, Serfaty JM, Laissy JP, Lavallée PC, et al. Impact of a combined intravenous/intra-arterial approach in octogenarians. Cerebrovasc Dis. 2011;31:559–565. doi: 10.1159/000324626. [DOI] [PubMed] [Google Scholar]
- 31.Chandra RV, Leslie-Mazwi TM, Oh DC, Chaudhry ZA, Mehta BP, Rost NS, et al. Elderly patients are at higher risk for poor outcomes after intra-arterial therapy. Stroke. 2012;43:2356–2361. doi: 10.1161/STROKEAHA.112.650713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.