Abstract
The biochemical pathways underlying major depressive disorder (MDD) and chronic stress are not well understood. However, it has been reported that monoamine oxidase A (MAO A, a major neurotransmitter-degrading enzyme) is significantly increased in the brains of human subjects affected with MDD and rats exposed to chronic social defeat (CSD) stress, which is used to model depression. In the current study, we compared the protein levels of a MAO A-transcriptional activator, Kruppel-like factor 11 (KLF11 , also recognized as transforming growth factor-beta-inducible early gene 2) between the brains of 18 human subjects with MDD and 18 control subjects. We found that, indeed, the expression of KLF11 is increased by 36% (p<0.02) in the postmortem prefrontal cortex of human subjects with MDD compared with controls. We also observed a positive correlation between KLF11 levels and those of its target gene, MAO A, both in association with MDD. KLF11 protein expression was also increased by 44% (p<0.02) in the frontal cortex of KLF11 wild-type mice (Klf11+/+) vs Klf11−/− when both exposed to CSD stress. In contrast, locomotor activities, central box duration and sucrose preference were significantly reduced in the stressed Klf11+/+ mice, suggesting that Klf11+/+ mice are more severely affected by the stress model compared with Klf11−/− mice. These results serve to assign an important role of KLF11 in upregulating MAO A in MDD and chronic social stress, suggesting that inhibition of the pathways regulated by this transcription factor may aid in the therapeutics of neuropsychiatric illnesses. Thus, the new knowledge derived from the current study extends our understanding of transcriptional mechanisms that are operational in the pathophysiology of common human diseases and thus bears significant biomedical relevance.
INTRODUCTION
Major depressive disorder (MDD) is a highly prevalent psychiatric illness and is considered as one of the most burdensome diseases in the world (World Health Organization, 2002), which has become a leading cause of death and disability in middle-to-high income nations (World Health Organization, 2008). The average lifetime prevalence of MDD is 17% for all Americans (Krishnan, 2010), affecting a variety of people ranging from adolescents to senior citizens with different employment statuses, education levels, marital statuses, and race/ethnicity across the United States (Kessler et al, 2003). The Sequenced Treatment Alternatives to Relieve Depression study has revealed that the disorder is highly resistant to treatment, requiring new therapeutic approaches (Gaynes et al, 2008). Thus, many laboratories, including ours, are devoting significant efforts to identify novel relevant pathophysiological mechanisms underlying the development and progression of MDD, which can be targeted for therapeutic purposes.
The current study seeks to shed light on the potential contribution of the Monoamine oxidase A-Kruppel-like factor 11 (MAO A-KLF11) pathway to MDD using a design that includes analyses of the brain tissue from affected human subjects and a relevant animal model. MAO A, an enzyme that degrades monoamine neurotransmitters such as serotonin, norepinephrine, and dopamine (Bach et al, 1988), has a well-documented implication in major depressive disorder (Du et al, 2004; Lung et al, 2011; Meyer et al, 2006, 2009 ). Significant increase in the levels of MAO A is found in association with the pathogenesis of MDD (Meyer et al, 2006, 2009; Sherif et al, 1991). As neurotransmitters are oxidized by MAO, reactive oxygen species, such as hydrogen peroxide, are produced causing oxidative stress that impairs neuronal homeostasis (Maurel et al, 2003). Stress-induced MAO A expression is mediated by transcription factors, in particular, KLF11 (also identified as transforming growth factor-beta-inducible early gene 2 (TIEG2) (Grunewald et al, 2012).
KLF11 regulates the transcription of neuronal genes by binding to distinct sequences within their promoter region (Cook et al, 1998; Tachibana et al, 1997), triggering Pol II-mediated transcriptional initiation. Through this key biochemical mechanism KLF11 regulates multiple cellular processes including cell growth, differentiation, apoptosis, endocrine disorders, and malignancy (Buck et al, 2006; Cook et al, 1998; Fernandez-Zapico et al, 2003; Tachibana et al, 1997). Relevant to the current study, we have previously shown that KLF11 (Fernandez-Zapico et al, 2003; Zhang et al, 2001) is a robust transcriptional activator for MAO B (Lu et al, 2008; Ou et al, 2004), increasing the transcription of this gene via distinct GC-rich sites, which are located adjacent to its transcription start sites (Ou et al, 2004). Similarly, the MAO A promoter also contains Sp1-binding sites which have been recently reported to be a target of KLF11, increasing the expression of this gene (Grunewald et al, 2012). Unfortunately, however, in spite of this valuable biochemical information, the role that this protein plays in MDD remains to be defined.
Consequently, the current study was designed with the goal of helping to fill this important gap of knowledge in the pathogenesis of this common neuropsychiatric disorder. Our experiments find that: (1) KLF11 protein levels are elevated in human individuals with MDD; (2) Changes in KLF11 expression correlate with changes in MAO A, which has been shown to be elevated in human MDD; and (3) chronic social defeat (CSD) stress, in genetically engineered Klf11 mice, which is used as a model for MDD, increases both KLF11 and MAO A expression. Notably, our results support a pathophysiological role of the KLF11-MAO A pathway in the pathogenesis of MDD, a fact that should be taken into consideration for the future design of psychotherapeutic strategies for the treatment of this disorder.
MATERIALS AND METHODS
Human Subjects and Tissue Collection
The study was performed in concurrence with the declaration of Helsinki (Stockmeier et al, 2009) and Institutional Review Board policies at University Hospitals of Cleveland and the University of Mississippi Medical Center. Prefrontal cortex samples (Brodmann area 8/9; right hemisphere) were collected upon autopsy at the Cuyahoga County Coroner's Office (Cleveland, Ohio). The next-of-kin for the subjects provided written consent (Miguel-Hidalgo et al, 2006; Ou et al, 2010; Stockmeier et al, 2009). A trained interviewer administered the Structured Clinical Interview for DSM-IV Psychiatric Disorders to knowledgeable informants to determine current and lifetime Axis I psychopathology (First et al, 1996). The validity of diagnoses resulting from retrospective interviews concurs with diagnoses based on reviewing the subject's medical records (Deep-Soboslay et al, 2005; Dejong and Overholser, 2009; First et al, 1996; Johnson et al, 2011; Kelly and Mann, 1996).
Two groups were formed: 18 subjects that met the DSM-IV criteria for MDD (American Psychiatric Association (Table 1 and Supplementary Data Table 1), (Johnson et al, 2011), and psychiatrically normal control subjects (18) were matched intently to MDD subjects (Table 1 and Supplementary Data Table 1) (Johnson et al, 2011).
Table 1. Demographics, Sample Conditions, and Clinical Characteristics Data of Subjects with Major Depressive Disorder and Corresponding Psychiatrically Normal Control Subjects.
| Control (n=18) mean±SEM | MDD (n=18) mean±SEM | Statistica | P-value | |
|---|---|---|---|---|
| Age of onset (years) | NA | 46.4±4.7 | ||
| Deaths due to suicide | 0 | 12 (66.7%) | ||
| Age (years) | 49.6±3.4 | 54.6±4.7 | t34=0.87 | 0.3908 |
| Gender (%male) | 61.1% | 66.7% | χ21=0.12 | 0.7286 |
| Race (%AA) | 44.4% | 27.8% | χ21=1.08 | 0.2979 |
| PMI (h) | 19.8±2.0 | 20.7±1.6 | t34=0.34 | 0.7340 |
| Tissue pH | 6.60±0.08 | 6.56±0.07 | t34=0.35 | 0.7285 |
| Storage time (years) | 13.3±0.8 | 13.5±1.2 | t17=0.15 | 0.8782 |
| Smokers (%yes) | 9 (50.0) | 7(38.9) | χ21=0.45 | 0.5023 |
| KLF11/actin | 1.40±0.15 | 2.17±0.25 | t34=2.62 | 0.0131 |
Abbreviations: MDD, major depressive disorder; n, number of subjects; NA, not applicable; PMI (h), postmortem interval in hours; SEM, standard error of the mean; y, years.
Categorical data compared with χ2-test with 1 degree of freedom. General outcomes: there were no significant differences between cohorts for demographic variables (age, gender, race, PMI, pH, storage time, and smoking history). The detailed analysis for KLF11 (TIEG2): in the univariate analysis using Student's t-test and KLF11/actin ratio as the outcome, the mean value for subjects with MDD was significantly greater than control subjects (t34=2.62, p-value=0.0131). KLF11 levels were not significantly correlated with age, PMI, tissue pH, or storage time, nor did gender, race, or smoking status significantly affect KLF11 levels. There was no significant correlation between KLF11 levels and either age of onset, duration of illness, or number of depressive episodes (data not included). Among subjects with MDD, there is no significant difference in KLF11 levels between those dying by suicide (n=12, 2.15±0.31) and those not dying by suicide (n=6, 2.21±0.50)(t16=0.09, p-value=0.9256).
Continuous data compared with t-statistic with 34 degrees of freedom. Bold entries indicate that statistically significant elevation in KLF11 protein levels in the prefrontal cortex of MDD samples compared to controls was not accompanied by any change in demographic data between the groups.
MDD Subjects with Corresponding Control Subjects
Eighteen (18) psychiatrically normal control subjects
The average age (years) for this control group was 49.6±3.4, 11 subjects were male and 8 subjects were smokers (Table 1 and Supplementary Data Table 1A). Eight subjects were African American and 10 subjects were Caucasian. The average postmortem interval (PMI) prior to the collection of brain specimens was 19.6±1.89 (hours). The average pH of the brain tissue for this group was 6.58±0.08. The average freezer storage time at the time of this investigation was 11.67±3.40 (years). Assessment of postmortem blood and urine for the normal control subjects did not reveal the presence of psychoactive drugs. These control subjects were matched as closely as possible to the 18 subjects with MDD taking into account age, sex, race, PMI, pH, toxicology, smoking status, and freezer storage time (Supplementary Data Tables 2).
Eighteen (18) untreated subjects with MDD
The average age for subjects with MDD is 54.6±4.7 and the average age of onset for MDD in subjects was 46.41±4.7. Twelve subjects were male (Table 1 and Supplementary Data Table 1B). Sixty-seven percent (12/18) of the MDD subjects were victims of suicide (data not shown). All MDD subjects had postmortem toxicology screenings that were negative for the presence of antidepressant drug therapy. Fifteen MDD subjects (15/18) had not taken antidepressant drugs in the last 3 years before death. The remaining three MDD subjects were prescribed antidepressants within the last 30 days before death but were considered noncompliant as these medications were not detected in the postmortem toxicology screen (Johnson et al, 2011). Toxicology reports revealed positive blood alcohol content in three MDD subjects at the time of their deaths; nevertheless, these subjects were not diagnosed with alcohol use disorders, including alcohol dependence.
Additional information is disclosed for subjects with MDD (Supplementary Data Table 1C). The MDD exhibited by these subjects included classifications such as single/recurrent episodes with/without psychotic and/or melancholic features. Among the 18 MDD subjects, six subjects had recurrent major depressive episodes and 12 had MDD with a single depressive episode throughout their lifetime. In addition, eight of the MDD subjects were actively in a major depressive episode at the time of death and five MDD subjects were likely in a major depressive episode at death based on correlating onset, duration, and time of death; the remaining five MDD subjects were either not in a depressive episode at the time of death or relevant information was unavailable. The duration of illness (depression) for these subjects ranged from 1 month to 34 years. The duration of individual episodes ranged from 3 weeks to 15 years. The average number of depressive episodes was 1.4 (Supplementary Data Table 1C) (Johnson et al, 2011).
Western Blot Analysis
Protein lysates were obtained from the brain tissue samples of each subject (human) following homogenization in a 0.5 ml solution containing 1 mM EDTA, 10 mM Tris-HCL, and fresh protease inhibitor (Sigma), and centrifuged at 4 °C (550 g) for 10 min. Forty micrograms (40 μg) of total protein were separated by 10.5% SDS-polyacrylamide gel electrophoresis. After transfer, membranes were incubated with mouse anti-TIEG2 antibody (1:500; BD Transduction Laboratory; 611402) or mouse anti-MAO A antibody (clone G-10, 1: 250; Santa Cruz Biotechnology) overnight at 4 °C and anti-mouse secondary antibody at room temperature for 2 h.
An equal number of samples from each group (control and MDD human subjects) were immunoblotted on separate membranes as duplicates. Protein bands were visualized by the ChemiDoc XRS+ Imaging System (Bio-Rad). Expression of β-actin was also quantified from stripped membranes (immunoblotted for the determination of KLF11, or MAO A, respectively) to establish loading controls. The band intensities for KLF11 or MAO A were calculated and normalized to the band intensities of β-actin using Quantity One analysis software. Using increasing protein concentrations from a human control subject, a standard curve was established for KLF11, which exhibited a linear relationship relative to the total protein concentration as shown in Supplementary Data Figure 1 (Johnson et al, 2011; Ou et al, 2010).
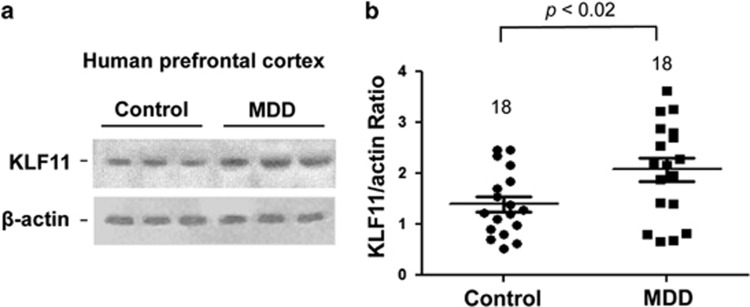
Figure 1.
Western blot analysis of KLF11 (TIEG2) protein levels in the human postmortem prefrontal cortex of 18 subjects with major depressive disorder (MDD) vs 18 non-affected, psychiatrically normal control subjects. (a) A representative immunoblot showing three healthy controls and three MDD subjects is shown. β-actin was used as the loading control. (b) Quantitative analysis. Each KLF11 band was evaluated by its relative intensity and normalized to the density of β-actin. Graphs of the average optical density of KLF11/actin for the individual subjects (solid circles or squares) and mean values (horizontal lines) are shown.
Statistical Analysis of Human Subjects
Student's t-test for two group comparison was utilized in the evaluation of statistical significance. The data were reported as mean±SEM, and a value of p<0.05 was considered statistically significant. The potential influence of age, sex, race, smoking habit, PMI, tissue pH, tissue storage time on KLF11, duration of illness, and number of depressive episodes were examined by linear regression (Table 1).
Animal Subjects
The Klf11 homozygous knockout (Klf11−/−) model was generated at the University of Washington (Seattle, WA) following homologous recombination techniques to inactivate the endogenous Klf11 gene in embryonic stem cells, generating chimeras, and isolating colony founders carrying the knockout gene (Bonnefond et al, 2011). These animals were originally generated in a mixed background and subsequently transferred to the Mayo Animal Facilities (Rochester, MN) where it was crossed back into a pure C57BL/6 background for >20 generations to produce the inbred strain used in this study. Klf11 wild-type (Klf11+/+) and knockout mice (Klf11−/−)(C57BL6) were obtain from Mayo Clinic. CD1 breeder mice were obtained from Harlan Laboratories. Mice were housed at a constant temperature with a 12 h light/dark cycle with ad libitum food and water. In all of the experiments, male Klf11−/− animals were compared with age-matched male Klf11+/+ littermates. All procedures were in accordance with the University of Mississippi Medical Center IACUC (protocol # 1316).
CSD Stress
Mice (2–3 months old) were subjected to social defeat stress for 10 consecutive days. Each mouse was introduced into the home cage of an unfamiliar resident for 5 min; during this time the mice were physically defeated. Unfamiliar resident mice were CD1 breeders; these mice are well-known for their consistently short-attack latencies. Once submissive posturing was exhibited, mice were kept in the same cages with the unfamiliar resident for 24 h separated by perforated plexiglass partitions in the resident's home cage allowing for sensory contact. Each day, experimental mice were exposed to new resident mice (Berton, 2006).
Behavioral Evaluation
Open field
One day after the last session of social defeat stress, mice were placed in a 40 × 40 × 40 cm box with opaque walls and allowed to roam freely for 30 min during which their activities were recorded with a digital camera and analyzed with the Noldus Ethovision 8.5 Software. Total distance travelled, immobility time, and duration in the central box were analyzed to indicate the depressive behaviors (Prut and Belzung, 2003; Rygula et al, 2005)
Sucrose preference
Sucrose preference was performed during the dark phase. The mice were trained to task by three presentations of sweetened vs tap water during the course of 1 week. Following 4 h water restriction, mice were given 1 h access to two bottles: sucrose (1% during training initiation for 3 days before chronic social defeat; mice were then transitioned to 2% sucrose for testing during 10-day's CSD on day 1, day 5, and finally on day 10) and tap water placed side by side. Bottle position was reversed with each presentation (Peng et al, 2012; Rygula et al, 2005). Fluid consumption was measured by subtracting the drinking bottle weight following presentation from the initial filled bottle weight. Baseline preference was determined 1 h prior to the onset of stress (day 1) and the final preference tests were conducted on day 10 of CSD stress.
Quantitative Real-time RT-PCR
Using mice brain tissue, mRNA was extracted with TRIzol reagent (Invitrogen) and reverse transcription was conducted with the SuperScript III first-strand synthesis system (Invitrogen). Resultant cDNA was quantified with the iCycler MyiQ real-time PCR detection system (Bio-Rad) using mice KLF11 and MAO A primers and the 18 S ribosomal RNA primer as an internal control (Grunewald et al, 2012).
MAO A Catalytic Activity Assay
Mice brain tissue was homogenized in 50 mM sodium phosphate buffer. For each sample, 100 μl of total protein was incubated with 100 μM [14C]5-hydroxytryptamine (in sodium phosphate buffer) at 37 °C for 20 min. One hundred microliter of 6 N HCl was added to discontinue the reaction. Subsequently, reaction products were extracted with a benzene/ethyl acetate mixture and radioactivity was determined by liquid scintillation spectroscopy (Grunewald et al, 2012).
Triple-label Immunofluorescence
Triple immune-labelling was performed to reveal the cell-type-specific expression of MAO A, with specific cell phenotype markers NeuN (for neuronal) and GFAP (for astrocytes) in human prefrontal cortex. Sections were blocked with 5% goat serum and incubated with rabbit anti-MAO A (H-70, 1:100, Santa Cruz, CA) antibody with mouse anti-NeuN monoclonal (1:500; Millipore, Billerica, MA) and chicken anti-GFAP polyclonal (1:1000; Abcam, Cambridge, England). Following primary antibody incubation, sections were visualized by incubation with FITC-conjugated Goat anti-Rabbit IgG (1:1000, Vector Laboratories, Burlingame, CA), Cy3-conjugated AffiniPure Goat anti-Chicken (1:1000, Jackson ImmunoResearch, West Grove, PA), and Cy5-conjugated Goat anti-mouse IgG. Last, all sections were rinsed with PBS and mounted with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA).
Statistical Analysis of Animal Subjects
Statistical significance was evaluated using student t-test for two group comparison or analysis of variance followed by Bonferroni adjusted tests when comparing more than two groups. A value of p<0.05 was considered significant.
RESULTS
Upregulation of the KLF11-MAO A Pathway in the Prefrontal Cortex is a Biochemical Feature of MDD in Humans
KLF11 is known to inhibit cell growth (Buck et al, 2006; Cook et al, 1998), and increase oxidative stress (Neve et al, 2005) and apoptosis (Tachibana et al, 1997; Wang et al, 2007); which are mechanisms known to contribute to the development and progression of MDD. We hypothesized that KLF11 may be elevated in postmortem brain tissue from subjects with MDD because of the reduced prefrontal cortex volume, signs of cell death, and oxidative damage that have been reported in the brains of depressive patients (Andreazza et al, 2010; Duman and Monteggia, 2006; Dwivedi et al, 2009), including patients with MDD (Duman and Monteggia, 2006; Dwivedi et al, 2009; Gawryluk et al, 2011; Kang et al, 2007). In addition, the significantly increased MAO A levels have been observed in the brains of human MDD subjects compared with healthy control subjects (Du et al, 2004; Johnson et al, 2011; Lung et al, 2011; Meyer et al, 2006, 2009). However, whether the expression of KLF11 is altered in this disease remains to be defined.
Consequently, we measured the levels of the KLF11 protein in both the postmortem prefrontal cortex of 18 subjects with MDD and 18 psychiatrically normal control subjects matched closely for age, sex, smoking habits, and other conditions (Johnson et al, 2011). The results of these experiments show that KLF11 levels were increased by 36% (p<0.02) in subjects with MDD compared with control subjects (Figure 1a and b) as determined by western blot analysis. In additon, using linear regression analyses, we evaluated the potential influence of age, sex, race, smoking habit, PMI, tissue pH, tissue storage time on KLF11, duration of illness, number of depressive episodes, and suicide. Interestingly, we find that KLF11 levels do not correlate with any demographic features of MDD (Table 1). Thus, KLF11 is a prevalent change of MDD regardless of the disease stage and/or patient characteristics.
We sought to determine the relationship between the levels of KLF11 and MAO A in MDD subjects using western blot analyses. Figure 2a shows that the levels of the MAO A protein are increased (28% increase, p<0.02) in the prefrontal cortex of subjects with MDD. Interestingly, statistical analysis shows a significant positive correlation between the protein expression levels of KLF11 and MAO A (p<0.03, Figure 2b).
Figure 2.
Western blot analysis of MAO A in the human postmortem prefrontal cortex of 18 subjects with major depressive disorder (MDD) vs 18 non-affected control subjects. (a) Quantitative analysis. Each MAO A band was evaluated by its relative intensity and normalized to the density of β-actin. Graphs of the average optical density of MAO A/actin for the individual subjects (solid circles or squares) and mean values (horizontal lines) are shown. (b) Graphic representation demonstrating the positive correlation between KLF11 and MAO A levels in psychiatrically normal control subjects (black circles) and subjects with MDD. (c) Representative images showing cell phenotype-specific localization of MAO A in neurons (NeuN positive) and astrocytes (GFAP positive) in the prefrontal cortex of the postmortem human brain.
We have recently demonstrated that KLF11 is specifically expressed in both neurons and astrocytes in prefrontal cortex of postmortem brain (Udemgba et al, 2014). In this study, we identified that the majority of MAO A immunoreactivity is localized in astrocytes with a weaker signal in neurons in the prefrontal cortex of controls and those with MDD (Figure 2c). This result is consistent with others showing that MAO A is expressed in astrocytes and neurons (Konradi et al, 1987; Riederer et al, 1987; Saura et al, 1992).
Evidence for the Upregulation of the KLF11-MAO A Pathway in the Brains of a relevant murine model of MDD
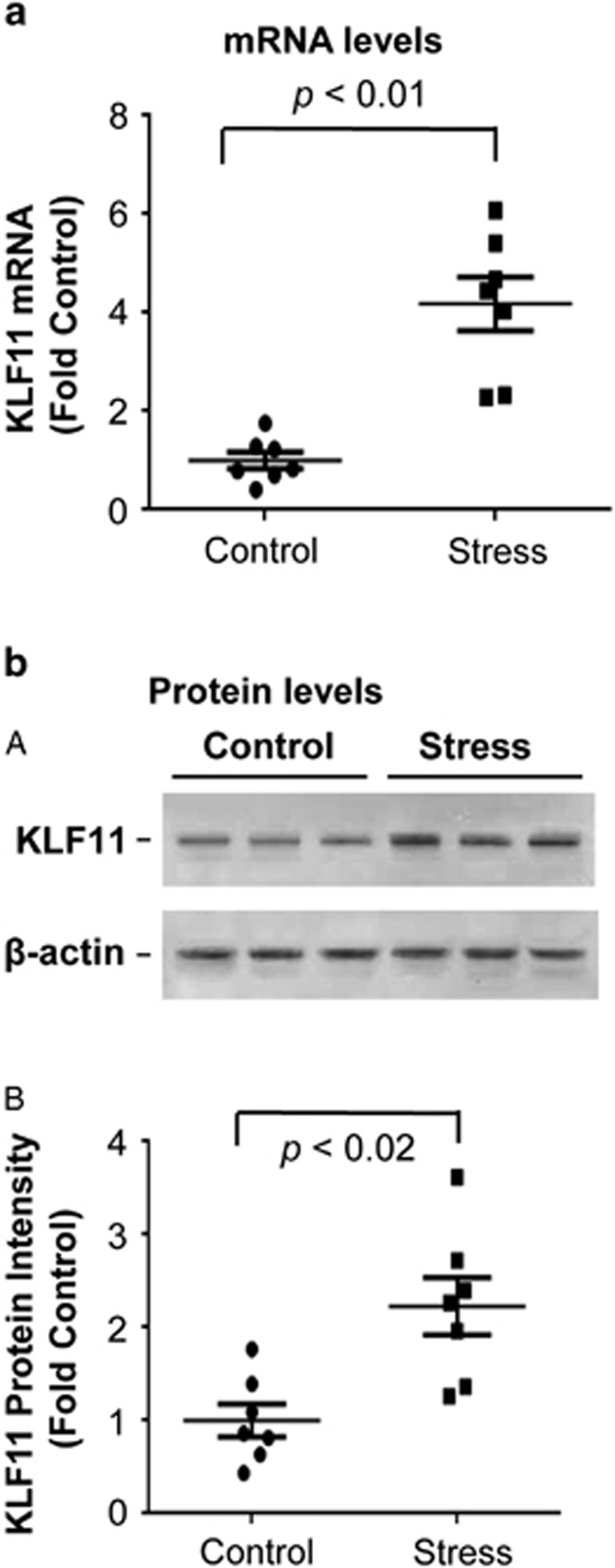
The expression of mRNA of KLF11 and MAO A in the prefrontal cortex of postmortem brains of subjects with MDD would provide important insights for MDD-related regulation on this pathway at transcriptional level. Owing to the limitation to obtain high-quality mRNA from the long term-freezer storage postmortem samples, we analyzed the stress-regulated KLF11-MAO A pathway using the mRNA and protein from the prefrontal cortex of mice following exposure to CSD stress (Figure 3). We found that KLF11 mRNA levels are increased by approximately fourfold (p<0.01) in the frontal cortex of mice following CSD stress compared with control mice (Figure 3a, n=7 mice per group). Congruently, the levels of KLF11 protein were more than doubled of the basal control values (p<0.02) in the frontal cortex of stressed mice (Figure 3b). In parallel, both MAO A mRNA and enzymatic activity were increased in the frontal cortex of stressed mice (Figure 4, lane 2 vs 1 in A and B). Together, these results reveal that the regulation of the normal levels of both KLF11 and its target, MAO A are impaired in diseased human brain in a manner that is recapitulated by a murine model for this disorder.
Figure 3.
Analysis of KLF11 in the frontal cortex of seven mice following exposure to chronic social defeat (CSD) stress vs seven control mice. (a) Quantitative analysis. Real-time RT-PCR results of KLF11 mRNA are shown for mice exposed to CSD stress. (b) Quantitative analysis. (A) A representative immunoblot of KLF11 and β-actin is shown for three control mice and three stressed mice is shown. (B) Each KLF11 band was assessed based on its relative intensity and normalized to β-actin.
Figure 4.
Analysis of MAO A in the frontal cortex of KLF11-wild-type and knockout mice following exposure to chronic social defeat (CSD) stress. (a) Quantitative analysis. Real-time RT-PCR results assessing MAO A mRNA are shown. (b) Quantitative analysis. Catalytic activity levels are illustrated for both control and stressed mice.
Genetic Inactivation of KLF11 results in reduced MAO A Expression After CSD Stress
To better elucidate the role of KLF11 in stress-induced MAO A expression, we assessed the levels and enzymatic activity of the MAO A protein in the frontal cortex of seven KLF11-wild-type Klf11 +/+ and seven Klf11 −/− mice following CSD stress (Figure 4). Figure 4a illustrates that, MAO A mRNA levels were increased (by 3.7-fold, p<0.01, lane 2 vs 1) in Klf11 +/+ mice after exposure to CSD. In addition, we observe that Klf11−/− mice exhibited reduced MAO A mRNA levels compared with wild-type mice following CSD (by 3.4-fold, p<0.01, lane 4 vs 2). Likewise, MAO A catalytic activity was significantly increased in Klf11 +/+ mice upon exposed to chronic social stress compared with stress-free Klf11 +/+ mice by 24% (p<0.05, Figure 4b, lane 2 vs 1). However, MAO A catalytic activity was not significantly increased (only by 12%) in Klf11−/− mice after CSD compared with control Klf11−/− mice (Figure 4b, lanes 4 vs 3). Last, CSD significantly reduced MAO A catalytic activity in Klf11−/− mice as compared with Klf11+/+ by 36% (p<0.02, Figure 4b, lane 4 vs 2). Therefore, these mechanistic experiments demonstrate that the inactivation of KLF11 leads to an impairment in its target gene, MAO A.
Mice Carrying a Genetic Inactivation of KLF11 Exhibit Significantly Less Depressive-like Behavior Following Chronic Stress Exposure
Experimental mice live in social groups and small cages. In the open-field test, mice are separated from their social group and in an anxiety-provoking condition. Therefore, the CSD-stressed mice decrease their locomotor activities and exploratory behavior (Prut and Belzung, 2003; Rygula et al, 2005). In addition, stress-induced reduction of sucrose preference is another common characteristic that assesses anhedonia in animal models of chronic stress (Peng et al, 2012; Rygula et al, 2005; Tang et al, 2013). To further establish whether KLF11 behaves as a gene modifier for the manifestation of stress and depression, we determined the effect of CSD stress on locomotor activities, central box exploration, and sucrose preference in both Klf11−/− and Klf11+/+ mice.
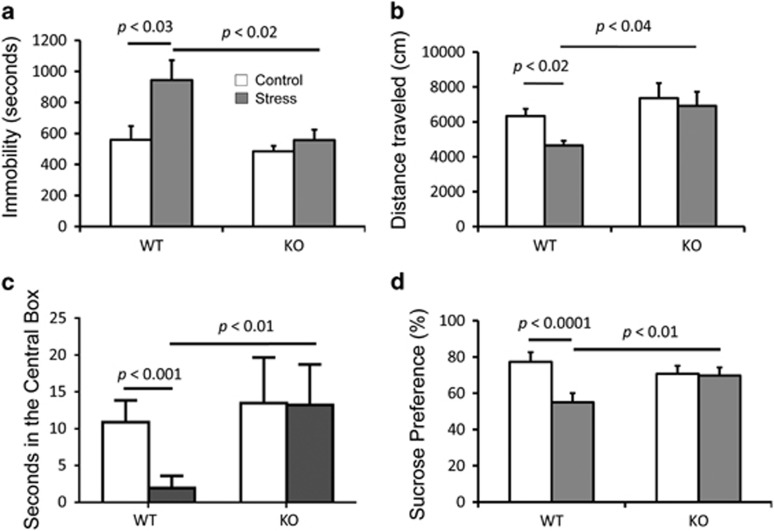
These experiments show that Klf11+/+mice display a significant increase in immobility (Figure 5a, p<0.01, n=7 mice per group), decreased total distance traveled (Figure 5b, p<0.02), and reduced time in the central box (Figures 5c, p<0.001) in the open-field test. The Klf11+/+mice also showed a significantly reduced sucrose preference upon completion of the CSD period (Figures 5d, p<0.0001). In contrast, Klf11−/− mice displayed no change in depressive-like behavior following CSD stress compared with control. There was also a slight reduction in sucrose preference in control Klf11−/− mice compared with control Klf11 +/+ mice, nonetheless, this difference was not statistically significant. Thus, as predicted by our hypothesis, disruption of the key MAO A regulator KLF11 confers resilience to depression-like symptoms in an animal model relevant to the study of MDD.
Figure 5.
Evaluation of the depressive behaviors. (a) Immobility, (b) total distance traveled, and (c) time in central box in open-field tests and (d) sucrose preference of KLF11-wild-type and knockout mice following exposure to chronic social defeat (CSD) stress. Open-field tests were performed 1 day after the sucrose preference tests that were performed on day 10 of the chronic social defeat procedure and compared with baseline.
DISCUSSION
Abnormal monoamine oxidase expression is associated with several common psychiatric disorders and neurodegenerative diseases (Cases et al, 1995; Du et al, 2002, 2004; Meyer et al, 2006, 2009; Ou et al, 2010; Sacher et al, 2010; Shih et al, 1999; Youdim et al, 2006). Therefore, understanding the molecular mechanisms that regulate MAO A expression and enzymatic function is crucial for advancing the clinical treatment of depressive disorders. This study supports the role of the transcription factor, KLF11, in the manifestation of MDD by gathering data directly from human postmortem brain of subjects diagnosed with MDD and the brains of mice exposed to CSD.
Previous evidence has characterized KLF11 as a transcriptional regulator of MAO A via the binding of this protein to GC-rich, Sp1-like binding sites located on the promoter of this gene (Grunewald et al, 2012). Various studies have implicated alterations in MAO A and depressive disorders (Du et al, 2002, 2004; Fan et al, 2010; Sacher et al, 2010). More specifically, positron emission tomography (PET) and carbon 11-labeled harmine measurements in the brain from living patients with MDD show that MAO A is significantly elevated in major depressive episodes (Meyer et al, 2006, 2009). The results reported in the current study illustrates that KLF11 protein levels are also significantly increased in the brains of postmortem human subjects with MDD, implicating an upregulation of KLF11 in the pathogenesis of MDD. Moreover, the increased KLF11 seen in MDD positively correlates with MAO A expression, further supporting our previous findings that KLF11 is a transcriptional activator for MAO A.
It has been reported that cigarette smoke reduces brain MAO A activity (Bacher et al, 2011; Berlin et al, 1995; Fowler et al, 1996). In the current study, we have not observed a significant reduction of MAO A protein expression in smokers either in the MDD cohort or the controls with non-smokers (Supplementary Figure 2C, D). The reason for this discrepancy may be because in the current work, we measured the content of MAO A protein in homogenates of frontal cortex from postmortem brain. While those who found reduction of MAO A activity in heavy smokers analyzed the binding of MAO A inhibitors, such as the [11C]clorgyline (Fowler et al, 1996) or [11C]harmine (Bacher et al, 2011). Cigarettes contain harmines, and harmine has a Ki of ∼50 nM for MAO-A (Bacher et al, 2011). Therefore, there is some occupancy of MAO-A by Harmine in the brains of smokers. When the level of brain MAO A was measured using [11C]clorgyline or [11C]harmine binding with PET, it was likely reduced owing to occupancy by harmine from cigarette smoke. Therefore, our results may suggest that cigarette smoking may not alter MAO A content itself, but inhibitors in cigarette smoke reduce the amount of free MAO A available to bind to PET ligands in vivo. In addition, it is reported that, in withdrawal, heavy cigarette-smoking subjects (⩾25 cigarettes/day) did not show a reduction of MAO-A binding as compared with healthy subjects (Bacher et al, 2011). This implies that in withdrawal, more free MAO A is available for binding to the PET ligand. In our study, the average amount of cigarettes smoked in the control group (1.5 packs/day, n=8) and in the MDD cohort (1.38 packs/day, n=6, one of the smoker has no record for the amount of cigarettes smoked) are similar. Therefore, we have not observed a significant effect of cigarettes smoked on MAO A protein expression after rigorous analysis.
This study also demonstrates that the levels of both the KLF11 mRNA and protein are upregulated in the frontal cortex of mice following CSD stress, an accepted animal model for depression (Czeh et al, 2007; Rygula et al, 2005; Vialou et al, 2010). Moreover, MAO A levels are decreased in KLF11 knockout mice after exposure to CSD stress, further supporting the role of this transcription factor as an activator for MAO A. Last, KLF11-wild-type mice had reduced locomotor activities, sucrose preference, increased anxiety (less time in the central box of an open-field) compared with KLF11-knockout mice following the CSD stress, indicating that KLF11-knockout mice exhibit less depressive-like behavior. Collectively, these findings document that the KLF11-MAO A pathway is altered at the level of both the neurotransmitter metabolism enzyme and its regulator, which bears implications for better understanding pathophysiological mechanisms underlying MDD. In addition, it is likely that compounds to modulate the levels of expression and function of the KLF11-MAO-mediated pathway may increase neuroprotection, neuroplasticity, and synaptic activities. Maximizing the therapeutic effects upon these targets is also essential toward achieving comprehensive management of stress-induced, frequently treatment-resistant, psychiatric illnesses, and addictions (Barr et al, 2004; Beasley et al, 2005; Dwivedi et al, 2006; Frazer, 1997; Mitchell et al, 2012; Sanacora, 2008; Sawada et al, 2005; Silberman et al, 2009; Wallace et al, 2007). Thus, the new knowledge generated by the current study has mechanistic relevance and also provides the rationale for targeting this pathway to develop potential novel therapeutics approaches in the treatment of MDD.
FUNDING AND DISCLOSURE
This research was supported by Public Health Service Grants AA020103, P30 GM103328, MH67996, The Brain & Behavior Research Foundation (NARSAD), The Canadian Institutes of Health Research, and an Intramural Research Support grant from the University of Mississippi Medical Center. Dr Meyer has applied for a patent to use MAO measures to diagnose or identify subtypes of MDD. He has had operating grant support/consultation with GlaxoSmithKline, BristolMyersSquibb, Lundbeck, SK Life Sciences, Teva, and Eli-Lilly. The remaining authors declare no conflict of interest.
Acknowledgments
We appreciatively acknowledge the invaluable contributions made by the families consenting to brain tissue donations and interviews and the Cuyahoga County Coroner's office, Cleveland, Ohio. We thank Dr Herbert Y Meltzer for assistance in the psychiatric assessment of volunteers, as well as Nicole Herbst, Timothy De Jong, Shawnnette Nelson, and Nicole Peak for their assistance with human tissue and written consents and Dr Gouri Mahajan for facilitating tissue preparation.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA, et al. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch Gen Psychiatry. 2011;68:817–826. doi: 10.1001/archgenpsychiatry.2011.82. [DOI] [PubMed] [Google Scholar]
- Barr AM, Young CE, Sawada K, Trimble WS, Phillips AG, Honer WG. Abnormalities of presynaptic protein CDCrel-1 in striatum of rats reared in social isolation: relevance to neural connectivity in schizophrenia. Eur J Neurosci. 2004;20:303–307. doi: 10.1111/j.0953-816X.2004.03457.x. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honer WG, Bergmann K, Falkai P, Lutjohann D, Bayer TA. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7:449–455. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase A and B activities in heavy smokers. Biol Psychiatry. 1995;38:756–761. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Lomberk G, Buttar N, Busiah K, Vaillant E, Lobbens S, et al. Disruption of a novel Kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 INS mutation found in neonatal diabetes mellitus. J Biol Chem. 2011;286:28414–28424. doi: 10.1074/jbc.M110.215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck A, Buchholz M, Wagner M, Adler G, Gress T, Ellenrieder V. The tumor suppressor KLF11 mediates a novel mechanism in transforming growth factor beta-induced growth inhibition that is inactivated in pancreatic cancer. Mol Cancer Res. 2006;4:861–872. doi: 10.1158/1541-7786.MCR-06-0081. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-beta-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–25936. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, et al. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dejong TM, Overholser JC. Assessment of depression and suicidal actions: agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39:38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Ravindran A, Hrdina PD. MAO-A gene polymorphisms are associated with major depression and sleep disturbance in males. Neuroreport. 2004;15:2097–2101. doi: 10.1097/00001756-200409150-00020. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, Sotonyi P, Bakish D, Hrdina PD. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, et al. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch Gen Psychiatry. 2006;63:639–648. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, et al. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Liu B, Jiang T, Jiang X, Zhao H, Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Mladek A, Ellenrieder V, Folch-Puy E, Miller L, Urrutia R. An mSin3A interaction domain links the transcriptional activity of KLF11 with its role in growth regulation. EMBO J. 2003;22:4748–4758. doi: 10.1093/emboj/cdg470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW.1996Structured Clinical Interview for DSM-IV Axis I Disorders—Patient EditionVersion 2.0. Biometrics Research Department, New York State Psychiatric Institute: New York, NY, USA [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17 (Suppl 1:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75:57–66. doi: 10.3949/ccjm.75.1.57. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Stockmeier CA, Meyer JH, Austin MC, Albert PR, Wang J, et al. The reduction of R1, a novel repressor protein for monoamine oxidase a, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Konradi C, Riederer P, Jellinger K, Denney R. Cellular action of MAO inhibitors. J Neural Transm Suppl. 1987;25:15–25. [PubMed] [Google Scholar]
- Krishnan R.2010Epidemiology, Pathogenesis, and Neurobiology of DepressionIn: Basow DS edsUpToDate: Waltham, MA, USA [Google Scholar]
- Lu D, Johnson C, Johnson S, Tazik S, Ou XM. The neuroprotective effect of antidepressant drug via inhibition of TIEG2-MAO B mediated cell death. Drug Discov Ther. 2008;2:289–295. [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Tzeng DS, Huang MF, Lee MB. Association of the MAOA promoter uVNTR polymorphism with suicide attempts in patients with major depressive disorder. BMC Med Genet. 2011;12:74. doi: 10.1186/1471-2350-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, et al. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol. 2003;284:H1460–H1467. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. 2009;66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Grossman LE, Coker AR, Messing RO. The anticonvulsant levetiracetam potentiates alcohol consumption in non-treatment seeking alcohol abusers. J Clin Psychopharmacol. 2012;32:269–272. doi: 10.1097/JCP.0b013e318248ba69. [DOI] [PubMed] [Google Scholar]
- Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci USA. 2005;102:4807–4812. doi: 10.1073/pnas.0409177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279:21021–21028. doi: 10.1074/jbc.M312638200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, et al. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatry. 2010;67:855–863. doi: 10.1016/j.biopsych.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YL, Liu YN, Liu L, Wang X, Jiang CL, Wang YX. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflammation. 2012;9:75. doi: 10.1186/1742-2094-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Riederer P, Konradi C, Schay V, Kienzl E, Birkmayer G, Danielczyk W, et al. Localization of MAO-A and MAO-B in human brain: a step in understanding the therapeutic action of L-deprenyl. Adv Neurol. 1987;45:111–118. [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry. 2010;67:468–474. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- Sanacora G. New understanding of mechanisms of action of bipolar medications. J Clin Psychiatry. 2008;69 (Suppl 5:22–27. [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3 H-Ro 41-1049 and 3 H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, et al. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2005;62:263–272. doi: 10.1001/archpsyc.62.3.263. [DOI] [PubMed] [Google Scholar]
- Sherif F, Marcusson J, Oreland L. Brain gamma-aminobutyrate transaminase and monoamine oxidase activities in suicide victims. Eur Arch Psychiatry Clin Neurosci. 1991;241:139–144. doi: 10.1007/BF02219712. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, et al. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, Clarke G, Friedman L, et al. Antagonist but not agonist labeling of serotonin-1 A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43:887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana I, Imoto M, Adjei PN, Gores GJ, Subramaniam M, Spelsberg TC, et al. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Investig. 1997;99:2365–2374. doi: 10.1172/JCI119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Lei J, Sun X, Liu G, Zhao S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013;1513:127–134. doi: 10.1016/j.brainres.2013.03.042. [DOI] [PubMed] [Google Scholar]
- Udemgba C, Johnson S, Stockmeier CA, Luo J, Albert PR, Wang J, et al. The expression of KLF11 (TIEG2), a monoamine oxidase B transcriptional activator in the prefrontal cortex of human alcohol dependence. Alcohol Clin Exp Res. 2014;38:144–151. doi: 10.1111/acer.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, et al. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J Neurosci. 2010;30:14585–14592. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, et al. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- Wang Z, Spittau B, Behrendt M, Peters B, Krieglstein K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-XL expression. J Neural Transm. 2007;114:867–875. doi: 10.1007/s00702-007-0635-6. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.