Abstract
Uncontrolled canonical Wnt signaling supports colon epithelial tumor expansion and malignant transformation. Understanding the regulatory mechanisms involved is crucial for elucidating the pathogenesis of and will provide new therapeutic targets for colon cancer. Epsins are ubiquitin-binding adaptor proteins upregulated in several human cancers; however, epsins’ involvement in colon cancer is unknown. Here we show that loss of intestinal epithelial epsins protects against colon cancer by significantly reducing the stability of the crucial Wnt signaling effector, dishevelled (Dvl2), and impairing Wnt signaling. Consistently, epsins and Dvl2 are correspondingly upregulated in colon cancer. Mechanistically, epsin binds Dvl2 via its epsin N-terminal homology domain and ubiquitin-interacting motifs and prohibits Dvl2 polyubiquitination and degradation. Our findings reveal an unconventional role for epsins in stabilizing Dvl2 and potentiating Wnt signaling in colon cancer cells to ensure robust colon cancer progression. Epsins’ pro-carcinogenic role suggests they are potential therapeutic targets to combat colon cancer.
Colon cancer is one of the most commonly diagnosed cancers in the United States and, although significant advances in early diagnosis and treatment have been made in the last several years, it remains a leading cause of cancer-related death (American Cancer Society). Future advances in the treatment of this disease depend heavily on a better understanding of the signaling cascades and molecular mechanisms involved in tumor development and progression. The canonical Wnt signaling cascade is known to play an important role in this process1,2. Under physiologic conditions, canonical Wnt signaling activation disassembles the Axin/GSK-3/APC destruction complex, ultimately resulting in β-catenin stabilization and nuclear accumulation3–7. Nuclear β-catenin binds to and activates the TCF/LEF transcription factors resulting in the expression of important cell proliferation factors, including cMyc and cyclin D18–11. Several mutations in Axin, GSK-3, APC and β-catenin genes cause significant deregulated nuclear β-catenin accumulation, uncontrolled cell proliferation and malignant transformation in the colon epithelium4,12–17.
In addition to deregulated nuclear β-catenin accumulation, other alterations in canonical Wnt signaling are also associated with cancer development such as the overexpression of several key upstream regulators including Wnt receptors (Frizzled (Fzd) and low density lipoprotein receptor-related protein (LRP)) and cytoplasmic signaling adaptor, dishevelled (Dvl)18–22. Dvl is recruited to the heterodimeric Wnt receptor complex, consisting of a single Fzd and LRP, in response to ligand stimulation and plays a crucial role in mediating Axin/GSK-3/APC destruction complex disassembly23,24. Given that both the Wnt receptors and Dvl are upregulated in cancer and result in elevated canonical Wnt signaling, it has been hypothesized that alterations in the stabilization and/or internalization of the Fzd:LRP:Dvl complex may contribute to the aberrant canonical Wnt signaling linked to colon cancer development25–27.
Previous studies reported that Wnt signaling is activated by endocytosis25,27,28. In 2010, Dr. Robertis’s group demonstrated that Wnt-dependent internalization of the Fzd:LRP:Dvl complex is an important step in activating Wnt signaling; however, the regulatory mechanisms involved are still unknown26. Our lab has a long-standing interest in the regulated internalization of cell surface receptors, specifically epsins’ role in this mechanism. We speculated that epsins may activate Wnt signaling by regulating Wnt-induced receptor complex endocytosis. Epsins are classically defined as clathrin-mediated endocytic adaptors that facilitate the internalization of ubiquitinated cell surface receptors29–33. Mammals express three highly conserved epsins; epsins 1 and 2, which are ubiquitously expressed in most cell types, and epsin 3, which is primarily expressed in the skin and stomach30,31,34–36. Epsins 1 and 2 are redundant in function as indicated by the fact that mice lacking either epsin 1 or 2 survive normally with no obvious phenotypic alterations while mice lacking both epsins 1 and 2 die in utero34. In order to bypass embryonic lethality, we previously generated a conditional epsin 1 mouse on an epsin 2 null background37. Importantly, these mice, which undergo post-developmental epsin 1 deletion, survive normally under physiologic conditions. Interestingly, we recently reported that in pathological conditions, such as cancer, loss of endothelial epsins altered tumor angiogenesis resulting in reduced tumor growth and progression37,38. In addition to playing a role in regulating the tumor microenvironment, epsins are upregulated in skin, mammary, and prostate tumors36,39–41. However, whether and how epsins promote colon cancer development are important and open questions.
In this study, we investigate the potential role epsins play in regulating colon cancer development using a unique colon cancer mouse model with inducible intestinal epithelial cell (IEpC)-specific epsin deficiency. We discover that epsin deficiency impairs canonical Wnt signaling and results in a colon cancer-resistant phenotype. Surprisingly, mechanistic analyses reveal that epsins are dispensable for the Wnt-induced endocytosis of receptors. Instead, epsins constitutively interact with and protect Dvl2 from polyubiquitination and proteasomal degradation, making them an essential regulator of Dvl2 stability. Without epsins, the Dvl2 protein pool is diminished thus preventing downstream canonical Wnt signaling activation. This novel role for epsins as a critical chaperone protein is completely unexpected. Because Dvl2 is reportedly upregulated in and contributes to cancer progression19,21,22, we propose a model in which epsin upregulation during the early stages of tumorigenesis promotes Dvl2 stabilization, deregulates canonical Wnt signaling and facilitates colon cancer development21,22.
Results
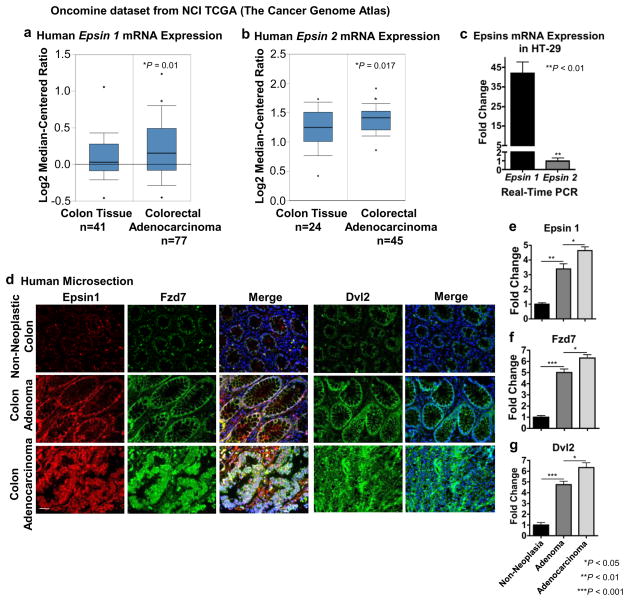
Epsins are upregulated in human colon cancer
We previously established that epsins play an important oncogenic role in regulating tumor microenvironments37; however, whether epsins played a specific role in promoting tumor cell transformation and proliferation was largely unknown. Using an Oncomine database from The National Cancer Institute (NCI) Cancer Genome Atlas containing publically available cDNA microarray data, we found that the gene expression of epsin 1 and epsin 2 were significantly upregulated in human colon cancer (Fig. 1a,b). Given that epsin 1 gene expression was significantly higher than epsin 2 in the HT-29 human colorectal adenocarcinoma cell line (Fig. 1c)14,42, we used immunofluorescence to analyze epsin 1 protein in human tissue microarrays containing colon adenomas, adenocarcinomas and corresponding non-neoplastic tissues (Fig. 1d). Epsin 1 upregulation correlated strongly with increased colon cancer severity (Fig. 1d,e)43. Epsin 1 upregulation also correlated strongly with increased levels of the Wnt receptor, Fzd7 (Fig. 1d,f), and downstream effector, Dvl2 (Fig. 1d,g).
Figure 1. Epsins were augmented in human colon cancer.
(a,b) Human epsin 1 (a) and epsin 2 (b) mRNA expression levels in normal colon and colorectal adenocarcinoma tissues. Data was retrieved from NCI The Cancer Genome Atlas (TCGA) Oncomine dataset. (c) qRT-PCR analysis of epsin 1 and epsin 2 mRNA expression in HT-29 human colon cancer cells, n=9. (d) Representative immunofluorescence images of epsin 1, Fzd7, and Dvl2 in peripheral non-neoplastic, adenoma and adenocarcinoma colon tissues from an AccuMax human tissue microarray. Scale bar: 50 μM. (e,f,g) Quantification of d, n=12. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
Intestinal epithelial epsins loss prohibits colon cancer
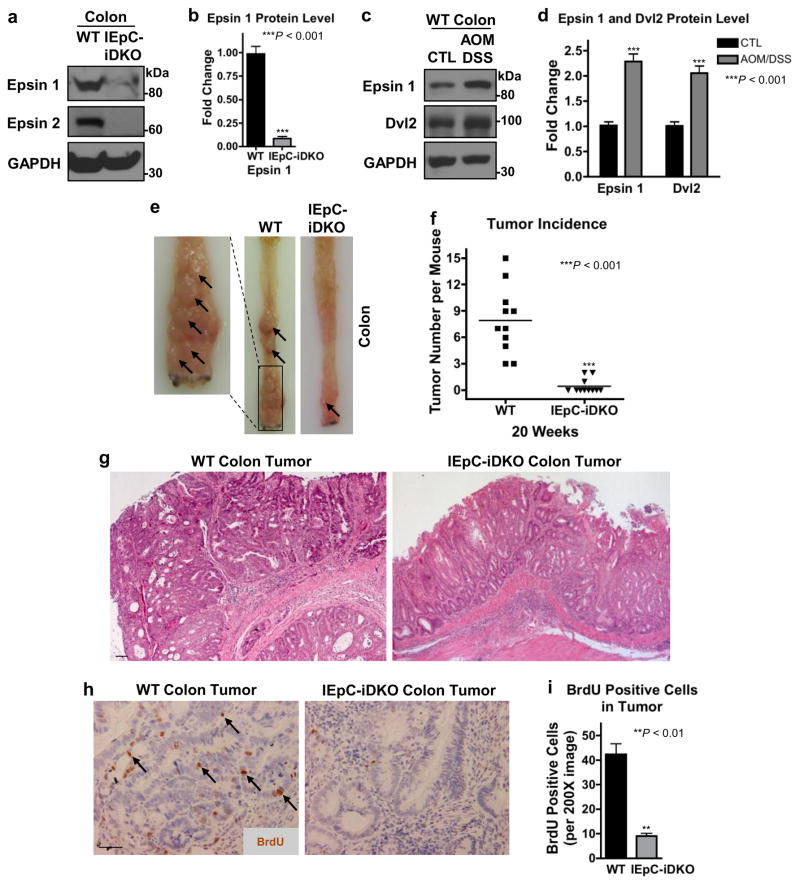
Encouraged by the upregulation of epsins in human colon cancer, we hypothesized that epsins, specifically intestinal epithelial epsins, may play a crucial role in propagating colon cancer cell transformation and/or proliferation. To test this, we generated a novel mouse model selectively lacking epsin 1 in IEpCs on an epsin 2 null background by crossing Epn1fl/fl;Epn2−/− mice34,37 with mice expressing tamoxifen-inducible Cre recombinase under control of the Villin promoter (Villin-ERT2 Cre)44. To produce inducible IEpC-iDKO mice, gender and genetic background-matched 10-week old WT or Epn1fl/fl;Epn2−/−;Villin-ERT2 Cre mice were intraperitoneal-injected with tamoxifen every-second-day for 2 weeks. Deletion of epsins 1 and 2 in the colon of IEpC-iDKO mice was validated by Western blotting (Fig. 2a,b).
Figure 2. Intestinal epithelial epsin deficiency reduced tumor growth in a colon cancer mouse model.
(a) Western blotting of epsins 1 and 2 in colon epitheliums from wild type (WT) and IEpC-iDKO mice. (b) Quantification of a, n=6. (c) Western blotting of epsin 1 and Dvl2 in colon epitheliums from control (CTL) and AOM/DSS-treated WT mice. (d) Quantification of c, n=6. (e) Longitudinally cut colons and rectums of WT and IEpC-iDKO mice sacrificed 20 weeks post-AOM/DSS treatment; arrows indicate tumors in the colon and rectal regions. (f) Quantification of tumor incidence in e, n=11. (g) H&E staining from representative WT and IEpC-iDKO colons from e, n=11; 10X objective. Scale bar: 50 μm. (h) In vivo BrdU immunohistochemistry of WT and IEpC-iDKO colons 20 weeks post-AOM/DSS treatment; 20X objective; arrows indicate BrdU-positive cells. Scale bar: 50 μm. (i) Quantification of BrdU immunohistochemistry in h, n=11. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
We utilized the IEpC-iDKO mice in an AOM/DSS-induced colon cancer model to investigate epsins’ role in colon cancer development45,46. Similar to human colon cancer, AOM/DSS-induced colon cancer in wild type (WT) mice 20 weeks post-treatment exhibited significantly increased epsin 1 and Dvl2 protein levels (Fig. 2c,d), indicative of epsins having a potential pro-carcinogenic role. In contrast, loss of epsins in the IEpC-iDKO colons revealed a striking tumor-resistant phenotype with significantly fewer tumors, indicated by arrows (Fig. 2e,f). H&E staining of isolated colons revealed only moderately differentiated adenomas in IEpC-iDKO mice but typical tubular adenocarcinomas with metastasis across the basement membrane in WT mice (Fig. 2g)43. We used in vivo BrdU labeling and immunohistochemical (IHC) staining to determine if the tumor-resistant phenotype in IEpC-iDKO colons resulted from impaired colon epithelial tumor cell proliferation. We found that IEpC-iDKO colons incorporated significantly less BrdU, indicated by arrows (Fig. 2h,i). Collectively, these data showed that specific loss of epsins in the colon epithelium hindered tumor cell proliferation and colon cancer progression in vivo.
Canonical Wnt signaling is impaired in IEpC-iDKO mice
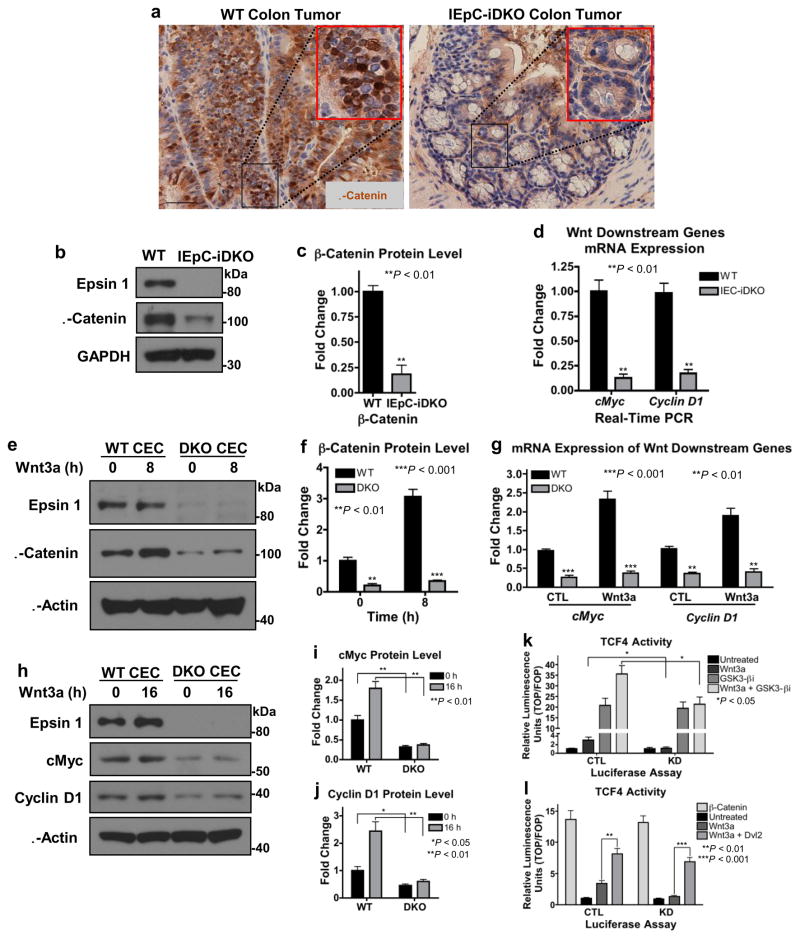
We hypothesized that loss of colon epithelial epsins protected against colon cancer by interfering with the aberrant canonical Wnt signaling associated with human colon cancer1,2,47. First, we measured nuclear β-catenin accumulation, the hallmark of canonical Wnt signaling, in colon tumors isolated from WT and IEpC-iDKO mice. WT colon tumors exhibited heavy nuclear β-catenin staining, as indicated by its colocalization with the nuclear hematoxylin stain, while the moderately differentiated IEpC-iDKO colon tumors exhibited very little staining (Fig. 3a). Western blotting confirmed that colon epithelial epsin deficiency significantly hindered β-catenin accumulation (Fig. 3b,c). Accumulated β-catenin transports to the nucleus where it binds to and activates the TCF/LEF transcription factors resulting in the expression of cMyc and cyclin D18–10. Using quantitative real-time PCR (qRT-PCR), we confirmed that IEpC-iDKO colon epithelium exhibited significant deficiencies in both cMyc and cyclin D1 mRNA expression (Fig. 3d). In summary, our in vivo data strongly indicated that epsin loss protected against colon cancer development by hindering aberrant canonical Wnt signaling.
Figure 3. Epsin deficiency significantly inhibited downstream canonical Wnt signaling.
(a) β-Catenin immunohistochemistry of WT or IEpC-iDKO colon tumors isolated 20 weeks post-AOM/DSS treatment; 20X objective. Scale bar: 50 μM, n=11. (b,c,d) Lysates from isolated colon epitheliums of adult WT and IEpC-iDKO mice analyzed by Western blotting for epsin 1 deletion and β-catenin protein accumulation (b, c) or by qRT-PCR for cMyc and cyclin D1 mRNA expression (d), n=9. (e,f) Primary WT and epsins 1 and 2 double knockout (DKO) CECs isolated and cultured from WT or IEpC-iDKO pups, respectively, then stimulated with Wnt3a (100 ng mL−1) for 8 h were analyzed by Western blotting for β-catenin, n=8. (g) CECs treated with Wnt3a for 12 h were analyzed by qRT-PCR for cMyc and cyclin D1 mRNA expression, n=9. (h,i,j) CECs treated with Wnt3a for 16 h were analyzed by Western blotting for cMyc and cyclin D1, n=8. (k,l) β-Catenin-dependent TCF4 transcriptional activation in response to Wnt3a-stimulation without or with the GSK3 inhibitor, GSK3-βi, (k) or in the presence of unphosphorylatable β-catenin (β-catenin) or Dvl2 (l) was measured in control (CTL) or epsins 1 and 2 knockdown (KD) HEK 293T cells transfected with TOPflash or FOPflash luciferase expression vectors and Renilla, n=8. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
Canonical Wnt signaling is attenuated in colon cancer cells
To understand the mechanism(s) by which increased epsin expression promoted colon epithelial cell (CEC) transformation and colon tumor proliferation, it was important to first understand the physiologic role of epsins. Towards this end, we isolated and cultured primary CECs from WT and Epn1fl/fl;Epn2−/−;Villin-ERT2 Cre pups. Epsin 1 deletion was induced ex vivo by tamoxifen-treatment (DKO CEC) and confirmed by Western blotting (Fig. 3e). Consistent with our in vivo findings, DKO CECs exhibited significantly less β-catenin stabilization (Fig. 3e,f) and downstream target gene expression (Fig. 3g–j) compared to WT CECs. Importantly, epsin deficiency significantly reduced β-catenin accumulation and transcriptional activation independent of Wnt stimulation (Fig. 3e–j), suggesting loss of epsins hindered canonical Wnt signaling upstream of β-catenin stabilization. To test this, we employed a luciferase assay using the β-catenin-dependent TOPflash reporter as readout of TCF4 activity. Prior to lysis, control and siRNA-mediated epsin-deficient (KD; knockdown) HEK 293T cells transfected with the luciferase vector system were treated without or with a GSK3-β inhibitor, GSK3-βi, and Wnt3a. As expected, loss of epsins significantly impaired Wnt-mediated TCF4 transcriptional activation (Fig. 3k,l); however, GSK3-βi treatment, which disassembled the β-catenin destruction complex, significantly increased TCF4 transcriptional activity in both control and KD cells under resting condition (Fig. 3k). Importantly, while Wnt stimulation further increased TCF4 transcriptional activity in GSK3-βi-treated control cells, it failed to do so in KD cells, thus suggesting epsins regulated Wnt-dependent signaling upstream of the destruction complex. Consistently, overexpression of constitutively active unphosphorylatable β-catenin produced maximal transcriptional activity in both control and KD cells regardless of stimulation (Fig. 3l). Further, overexpression of Wnt receptor effector, Dvl2, rescued Wnt-dependent signaling in KD cells, supporting that the action of epsin-mediated regulation of Wnt signaling is at the receptor level (Fig. 3l).
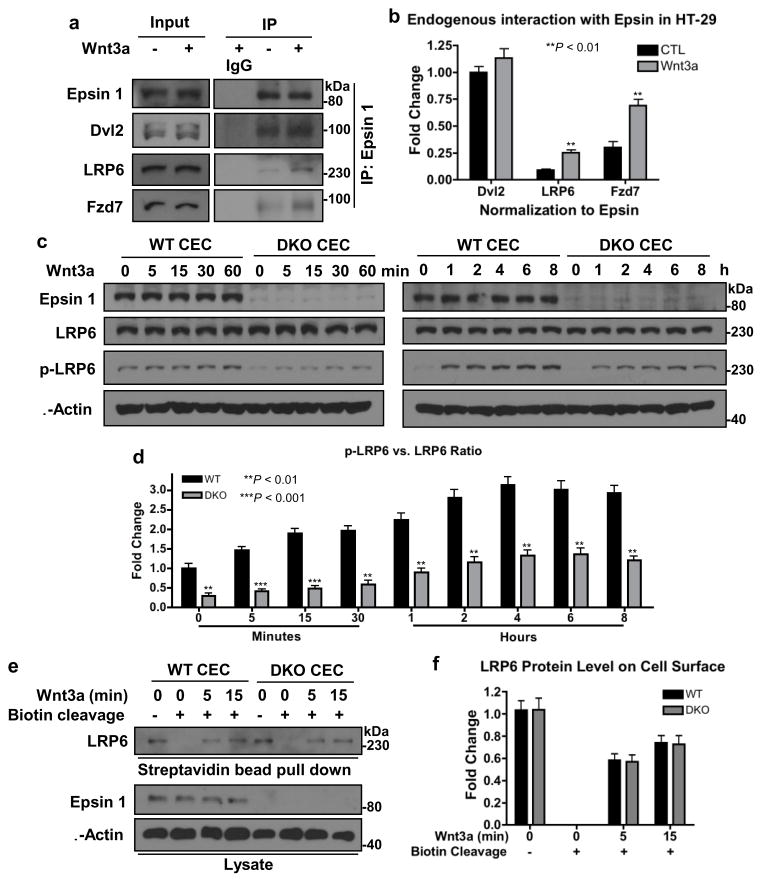
Wnt receptors endocytosis is independent of epsins
Given epsin’s classic role as an endocytic adaptor protein and that Wnt signaling is reportedly activated by receptor internalization25–28,30,31,48, we originally hypothesized that epsins might potentiate Wnt signaling by facilitating Wnt receptor endocytosis. To determine if epsin interacted with Wnt receptors endogenously, we performed co-immunoprecipitation assays in HT-29 cells using an epsin 1-specific antibody. We found that endogenous epsin 1 bound endogenous Wnt receptors, Fzd7 and LRP6, in a Wnt-dependent manner (Fig. 4a,b); a finding consistent with Wnt-dependent recruitment of epsin 1 to the Wnt receptor complex. Epsin loss also significantly hindered Wnt-dependent LRP6 phosphorylation in CECs (Fig. 4c,d), further suggesting impaired Wnt signaling activation. Given the cumulative evidence supporting a role for epsins in regulating Wnt receptor internalization and activation, we were surprised to find that loss of epsins failed to impair Wnt-mediated LRP6 internalization in CECs (Fig. 4e,f). Briefly, we used a cleavable biotinylation kit to label receptors on the surface of chilled WT and DKO CECs. CECs were washed with warm media, stimulated for various times with Wnt3a then chilled again; any remaining biotinylation on the surface was cleaved. Internalized biotin-labeled receptors were immunoprecipitated with streptavidin and analyzed by Western blotting using a LRP6-specific antibody. LRP6 was internalized with similar efficiency after 5 min of Wnt stimulation in both WT and DKO CECs (Fig. 4e,f), suggesting that Wnt-dependent internalization of the Wnt receptor complex was not regulated by or dependent on epsin.
Figure 4. Epsin interacted with Dvl2 to regulate Wnt receptor signaling independent of receptor-mediated endocytosis.
(a) Co-immunoprecipitation of endogenous Dvl2, LRP6 and Fzd7 by anti-epsin 1 antibody in Wnt3a-stimulated (100 ng mL−1, 5 min) HT-29 cells analyzed by Western blotting. (b) Quantification of a, n=8. (c) WT and DKO CECs were stimulated with Wnt3a (100 ng mL−1) as indicated then Wnt signaling was analyzed by Western blotting with LRP6 and phospho-LRP6 antibodies. (d) Quantification of phospho-to-total LRP6 fold change in c, n=8. (e) Cell surface of WT and DKO CECs were labeled with cleavable biotin, incubated with Wnt3a at 37°C as indicated then surface biotin was cleaved. Internalized biotinylated-LRP6 was determined by streptavidin bead pull-down and Western blotting. (f) Quantification of e, n=8. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
How then was epsin regulating the activation of canonical Wnt signaling? To answer this, we returned to our co-immunoprecipitation assay and found that endogenous epsin 1 constitutively interacted with the crucial cytoplasmic signaling effector, Dvl2, independent of Wnt stimulation (Fig. 4a,b). Notably, Dvl2 was not immunoprecipitated in epsin-deficient HT-29 cells, indicating the specificity of the epsin:Dvl2 interaction (Supplementary Fig. 1). Dvl interacts with the cytoplasmic tail of LRP in response to Wnt stimulation and plays a crucial role in the recruitment, phosphorylation and disassembly of the β-catenin destruction complex6,23,24,49. In light of this new finding, we hypothesized that epsins interacted with and stabilized Dvl2, thus protecting it from degradation to ensure Wnt signaling activation.
Epsin regulates Dvl2 stability
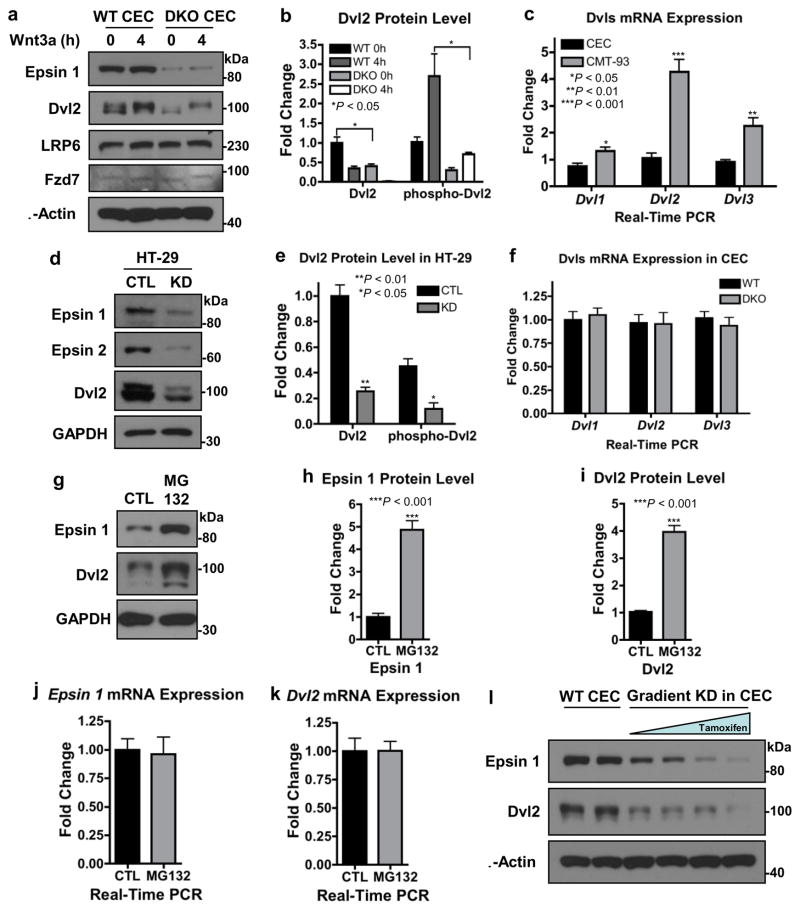
To test the hypothesis that epsins regulated Dvl2 stability, we used Western blotting to determine if the loss of epsins would reduce total Dvl2 protein. Given that Dvl2 overexpression is strongly associated with colon cancer19,22, a result we confirmed using qRT-PCR of the CMT-93 mouse colon cancer cell line compared to wild type CECs (Fig. 5c), we focused our attention on the relationship between epsins and Dvl2. We found that epsin deficiency, either in DKO CECs (Fig. 5a,b) or in siRNA-mediated knockdown in HT-29 cells (Fig. 5d,e), significantly reduced total Dvl2 protein levels. Importantly, epsin loss did not hinder Wnt-dependent Dvl2 phosphorylation as indicated by the molecular weight shift in the Western blotting (Fig. 5a,b,d,e). Further, loss of epsins did not alter mRNA expression of Dvl1, Dvl2, or Dvl3 in CECs (Fig. 5f), suggesting that loss of Dvl2 protein was due to increased degradation not decreased transcription. To confirm, we treated WT CECs with the proteasomal inhibitor, MG132, to impair Dvl2 degradation. Surprisingly, MG132 treatment significantly increased both epsin 1 and Dvl2 protein levels (Fig. 5g–i) without altering gene expression (Fig. 5j,k). These results demonstrated that epsin 1 and Dvl2 can be degraded via the proteasome and indicated a correlation between epsin 1 protein levels and Dvl2 stability. To confirm that Dvl2 protein levels were directly regulated by epsin 1, we created a gradient of epsin 1 depletions in the epsin 2 null CECs by adjusting tamoxifen exposure and measured Dvl2 protein levels. We found that greater loss of endogenous epsin 1 resulted in greater loss of endogenous Dvl2 (Fig. 5l). Cumulatively, these data uncovered a novel role for epsins in the stabilization of Dvl2 and Wnt signaling.
Figure 5. Epsin interacted with and stabilized Dvl2 to promote Wnt signaling.
(a) CECs were stimulated with Wnt3a (100 ng mL−1) for 4 h, then endogenous epsin 1, Dvl2, LRP6 and Fzd7 proteins were analyzed by Western blotting. (b) Quantification of total and phospho-Dvl2 in a, n=8. (c) qRT-PCR analyses of Dvl1, Dvl2 and Dvl3 mRNA expressions in WT CECs and the CMT-93 mouse colon cancer cell line, n=9. (d) Western blotting of epsins 1 and 2 and Dvl2 in control (CTL) and siRNA-mediated epsin-deficient (KD) HT-29 human colon cancer cells. (e) Quantification of total and phospho-Dvl2 in d, n=8. (f) qRT-PCR analyses of Dvl1, Dvl2 and Dvl3 mRNA expressions in WT and DKO CECs, n=9. (g) Western blotting of epsin 1 and Dvl2 in WT CECs after 4h treatment with 10 μM proteasomal inhibitor, MG132. (h, i) Quantifications of epsin 1 (h) and Dvl2 (i) in g, n=8. (j, k) qRT-PCR analyses of epsin 1 (j) and Dvl2 (k) mRNA expressions in WT CECs after MG132 treatment, n=9. (l) Western blotting of epsin 1 and Dvl2 in CECs with a variable tamoxifen-induced gradient of epsin 1 knockdown (KD), n=8. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
Epsin ENTH and UIM domains interact with Dvl2
Epsins are multi-domain proteins with the most notable domains being the two highly conserved ubiquitin-interacting motifs (UIM) responsible for epsin interaction with ubiquitinated cell surface receptors29,32,37,50–52. Given that proteasomal degradation of proteins such as Dvl2 is dependent on polyubiquitination53, we hypothesized that the epsin:Dvl2 interaction may be facilitated by epsin UIM binding to polyubiquitinated Dvl2. To determine if the epsin UIM was responsible for the epsin:Dvl2 interaction, we co-immunoprecipitated Dvl2 using either full length epsin 1 or an epsin 1 construct lacking the UIM region (epsin 1 ΔUIM) and found that the epsin 1 ΔUIM only partially inhibited the interaction between epsin 1 and Dvl2 suggesting that, while it does play a role, the UIM was not sufficient to mediate epsin:Dvl2 interaction (Fig. 6a; Supplementary Fig. 2a). Interestingly, the UIM did mediate the Wnt-dependent interaction between epsin 1 and the Wnt receptors (Supplementary Fig. 2b–d); a finding that suggests a multifaceted nature for epsin, and its UIM domain, in canonical Wnt signaling.
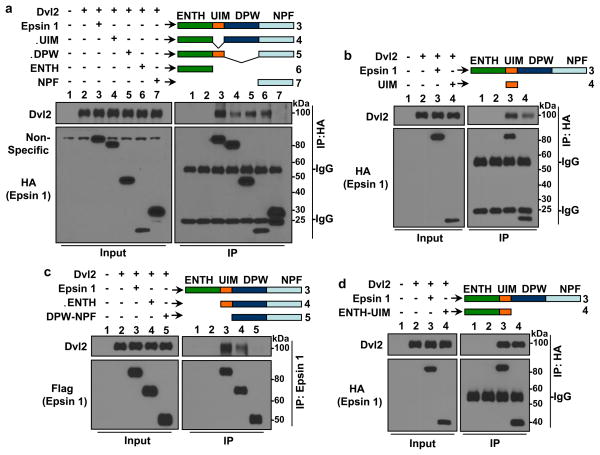
Figure 6. Epsin 1 interacted with Dvl2 via its ENTH and UIM domains.
Epsin 1 full length (Epsin 1) and the following truncated epsin 1 constructs were co-transfected with full length Dvl2 into HEK 293T cells: HA-tagged full length epsin 1 (Epsin 1) (a,b,d), Epsin 1 ΔUIM (ΔUIM) (a), Epsin 1 ΔDPW (ΔDPW) (a), ENTH alone (ENTH) (a), NPF alone (NPF) (a), UIM alone (UIM) (b) and ENTH-UIM alone (ENTH-UIM) (d); Flag-tagged Epsin 1 full length (Epsin 1) (c), Epsin 1 ΔENTH (ΔENTH) (c) and DPW-NPF alone (DPW-NPF) (c). (a–d) Dvl2 was co-immunoprecipitated by specified epsin 1 constructs using anti-HA (a,b,d) or anti-epsin 1 (c) antibodies, then analyzed by Western blotting. All are representative blots from n=8.
Epsin contains several other potential binding domains that may work in combination with the UIM to efficiently interact with and protect Dvl2 including the ENTH, DPW and NPF domains29,30,51. To determine the role these domains play in the epsin:Dvl2 interaction, we utilized epsin 1 truncation constructs lacking the ENTH domain (epsin 1 ΔENTH) or DPW motif (epsin 1 ΔDPW) or containing a single binding domain (ENTH, UIM, or NPF) to co-immunoprecipitate Dvl2. We found that ENTH alone (Fig. 6a) and UIM alone (Fig. 6b) or constructs containing ENTH (ΔUIM and ΔDPW (Fig. 6a)) or UIM (ΔDPW (Fig. 6a) and ΔENTH (Fig. 6c)) all partially immunoprecipitated Dvl2. In contrast, neither NPF alone (Fig. 6a) nor DPW-NPF (Fig. 6c) interacted with Dvl2. From these binding data, we proposed that epsin interacted constitutively with Dvl2 through cooperative binding by the ENTH and UIM domains; a hypothesis supported by the fact that an ENTH-UIM construct was the only one tested that co-immunoprecipitated Dvl2 comparably to full length epsin 1 (Fig. 6d).
Dvl2 ubiquitination is reduced by Epsin ENTH-UIM binding
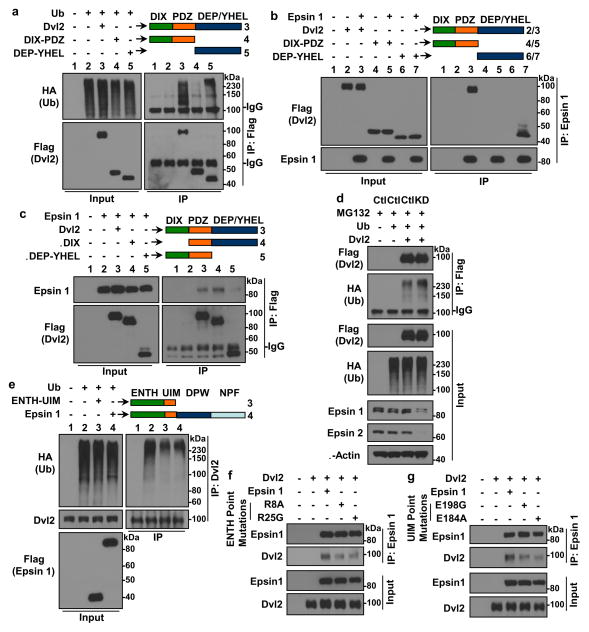
It is well established that Dvl2 polyubiquitination is facilitated by the interaction of the Dvl2 C-terminus with various E3 ligases53–57. We hypothesized that epsin may protect Dvl2 from degradation by competitively interfering with E3 ligase binding at the C-terminus of Dvl2. To test this and map the epsin 1 binding domains within Dvl2, we generated Dvl2 truncation constructs containing either the N-terminal DIX and PDZ domains (DIX-PDZ) or C-terminal DEP and YHEL domains (DEP-YHEL). Only Dvl2 constructs containing the C-terminal DEP and YHEL domains were polyubiquitinated (Fig. 7a). Epsin 1 interacted with and co-immunoprecipitated full length Dvl2 and the DEP-YHEL construct but failed to interact with the DIX-PDZ construct (Fig. 7b). Reciprocally, Dvl2 lacking only the DIX domain (ΔDIX) was sufficient to immunoprecipitate epsin 1 comparably, if not more efficiently, than full length Dvl2 (Fig. 7c). Collectively, our binding studies suggested that the N-terminal ENTH-UIM domain of epsin interacted with the C-terminal domains of Dvl2.
Figure 7. Epsin ENTH-UIM interacted with and impaired polyubiquitination of the Dvl2 C-terminus.
(a) Polyubiquitinated full length or truncated flag-tagged Dvl2 was immunoprecipitated using anti-flag antibody in HEK 293T cells transfected with Flag-tagged Dvl2 full length (Dvl2), DIX-PDZ alone (DIX-PDZ) or DEP-YHEL alone (DEP-YHEL) constructs and HA-tagged ubiquitin and then analyzed by Western blotting. (b) Specified flag-tagged Dvl2 constructs were co-immunoprecipitated using anti-epsin 1 antibody in HEK 293T cells transfected with specified flag-tagged Dvl2 constructs and full length epsin 1 and then analyzed by Western blotting. (c) Epsin 1 was co-immunoprecipitated using anti-flag antibody in HEK 293T cells transfected with Flag-tagged Dvl2 full length (Dvl2), Dvl2 ΔDIX (ΔDIX) or Dvl2 ΔDEP-YHEL (ΔDEP-YHEL) constructs and full length epsin 1 and then analyzed by Western blotting. (d) Polyubiquitinated full length flag-tagged Dvl2 was immunoprecipitated using anti-flag antibody in MG132-treated control (CTL) or epsins 1 and 2 knockdown (KD) HEK 293T cells transfected with flag-tagged Dvl2 and HA-tagged ubiquitin and then analyzed by Western blotting. (e) Polyubiquitinated endogenous Dvl2 was immunoprecipitated using anti-Dvl2 antibody in HEK 293T cells transfected with specified flag-tagged epsin 1 constructs and HA-tagged ubiquitin and then analyzed by Western blotting. (f,g) Dvl2 was co-immunoprecipitated using anti-epsin 1 antibody in HEK 293T cells transfected with specified mutant epsin 1 constructs and Dvl2 and then analyzed by Western blotting. All are representative blots from n=8.
To capture the polyubiquitinated state of Dvl2, we treated HEK 293T cells overexpressing Dvl2 and HA-tagged ubiquitin with MG132, then lysed the cells, immunoprecipitated Dvl2 and analyzed Dvl2 ubiquitination by Western blotting. Consistent with previous reports, Dvl2 was constitutively polyubiquitinated (Fig. 7d). Notably, epsin depletion increased Dvl2 polyubiquitination after MG132 treatment. To determine if epsin ENTH-UIM could protect Dvl2 from polyubiquitination, we overexpressed full length epsin 1 or ENTH-UIM in combination with HA-tagged ubiquitin and measured the endogenous Dvl2 ubiquitination status. Consistent with the epsin:Dvl2 interaction interfering with E3 ligase binding to Dvl2, both full length epsin 1 and ENTH-UIM constructs were sufficient to reduce Dvl2 polyubiquitination (Fig. 7e). For further confirmation, we used molecular modeling to identify critical residues within the ENTH (R8 and R25) and UIM (E184 and E198) domains of epsin 1 predicted to facilitate Dvl2 binding. Individual point mutations (R8A, R25G, E184A or E198G) and subsequent co-immunoprecipitation assays revealed significant reductions in epsin 1:Dvl2 binding (Fig. 7f,g) by all four mutants. To summarize our detailed domain mapping data, epsin ENTH-UIM interacted with the C-terminus of Dvl2 to impair Dvl2 polyubiquitination. Impaired polyubiquitination and increased stabilization of Dvl2 in the presence of epsins strongly suggested that epsins may competitively impede E3 ligase binding to the Dvl2 C-terminus.
Epsin ENTH-UIM or Dvl2 overexpression rescues tumor growth
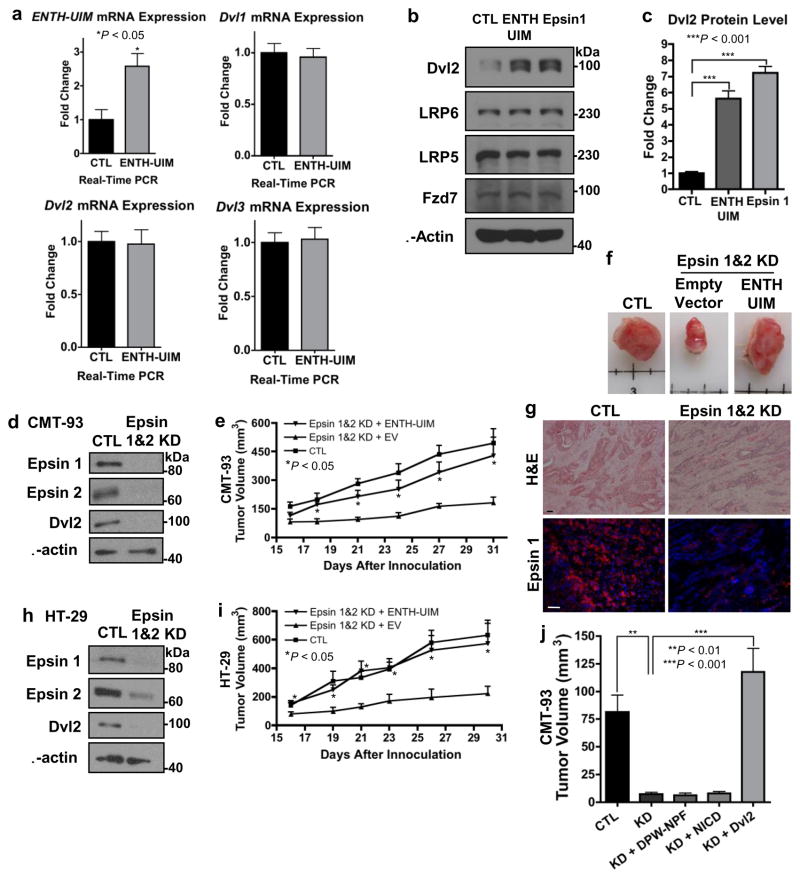
To directly test whether epsins facilitated canonical Wnt signaling by interacting with and stabilizing Dvl2, we subcloned the ENTH-UIM fragment into a lentiviral vector for stable infection and expression in mammalian cells. Epsin ENTH-UIM expression was confirmed after infection of HEK293T cells by qRT-PCR (Fig. 8a). Importantly, epsin ENTH-UIM fragment expression stabilized Dvl2 protein to levels similar to full length epsin 1 overexpression (Fig. 8b,c). The lentiviral epsin ENTH-UIM vector was subsequently used to create stable epsin-depleted CMT-93 and HT-29 cells without or with expression of the ENTH-UIM fragment. Epsin depletion was confirmed by Western blotting prior to lentiviral infection (Fig. 8d,h). Cells were subcutaneously implanted into SCID mice and tumor growth monitored. Based on our binding data, we hypothesized that the ENTH-UIM fragment would stabilize Dvl2 thus facilitating canonical Wnt signaling and tumor growth. Furthermore, by deleting the epsin 1 C-terminus we limited the fragment’s clathrin-binding capabilities and, thus, its ability to modulate receptor internalization. Similar to our IEpC-iDKO mice, we determined that epsin depletion significantly hindered growth of both CMT-93 and HT-29 colon cancer cell xenografts (Fig. 8e–g,i). Expression of the epsin ENTH-UIM fragment was sufficient to recover tumor growth (Fig. 8e–g,i). In contrast, lentiviral expression of the C-terminal epsin DPW-NPF fragment failed to rescue tumor growth (Fig. 8j). These findings provided further evidence that epsins function to promote colon cancer growth, likely by stabilizing Dvl2. As proof, Dvl2 overexpression in epsin-deficient cells rescued Wnt signaling activation (Fig. 3l) and likewise, overexpression of Dvl2 by retroviral infection in epsin-deficient CMT-93 colon cancer cells rescued tumor growth (Fig. 8j). In contrast, overexpression of active Notch, NICD, by retroviral infection did not rescue epsin-deficient tumor inhibition (Fig. 8j). The fact that either the ENTH-UIM fragment or Dvl2 expression, but not DPW-NPF fragment or NICD expression, was sufficient to recover tumor growth after epsin deficiency emphasized the importance of this novel mechanism for canonical Wnt signaling, independent of any role for epsin in Wnt receptor internalization or Notch signaling.
Figure 8. Epsin ENTH-UIM fragment was sufficient to recover tumor growth.
(a) qRT-PCR analyses of Epsin ENTH-UIM, Dvl1, Dvl2 and Dvl3 mRNA expressions in HEK 293T cells infected with empty vector (EV)- or ENTH-UIM-expressing lentivirus, n=9. (b) Western blotting of HEK 293T cells infected with EV-, ENTH-UIM- or full length epsin 1-expressing lentivirus. (c) Quantification of Dvl2 in b, n=6. (d) Western blotting of epsins 1 and 2 and Dvl2 in CMT-93 cells infected with EV or epsins 1 and 2 shRNA-expressing viruses, n=6. (e) Xenograft tumor volume analyses of control, epsin-depleted or epsin-depleted ENTH-UIM-expressing CMT-93 cells subcutaneously implanted in SCID mice, n=6. (f) Representative images at 31 d post-implantation. (g) H&E and immunofluorescence staining of epsin 1 in representative CMT-93 xenograft tumors from (f). Scale bars: 50 μm. (h) Western blotting of epsins 1 and 2 and Dvl2 in HT-29 cells infected with EV or epsins 1 and 2 shRNA-expressing viruses, n=6. (i) Xenograft tumor volume analysis of control, epsin-depleted or epsin-depleted ENTH-UIM-expressing HT-29 cells subcutaneously implanted in SCID mice, n=6. (j) Final xenograft tumor volumes of control, epsin-depleted or epsin-depleted DPW-NPF-, NICD-myc- or Dvl2- expressing CMT-93 cells 18 days post-subcutaneous implantation in SCID mice, n=6. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
Epsin is upregulated in mouse colon cancer in vivo
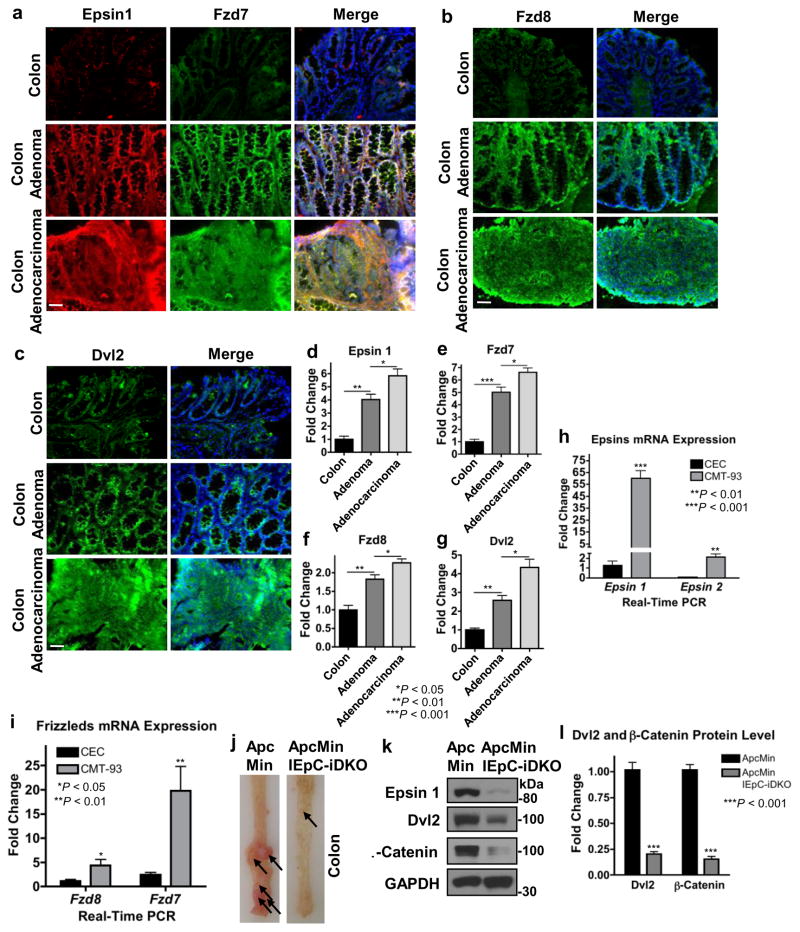
To confirm epsins were upregulated in mouse colon cancer, and that this upregulation correlated with upregulated Dvl2 and Wnt receptor expression, we used immunofluorescence to stain normal and tumorous colon tissue sections from control and AOM/DSS-treated WT mice. We found that epsin 1, Dvl2, Fzd7 and Fzd8 were upregulated in mouse colon tumors, compared to normal tissues (Fig. 9a–c). Furthermore, and similar to human colon cancer (Fig. 1), these expression levels correlated strongly with tumor severity such that expressions in malignant adenocarcinomas were higher than benign adenomas which were in turn higher than normal tissues (Fig. 9d–g). We focused our staining on epsin 1, Dvl2, and Fzd7 after determining that epsin 1 mRNA expression was dominant over epsin 2 (Fig. 9h), Dvl2 over Dvl1 or Dvl3 (Fig. 5c) and Fzd7 over Fzd8 (Fig. 9i) in mouse CMT-93 cells.
Figure 9. Epsin 1, Dvl2 and Wnt receptors were upregulated in mouse colon cancer.
(a–c) Epsin 1 and Fzd7 (a), Fzd8 (b), and Dvl2 (c) were analyzed by immunofluorescence staining of peripheral normal, adenoma and adenocarcinoma colon tissues from control and AOM/DSS-treated WT mice. Scale bar: 50 μm. (d–g) Quantifications of epsin 1 (d), Fzd7 (e), Fzd8 (f) and Dvl2 (g) staining in (a–c), n=11. (h,i) qRT-PCR analyses of epsin 1 and epsin 2 (h) or Fzd7 and Fzd8 (i) mRNA expressions in WT CECs and the CMT-93 mouse colon cancer cell line, n=9. (j) Representative image of longitudinally cut colons and rectums of ApcMin and ApcMin/IEpC-DKO mice sacrificed at 20 weeks of age; arrows indicate tumors in colon and rectal regions, n=11. (k) Western blotting of epsin 1, Dvl2 and β-catenin in the colon epitheliums from ApcMin and ApcMin/IEpC-DKO mice. (l) Quantification of Dvl2 and β-catenin in k, n=6. All statistical values were calculated using a Student’s t test; P values are indicated. Error bars indicate the mean ± s.e.m.
Epsin loss reduces tumor rate in ApcMin colon cancer model
Familial adenomatous polyposis (FAP), a hereditary human intestinal cancer syndrome, as well as many forms of sporadic human colon cancers are caused by genetic mutations that promote β-catenin stabilization downstream of Wnt receptor activation and Dvl23,14,22,42,58. Given the importance of these downstream alterations, it remained unclear what implications epsins’ novel role in stabilizing Dvl2, and the potential disruption of this interaction, would have on genetic models of colon cancer. To investigate, we engineered an epsin-deficient genetic model for colon cancer by crossing our Epn1fl/fl;Epn2−/−;Villin-ERT2-Cre mice with the well-characterized FAP-model ApcMin mice which are heterozygous for a truncation mutation of the Apc gene59. Postnatal epsin 1 deletion was induced as described above. Tumor incidence was evaluated in WT or ApcMin/IEpC-iDKO colons isolated 20 weeks after tamoxifen-induced epsin deletion. Epsin deficiency was found to protect against tumor development in the colons (Fig. 9j) and small intestines (Supplementary Fig. 3) of ApcMin/IEpC-iDKO mice. Consistent with epsins’ role in Dvl2 stabilization, loss of epsins in ApcMin mice significantly reduced Dvl2 protein (Fig. 9k,l). Reduced Dvl2 protein directly correlated with significant loss of β-catenin (Fig. 9k,l) despite the genetic destabilization of the β-catenin destruction complex. This phenotype closely mimicked the phenotype of the previously reported tumor resistant APCMin/Dvl2−/− mice22. The similarities of our APCMin/IEpc-iDKO mice in which epsin loss destabilized Dvl2 to prevent colon cancer and the tumor resistant phenotype after genetic ablation of Dvl2 (APCMin/Dvl2−/− mice) provided strong evidence that β-catenin activation can be modulated despite alterations of the β-catenin destruction complex. Therefore, we propose that Dvl2 stability, modulated in part by epsins, is critical for the aberrant Wnt-dependent stabilization of β-catenin responsible for excessive tumor cell proliferation and colon cancer development. Furthermore, our finding emphasized the potential usefulness of epsin inhibition in preventing FAP by destabilizing Dvl2 and β-catenin.
Discussion
We previously reported that epsins, specifically endothelial epsins, played a critical role in regulating the tumor microenvironment to promote cancer progression37,38. However, epsins are also reportedly upregulated in tumors, but the critical role for this upregulation within a given tumor cell type was still unknown36,39–41. In this study, we revealed that epsins were significantly upregulated in both human and mouse colon cancer. Further, we discovered a novel role for epsins in the regulation of canonical Wnt signaling independent of its classical role as an endocytic adaptor.
As discussed previously, deregulated canonical Wnt signaling is an established pathway involved in colon cancer development and progression1,2,14. Although several genetic mutations within β-catenin and its destruction complex are known to cause deregulation, other mechanisms of regulation including Wnt receptor expression, activation and internalization have also been implicated4,12,13,16,18,19. Consistently, we found that both human and mouse colon cancers exhibited parallel upregulation of epsins, Dvl2and Fzd (Fig. 1, Fig 9). Furthermore, epsin expression levels tightly correlated with the aggressiveness of the tumor. These findings, in combination with the fact that loss of intestinal epithelial epsins inhibited both canonical Wnt signaling and colon cancer progression (Fig. 2, Fig. 3, Fig. 9), provided strong evidence suggesting intestinal epithelial epsins play an important role in regulating Wnt signaling; however, given epsin’s classic role as an endocytic adaptor, we were surprised to find that, while epsins were necessary for canonical Wnt signaling activation and colon cancer development, they were dispensable for internalization-dependent activation of the Wnt receptors (Fig. 4e,f). We had already determined via the luciferase assay that loss of epsins inhibited canonical Wnt signaling upstream of β-catenin stabilization and was dependent in part on Dvl2 (Fig.3k,l). Our internalization data further indicated that the inhibitory effect was downstream of receptor internalization. Given that Dvl2 is the signaling molecule that connects Wnt receptor activation with β-catenin stabilization through regulated disassembly of the destruction complex, we investigated the effects of epsin deficiency on Dvl223,24. Our discovery that epsins constitutively associated with Dvl2 (Fig. 4a), that loss of epsins significantly diminished Dvl2 protein levels (Fig 5l) and that Dvl2 overexpression rescued the epsin-deficient phenotype (Fig. 3l, Fig. 8j) provided a much needed explanation for how epsin deficiency abolished canonical Wnt signaling.
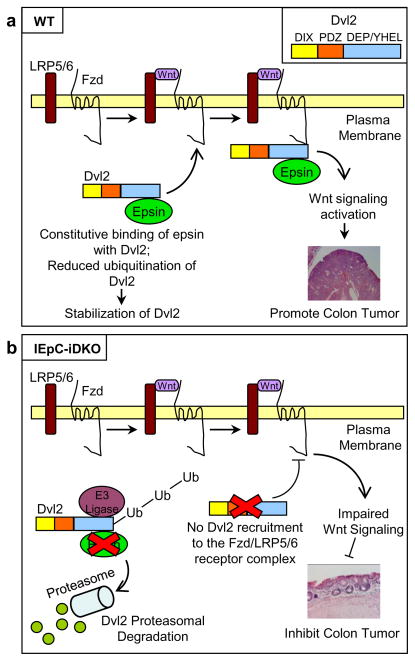
For the first time, we have identified a novel role for epsin as a cytosolic chaperone protein; a role completely independent of its classical role as a membrane-associated endocytic adaptor. In light of our extensive mechanistic binding analyses, we propose a model in which epsin interacts constitutively with the C-terminus of Dvl2 via its ENTH and UIM domains, presumably prohibiting E3 ligase binding, and thus reducing Dvl2 ubiquitination and degradation (Fig. 10). The epsin:Dvl2 complex is subsequently recruited to the Wnt receptor complex thus explaining the Wnt-dependent association between epsin and the Wnt receptors. As such, we propose epsins are critical regulators for Wnt signaling activation and colon cancer progression by protecting Dvl2 from proteasomal degradation (Fig. 10). Our proposed mechanism explains how the loss of epsins leads to the loss of Dvl2 and decreased tumor progression in IEpC-iDKO mice, and further provides a potential explanation for why epsins and Dvl2 are correspondingly upregulated in both mouse and human colon cancers.
Figure 10. Schematic model of the interaction between epsin and Dvl2.
(a) Epsins bind to the C-terminus of Dvl2 independent of Wnt stimulation, thus protecting Dvl2 from E3 ligase-mediated polyubiquitination and degradation. Wnt stimulation, and subsequent activation of the Fzd:LRP receptor complex, recruits epsin-associated Dvl2 to the complex. Complex-associated epsins may further stabilize the complex and promote its internalization, a necessary event for Wnt signaling, through interactions between epsin UIM and the Wnt receptors. Activated Wnt signaling promotes colon epithelial tumor progression. (b) Epsin deficiency reduces Dvl2 stability resulting in reduced Wnt-dependent signaling activation. Impaired Wnt signaling activation results in reduced colon epithelial tumor progression.
Since its original discovery in 199830, epsins have been implicated in several proliferative signaling pathways including Notch34,37,38,50,60. In Drosophila, Notch and Wnt signaling pathways crosstalk to ensure proper development60. Notably, epsins (liquid facets) play a pivotal role in Drosophila development through the regulation of Notch signaling, but do not appear to alter Wnt signaling as evident by the lack of a wingless phenotype when epsins were depleted34,60–62. Our data however strongly indicate that epsin is a critical regulator for Wnt signaling in mammalian vertebrates because in our novel IEpC-iDKO mice Wnt signaling is dramatically impaired by loss of epsins. Nonetheless, it remained a possibility that intestinal epithelial epsin loss also impaired tumor growth by modulating Notch signaling given the previously determined role of epsins as promoters of Notch signaling63–66. However, in colon cancer, Notch signaling counteracts Wnt signaling resulting in reduced expression of β-catenin target genes and impaired colon cancer cell proliferation67. The fact that epsin depletion in the intestinal epithelial cells significantly impaired, instead of promoted, colon cancer progression supposedly produced by Notch signaling inhibition suggested that, at least in colon cancer, epsin-mediated Notch signaling does not play a significant role in regulating cancer progression. This is further supported by that fact that expression of the active form of Notch, NICD, did not rescue tumor growth in our epsin-deficient subcutaneous colon cancer model (Fig. 8j).
In our final observations, we found that, in addition to colitis-induced colon cancer, intestinal epithelial epsin deficiency also sufficiently protected against colon cancer development in the well-characterized genetic ApcMin model of FAP. These findings mimicked those previously reported in ApcMin/Dvl2−/− mice including reduced β-catenin activity, canonical Wnt signaling and colon cancer development despite genetic destabilization of the β-catenin destruction complex22. This previous report also determined that loss of Dvl2 protected against tumor development by reducing canonical Wnt signaling in ApcMin/Dvl2−/− mice. Although the ApcMin mutation promotes constitutive β-catenin stabilization downstream of Dvl2 stabilization and Wnt-dependent recruitment, our discovery that epsin loss destabilized both Dvl2 and β-catenin despite the mutated destruction complex emphasized the importance of epsin-mediated stabilization of Dvl2 to Wnt signaling, intestinal epithelial cell proliferation and colon cancer progression. Furthermore, this finding supports a potential use for targeted inhibition of epsins in the therapeutic treatment of a variety of colon cancers despite the fact that a significant percentage of them result from genetic mutations that directly stabilize β-catenin through inhibition of the destruction complex. A significant amount of effort was previously spent on developing anti-Wnt therapies to combat cancer but progress has been limited due to high toxicity, minimal efficacy and/or off-target effects68,69. Our findings suggest that targeting epsins represents a potential novel strategy to combat colon cancer and provides hope for patients who are resistant or unresponsive to current anti-colon cancer therapies.
Methods
Generation of conditional IEpC-iDKO and APCMin mice
We reported a strategy to generate an epsins 1 and 2 global double knockout (DKO) mouse model34. We used a similar strategy with modifications to create a conditional knockout of epsin 1 (Epn1fl/fl mice)34,37. Epn1fl/fl mice were mated with Epn2−/− null mice to generate Epn1fl/fl;Epn2−/− mice. Tamoxifen-inducible intestinal epithelial cell-specific DKO (IEpC-iDKO) mice were obtained by crossing Epn1fl/fl;Epn2−/− mice with Villin-ERT2 Cre deleter mice which express Cre recombinase specifically in the IEpCs44. To induce postnatal deletion of IEpC epsin 1, we administered 5 mg kg-1 (body weight) of 4-hydroxytamoxifen by intraperitoneal-injection into 10-week-old WT or Epn1fl/fl;Epn2−/−;Villin-ERT2 mice every-second-day for two weeks. To generate the ApcMin/IEpC-iDKO mice, we crossed our Epn1fl/fl;Epn2−/−;Villin-ERT2 mice with ApcMin mice purchased from Jackson Laboratories. All vertebrate procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Oklahoma Medical Research Foundation.
Antibodies and Reagents
Unless specified otherwise, common laboratory chemicals and reagents were from either Sigma-Aldrich or Fisher Scientific. Media and additives for cell culture were from Gibco. The reagents and materials for RNA isolation, qRT-PCR, transfection reagents, primers and siRNA were from Invitrogen. Dual-Luciferase Reporter Assay Kit was from Promega. QuikChange II Site-Directed Mutagenesis Kit was from Agilent Technologies. Bromodeoxyuridine (BrdU), 4-hydroxytamoxifen, and Azoxymethane (AOM) were from Sigma-Aldrich. Dextran Sulfate Sodium (DSS) was from MP Chemicals, Inc. Recombinant mouse and human Wnt3a were from R&D systems. GSK3-β inhibitor (GSK3-βi; CHIR-99021) was from Selleckchem. MG132 was from Calbiochem. Polyclonal rabbit antibodies for epsin 1 (1:2000 Western blotting dilution) and epsin 2 (1:4000 Western blotting dilution) were obtained as described30,31. Anti-Epsin 1 (1:1000 Western blotting dilution; 1:200 immunofluorescence dilution; 1:200 immunoprecipitation dilution), -GAPDH (Glyceraldehyde-3-phoshate Dehydrogenase) (1:3000 Western blotting dilution), -LRP5 (1:500 Western blotting dilution), -LRP6 (1:500 Western blotting dilution), -Fzd7 (1:500 Western blotting dilution; 1:50 immunofluorescence dilution), -Fzd8 (1:500 Western blotting dilution; 1:50 immunofluorescence dilution), -cMyc (1:1000 Western blotting dilution) and -cyclin D1 (1:1000 Western blotting dilution) antibodies were from Santa Cruz Biotechnologies; anti-β-catenin antibody (1:3000 Western blotting dilution; 1:100 Immunohistochemistry) was from BD; anti-Dvl2 (1:2000 Western blotting dilution; 1:100 immunofluorescence dilution; 1:200 immunoprecipitation dilution) and anti-phospho-LRP6 (Ser1490) (1:1000 Western blotting dilution) antibodies were from Cell Signaling Technology; anti-β-actin antibody (1:3000 Western blotting dilution) was from Invitrogen; anti-HA antibody (1:2000 Western blotting dilution; 1:200 immunoprecipitation dilution) was from Covance; anti-Flag antibody (1:2000 Western blotting dilution; 1:200 immunoprecipitation dilution) was from Sigma-Aldrich.
Colitis-induced mouse colon cancer model
Colitis-inducible colon cancer mouse model was created as described45. Briefly, 12–13 week-old WT or IEpC-iDKO mice were given a single intraperitoneal-injection of AOM (10 mg kg−1 body weight). One week after injection, mice received 2% DSS in drinking water for 7 days followed by no further treatment. Mice were sacrificed 20 weeks after the initial AOM injection. The colons and rectums were cut open longitudinally and examined for tumors. The number and size of tumors were recorded.
In vivo Colon Tumor Xenograft Assay
Control, epsin-deficient or epsin-deficient ENTH-UIM-, DPW-NPF-, Dvl2- or NICD-expressing CMT-93 or HT-29 colon cancer cells were implanted subcutaneously (5x106 cells per injection) in twelve-week old SCID mice. We recognized tumors more than 2 mm in diameter as positive. Tumor growth was monitored by measuring tumor size using a digital caliper. Tumor volumes were calculated based on the formula: 0.5326 (length [mm] x width2 [mm2]). Tumors were harvested, photographed, embedded and processed for staining as described below.
Histological examination
AccuMax human tissue microarray test slides of twelve colon adenomas and adenocarcinomas with corresponding non-neoplastic tissues (A713 VIII) were obtained from ISU ABXIS. Mouse tissues were harvested, fixed in 10% formalin and embedded in paraffin. All sections were stained with H&E according to standard procedures or by immunohistochemistry or immunofluorescence as described below.
In vivo BrdU labeling
Mice were intraperitoneal-injected with BrdU (100 mg kg−1 body weight). Colon tumors from AOM/DSS-treated mice were carefully harvested 3 h post-injection, fixed in 10% formalin and paraffin embedded. Paraffin sections were deparaffinized with xylene and alcohol series. After inactivation of endogenous peroxidase with 0.3% hydrogen peroxide and blocking with normal blocking serum, the sections were labeled using a mouse anti-BrdU antibody. After washing, the sections were incubated with biotinylated secondary antibody in the Vectastain Elite ABC staining kit (Vector Laboratories) for 1 h at room temperature, followed by treatment with the ABC reagent for 30 min. Aminoethyl carbazole (AEC) was used as the peroxidase substrate. Sections were washed, counterstained with hematoxylin, mounted and photomicrographs obtained.
Immunohistochemistry and immunofluorescence staining
For immunohistochemical staining: Sections were deparaffinized with xylene and alcohol series. After inactivation of endogenous peroxidase with 0.3% hydrogen peroxide and blocking with normal blocking serum, the sections were incubated with anti-β-catenin antibody overnight at 4°C. After washing, the sections were incubated with biotinylated secondary antibody (1:500) in the Vectastain Elite ABC staining kit (Vector Laboratories) for 1 h at room temperature, followed by treatment with the ABC reagent for 30 min. Aminoethyl carbazole (AEC) was used as the peroxidase substrate. Sections were then washed, counterstained with hematoxylin, mounted and photomicrographs obtained. Tissues stained with omission of primary antibody were captured using the same settings and used as negative controls. For immunofluorescence staining: Cryosections were blocked as described above then incubated with primary antibody overnight at 4°C, followed by incubation with the respective secondary antibodies conjugated to fluorescent labels (Alexa Flour 594 or 488; 1:500) (Invitrogen) for 1 h at room temperature. Slides were washed in PBS, stained with DAPI (100 ng mL−1), mounted and immunofluorescence images obtained using Olympus Fluorescence Microscopy equipped with Slidebook 5.0 analysis software37. Tissues stained with omission of primary antibody were captured using the same settings and used as negative controls.
Isolation of adult mouse colon epithelium
Colons of adult mice were isolated and the epithelium extracted by shaking the chopped colon in Dulbecco’s PBS containing 30 mM EDTA, 10% Fetal Bovine Serum (FBS), 1 mM DTT, pH 8.0 at 4°C for 30 to 40 min followed by centrifugation at 900 x g at 4°C for 10 min. Colon epitheliums were used to confirm epsins knockdown via Western blotting and detect transcription of Wnt pathway downstream genes via qRT-PCR.
Cell culture
Isolation and culture of primary mouse CECs from 4 to 6 day old WT or Epn1fl/fl;Epn2−/−;Villin-ERT2 Cre pups was performed as described70. Briefly, colons were harvested, split longitudinally, diced into very small fragments and washed in PBS-Glucose (PBS supplemented with 2% glucose and 1% penicillin/streptomycin). After excess fluid removal, colon fragments were digested for 1 h at 37 °C in PBS-Glucose supplemented with 100 μg mL−1 Dispase type II and 2 mg mL−1 Crude Collagenase type I. After crypt isolation was confirmed by microscopy, digestion enzymes were neutralized in DMEM-sorbitol (DMEM supplemented with 5% fetal calf serum (FCS) and 2% sorbitol). Supernatant containing crypts was obtained after a series of 1 min gravity sedimentation cycles. Single cell contaminants were removed by centrifugation at 300 rpm for 3 min. After isolation, colon epithelial progenitor cell-containing crypt aggregates were seeded on culture dishes pre-coated with 0.2% gelatin and cultured at 37°C with 5% CO2 in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 0.25 IU mL−1 insulin, 20 ng mL−1 epidermal growth factor (EGF), 50 μg mL−1 heparin and 1% penicillin/streptomycin. After 2 to 3 days, CECs were treated with 5 μM 4-hydroxytamoxifen for 72 h to induce epsin 1 deletion. Freshly isolated primary CECs were used for all experiments without further passage.
The HT-29 (human colorectal adenocarcinoma cell line, ATCC#HTB-38) and CMT-93 (mouse rectum carcinoma cell line, ATCC#CCL-223) cell lines were cultured at 37°C with 5% CO2 in high-glucose DMEM supplemented with 10% FBS and 1% penicillin/streptomycin.
The HEK 293T cell line (ATCC#CRL-11268) was cultured at 37°C with 5% CO2 in high-glucose DMEM supplemented with 10% FBS and 1% penicillin/streptomycin.
RNA interference
HT-29 cells were transfected by RNAiMAX according to the manufacturer’s instructions with siRNA duplexes of scrambled or human epsin 1 (UGCUCUUCUCGGCUCAAACUAAGGG) and epsin 2 (AAAUCCAACAGCGUAGUCUGCUGUG), designed using Ambion® Silencer® Select Predesigned siRNAs (Invitrogen). At 48 h post-transfection, cells were processed for Western blotting.
Plasmids and transfection
Flag-tagged Dvl2, unphosphorylatable β-catenin, HA-ubiquitin and NICD-myc plasmids were obtained from Addgene. Retroviral pLEX expression system was from Thermoscientific. Epsin 1 and epsin 1 ΔUIM plasmids and lentiviral pLL3.7 RNA interference vectors encoding epsins 1 and 2 shRNA were described previously29. All remaining truncation and single domain constructs were generated using standard subcloning procedures. Epsin 1 point mutations were made using QuikChange Site-Directed Mutagenesis Kit per manufacturer’s instruction. Epsin 1 point mutations were predicted using three-dimensional modeling by PEP-FOLD (http://bioserv.rpbs.univ-paris-diderot.fr/PEP-FOLD). Cloning and mutagenesis primers are available upon request. HEK 293T cells were transfected using Lipofectamine 2000 as instructed by the manufacturer. Lentiviral production was accomplished by transfecting Phoenix ECO cells (ATCC#CRL-3214) using Lipofectamine 2000. Viruses were harvested 48 hours post-transfection.
RNA isolation and quantitative real-time PCR
Total RNA was extracted using Trizol Reagent. One microgram of total RNA was treated with 1 U RNase-free DNase I to eliminate genomic DNA. The first strand cDNA was synthesized using the SuperScript III First-Strand Synthesis SuperMix. An aliquot of 1 μl of the product was subjected to qRT-PCR in a 7300 Real-Time PCR System (Applied Biosystems) using SYBR Green PCR Master Mix reagent as the detector. PCR amplification was performed in triplicate on 96-well optical reaction plates and replicated in three independent experiments. Gel electrophoresis and melting curve analyses were performed to confirm correct PCR product sizes and absence of nonspecific bands.
Immunoprecipitation and Western blotting
For Wnt signaling assays, CECs that had been starved overnight in serum free medium were treated with 100 ng mL−1 of Wnt3a for 0, 5, 15 or 30 min or 1, 2, 4, 6 or 8 h at 37°C, then washed twice with ice-cold PBS, lysed in Laemmli buffer and processed for Western blotting. For the MG132 experiments, cells were serum starved overnight in DMEM, pretreated for 4 h with 10 μM MG132, stimulated without or with Wnt3a as indicated, then prepared for Western blotting as described above. For immunoprecipitation, transfected HEK 293T cells were lysed with RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholic acid, 5 mM tetrasodium pyrophosphate, 50 mM sodium fluoride, 5 mM EDTA, 150 mM NaCl, 25 mM Tris, pH 7.5, 4 mM Na3VO4, 5 mM N-ethylmaleimide and protease inhibitor cocktail). For Dvl2 ubiquitination experiments, cells were lysed using one volume of denaturing buffer (1% SDS in 50 mM Tris, pH 7.5) and boiled for 10 min at 100 °C to denature protein complexes. Lysates were re-natured using nine volumes of ice-cold RIPA buffer then prepared for immunoprecipitation as follows. Cell lysates were pre-cleared with mouse IgG and protein G Sepharose beads for 2 h at 4 °C followed by incubation with antibodies against epsin 1, Dvl2, Flag or HA for 4 h at 4 °C. For negative controls, equal concentration of mouse IgG was added instead of specific antibodies. Precipitated proteins were eluted from beads using 2% SDS in 50 mM Tris, pH 7.5 and diluted 1:20 with RIPA buffer followed by Western blotting. Proteins were resolved by SDS-PAGE (7.5% acrylamide) followed by electroblotting to a polyvinylidene difluoride membrane and blocking with 3% milk (weight per volume). Primary antibodies were incubated at 4°C overnight, followed by incubation at room temperature with the respective horseradish peroxidase-conjugated secondary antibodies (1:2000) for 1 h. The immunoreactive proteins were detected by enhanced chemiluminescence with autoradiography. Western blots were quantified using NIH ImageJ software. Uncropped images of the Western blots depicted in the main figures are available in Supplementary Figure 4.
Luciferase reporter assay
HEK 293T cells were serum starved overnight and co-transfected with either TOPflash (containing TCF binding sites) or FOPflash (mutant, inactive TCF binding sites) luciferase expression vectors and with Renilla luciferase vector (control) using Lipofectamine 200054. Sixteen hours post-transfection, cells were incubated with recombinant human Wnt3a and/or CHIR-99021, an inhibitor of GSK3-β (GSK3-βi). After 8 h, luciferase activity in total cell lysates was measuring using Dual-Luciferase Reporter Assay Kit (Promega) and normalized for transfection efficiency via dividing by the Renilla luciferase activity. The TOP/FOP ratio was used as a quantitative measure of β-catenin-mediated TCF4 activity.
Internalization of biotinylated LRP6
Primary CECs were serum starved overnight and incubated with 1 nM cleavable EZ-Link Sulfo-NHS-S-S-Biotin dissolved in cold PBS at 4°C for 30 min with gentle shaking. Cells were washed with cold PBS plus 50 mM glycine to stop biotinylation. Cells were then changed to warm media with 100 ng mL−1 Wnt3a and incubated at 37°C for 0, 5, 15, or 30 min. Remaining surface biotin was cleaved by incubating with cleavage buffer (23 mM NaH2PO4, 27 mM Na2HPO4, 75 mM NaCl, 1% BSA, 10 mM EDTA, pH 8.0, 50 mM DTT) three times for 15 min each at 4°C with gentle shaking. Cells were lysed in RIPA buffer and processed for streptavidin bead pull-down. Pull-downs were separated by SDS-PAGE and analyzed by Western blotting. Endocytosed LRP6 was visualized by Western blotting using anti-LRP6 antibody.
Statistical analysis
We used 11 animals in all groups to detect at the 0.05 significance level with power of 0.80–0.90, an effect size of 1.55–1.65 SD units with the two-tailed Student’s t-test. Immunoprecipitation, Western blotting, IF and IHC staining, and luciferase reporter assays were replicated 6–8 times to achieve similar 0.05 significance using two-tailed Student’s t-tests. Quantitative RT-PCR was done in triplicate and replicated 3 times to achieve 0.05 significance using two-tailed Student’s t-test. All data are represented as the mean ± s.e.m and the P values indicated. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Kirk Bergstrom in Dr. Lijun Xia’s lab in our department and Dr. Bruce Vallance at the University of British Columbia in Canada, for sharing the CMT-93 cell line. We also thank the Oklahoma Medical Research Foundation imaging core for help with the histological studies. This work was supported in part by NIH grants R01HL-093242, R01HL-118676, P20 RR018758, a National Scientific Development Grant from the American Heart Association (AHA) (0835544N), grant from the Oklahoma Center for Advanced Science and Technology (OCAST) HR09-116, and a grant from the Department of Defense W81XWH-11-1-00226 to H. Chen; NIH grants R01DK085691 and P01HL-085607 to L. Xia; grants from OCAST AR11-043 and AH14-056 and from AHA-SDG 12SDG8760002 to Y. Dong; AHA Postdoctoral fellowships 13POST16940008 to K.L. Tessneer and 13POST17270006 to S. Pasula; AHA Predoctoral fellowship RSRCH016952 to X. Liu.
Footnotes
Author Contributions
B.C. and K.L.T. developed the project, performed experiments, analyzed data and wrote the manuscript together with the supervision of H.C.; J.M., X.L., S.H., S.P., H.W., H.S., Y.C., X.C., Y.D., M.L.B. and R.R. performed experiments, analyzed data and assisted in concept development; J.X.M., L.X. and H.C. provided key reagents, expert advice in the development of the project and assisted in editing the manuscript.
Competing Financial Interests
All authors declare no competing financial interests.
References
- 1.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Rubinfeld B, et al. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 4.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 5.Rubinfeld B, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 6.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes & development. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 8.Batlle E, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 9.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature genetics. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 10.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 11.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 12.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Frontiers in bioscience : a journal and virtual library. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 13.Kinzler KW, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 14.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 15.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 16.Rustgi AK. The genetics of hereditary colon cancer. Genes & development. 2007;21:2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 17.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 18.Ueno K, et al. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. British journal of cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You XJ, Bryant PJ, Jurnak F, Holcombe RF. Expression of Wnt pathway components frizzled and disheveled in colon cancer arising in patients with inflammatory bowel disease. Oncology reports. 2007;18:691–694. [PubMed] [Google Scholar]
- 20.Gupta S, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer research. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q, et al. Dishevelled family proteins are expressed in non-small cell lung cancer and function differentially on tumor progression. Lung Cancer. 2008;62:181–192. doi: 10.1016/j.lungcan.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Metcalfe C, et al. Dvl2 promotes intestinal length and neoplasia in the ApcMin mouse model for colorectal cancer. Cancer research. 2010;70:6629–6638. doi: 10.1158/0008-5472.CAN-10-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. Journal of cell science. 2007;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 25.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC cell biology. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Developmental cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, He X, Howe PH. Disabled-2 (Dab2) inhibits Wnt/beta-catenin signalling by binding LRP6 and promoting its internalization through clathrin. The EMBO journal. 2012;31:2336–2349. doi: 10.1038/emboj.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal JA, et al. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. The Journal of biological chemistry. 1999;274:33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 32.Shih SC, et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nature cell biology. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 33.Wendland B. Epsins: adaptors in endocytosis? Nature reviews. Molecular cell biology. 2002;3:971–977. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko G, et al. Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21511–21516. doi: 10.1073/pnas.1016390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. The Journal of biological chemistry. 2001;276:29257–29267. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 37.Pasula S, et al. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. The Journal of clinical investigation. 2012;122:4424–4438. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tessneer KL, et al. Genetic Reduction of Vascular Endothelial Growth Factor Receptor 2 Rescues Aberrant Angiogenesis Caused by Epsin Deficiency. Arteriosclerosis, thrombosis, and vascular biology. 2013 doi: 10.1161/ATVBAHA.113.302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessneer KL, et al. Endocytic Adaptor Protein Epsin Is Elevated in Prostate Cancer and Required for Cancer Progression. ISRN Oncology. 2013;2013:8. doi: 10.1155/2013/420597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, et al. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. The Journal of biological chemistry. 2006;281:14129–14135. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 41.Pawlowski KM, et al. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2009;60 (Suppl 1):85–94. [PubMed] [Google Scholar]
- 42.Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cammarota R, et al. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. Journal of translational medicine. 2010;8:112. doi: 10.1186/1479-5876-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 45.An G, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. The Journal of experimental medicine. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer science. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clevers H. Wnt breakers in colon cancer. Cancer cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 48.Hawryluk MJ, et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 49.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 50.Kazazic M, et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 51.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama S, Kishida S, Chayama K, Koyama S, Kikuchi A. Ubiquitin-interacting motifs of Epsin are involved in the regulation of insulin-dependent endocytosis. Journal of biochemistry. 2005;137:355–364. doi: 10.1093/jb/mvi044. [DOI] [PubMed] [Google Scholar]
- 53.Gao C, et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nature cell biology. 2010;12:781–790. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Gao X, Wen J, Ning Y, Chen YG. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. The Journal of biological chemistry. 2006;281:8607–8612. doi: 10.1074/jbc.M600274200. [DOI] [PubMed] [Google Scholar]
- 55.Jung H, et al. Deubiquitination of Dishevelled by Usp14 is required for Wnt signaling. Oncogenesis. 2013;2:e64. doi: 10.1038/oncsis.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cellular signalling. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Ding Y, Zhang Y, Xu C, Tao QH, Chen YG. HECT domain-containing E3 ubiquitin ligase NEDD4L negatively regulates Wnt signaling by targeting dishevelled for proteasomal degradation. The Journal of biological chemistry. 2013;288:8289–8298. doi: 10.1074/jbc.M112.433185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 59.Su LK, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 60.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 61.DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–833. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- 62.Xie X, Cho B, Fischer JA. Drosophila Epsin’s role in Notch ligand cells requires three Epsin protein functions: the lipid binding function of the ENTH domain, a single Ubiquitin interaction motif, and a subset of the C-terminal protein binding modules. Developmental biology. 2012;363:399–412. doi: 10.1016/j.ydbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Overstreet E, Chen X, Wendland B, Fischer JA. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of delta in the developing eye. Current biology : CB. 2003;13:854–860. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 64.Eun SH, et al. Identification of genes that interact with Drosophila liquid facets. Genetics. 2007;175:1163–1174. doi: 10.1534/genetics.106.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 67.Kim HA, et al. Notch1 counteracts WNT/beta-catenin signaling through chromatin modification in colorectal cancer. The Journal of clinical investigation. 2012;122:3248–3259. doi: 10.1172/JCI61216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lepourcelet M, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 69.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nature reviews. Drug discovery. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 70.Campbell CF. Isolation and culture of mouse intestinal cells. Methods Mol Biol. 2010;633:197–206. doi: 10.1007/978-1-59745-019-5_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.