Abstract
Recent studies suggest that because of their energy value, sugars are more rewarding than non-caloric sweeteners. However, intragastric infusion data indicate that sugars differ in their postoral appetite-stimulating effects. We therefore compared the preference for isocaloric 8% sucrose, glucose, and fructose solutions with that of a non-caloric sweetener solution (0.8% sucralose) in C57BL/6J mice. Brief 2-bottle tests indicated that sucralose was isopreferred to sucrose but more preferred than glucose or fructose. Yet, in long-term tests, the mice preferred sucrose and glucose, but not fructose to sucralose. Additional experiments were conducted with a non-caloric 0.1% sucralose + 0.1% saccharin mixture (S + S), which does not have the postoral inhibitory effects of 0.8% sucralose. The S + S was preferred to fructose in brief and long-term choice tests. S + S was also preferred to glucose and sucrose in brief tests, but the sugars were preferred in long-term tests. In progressive ratio tests, non-deprived and food-deprived mice licked more for glucose but not fructose than for S + S. These findings demonstrate that the nutrient-specific postoral actions, not calories per se, determine the avidity for sugar versus non-caloric sweeteners. Furthermore, sweet taste intensity and potential postoral inhibitory actions must be considered in comparing non-caloric and caloric sweeteners.
Key words: fructose, postoral conditioning, progressive ratio test, sucralose, sucrose
Introduction
The attraction to natural sugars displayed by many mammalian species starts with the activation of sweet taste receptors in the mouth (Bachmanov and Beauchamp 2007). Sweet taste alone, however, does not determine sugar appetite, and numerous studies demonstrate an important postoral influence on sugar intake and preference. In rodents, intragastric (IG) infusions of sucrose, glucose, or glucose polymers (e.g., polycose) can stimulate the intake of and preference for flavored solutions (the conditioned stimulus, CS+) compared with different flavored solutions (the CS−) paired with IG water infusions (Sclafani and Ackroff 2012b). These postoral appetite-stimulating and preference conditioning effects of sugars are referred to as “appetition” to distinguish them from the postoral satiation process that suppresses intake (Sclafani 2013). IG sugar infusions can condition preferences for inherently unpalatable flavors (e.g., bitter or sour tastes) but produce stronger appetition effects with palatable sweet flavors, demonstrating that oral (taste) and postoral factors contribute to the potent reward effects of sugars (Sclafani and Ackroff 2012b). Consistent with these findings, sweet ageusic knockout mice (T1r3 KO, Trpm5 KO, Calhm1 KO) are indifferent to sugars in brief access tests but develop robust preferences for sucrose and glucose over water in 24-h two-bottle tests, although they consume significantly less sugar than do sweet-sensitive wild-type mice (Zukerman et al. 2009; Zukerman et al. 2013c; Sclafani et al. 2014a).
Taste and postoral factors in sugar intake and preference have long been investigated by comparing the ingestive response of rodents to sugar versus non-caloric sweeteners (Hausmann 1933). Based on the results of intake, preference, and operant tests, recent studies have concluded that sugars are more rewarding than non-caloric sweeteners (saccharin and sucralose) (de Araujo et al. 2008; Domingos et al. 2011; Beeler et al. 2012; Scheggi et al. 2013; Tellez et al. 2013). An important consideration in comparing sugars with non-caloric sweeteners is differential taste palatability. Saccharin, the most widely used non-caloric sweetener in rodent research, has limited palatability compared with sucrose. The most attractive saccharin solutions (0.2–0.4%) to rats are “isopreferred” only to dilute sucrose solutions (2–4%, Young and Madsen 1963; Smith and Sclafani 2002). Rats have been tested with other non-caloric sweeteners (acesulfame K, stevia, sucralose, and aspartame), but none appear to be more attractive than saccharin and several are less so (Sclafani and Abrams 1986; Smith and Sclafani 2002; Sclafani and Clare 2004; Bello and Hajnal 2005; Dess et al. 2009; Sclafani et al. 2010a). Sucralose is a peculiar sweetener because most rats avoid rather than prefer it (Sclafani and Clare 2004; Bello and Hajnal 2005; Loney et al. 2011). In contrast, mice (C57BL/6J, 129) display significant preferences for sucralose as well as saccharin (Bachmanov et al. 2001). Recent studies have reported equal preferences for sucralose and sucrose in mice at selected concentrations (de Araujo et al. 2008; Sclafani et al. 2010b; Domingos et al. 2011). A drawback of sucralose, however, is that at higher concentrations it has postoral inhibitory actions that limit intake (Sclafani et al. 2010b).
Studies comparing caloric versus non-caloric sweeteners usually use sucrose, the prototypical sugar used in taste and feeding studies. However, common food sugars differ in their postoral appetition effects at isocaloric concentrations, which has implications for the interpretation of findings obtained with sucrose versus non-caloric sweeteners. In particular, IG glucose infusions are much more effective than isocaloric fructose infusions in stimulating intake and conditioning preferences in rodents (Sclafani and Ackroff 2012b). Also, although sweet ageusic KO mice develop strong sucrose and glucose preferences in 24-h tests, they fail to prefer fructose to water (Zukerman et al. 2009; Zukerman et al. 2013c). Based on these findings, the postoral appetition action of sucrose, a glucose + fructose disaccharide, is attributed primarily to its glucose moiety. If sweetener preferences are determined primarily by the energy value of sugars, as suggested by some investigators (Beeler et al. 2012; Scheggi et al. 2013), then mice should prefer all 3 sugars over non-caloric sweetener alternatives. On the other hand, if sugar-specific appetition effects are the critical factor, than mice should prefer sucrose and glucose, but not fructose, over non-caloric sweeteners.
In this study, we compared caloric versus non-caloric sweetener preference and reward in C57BL/6J (B6) mice using different caloric sugar and non-caloric sweetener solutions. The first 2 experiments compared the preference for 8% sucrose, glucose, or fructose with that of 0.8% sucralose to determine the role of caloric versus specific nutritive effects on sweetener preference. Experiment 3 further compared sweetener preferences using 8% sugars but replaced 0.8% sucralose with a mixture of 0.1% sucralose and 0.1% saccharin, which has a palatable taste but minimal postoral inhibitory effects. Non-caloric sweetener blends are commonly used in diet drinks but rarely in animal studies (Powers 1994; Franz 2010). In 2 final experiments, we compared the reward value of glucose, fructose, and sucralose + saccharin mixture using a progressive ratio (PR) task. The results revealed that sweetener preference and reward are not determined by caloric value per se but by the specific postoral nutritive and inhibitory actions of the sweeteners.
Experiment 1: 8% sucrose versus 0.8% sucralose preference tests
In this experiment, we measured preferences for 8% sucrose versus 0.8% sucralose. The 8% sucrose concentration was selected because it produces peak intakes in 2-day (i.e., 48-h) sugar versus water tests and has potent postoral conditioning effects (Bachmanov et al. 2001; Sclafani and Glendinning 2005; Zukerman et al. 2009). The 0.8% sucralose concentration was used because recent findings indicated that it is closely matched to 8% sucrose in palatability (Sclafani et al. 2010b). These results, however, were obtained with mice that had prior experience with non-caloric sweeteners, which may have influenced their sweetener preferences. The present experiment, therefore, evaluated sucralose versus sucrose preference in naive mice given brief access choice tests, which minimize postoral influences, and in other mice given 2-day choice tests, which allow for postoral influences. Experiential influences on sweetener preference were also measured by determining how separate 2-day tests with sucrose versus water and sucralose versus water altered the preference for sucrose versus sucralose. These are referred to as “1-sweetener” tests and allowed the animals to associate the taste of the sweetener unambiguously with its postoral actions.
Materials and methods
Animals
Adult male C57BL/6J (B6) mice born in the laboratory from Jackson Lab stock were used in Experiments 1A (n = 10, 9 weeks old) and 1B (n = 10, 11 weeks old). The animals were singly housed in plastic tub cages in a room maintained at 22 °C with a 12:12-h light:dark cycle and given ad libitum access to chow (5001; PMI Nutrition International) and water except where noted. Experimental protocols were approved by the institutional animal care and use committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Apparatus
Experiment 1A.
Short (1-min) 2-bottle tests were conducted in clear plastic cages (15×15×32cm) with a stainless-steel perforated floor. Fluid was available from 2 stainless-steel sipper spouts through slots (5×20mm, 32mm apart) in a stainless-steel plate at the front of the cage. The sipper spouts were attached to 50-mL glass tubes with a rubber stopper. The tubes were mounted on motorized bottle holders (ENV-252M; Med Associates) that positioned the spouts 1mm in front of the cage at the start of a trial and retracted them at the end of the trial. Licks were monitored with electronic lickometers (ENV-250B; Med Associates) interfaced to a microcomputer. Intakes were not measured because the low intakes precluded accurate measurements.
Experiment 1B.
Two-day tests were conducted in the home cages. The solutions were available through stainless-steel sipper spouts with a 1.5-mm hole attached to 50-mL plastic tubes that were placed on the stainless-steel grid top of the home cage. The 2 sipper spouts were inserted through holes positioned 3.7cm apart, and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1g by weighing the drinking bottles on an electronic balance interfaced to a laptop computer. Intakes were corrected for spillage, which was estimated by recording the change in weight of 2 bottles that were placed on an empty cage.
Test solutions
Solutions were prepared using food-grade sucralose (Tate & Lyle) and sucrose (Domino Foods). The solutions were prepared with deionized water on a wt/wt basis because intakes were measured by weight.
Procedure
Experiment 1A.
Naive mice were adapted to the test cages overnight with ad libitum access to food and water. They were then given restricted access to water (1h/day) in their home cages and trained to drink water from 2 bottles in the test cages for 5min. The next day they were given 1-min choice tests with 8% sucrose versus 0.8% sucralose while still water deprived. The mice were then given ad libitum access to water but restricted to a food ration (1–2.5g/day) that maintained their body weights at 85–90% of free-feeding levels. Fixed-size chow pellets (0.5 or 1g, F0171, F0173; Bio-Serv) were used to precisely adjust the daily rations. On the following 2 test days, they were given 1-min tests with 8% sucrose versus 0.8% sucralose. In these tests, the mice were first given 5-s access to 1 sipper tube and then 5-s access to the other sipper tube to allow them to sample the contents of each tube before being presented with both tubes for 1min. The timing of each session for each mouse began with its 10th lick and the bottles were automatically retracted 5 s or 1min later. The mice were returned to their home cages after the 1-min test. One hour later, they were given a second 1-min test with the left–right position of the sucrose and sucralose solutions reversed. Food rations were placed in the home cages 1h after the last test.
Experiment 1B.
Naive mice were adapted to their home cages with 2 water bottles and ad libitum chow for 1 week. They were then given a series of 2-day, 2-bottle choice tests as follows: Test 1 (days 1–2) 8% sucrose versus 0.8% sucralose, Test 2 (days 3–4) 8% sucrose versus water, Test 3 (days 5–6) 0.8% sucralose versus water, and Test 4 (days 8–9) 8% sucrose versus 0.8% sucralose. The mice were given water only on day 7 between Tests 3 and 4. Water was available in Tests 2 and 3 so that the animals were not forced to drink the sweetener, but they consumed little or no water in these tests. The left–right position of the sweetener and water bottles were switched from the first to second day of each test.
Data analysis
In Experiment 1A, 1-min licks of sucrose and sucralose were averaged over the 1-min tests conducted, when the animals were food restricted and analyzed with a t-test. In Experiment 1B, the 24-h solution intakes were averaged over the 2 days of each test and sweetener preferences were expressed as percent solution intakes (e.g., sucralose solution intake/total intake × 100). Intakes were analyzed using a mixed model analysis of variance (ANOVA) with test and solution as repeated factors. The first ANOVA included results from Tests 1 and 4 and asked whether relative intakes of sugar and sucralose changed across the 2 tests. A second ANOVA included results from Tests 2 and 3 and compared the preference for each sweetener over water. Percent sweetener intakes were analyzed with t-tests.
Results and discussion
Experiment 1A
The mice licked slightly more sucralose than sucrose in the 1-min tests, but the difference was not significant and their sucralose preference was 59% (Table 1).
Table 1.
One-minute 2-bottle sugar versus sweetener tests (licks)
| Sweetener | 8% Sugar | Sweetener preference, Percent of total licks | |
|---|---|---|---|
| Experiment 1: 0.8% sucralose | |||
| Sucrose | 91.0±12.5 | 59.8±7.7 | 59.0±5.5 |
| Experiment 2: 0.8% sucralose | |||
| Glucose | 161.6±23.7* | 39.3±18.3 | 80.2±4.8 |
| Fructose | 137.1±13.2* | 68.6±11.1 | 66.6±13.5 |
| Experiment 3A: 0.1% sucralose + saccharin | |||
| Glucose | 122.1±14.5* | 40.9±9.1 | 74.9±4.6 |
| Fructose | 93.2±9.0* | 42.2±4.7 | 66.2±1.6 |
| Sucrose | 172.4±19.5* | 86.5±17.4 | 66.4±6.0 |
Values are mean 1-min licks ± SEM. Experiment 3A Glucose and Fructose tests were 5min in duration, but data are expressed as licks/min.
*Significant sweetener versus sugar difference, P < 0.05.
Experiment 1B
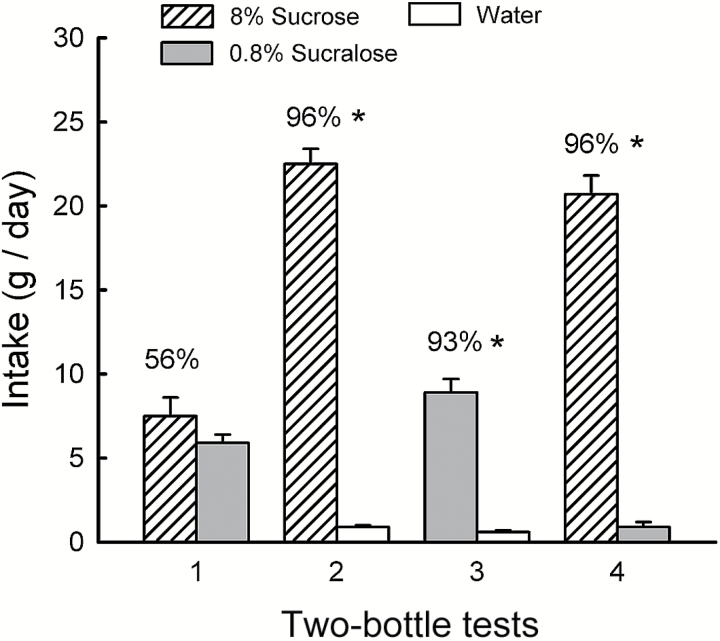
In the 2-day choice tests, the mice consumed slightly more sucrose than sucralose in Test 1 but significantly more in Test 4 (Sweetener × Test interaction, F 1,9 = 92.1, P < 0.001; Figure 1). Sucralose intake decreased from the first to last test, whereas sucrose intake and percent preference substantially increased (56% to 96%, t 9 = 7.3, P < 0.001). In the sweetener versus water Tests 2 and 3, the mice consumed significantly more sucrose and sucralose than water (F 1,9 = 556.7, P < 0.001), and more than twice as much sucrose as sucralose (Sweetener × Test interaction, F 1,9 = 204.5, P < 0.001). The percent sucrose intake over water was marginally greater than the percent sucralose intake (97% vs. 93%, t 9 = 2.02, P < 0.06).
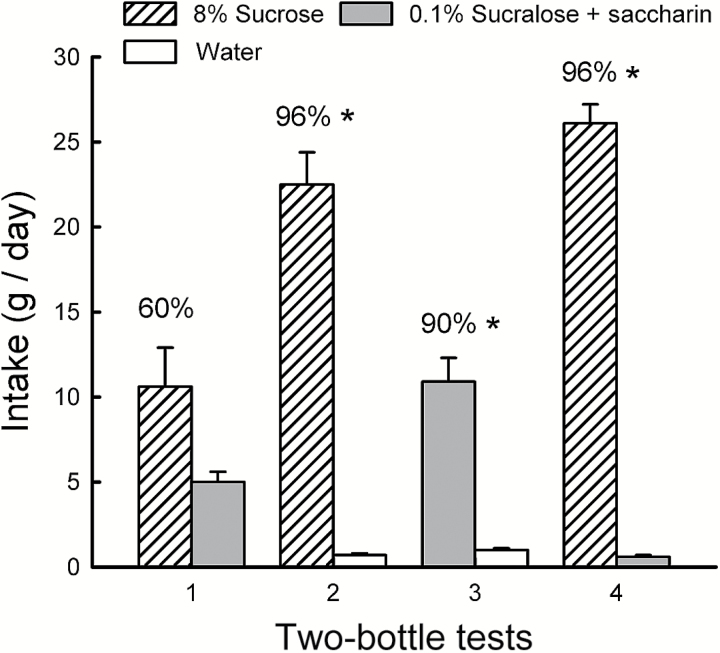
Figure 1.
Experiment 1B. Mean (+SEM) intakes of 8% sucrose and 0.8% sucralose in 2-day 2-bottle Tests 1–4. The mice were given the choice of sucrose versus sucralose in Tests 1 and 4, and sucrose versus water and sucralose versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
The results of the 1-min and initial 2-day test with sucrose versus sucralose indicate that at the concentrations tested the 2 sweeteners are closely matched in palatability to naive B6 mice. The increase in sucrose preference from Tests 1 to 4 can be explained by the differential postoral actions of sucrose and sucralose experienced during the separate sweetener versus water tests. This can also account for the greater intake of sucrose than sucralose in Tests 2 and 3. This interpretation is based on our findings that IG sucrose infusions had an appetition effect, increasing the intake of and preference for a CS+ flavor in B6 mice, whereas IG sucralose infusions had a postoral inhibitory action that decreased CS+ intake and preference (Sclafani et al. 2010b).
Experiment 2: 8% glucose and fructose versus 0.8% sucralose preference tests
Ingested sucrose is rapidly digested in the gut to glucose and fructose, which have substantially different postoral appetition effects in B6 mice: IG infusions of glucose but not fructose stimulate the intake of and preference for a CS+ solution (Sclafani and Ackroff 2012a; Zukerman et al. 2013a). We predicted, therefore, that B6 mice would develop a strong preference for glucose but not fructose over sucralose in long-term choice tests. However, if energy value alone determines sugar versus sucralose preference, then the mice should come to prefer both sugars over the non-caloric sweetener.
Materials and methods
Experiment 2A
Naive B6 mice (n = 10) were adapted and trained to drink water in the test cages as in Experiment 1A. They were then given a series of 1-min 2-bottle tests, while food restricted as follows: 8% glucose versus 8% fructose; 0.8% sucralose versus water, 8% glucose versus 0.8% sucralose, and 8% fructose versus 0.8% sucralose. Half of the mice were tested with glucose versus sucralose first followed by fructose versus sucralose second; the remaining animals were tested in the reverse order.
Experiment 2B
Naive B6 mice were given a series of 2-day sweetener versus sweetener and sweetener versus water tests as described in Experiment 1B. The Glucose group (n = 8) was tested with 8% glucose and 0.8% sucralose, whereas the Fructose group (n = 9) was tested with 8% fructose and 0.8% sucralose.
Results
Experiment 2A
In the initial 1-min choice test, the mice licked similarly for glucose and fructose and their fructose preference was 54%. When next offered sucralose versus water (to familiarize them with the sweetener), the mice displayed a near total preference for the sweetener (96%) and sucralose licks were comparable to the total licks for glucose and fructose in the first test (195.2 vs. 192.0 licks/min). In sucralose versus sugar 1-min tests, the mice licked more for 0.8% sucralose than for glucose and fructose (F 1,9 = 23.4, P < 0.001; Table 1). The percent sucralose preference was numerically greater in the glucose test (80.2%) than in the fructose test (66.6%), but this difference was not significant.
Experiment 2B
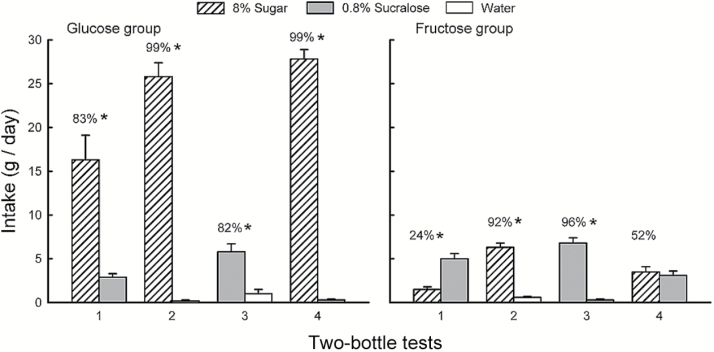
In the 2-day tests, the mice consumed significantly more glucose than sucralose in both Tests 1 and 4 (F 1,7 = 15.2, P < 0.01), and this difference increased from the first to last test (Sweetener × Test interaction, F 1,7 = 29.8, P < 0.001; Figure 2). The percent glucose intakes also increased from Test 1 to 4 (83% to 99%; t 7 = 4.9, P < 0.01). In the sweetener versus water Tests 2 and 3, the mice consumed significantly more glucose and sucralose than water (F 1,7 = 317.8, P < 0.001) but 4 times more glucose than sucralose (Sweetener × Test interaction, F 1,7 = 75.0, P < 0.001). The percent glucose intake over water was greater than the percent sucralose intake (99% vs. 82%), but this difference was not significant because of the variability in the percent sucralose data (i.e., 1 mouse preferred water to sucralose).
Figure 2.
Experiment 2B. Mean (+SEM) intakes of 8% glucose, 8% fructose, and 0.8% sucralose in 2-day 2-bottle Tests 1−4. The left graph presents the Glucose group data and the right graph presents the Fructose group data. The mice were given the choice of sugar versus sucralose in Tests 1 and 4, and sugar versus water and sucralose versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
The Fructose group consumed significantly more (P < 0.01) sucralose than sugar in Test 1 and then similar amounts of fructose and sucralose in Test 4 (Sweetener × Test interaction, F 1,8 = 13.9, P < 0.01; Figure 2). The percent fructose intake increased from a low of 24% in Test 1 to indifference (52%) in Test 4 (t 8 = 4.2, P < 0.01). The mice consumed significantly more fructose and sucralose than water in Tests 2 and 3 (F 1,8 = 148.8, P < 0.001), and their absolute and percent intakes of fructose and sucralose did not differ (6.3 vs. 6.8g/day; 92% vs. 96%, respectively).
Discussion
In the 1-min tests, 8% glucose and 8% fructose were less preferred than 0.8% sucralose suggesting that sucralose is sweeter than the 2 sugars at these concentrations. Yet, despite its lower palatability, glucose was significantly preferred to sucralose in the 2-day sweetener Tests 1 and 4. We attribute this to the animals rapidly learning to prefer glucose based on its potent postoral appetition effects (Sclafani and Ackroff 2012a; Zukerman et al. 2013a). Consistent with this interpretation, glucose preference increased from 66% to 93% from the first to second days of Test 1. The much greater intake of glucose than sucralose in the “1-sweetener” tests is also consistent with the differential postoral intake stimulating actions of the 2 sweeteners (Sclafani et al. 2010b; Sclafani and Ackroff 2012a; Zukerman et al. 2013a).
In contrast, the mice tested with fructose preferred sucralose to the sugar in both the 1-min test (by 67%) and first 2-day test (by 76%). However, after the “1-sweetener” experience provided by Tests 2 and 3, the mice were indifferent to fructose and sucralose in Test 4. The increase in fructose preference from Tests 1 to 4 may reflect a postoral appetition effect of the caloric sugar. Yet, in 24-h conditioning experiments, IG self-infusions of 8% and 16% fructose did not stimulate CS+ intake or condition a preference (Sclafani and Ackroff 2012a). Alternatively, the loss of a sucralose preference from Test 1 to 4 may be due to the postoral inhibitory actions of sucralose (Sclafani et al. 2010b). Mice trained with a saccharin-sweetened CS+ flavor paired with IG infusions of 1.6% sucralose, diluted to 0.8% sucralose in the stomach by the consumed CS+ solution, preferred the CS− flavor in a choice test. Nevertheless, they preferred the CS+ flavor to plain water, indicating that the IG sucralose did not produce a CS+ aversion per se. Thus, the sucralose-only experience in Test 3 reduced the animals’ attraction to this otherwise sweeter solution to that of the fructose solution. This explains why the animals consumed similar amounts of fructose and sucralose in Tests 2 and 3.
Note that fructose is sometimes assumed to have a sweeter taste than sucrose to rodents, as suggested by human data, see Glendinning et al. (2010). Our findings that 0.8% sucralose was preferred to 8% fructose but not 8% sucrose in 1-min choice tests and other short-term licking results suggests that 8% sucrose is sweeter than 8% fructose to B6 mice (Glendinning et al. 2010).
Experiment 3: 8% sugar versus 0.1% sucralose + saccharin preference tests
In view of the possibility that the postoral inhibitory actions of 0.8% sucralose contributed to the experience-induced changes in sweetener preferences observed in Experiments 1 and 2, we attempted to identify a lower sucralose concentration that was preferred (or isopreferred) to glucose and fructose but did not have postoral inhibitory effects. One-minute tests revealed that 0.4–0.6% sucralose solutions were less preferred than 8% glucose, whereas 0.7% sucralose was more preferred (Supplementary Figure S1). It seemed unlikely that the postoral actions of 0.7% and 0.8% sucralose differed by much. We therefore attempted to enhance the preference for a lower sucralose concentration by mixing it with saccharin. There are reports of taste synergism in humans with binary mixtures of non-caloric sweeteners (Powers 1994; Schiffman et al. 1995). Brief access tests in B6 mice revealed that a 0.1% sucralose + 0.1% saccharin (S + S) mixture was preferred to 8% glucose (Supplementary Figure S1). We next determined that the 0.1% S + S mixture, unlike 0.8% sucralose, did not suppress the intake of glucose when mixed with the sugar or given by IG co-infusion (Supplementary Figures S2 and S3). These findings indicated that the 0.1% S + S solution is very palatable and has no or minimal postoral inhibitory actions in B6 mice. A brief access lick test with 0.8% sucralose versus 0.1% S + S revealed no preference (Supplementary Figure S3), which suggests that the solutions are equally sweet. Alternatively, the 0.8% solution may have not only a sweeter taste but also a bitter off-taste that reduces its palatability.
In Experiment 3, we evaluated the relative preference of 0.1% S + S versus 8% sugar in naive B6 mice and determined if separate experience with each sweetener modified long-term sweetener preferences. We also investigated the effects of food deprivation on sweetener preference.
Materials and methods
Experiment 3A
Naive B6 mice (n = 10) were trained to drink water and then sweeteners as in Experiments 1A and 2A. While they were food restricted, 5 mice were tested first with 8% glucose versus 0.1% S + S and then 8% fructose versus 0.1% S + S for 4 sessions each. The remaining mice were tested in the reverse order and data for the 2 subgroups were combined. Due to an experimenter error, the sessions were 5min rather than 1min in duration, but the preference results were comparable to pilot data obtained with 1-min tests. A second group of naive B6 mice (n = 10) were given 1-min tests with 0.1% S + S versus 8% sucrose.
Experiment 3B
Naive male B6 mice were given a series of 2-day sweetener versus sweetener and sweetener versus water tests as described in Experiments 1B and 2B. The Glucose group (n = 10) was tested with 8% glucose and 0.1% S + S, whereas the Fructose group (n = 10) was tested with 8% fructose and 0.1% S + S. Because the Fructose mice displayed low sugar preferences in fructose versus S + S tests, they were given a second series of tests, while they were food restricted to determine if hunger enhanced their preference for the caloric fructose over the non-caloric sweetener. The mice were given limited food rations and maintained at 90% of ad libitum body weight for 3 days and then given a series of four 2-bottle tests (Tests 5–8) as above. They were then returned to ad libitum food and 3 days later given a final 2-day fructose versus S + S choice test.
A third group of naive male B6 mice (n = 10) were given 2-day tests as described above but with 8% sucrose versus 0.1% S + S.
Results
Experiment 3A
The results of the 5-min lick tests are expressed in Table 1 as licks/min to be comparable to the 1-min lick data reported for Experiments 1A and 1B. The mice licked significantly more for the 0.1% S + S solution than for 8% glucose or 8% fructose (F 1,9 = 27.9, P < 0.001). Their glucose and fructose licks did not differ, but they licked more (P < 0.01) S + S in the glucose test than in the fructose test (Solution × Test interaction, F 1,9 = 5.3, P < 0.05).
The Sucrose mice licked significantly more 0.1% S + S than 8% sucrose in the 1-min tests (t 9 = 2.8, P < 0.05) and had a sucralose preference of 65% (Table 1). The percent preference for S + S over glucose was numerically greater than that for S + S over fructose or sucrose, but these differences were not significant.
Experiment 3B
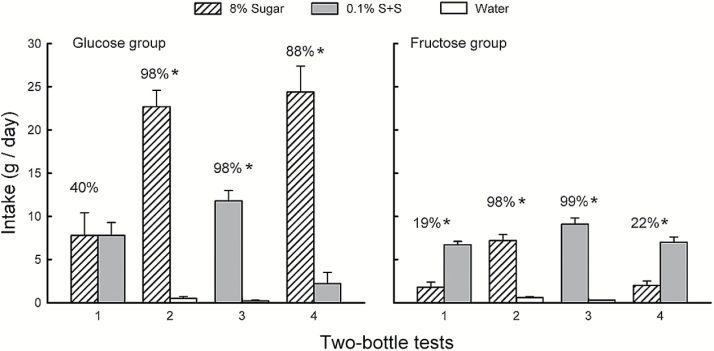
The mean intakes of glucose and S + S were identical in Test 1, but the mice consumed much more glucose in Test 4 (Sweetener × Test interaction, F 1,9 = 23.0, P < 0.001; Figure 3). In addition, glucose intake increased (P < 0.001) from Test 1 to 4, whereas S + S intake declined, although this difference was not significant. The percent glucose intake increased from 40% to 88% in Tests 1–4 (t 9 = 4.0, P < 0.01). There was a bimodal response to the sweeteners in Test 1; 6 mice preferred the S + S (86%), 3 mice preferred glucose (87%), and 1 mouse was indifferent. This accounts for the group mean percent intake of 40% despite identical group mean intakes of glucose and S + S. In Test 4, 9 of the 10 mice strongly preferred glucose, whereas 1 mouse preferred the S + S. In the sweetener versus water Tests 2 and 3, the mice consumed significantly more glucose and S + S than water (F 1,9 = 189.5, P < 0.001) but twice as much (P < 0.001) glucose as S + S (Sweetener × Test interaction, F 1,9 = 22.3, P < 0.001). The percent intakes of glucose and S + S over water were identical at 98%.
Figure 3.
Experiment 3B. Mean (+SEM) intakes of 8% glucose, 8% fructose, and 0.1% sucralose + 0.1% saccharin (S + S) in 2-day 2-bottle Tests 1–4. The left graph presents the Glucose group data and the right graph presents the Fructose group data. The mice were given the choice of sugar versus S + S in Tests 1 and 4, and sugar versus water and S + S versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
In contrast, the Fructose mice consumed significantly more S + S than fructose in both Tests 1 and 4 (F 1,9 = 246.0, P < 0.01] and the intake of the 2 solutions did not change across tests (Figure 3). The percent fructose intakes remained low at 19% and 22%, respectively, in the 2 tests; except for 1 or 2 mice that were indifferent, all the mice preferred S + S to fructose. In Tests 2 and 3, the mice consumed more fructose and S + S than water (F 1,9 = 155.5, P < 0.001), but they consumed more (P < 0.01) S + S than fructose (Sweetener × Test interaction, F 1,9 = 9.7, P < 0.05). Percent intakes of fructose and S + S, however, were similar (98% vs. 99%).
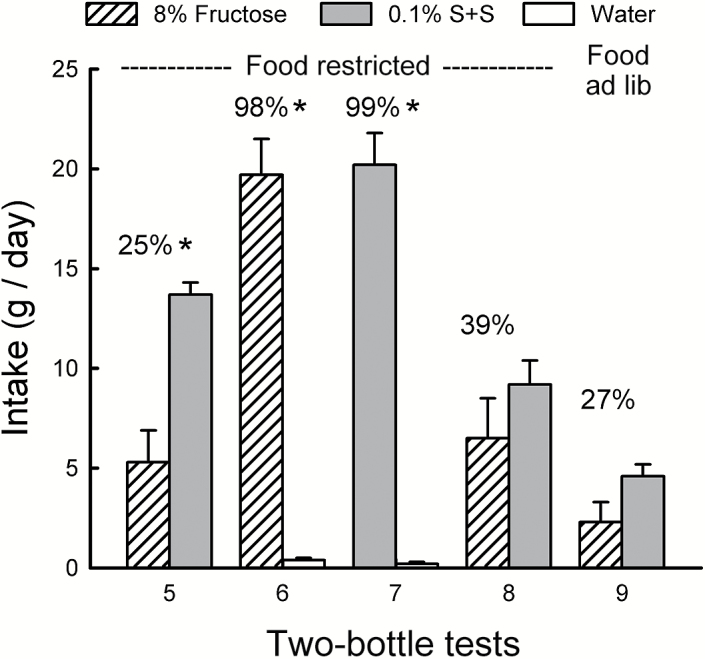
In Test 5, the now-food-restricted mice preferred S + S to fructose and their percent fructose preference did not differ from that of the ad libitum Test 4 (25% vs. 22%; Figure 4). Overall, however, the mice consumed twice as much total solution in Test 5 than in Test 4, demonstrating their sensitivity to the deprivation state (F 1,9 = 35.9, P < 0.001). After the separate 1-sweetener tests, the mice increased fructose preference somewhat to 39% in Test 8 compared with 25% in Test 5 (t 9 = 2.2, P < 0.06). Analysis of the intake data indicated that the mice consumed less (P < 0.01) fructose than S + S in Test 5 but not significantly so in Test 8 (Sweetener × Test interaction, F 1,9 = 5.8, P < 0.05). Note that S + S intake significantly (P < 0.01) declined from Test 5 to 8, whereas fructose intake did not significantly change. Yet, in Tests 6 and 7, the mice strongly preferred both fructose and S + S to water (F 1,9 = 186.4, P < 0.001) and their absolute and percent intakes of the 2 sweeteners did not differ. Compared with their sweetener intakes when fed ad libitum (Tests 2 and 3), the mice consumed more than twice as much fructose and S + S while they were food restricted in Tests 6 and 7 (F 1,9 = 84.9, P < 0.001). In the final preference Test 9, the mice were again fed ad libitum and they consumed more S + S than fructose, but the difference was not significant. However, percent fructose preference in Test 9 was not significantly higher than that in the previous ad libitum Test 4 (27% vs. 22%).
Figure 4.
Experiment 3B. Mean (+SEM) intakes of 8% fructose and 0.1% sucralose + 0.1% saccharin (S + S) in 2-day 2-bottle Tests 5–9. The Fructose mice were food restricted during Tests 5–8. They were given the choice of fructose versus S + S in Tests 5 and 8, and fructose versus water and S + S versus water in Tests 6 and 7. In Test 9, the mice were given food ad libitum and tested with fructose versus S + S. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
The Sucrose mice drank more sucrose than S + S in Tests 1 and 4 but only the Test 4 difference was significant (Sweetener × Test interaction, F 1,9 = 58.9, P < 0.001; Figure 5). In addition, sucrose intake increased (P < 0.001) from Test 1 to 4, whereas S + S intake declined (P < 0.05). The percent sucrose intake increased from 60% to 88% from Test 1 to 4 (t 9 = 4.4, P < 0.01). In Test 1, 3 mice preferred S + S (73%), 6 mice preferred sucrose (78%), and 1 mouse was indifferent; all mice preferred sucrose in Test 4. In the sweetener versus water Tests 2 and 3, the mice consumed significantly more sucrose and S + S than water (F 1,9 = 138.8, P < 0.001) but twice as much (P < 0.001) sucrose as S + S (Sweetener × Test interaction, F 1,9 = 36.1, P < 0.001). The percent intake of sucrose over water was greater than S + S over water (96% vs. 90%, t 9 = 3.0, P < 0.05).
Figure 5.
Experiment 3B. Mean (+SEM) intakes of 8% sucrose and 0.1% sucralose + 0.1% saccharin (S + S) in 2-day 2-bottle Tests 1–4. The mice were given the choice of sucrose versus S + S in Tests 1 and 4, and sucrose versus water and S + S versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
Discussion
In the brief access choice tests, the 0.1% S + S solution was preferred to the 8% sugar solutions, suggesting that the S + S mixture was sweeter than the sugar solutions. Yet, the mice consumed similar amounts of 8% glucose and somewhat more 8% sucrose than S + S in the first 2-day test (Test 1) and significantly more of these sugars in Test 4. They also consumed significantly more glucose and sucrose than sucralose in Tests 2 and 3, although all sweeteners were strongly preferred to water. The elevated intake and preference for glucose and sucrose in the 1- and 2-sweetener tests are consistent with the potent postoral appetition actions of these sugars in B6 mice (Sclafani and Glendinning 2005; Zukerman et al. 2011; Sclafani and Ackroff 2012a; Zukerman et al. 2013b).
In contrast, the mice strongly preferred 0.1% S + S to 8% fructose in 2-day Tests 1 and 4 and consumed more S + S than fructose in Tests 2 and 3. The persistent S + S preference displayed in Tests 1 and 4 indicates that the energy value of 8% fructose was not sufficient to alter sweetener preference in ad libitum fed B6 mice. Tests 5–8 were conducted to determine if food restriction would enhance the preference for the caloric fructose solution over the non-caloric S + S solution. The results revealed only a relatively small effect. Food restriction increased total sweetener intake in Test 5, but it did not significantly increase fructose preference compared with the food ad libitum Test 4. In addition, the hungry mice did not consume more fructose than S + S in Tests 6 and 7. The 1-sweetener experience of the hungry mice with the caloric and non-caloric sweeteners failed to induce them to consume more fructose than S + S in Test 8 but rather only marginally increased their fructose preference from 25% (Test 5) to 39% (Test 8). These findings are consistent with our prior report that IG fructose infusions did not condition a significant CS+ flavor preference in hungry B6 mice (Zukerman et al. 2013a). Yet, after the 1-sweetener experience, the mice consumed less S + S in Test 8 than Test 5, which indicates that experience with the S + S and fructose solutions while food restricted reduced the animals’ avidity for S + S. This possibility requires further study.
The finding that the Fructose mice did not increase their fructose preference from Test 1 to 4 as they did in Experiment 1 indicates that the preference change in the first experiment was not due to postoral stimulatory actions of the sugar but rather to the postoral inhibitory action of 0.8% sucralose, which is not present with the 0.1% S + S (Supplementary Figure S4). The differential postoral actions of the 2 non-caloric sweetener solutions can also account for why, in Test 1, the mice preferred glucose to 0.8% sucralose but not to 0.1% S + S. This does not explain, however, why in Test 1 the mice displayed similar preferences for sucrose over sucralose and S + S (56% vs. 60%). The 1-min test data suggest that the palatability of sucrose is more closely matched than that of glucose to the sucralose and S + S solutions, which may have made it more difficult for the mice to distinguish the postoral actions of sucrose versus non-caloric sweeteners when both were presented together.
Experiment 4: PR responding for 8% glucose, 8% fructose, and 0.1% sucralose + sucralose in free-feeding mice
Experiment 3 revealed that the non-caloric 0.1% S + S is a potent sweetener mixture that is preferred to 8% glucose and fructose in 1-min tests. Experienced mice, however, switched their preference to glucose in 2-day tests, but they failed to prefer fructose to the non-caloric S + S mixture even when food restricted. The present experiment evaluated the relative reward value of these caloric and non-caloric sweeteners using a PR operant task. Recent studies report that rats and mice expend more effort on a PR schedule for sucrose than for non-caloric saccharin or sucralose, which was taken as evidence that caloric value is the primary determinant of sweet food reward value (Beeler et al. 2012; Scheggi et al. 2013). The 2-day preference results obtained with isocaloric glucose and fructose solutions versus the non-caloric S + S solution suggests that this may not be the case with all caloric food sources.
Materials and methods
Apparatus
The mice were housed in clear plastic cages with 2 drinking bottles similar to those in Experiment 1A. After initial bottle tests, 1 bottle was replaced with a sipper spout connected via Tygon tubing (06419-14; Cole Parmer) to a 30-mL plastic syringe mounted in a syringe pump (A-99; Razel Scientific Instruments) set at a 0.5-mL/min pump speed (Sclafani 2006). As the mouse licked the sipper spout, a microcomputer counted all licks and activated the syringe pump for 3 s when the accumulated licks equaled the PR requirement, which delivered approximately 0.025mL of solution (a “sip”) to the tip of the sipper tube.
Procedure
Naive male B6 mice (n = 8) were housed in the test cages with ad libitum access to food and water throughout the experiment. After 4 days of water only, they were given 2-bottle access to 0.1% S + S versus water for 3 days, followed by another 3 days of water only. PR testing then began by giving the mice access to a sipper tube that delivered a 0.1% S + S sip (0.025mL) to the spout when the required numbers of licks were emitted. Licks during the 3-s sip period counted for the next reinforcement. For 3 days each, the S + S solution was available on ratio schedules of PR8-1, PR4-1, and PR2-1. On the PR8-1 schedule, the number of times the mouse had to lick to obtain a sip was increased by 1 (starting at 20 licks) after every 8 sips. On the PR4-1 and PR2-1 schedules, the lick requirement increased by 1 after every 4 and 2 sips, respectively. For example, the cost of successive sips on the PR2-1 schedule was 20, 20, 21, 21, 22, 22, etc., licks. The highest lick ratio reached during the 23-h test session was defined as the break point. The initial lick requirement was reset to 20 at the start of each test day. Water was always freely available from a sipper spout attached to a bottle. The left–right positions of S + S and water spouts were alternated daily to control for side preferences. Following the last PR2-1 S + S test day, the mice were given 3 test days with 8% fructose and then 3 test days with 8% glucose on the PR2-1 schedule. Water only was available for 1 day, followed by 2-day, 2-bottle (no PR cost) choice tests with 0.1% S + S versus water, 8% fructose versus water, and 8% glucose versus water.
Fluid intakes and lick data were averaged over the last 2 days of each test (bottle, PR) and were evaluated using repeated measures ANOVA. Sweetener preferences relative to water were also expressed as percent intakes (sweetener intake/total intake × 100). Drinking bout patterns during the final bottle tests were analyzed with a drinking bout defined as a period of drinking containing at least 30 licks and with interlick intervals no longer than 5min (Gannon et al. 1992; Sclafani 2006).
Results
In the initial bottle test and subsequent PR tests with 0.1% S + S, the mice consumed more sweetener than water. Their S + S intakes declined (P < 0.05), however, from the bottle test to the PR8, PR4, and PR2 tests (9.1 > 7.2 ≥ 6.2 > 4.3g/day, respectively, F 2,21 = 30.7, P < 0.001). The percent S + S preference over water was similar in the bottle, PR8, and PR4 tests but declined (P < 0.05) in the PR2 test (92 = 90 = 94 > 75%, respectively, F 2,21 = 7.9, P < 0.01).
In the PR2 tests with the 3 different sweeteners, the mice consumed more (P < 0.05) glucose and less (P < 0.05) fructose than S + S (F 2,21 = 86.3, P < 0.001), which paralleled the number of sips earned with each sweetener (F 2,21 = 74.9, P < 0.001; Figure 6). The mice consumed more (P < 0.05) water during the fructose test than the glucose and S + S tests (F 2,21 = 5.3, P < 0.05), and their percent sweetener intake was lower (P < 0.01) in the fructose test than in the S + S and glucose tests (62% < 75% ≤ 83%, F 2,21 = 15.8, P < 0.001). The mice licked considerably more (P < 0.001) for glucose than for S + S and more for S + S than fructose although this difference was not significant (F 2,21 = 32.3, P < 0.001). Separate analyses compared the sweetener licks as a function of days and revealed that S + S and fructose licks did not vary from test days 1 to 3, whereas the mice increased their glucose licks from day 1 to 2 and 3 (21864 to 31504 and 32937 licks/day; F 2,14 = 12.5, P < 0.001). The lick break point (the highest PR cost reached in a test) was higher (P < 0.01) with glucose than S + S, and higher (P < 0.01) with S + S than fructose (F 2,21 = 75.0, P < 0.001).
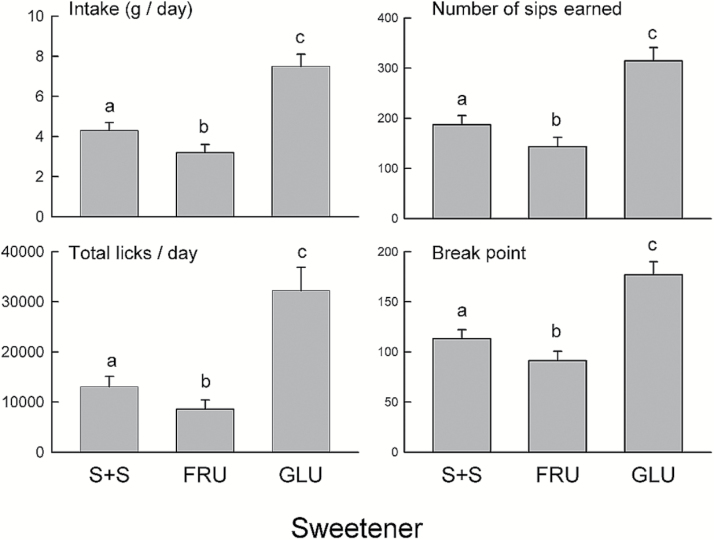
Figure 6.
Experiment 4. Mean (+SEM) sweetener intake, number of sweetener sips earned, total sweetener licks/day, and lick break points during the 24-h PR2-1 tests with 0.1% sucralose + saccharin (S + S), 8% fructose (FRU), and 8% glucose (GLU). In each panel, bars with different letters differ significantly from one another (P < 0.05).
In the bottle tests, the mice consumed considerably more (P < 0.001) glucose than S + S and more (P < 0.05) S + S than fructose (F 2,21 = 76.5, P < 0.001; Figure 7), but their water intakes did not differ (0.7–0.8g/day). The mice took more (P < 0.01) bouts per day with glucose than S + S, and more (P < 0.01) with S + S than fructose (F 2,21 = 48.9, P < 0.001). Mean bout sizes with glucose exceeded (P < 0.01) those with S + S and fructose, which did not differ (F 2,21 = 8.0, P < 0.01).
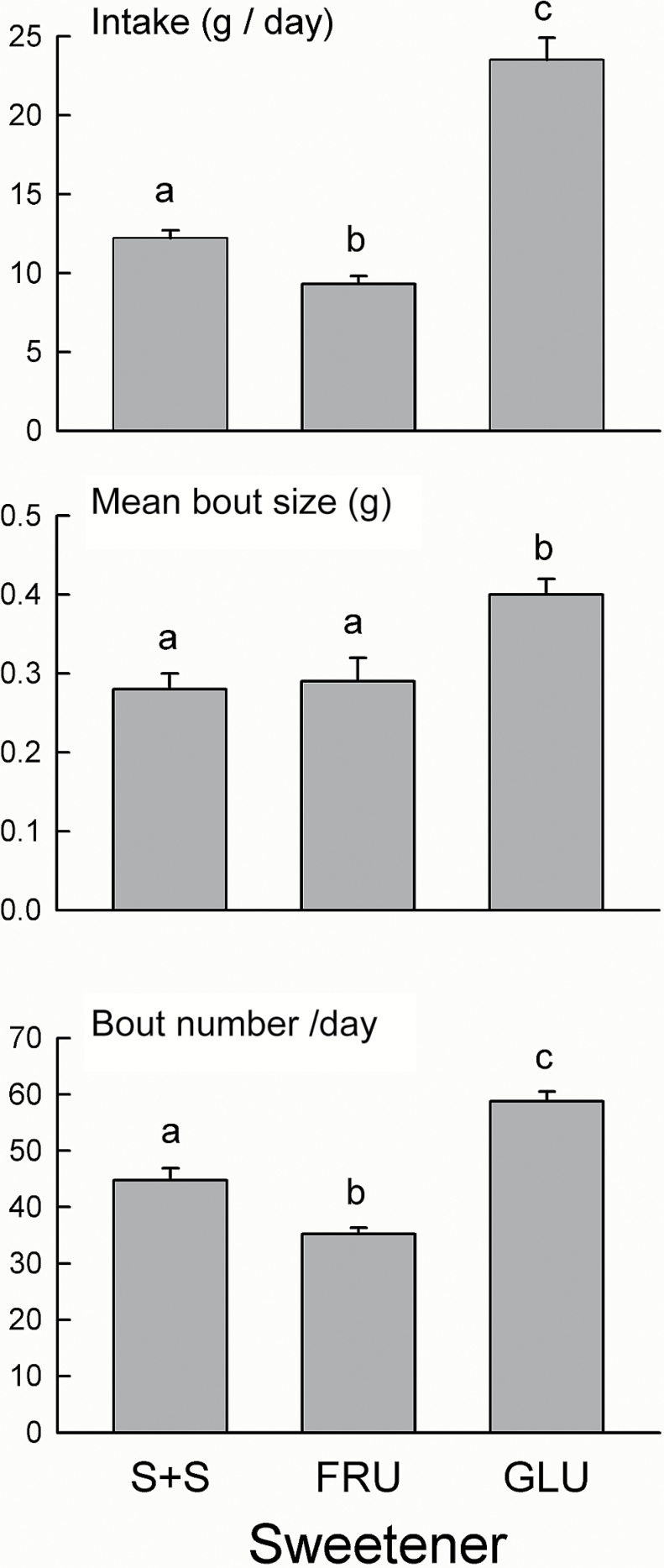
Figure 7.
Experiment 4. Mean (+SEM) sweetener intake (g/day), bout size (g/bout), and bout number per day during the 24-h bottle tests with 0.1% sucralose + saccharin (S + S), 8% fructose (FRU), and 8% glucose (GLU). In each panel, bars with different letters differ significantly from one another (P < 0.05).
Discussion
In confirmation of Experiment 3, the mice consumed substantially more glucose than S + S and more S + S than fructose when the sweeteners were freely available in 2-bottle tests versus water. The elevated intake of glucose was due to both increased mean bout size and number, whereas the differences in S + S and fructose intakes were due to differences in bout number only. When the mice were required to lick for the sweeteners on a PR schedule, they showed a similar response pattern: sips earned, total licks, and break points were higher with glucose than S + S and higher with S + S than fructose. Thus, the PR reward value of the 3 sweeteners was not related to their caloric content (glucose = fructose >> S + S) or their sweet taste (S + S > glucose ~ fructose), but rather to their postoral appetition effects (glucose >> fructose ~ S + S). It is also possible that postoral satiety effects of fructose contributed to the reduced avidity for this sweetener. The similar bout sizes but dissimilar bout numbers displayed by the Fructose and S + S groups in the bottle test implicate postmeal satiety rather than within-meal satiation processes.
Experiment 5: PR responding for 8% glucose, 8% fructose, and 0.1% sucralose + sucralose in food-deprived mice
In Experiment 4, PR responding for the 3 sweeteners was not related to their energy value. The mice had ad libitum access to chow during these tests, and it is possible that food-restricted mice would respond more for both caloric sugars than the non-caloric sweetener. This is suggested by the recent report of similar PR responding for sucrose and saccharin solutions in freely fed rats but higher PR responding for sucrose in food-deprived rats (Scheggi et al. 2013). Food-restricted mice were also observed to respond more in a PR task for sucrose pellets than for sucralose or saccharin pellets (Beeler et al. 2012). In the present experiment, therefore, we compared PR licking in food-restricted mice reinforced with glucose, fructose, or S + S solutions.
Materials and methods
Naive B6 mice (12 males and 12 females) were housed in tub cages. The animals were water restricted (1h/day) and trained to drink water in the test cages described in Experiment 4 for three 1-h/day sessions. Water was available from the sipper spout on a fixed ratio 20 (FR20) schedule, which delivered 0.025mL of water for every 20 licks. On day 4, the animals were divided into 3 groups (4 males and 4 females each) equated for their 1-h water licks and were given a FR20 session with 8% glucose, 8% fructose, or 0.1% S + S; the test session was only 5min in length to limit their sweetener intakes while thirsty. They were then given ad libitum access to water but restricted food rations that maintained them at approximately 90% of their free-feeding body weight levels. The Glucose, Fructose, and S + S groups were given 5 daily 1-h FR20 tests with their respective sweeteners. This was followed by another 5 daily tests with the sweeteners available on a PR1-1 schedule.
Results
In the 1-h FR20 sessions, the mice licked and consumed slightly but not significantly more glucose than S + S, and more (P < 0.01) glucose and S + S than fructose (F 2,21 = 16.9, P < 0.001; Figure 8). In the PR-licking sessions, the mice consumed more (P < 0.05) glucose than S + S and fructose, and somewhat more S + S than fructose (F 2,21 = 11.0, P < 0.001), which mirrored the analysis of the number of sips earned (F 2,21 = 11.0, P < 0.001). The mice licked considerably more (P < 0.001) for glucose than for S + S and fructose and somewhat more S + S than fructose (F 2,12 = 11.6, P < 0.001). A separate analysis compared PR total licks as a function of test days and revealed no change over the 5 test sessions with each sweetener. The lick break point was higher (P < 0.05) with glucose than S + S and fructose, and somewhat higher with S + S than fructose (F 2,21 = 10.9, P < 0.001).
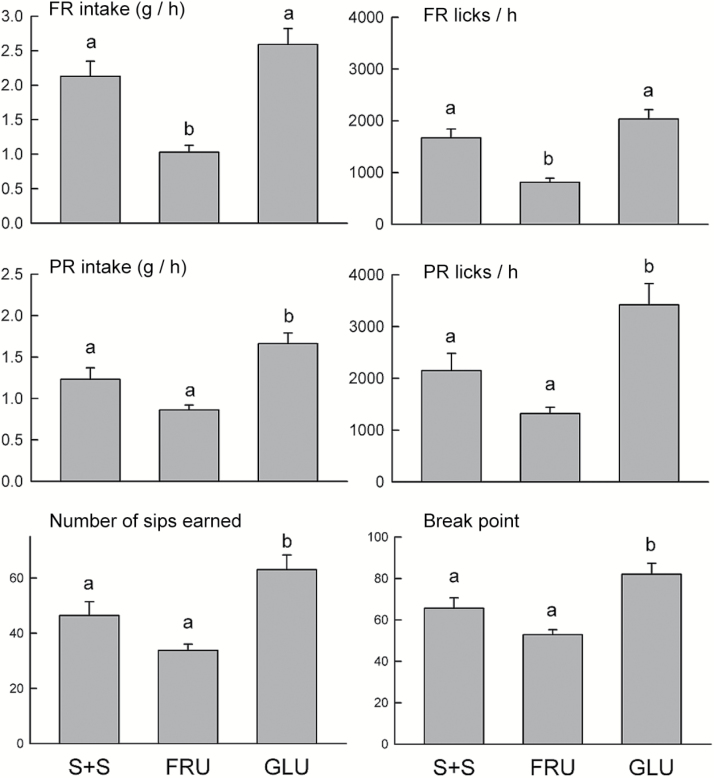
Figure 8.
Experiment 5. Mean (+SEM) FR sweetener intake (g/h) and FR licks/h during the FR tests with the sweeteners in the 0.1% sucralose + 0.1% saccharin (S + S) group, 8% Fructose (FRU) group, and 8% Glucose (GLU) groups. Mean (+SEM) PR sweetener intake, PR licks, number of PR sweetener sips earned, and PR lick break points during the 1-h/day PR1-1 tests. In each panel, bars with different letters differ significantly from one another (P < 0.05).
Discussion
The PR results obtained in this experiment were quite similar to those observed in Experiment 4 despite the procedural differences between the 2 experiments (1- vs. 24-h tests, food restricted vs. food ad libitum, between vs. within group design). As in the prior PR experiment, the mice responded more for 8% glucose than S + S and more for S + S than fructose. In the present experiment, however, the S + S versus fructose differences obtained with the food-restricted mice were not statistically significant. This is consistent with the findings of Experiment 4 in which food restriction reduced the magnitude of the preference for S + S over fructose in a 2-day choice test compared with that observed with freely fed animals. These differences aside, the PR results obtained with food-restricted mice are similar to those obtained with freely fed animals: the PR responding for the 3 sweeteners was determined by their postoral appetition effects, not by their caloric content or sweet taste. The enhanced PR responding displayed by the mice to glucose relative to the non-caloric S + S solution in this and the prior experiment is consistent with prior PR findings obtained with sucrose, sucralose, and saccharin reward (Beeler et al. 2012; Scheggi et al. 2013). However, the present findings indicate that it is not the caloric value of sucrose that is responsible for its reinforcing action but rather its postoral appetition effects related to its glucose moiety.
General discussion
This study investigated the reward value of caloric and non-caloric sweeteners in mice as measured in 2-bottle preference and PR operant lick tests. This has been a topic of recent interest with some investigators concluding that sweet taste in the absence of calories provides only a weak reward (Beeler et al. 2012; Scheggi et al. 2013). However, our findings revealed that the specific postoral nutritive effect, not caloric value per se, determines sugar reward. Furthermore, both the palatability and postoral actions of non-caloric sweeteners must be considered in comparing caloric and non-caloric sweeteners.
Sucrose versus sucralose
Experiment 1 demonstrated that 8% sucrose and 0.8% sucralose are nearly equally preferred by naive B6 mice in 1-min as well as in initial 2-day 2-bottle tests. The isopreference of the naive mice for 8% sucrose and 0.8% sucralose is compatible with the report that B6 mice equally preferred 140mM (4.8%) sucrose and 10mM (0.4%) sucralose in a brief access test (Domingos et al. 2011) but questions the assumption (Beeler et al. 2012), based on sweetener versus water comparisons, that sucrose and sucralose are “isosweet” at 5% versus 0.05% or 10% versus 0.1% concentrations, respectively. Experiment 1 further demonstrated that separate experience with sucrose and sucralose in 2-day sweetener versus water tests resulted in B6 mice strongly preferring sucrose to sucralose, which confirms our prior findings (Sclafani et al. 2010b). The 96% preference for sucrose over sucralose displayed by the experienced B6 mice can be attributed to the differential postoral conditioning actions of the 2 sweeteners. This is indicated by our finding that B6 mice acquired a 92% preference for a CS+ flavor paired with 16% sucrose IG infusions over a water-paired CS-flavor but only a 36% “preference” (i.e., avoidance) for a CS+ flavor paired with 1.6% sucralose IG infusions (Sclafani et al. 2010b). The sucrose and sucralose infusions were diluted to 8% and 0.8%, respectively, by the orally consumed CS+ solutions, which makes the IG findings directly comparable to the preference results obtained with the 8% sucrose and 0.8% sucralose solutions in this study.
In Experiment 3, the mice displayed a weak (66%) but significant preference for 0.1% S + S over 8% sucrose in the 1-min test but significantly preferred sucrose to S + S in the 2-day Test 4 after separate experience with the 2 sweeteners. The strong Test 4 sucrose preference (96%) indicates that even in the absence of a postoral inhibitory action of concentrated sucralose, the postoral conditioning action of sucrose is sufficient to support a near total sugar preference.
Glucose and fructose versus sucralose
The second experiment compared the preference of B6 mice for 8% glucose and fructose, the constituent sugars of sucrose, versus 0.8% sucralose. In the 1-min choice tests, naive B6 mice significantly preferred sucralose to both sugars, suggesting that sucralose is sweeter than the sugars at these concentrations.
Although B6 mice preferred 8% glucose less than 0.8% sucralose in 1-min tests, the mice in Experiment 2B preferred glucose to sucralose in the very first 2-day test (Test 1). This contrasts with the 0.8% sucralose preference over 8% fructose in the first 2-day test. The different long-term sugar versus sucralose preferences can be attributed to the differential postoral conditioning actions of glucose and fructose. In IG conditioning studies, infusions of 16% glucose conditioned a significant CS+ preference, whereas infusions of 16% fructose were ineffective (Sclafani and Ackroff 2012a). Thus, we expected mice to reverse their preference for sucralose over glucose but not for sucralose over fructose in long-term tests.
Although the mice in the second experiment preferred 0.8% sucralose to 8% fructose in the 1-min test and initial 2-day test, they lost this preference in Test 4 after separate experience with the 2 sweeteners in Tests 2 and 3. The preference shift was not predicted based on the equal intakes and preferences for fructose and sucralose over water in Tests 2 and 3. However, the preference shift is consistent with our earlier report that IG infusion of 1.6% sucralose (diluted to 0.8% in the stomach) limits the intake of and preference for a flavored CS+ solution (Sclafani et al. 2010b). The finding that the intake of a glucose solution is reduced by the addition of 0.8% sucralose (Supplementary Figure S2) provides further evidence for the postoral inhibitory actions of concentrated sucralose. In contrast, the mixture of 0.1% sucralose + saccharin did not have these inhibitory actions (Supplementary Figure S2 and S3) and was therefore used to compare caloric versus non-caloric sweeteners in Experiment 3–5.
Experiment 3 revealed a persistent long-term preference for the S + S mixture over 8% fructose, indicating that in the absence of inhibitory effects of concentrated sucralose, non-deprived mice prefer a sweeter non-caloric solution over the less-sweet but caloric fructose solution. Even when food restricted, the mice did not develop a preference for fructose over S + S. Food restriction, however, reduced the S + S preference over fructose, suggesting that the sugar’s metabolic actions may have enhanced fructose preference to some degree. Yet, in other experiments, food-restricted B6 mice did not develop a preference for a CS+ flavor paired with IG fructose infusions (Zukerman et al. 2013a). The glucose mice, however, developed a strong glucose preference over sucralose, consistent with the potent postoral conditioning actions of this sugar (Sclafani and Ackroff 2012a; Zukerman et al. 2011, 2013a, 2013b). However, in contrast to Experiment 2, this glucose preference was observed in Test 4 but not in Test 1. This indicates that the Test 1 preference for 8% glucose over 0.8% sucralose was due to the combined effects of the sugar’s postoral appetite-stimulating actions and the sucralose’s postoral inhibitory actions. Substitution of 0.1% S + S for 0.8% sucralose retarded the ability of mice to distinguish the postoral appetition effects of the glucose versus the non-caloric sweetener in Test 1.
In addition to the significant differences in glucose and fructose versus sweetener preferences observed in Experiments 2 and 3, the mice consumed substantially more glucose than fructose and sweetener solutions in the 2-day tests. This is due not to the taste of glucose, given that the sugar was not preferred to fructose or sucralose in 1-min tests, but to its postoral appetition effects. In IG studies, glucose infusions greatly stimulated the intake of sweetened CS+ solutions, whereas fructose infusions had no effect or suppressed CS+ intakes (Sclafani and Ackroff 2012a; Zukerman et al. 2013a).
Experiments 4 and 5 further demonstrate that the differential attraction of B6 mice to glucose, fructose, and the non-caloric S + S solution is expressed in PR operant lick tests as well as ad libitum drinking tests. In 24-h/day PR tests, the mice licked substantially more for glucose than S + S and more for S + S than fructose. When food restricted, hungry mice licked more for glucose than S + S and fructose in 1-h/day tests. It is notable that the hungry mice licked somewhat more for S + S rather than more for the caloric fructose solution. As in the bottle tests, the avid PR-licking response to glucose compared with the other sweeteners is attributed to the postoral, not the taste properties of the sugar. In a prior rat study, we reported higher PR licks for a sweet CS+ solution paired with IG glucose infusions over an equally sweet CS− solution paired with IG water infusions (Sclafani and Ackroff 2006).
Postoral sugar appetition
Although it is common to attribute postoral sugar conditioning to caloric effects (e.g., Beeler et al. 2012, Scheggi et al. 2013), the preference and PR results obtained in this study do not support this interpretation. The differential IG conditioning actions of glucose versus fructose in rodents also refute the caloric-reward hypothesis (Sclafani et al. 1999; Sclafani and Ackroff 2012a; Zukerman et al. 2013a). We recently reported that CS+ flavor preferences are conditioned by IG infusions of glucose and by a non-metabolizable glucose analog, α-methyl-D-glucopyranoside (MDG), but not fructose in food-restricted B6 mice (Zukerman et al. 2013a). Glucose and MDG, but not fructose, are ligands for intestinal SGLT1 and SGLT3, which function as sugar transporters and/or sensors, implicating these sensors in postoral sugar appetition. This does not preclude the involvement of other sugar and/or metabolic sensors that may contribute to food reward (Oliveira-Maia et al. 2011). Note that although fructose appears to have no postoral appetition actions in B6 mice, this is not the case with other rodents. IG fructose infusions fail to condition flavor preferences in Sprague-Dawley rats trained 30 min/day, but are effective in rats trained 20–24-h/day depending upon specific test parameters (Sclafani et al. 1993; Ackroff et al. 2001). We also recently observed fructose-conditioned preferences in FVB mice (Sclafani et al. 2014b). Nevertheless, glucose-conditioned preferences were much stronger than fructose-conditioned preferences in rats and FVB mice.
Several studies indicate that the postoral actions of glucose and sucrose, but not non-caloric sweeteners, activate brain dopamine reward circuits that are thought to enhance the reward value of these sugars (de Araujo et al. 2008; Tsurugizawa et al. 2009; Beeler et al. 2012; Scheggi et al. 2013; Tellez et al. 2013; Tsurugizawa and Uneyama 2014). The present and prior IG conditioning findings predict that the postoral actions of fructose are much less effective than those of isocaloric sucrose and glucose in activating brain reward circuits in rodents. In contrast, recent functional magnetic resonance imaging data were taken as evidence that fructose is more effective than glucose in activating reward circuits in humans, but this requires confirmatory behavioral findings (Page et al. 2013).
Non-caloric sweetener taste and postoral properties
As noted in the Introduction, a potential problem with comparing non-caloric sweeteners with sugars in rats is the limited palatability of available sweeteners. Saccharin, the most commonly studied sweetener, is at best isopreferred to only dilute sucrose solutions (2–4%) in rats, which limits its usefulness to study sugar reward (Smith and Sclafani 2002). This is often overlooked, however, and many studies compare saccharin (0.1–0.8%) and sucrose (10–30%) solutions that are not matched in sweet taste intensity (Messier and White 1984; Stephens and Brown 1999; Davidson and Swithers 2004; Scheggi et al. 2013). Commercially available sucrose and saccharin pellets have also been assumed to be isosweet to rodents (Beeler et al. 2012). Yet, we found that naive B6 mice strongly preferred sucrose pellets to saccharin pellets in a brief access choice test (Supplementary Figure S4).
Although sucralose is avoided by most rats (Sclafani and Clare 2004; Bello and Hajnal 2005; Loney et al. 2011), it is a very effective sweetener in mice and is preferred to saccharin over a range of concentrations by B6 mice (Sclafani A, unpublished data). In addition, as reported here and elsewhere, there are concentrations of sucralose that are isopreferred or more preferred to sucrose, glucose, and fructose solutions (Domingos et al. 2011). A problem with sucralose, however, is that at higher concentrations (e.g., 0.8%), it has a postoral inhibitory action that limits its intake and preference. Thus, IG infusions of 1.6% sucralose limit the intake of saccharin and glucose solutions and condition a CS+ flavor avoidance (Sclafani et al. 2010b; Supplementary Figure S3). This postoral inhibitory action can be “hidden” because B6 mice strongly prefer 0.8% and 1.6% sucralose (as well as lower concentrations) in sweetener versus water choice tests (Experiments 1 and 2; unpublished findings). This was the case in Experiment 2B in which the mice preferred 0.8% sucralose to water, yet lost their preference for 0.8% sucralose over 8% fructose. Thus, findings obtained with concentrated sucralose solutions must be interpreted with caution. The source of postoral sucralose inhibition is not known but it does not appear to be mediated by gut T1r3 sweet receptors; IG sucralose infusions inhibited CS+ intakes to the same degree in T1r3 KO mice and wild-type mice (Sclafani et al. 2010b).
The present findings demonstrated that an alternate strategy for matching sugar and non-caloric sweetener palatability is to use a mixture of sucralose and saccharin. The 0.1% S + S mixture was highly palatable but did not have the postoral inhibitory action of 0.8% sucralose. It was also significantly preferred to 8% glucose, fructose, and sucrose in 1-min tests and persistently preferred to fructose in 24-h/day tests. Non-caloric sweetener mixtures (“blends”) are commonly used in diet drinks and foods to enhance sweetness and improve the flavor profile of products (Powers 1994; Schiffman et al. 1995; Franz 2010). This taste synergism of different sweeteners presumably occurs because they bind to different sites on the T1r2/T1r3 receptor complex (Morini et al. 2008). Non-caloric sweetener mixtures, however, are rarely used in rodent research. One early study reported that adding cyclamate to a saccharin solution reduced rather than increased its preference to rats, which is not surprising as rats do not prefer cyclamate solutions to water (Wagner 1971). Similarly, we observed that adding sucralose to a saccharin solution decreased preference, consistent with the sucralose aversion displayed by most rats (Sclafani and Clare 2004; Bello and Hajnal 2005; Loney et al. 2011). It should be noted that in consumer sweetener packets (e.g., Spenda), sucralose is mixed with maltodextrin, a carbohydrate that is highly preferred by rats, explaining why rats prefer Spenda solutions but not pure sucralose solutions (Dess et al. 2009). Perhaps mixing other preferred sweeteners (acesulfame and stevia) with saccharin might enhance sweet taste intensity to rats. An alternative approach is to mix a small amount of glucose and saccharin to enhance palatability. Warwick (Warwick and Weingarten 1994) reported that a 0.125% saccharin + 1% glucose mixture was isopreferred to 6% glucose, but less preferred than 8–16% glucose solutions in sham-feeding tests that minimized postoral influences. However, the sugar concentration must be kept very low to minimize the postoral conditioning actions of glucose (Ackroff and Sclafani 1994; Ramirez 1994; Zukerman et al. 2013b).
In summary, the present findings are consistent with reports that sucrose and glucose are more rewarding than non-caloric sweeteners based on the sugars’ postoral actions (de Araujo et al. 2008; Beeler et al. 2012; Tellez et al. 2013; Scheggi et al. 2013). However, the present data and related IG conditioning findings (Sclafani and Ackroff 2012a; Zukerman et al. 2013a) indicate that this is not due to the caloric effects of sugars given that fructose, unlike glucose and sucrose, was not more rewarding than the non-caloric sweeteners. Recent findings suggest that postoral sugar appetition is mediated in part by specific intestinal sugar receptors rather than gut sweet taste or “caloric” receptors (Zukerman et al. 2013a). How these receptors signal the brain is not known although a recent study suggests that hypothalamic melanin concentrating hormone neurons are an important signaling relay to brain reward systems (Domingos et al. 2013).
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK-31135).
Supplementary Material
Acknowledgements
The authors thank Mohammad Riad and Martin Zartarian for their expert technical assistance.
References
- Ackroff K, Sclafani A. 1994. Flavor preferences conditioned by intragastric infusions of dilute polycose solutions. Physiol Behav. 55(5):957–962. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Touzani K, Peets TK, Sclafani A. 2001. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 72(5):691–703. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. 2007. Taste receptor genes. Annu Rev Nutr. 27:389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. 2001. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 26(7):905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. 2012. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 36(4):2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. 2005. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr Res. 25(7):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. 2004. A Pavlovian approach to the problem of obesity. Int J Obes. 28(7):933–935. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. 2008. Food reward in the absence of taste receptor signaling. Neuron. 57(6):930–941. [DOI] [PubMed] [Google Scholar]
- Dess NK, Chapman CD, Monroe D. 2009. Consumption of SC45647 and sucralose by rats selectively bred for high and low saccharin intake. Chem Senses. 34(3):211–220. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, et al. 2013. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife Sciences. 2:e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. 2011. Leptin regulates the reward value of nutrient. Nat Neurosci. 14(12):1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M. 2010. Diet soft drinks: how safe are they? Diabetes Self Manag. 27(2):8, 11–13. [PubMed] [Google Scholar]
- Gannon KS, Smith JC, Henderson R, Hendrick P. 1992. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 51(3):515–521. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Breinager L, Kyrillou E, Lacuna K, Rocha R, Sclafani A. 2010. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiol Behav. 101(3):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann MF. 1933. The behavior of albino rats in choosing foods: II. Differentiation between sugar and saccharin. J Comp Psychol. 15:419–428. [Google Scholar]
- Loney GC, Torregrossa AM, Smith JC, Sclafani A, Eckel LA. 2011. Rats display a robust bimodal preference profile for sucralose. Chem Senses. 36(8):733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C, White NM. 1984. Contingent and non-contingent actions of sucrose and saccharin reinforcers: effects on taste preference and memory. Physiol Behav. 32(2):195–203. [DOI] [PubMed] [Google Scholar]
- Morini G, Bassoli A, Temussi PA. 2008. Multiple receptors or multiple sites? Modeling the human T1R2-T1R3 sweet taste receptor. In: Weerasinghe DL, DuBois GE, editors. Sweetness and sweeteners. Washington (DC): American Chemical Society: p. 147–161. [Google Scholar]
- Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. 2011. Intravascular food reward. PLoS One. 6(9):e24992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, et al. 2013. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 309(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MA. 1994. Sweetening our foods: blending sweeteners. Diabetes Educ. 20(3):243–244. [DOI] [PubMed] [Google Scholar]
- Ramirez I. 1994. Stimulation of fluid intake by carbohydrates: interaction between taste and calories. Am J Physiol. 266:R682–R687. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Secci ME, Marchese G, De Montis MG, Gambarana C. 2013. Influence of palatability on motivation to operate for caloric and non-caloric food in non food-deprived and food-deprived rats. Neuroscience. 236:320–331. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Booth BJ, Carr BT, Losee ML, Sattely-Miller EA, Graham BG. 1995. Investigation of synergism in binary mixtures of sweeteners. Brain Res Bull. 38(2):105–120. [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2006. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 87(4):734–744. [DOI] [PubMed] [Google Scholar]
- Sclafani A. 2013. Gut-brain nutrient signaling. Appetition vs. satiation. Appetite. 71:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Abrams M. 1986. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 37(2):253–256. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2006. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol Behav. 88(1-2):88–94. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2012a. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 106(4):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 2012b. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 302(10):R1119–R1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Bahrani M, Zukerman S, Ackroff K. 2010a. Stevia and saccharin preferences in rats and mice. Chem Senses. 35(5):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. 1993. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 265:R320–R325. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Clare RA. 2004. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 29(6):523–528. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. 1999. Conditioned flavor avoidance, preference, and indifference produced by intragastric infusions of galactose, glucose, and fructose in rats. Physiol Behav. 67(2):227–234. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, Glendinning JI. 2010b. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 299(6):R1643–R1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. 2005. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 289(3):R712–R720. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Marambaud P, Ackroff K. 2014a. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 126:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Zukerman S, Ackroff K. 2014b. Fructose- and glucose-conditioned preferences in FVB mice: strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol. 307(12):R1448–R1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Sclafani A. 2002. Saccharin as a sugar surrogate revisited. Appetite. 38(2):155–160. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G. 1999. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 23(12):1914–1920. [PubMed] [Google Scholar]
- Tellez LA, Ren X, Han W, Medina S, Ferreira JG, Yeckel CW, de Araujo IE. 2013. Glucose utilization rates regulate intake levels of artificial sweeteners. J Physiol. 591:5727–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K. 2009. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology. 137(1):262–273. [DOI] [PubMed] [Google Scholar]
- Tsurugizawa T, Uneyama H. 2014. Differences in BOLD responses to intragastrically infused glucose and saccharin in rats. Chem Senses. 39(8):683–691. [DOI] [PubMed] [Google Scholar]
- Wagner MW. 1971. Comparative rodent preferences for artificial sweeteners. J Comp Physiol Psychol. 75(3):483–490. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Weingarten HP. 1994. Dissociation of palatability and calorie effects in learned flavor preferences. Physiol Behav. 55(3):501–504. [DOI] [PubMed] [Google Scholar]
- Young PT, Madsen CH., Jr 1963. Individual isohedons in sucrose-sodium chloride and sucrose-saccharin gustatory areas. J Comp Physiol Psychol. 56:903–909. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2011. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 301(6):R1635–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2013a. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 305(7):R840–R853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2013b. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav. 109:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. 2009. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 296(4):R866–R876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. 2013c. Impact of T1r3 and Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses. 38(5):421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.