Abstract
Squamous cell carcinoma of the oral tongue (SCCOT) exhibits high risk for recurrence and regional metastasis even after surgical resection. We assessed the clinicopathologic and prognostic significance of a group of functionally related biomarkers. We used a tissue microarray consisting SCCOT from 32 patients for this study. These patients were treated at the UT- M.D. Anderson Cancer Center from 1995 to 2008. Biomarker expression levels were examined by immunohistochemistry and graded semiquantitatively to determine their prognostic significance. CD147 and Tp63 expressions were significantly associated with a higher T-stage and Ki-67 labelling index as well as shorter overall survival (OS). Expression of Tp63 associated positively with poorly-differentiated histology. There was significant association of Tp63 with the expression levels of CD147 and Glut-1. Glut-1 overexpression was marginally associated with a higher T-stage. There was no prognostic significance of CD44v6 expression in SCCOT. SCCOT with CD147 overexpression in combination with high Ki-67 labelling index had poor OS. CD147 and Ki-67 overexpression is associated with aggressive disease with poor prognosis in SCCOT.
Keywords: Oral cancer, Squamous cell carcinoma of oral tongue, Biomarkers, CD147, Tp63, CD44v6, Glut-1, Ki-67, Overall and recurrence free survival
Introduction
Oral squamous cell carcinoma (OSCC) arises in the lip and oral cavity, representing one of the ten most frequently diagnosed cancers globally1. Countries with the highest incidence of OSCC are India, Pakistan and Sri Lanka2. In these countries, OSCC is the most common cancer in men and the third most common cancer in women2. OSCC exhibits a high mortality rate of 40–50% which is due to the high incidence of disease recurrence and subsequent spread to local and distant sites3. The tongue is the most common intraoral site of OSCC in most countries and accounts for 25–40% of all OSCC3. The anterior two-thirds of the tongue, also known as the mobile tongue, is a sub-site of the oral cavity; cancers arising from this portion of the tongue are designated as squamous cell carcinomas of oral tongue (SCCOT). The posterior one third or base of the tongue is a sub-site of the oropharynx; cancers arising from this portion of the tongue are designated as squamous cell carcinomas of the base of the tongue. Tobacco and alcohol consumption are major risk factors for SCCOT which typically occurs in patients older than 50-years of age4, 5. On the other hand, oncogenic human papilloma virus (HPV) infection is a major risk factor for squamous cell carcinomas of the base of the tongue6. The incidence of SCCOT has been steadily increasing since 1975, whereas the incidence of OSCC of other intraoral sites has been decreasing7–9. SCCOT represents 75% of all tongue cancers and approximately 7,100 new SCCOT cases are diagnosed each year in the United States10. Furthermore, recent studies report an increased incidence of SCCOT in young white females who were never-smokers or never-drinkers7, 11. SCCOT occurring in young patients without the traditional risk factors of tobacco and/or alcohol abuse exhibit a more aggressive clinical course characterized by higher rates of loco-regional recurrences, shorter RFS and OS7, 12. Recent cancer exome sequencing studies revealed that SCCOT in young non-smokers and older smokers had similar genomic profiles and mutational spectrum except for the frequency of Tp53 mutation, which is more common in SCCOT of older smokers than young non-smokers13, 14.
SCCOT is the most aggressive of all OSCC subtypes with a poor 5-year survival rate of < 50%15. Despite recent advances in cancer research and treatment modalities, prognosis of SCCOT remains poor, primarily due to high rates of distant metastasis and locoregional recurrence15. Advanced local disease and/or lymph node metastasis are associated with the poor prognosis in SCCOT patients10. SCCOT exhibits extremely high rates of occult lymph node metastases which cannot be identified by clinical and radiographic imaging studies15. The rate of occult cervical lymph node metastasis in patients diagnosed with early stage SCCOT has been reported to range from 24–42%16–19. More importantly, relapse from the subclinical nodal disease is the most common cause of treatment failure in SCCOT15. Rusthoven and colleagues demonstrated that patients of early stages of SCCOT had worse survival rates, even after aggressive therapy20. Most clinicians advocate elective neck dissection, even in clinically node negative (N0) patients with early stage (T1 and T2) SCCOT, to accurately determine the stage for effective treatment21–23. However, elective neck dissection represented an overtreatment in >70% of early stage SCCOT patients (T1/T2) who were found to have pathologically negative nodes24, 25.
Hence, there is increased interest in identifying molecular biomarkers that are associated with disease specific survival and can be incorporated into the staging in guiding therapeutic decision making in patients with SCCOT. Tumor biomarkers are molecules produced either by tumor or the surrounding host tissue in response to tumorigenesis, which have various clinical applications that include cancer risk assessment, diagnosis and staging of cancer, prognostication and therapeutic selection26, 27. Numerous studies have examined an extensive list of tumor biomarkers in SCCOT28. Conflicting or unconfirmed associations for the same biomarker were found among different studies28. The sample size for a single SCCOT study is usually relatively small and differences existed in the study design, patient population, and variability of the quantitative measurements of biomarkers. Moreover, a majority of these independent studies have tested only one or two biomarkers, reporting limited success in predicting the overall survival (OS) and recurrence free-survival (RFS) and have not been adapted for clinical practice. There is emerging evidence that a panel of biomarkers, rather than single markers, will be required to have sufficient sensitivity and specificity for accurate cancer staging and prediction of treatment response at the time of diagnosis26.
In this study, we tested a group of functionally related biomarkers namely, CD147, CD44v6, Glut-1, Tp63 and Ki-67, which are reported to have prognostic implications in head and neck squamous cell carcinomas29–34. CD147 (also known as EMMPRIN or BSG) is a multifaceted molecule that facilitates tumor progression by several mechanisms that include tumor invasion, angiogenesis, metastasis, suppression of tumor immunity and resistance to therapy. CD44 is a cell surface glycoprotein involved in regulating cell-cell and cell-ECM (extracellular matrix) interactions. Overexpression of CD44, a putative cancer stem cell maker, confer adverse prognosis and poor response to radiation therapy in laryngeal dysplasias and carcinomas, respectively35, 36. Tp63 belongs to the p53 transcription factor family (p53, Tp63 and p73) and contains a middle region of DNA binding domain that is constant among these members to regulate cell cycle arrest and apoptosis37, 38.. Tp63 is one of the most frequently mutated gene in head and neck SCC39. The predominant Tp63 isoform expressed in head and neck SCC lacks the full N-terminal domain and is referred to as ΔNp6340. The transcriptionally inactive isoform ΔNp63 promotes oral tumorigenesis by functioning as a trans-dominant negative mutant and inactivating tumor suppressor gene p5337. Glucose transporter Glut-1 is frequently overexpressed in many solid malignancies to increase glucose uptake in response to aerobic and anaerobic glycolysis41. Overexpression of Glut-1 in oral squamous cell carcinomas is associated with poor prognosis42, 43. The Ki67 is a nuclear protein expressed during the G2/M phase of the cell cycle and is widely used to determine the proliferative fraction in solid malignancies44. We assessed the expression of these biomarkers in tissue microarrays of SCCOT and correlated their expression levels with clinicopathologic factors, OS and RFS.
Material and methods
Patient population and specimens
Archival SCCOT specimens with adequate clinical follow up and tissue samples were selected for the fabrication of these tissue microarrays (TMAs). Patients with SCCOT treated at the M.D. Anderson Cancer Center with archived tissue and adequate clinical data were included in this study. Clinical data obtained from chart review include tumor site, size, regional and distant metastasis status, extracapsular spread, treatment type and the disease status at the time of last follow-up. Patients’ samples were not included if there were missing data. Histopathological grading of hematoxylin stained sections of primary SCCOT was made using the standard criteria into well- (Grade 1), moderately- (Grade 2) and poorly- (Grade 3) differentiated tumors45.
This SCCOT patient population is composed of thirty-two patients with operable tongue cancers who were treated and followed up at the M.D. Anderson Cancer Center (MDACC), Houston, TX between 1995 and 2008.This research follows the tenets of the Declaration of Helsinki and all samples were obtained after informed consent from the patients. All protocols using tissue specimens were approved by the Institutional Review Boards of MDACC. Patient tissue samples were spotted on glass slides for tissue microarray immunohistochemistry (TMA-IHC) staining. Among the 32 patients, there were sixteen patients who had one specimen, fifteen patients who had two specimens, and one patient who had three specimens for the TMA-IHC analysis.
Tissue microarray (TMA)
Tumor tissues were formalin fixed, embedded in paraffin and incised into sections for the histological diagnosis and microscopic characterization. The staging was evaluated according to the American Joint Committee on Cancer (AJCC) system for the tumor (T), nodal invasion (N), and distant metastasis (M). Representative tumors were identified and a 1 mm-in-diameter tissue core was punched out and transferred to a recipient block with 1.5 mm between each core. Each tissue microarray also contained sections of normal oral mucosa, dysplastic oral mucosal, spleen and lymph nodes as internal controls. Two sets of slides were used labeled TMA-1, TMA-2, with a positive and negative control for each biomarker.
Immunohistochemical staining for biomarkers
Consecutive TMA sections were deparaffinized, rehydrated and subjected to antigen retrieval by boiling in ANTIGEN DECLOAKER (Biocare Medical, Concord, CA) solution for ten minutes. Endogenous peroxidase activity was blocked by incubating the tissue section with 3% H2O2 in methanol for ten minutes. Non-specific binding sites were blocked by incubating the tissue sections in BACKGROUND TERMINATOR (Biocare Medical, Concord, CA) solution for ten minutes. Tissue sections were treated with normal horse serum for ten minutes to block non-specific binding sites and then incubated diluted primary antibodies overnight at room temperature. Source and working dilutions of the primary antibodies specific for each biomarkers are provided in Table 1. Primary antibody binding sites were detected using the Vectastain Elite mouse/rabbit/goat ABC kits (Vector Labs, Burlingame, CA) according to the manufacturer’s protocol. Peroxidase reactivity was visualized using 3-3’-diaminobenzidine tetrachloride as chromogenic substrate and counterstained with hematoxylin.
Table 1.
Sources of antibodies and the working dilutions that were used for immunohistochemical analysis of tissue microarrays
| Antibody | Type and Dilution |
Source |
|---|---|---|
| Tp63 | Mouse Ig 1:100 |
Santa Cruz, Dallas, TX, USA (4A4 clone; sc-8431) |
| Ki-67 | Rabbit Ig 1:100 |
Life Technologies, Grand Island, New York, USA (18-0191Z) |
| CD44var (v3–v10) | Goat Ig 1:200 |
eBioscience Inc, San Diego, CA, USA (BMS 124) |
| CD147 | Goat Ig 1:200 |
R & D Systems, Minneapolis, MN, USA AF972 |
| Glut-1 | Rabbit Ig 1:250 |
Thermo Scientific. Waltham, MA, USA RB-9052 |
Scoring of immunohistochemical staining
Expression levels of CD147, CD44v6, and Glut-1 were evaluated semiquantitatively based on the staining intensity and the percentage of tumor cells with membranous and cytoplasmic staining as previously reported46. Immunoreactive scores (range 0 – 4) were derived by multiplying the percentage of positive cells times staining intensity score. Immunoreactive scores for Tp63 were determined based on the percentage of nuclei positive cells as follows: grade 0, <5%; grade 1, 5%–25%; grade 2, 26%–50%; grade 3; 51%–75%; and grade 4; 76%–100%. The growth fraction of SCCOT samples was determined using Ki-67 immunolabeling and the Ki-67 Immuno scoring was done as follows: grade 1, < 25% of tumor cells are positive; grade 2, > 25% of tumor cells are positive.
Statistical analysis
Frequencies and percentages are reported for categorical variables (such as gender, smoking status, and stage). To evaluate the association between histology grade and marker expression and the association between two markers, regression models using generalized estimating equations (GEE) were used to take the intra-patient correlation into account. Kaplan-Meier method was used to analyze the time-to-event outcomes, including OS and RFS. The log-rank test was used to evaluate the difference in time-to-event free rate between patient groups. Multi-covariate Cox proportional hazards model was fitted to evaluate the effect of a marker on time-to-event outcome with adjustment of other prognostic variables in the model. All tests were two-sided. P-values less than 0.05 were considered statistically significant. Statistical software SAS 9.1.3 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA) were used for all the analyses.
RESULTS
Clinicopathological characteristics of the TMA samples
We analyzed the expression levels of CD147, CD44v6, Glut-1, Tp63 and Ki-67 in TMA composed primary tumor sections from 32 patients with SCCOT (Table 2). There were 27 males and 5 females, with a median age of 60-years (range: 22–77 years). Twenty-eight patients were either current or former smokers and the remaining four patients had no history of tobacco use. Distribution of these patients according to their tumor size was as follows: T1=6%; T2=41%; T3=34%; T4=19%. Twenty patients (62.5%) had pathologically confirmed regional lymph node metastatic spread and all of these lymph node metastases revealed extracapsular spread (ECS) at the time of diagnosis. Four of these patients also developed distant metastasis. The remaining 12 patients were staged as N0 and none of the 20 ECS (−) patients presented with distant metastasis. These tumors were graded histologically as well differentiated or Grade 1 (n= 8; 25%), moderately differentiated or Grade 2 (n= 19; 59%) and poorly differentiated Grade 3 (n=5; 16%) (Figure 1). These patients were treated with surgery alone (n=11; 34%), surgery with radiation therapy (n=20; 63%) and surgery with radiation and chemotherapies (n=1; 3%). In all, 9 (28%) patients had tumor recurrences which included both local and nodal recurrence and the remaining patients were free of tumor recurrence during the follow-up period. At the time of last follow-up, 12 patients were alive and 20 patients had died. Among the patients who died during the follow-up period, 13 of them died of SCCOT, one patient died due to treatment related complications and six of them died of unknown disease status. The median OS time was 18.3 months, with the lower bound of the 95% confidence interval (CI) at 7.56 months and upper bound was not reached.
Table 2.
Clinicopathologic characteristics of the SCCOT samples in the tissue microarrays
| Variables | N (%) | |
|---|---|---|
| Age | ||
| < 40 | 1 (3) | |
| 40–49 | 5 (16) | |
| 50–59 | 8 (25) | |
| 60–70 | 12 (38) | |
| >70 | 6 (18) | |
| Gender | ||
| Male | 27 (84) | |
| Female | 5 (16) | |
| Smoking | ||
| Current | 14 (44) | |
| Never | 4 (12) | |
| Former | 14 (44) | |
| T Stage | ||
| T1–2 | 15 (47) | |
| T3–4 | 17 (53) | |
| N Stage | ||
| N0 | 12 (38) | |
| N1–2C | 20 (62) | |
| Extracapsular Spread | ||
| No | 20 (62) | |
| Yes | 12 (38) | |
| Treatment | ||
| Surgery | 11 (34) | |
| Surgery+RT | 20 (63) | |
| Surgery+CRT | 1 (3) | |
| Histology Grade | ||
| 1 (Well differentiated) | 8 (25) | |
| 2 (Moderately differentiated) | 19 (59) | |
| 3 (Poorly differentiated) | 5 (16) | |
| Distant Metastasis | ||
| M0 | 24 (75) | |
| M1-X | 4 (12.5) | |
| Missing | 4 (12.5) | |
| Survival | ||
| Alive | 12 (37) | |
| Dead | 20 (63) | |
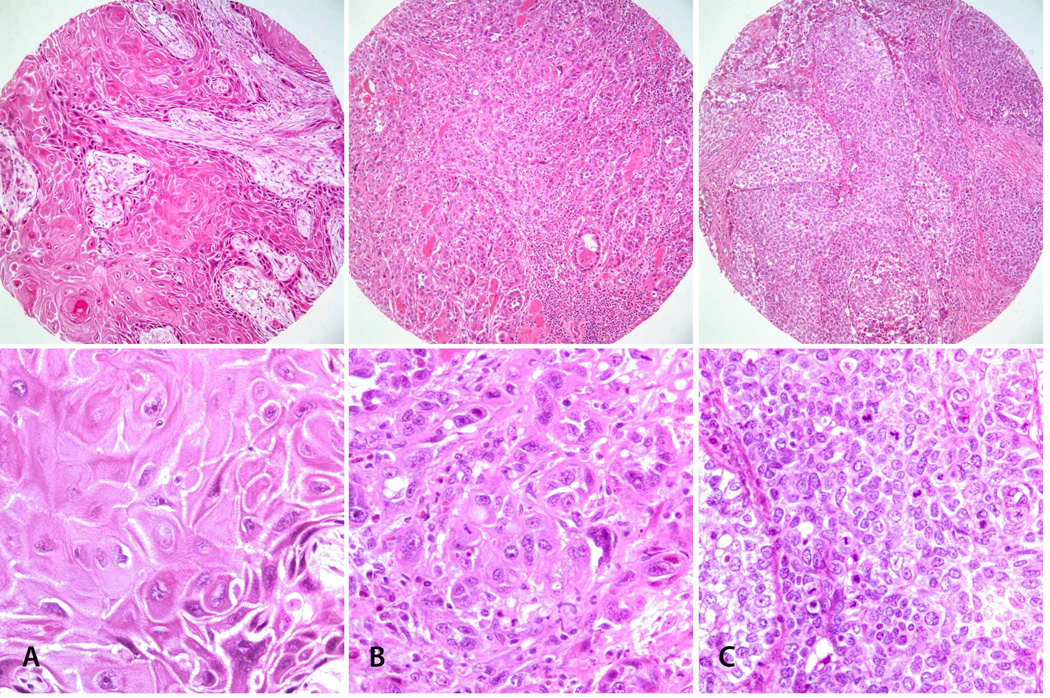
Figure 1.
Hematoxylin and eosin (H&E) stained images of TMA cores. Representative images of SCCOT with well differentiated (A: Grade 1), moderately differentiated (B: Grade 2) and poorly differentiated (C: Grade 3) histologic grades. Top: Low-power view (original magnification × 100); Bottom: High-power view of the same tumors (original magnification × 200).
Expression patterns of CD147, CD44v6, Tp63 and Glut-1 in SCCOT
All of the tumor samples in the TMA revealed positivity for CD147, CD44v6, and Glut-1, albeit at different expression levels. Immunohistochemical stainings for CD147, CD44v6, and Glut-1 revealed prominent cell surfaces, diffuse and weak cytoplasmic patterns in individual tumor cells (Figures 2–4) We found high levels (Immunoscore >3) of CD147, CD44v6 and Glut-1 expression in 32%, 18%, and 22% of SCCOT samples, respectively (Figures 2–4). SCCOT tumor cells revealed nuclear positivity for Tp63 and Ki-67 at varying proportions (Figures 5 & 6). Twenty-six percent of SCCOT expressed high-levels of Tp63 (Immunoscore >3) (Figure 5). Twelve of 49 SCCOT (24%) cases in the TMA revealed a higher proliferation rate (Immunoscore 2 = > 25% Ki-67 positive tumor cells) as measured by Ki-67 immunolabeling.
Figure 2.
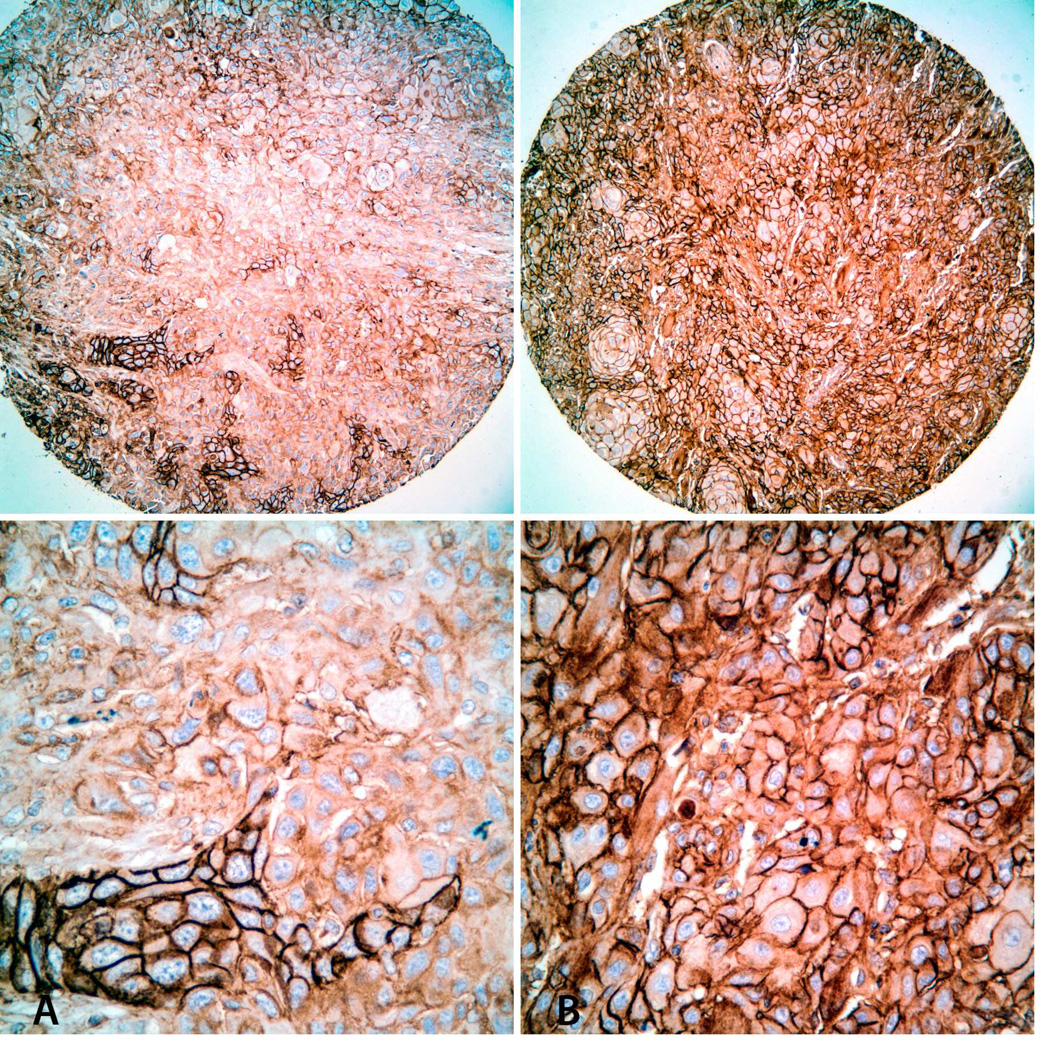
Immunohistochemical staining for CD147 in TMA cores. Representative images of SCCOT with low (A: Immunoscore = 1) and high (B: Immunoscore = 4) CD147 expression levels. Top: Low-power view (original magnification × 100); Bottom: High-power view of the same tumors (original magnification × 200).
Figure 4.
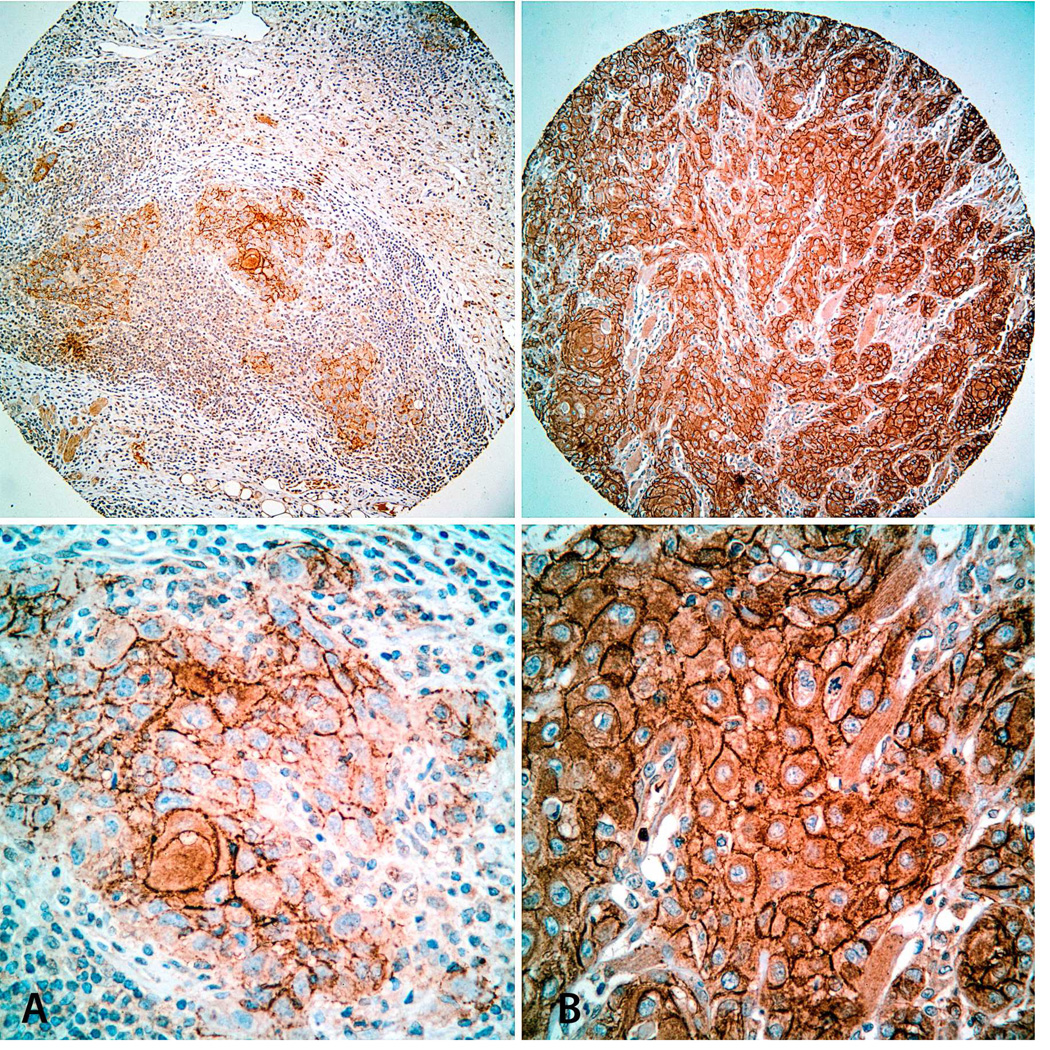
Immunohistochemical staining for CD44v6 in TMA cores. Representative images of SCCOT with low (A: Immunoscore = 1) and high (B: Immunoscore = 4) CD44v6 expression levels. Top: Low-power view (original magnification × 100); Bottom: High-power view of the same tumors (original magnification × 200).
Figure 5.
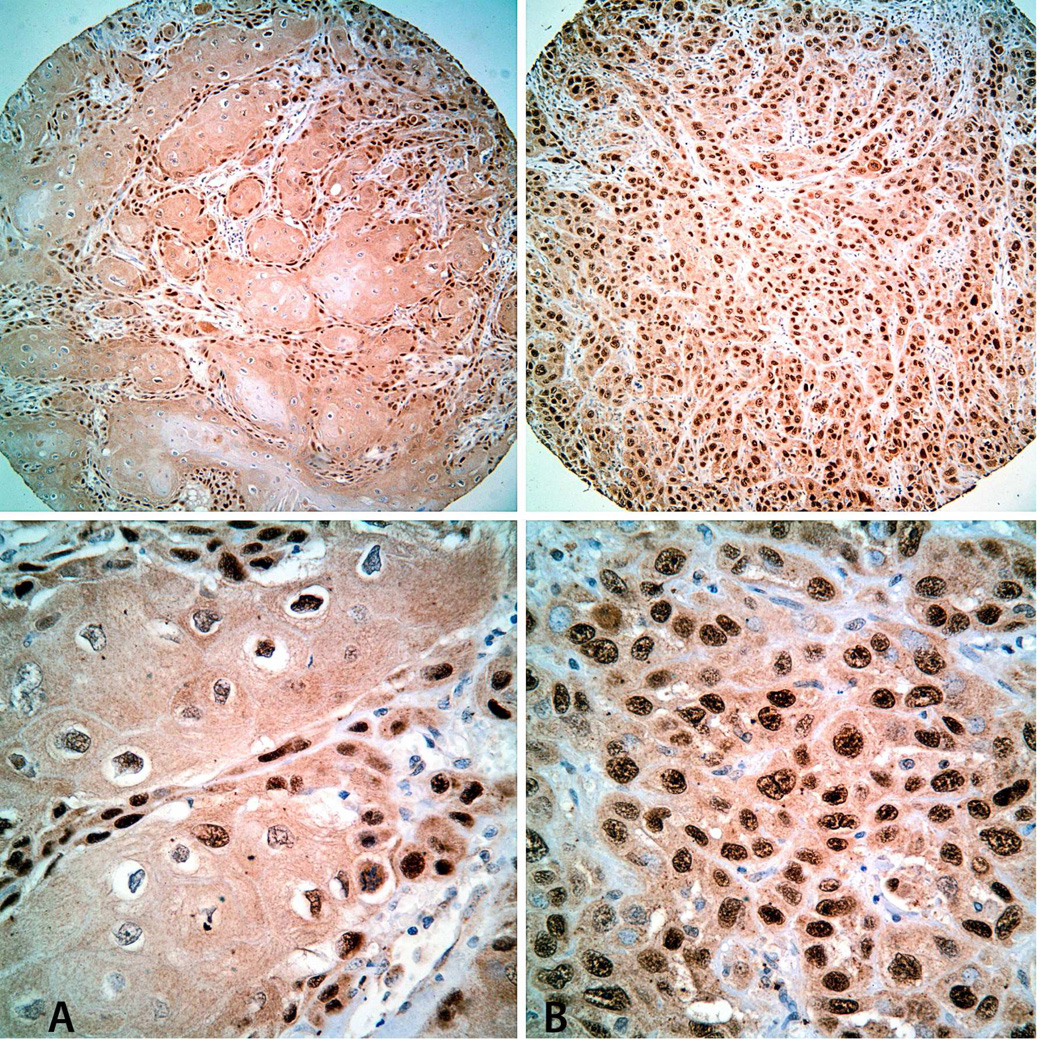
Immunohistochemical staining for Tp63 in TMA cores. Representative images of SCCOT with low (A: Immunoscore = 1) and high (B: Immunoscore = 4) Tp63 expression levels. Top: Low-power view (original magnification × 100); Bottom: High-power view of the same tumors (original magnification × 200).
Figure 6.
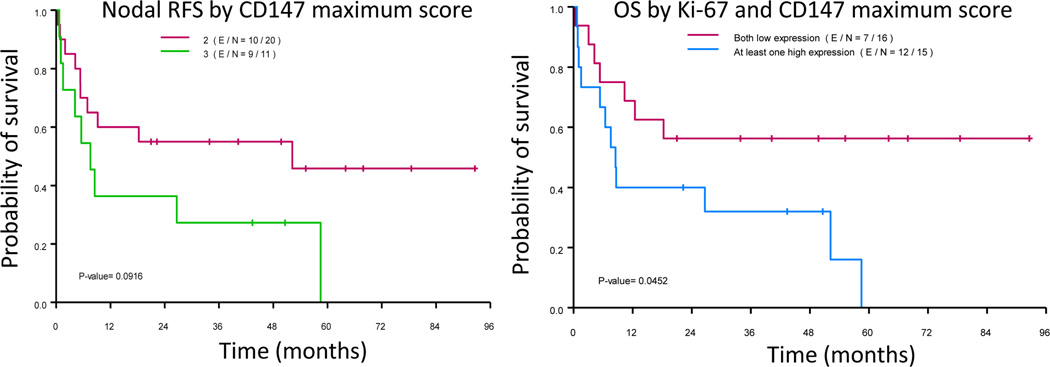
Kaplan-Meier analysis of recurrence free survival (RFS; right; p = 0.0916) and overall survival (OS; left) of patients with squamous cell carcinoma of the tongue according to CD147 and CD147 + Ki-67 expression levels, respectively (p = 0.0452).
Association between biomarkers expression and clinicopathologic variables
Increased expression of CD147 correlated significantly with advanced tumor stage (p = 0.004) (Table 3). Interestingly, high levels of CD147 expression (p = 0.058) is observed more often in SCCOT of non-smokers (71.4%) than SCCOT of current (36.4%) and former smokers (15%). SCCOT with high Glut-1 expression levels (Immunoscore ≥ 3) presented with higher T-stages, but this association is only marginally significant (p=0.058). Overexpression of Tp63 in SCCOT was positively associated with large tumor size (T3/T4; p=0.04) and poor histologic differentiation (p = 0.001) (Table 3). Tumor size and histologic grading of SCCOT were also significantly (p < 0.5) associated with the percentage of tumor cells positive for Ki-67 antigen (growth fraction) (Table 3). Expression of CD44v6 was not significantly associated with any of the examined clinicopathologic features (Table 3). Expression levels of neither of these biomarkers revealed positive association with either pathological N stage or extracapsular spread (ECS) (Table 3).
Table 3.
Correlation between the traditional prognostic parameters and biomarkers
| Variables | Biomarkers association (p-value from GEE model) | ||||
|---|---|---|---|---|---|
| p63 | Ki-67 | CD147 | CD44v6 | Glut1 | |
| T-stage | 0.042 | 0.048 | 0.004 | NS | NS |
| N-stage | NS | NS | NS | NS | NS |
| ECS-Stage | NS | NS | NS | NS | NS |
| Histology grade | 0.001 | 0.022 | NS | NS | NS |
Associations among biomarkers in SCCOT
Using the total 49 TMA-IHC records, we evaluated the association of IHC scoring between pairs of biomarkers (Table 4). CD147 expression levels in SCCOT associated positively with the expression of Tp63 and Ki-67 (p < 0.05). Similarly, there were significant positive associations between the expression levels of Tp63 and Ki-67 as well as Tp63 and Glut-1 (p < 0.05). We did not find any significant association between the expression of CD44v6 and other biomarkers.
Table 4.
Positive correlation between biomarkers expression levels in SCCOT-TMA
| Biomarker | (p-value) |
|---|---|
| Ki-67 & p63 | 0.018 |
| Ki-67 & CD147 | 0.035 |
| Ki-67& CD44v6 | NS |
| Ki-67 & Glut1 | NS |
| p63 & CD147 | 0.003 |
| p63 & CD44v6 | NS |
| p63 & Glut1 | 0.035 |
Association between biomarker expression levels and RFS and OS
The follow-up period ranged from 0.33 to 93 months. A total of 5 patients had nodal recurrence and 20 patients died. The associations of pathological N- stage and extracapsular spread (ECS) on OS or RFS were significant (p < 0.05). With regard to the TMA-IHC grading of biomarkers, SCCOT with high CD147 expression levels had marginally significant association with poor OS (p = 0.094) and poor RFS (p=0.092) (Figure 6); SCCOT with high Ki-67 expression levels had marginally significant association with poor OS (p = 0.06) and significant association with poor RFS (p=0.041) (Figure 6). Moreover, CD147 expression levels, when combined with Ki-67 labelling indices, were significantly associated with OS (p = 0.045) and RFS (p = 0.047). We also fitted multi-covariate Cox proportional hazards model to evaluate the effect of CD147 or CD147 and Ki-67 combining score on OS and RFS. With the adjustment of T stage (1/2 vs. 3/4) in the model, the effect of the marker on OS or RFS was no longer significant. However, the trend persisted.
DISCUSSION
Squamous cell carcinoma of the head and neck is the sixth most common cancer worldwide and the tongue remains the most common site in the United States. The tongue has rich lymphatic drainage resulting in a high incidence of occult nodal metastases with SCCOT at the time of diagnosis than the SCCs of any other site in the oral cavity47, 48. Nodal metastasis remains the most important independent prognostic factor for tongue cancer patients15. Therapeutic decisions for SCCOT are currently made based on the standard clinicopathologic variables such as the tumor size, depth of invasion, cervical lymph nodes status, extracapsular spread (ECS) and histologic grade28, 49. However, the accuracy of these clinicopathologic parameters in predicting the SCCOT response to therapy remains uncertain18, 50. There is a critical need to avoid overtreatment in SCCOT patients which may provide only modest benefit while causing significant morbidity. On the other hand, under-treatment or incorrect treatment that increases the risk of local recurrence and metastasis should be also avoided. There is a need to incorporate predictive biomarkers into the current tumor staging to identify SCCOT patients who are at risk for recurrence and poor survival. Such identification could aid clinicians in identifying patients who need more aggressive treatment and close monitoring. In this study, we tested the technical validity and clinical utility of five biomarkers in predicting the prognosis of SCCOT. By testing five biomarkers in the same set of tumor samples, we were able to explore the correlations and interplays among these functionally related biomarkers that are reported to be prognostic predictors in head and neck squamous cell carcinomas in previous studies29, 31–34.
Our results demonstrated that CD147 and Tp63 expression levels in SCCOT were associated with a large tumor size, a high proliferation rate (Ki-67 labeling index) and shorter OS and RFS. Increased expression of CD147 in combination with high Ki-67 labeling index associated with poor OS. Moreover, poorly differentiated SCCOTs (Grade 3) revealed significantly higher nuclear labeling of Tp63 compared with well to moderately (Grade 1 and 2) differentiated tumors. Most importantly, SCCOT with elevated CD147 expression possessed significantly higher levels of Tp63. Previous studies have reported that high expression of CD147 and Tp63 is associated with poor clinical outcomes in head and neck SCC51, 52. Our study confirms the previous reports and suggests that overexpression of CD147 significantly correlated with aggressive tumor phenotype and poor clinical outcome in SCCOTs.
CD147 is a cell surface transmembrane protein that regulates cancer development and progression by mediating tumor-microenvironmental cross-talk. CD147 induces peritumoral fibroblasts to produce matrix metalloproteinases (MMPs) which initiate tumor invasion53. Tumor cell surface CD147 functions as a receptor for α3β1 and α6β1 integrins that facilitate cancer metastasis54–56. Tumor cell surface CD147 stimulates the production of vascular endothelial growth factor (VEGF) by both tumor and stromal cells and promotes tumor angiogenesis57, 58. Moreover, CD147 acts as chaperone for the cell surface expression of monocarboxylate transporters-1&4, which in turn promotes MMP induction, lactate-induced angiogenesis and hyaluronan production59. In a previous study, we reported that CD147 is a promising molecular biomarker of oral mucosal carcinogenesis60. CD147 is the most constantly upregulated mRNA in metastatic cells61 and plays critical roles in tumor metastasis and resistance to chemoradiation therapy53, 62–65. CD147 is overexpressed in various solid malignancies and its overexpression is reported to correlate with tumor progression and poor prognosis51, 66–69. Tumor cells engineered to overexpress CD147 demonstrate increased invasiveness in vitro and accelerated growth rate and metastatic progression in animal models65. RNAi mediated silencing of CD147 inhibits proliferation, invasion, angiogenesis and metastatic propensity of malignant melanoma cells70. In concordance with these published reports, our study reveals CD147 overexpression is a reliable predictor of loco-regional failure and poor OS in SCCOT, lending credence to the proposed link between CD147 and maintenance of cancer stem cells niche and poor response to therapy71, 72. CD147 stimulates the production of hyaluronan by tumors cells, which not only promotes tumor cell survival during anchorage-independent growth,73 but also aids in the maintenance of cancer stem cell niche by being the primary ligand for cancer stem cell marker CD4472, 74–77.
The expression levels of CD44, which is a putative marker of cancer stem cells in head and neck carcinomas, failed to show prognostic significance in SCCOT patients78. CD44 is encoded by at least 20 exons and 10 variant exons (v1-10) which can be alternatively spliced in various combinations, thereby producing numerous spliced variants (VD44v1-10) from a single gene79, 80. The standard CD44 (CD44s), which is not encoded by variant exons, is exclusively expressed by hematopoietic and mesenchymal cells79, 80. On the other hand, CD44v6, which is a spliced variant of CD44 containing variant exon 6, is frequently overexpressed in carcinomas and is reported to be associated with tumor progression and metastasis81. However, there are contradictory data in validating the association between the expression levels of CD44v6 and poor prognosis in several types of malignancies including head and neck SCC32, 75, 82, 83. Although CD44v6 was expressed in about > 80% (Immunoscore >2) of our tumor samples, it surprisingly did not show any significant correlations with the main clinical prognostic factors for SCCOT nor with the survival functions.
Expression of another stem cell related molecular marker, Tp63, is associated with aggressive tumor phenotype in SCCOT. Tp63 gene is expressed as multiple isoforms due to alternative splicing and differential promoter usage and these isoforms have variable N- and C-terminal regions37, 38. Tp63 is crucial for the proliferation of stem cells in stratified squamous epithelium84 and is one of the most frequently mutated gene in head and neck SCC39. The predominant Tp63 isoform expressed in head and neck SCC lacks the full N-terminal domain and is referred to as ΔNp6340. This N-terminal region of Tp63 harbors the transactivation domain (TA) which is present in transcriptionally active isoforms but is absent in the ΔNp63 isoform37. Deregulated expression of Tp63 leading to an increased ΔNp63/Tp63 ratio promotes tumor development by inhibiting cellular senescence85. Inactivation of p53 function, combined with the activation of Wnt/β-catenin pathway, results in the overexpression of ΔNp63 that promotes oral mucosal tumorigenesis and early metastatic progression30, 86. Furthermore, in squamous cell carcinomas, the transcription factor SOX2, which is critical for the maintenance of embryonic and adult stem cells, preferentially interacts with ΔNp63 and regulates gene expression that governs undifferentiated cancer stem cells-like properties87.
Interestingly, there is a coordinated expression of CD147 and Tp63 limited to the basal cells of normal oral mucosa; the expression of both of these markers is downregulated during maturation of normal oral squamous epithelium. In contrast, both CD147 and Tp63 are aberrantly overexpressed spreading to the upper layers of the epithelium during the early stages of oral carcinogenesis suggesting that they may act to maintain an immature cell state in SCCOT and its precursors30, 60. In this study, we document a close correlation between the expression levels of CD147 and Tp63 in SCCOT which has significant prognostic implication.
Moreover, Tp63 expression was significantly correlated with the expression levels of Glut-1 which is functionally related to CD147. Most solid cancers, including SCCOT, switch from oxidative to glycolytic metabolism for energy production resulting in increased glucose uptake and enhanced production of intracellular lactic acid. Glut-1 is the main transporter involved in glucose influx, whereas monocarboxylate transporters (MCT) mediate the efflux of lactic acid from the tumor cells. Maturation and tumor cell surface expression of MCT are dependent on CD147 expression59. Moreover, increased expression of Glut-1 in association with CD147 renders poor prognosis and resistance to radiation therapy in cervical squamous cell carcinomas88. Our data shows that Glut-1 expression associates marginally with tumor size (T-stages) but did not show any significant association with RFS or OS. We observed that the majority of the T1 and T2 SCCOT (>50%) had low levels of Glut-1 (immunoscore =1) expression, while most (> 70%) of T3 and T4 cases were intensely positive (immunoscore 2 & 3) for Glut-1. The association between Glut-1 expression levels and tumor size in SCCOT is consistent with previous studies in oral squamous cell carcinomas33.
CONCLUSION
Our data show that SCCOT with high expressions of CD147 and Ki-67 have increased risk for loco-regional recurrence resulting poor OS and aggressive tumor phenotypes, respectively. Sample size is the major limitation of this study and further studies on larger cohorts of SCCOT are needed to determine the prognostic value of CD147 and Tp63. In addition to their prognostic relevance, correlation between the expression levels of CD147 and Ki-67 within the same dataset suggests a potential functional cross-talk between these two molecules. Further research is needed to elucidate the underlying biologic mechanisms.
Figure 3.
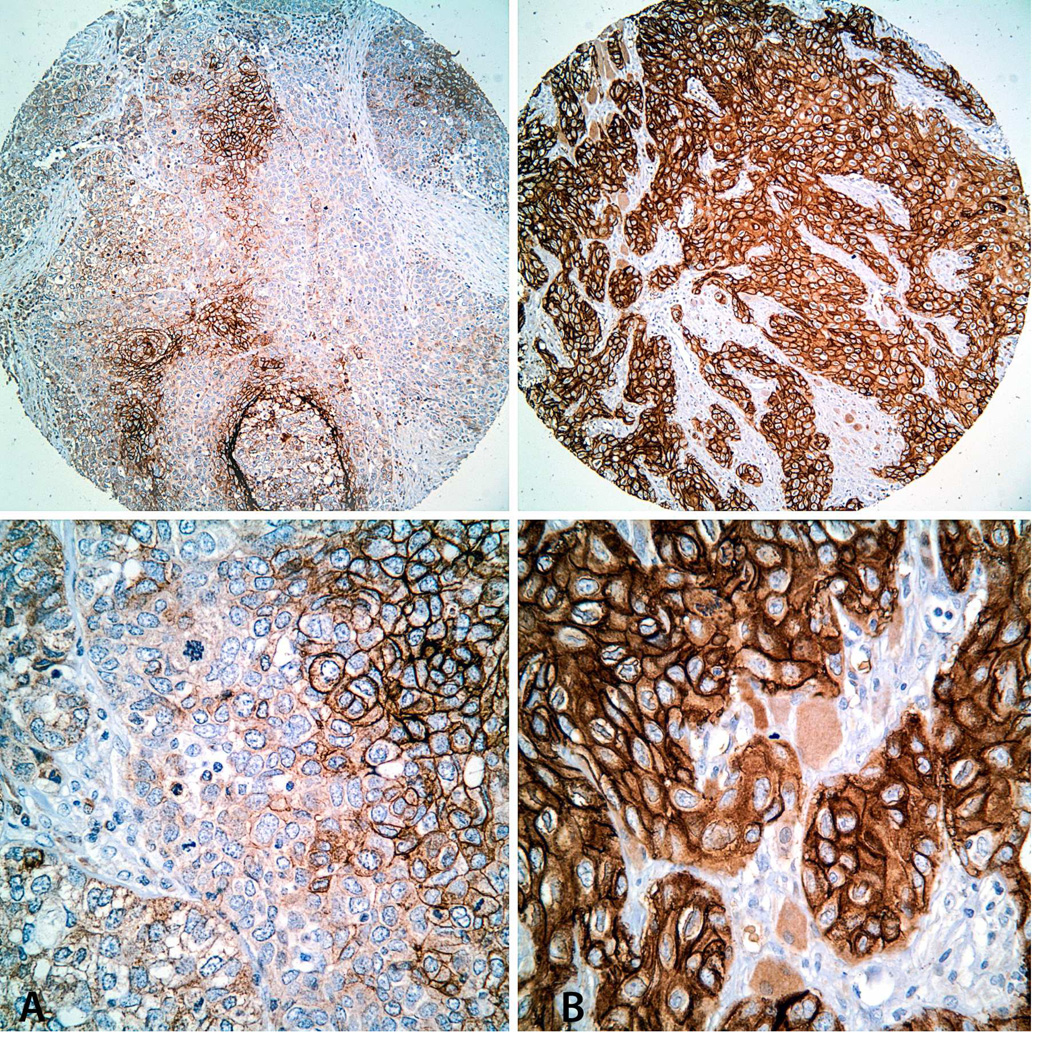
Immunohistochemical staining for Glut-1 in TMA cores. Representative images of SCCOT with low (A: Immunoscore = 1) and high (B: Immunoscore = 4) Glut-1 expression levels. Top: Low-power view (original magnification × 100); Bottom: High-power view of the same tumors (original magnification × 200).
Table 5.
Prognostic markers associated with worse overall and recurrence free survival
| Variables | Overall survival (p-value) |
Recurrence-free survival (p-value) |
|---|---|---|
| N-stage | 0.005 | 0.010 |
| ECS-status | 0.0005 | 0.001 |
| p63 | 0.022 | 0.011 |
| CD147 | 0.094 | 0.092 |
| Ki-67 ± CD147 | 0.045 | 0.047 |
Statement of Clinical Relevance: CD147, Tp63, as well as CD147 combined with Ki-67 are potential predictive biomarkers in early stages of squamous cell carcinomas of oral tongue and can be used as adjunct for conventional tumor staging for optimal therapeutic management.
ACKNOWLEDGEMENT
Abstract of this study was presented at the Annual Meeting of the American Academy of Oral and Maxillofacial Pathology, in May 2011. This study was supported by NIH/NIDCR grants R21DE019956 & R01DE024392, a grant from the Cancer Prevention & Research Institute of Texas (CPRIT; RP101382) and The University of Puerto Rico Comprehensive Cancer Center (UPRCCC) and The University of Texas M. D. Anderson Cancer Center (MDACC) Partnership for Excellence in Research.
Abbreviations
- OSCC
Oral squamous cell carcinoma
- SCCOT
Squamous cell carcinoma of the oral tongue
- TMA
Tissue microarray
- OS
Overall survival
- RFS
Recurrence free survival
- ECS
Extracapsular spread
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors confirm that they have no current or potential conflicts of interest, including financial, personal, or other relationships with individuals or organizations that could inappropriately influence this study.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Dec 15;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009 Apr-May;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Myers JN. Cancer of the oral cavity. Dis Mon. 2001 Jul;47(7):275–361. doi: 10.1067/mcd.2001.109374. [DOI] [PubMed] [Google Scholar]
- 4.Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992 Mar-Apr;70(3–4):320–327. doi: 10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 5.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008 Apr;83(4):489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 6.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008 Dec;22(6):1125–1142. vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011 Apr 10;29(11):1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 8.Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002 Mar;128(3):268–274. doi: 10.1001/archotol.128.3.268. [DOI] [PubMed] [Google Scholar]
- 9.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005 May 1;103(9):1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 10.Ridge JA, Glisson BS, Lango MN, Feigenberg S. Head and Neck Tumors. In: Haller DE, Wagman LD, Camphausen KA, Hoskins WJ, editors. Cancer Management: A Multidisciplinary Approach, Medical, Surgical & Radiation Oncology. 14th ed. NY, USA: UBM Medica; 2011. pp. 1–42. [Google Scholar]
- 11.Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010 Apr;32(4):499–503. doi: 10.1002/hed.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas H, Pitman KT, Johnson JT, Galati LT. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope. 2000 Oct;110(10 Pt 1):1623–1626. doi: 10.1097/00005537-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Faden DL, Fakhry C, Langelier C, Jiao Y, Wang Y, et al. Clinical, Genomic and Metagenomic Characterization of Oral Tongue Squamous Cell Carcinoma in Patients Who Do Not Smoke. Head Neck. 2014 Jun 21; doi: 10.1002/hed.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering CR, Zhang J, Neskey DM, Zhao M, Jasser SA, Wang J, et al. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin Cancer Res. 2014 Jul 15;20(14):3842–3848. doi: 10.1158/1078-0432.CCR-14-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007 Dec;26(3–4):645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 16.Byers RM, El-Naggar AK, Lee YY, Rao B, Fornage B, Terry NH, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998 Mar;20(2):138–144. doi: 10.1002/(sici)1097-0347(199803)20:2<138::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984 Oct;7(1):15–21. doi: 10.1002/hed.2890070105. [DOI] [PubMed] [Google Scholar]
- 18.El-Naaj IA, Leiser Y, Shveis M, Sabo E, Peled M. Incidence of oral cancer occult metastasis and survival of T1-T2N0 oral cancer patients. J Oral Maxillofac Surg. 2011 Oct;69(10):2674–2679. doi: 10.1016/j.joms.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Brugere JM, Mosseri VF, Mamelle G, David JM, Buisset E, Vallicioni J, et al. Nodal failures in patients with NO N+ oral squamous cell carcinoma without capsular rupture. Head Neck. 1996 Mar-Apr;18(2):133–137. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<133::AID-HED4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008 Jan 15;112(2):345–351. doi: 10.1002/cncr.23183. [DOI] [PubMed] [Google Scholar]
- 21.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989 Oct;158(4):309–313. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 22.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997 Oct;19(7):583–588. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Peng KA, Chu AC, Lai C, Grogan T, Elashoff D, Abemayor E, et al. Is there a role for neck dissection in T1 oral tongue squamous cell carcinoma? The UCLA experience. American journal of otolaryngology. 2014 Jul 10; doi: 10.1016/j.amjoto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris RL, Xi L, Seethala RR, Chan J, Desai S, Hoch B, et al. Intraoperative qRT-PCR for detection of lymph node metastasis in head and neck cancer. Clin Cancer Res. 2011 Apr 1;17(7):1858–1866. doi: 10.1158/1078-0432.CCR-10-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitman KT, Johnson JT, Myers EN. Effectiveness of selective neck dissection for management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1997 Sep;123(9):917–922. doi: 10.1001/archotol.1997.01900090023004. [DOI] [PubMed] [Google Scholar]
- 26.Tainsky MA. Genomic and proteomic biomarkers for cancer: a multitude of opportunities. Biochim Biophys Acta. 2009 Dec;1796(2):176–193. doi: 10.1016/j.bbcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005 Nov;5(11):845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 28.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II) Oral Oncol. 2010 Sep;46(9):636–643. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Ayala FR, Rocha RM, Carvalho KC, Carvalho AL, da Cunha IW, Lourenco SV, et al. GLUT1 and GLUT3 as potential prognostic markers for Oral Squamous Cell Carcinoma. Molecules. 2010 Apr;15(4):2374–2387. doi: 10.3390/molecules15042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foschini MP, Gaiba A, Cocchi R, Pennesi MG, Gatto MR, Frezza GP, et al. Pattern of p63 expression in squamous cell carcinoma of the oral cavity. Virchows Arch. 2004 Apr;444(4):332–339. doi: 10.1007/s00428-003-0969-x. [DOI] [PubMed] [Google Scholar]
- 31.Kunkel M, Moergel M, Stockinger M, Jeong JH, Fritz G, Lehr HA, et al. Overexpression of GLUT-1 is associated with resistance to radiotherapy and adverse prognosis in squamous cell carcinoma of the oral cavity. Oral Oncol. 2007 Sep;43(8):796–803. doi: 10.1016/j.oraloncology.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Lindquist D, Ahrlund-Richter A, Tarjan M, Tot T, Dalianis T. Intense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancer. Anticancer Res. 2012 Jan;32(1):153–161. [PubMed] [Google Scholar]
- 33.Roh JL, Cho KJ, Kwon GY, Ryu CH, Chang HW, Choi SH, et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009 Jan;45(1):63–68. doi: 10.1016/j.oraloncology.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Wangsa D, Ryott M, Avall-Lundqvist E, Petersson F, Elmberger G, Luo J, et al. Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. Br J Cancer. 2008 Oct 7;99(7):1121–1128. doi: 10.1038/sj.bjc.6604633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jong MC, Pramana J, van der Wal JE, Lacko M, Peutz-Kootstra CJ, de Jong JM, et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 2010 Nov 1;16(21):5329–5338. doi: 10.1158/1078-0432.CCR-10-0799. [DOI] [PubMed] [Google Scholar]
- 36.Staibano S, Merolla F, Testa D, Iovine R, Mascolo M, Guarino V, et al. OPN/CD44v6 overexpression in laryngeal dysplasia and correlation with clinical outcome. Br J Cancer. 2007 Dec 3;97(11):1545–1551. doi: 10.1038/sj.bjc.6604070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcel V, Hainaut P. p53 isoforms - a conspiracy to kidnap p53 tumor suppressor activity? Cell Mol Life Sci. 2009 Feb;66(3):391–406. doi: 10.1007/s00018-008-8336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moll UM, Slade N. p63 and p73: roles in development and tumor formation. Mol Cancer Res. 2004 Jul;2(7):371–386. [PubMed] [Google Scholar]
- 39.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 Aug 26;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nylander K, Coates PJ, Hall PA. Characterization of the expression pattern of p63 alpha and delta Np63 alpha in benign and malignant oral epithelial lesions. Int J Cancer. 2000 Aug 1;87(3):368–372. [PubMed] [Google Scholar]
- 41.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007 Jun;26(2):225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 42.Grimm M, Munz A, Teriete P, Nadtotschi T, Reinert S. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral surgery, oral medicine, oral pathology and oral radiology. 2014 Jun;117(6):743–753. doi: 10.1016/j.oooo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Kilic M, Kasperczyk H, Fulda S, Debatin KM. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007 Mar 29;26(14):2027–2038. doi: 10.1038/sj.onc.1210008. [DOI] [PubMed] [Google Scholar]
- 44.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- 45.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral and maxillofacial surgery clinics of North America. 2014 May;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vigneswaran N, Zhao W, Dassanayake A, Muller S, Miller DM, Zacharias W. Variable expression of cathepsin B and D correlates with highly invasive and metastatic phenotype of oral cancer. Hum Pathol. 2000 Aug;31(8):931–937. doi: 10.1053/hupa.2000.9035. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T, Tanaka R, Taira S, Koyama J, Katsura K, Kobayashi F. Non-contrast-enhanced CT findings of high attenuation within metastatic cervical lymph nodes in patients with stage I or II tongue carcinoma during a follow-up period. AJNR Am J Neuroradiol. 2003 Aug;24(7):1330–1333. [PMC free article] [PubMed] [Google Scholar]
- 48.Okura M, Iida S, Aikawa T, Adachi T, Yoshimura N, Yamada T, et al. Tumor thickness and paralingual distance of coronal MR imaging predicts cervical node metastases in oral tongue carcinoma. AJNR Am J Neuroradiol. 2008 Jan;29(1):45–50. doi: 10.3174/ajnr.A0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997 May;19(3):205–210. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005 Dec;27(12):1080–1091. doi: 10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- 51.Huang Z, Huang H, Li H, Chen W, Pan C. EMMPRIN expression in tongue squamous cell carcinoma. J Oral Pathol Med. 2009 Jul;38(6):518–523. doi: 10.1111/j.1600-0714.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 52.Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol. 2005 Feb;36(2):187–194. doi: 10.1016/j.humpath.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005 Feb;93(2):199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 54.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer Metastasis Rev. 2005 Jan;24(1):35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 55.Shintani S, Li C, Mihara M, Nakashiro K, Hamakawa H. Gefitinib ('Iressa'), an epidermal growth factor receptor tyrosine kinase inhibitor, mediates the inhibition of lymph node metastasis in oral cancer cells. Cancer Lett. 2003 Nov 25;201(2):149–155. doi: 10.1016/s0304-3835(03)00464-6. [DOI] [PubMed] [Google Scholar]
- 56.Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci. 2005 Jun 15;118(Pt 12):2649–2660. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- 57.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005 Apr 15;65(8):3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 58.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006 Nov 20;95(10):1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007 May 1;67(9):4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 60.Vigneswaran N, Beckers S, Waigel S, Mensah J, Wu J, Mo J, et al. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp Mol Pathol. 2006 Apr;80(2):147–159. doi: 10.1016/j.yexmp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O, et al. Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol. 2002 Apr;20(4):387–392. doi: 10.1038/nbt0402-387. [DOI] [PubMed] [Google Scholar]
- 62.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007 Dec;83(3):283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM, et al. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007 Jul;98(7):1064–1069. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, et al. Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy. 2008;54(4):291–301. doi: 10.1159/000151225. [DOI] [PubMed] [Google Scholar]
- 65.Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol. 2001 Jun;158(6):1921–1928. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003 Aug;113(8):1406–1410. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002 Jun 1;99(4):520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 68.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer. 2000 Feb 1;85(3):347–352. [PubMed] [Google Scholar]
- 69.Ishibashi Y, Matsumoto T, Niwa M, Suzuki Y, Omura N, Hanyu N, et al. CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004 Nov 1;101(9):1994–2000. doi: 10.1002/cncr.20593. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006 Dec 1;66(23):11323–11330. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 71.Kang MJ, Kim HP, Lee KS, Yoo YD, Kwon YT, Kim KM, et al. Proteomic analysis reveals that CD147/EMMPRIN confers chemoresistance in cancer stem cell-like cells. Proteomics. 2013 May;13(10–11):1714–1725. doi: 10.1002/pmic.201200511. [DOI] [PubMed] [Google Scholar]
- 72.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008 Jun;11(3):110–121. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, et al. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004 Feb 15;64(4):1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 74.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi: 10.1155/2012/708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tzircotis G, Thorne RF, Isacke CM. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J Cell Sci. 2005 Nov 1;118(Pt 21):5119–5128. doi: 10.1242/jcs.02629. [DOI] [PubMed] [Google Scholar]
- 77.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 78.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. 2006 Jan;26(1):362–370. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- 81.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 82.Andratschke M, Chaubal S, Pauli C, Mack B, Hagedorn H, Wollenberg B. Soluble CD44v6 is not a sensitive tumor marker in patients with head and neck squamous cell cancer. Anticancer Res. 2005 Jul-Aug;25(4):2821–2826. [PubMed] [Google Scholar]
- 83.Mack B, Gires O. CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 2008;3(10):e3360. doi: 10.1371/journal.pone.0003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007 May 4;129(3):523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 85.Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W, Vogel H, et al. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell. 2011 Feb 4;8(2):164–176. doi: 10.1016/j.stem.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruptier C, De Gasperis A, Ansieau S, Granjon A, Taniere P, Lafosse I, et al. TP63 P2 promoter functional analysis identifies beta-catenin as a key regulator of DeltaNp63 expression. Oncogene. 2011 Nov 17;30(46):4656–4665. doi: 10.1038/onc.2011.171. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014 Apr 1;124(4):1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang XQ, Chen X, Xie XX, Zhou Q, Li K, Li S, et al. Co-expression of CD147 and GLUT-1 indicates radiation resistance and poor prognosis in cervical squamous cell carcinoma. International journal of clinical and experimental pathology. 2014;7(4):1651–1666. [PMC free article] [PubMed] [Google Scholar]