Abstract
Leptin, a peptide hormone released by adipose tissue, acts on the hypothalamus to control cravings and appetite. Leptin also acts to decrease taste responses to sweet substances, though there is little detailed information regarding where leptin acts in the taste transduction cascade. The present study examined the effects of leptin on sweet-evoked responses and neuro transmitter release from isolated taste buds. Our results indicate that leptin moderately decreased sweet-evoked calcium mobilization in isolated mouse taste buds. We also employed Chinese hamster ovary biosensor cells to examine taste transmitter release from isolated taste buds. Leptin reduced ATP and increased serotonin release in response to sweet stimulation. However, leptin has no effect on bitter-evoked transmitter release, further showing that the action of leptin is sweet specific. Our results support those of previous studies, which state that leptin acts on taste tissue via the leptin receptor, most likely on Type II (Receptor) cells, but also possibly on Type III (Presynaptic) cells.
Key words: ATP, leptin, serotonin, taste bud
Introduction
It is well established that appetite is regulated primarily by neuronal circuits in the central nervous system (CNS) (the hypothalamus, brain stem, and reward centers) and their communication with the gut (Ahima and Antwi 2008). Hormones secreted by the gut, adipose tissue, and other peripheral organs target these CNS centers to help regulate feeding. These hormones include leptin, ghrelin, insulin, and others. Hormones that regulate appetite can also affect taste (Shin et al. 2008; Horio et al. 2010; Martin et al. 2010; Yoshida 2012; Dotson et al. 2013). Interestingly, some of these hormones influence taste by binding directly to receptors on taste bud cells (Dando 2010; Dotson et al. 2013). Taste lies at the initial step of feeding and is used to identify potential food items (Norgren et al. 2006; Duffy 2007). Thus, appetite-regulating hormones likely influence the selection, consumption, and palatability of food.
Sweet, bitter, and umami stimuli bind to and activate taste G protein-coupled receptors, leading to a PLC-IP3-mediated rise in intracellular Ca2+ concentration and membrane depolarization in taste cells (reviewed in Chaudhari and Roper 2010). These events initiate secretion of the taste transmitter ATP via pannexin 1 hemichannels or calcium homeostasis modulator 1 (CALHM1) channels (Huang et al. 2007; Romanov et al. 2007; Taruno et al. 2013). ATP activates gustatory sensory afferent fibers (Rong et al. 2000; Finger et al. 2005). ATP released during sweet, bitter, or umami stimulation also causes neighboring Presynaptic (Type III) cells to release serotonin (5-HT) (Huang et al. 2007). Serotonin provides negative para crine feedback onto Receptor cells, reducing ATP secretion (Huang et al. 2009). These cell–cell interactions within the taste bud all contribute to shaping the final output signal to the afferent taste fibers (Roper 2013; Chaudhari 2014).
Leptin is a peptide hormone released by adipose tissue that acts in the hypothalamus to inhibit food intake. Its role in appetite regulation is critical; leptin deficiency is associated with severe obesity (Schwartz et al. 2000). In addition to its known targets in the hypothalamus, leptin receptors have been identified in peripheral tissue, including liver, lungs, muscle, and pancreatic β-cells, suggesting important roles for leptin along with its role in appetite regulation (Ghilardi et al. 1996; Kieffer et al. 1996; Hoggard et al. 1997). Importantly, leptin receptor (Ob-Rb) expression has been reported in taste tissue and is suggested to play an integral role in the regulation of sweet stimuli (Kawai et al. 2000; Shigemura et al. 2003). Further, diurnal variations in circulating plasma leptin levels have been correlated with sweet taste thresholds in humans (Nakamura et al. 2008) and mice (Kawai et al. 2000).
There is little detailed information regarding where leptin acts in the taste transduction cascade. Kawai et al. (2000) reported that leptin hyperpolarizes a population of taste cells, though they were only able to indirectly infer that these cells were sweet-sensing receptor cells. Also, intraperitoneal administration of leptin reduced sweet-evoked chorda tympani (CT) and glossopharyngeal nerve responses (Kawai et al. 2000). However, a more recent study reported that in some conditions, leptin increases CT nerve responses to sweet stimulation (Lu et al. 2012). We undertook a study to examine the effects of leptin on sweet-evoked responses and neurotransmitter release from isolated taste buds. Our results indicate that leptin reduces ATP and increases serotonin release in response to sweet stimulation. Leptin has no effect on bitter-evoked transmitter release.
Materials and methods
Animals
All experimental protocols in this study were approved by the University of Miami Animal Care and Use Committee. Adult C57BL/6J mice (wild-type) mice of both sexes were sacrificed by CO2 exposure followed by cervical dislocation.
Isolating taste buds
To isolate taste buds, the tongues were dissected from C57BL/6J mice. The oral epithelium was peeled from the surface after incubating with an enzyme mixture (1mg/mL collagenase A, Roche; 2.5mg/mL dispase II, Roche; 0.25mg/mL elastase, Worthington; and 1mg/mL trypsin inhibitor, Sigma) (Huang et al. 2007; 2011). Taste buds were removed from the circumvallate (CV) papillae with a fire-polished glass micropipette. For imaging taste-evoked Ca2+ mobilization, isolated taste buds were loaded with the calcium-sensitive dye, Fura-2AM (5 µM, Invitrogen), following a previously described protocol (Huang et al. 2005; DeFazio et al. 2006; Dando et al. 2012). Taste buds were then placed in a recording chamber, secured with Cell-Tak (BD Biosciences) and superfused with Tyrode’s buffer (140mM NaCl, 5mM KCl, 2mM CaCl2, 1mM MgCl2, 10mM HEPES, 10mM glucose, 10mM Na-pyruvate, 5mM NaHCO3, pH 7.2, 300–330 mOsm).
Transmitter release using biosensors
Taste buds were isolated from mouse circumvallate papillae of C57BL/6J mice as described above and transferred to a recording chamber where they were superfused with physiological buffer. Genetically engineered Chinese hamster ovary cells expressing 5-HT2c (serotonin) and P2X2/X3 receptors, as well as endogenous P2Y (ATP) receptors, were loaded with 5 µM Fura-2AM as described by Huang et al. (2007) and transferred into the recording chamber with the taste buds. Biosensor sensitivity was screened by testing their responses to either 5-HT (3nM) or ATP (1 µM). Responsive biosensors were moved with a fire-polished glass micropipette adjacent to isolated taste buds to monitor transmitter release from the taste buds. When recording 5-HT secretion, ATP receptors on the biosensors were desensitized by application of 500 µM ATP for 30min. Conversely, when recording ATP secretion, biosensor 5-HT2c receptors were desensitized with 10 µM 5-HT for 20min (Huang Y, personal communication). 5-HT or ATP release was detected by sharp increases in intracellular Ca2+ effected through purino- or serotonin receptors, monitored with Fura-2 imaging (Huang et al. 2007). In separate control experiments, we confirmed that the biosensor cells were unaffected by KCl, sweet or bitter taste stimuli, leptin, and the leptin receptor antagonist (superactive mouse leptin antagonist [SMLA]), at concentrations used in this study.
Ca2+ imaging
Images were recorded at ×20 using an Olympus IX71 inverted fluorescence microscope. Fluorescence of Fura-2AM-loaded cells was captured using Indec Workbench v5 software. Cells were excited at 340 and 380nm and emission was recorded at 510nm. The F340/F380 ratio was converted to [Ca2+]i using a Fura-2AM calcium imaging calibration kit (Invitrogen) (Grynkiewicz et al. 1985).
To test the actions of leptin on taste-evoked calcium responses of taste buds, we perfused 1 µg/mL leptin (Kawai et al. 2000) over taste buds during stimulation with tastants. Taste-evoked Ca2+ responses were determined in the presence and absence of leptin. Responses in the presence of leptin were normalized to the responses in the absence of leptin from the same experiment and compared using paired t-tests. All statistical analyses were conducted using GraphPad Prism version 5 for Windows (GraphPad Software).
To test the effects of leptin on taste transmitter secretion, we measured taste-evoked ATP and 5-HT release from CV taste buds in the presence and absence of 100–1000ng/mL leptin. For 5-HT secretion experiments, isolated taste buds were preloaded with 500 µM 5-OH-l-tryptophan (5-HT precursor; Sigma) for 30min to maximize the signal (Huang et al. 2005). Biosensor responses were normalized to responses in the absence of leptin from the same experiment. We analyzed the effect of leptin on ATP and 5-HT release by comparing normalized biosensor responses in the presence and absence of leptin, using paired t-tests.
Stimuli
Taste stimuli for sweet consisted of sucralose (1mM) and SC45647 (0.1mM); for bitter, a mixture of denatonium (1mM) and cycloheximide (10 µM) (Sigma). Other stimuli included KCl-Tyrode’s (50mM KCl substituted equimolar for NaCl), 1–10 µM ATP (Sigma), 3nM 5-HT (Sigma), 100–1000ng/mL leptin (Invitrogen), and 2.5–25 µg/mL SMLA (Protein Laboratories Rehovot). All stimuli and pharmacological agents were diluted in Tyrode’s buffer. SMLA was diluted in 0.01% bovine serum albumin Tyrode’s.
Results
Effect of leptin on taste-evoked Ca2+ responses
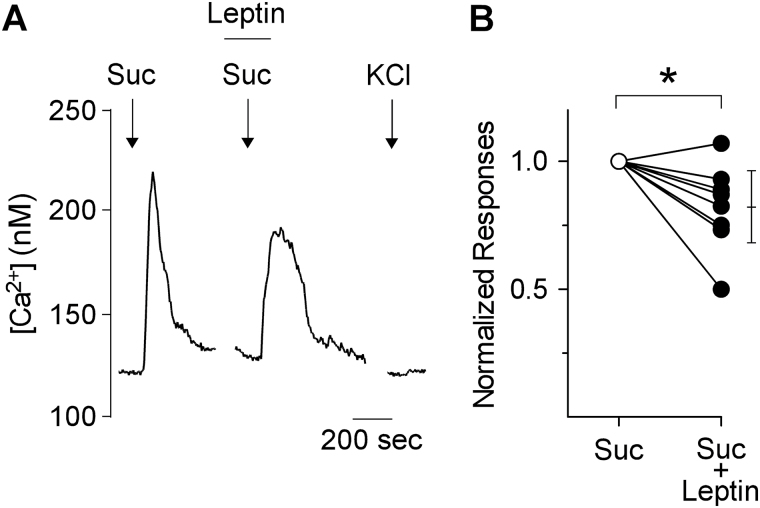
A recent report cited unpublished findings that leptin directly acts on sweet-evoked Ca2+ mobilization (Jyotaki et al. 2010). To test this hypothesis, we examined the effect of leptin on sucralose-evoked Ca2+ responses in isolated mouse circumvallate taste buds. We found that at very high concentrations (1000ng/mL), leptin caused a small but statistically significant depression (0.82±0.05, n = 8) of sucralose-evoked Ca2+ mobilization in sweet-sensitive taste cells (paired t-test, 2-tailed, P = 0.02) (Figure 1). None of these cells responded to 50mM KCl, consistent with their being Type II Receptor cells (DeFazio et al. 2006).
Figure 1.
Calcium responses of isolated circumvallate taste buds to sucralose in the absence and presence of leptin. (A) Stimulation of mouse taste buds with 1mM sucralose (arrows) elicits Ca2+ responses that are significantly decreased by 1000 ng/mL leptin (bar). (B) Summary of data from 8 taste buds during stimulation with sucralose alone (open circle) and in the presence of leptin (filled circles). Leptin caused a moderate, yet significant decrease in the sucralose-evoked calcium responses (a decrease to 0.82±0.05). Paired t-test, 2-tailed, P < 0.02. *indicates statistical significance. Bar on right shows the mean and 95% confidence interval. Responses for each experiment were normalized to the sucralose-alone response. None of the cells that responded to sucralose were responsive to 50mM KCl.
Effect of leptin on taste-evoked transmitter secretion
The modest effect of leptin on sweet-evoked Ca2+ mobilization in taste cells was unexpected, given its suppression of taste nerve response to sweet substances (Kawai et al. 2000). Thus, we next explored taste mechanisms downstream of Ca2+ mobilization and specifically whether leptin reduced taste-evoked transmitter secretion. Yoshida et al. (2013) had proposed a hypothetical model for the action of leptin on sweet-receptive taste cells that included suppression of ATP release, but this had not yet been explored experimentally.
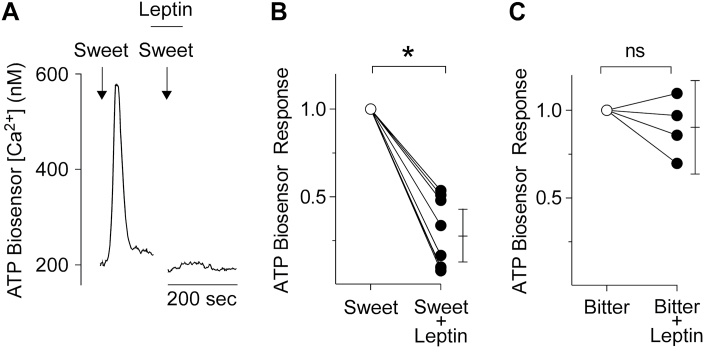
First, we used transmitter biosensors to test the effect of leptin on taste-evoked ATP secretion from isolated taste buds. ATP release elicited by sweet stimulation decreased significantly in the presence of 200–1000ng/mL leptin (paired t-test, 2-tailed, P < 0.0001) (Figure 2A and 2B). In contrast, leptin, even at 1000ng/mL, had no significant effect on bitter-evoked ATP release (paired t-test, 2-tailed, P = 0.344) (Figure 2C).
Figure 2.
Sweet-evoked neurotransmitter secretion from taste buds, measured with ATP-biosensors. (A) ATP biosensor responses to sweet taste stimulation with 1mM sucralose + 0.1mM SC45647 (arrows) before and during bath application of 1000ng/mL leptin (bar). (B) Summary of data from 9 taste buds. For each data point, the biosensor response during exposure to leptin was normalized to the corresponding response before adding leptin. The ATP biosensor response during sweet stimulation (open circle) decreased significantly in the presence of 200–1000ng/mL leptin (filled circles). Paired t-test, 2-tailed, P < 0.0001. (C) 1000ng/mL leptin did not affect bitter-evoked (10 µM cycloheximide + 1mM denatonium) ATP secretion from 4 taste buds. Paired t-test, 2-tailed, P = 0.344. Responses were normalized to the stimulus-alone response. Bars on right shows means ± 95% confidence intervals (ns, nonsignificant; *indicates statistical significance.).
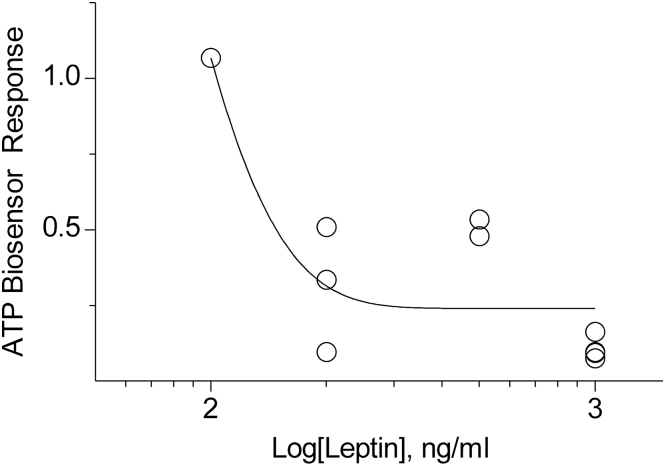
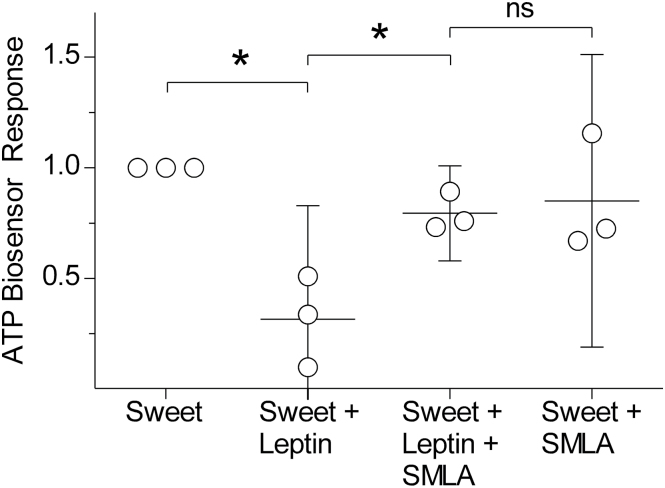
Leptin suppressed sweet-evoked ATP secretion in a roughly concentration-dependent manner, with little to no effect seen at 100ng/mL and unambiguous suppression at or near 1000ng/mL (Figure 3). Because leptin is proposed to act on taste cells via the leptin receptor, we tested whether the specific antagonist SMLA reduced the actions of leptin (Shpilman et al. 2011). In 3 experiments, we were able to record ATP release before, during, and after leptin treatment (200ng/mL), with and without SMLA (5 µg/mL). The results showed that SMLA reversed the inhibitory effect of leptin on sweet-evoked ATP secretion (ANOVA, P < 0.01; Tukey test, P < 0.05 for all comparisons) (Figure 4). SMLA on its own had no effect on the biosensors nor on ATP release from taste buds.
Figure 3.
Sweet-evoked ATP secretion from isolated CV taste buds measured using biosensors during application of varying concentrations of leptin (100–1000ng/mL). We observed little if any effect at 100ng/mL and unambiguous suppression at 1000ng/mL. Responses were normalized to the stimulus-alone response and fit with an exponential curve.
Figure 4.
ATP secretion from isolated CV taste buds in response to sweet, leptin (200ng/mL), and SMLA (5 µg/mL) measured using biosensors. Leptin significantly reduced sweet-evoked ATP secretion, and the addition of SMLA recovered the sweet-evoked response. Data were analyzed with a one-way ANOVA followed by Tukey post hoc test for pairwise comparisons, P < 0.05 for significant differences. *indicates statistical significance. Responses were normalized to the sweet-alone response. Bars indicate the mean and 95% confidence interval (ns, nonsignificant).
During gustatory stimulation, Type II Receptor cells release ATP which excites neighboring Type III Presynaptic cells to secrete serotonin (Huang et al. 2007). Serotonin provides negative (paracrine) feedback onto Type II cells, reducing their ATP output (Huang et al. 2009). Thus, we investigated whether leptin also alters taste-evoked 5-HT release. Unexpectedly, leptin (1000 ng/mL) significantly increased sweet-evoked 5-HT secretion (paired t-test, 2-tailed, P = 0.01) (Figure 5). In marked contrast, leptin had no effect on bitter-evoked 5-HT secretion (paired t-test, 2-tailed, P = 0.38) (Figure 6A).
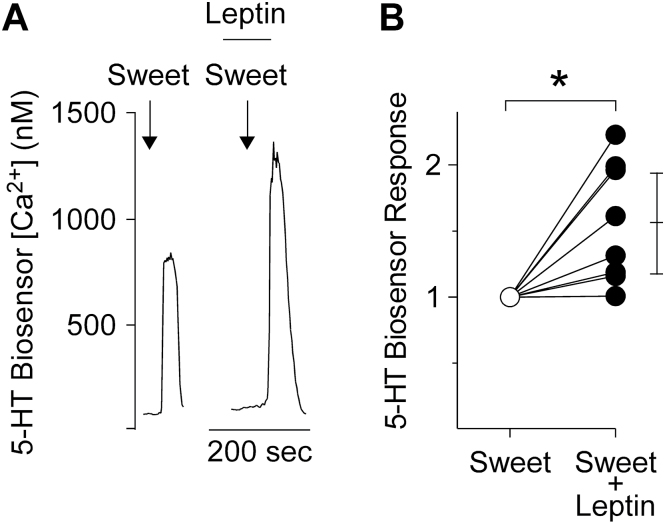
Figure 5.
Sweet-evoked serotonin (5-HT) secretion from taste buds, measured with biosensors. (A) 5-HT biosensor responses to sweet taste stimulation with sweet (arrows) before and during bath application of 1000ng/mL leptin (bar). 5-HT secretion is significantly enhanced in the presence of leptin. (B) Summary of data from 8 taste buds. The 5-HT biosensor response during sweet stimulation (open circle) increased significantly in the presence of leptin (filled circles). Paired t-test, 2-tailed, P = 0.01. *indicates statistical significance. Bar on right shows the mean and 95% confidence interval. Responses for each experiment were normalized to the sweet-alone response.
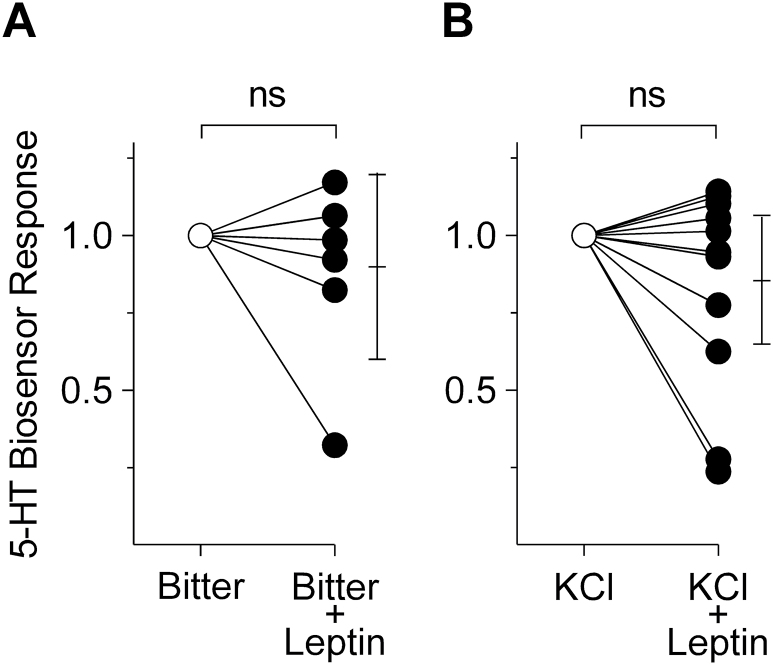
Figure 6.
Leptin had no effect on bitter- and depolarization-evoked 5-HT secretion from taste buds. (A) There was no significant difference in bitter-evoked (10 μM cycloheximide + 1 mM denatonium) 5-HT secretion before (open circle) and during 1000 ng/mL leptin (filled circles) (n = 6 taste buds). (B) Leptin did not affect depolarization-evoked (50 mM KCl) 5-HT secretion from 11 taste buds. Responses were normalized to the stimulus-alone response. Paired t-test, 2-tailed, P > 0.13. Bar on right shows the mean and 95% confidence intervals (ns, nonsignificant).
As stated, Type III (Presynaptic) cells are indirectly stimulated to secrete 5-HT when taste buds are exposed to sweet or bitter tastants due to ATP released from Receptor (Type II) cells. Presynaptic (Type III) cells are also depolarized by KCl. Depolarization elicits Ca2+ influx through voltage-gated Ca2+ channels in these cells and directly stimulates 5-HT exocytosis (Huang et al. 2007; 2009). We tested whether leptin alters direct, depolarization-evoked 5-HT secretion. Figure 6B shows that even a high concentration of leptin (1000ng/mL) wdid not significantly affect KCl-evoked 5-HT release (paired t-test, 2-tailed, P = 0.13).
Discussion
This study was designed to test the effects of leptin on chemotransduction mechanisms in isolated taste buds from mouse circumvallate papillae, particularly for sweet and bitter tastes. Our findings show that leptin produces only modest reduction of taste-evoked Ca2+ mobilization in taste bud cells but has a striking action on taste-evoked transmitter secretion, specifically for sweet stimuli.
Previous studies have reported that leptin hyperpolarizes taste cells via activation of K+ channels (Kawai et al. 2000; Yoshida et al. 2013). However, the identity of the cells responding to leptin was not established; it is uncertain that they were sweet-sensing taste receptor cells. More recent studies found that impulse activity in sweet-sensing taste cells was depressed by leptin (Yoshida et al. 2013). Our report firmly establishes that leptin caused a small but significant depression of taste-evoked Ca2+ mobilization in sweet-sensitive, Type II (Receptor) taste cells, at least for high leptin concentrations. The mechanism by which leptin decreased Ca2+ responses is unknown. Activation of Ob-Rb induces numerous downstream signals such as signal transducer and activator of transcription 3 (STAT3) (Shigemura et al. 2003). It seems unlikely that the observed, leptin-mediated, reduction in sweet-evoked calcium mobilization is mediated by transcription factors. However, leptin-induced activation of Ob-Rb has been shown to stimulate numerous kinases including ERK1/2 and PI3K (Lin et al. 2014). Increased activation of cytosolic kinases may regulate the function of either ryanodine receptors or TRP channels to decrease intracellular [Ca2+]. Jyotaki et al. (2010) describe unpublished findings that leptin suppresses sweet-evoked Ca2+ responses in enteroendocrine STC-1 cells and drew parallels with taste cells. Clearly, the mechanisms by which leptin reduces sweet-evoked Ca2+ mobilization require further study.
The main effect of leptin appeared to be on the release of taste transmitters, ATP and serotonin (5-HT). Leptin significantly decreased sweet-evoked ATP secretion from taste buds but had the converse effect on 5-HT release evoked by sweet stimuli. Leptin did not alter transmitter release in response to stimulation with a mixture of denatonium and cycloheximide, potent bitter taste stimuli. Though we did not test all classes of taste stimuli over an expansive concentration range, our results support the current body of evidence that leptin’s effect on taste is sweet-specific. Taste stimulation directly elicits ATP release by exciting Type II (Receptor) taste cells, and indirectly by cell–cell interactions in the taste bud causes Type III cells to secrete 5-HT release. We surmise that the principal action of leptin is on sweet-sensitive Receptor cells, although there is no direct evidence for the expression of leptin receptors specifically on those cells. In principal, because 5-HT is an inhibitory transmitter in the taste bud (Huang et al. 2009), leptin may instead have a principal action on Presynaptic (Type III) cells, increasing 5-HT secretion (as we show), and thereby inhibiting Receptor cells and ATP release. Because 5-HT release is itself initiated by Receptor cells, such an enhanced negative feedback mechanism would depend on the ability of leptin to markedly increase the gain of ATP-induced 5-HT release, and this effect would need to be specific for sweet-evoked ATP release. Our study does not examine this conundrum, and how leptin enhances serotonergic mechanisms in taste buds remains unanswered. Though the specific cell target of leptin is unclear, the end result of a leptin-mediated decrease in ATP secretion, shown in this study, would affect transmission of the signal to the taste afferent fibers, in accordance with the results of Kawai et al. (2000). This lends support to the argument that leptin modulates food intake not only at the level of the CNS through its action in the hypothalamus, but also in the periphery by influencing sweet taste.
Our findings on isolated taste buds are consistent with leptin having a negative action on sweet-evoked responses in gustatory nerves, as originally reported by Kawai et al. (2000). However, data in Kawai et al. (2000) differ from findings by Lu et al. (2012) who reported that administering leptin enhanced sweet-evoked responses in the CT nerve if taste stimuli were warmed to 35 °C. It is important to note that our studies and those of Kawai et al. (2000) were conducted at room temperature, which might readily explain the different results.
Leptin concentrations that we and others (Kawai et al. 2000) used on isolated taste buds are relatively high compared to circulating plasma levels in mice (~2–15ng/mL [Ahrén 2000]). Nonetheless, our experiments with the leptin receptor antagonist, SMLA, support that leptin acts on taste tissue via the leptin receptor (Figure 4). Studies show that taste buds express the leptin receptor, Ob-Rb (Kawai et al. 2000; Shigemura et al. 2003; 2004). Yoshida et al. (2013) cite unpublished observations that 30%–40% of taste cells that express a sweet receptor also express the leptin receptor. Our results suggest an alternate or at least additional potential target for leptin, Type III (Presynaptic) cells. Future studies should examine leptin receptor expression and leptin-mediated changes in transmitter release from isolated single taste cells to further resolve the specific cell target(s) for leptin in taste buds and the mechanism of leptin’s sweet-specific action.
Funding
This work was supported by the National Institutes of Health - National Institute on Deafness and Other Communication Disorder [1R01DC007630 and 2R01DC000374 to S.D.R.].
Acknowledgements
We thank the Nirupa Chaudhari providing the Chinese hamster ovary cells and cell culture facilities and the Roper Lab, Chaudhari Lab, and Dr Anthony Huang for helpful feedback on experimental design.
References
- Ahima RS, Antwi DA. 2008. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 37:811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrén B. 2000. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol Scand. 169:325–331. [DOI] [PubMed] [Google Scholar]
- Chaudhari N. 2014. Synaptic communication and signal processing among sensory cells in taste buds. J Physiol.592:3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R. 2010. Endogenous peripheral neuromodulators of the mammalian taste bud. J Neurophysiol. 104:1835–1837. [DOI] [PubMed] [Google Scholar]
- Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2012. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 32:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. 2006. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 26:3971–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Geraedts MC, Munger SD. 2013. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 24:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB. 2007. Variation in oral sensation: implications for diet and health. Curr Opin Gastroenterol. 23:171–177. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. 2005. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310:1495–1499. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. 1996. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 93:6231–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 260:3440–3450. [PubMed] [Google Scholar]
- Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM. 1997. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun. 232:383–387. [DOI] [PubMed] [Google Scholar]
- Horio N, Jyotaki M, Yoshida R, Sanematsu K, Shigemura N, Ninomiya Y. 2010. New frontiers in gut nutrient sensor research: nutrient sensors in the gastrointestinal tract: modulation of sweet taste sensitivity by leptin. J Pharmacol Sci. 112:8–12. [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. 2009. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 29:13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2007. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 104:6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. 2005. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 25:843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E, Roper SD. 2011. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One. 6:e25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotaki M, Shigemura N, Ninomiya Y. 2010. Modulation of sweet taste sensitivity by orexigenic and anorexigenic factors. Endocr J. 57:467–475. [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. 2000. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 97:11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Habener JF. 1996. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun. 224:522–527. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yang SH, Tang HY, Cheng GY, Davis PJ, Grasso P. 2014. Biologically active leptin-related synthetic peptides activate STAT3 via phosphorylation of ERK1/2 and PI-3K. Peptides. 57:95–100. [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. 2012. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav. 107:533–539. [DOI] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, et al. 2010. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 59:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Sanematsu K, Ohta R, Shirosaki S, Koyano K, Nonaka K, Shigemura N, Ninomiya Y. 2008. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes. 57:2661–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. 2006. Gustatory reward and the nucleus accumbens. Physiol Behav. 89:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. 2007. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 26:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Burnstock G, Spyer KM. 2000. P2X purinoceptor-mediated excitation of trigeminal lingual nerve terminals in an in vitro intra-arterially perfused rat tongue preparation. J Physiol. 524(Pt 3):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. 2013. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 24:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature. 404:661–671. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Miura H, Kusakabe Y, Hino A, Ninomiya Y. 2003. Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch Histol Cytol. 66:253–260. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. 2004. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 145:839–847. [DOI] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, et al. 2008. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, Boder E, Halpern Z, Elinav E, Gertler A. 2011. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 286:4429–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R. 2012. Hormones and bioactive substances that affect peripheral taste sensitivity. J Oral Biosci. 54:67–72. [Google Scholar]
- Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, Ninomiya Y. 2013. Modulation of sweet responses of taste receptor cells. Semin Cell Dev Biol. 24:226–231. [DOI] [PubMed] [Google Scholar]