Abstract
Drosophila melanogaster is an emerging model to study different aspects of social interactions. For example, flies avoid areas previously occupied by stressed conspecifics due to an odorant released during stress known as the Drosophila stress odorant (dSO). Through the use of the T-maze apparatus, one can quantify the avoidance of the dSO by responder flies in a very affordable and robust assay. Conditions necessary to obtain a strong performance are presented here. A stressful experience is necessary for the flies to emit dSO, as well as enough emitter flies to cause a robust avoidance response to the presence of dSO. Genetic background, but not their group size, strongly altered the avoidance of the dSO by the responder flies. Canton-S and Elwood display a higher performance in avoiding the dSO than Oregon and Samarkand strains. This behavioral assay will allow identification of mechanisms underlying this social behavior, and the assessment of the influence of genes and environmental conditions on both emission and avoidance of the dSO. Such an assay can be included in batteries of simple diagnostic tests used to identify social deficiencies of mutants or environmental conditions of interest.
Keywords: Neuroscience, Issue 94, social behavior, social avoidance, Drosophila melanogaster, Drosophila, Stress Odorant - dSO, T-maze apparatus, neurogenetics
Introduction
The goal of this method is to easily quantify a new aspect of simple social behavior in Drosophila melanogaster, independent from courtship and aggression.
Social interactions are crucial to the proper development and health of individuals within a society, as well as the functionality of a social group as a whole. The high complexity of these interactions necessitates large sample sizes and a system that allows for simplification of the behavior as the genetic and neural bases of social behavior are still poorly understood. Drosophila melanogaster is a powerful genetic model that can be used to identify the genetic and neural bases of social interactions. Indeed, D. melanogaster has a repertoire of complex social behaviors and some direct measurements of socialization have already been done1-7. However, most of these efforts have been focused on relatively complex social behaviors, such as aggressive interactions3,6, various aspects of courtship3,8-12, and how social experience affects other behaviors such as learning, or circadian rhythm13-17. In addition, many of these assays rely on analyzing complex interaction patterns of groups of flies, using video tracking and computer software to analyze the resulting abundance of data. Such analyses are invaluable, and lead to important new insights such as the dynamic of fly-fly interactions in groups7. One limitation, however, is the inaccessibility of these assays to the community at large, and the limited knowledge of the mechanisms underlying recognition of others. In other words, the basis of the emission of a signal by one individual and its recognition by another is still poorly understood18.
In contrast, flies also exhibit a simple behavior, social avoidance, where individuals move away from a signal emitted by stressed flies: the D. melanogaster Stress Odorant or dSO19. In a high-throughput assay, this behavior can be quantified as the avoidance of a stress signal emitted by other flies, or social avoidance19. Flies are placed in a T-maze apparatus and given the choice to avoid a vial containing dSO. Using this assay, CO2 was shown to be a component of the dSO, and part of the neural circuitry necessary to respond to CO2 was dissected19.
The social avoidance assay presented here is similar conceptually to the simple behavioral assays developed in Seymour Benzer’s laboratory that enabled generations of researchers to dissect complex behaviours20. Analysis of social avoidance can be carried out cost-effectively using the T-maze assay, allowing for more widespread study of social behavior. For example, using this assay we recently demonstrated that different genetic risks for autism have contrasting effects in social behavior assays. Mutants for a candidate gene for autism — neurobeachin21,22 — present deficiencies both in social space (described elsewhere23) and social avoidance24. Abnormal dopaminergic signaling is also proposed to play a role in the etiology of autism in humans25,26. In contrast to the results obtained with neurobeachin, we found that social avoidance performance was unaffected by increased or decreased levels of the Drosophila Vesicular Monoamine Transporter (VMAT) in dopaminergic cells, although social space was directly correlated to these levels of VMAT27. The contrasting results obtained with neurobeachin and VMAT underscore the possibility of identifying various forms of asocial behavior, and thus the different underlying neural circuitries modulating the response to others.
Protocol
1. Equipment and Reagents Created In-house (See List of Material for Others)
- Prepare a Drosophila cold anesthesia apparatus to perform fly work.
- Cut a porous polyethylene sheet to cover a small plastic box (12.7 cm long, 10.2 cm wide), typically the top cover of a pipette tips box (12.7 cm long, 10.2 cm wide, 3.8 cm deep).
- Fill the box with crushed ice, cover with the porous polyethylene sheet.
Prepare a T-maze apparatus; adapt the apparatus previously described in details 28-31.
Prepare a fly aspirator as described 32.
- Prepare a phototaxis response apparatus to displace flies in and out of test vials without stress:
- Use a countercurrent apparatus, as described by29,33.
NOTE: The T-maze can also be used for other purposes, as described by28.
Ensure homogeneous lighting conditions: perform the experiment on a bench covered with a white bench cover, and in front of a white board. NOTE: For these experiments, the fly strains used were Drosophila melanogaster wild-types: Canton-S, Oregon and Samarkand flies from our laboratory stocks34; Elwood flies were collected in Fall 2011 in the Elwood neighborhood of Huntington, on Long Island, New York, USA.
2. Preparing the Flies before the Experiment
1-2 days before performing the experiment:
Maintain flies on standard cornmeal-agar-molasses-yeast medium at 25 °C on a 12 hr light/dark cycle.
- Collect the responder flies under cold anesthesia 1-2 days prior to performing the experiment
- Transfer the flies from the bottles in which they have been raised into 50 ml Falcon tubes, using a funnel.
- Place the falcon tube with the flies under ice level, into an insulated ice bucket.
- Place the cold anesthesia apparatus with crushed ice at -4 °C to chill.
- Wait 5 min for the flies to enter a chill coma.
- Transfer the flies onto the porous polyethylene sheet of the cold anesthesia apparatus.
- Collect the responder flies on the cold anesthesia apparatus. At RT, under a stereomicroscope, separate 3-7 days old male from female flies and maintain these flies in groups of 40.
- Collect the emitter flies 1-2 days prior to performing the experiment.
- Gently collect Canton-S mixed-sex flies from their bottles, using a mouth aspirator, controlling for their number.
- Maintain these flies in group of 60-100, as they will be the emitters.
- Prepare as many samples of emitters as data points needed.
Allow at least a night of recovery after collection of the responders and emitters, to minimize any confounding effect of the cold anesthesia and mouth aspirator collection on behavior.
Open the bag of test vials, to allow for the stale plastic-smelling air in the bag to be replaced by fresh air, at least 1 day prior to the experiment.
2 hr before performing the experiment:
Ensure that the temperature in the room in which the experiment is performed is around 23-25 °C, with even light, and humidity above 30%.
Transfer the responders and emitters fruit flies into fresh food vials to ensure that these flies are not starved.
Let the fruit flies adjust to the environment for 2 hr, on the bench on which the experiment will be performed.
3. Performing the Social Avoidance Experiment
Perform each experiment at the same time of the day, in a range of 3–4 hr in the afternoon between Zeitgeber time ZT5 and ZT9.
Place the T-maze on the pounding pad, and tighten the screw clip so the elevator is stable.
Transfer the responder flies into a fresh test vial. Snap this vial containing the responder flies onto the top part of the T-maze.
Slant the T-maze apparatus and tap it on the pounding mat so the fruit flies fall in the elevator.
Move the top part of the elevator down so the flies are between the top and bottom section of the T-maze, but pay attention to keep the elevator above the choice point – to prevent the flies from escaping.
- Obtain vials with dSO (see Section 5 to generate dSO-free vials that were previously occupied by flies).
- While the responder flies are adjusting to this new environment, place the emitter flies in a test vial.
- Place a piece of cotton to close the vial so the fruit flies do not escape.
- Mechanically agitate this vial on a mini-vortex as follows: vortex for 15 sec, remove the vial from the vortex for 5 sec. Repeat 3 times for a total of 55 sec.
- Remove the emitter flies out of the fresh vial — by transferring in a food vial — and quickly place this dSO filled vial into one of the two sides of the T-maze apparatus.
- Alternate the side of placement for the dSO filled vial in each new run.
Place a fresh test vial on the other side of the T-maze.
Bring the elevator completely down, so the fruit flies can chose between the fresh test vial and the test vial with dSO, and start the timer.
Let the flies choose between the fresh vial and the dSO vial, for 1 min, unless noted otherwise.
After 1 min, move the elevator up to separate the flies in the fresh vial, dSO vial, and those stuck in the elevator.
Count the number of flies in each vial, and in the elevator.
Repeat this for each genotype/condition.
4. Analyzing the Social Avoidance Data
Count the number of fruit flies in each vial for each genotype, and transfer the data to a spreadsheet.
Using a spreadsheet program, calculate the Performance Index (PI) for each genotype by subtracting the number of flies in the dSO vial from the number of flies in the dSO-free vial, and then dividing by the total number of flies.
- Compare the PIs’ means using statistical analysis software for analysis of the results.
- Ideally, prepare 2-3 internal repeats for each experiment, and 3 independent repeats, performed on different days, with different bottles or crosses30.
- Use Gaussian distribution statistical tests, as the data follow a normal distribution.
- When testing one effect (i.e., density — Figure 2), use a one-way ANOVA, with post-test for multiple comparison (column by column), or a simple t-test to compare each result to a control.
- When group experiments are performed, test both conditions and genders (empty vials, stressed and un-stressed flies; and gender effect — Figure 1), using a two-way ANOVA.
5. Generate Vials Previously Occupied by Unstressed Flies — Perform Instead of 3.5 to 3.6
Use phototaxis to prepare vials occupied by flies that have not been stressed, as a control.
Transfer the flies in a test vial, and snap it into the countercurrent apparatus, with fresh empty vials in the opposite location.
Lay the apparatus horizontally with the distal vial directly in front of a 15 W fluorescent cool-white light, and cover with a black cloth.
Let the flies gently move in the fresh vial. This might take several minutes. Once most of the flies are walking toward the light in the distal vial, start the timer for 1 min.
Gently remove the black cloth, switch the location of the light, recover with the black cloth, and allow the flies to leave the test vial.
At that time, initiate the choice experiment as in 3.2 to 3.5. Skip 3.6 and 3.7.
Move the slider of the apparatus, to separate the flies that vacated the test vial from the other side.
Use the vacated test vial in the T-maze right away for the choice experiment, as in 3.7.
Representative Results
The social avoidance assay is a robust test quantifying the ability of Drosophila melanogaster to recognize a stress signal (dSO) emitted by other flies, and thus assessing one aspect of social interactions. The assay is performed using an apparatus commonly used in various behavior assays known as a T-maze, which present the flies with a choice between two different options — left or right19,28-31. In this case, the efficiency at which the flies avoid the dSO is quantified by calculating and comparing their performance Index (PI). A PI of zero indicates a 50-50 distribution or no avoidance, while a PI of 100 indicates that all the flies avoided the dSO. And a PI of -100 would indicate that all the flies went inside the dSO vial.
The flies are presented with a vial containing dSO emitted by mechanically agitated flies, and one fresh (dSO-free) vial. Representative results presented here underscore the strength of this assay, as well as some known conditions necessary to obtain best results.
Avoidance of dSO is not sex specific but depends on flies being stressed. The sex of responder flies has no effect on their avoidance of dSO (Figure 1). However, it is not sufficient for flies to have occupied the test vial to elicit an avoidance of that vial, the flies must have been stressed — in this case mechanically agitated (Figure 1). If no flies had occupied the test vial, or if the flies have entered and exited the vial without stress using phototaxis, the responders elicit no preference as was previously reported19.
Although Figure 1 reproduces known results, it is also an example of the kind of data obtained with sub-optimal conditions. With only 2 to 5 internal repeats, and no independent trials, the error bars represent the range. However, there is a significant difference between vials previously occupied by stressed flies and non-stressed flies or empty, as previously reported19. In this context, the flies still display a strong PI of avoidance. This underscores the robustness of the assay. In addition, the PI for the choice between two fresh vials is negative (different from 0, for females p < 0.05, and pooled sex p < 0.01). This indicates a preference for one side of the T-maze, or a bias due to a small sample size. This limitation can be countered by alternating the side on which the test vial is placed on the T-maze in the internal replicates, as done in the subsequent experiments presented.
Despite the lack of sex effect in the response to the dSO, responders were separated by sex to prevent possible confounding effects. For example, in the case of mutations that could have sex-specific effects, such as those affecting genes involved in olfactory signaling36. The following representative results focus on male responders, and mixed sex emitters.
Avoidance of dSO is independent of the number of responder flies tested.
Previous experiments19 have used a standard of 40 Canton-S flies in testing social avoidance, which showed a high PI, indicating that most flies preferred the fresh dSO-free vial. To test the effect of group size on the PI of the responders, a range of 10-80 flies was assayed, as seen in Figure 2. A minimum of two independent trials and three internal replicates per trial was performed. A t-test showed no statistical significance when comparing the PI of each of the different number of responder flies to the performance index of the control (forty responder flies). Thus, the number of responder flies has no effect on the PI of dSO avoidance and the effect that mutations have on avoidance can be tested even if mutants are available in small numbers.
Higher number of emitters flies leads to higher avoidance of dSO.
To determine the extent to which the number of emitter flies affected the dSO and its avoidance, we also tested the effect of amount of stressed flies. In past experiments19, and this protocol, have used a standard of 70 Canton-S flies. We now tested 10 to 160 emitter flies (Figure 3). The performance of avoidance is directly related to the number of emitters, although there is no increase in avoidance with more than 80 emitters. Interestingly, a substantial avoidance was still detected with only 10 stressed flies. Again, this would allow testing the emission capabilities of few flies at a time.
Genetic background affects the dSO avoidance performance.
Drosophila melanogaster is a strong model organism for genetic studies. Thus it was important to determine whether the assay could be used to discriminate different genetic backgrounds. The response of four different wild types was measured (Canton-S, Oregon-R, Samarkand and Elwood) with respect to their avoidance of dSO emitted by Canton-S (Figure 4). Canton-S and Elwood have a higher PI compared to Oregon and Samarkand (p < 0.05). These results demonstrate that there is a genetic component underlying the avoidance of the dSO, and that this assay has the power to perform a study of this genetic influence.
Length of choice time affect performance.
The set of representative data presented in Figures 1, 2, 3 and 4 also illustrate the effect of length of time given to the responders to decide which vial they avoid. When Canton-S responders are given 30 to 45 sec to choose their preferred vial, they typically reach performances around PI = 55 (Figure 2, respectively PI males = 57 ± 2; and 52 ± 11). When given 60 sec, their performance is higher, as seen in Figure 4 (PI = 77 ± 6.5).
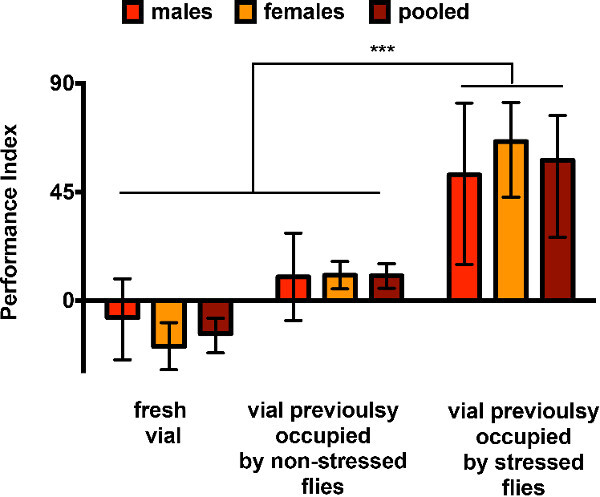 Figure 1. Avoidance of dSO is not sex specific but depends on flies being stressed. Bar graphs represent the average ± range of the PI of avoidance of a vial the stress odor left by agitated flies (dSO). Males (in red), and females (orange) were tested together (brown), and then counted after the experiment. The flies performed similarly, regardless of their sex (two-way ANOVA). But they only avoided a dSO-filled vial in which flies had been previously stressed (mechanically agitated), and not a vial in which flies had not been stressed (entering and exiting the vial by phototaxis, p < 0.05, Tukey’s multiple comparison post-test), or fresh vial (p < 0.001, Tukey’s multiple comparison post-test). In addition, there was no statistical differences between the fresh vial and non-stressed flies conditions, despite a bias the fresh vial experiment — n = 2-5 replicates with 40 mixed sex flies each, with 45 sec choice time (n = 3 for fresh vials, n = 2 for vial with non-stress files, n = 5 for vial with stressed flies).
Figure 1. Avoidance of dSO is not sex specific but depends on flies being stressed. Bar graphs represent the average ± range of the PI of avoidance of a vial the stress odor left by agitated flies (dSO). Males (in red), and females (orange) were tested together (brown), and then counted after the experiment. The flies performed similarly, regardless of their sex (two-way ANOVA). But they only avoided a dSO-filled vial in which flies had been previously stressed (mechanically agitated), and not a vial in which flies had not been stressed (entering and exiting the vial by phototaxis, p < 0.05, Tukey’s multiple comparison post-test), or fresh vial (p < 0.001, Tukey’s multiple comparison post-test). In addition, there was no statistical differences between the fresh vial and non-stressed flies conditions, despite a bias the fresh vial experiment — n = 2-5 replicates with 40 mixed sex flies each, with 45 sec choice time (n = 3 for fresh vials, n = 2 for vial with non-stress files, n = 5 for vial with stressed flies).
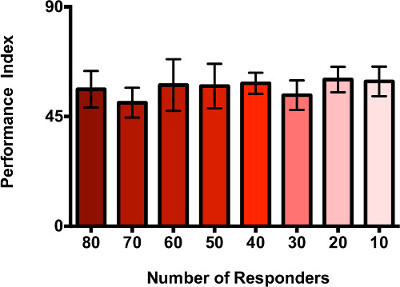 Figure 2. Avoidance of dSO is independent of the number of flies tested. Representative data shows that the number of Canton-S responder flies in the T-maze has no effect on social avoidance of the dSO. Responder flies were given 30 sec to decide between a fresh vial and a vial containing dSO (n = 2-6 internal replicates per trial, 1-2 trials, minimum of 5 data points per condition, bar graphs represent the average ± s.e.m.)
Figure 2. Avoidance of dSO is independent of the number of flies tested. Representative data shows that the number of Canton-S responder flies in the T-maze has no effect on social avoidance of the dSO. Responder flies were given 30 sec to decide between a fresh vial and a vial containing dSO (n = 2-6 internal replicates per trial, 1-2 trials, minimum of 5 data points per condition, bar graphs represent the average ± s.e.m.)
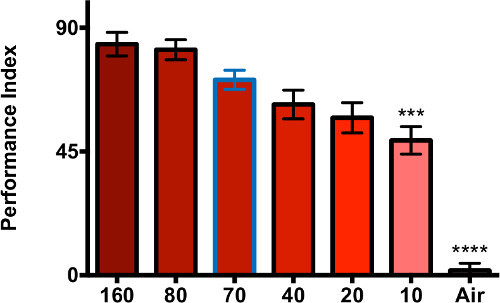 Figure 3. Increased number of emitters leads to increase dSO avoidance. Representative data shows that the number of Canton-S emitter flies affects the dSO and its avoidance (increasing shade of red indicates increased number of emitter flies, with 70 emitters outlined in blue). When compared to 70 emitters, only the vials that contained 10 emitters or air showed a significant difference in performance (one-way ANOVA p = 0.0001, Dunnett’s multiple comparison post-test). The 40 responder flies were given 1 min. to decide between a fresh vial and a vial containing dSO (n = 3 internal replicates per trial, 3 independent trials, bar graphs represent the average ± s.e.m.)
Figure 3. Increased number of emitters leads to increase dSO avoidance. Representative data shows that the number of Canton-S emitter flies affects the dSO and its avoidance (increasing shade of red indicates increased number of emitter flies, with 70 emitters outlined in blue). When compared to 70 emitters, only the vials that contained 10 emitters or air showed a significant difference in performance (one-way ANOVA p = 0.0001, Dunnett’s multiple comparison post-test). The 40 responder flies were given 1 min. to decide between a fresh vial and a vial containing dSO (n = 3 internal replicates per trial, 3 independent trials, bar graphs represent the average ± s.e.m.)
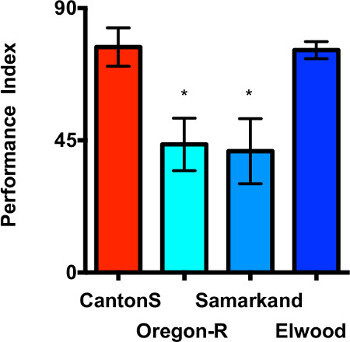 Figure 4. Choice time and genetic background of fruit flies affect the response to dSO. Canton-S and Elwood genotypes display a stronger avoidance of the dSO than Oregon and Samarkand (p < 0.05*, t-test comparing to Canton-S). Responder flies were given 1 min. to decide between a fresh vial and a vial containing dSO (n = 4-5 internal replicates per trial, 2 independent trials, bar graphs represent the average ± s.e.m.)
Figure 4. Choice time and genetic background of fruit flies affect the response to dSO. Canton-S and Elwood genotypes display a stronger avoidance of the dSO than Oregon and Samarkand (p < 0.05*, t-test comparing to Canton-S). Responder flies were given 1 min. to decide between a fresh vial and a vial containing dSO (n = 4-5 internal replicates per trial, 2 independent trials, bar graphs represent the average ± s.e.m.)
Discussion
This protocol describes a detailed procedure for the social avoidance assay. Canton-S will only avoid a vial in which flies have previously been mechanically stressed, and that sex and number of responders does not affect that social avoidance performance. However, genetic background of the responders has a major influence.
The following are several critical steps for performing this experiment successfully: 1) always transfer the flies 2 hr before the experiment and make sure not to disturb their environment; 2) perform the experiment always at the same time of the day, ideally between Zeitgeber time ZT5 (hours after the onset of light) and ZT9 to reduce variation in performance linked to circadian rhythm and thus possible variation in locomotion and activity levels; 3) note the temperature and humidity conditions when performing the experiment; a temperature around 23-25 °C and humidity above 30% are best; 4) make sure not to disturb the T-maze apparatus during the flies decision time; 5) make sure to air out the fresh vials at least 24 hr before using them; and 6) during the experiment it is important to always change the location of the test vial in the T-maze apparatus to avoid preference to one side of the T-maze. Although additional tests need to be done to confirm this, empirically it seems that the dSO escapes the “stress” vial in less than 5 min. In contrast, it seems that flies are able to emit the dSO up to 1 hr after being stressed.
There are several considerations that must be taken into account in the protocol. The correlation between the age of the fly and social avoidance is currently unknown. Therefore, it is recommended to perform the experiment with no more than two week old flies at 25 °C, as behavioral changes associated with aging do not appear prior to that age37. Mated flies (housed mixed sexes) are collected for the experiment as it is unknown how the dSO emission is affected by the mating status of the flies. Although the behavioral output of dSO avoidance is not affected by sex, the underlying brain circuitry may be sexually dimorphic, and it is advisable not to mix male and female flies. Finally, a reduction in the number of emitter flies leads to a decrease in avoidance, probably linked to a decrease in total dSO emitted. However, enough dSO is still present with only 10 flies, leading to a robust and reproducible avoidance.
Reduction of decision time of the responder flies from 60 sec to 30 sec, also results in a reduction of the PI for Canton-S flies. This can be used as a tool to discriminate for conditions or mutations in which flies perform better than Canton-S. Thus, in its current state, the social avoidance assay can be used as a behavioral paradigm to test the effect of genes and the environment on dSO avoidance. After mastering the technique, future steps can be taken to incorporate the ideal decision time for dSO avoidance and the exact number of emitter flies that must be used to get the social avoidance assay to work optimally.
A limitation that would arise in testing mutants can occur if the responders have poor locomotion. Experimentally determining a longer choice time for these mutants may counter such poor locomotion. Similarly, flies with sensory deficiencies that would prevent detection of CO2 — a major component of the dSO, or the other unknown component of the dSO would not be able to be tested in this assay.
There is currently no other assay allowing quantification of this response. Although it was shown that CO2 is a component of the dSO19, other components of the signal, as well as neurotransmitters involved in the choice made to avoid other stressed individuals have not yet been determined. Similarly, the mechanism that drives the flies to emit the dSO is not known. This assay can currently be used as an additional bench top diagnostic assay for determining the social and overall behavioral profile of a mutant, or environment treatment. For example, one can compare and contrast the quantification of social space, i.e., the distance to the closest neighbor23, to the social avoidance of stressed flies. Both behaviors require responding to the presence of other individuals, and making the decision to come close or to avoid them. These are very simple yet powerful ways to dissect brain circuitry and neurotransmitters involved. These behavioral paradigms also have a tremendous potential to be used for screening purposes.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We thank Rachelle Kanippayoor for her help in identifying the new wild-type strain as being of the melanogaster species. R.W.F, O.F. and A.F.S were responsible for research design; R.W.F, M.N. and O.F. performed the experiments. R.W.F, M.N., O.F. and A.F.S. analyzed the data; R.W.F., I.S.M. and A.F.S. wrote the manuscript.
This work was supported by PSC-CUNY research awards, jointly funded by The Professional Staff Congress and The City University of New York to A.F.S.; by internal funding from Western University to A.F.S. and I.S.M.; by a training support from the National Alliance for Hispanic Health’s Alliance/Merck Ciencia (Science) Hispanic Scholars Program and a University Fellowship from the Yale Graduate School of Arts and Sciences to R.W.F.
References
- Fry SN, Rohrseitz N, Straw AD, Dickinson MH. TrackFly: virtual reality for a behavioral system analysis in free-flying fruit flies. J Neurosci Methods. 2008;171:110–117. doi: 10.1016/j.jneumeth.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Slawson JB, Kim EZ, Griffith LC. High-resolution video tracking of locomotion in adult Drosophila melanogaster. J Vis Exp. 2009;24:1096. doi: 10.3791/1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JC, Dickinson MH. A new chamber for studying the behavior of Drosophila. PLoS One. 2010;5:8793. doi: 10.1371/journal.pone.0008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. Inaugural Article: A common genetic target for environmental and heritable influences on aggressiveness. in Drosophila. Proceedings of the National Academy of Sciences. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Dickinson MH, Levine JD. Social structures depend on innate determinants and chemosensory processing in Drosophila. Proceedings of the National Academy of Sciences. 2012;2:17174–17179. doi: 10.1073/pnas.1121252109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A, Hall JC, Jeffrey CH. Chapter 3 Neurogenetics of Courtship and Mating in Drosophila. Advances in Genetics. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Ejima A, Griffith LC. Courtship Initiation Is Stimulated by Acoustic Signals in Drosophila melanogaster. PLoS ONE. 2008;3:3246. doi: 10.1371/journal.pone.0003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F, et al. Public Versus Personal Information for Mate Copying in an Invertebrate. Current Biology. 2009;19:730–734. doi: 10.1016/j.cub.2009.02.064. [DOI] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter J-C, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, et al. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- Kent C, Azanchi R, Smith B, Formosa A, Levine JD. Social context influences chemical communication in D. melanogaster males. Curr Biol. 2008;18:1384–1389. doi: 10.1016/j.cub.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Resetting the Circadian Clock by Social Experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking Experience Affects Sleep Need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- Billeter J-C, Levine JD. Who is he and what is he to you? Recognition in Drosophila melanogaster. Curr. Opin. Neurobiol. 2013;23:17–23. doi: 10.1016/j.conb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Bonini N. A Tribute to Seymour Benzer 1921-2007. 2008;180:1265–1273. doi: 10.1534/genetics.104.97782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castermans D, et al. The neurobeachin gene is disrupted by a translocation in a patient with idiopathic autism. Journal of Medical Genetics. 2003;40:352–356. doi: 10.1136/jmg.40.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L, et al. Neurobeachin, a protein implicated in membrane protein traffic and autism, is required for the formation and functioning of central synapses. J Physiol. 2009;587:5095–5106. doi: 10.1113/jphysiol.2009.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, et al. A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav. 2012;11:243–252. doi: 10.1111/j.1601-183X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh T, et al. Cold Spring Harbor Meeting: From Molecules to Circuit Behavior. Cold Spring Harbor, NY: Cold Spring Harbor Laboratories; 2013. [Google Scholar]
- Hamilton PJ, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, et al. Association of dopamine gene variants, emotion dysregulation and ADHD in autism spectrum disorder. Research in Developmental Disabilities. 2014;35:1658–1665. doi: 10.1016/j.ridd.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RW, Akinleye AA, Nurilov M, Rouzyi Z, Simon AF. 54th Annual Drosophila Research Conference.2014. [Google Scholar]
- Ali YO, Escala W, Ruan K, Zhai RG. Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration. J Vis Exp. 2011. p. 2504. [DOI] [PMC free article] [PubMed]
- Connolly JB, Tully T. In: Drosophila: A Practical Approach. Roberts DB, editor. Vol. 1. New York, NY: IRL; 1998. pp. 265–317. [Google Scholar]
- Tully T, Quinn WG. Classical-conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Waddell S. Drosophila Aversive Olfactory Conditioning. Cold Spring Harbor Protocols. 2011;2011 doi: 10.1101/pdb.prot5608. [DOI] [PubMed] [Google Scholar]
- Ejima A, Griffith LC. Ch. 30. In: Zhang B, Freeman MR, Waddell S, editors. Drosophila Neurobiology, A Laboratory Manual. Cold Spring, NY: Cold Spring Harbor Laboratory Press; 2010. pp. 475–481. [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila melanogaster isolated by countercurrent distribution. PNAS. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- Vaux DL. Research methods: Know when your numbers are significant. Nature. 2012;492:180–181. doi: 10.1038/492180a. [DOI] [PubMed] [Google Scholar]
- Stowers L, Logan DW. Sexual dimorphism in olfactory signaling. Curr. Opin. Neurobiol. 2010;20:770–775. doi: 10.1016/j.conb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Liang DT, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mechanisms of Ageing and Development. 2006. pp. 127–647. [DOI] [PubMed]


