Abstract
Hepatic ischemia-reperfusion injury (IRI), an innate immunity-driven inflammation response, occurs in multiple clinical settings including liver resection, transplantation, trauma, and shock. TIM-4, the only TIM protein not expressed on T cells, is found on macrophages and dendritic cells. The regulatory function of macrophage TIM-4 in the engulfment of apoptotic/necrotic bodies in innate immunity-mediated disease states remains unknown. This study focuses on putative role of TIM-4 signaling in a model of liver warm ischemia (90min) and reperfusion. The ischemia insult triggered TIM-4 expression by stressed hepatocellular phosphatidylserine (PS) presentation, peaking at 6h of reperfusion, and coinciding with the maximal hepatocellular damage. TIM-4-deficient or WT mice treated with antagonistic TIM-4 mAb were resistant against liver IRI, evidenced by diminished sALT levels and well-preserved hepatic architecture. Liver hepatoprotection rendered by TIM-4 deficiency was accompanied by diminished macrophage infiltration/chemoattraction, phagocytosis and activation of TLR2/4/9-dependent signaling. Correlating with in vivo kinetics, the peak of TIM-4 induction in LPS-activated bone marrow derived-macrophages (BMM) was detected in 6h cultures. To mimic liver IRI, we employed hydrogen peroxide-necrotic hepatocytes, which readily present PS. Indeed, necrotic hepatocytes were efficiently captured/engulfed by WT (TIM-4+) but not by TIM-4-deficient BMM. Finally, in a newly established model of liver IRI, adoptive transfer of WT but not TIM-4 deficient BMM readily recreated local inflammation response/hepatocellular damage in the CD11b-DTR mouse system. Conclusion: Our novel findings document the importance of macrophage-specific TIM-4 activation in the mechanism of hepatic IRI. Macrophage TIM-4 may represent a therapeutic target to minimize innate inflammatory responses in IR-stressed organs.
Keywords: Inflammation, Hepatocytes, Macrophages, TIM-4, CD11b-DTR
Ischemia-reperfusion injury (IRI) is a major complication of hemorrhagic shock, tissue resection and transplantation (1). Resulting from organ retrieval, cold preservation and warm ischemia during surgery, IRI represents one of the most challenging problems in transplantation. Indeed, IRI often leads to primary graft non-function, may predispose to late chronic rejection, and contributes to a shortage of organs available for transplantation. In the liver, IRI represents a continuum of immune processes, which include endothelial activation, increased expression of adhesion molecules, Kupffer cell/neutrophil activation, and cytokine release, followed by hepatocyte death. Our group was among the first to document that activation of TLR4 is central to the propagation of sterile inflammation (2), and its molecular signature (TNF-α, IFN-β, and CXCL-10) (3) in IR-stressed liver. Others have reported on the essential role of phagocytosis in liver IRI, with depletion of phagocytic cells attenuating (4), while endotoxin-mediated phagocyte priming exacerbating (5) local inflammation.
TIM (T cell Ig and mucin domain) gene family of cell surface proteins consists of eight members in mice (TIMs 1-8) and three in humans (TIM-1, TIM-3, and TIM-4) (7). TIM-4, the only non-T cell TIM protein, is expressed on macrophages, a population of CD11c+ DCs (6-9), CD169+ (MOMA-1+) marginal metallophilic macrophages (9), and some peritoneal B cells (10). TIM-4 was initially identified as the phosphatidylserine (PS) ligand, capable of binding/engulfing apoptotic bodies (6,8,11,12), the key phagocytosis step in innate immune reactions (8). By acting as TIM-1 ligand on T cells, TIM-4 may function as a costimulatory molecule (7) that controls adaptive immunity (9,13,14). TIM-4 upregulation on intestinal mucosal and bone marrow-derived DCs after exposure to Staphylococcus enterotoxin B or cholera toxin and peanut extract, promoted Th2 polarization and intestinal allergy, respectively (13,14). TIM-4 was proven essential for the maintenance of the homeostatic state (15), with aberrant persistence of apoptotic bodies leading to dysregulated lymphocyte activation and autoimmunity (10).
Despite expression on mature APCs, no data exists on the impact of TIM-4 signaling in innate immunity-driven disease states. For instance, whether macrophage TIM-4-mediated phagocytosis promotes IR-stress, or negatively regulates immune responses to maintain homeostasis, remains to be determined. In the present study, we determined hepatocellular PS presentation and kinetics of endogenous TIM-4 expression during liver IRI in a mouse model. Then, we asked whether TIM-4 is essential in macrophage activation/phagocytic, and whether macrophage-specific TIM-4 signaling is sufficient to facilitate hepatic IRI. By providing evidence for previously unrecognized role of TIM-4 in innate activation, this study provides new insights into the pathogenesis of, and identifies potential molecular targets against liver IRI.
Materials and Methods
Animals
Male 8-12 weeks old wild-type (WT); CD11b-DTR (Jackson Laboratory, Bar Harbor, ME) and TIM-4 deficient (KO) (10) (gift from Dr. Vijay K. Kuchroo, Harvard University, Boston, MA) mice (C57BL/6) were used. Animals were housed in the UCLA animal facility under specific pathogen-free conditions and received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; NIH publication 86-23, revised 1985).
Liver IRI Model
We used an established mouse model of segmental (70%) hepatic IRI (16,17). After 90min of warm ischemia, animals were sacrificed at reperfusion, and liver/serum samples were collected. Sham-operated controls underwent the same procedures, but without vascular occlusion. In the treatment groups, WT mice were infused at 1h prior to ischemia with a blocking TIM-4 mAb (RMT4-53; 0.5mg/mouse i.v.; Bio X Cell, West Lebanon, NH) (18); control Ig; or Diannexin (200μg/kg i.v.; Alavita Inc., Mountain View, CA), a novel annexin V homodimer, which binds PS with high affinity (19). To focus at macrophage-specific TIM-4, we used a conditional ablation transgenic system in which CD11b-DTR mice were treated with diphtheria toxin (DT 25ng/g i.p. at day -2) to deplete CD11b+ cells (20). Separate groups of CD11b-DTR mice were infused with bone marrow-derived macrophages (BMM; 5×106 cells i.v. at -1h) from WT or TIM-4 KO donors. DT treatment does not trigger hepatotoxicity in WT mice (Supplementary Fig. 1a).
Measurement of Hepatocellular Damage
Serum alanine aminotransferase (sALT) levels were measured by IDEXX Laboratory (Westbrook, ME).
Histopathology
Liver specimens (4μm), stained with hematoxylin/eosin (H&E) were analyzed blindly by modified Suzuki's criteria (16). Primary mAb against mouse macrophages CD68 (FA-11; AbD Serotec, Raleigh, NC) was used. Liver sections were evaluated blindly by counting labeled cells/10 HPF.
Quantitative RT-PCR
Quantitative PCR was performed with platinum SYBR green quantitative PCR kit (Invitrogen, Carlsbad, CA) by the Chromo 4 detector (MJ Research, Waltham, MA). Primers to amplify specific gene fragments were published (16). The sequence of TIM-4, SR-A, LOX-1, MARCO, IRF3, TLR2, TLR4 and TLR9 primers is shown (Supplementary Table 1). Target gene expressions were calculated by their ratios to the housekeeping hypoxanthine-guanine phosphoribosyl transferase (HPRT) gene.
Flow Cytometry and Immunofluorescence Imaging
Liver non-parenchyma cells or BMM were stained with FITC-F4/80 (BM8), FITC-CD11b, PE/Cy5-CD11b (M1/70), FITC-CD11c (N418), PE/Cy5-CD8 (53-6.7) and PE-TIM-4 (RMT4-54) (BioLegend, San Diego, CA); necrotic hepatocytes were stained with FITC-Annexin V (BD Biosciences).
Cell Cultures
Mouse BMMs from femurs/tibias were cultured (5×106/well) with 10% L929 medium for 6 days (94-99% CD11b+). Cultured BMM activated by LPS (10ng/ml, Sigma-Aldrich) were harvested for TIM-4/cytokine analysis (17). Mouse hepatocytes were isolated by in situ two-stage collagenase perfusion, cultured with complete William E medium plus maintenance supplement (Life Technologies, Grand Island, NY) for 24h. Hepatocyte viability was 95-99%. Hydrogen peroxide (H2O2, 0.1mM, Sigma-Aldrich) was used to trigger hepatocyte necrosis (17).
In vivo Phagocytosis Assay
CellTrace CFSE (Life Technologies) stained C57BL/6 thymocytes, treated with 1μM dexamethasone (Sigma) for 12h. FITC-Annexin V and propidium iodide (BD Biosciences) staining was used to confirm thymocyte apoptosis. Groups of WT and TIM-4 KO mice were infused with apoptotic bodies (20×106 cells/mouse, i.v.) 1h prior to ischemia. Some WT mice were given anti-TIM-4 mAb/control Ig (0.5mg/mouse, i.v.). Animals were sacrificed at 6h of reperfusion after 90min hepatic ischemia. Non-parenchyma cells from collagenase-digested livers were stained with PE-CD11b (M1/70, eBioscience). The frequency of CD11b+CFSE+ cells, representing macrophages that phagocytosed apoptotic bodies, was assessed.
In vitro Phagocytosis Assay
CellTracker Green CMFDA (Life Technologies) stained necrotic hepatocytes were co-cultured with BMM for 3h. After wash, the BMMs were stained with PE-CD11b (M1/70, eBioscience) for immunofluorescence imaging. Culture supernatants were assessed for TNF-α and IL-1β levels by ELISA (eBioscience).
Statistical Analysis
All values are expressed as the mean±SD. Data were analyzed with an unpaired, two-tailed Student's-t test and checked by ANOVA. P<0.05 was considered to be statistically significant.
Results
Stress-Induced PS Presentation Triggers TIM-4 Expression in Liver IRI
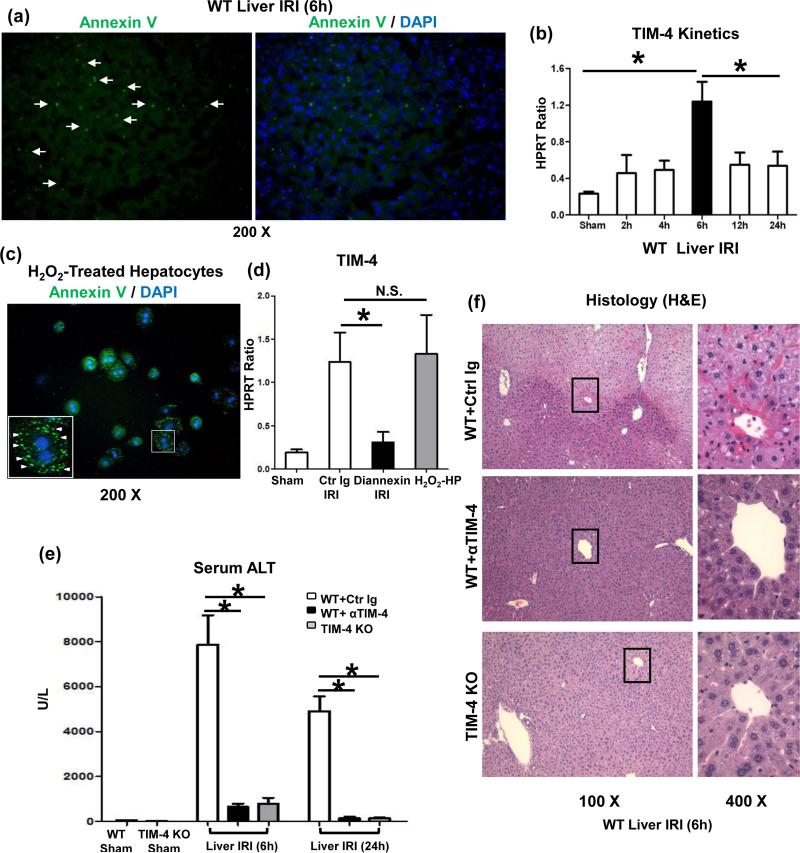
Phosphatidylserines (PS), exposed on cells undergoing necrosis/apoptosis, may signal macrophages to bind/engulf dying cells via TIM-4 signaling (6). First, by using FITC-Annexin V staining we detected very high frequency of PS+ cells in mouse livers subjected to 90min warm ischemia and 6h reperfusion (Fig. 1a). As TIM-4 was identified as PS ligand that mediates uptake of apoptotic cells (6), we determined the kinetics of endogenous TIM-4 induction in our model. Unlike in sham controls, ischemia triggered progressive TIM-4 mRNA expression, peaking by 6h of reperfusion and decreasing at 12-24h (Fig. 1b), which correlated with hepatocellular damage (sALT levels) and macrophage infiltration/activation in IR-stressed livers (16). Having documented that H2O2-induced necrotic hepatocytes readily present PS, as shown by FITC-Annexin V staining (Fig. 1c), we then transferred PS+ hepatocytes (10×106 cells/mouse) via intra-splenic route directly into recipient livers. Without concomitant IR insult, hepatic TIM-4 expression dramatically increased by 6h of PS+ cell infusion (Fig. 1d; H2O2-HP grp), comparable with that in liver IRI (Fig. 1d; Ctr Ig-IRI grp). Finally, we employed Diannexin, an Annexin V homodimer, which competitively binds apoptotic PS (19). Interestingly, disruption of PS–TIM-4 signaling not only decreased TIM-4 gene induction (Fig. 1d; Diannexin IRI grp) but also diminished hepatocellular damage (Supplementary Fig. 1b) in IR-stressed livers.
Figure 1.
Stressed PS presentation triggers TIM-4 expression in IR-livers. (a) Annexin V (green)-stained PS in liver IRI (90 min ischemia and 6h reperfusion; white arrows, magnification x200). (b) TIM-4 gene expression in livers sham-operated or undergoing warm ischemia (90min) and reperfusion (2-24h). (c) Annexin V (green) stained-PS in H2O2-induced necrotic hepatocytes (white triangles, magnification x200); (d) TIM-4 gene induction in Diannexin-treated IR-livers, and in livers infused with H2O2-necrotic hepatocytes, compared with Ig-treated IR-livers (*p<0.001; N.S. p>0.05). n=4/group.
TIM-4 deficiency ameliorates hepatic IRI. Livers in groups of WT, TIM-4 KO, and WT mice treated with control Ig, or anti-TIM-4 mAb were subjected to ischemia (90min), and hepatocellular damage was analyzed at 6h and 24h by: (e) sALT levels, and (f) liver histology (representative H&E staining; magnification ×100 and x400); (*p<0.001). n=10-12/group.
Disruption of TIM-4 Signaling Ameliorates Liver IRI
To assess the pathogenic role of TIM-4 signaling, we evaluated the impact of TIM-4 ablation or blockade by employing TIM-4 KO mice, or by treating WT mice with antagonistic TIM-4 mAb, respectively. Unlike Ig-treated controls, those with disrupted TIM-4 signaling were resistant against liver IRI, evidenced by reduced sALT levels at both 6h and 24h of reperfusion (p<0.001; Fig. 1e); preserved hepatic architecture (Fig. 1f: minimal sinusoidal congestion, no edema, vacuolization/necrosis), and decreased Suzuki's score of IR-mediated histological liver damage (p<0.001; Supplementary Fig. 2a).
TIM-4 Deficiency Depresses Macrophage/Neutrophil Sequestration in IR-Liver
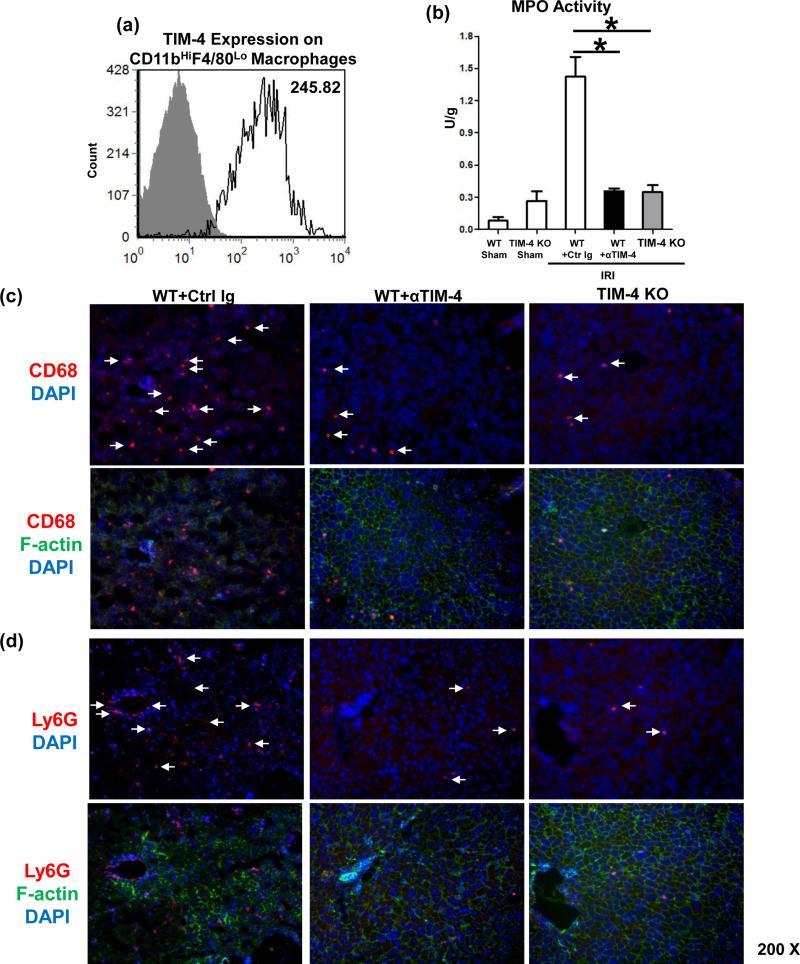
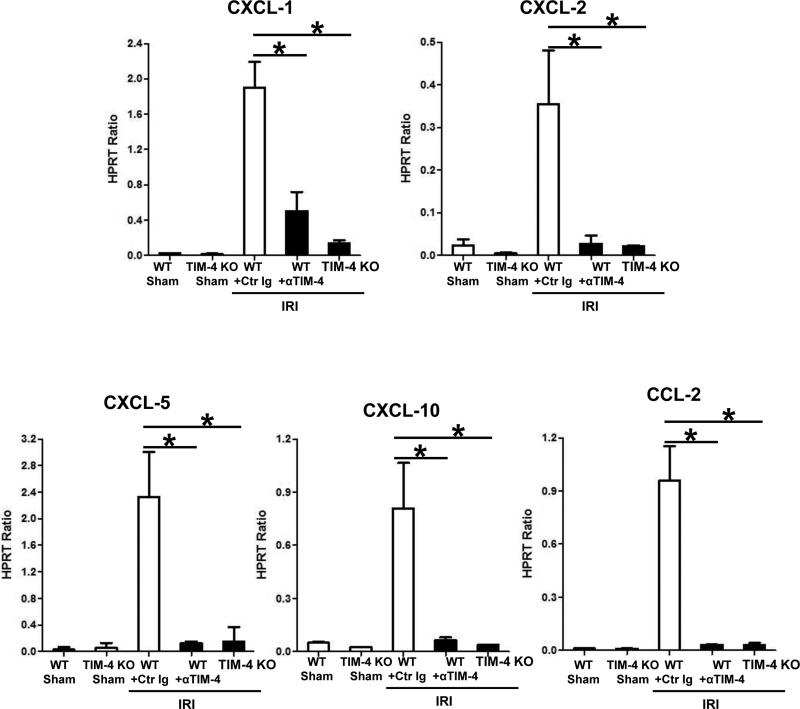
Large numbers of TIM-4 expressing CD11bHiF4/80Lo macrophages were sequestered by 6h of reperfusion in IR-stressed livers (Fig 2a: 258.6±18.1 vs. 6.2±1.5 isotype control). CD68+ macrophages (red) localized primarily in the necrotic areas (Fig. 2c). Unlike in untreated controls, TIM-4 blockade/ablation decreased CD68+ traffic (60±2.8 vs. 5.4±1.1 and 2.6±1.1, respectively; p<0.001) and preserved parenchyma cell integrity/cytoskeleton (green). The expression of CXCL-2 and CXCL-10 in TIM-4 deficient livers was diminished (Fig. 3; p<0.001). In addition, MPO-based neutrophil activity (U/g) was depressed after TIM-4 blockade/ablation (Fig. 2b: 1.42±0.18 vs. 0.35±0.02 and 0.34±0.06, resp.; p<0.001). These correlated with reduced frequency of neutrophils (Fig. 2d: 50±2.8 vs. 2.6±1.1 and 1.8±0.8, resp.; p<0.001), and decreased CXCL-1 and CXCL-5 chemokine levels in TIM-4 deficient IR-livers (Fig. 3; p<0.001).
Figure 2.
TIM-4 signaling in liver macrophage/neutrophil function (6h reperfusion after 90min warm ischemia). (a) Flow cytometry-assisted TIM-4 expression by liver-infiltrating CD11bHiF4/80Lo macrophages; (b) MPO levels; (c) CD68+ macrophages; and (d) Ly-6G+ neutrophils (magnification x200). n=4-6/group.
Figure 3.
Macrophage/neutrophil-associated chemoattractants in liver IRI (6h reperfusion after 90min warm ischemia): Quantitative RT-PCR-assisted detection of CXCL-1, CXCL-2, CXCL-5, CXCL-10, and CCL-2 (*p<0.001). n=4-6/group.
TIM-4 is Essential for Macrophage Phagocytosis in Liver IRI
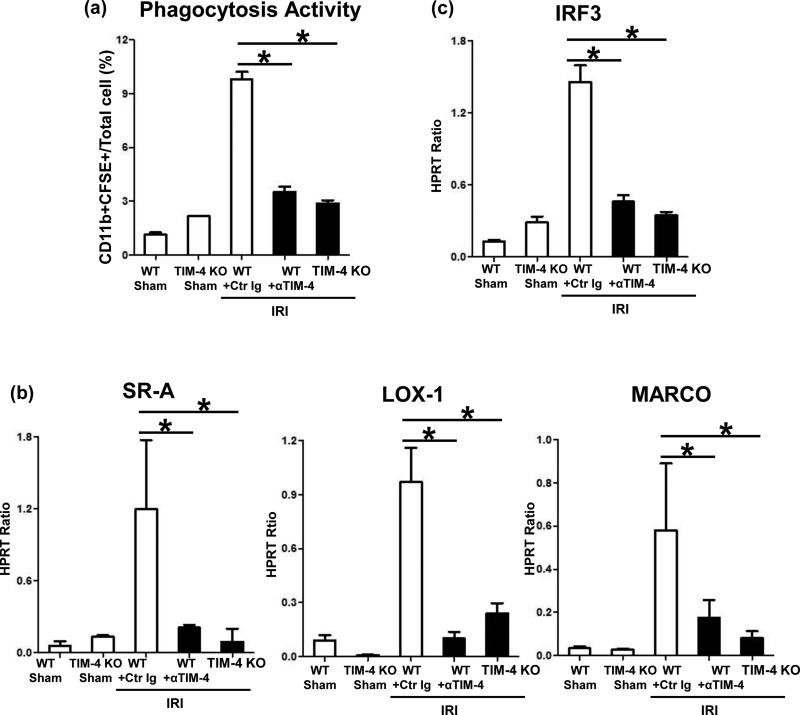
To further assess the role of TIM-4 in macrophage function, we analyzed hepatic phagocytosis in IR-stressed livers. First, we employed trackable exogenous apoptotic bodies to measure macrophage phagocytosis activity in vivo. CFSE-stained thymocytes were infused i.v. into groups of WT +/- anti-TIM-4 mAb or into TIM-4 KO mice. One hour later, experimental animals were subjected to a standard 90min liver ischemia procedure. By 6h of reperfusion, nonparechyma hepatic CD11b+CFSE+ phenotype macrophages, which engulfed apoptotic bodies, were assessed by flow cytometry. Indeed, the frequency of CD11b+CFSE+ cells significantly increased in control Ig-treated WT group, as compared with sham cohorts (Fig. 4a), confirming pathophysiological role of macrophage phagocytosis in liver IRI. Further, TIM-4 signaling blockade or ablation resulted in diminished phagocytic activity (Fig. 4a), implying macrophage phagocytosis in liver IRI is TIM-4 dependent. Next, we analyzed the expression of SR-A, LOX-1, and MARCO, the three major macrophage scavenger receptors (SRs) (21). Unlike in IR-stressed control livers, the hepatic expression of all three SRs was abolished after disruption of TIM-4 pathway (Fig. 4b; p<0.001). As phagocytosis is a receptor-mediated and actin-dependent process, in which activation of Interferon Regulatory Factor 3 (IRF3) is essential for phagosome formation/maturation (22), we determined the impact of TIM-4 signaling on IRF3 pathway. Consistent with its role in the mechanism of liver IRI (2), TIM-4 blockade/ablation diminished increased IRF3 levels seen otherwise in control IR-livers (Fig. 4c).
Figure 4.
TIM-4 signaling in liver macrophages (6h reperfusion after 90min ischemia): (a) In vivo phagocytosis activity (CD11b+CFSE+ / total cell ratio); Quantitative RT-PCR-assisted detection of hepatic: (b) SRs; and (c) IRF3 (*p<0.001). n=4-6/group.
TIM-4 Signaling is Required for Macrophage Activation in Liver IRI
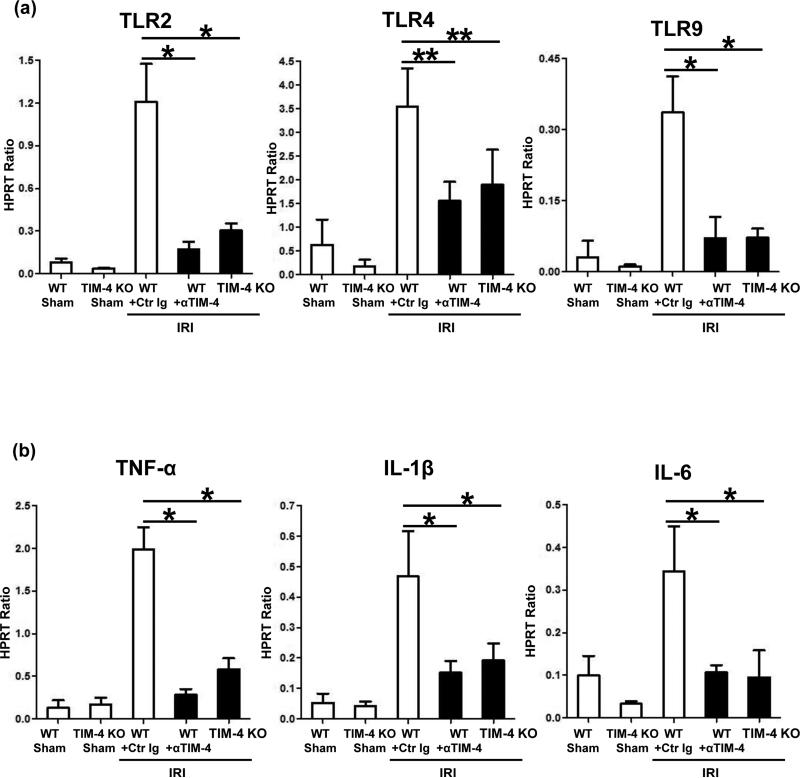
TLR signaling/downstream pro-inflammatory response are hallmarks of macrophage-mediated innate immune activation and tissue damage in IR-stressed livers (2,17). To test the effect of TIM-4 on macrophage activation, we studied the expression of TLR receptors and downstream cytokine programs. Although TLR2, TLR4, and TLR9 were all induced in control Ig-treated IR-stressed livers, their levels were suppressed in TIM-4 deficient groups (Fig. 5a). This correlated with diminished pro-inflammatory cytokine (TNF-α, IL-1β, and IL-6) programs when hepatic TIM-4 signaling was blocked or ablated (Fig. 5b).
Figure 5.
TIM-4 signaling in liver macrophages (6h reperfusion after 90min ischemia): (a) TLR expression; and (b) downstream TLR activation (**p<0.01, *p<0.001). n=4-6/group.
TIM-4 is Pivotal for Engulfment of Necrotic Hepatocytes by Macrophages
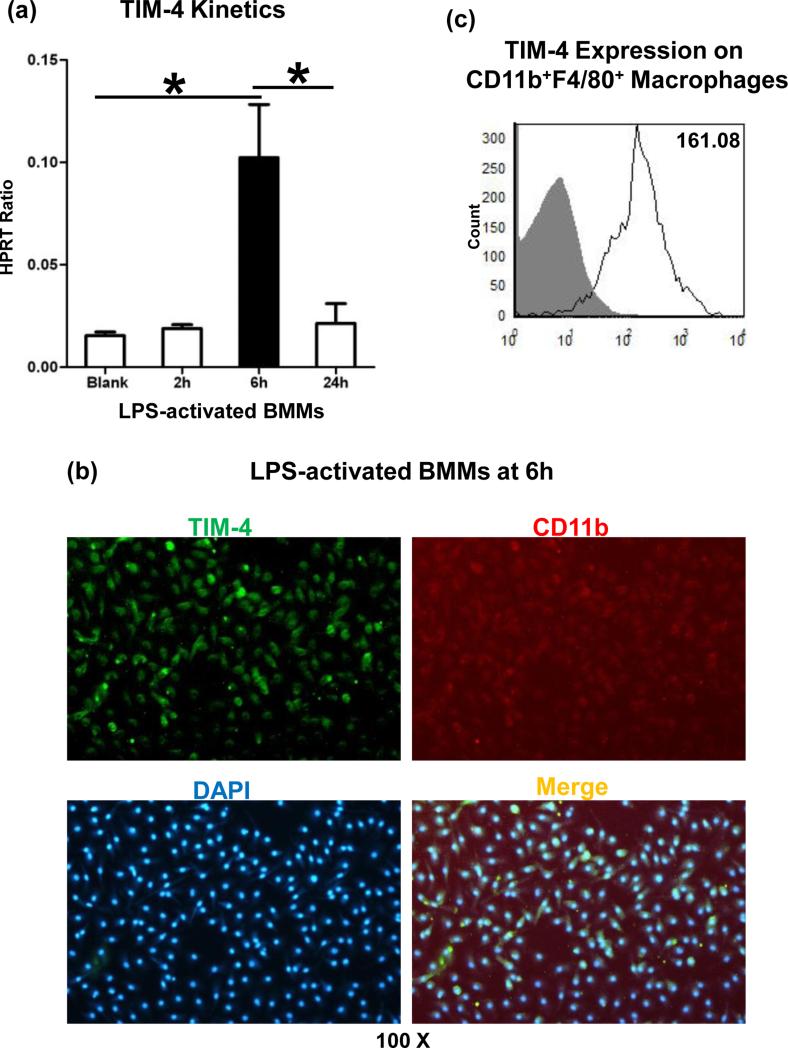
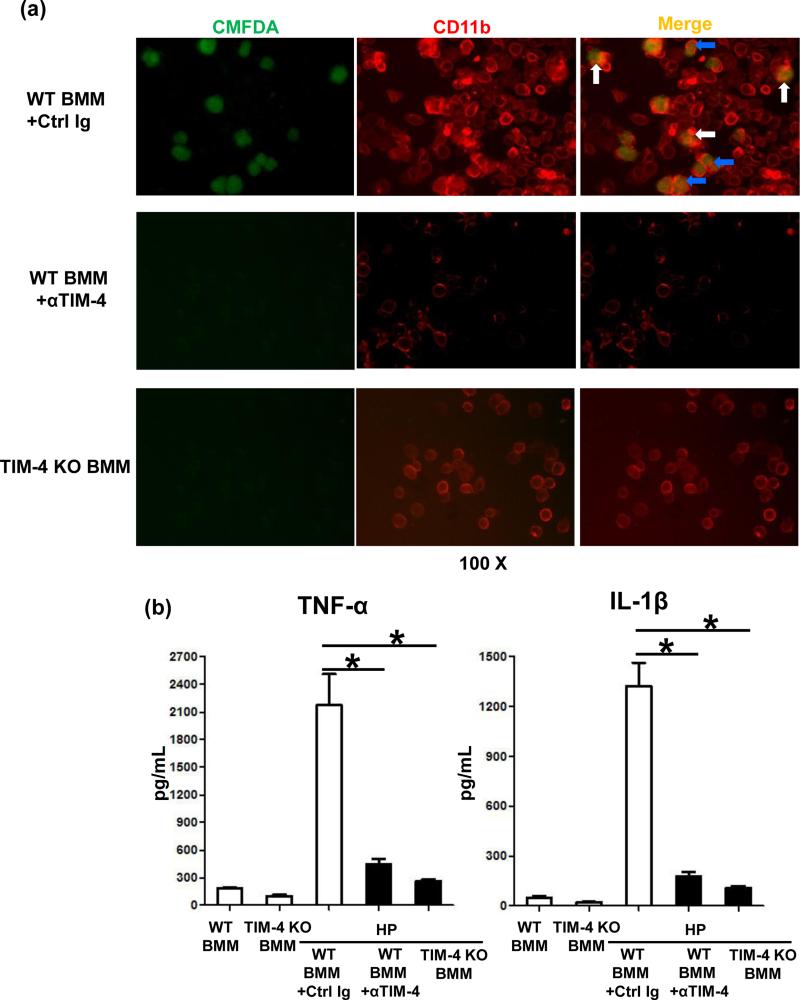
We employed BMM cultures to further analyze the role of TIM-4 signaling in innate immune responses in vitro. First, in agreement with our in vivo data (Fig. 1b), TIM-4 gene induction in LPS-stimulated BMM cultures peaked at 6h (Fig. 6a), coinciding with the maximal IR-hepatocellular damage. Then, by using flow cytometry immunostaining and microscopy imaging, we have documented that TIM-4 was expressed by CD11b+ F4/80+ BMM (Fig. 5b/c: 160.4±1.0 vs. 3.9±0.4 isotype control). As macrophage TIM-4 represents an essential PS receptor that mediates the uptake and phagocytosis of necrotic debris (11,12), we analyzed the phagocytic phenotype in our model. In a newly developed in vitro phagocytosis assay, we employed H2O2- native mouse hepatocytes to mimic in vivo liver IR-setting, Having documented that liver necrotic cells presented PS, as shown by FITC-Annexin V staining (Fig. 1c), we then co-cultured cell tracker CMFDA-necrotic hepatocytes with BMM from WT treated with control Ig; anti-TIM-4 mAb; or TIM-4 KO donors, and stained BMM with PE-CD11b (Fig. 7a). Indeed, H2O2-induced necrotic hepatocytes (green) were captured (white arrow) and engulfed (blue arrow) by TIM-4 proficient (WT) BMM (red), but not by BMM in which TIM-4 signaling was either deleted (KO) or blocked (mAb). These correlated with depressed TNF-α/IL-1β levels in co-culture supernatants (Fig. 7b). Thus, macrophage TIM-4 expression is required to efficiently phagocytose PS-expressing liver necrotic cells in vitro.
Figure 6.
Macrophage TIM-4 expression/activation in vitro (LPS-activated BMM cultures at 6h): (a) TIM-4 gene kinetics; (b) TIM-4 immunostaining (green); (c) TIM-4 expression by CD11b+F4/80+ macrophages (*p<0.001). Representative of n=3/group.
Figure 7.
TIM-4 signaling in macrophage phagocytosis/activation in vitro. (a) CMFDA-stained H2O2-necrotic hepatocytes (green) co-cultured with CD11b+ BMMs (red) from WT + control Ig vs. TIM-4 mAb treatment; or from TIM-4 KO donor mice. “White” and “blue” arrows represent captured and engulfed hepatic debris by BMMs, respectively. (b) ELISA-assisted TNF-α and IL-1β detection in co-culture supernatants (*p<0.001). Representative of n=3/group.
Macrophage TIM-4 Signaling is Sufficient to Facilitate Liver IRI
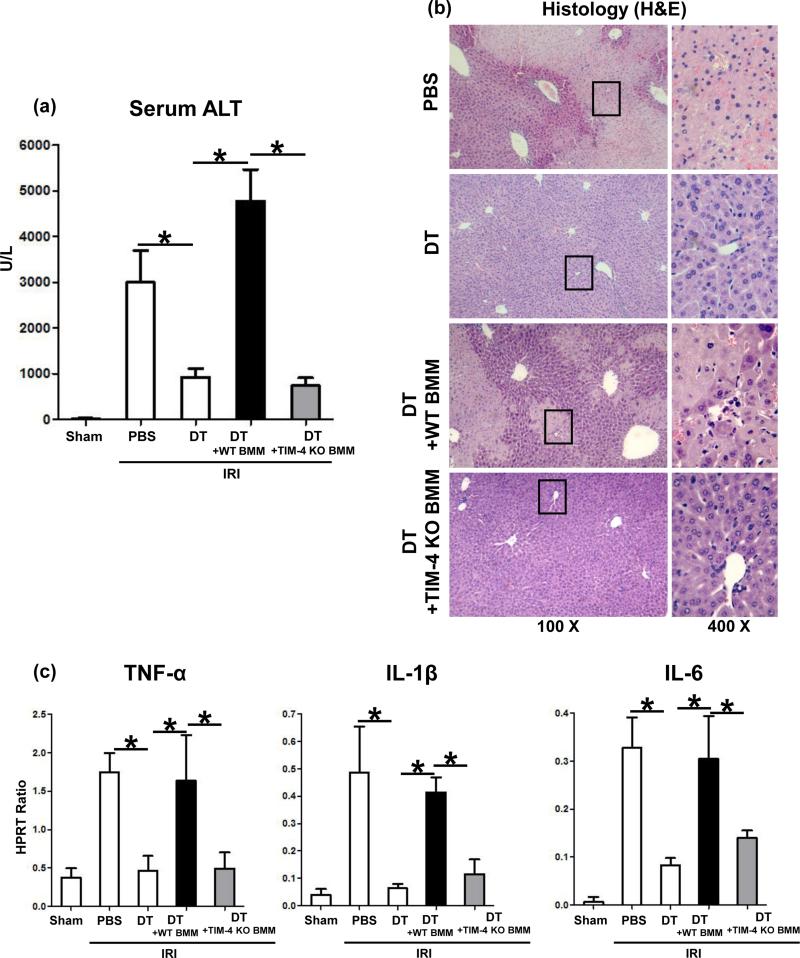
In addition to macrophages, TIM-4 is also expressed by DCs (6-9). However, the expression of TIM-4 in IR-stressed livers by CD11c+CD8+ DC was much lower as compared with that by CD11bHiF4/80Lo macrophages (Supplementary Fig. 3a: 19.9±2.5 vs. 258.6±18.1 p<0.001). Hence, to focus on the function of TIM-4+ macrophages, we developed a model of liver IRI in CD11b-DTR mice. We used CD11b-DTR mice for screening the function of adoptively transferred syngeneic BMM (5×106 cells i.v. at -1h). Without DT therapy, CD11b-DTR mice were susceptible to liver IRI (Fig. 8a). Importantly, after DT treatment at day -2 (frequency of circulating CD11b+ dropped to <0.2%, consistent with published data (20)), the CD11b-DTR mice remained resistant against liver IRI, evidenced by sALT levels (Fig. 8a; 926±187 vs. 3003±692; p<0.001) and histology (Fig. 8b; well-preserved liver architecture with no edema, vacuolization/ necrosis). Interestingly, unlike WT BMM, which recreated liver IRI after adoptive transfer, infusion of TIM-4-deficient BMM failed to induce IRI in CD11b-DTR mice, as shown by diminished sALT levels (Fig. 8a; 739±174 vs. 4777±689; p<0.001), well-preserved liver architecture (Fig. 8b), and decreased Suzuki's histological score of IR-damage (Supplementary Fig. 2b). Consistently heightened TNF-α, IL-1β, and IL-6 levels in CD11b-DTR mice repopulated with TIM-4 proficient BMM were abolished after infusion of TIM-4 deficient cells (Fig. 8c).
Figure 8.
TIM-4+ macrophages trigger liver IRI in CD11b-DTR mice. Adoptive transfer of TIM-4 proficient (WT) but not TIM-4 deficient BMM (5×106 cells i.v. at -1h) recreated hepatocellular damage in CD11b-DTR mice (6h reperfusion after 90min ischemia), evidenced by (a) sALT levels; (b) hepatic histology (representative H&E; magnification x100 and x400); and (c) TNF-α, IL-1β, and IL-6 expression (*p<0.001). n=4-6/group.
Discussion
TIM-4, a cell surface receptor expressed on mature APCs restricted to CD11b+ and CD11c+ subtypes, has the ability to engage TIM-1 ligand to co-stimulate T cell activation, and determining the fate of T cell differentiation in adaptive immunity (7,13). However, the role of TIM-4 signaling in innate immunity remains largely unknown. Here, by using a murine model of sterile inflammatory liver injury, we show that: i/ nonlethal ischemic insult triggered hepatic TIM-4 expression by stressed hepatocellular PS presentation, correlating with the severity of IR-damage; ii/ TIM-4 deficiency mitigated IRI, implying functional role of TIM-4 in this innate immunity-driven in vivo responses; iii/ disruption of TIM-4 signaling inhibited macrophage and neutrophil migration/function, evidenced by decreased sequestration/chemoattraction, diminished phagocytosis of necrotic hepatocytes, and suppressed pro-inflammatory activation in IR-stressed livers; iv/ macrophage-mediated inflammation/damage was TIM-4 dependent, data deriving from a newly developed model of liver IRI in CD11b-DTR system. Together with our data on the pathogenic role of CD4+ T cell-dependent TIM-1 signaling (23), and the ability of TIM-4 blockade to depress hepatic TIM-1 gene expression (Supplementary Fig. 4a), it becomes clear that intact TIM-4–TIM-1 costimulation pathway is required to facilitate hepatic IRI. Importantly, our screening of human liver transplants suffering from IRI supports a novel concept of TIM-4–TIM-1 signaling as: i/ a therapeutic target in IR-liver inflammation; ii/ a biomarker candidate to identify the severity of hepatic IRI; and iii/ a predictor of clinical outcomes in liver transplant patients (Kupiec-Weglinski, unpublished data).
TIM-4 may affect a diverse range of adaptive immune responses. Indeed, TIM-4 deficient mice display increased lymphocyte hyperactivity due to blunt apoptotic body clearance, consistent with TIM-4 ablation resulting in higher titers of antibody response to nuclear antigens, such as dsDNA (10). It was also shown that TIM-4 expressing APCs control the clearance of Ag-specific T cells, thereby regulating the size of Ag-specific memory pool (24). Blocking TIM-4– PS binding increased secondary T cell responses and frequency of Ag-specific CD4/CD8 T cells. Conversely, by enhancing clearance/reducing number of Ag-specific T cells, TIM-4 overexpression on APCs downregulated secondary T cell responses (24). To the best of our knowledge, TIM-4–PS mediated phagocytosis has never been analyzed in innate immune-mediated disease model. First, we found that warm ischemia triggered TIM-4 expression by stressed hepatocellular PS induction, the level of which increased gradually, peaking at 6h of reperfusion. We then adoptively transferred ex-vivo generated PS+ hepatocytes to recreate TIM-4 expression in the liver. Further, treatment with Diannexin, a novel competitive PS inhibitor (19), diminished hepatic TIM-4 gene levels, whereas disruption of PS–TIM-4 signaling ameliorated liver IR-damage. These findings support the pathogenic function of PS-TIM-4 axis in IRI. To focus on the requirement for TIM-4, we used animals with permanent TIM-4 deletion (KO) or transient blockade (WT + RMT4-53 mAb). Strikingly, disruption of TIM-4 signaling suppressed liver damage in both recipient groups, evidenced by decreased sALT levels and amelioration of histological features of liver IRI at 6h and 24h of reperfusion.
We found increased sequestration of CD68+, CD11b+, and F4/80+ macrophages in the initial hepatic inflammation phase, consistent with preferential pro-inflammatory chemotactic gene expression profile in IR-stressed livers. As Kupffer cells constitute the first liver defensive line, their activation and chemokine products, such as CXCL-2, CXCL-10, and CCL-2 are instrumental to recruit circulating macrophages and amplify pro-inflammatory cell cascades in the reperfusion phase (16). Although Kupffer cells express TIM-4 (Supplementary Fig. 3b), TIM-4 gene induction at 6h of reperfusion, as a result of macrophage infiltration, was significantly higher as compared with that in livers subjected to ischemia alone (Supplementary Fig. 4b). Although neutrophils do not express TIM-4, Ly-6G+ neutrophil infiltration/MPO activity increased sharply in Ig-treated IR-livers. In contrast, TIM-4-deficient livers were characterized by decreased neutrophil infiltration/MPO activity and depressed CXCL-1 and CXCL-5 levels, the key neutrophil chemoattractants. As neutrophil activation/tissue sequestration can be enhanced by macrophage-derived inflammatory products, TIM-4 targeting exerted its regulatory function through cytokine/chemokine networks.
We then studied the regulatory function of TIM-4 in macrophage-mediated phagocytosis, one of the hallmarks in innate immune-mediated liver damage. Indeed, phagocyte priming for ROS production has been reported to potentiate susceptibility for endotoxin-induced liver damage (5), whereas phagocyte depletion reduced hepatic IRI (4,10,15). However, a direct evidence for in vivo phagocytic activity in liver IRI has been missing. Here, by using trackable syngeneic apoptotic bodies, we first measured in vivo phagocytic activity of liver macrophages. As TIM-4 constitutes a surface PS receptor, enabling the uptake of apoptotic cells and maintenance of the homeostatic state (10,15), we found slightly increased phagocytic activity in TIM-4-deficient sham livers, as compared with WT sham counterparts. The hepatic macrophage phagocytic activity was augmented in control Ig-treated IR-livers, but abolished in both TIM-4 deficient groups, consistent with diminished expression of macrophage scavenger receptors, SR-A, LOX-1, and MARCO. Molecular mechanisms by which macrophages may use these scavenger receptors to recognize/engulf apoptotic cells warrant future studies. In agreement with our in vivo data, TIM-4-mediated phagocytosis was readily detectable in macrophage– necrotic hepatocyte co-cultures. Consistent with critical role of IRF3-mediated phagosomal infusion/maturation (22), we found that unlike in TIM-4 proficient IR-stressed livers, disruption of TIM-4 pathway mutated IRF3 expression. This supports an idea that phagocytic hepatocyte clearance may progress via IRF3 pathway and it does require intact TIM-4 signaling. Our findings suggest that under ischemic conditions liver-infiltrating macrophages exceed their homeostatic capacity to efficiently phagocytose parechyma debris, become hyperactivated, and programmed to facilitate the hepatocellular damage. TIM-4 blockade impairs macrophage intrinsic functions to phagocytose PS+ hepatic debris, which further mitigates TLR4-mediated inflammation in IR-stressed livers.
TLR activation promotes innate immune activation through MyD88- or TRIF-dependent pathway (1,2). We have shown that signaling via TRIF-IRF3 rather than MyD88 is instrumental for downstream NF-κB activation, local inflammation, and IR-hepatocellular damage (2). Indeed, TLR2 (25), TLR4 (2,26), and TLR9 (27) were all found to contribute in the pathogenesis of tissue IRI. Following TLR-induced NF-κB activation, pro-inflammatory IRI signature, i.e., TNF-α, IL-1β, IL-6 are essential to promote TNFR/IL-1R de novo activation (1,17). Here, TIM-4 ablation/blockade decreased TLR2/4/9 expression/activation, and their downstream pro-inflammatory cytokine programs. In agreement with our in vivo data, TIM-4 deficiency diminished pro-inflammatory cytokine profile in BMM-hepatocyte co-culture system.
Although TIM-4 serves as an endogenous ligand for DCs (6-9), frequency of hepatic TIM-4 expressing DCs was markedly lower as compared with that on macrophages (Supplementary Fig. 3a). To focus on macrophage-dependent TIM-4, we have developed a model of liver IRI in CD11b-DTR mice. If untreated, these animals suffered classic liver IRI, whereas a minute dose of DT made them IR-resistant. Importantly, after DT-mediated depletion of CD11b+ cells (i.e., MAC-1+ tissue macrophages, circulating monocytes, granulocytes and some NK cells), subsequent transfer of WT (TIM-4 proficient) BMM triggered IR-hepatocellular damage, accompanied by augmented TNF-α, IL-1β, and IL-6 levels in CD11b-DTR recipients. This highlights the pathogenic function of non-resident macrophages in our model. In contrast, infusion of TIM-4-deficient BMM failed to trigger IRI and proinflammatory signature in CD11b-DTR mice. These findings document for the first time the pathogenic role of TIM-4+ macrophages in the mechanism of liver IRI; and demonstrate the ability of TIM-4 ablation to recreate homeostasis in IR-stressed organ.
In conclusion, the current study is the first to document indispensible role of intrinsic macrophage-specific TIM-4 signaling in innate immunity-driven liver IRI. Our results show that TIM-4 is required for macrophage migration, phagocytosis, and activation, leading to hepatic inflammation/damage. Hence, targeting TIM-4 signaling should be considered as a therapeutic means to manage panoply of inflammatory responses in IR-stressed livers.
Supplementary Material
Acknowledgments
Financial Support: NIH Grant RO1 DK062357 (JWKW) ; W.M. Keck Foundation; The Diann Kim Foundation; The Dumont Research Foundation. HJ is a recipient of the Pilot and Feasibility Grant from CURE Digestive Diseases Research Center and Clinical/Translational Science Institute (CTSI) at UCLA. YZ is a recipient of National Natural Science Foundation of China Grants 81302547.
List of Abbreviations
- BMM
bone marrow-derived macrophages
- DT
diphtheria toxin
- IRF3
interferon regulatory factor 3
- IRI
ischemia-reperfusion injury
- MPO
myeloperoxidase
- PS
phosphatidylserine
- ROS
reactive oxygen species
- sALT
serum alanine aminotransferase
- TIM
T-cell Immunoglobulin and Mucin
- TLR
Toll-like receptor
- WT
wild-type
References
- 1.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Shen XD, Gao F, Zhao A, Freitas MC, Lassman C, Luster AD, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47:207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 4.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparechyma cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 5.Liu P, Vonderfecht SL, Fisher MA, McGuire GM, Jaeschke H. Priming of phagocytes for reactive oxygen production during hepatic ischemia-reperfusion potentiates the susceptibility for endotoxin-induced liver injury. Circ Shock. 1994;43:9–17. [PubMed] [Google Scholar]
- 6.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 8.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 9.Mizui M, Shikina T, Arase H, Suzuki K, Yasui T, Rennert PD, Kumanogoh A, et al. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int Immunol. 2008;20:695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci U S A. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savill J, Gregory C. Apoptotic PS to phagocyte TIM-4: eat me. Immunity. 2007;27:830–832. doi: 10.1016/j.immuni.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Feng BS, Chen X, He SH, Zheng PY, Foster J, Xing Z, Bienenstock J, et al. Disruption of T-cell immunoglobulin and mucin domain molecule (TIM)-1/TIM4 interaction as a therapeutic strategy in a dendritic cell-induced peanut allergy model. J Allergy Clin Immunol. 2008;122:55–61. 61, e51–57. doi: 10.1016/j.jaci.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, Yeh C. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–1533. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Valdez PA, Tan C, Yeh S, Hongo JA, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci U S A. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji H, Shen X, Gao F, Ke B, Freitas MC, Uchida Y, Busuttil RW, et al. Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology. 2010;52:1380–1389. doi: 10.1002/hep.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji H, Zhang Y, Shen XD, Gao F, Huang CY, Abad C, Busuttil RW, et al. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation by the cAMP-PKA pathway. Hepatology. 2013;57:1225–1237. doi: 10.1002/hep.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung MY, McGrath MM, Nakayama M, Shimizu T, Boenisch O, Magee CN, Abdoli R, et al. Interruption of Dendritic Cell-Mediated TIM-4 Signaling Induces Regulatory T Cells and Promotes Skin Allograft Survival. J Immunol. 2013;191:4447–4455. doi: 10.4049/jimmunol.1300992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen XD, Ke B, Zhai Y, Tsuchihashi SI, Gao F, Duarte S, Coito A, et al. Diannexin, a novel annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am J Transplant. 2007;7(11):2463–2471. doi: 10.1111/j.1600-6143.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- 20.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle SE, O'Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, et al. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, Batikian CM, et al. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant. 2013;13:56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, Kobayashi N, et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J Immunol. 2010;185(11):6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.