Abstract
Eighteen endophytic fungi with different colony morphologies were isolated from the roots of Nymphoides peltata growing in the Dalsung wetland. The fungal culture filtrates of the endophytic fungi were treated to Waito-c rice seedling to evaluate their plant growth-promoting activities. Culture filtrate of Y2H0002 fungal strain promoted the growth of the Waito-c rice seedlings. This strain was identified on the basis of sequences of the partial internal transcribed spacer region and the partial beta-tubulin gene. Upon chromatographic analysis of the culture filtrate of Y2H0002 strain, the gibberellins (GAs: GA1, GA3, and GA4) were detected and quantified. Molecular and morphological studies identified the Y2H0002 strain as belonging to Aspergillus clavatus. These results indicated that A. clavatus improves the growth of plants and produces various GAs, and may participate in the growth of plants under diverse environmental conditions.
Keywords: Aquatic plant, Aspergillus clavatus, Fresh water, Gibberellin, Plant growth-promoting activity
Endophytic fungi are known to exist symbiotically with plants and to play a significant role in the growth of the host plant [1]. These types of fungi, isolated from plants native to various environments, produce several types of plant hormones such as gibberellins (GAs), abscisic acid, and auxins (such as indole-3-acetic acid) [2, 3]. Research on the GAs produced by endophytic fungi has increased. The phytohormone GAs are diterpenoid complexes that are involved in plant growth, stem elongation, proliferation, ripening, and seed germination [4]. Although GAs are known to be produced by various fungi such as those belonging to the genera Arthrinium [5], Cadophora [3], Cladosporium [6], Paecilomyces [2], Penicillium [4, 7], and Phoma [8], their production by Aspergillus species has rarely been studied. Although strains of Aspergillus section Clavati are known more as producers of mycotoxins [9] and bioactive natural products [9, 10], in this study, we primarily report that Aspergillus clavatus produces GAs as well.
Collection of aquatic plant and fungal isolation
The aims of this study were to determine the plant growthpromoting activities of endophytic fungi in symbiosis with aquatic plants and to analyze the secondary metabolites produced by these fungi. Nymphoides peltata, a native aquatic plant (35°49'38.29" N, 128°28'58.56" E) from the Dalsung wetlands in Daegu, Korea, was collected for this study. The roots of N. peltata were sterilized with Tween 80 solution and 1% (w/v) perchloric acid solution [7]. Then, the roots were cut into pieces of about 3 cm and incubated at 25℃ on Hagem minimal medium containing streptomycin until the emergence of fungi from the roots [3]. The fungal mycelia were purified from the root pieces via subculture on potato dextrose agar. In this way, 18 endophytic fungi of different morphological colonies were isolated from the roots of N. peltata. There is detailed information about sampling below: sampling site (Dalsung wetland); host plant species (Nymphoides peltata); collector (Young-Hyun You); date of collection (June 29, 2013); storage place (Microbial Genetics Lab., Kyungpook National University).
Screening of plant growth-promoting activity by endophytic fungi
The filtered culture media of the fungi were used on Waito-c rice (WR) seedlings to test for their plant growth-promoting capacity. The fungal strains were first incubated for 7 days at 25℃ and 180 rpm in Czapek broth medium (CBM) and then harvested by filtration [11]. The harvested fungal culture filtrates (FCFs) were lyophilized at -80℃. The lyophilized FCFs were then mixed with 1 mL of distilled water. The WR seeds were treated for 24 hr with uniconazole to minimize the activity of GAs in the seed coat. The WR seedlings were transplanted in glass tubes containing 0.5% water-agar and grown in a growth chamber [3]. When the WR seedlings had reached the two-leaf stage, the meristems were treated with distilled water, lyophilized CBM, or concentrated lyophilized FCFs. The shoot length (SL) and plant length (PL) were observed after the treatments [7]. The wild-type strain Gibberella fujikuroi provided by the Korean Culture Center of Microorganisms was used as a positive control in this study. Each group included a control to determine the shoot and plant lengths of the WR seedlings. The results were observed for a week. Statistical analysis was assessed using the Excel program with one-way ANOVA. A Duncan's multiple range test was conducted to analyze significant differences (p < 0.05) among the different groups (ver. 18.0; SPSS Inc., Chicago, IL, USA). Distilled water and CBM were used as the negative controls for comparison of the plant growth-promoting activities of the FCFs. The bioassay test result using the FCF of the Y2H0002 strain was superior to that of G. fujikuroi in regard to plant growth promotion of the SL (11.28 cm) and PL (20.78 cm) of WR seedlings (Table 1).
Table 1. Plant growth-promoting activity of WR seedlings by endophytic fungi isolated from the aquatic plant.
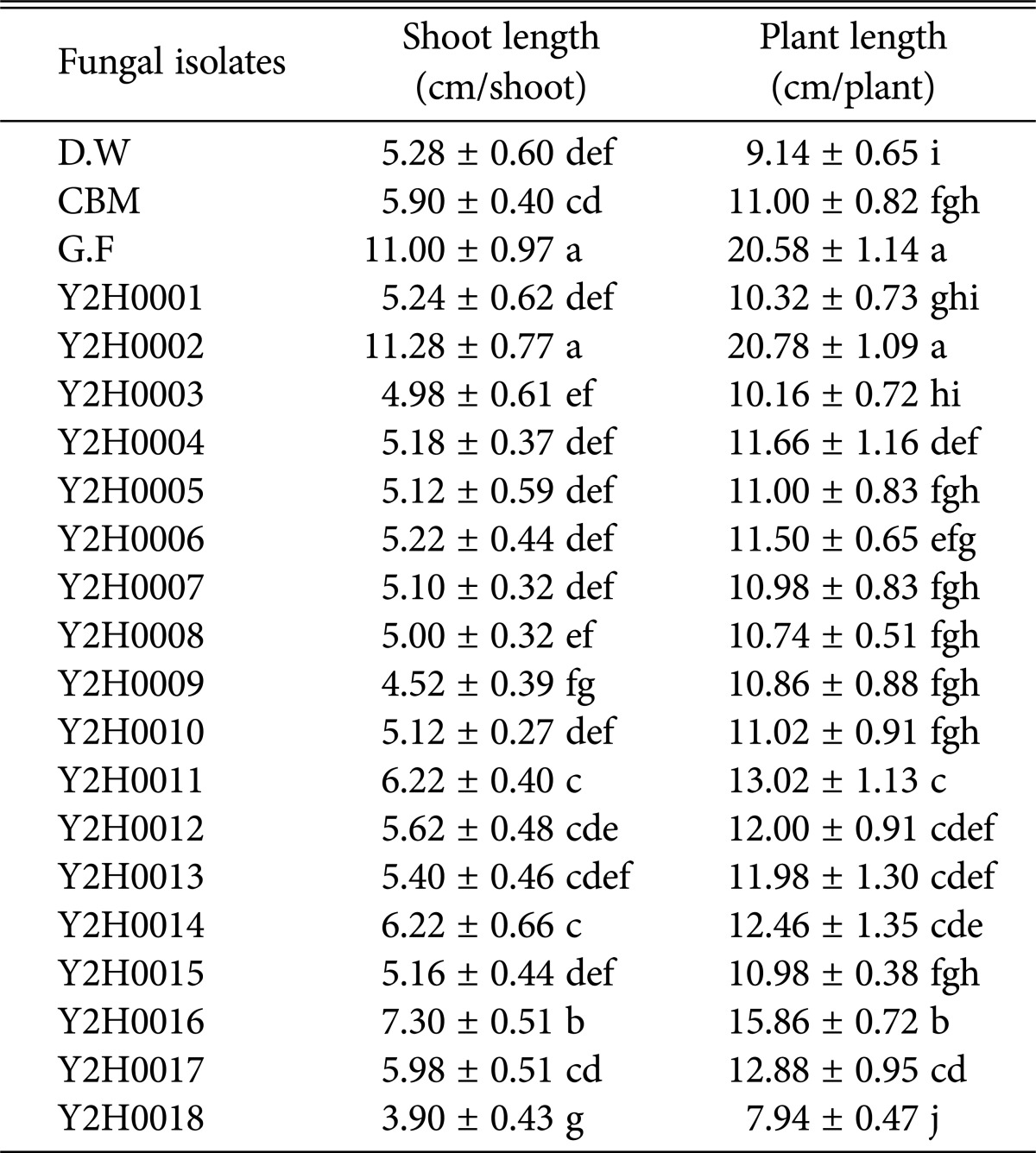
Ten microliters of lyophilized fungal culture filtrates was treated to Waito-c rice (WR) seedlings. The shoot length and plant length of the WR seedlings were measured after 7 days of treatment. According to Duncan's multiple range test (p < 0.05), the different letters in a row indicate significant differences. The letters indicate that values are not significantly different. Data are expressed as mean value ± standard deviation.
D.W, distilled water; CBM, lyophilized Czapek broth medium; G.F, culture filtrate lyophilized of wild-type Gibberella fujikuroi.
Quantitative analysis of GAs
CBM containing 1% (w/v) glucose and peptone was used for the production of secondary metabolites by the fungi [11]. Extraction of GAs from the FCFs was performed as reported previously. After incubation of the FCFs for 7 days, the extracted GAs were analyzed by reverse-phase C18 high-performance liquid chromatography. The fractions were collected and prepared for gas chromatography-mass spectrometry (GC-MS) with selected ion monitoring (SIM). After the GC-MS data had been collected and analyzed, three major ions of the supplemented [2H2] GA internal standard and GAs were monitored. To calculate the Kovats retention index value, standards were used to determine the retention time, and peak area ratios between non-deuterated and deuterated GAs were used to quantify the GAs [4, 7].
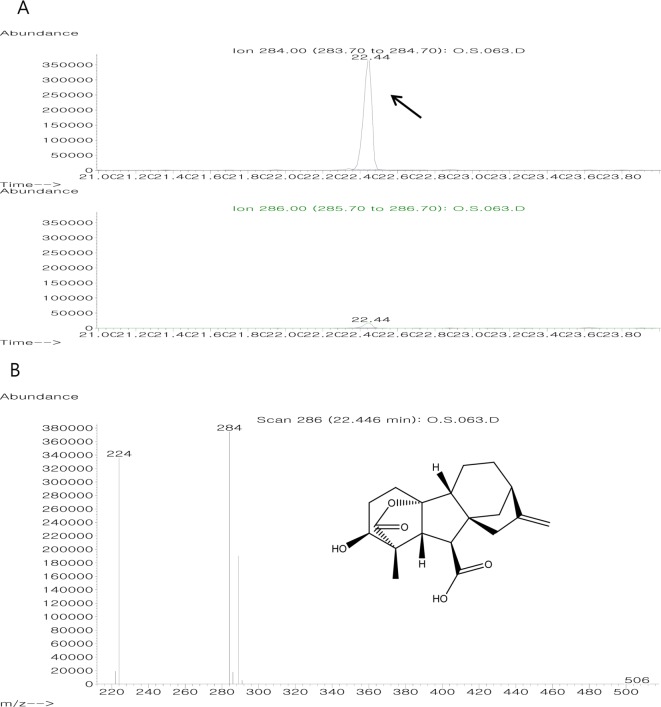
The plant growth-promoting activity of the FCF of strain Y2H0002 was confirmed on WR seedlings. The phytohormones, GA1 (1.241 ng/mL), GA3 (1.426 ng/mL), and GA4 (4.295 ng/mL), from the culture filtrate of strain Y2H0002 were detected by quantitative analysis using GCMS SIM (Fig. 1). These three detected GAs are physiologically active hormones. It was confirmed that strain Y2H0002 produced higher amounts of these GAs than did wild-type G. fujikuroi (GA1, 1.061 ng/mL; GA3, 1.142 ng/mL; and GA4, 3.12 ng/mL).
Fig. 1. On gas chromatography-mass spectrometry (GC-MS) selected ion monitoring (SIM) analysis extracted from culture filtrate of Y2H0002 strain, three bioactive gibberellins (GAs) were detected [4, 7]. The GC-MS SIM spectra for GA4 in culture filtrate of the Aspergillus clavatus Y2H0002. Arrow indicates the peak of fungal GA4 that coincides with that of internal standard GA4. GC-MS peak of A. clavatus Y2H0002: A, GC-MS peak of culture filtrate, and GC-MS peak of standard GA4; B, Ion value of standard GA4.
Analysis of sequences and phylogenetic analysis
The Y2H0002 strain was inoculated in an Erlenmeyer flask containing 50 mL of potato dextrose broth medium and incubated at 28℃ for 7 days in a shaker (120 rpm). The lyophilized Y2H0002 strain was obtained by vacuum filtration. The fungal genomic DNA was extracted by using a DNeasy plant mini kit (DNeasy Plant Mini Kit; Qiagen, Valencia, CA, USA). The rDNA internal transcribed spacer (ITS) [12] and β-tubulin [13] sequences were generated from the Y2H0002 and G. fujikuroi strains, and other species sequences in Aspergillus section Clavati were obtained from GenBank [14].
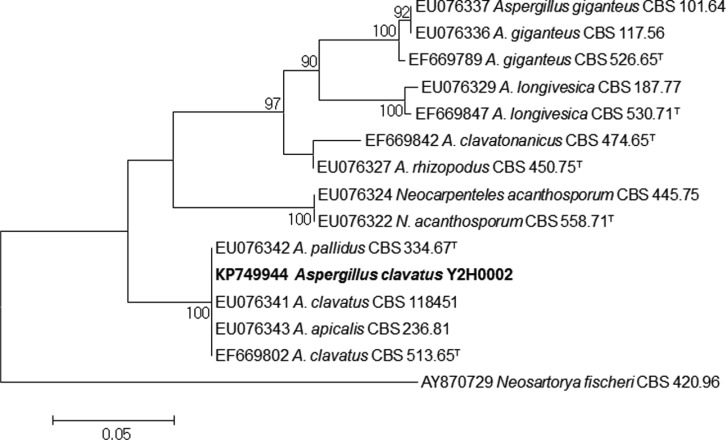
The DNA sequences were analyzed by ClustalW with the MEGA ver. 6.0 software program [15]. A phylogenetic tree based on the β-tubulin gene sequence was constructed using maximum likelihood and Kimura-2-parameter modeling (1,000 bootstrap replications). The data confirmed the phylogenetic placement of the strains in Aspergillus section Clavati (Fig. 2). The DNA sequences of the Y2H0002 strain were registered in the GenBank database of the National Center for Biotechnology Information (KP749943 for rDNA-ITS; KP749944 for β-tubulin).
Fig. 2. Phylogenetic position of Aspergillus clavatus Y2H0002 based on sequences data of partial β-tubulin. DNA sequences of the A. clavatus Y2H0002 strain was compared with type strains belonging to Aspergillus section Clavati [14]. Phylogenetic tree was constructed by the maximum likelihood method with MEGA 6 software. Bootstrap analysis was performed using 1,000 replications. The "T" after the collection number indicates the type strains of the species. Bar, 0.05 substitutions per nucleotide position. Only values above 70% are indicated.
Morphological characteristics
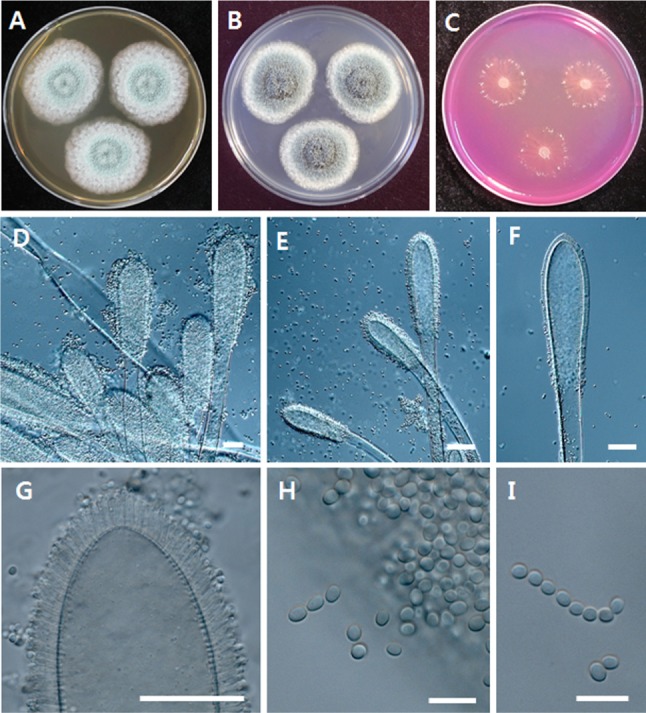
The macroscopic descriptions of the Y2H0002 strain are based on cultures grown at 25℃ on malt extract agar (MEA), Czapek yeast extract agar (CYA), and creatine sucrose agar (CREA), for 7 days in darkness. Microscopic characterization was determined by light microscopy (Zeiss Axioscope; Zeiss, Jena, Germany) of the colonies growing on MEA. The morphological features and structures of strain Y2H0002 are shown in Fig. 3.
Fig. 3. Aspergillus clavatus Y2H0002. A, Colonies on malt extract agar; B, Colonies on Czapek yeast extract agar; C, Colonies on creatine sucrose agar; D~G, Conidiophores and conodia; H~I, Conidia (scale bars: D~G = 40 µm, H, I = 10 µm).
Description of colony diameter (7 days)
MEA: 35~38 mm; CYA: 35~40 mm; CREA: 20~23 mm. Colonies on MEA: good growth, velvety, conidia light grey-green, colony reverse orange-brown. Colonies on CYA: very good growth, mostly velvety, sporulation moderate to strong, conidia dark grey-green, colony reverse beige. Colonies on CREA: good growth, little acid production. Conidiophores, clavate, stipes smooth-walled; 1,200~3,000 × 20~30 µm long; vesicles 180~250 × 60~70 µm in size. Conidia smooth or thick-walled, elliptical (3.0~4.0 × 2.5~3.0 µm).
Note
Many species of endophytic fungi are known to produce a variety of secondary metabolites, including phytohormones [2, 3]. In this study, the Y2H0002 strain was isolated from the roots of Nymphoides peltata growing in a fresh-water environment, and this strain proved to possess plant growth-promoting activity. The FCF of strain Y2H0002 bioassayed on WR seedlings showed excellent plant growth-promoting activity. This strain was confirmed to produce three bioactive GAs. In this study, clavate conidiophores belonging to typical Aspergillus section Clavati were found in strain Y2H0002 by morphological observation. As seen in the phylogenetic tree, A. clavatus Y2H0002 and A. clavatus CBS 513.65T were in the same group [14], and the homology of the β-tubulin sequences was 100%. The results suggested that the Y2H0002 strain produced GAs in the section of Clavati and thus it was named A. clavatus Y2H0002, as it was isolated from fresh water. This study emphasizes the importance of new endophytic fungal strain with plant growth-promoting activity and phytohormones under the fresh-water environment.
ACKNOWLEDGEMENTS
This subject is supported by Korea Ministry of Environment as "The Eco-Innovation Project".
References
- 1.Vandenkoornhuyse P, Baldauf SL, Leyval C, Straczek J, Young JP. Extensive fungal diversity in plant roots. Science. 2002;295:2051. doi: 10.1126/science.295.5562.2051. [DOI] [PubMed] [Google Scholar]
- 2.Khan AL, Hamayun M, Kang SM, Kim YH, Jung HY, Lee JH, Lee IJ. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol. 2012;12:3. doi: 10.1186/1471-2180-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You YH, Yoon H, Kang SM, Woo JR, Choo YS, Lee IJ, Shin JH, Kim JG. Cadophora malorum Cs-8-1 as a new fungal strain producing gibberellins isolated from Calystegia soldanella. J Basic Microbiol. 2013;53:630–634. doi: 10.1002/jobm.201200002. [DOI] [PubMed] [Google Scholar]
- 4.Khan SA, Hamayun M, Yoon H, Kim HY, Suh SJ, Hwang SK, Kim JM, Lee IJ, Choo YS, Yoon UH, et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008;8:231. doi: 10.1186/1471-2180-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Hamayun M, Kim HY, Yoon HJ, Seo JC, Choo YS, Lee IJ, Kim SD, Rhee IK, Kim JG. A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellin production. Biotechnol Lett. 2009;31:283–287. doi: 10.1007/s10529-008-9862-7. [DOI] [PubMed] [Google Scholar]
- 6.Hamayun M, Khan SA, Ahmad N, Tang DS, Kang SM, Na CI, Sohn EY, Hwang YH, Shin DH, Lee BH, et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J Microbiol Biotechnol. 2009;25:627–632. [Google Scholar]
- 7.You YH, Yoon H, Kang SM, Shin JH, Choo YS, Lee IJ, Lee JM, Kim JG. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J Microbiol Biotechnol. 2012;22:1549–1556. doi: 10.4014/jmb.1205.05010. [DOI] [PubMed] [Google Scholar]
- 8.Hamayun M, Khan SA, Khan AL, Rehman G, Sohn EY, Shah AA, Kim SK, Joo GJ, Lee IJ. Phoma herbarum as a new gibberellin-producing and plant growth-promoting fungus. J Microbiol Biotechnol. 2009;19:1244–1249. [PubMed] [Google Scholar]
- 9.Cole RJ, Cox RH. Handbook of toxic fungal metabolites. New York: Academic Press; 1981. [Google Scholar]
- 10.Lin A, Huang KC, Hwu L, Tzean SS. Production of type II ribotoxins by Aspergillus species and related fungi in Taiwan. Toxicon. 1995;33:105–110. doi: 10.1016/0041-0101(94)00140-4. [DOI] [PubMed] [Google Scholar]
- 11.Hasan HA. Gibberellin and auxin-production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol Immunol Hung. 2002;49:105–118. doi: 10.1556/AMicr.49.2002.1.11. [DOI] [PubMed] [Google Scholar]
- 12.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 13.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varga J, Due M, Frisvad JC, Samson RA. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud Mycol. 2007;59:89–106. doi: 10.3114/sim.2007.59.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]