SUMMARY
Introduction
Meniscal extrusion is thought to be associated with less meniscus coverage of the tibial surface, but the association of radiographic disease stage with quantitative measures of tibial plateau coverage is unknown. We therefore compared quantitative and semi-quantitative measures of meniscus position and morphology in individuals with bilateral painful knees discordant on medial joint space narrowing (mJSN).
Methods
A sample of 60 participants from the first half (2,678 cases) of the Osteoarthritis Initiative cohort fulfilled the inclusion criteria: bilateral frequent pain, Osteoarthritis Research Society International (OARSI) mJSN grades 1–3 in one, no-JSN in the contra-lateral (CL), and no lateral JSN in either knee (43 unilateral mJSN1; 17 mJSN2/3; 22 men, 38 women, body mass index (BMI) 31.3 ± 3.9 kg/m2). Segmentation and three-dimensional quantitative analysis of the tibial plateau and meniscus, and semi-quantitative evaluation of meniscus damage (magnetic resonance imaging (MRI) osteoarthritis knee score – MOAKS) was performed using coronal 3T MR images (MPR DESSwe and intermediate-weighted turbo spin echo (IW-TSE) images). CL knees were compared using paired t-tests (between-knee, within-person design).
Results
Medial tibial plateau coverage was 36 ± 9% in mJSN1 vs 45 ± 8% in CL no-JSN knees, and was 31 ± 9% in mJSN2/3 vs 46 ± 6% in no-JSN knees (both P < 0.001). mJSN knees showed greater meniscus extrusion and damage (MOAKS), but no significant difference in meniscus volume. No significant differences in lateral tibial coverage, lateral meniscus morphology or position were observed.
Conclusions
Knees with medial JSN showed substantially less medial tibial plateau coverage by the meniscus. We suggest that the less meniscal coverage, i.e., less mechanical protection may be a reason for greater rates of cartilage loss observed in JSN knees.
Keywords: Meniscus, Joint space narrowing, Radiographic osteoarthritis, Magnetic resonance imaging, Tibial coverage
Introduction
The meniscus is a fibrocartilage structure positioned between the tibial plateau and distal femoral knee cartilages, with all three structures being known to make up the radiographic joint space1. The meniscus transmits a substantial proportion of the forces across the femorotibial joint2–4, and keeps the forces encountered by the cartilage and subchondral bone in reasonable limits, by distributing loads and reducing knee joint contact stress3–6. Meniscus damage is frequent in the general population, occurs more often in the medial than in the lateral meniscus, and its prevalence increases with more severe joint space narrowing (JSN)7. Further, meniscal damage is known to be associated with meniscal extrusion8–11. Although meniscal extrusion is thought to be associated with lesser coverage of the tibial plateau and hence less mechanical protection of the articular surface, the association of radiographic disease stage (i.e., JSN) with quantitative measures of the meniscus, and particularly tibial plateau coverage, is unknown. In this context, it also is of interest that JSN is a diagnostic feature that is commonly used to classify knees as having advanced structural OA12, and that it has been shown that JSN predicts further structural deterioration of the knee, specifically femorotibial cartilage loss13–17.
Only few studies have quantitatively evaluated the position (extrusion) of the meniscus in two dimensions in one or more image slices1,18–23. More recently, several research groups have proposed three-dimensional (3D) technology for quantitative morphometric analysis of the meniscus24–28, with that developed by us including tibial plateau coverage, meniscus position, and meniscus size [e.g., volume, height, etc.] as key quantitative parameters29. In view of the above reasons, the aim of the current study therefore was to compare quantitative (and semi-quantitative) measures of the meniscus in painful knees with discordant medial JSN (mJSN) status, using a between-knee, within-person study design14,30–32. Specifically, we hypothesized that knees with mJSN have substantially less medial tibial coverage than contra-lateral (CL) knees without mJSN and we stratified the analysis between participants with mild unilateral mJSN vs no-JSN and advanced mJSN vs no-JSN. Further we explored whether only medial or also lateral meniscus coverage is affected by mJSN, whether meniscus extrusion or size differ between mJSN vs no-JSN knees, and we characterized meniscus damage and extrusion in these participants using the novel magnetic resonance imaging (MRI) osteoarthritis knee score (=MOAKS) grading system33.
Methods
Study participants
The subsample analyzed in the current study was drawn from the first half (2,678 cases) of the OA Initiative (OAI) cohort (baseline clinical data 0.2.1; http://www.oai.ucsf.edu/datarelease/)31. The OAI is a multi-center, population-based longitudinal cohort study, targeted at identifying risk factors associated with the onset and progression of knee OA, and at characterizing biomarkers of the disease. Participants in the OAI cohort were between 45 and 79 years old at baseline and included a diversity of ethnic backgrounds. Participants with rheumatoid or other inflammatory arthritis, bilateral end-stage knee OA, inability to walk without aids, or MRI contra-indications were excluded. Informed consent was obtained from all participants and the study was approved by the local ethics committees.
The subcohort for the current study was selected specifically to permit a between-knee, within-person comparison of painful knees with mJSN vs painful knees without mJSN or lateral JSN31. Briefly the subjects fulfilled the following inclusion criteria:
Body mass index (BMI) > 25 kg/m2,
Frequent knee pain (i.e., pain on most days in at least 1 month in the past 12 months) in both knees,
mJSN Osteoarthritis Research Society International (OARSI) grades 1–3 in one knee34,35 and no mJSN in the other (CL) knee,
No lateral JSN in either knee.
The primary selection was based on the radiographic readings performed at the OAI clinical sites and was complemented by either central OAI readings (when available at the time point of participant selection) or by consensus evaluation of two experienced readers (AG and DH)14,31. Compared to a previous study with n = 73 participants31, the current study excluded three participants with infrequent pain in the no-JSN knee, three participants with some degree of lateral JSN, and seven in whom the meniscus could not be segmented due to severe destruction (1 = mJSN1, 3 = mJSN2, 3 = mJSN3). Finally, 60 participants were included in the analysis, of which 22 were men and 38 women. The mean age was 61.3 ± 9.2 years, the body height 1.66 ± 0.96 m, the body weight 86.6 ± 13.0 kg, and the BMI 31.3 ± 3.9 kg/m2. Of the 60 mJSN knees, 43 knees were mJSN grade 1,14 mJSN grade 2, and 3 mJSN grade 3. As per inclusion/exclusion criteria, no lateral JSN was present.
MR images and segmentation
MR images were acquired for each knee with a 3 T Magnetom Trio magnet (Siemens Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH, USA)36,37. For the current study, the coronal multi-planar reconstruction of the sagittal double echo steady state sequence with water excitation was used (DESSwe: reconstructed slice thickness = 1.5 mm, in-plane resolution 0.37 mm × 0.7 mm, interpolated to 0.37 mm × 037 mm)38,39. Meniscus segmentation and morphometry from the DESS have been shown to yield acceptable inter-observer reliability and good agreement with measurements made from a coronal intermediate-weighted turbo spin echo (IW-TSE) sequence40. The advantage of the DESS, however, is that it provides greater spatial resolution and better delineation of the tibial plateau cartilage surface area and also has been validated for accurately depicting the tibial cartilage38.
Quantitative analysis
All images underwent initial quality control (KB). Manual segmentation of the medial and lateral tibial plateau area (i.e., the area of cartilage surface, including denuded areas of subchondral bone = ACdAB29,41), and the surfaces of the medial and lateral meniscus (tibial, femoral and external – Fig. 1) were performed by a single experienced operator (KB). Segmentation and quantitative analysis were performed using dedicated image analysis software (Chondrometrics GmbH, Ainring, Germany)29,41. Segmentation was started anteriorly and was ended posteriorly in the first/last image in which both the tibial cartilage and the menisci could be reliably identified. Internally, the borders of the menisci were defined by the internal margin of the cartilage surfaces of the medial and lateral tibia, respectively, because these are continuous with the transverse and menisco-femoral ligaments and because no intrinsic anatomical demarcation could be used to separate these structures. The size of the tibial plateau and of the total meniscus surface (i.e., the sum of the tibial, femoral and external surface), the meniscus volume, mean and maximal meniscus thickness, and the mean and maximal meniscus width were computed from the segmentations29. Meniscus position relative to the tibial plateau was measured by determining the percentage of tibial plateau covered by meniscus. The mean and maximal extrusion distance of the meniscus were measured as the distance between the external margin of the tibial plateau area and that of the tibial meniscus area (Figs. 1 and 2). A further measure of extrusion was the (relative, percent) area of the tibial meniscus surface not covering the tibial plateau. The mean and maximal overlap distance between the meniscus and tibial plateau were computed using the distance between the external margin of the tibial plateau and the internal margin of the meniscus (i.e., the intersection of its tibial and femoral area (Figs. 1 and 2)). Please note that a more negative value indicates a more Internal position relative to the external border of the tibial plateau29,41. In addition to the above 3D measures, meniscus width, extrusion and overlap distance were also determined for the central five slices, to more specifically evaluate the meniscus body (Figs. 1 and 2). Measures in this region also were shown to display superior inter-observer reproducibility40 and sensitivity to between-knee differences of pain frequency42.
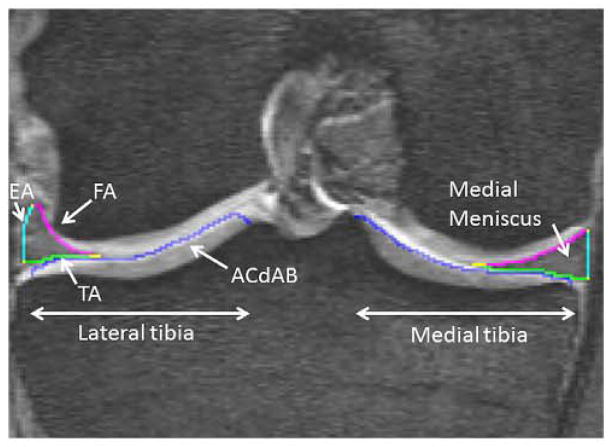
Fig. 1.
Coronal Reformat DESS MRI: showing the medial and lateral meniscus on the medial and lateral tibial plateau with segmentation of: FA – femoral meniscus area, TA – tibial meniscus area, EA – external meniscus area, ACdAB – articular surface of the medial tibial plateau area.
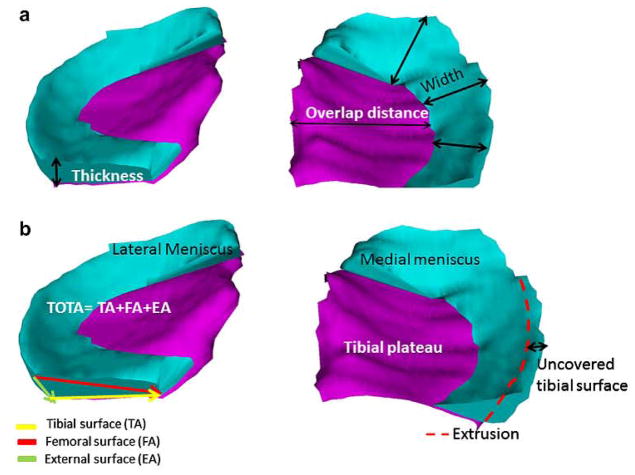
Fig. 2.
3D reconstruction of the medial (right) and lateral (left) meniscus; (a) meniscal thickness (Th), overlap distance (OvD) and width (Wid) are marked; (b) both menisci (turquoise) covering the tibial plateau (ACdAB; purple), Tibial (TA), femoral (FA) and external (EA) surface areas are marked, as well as the total surface area of the meniscus (TOT A); meniscal extrusion (Ex) and that part of the meniscus surface facing the tibia but extruding the tibial plateau margin (TA.uncovp) arc indicated schematically.
Semi-quantitative analysis
Semi-quantitative MR imaging readings of meniscal integrity and position were performed by an experienced musculoskeletal radiologist (AG) using the MOAKS scoring system33 based on fat-suppressed sagittal and non-fat-suppressed coronal IW-TSE images36. Meniscus morphology (damage) was evaluated for the medial and lateral meniscus in the anterior and posterior horn and the meniscus body and divided into seven different grades (0 = normal; 1 = signal change; 2 = radial tear; 3 = horizontal tear; 4 = vertical tear; 5 = complex tear; 6 = partial maceration; 7 = complete maceration; Fig. 3). Meniscal root tears were defined as being present (=1) or absent (=0). Meniscus position (extrusion) was also classified, with grade 0 representing <2 mm; grade 1 representing 2–2.9 mm; grade 2 representing 3–4.9 mm, and grade 3 representing >5 mm extrusion33.
Fig. 3.
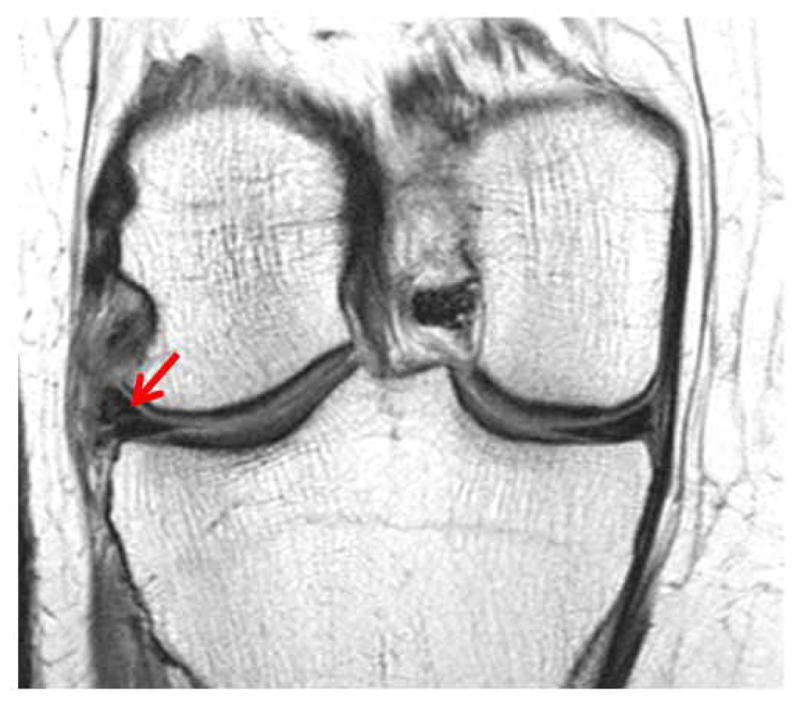
Coronal IW-TSE MRI or the Left Knee: showing a meniscus grade 3 tear (arrow), scored using the MOAKS system.
Statistical analysis
Mean values and standard deviations (SDs) were determined for all quantitative measures of meniscus position and size in knees with and without mJSN. Participants were stratified based on mJSN grade; mJSN2 and mJSN3 were combined due to the small number of the latter (see below). Hence, mJSN1 knee were compared vs CL no-JSN knees, and mJSN2/3 vs CL no-JSN knees, using 95% confidence intervals (CIs). Because statistical comparisons were performed between knees of the same subjects, differences were tested using paired t-tests. Medial tibial plateau coverage (ACdAB.Covp) by the medial meniscus was the primary analytical focus. Other quantitative measures of tibial coverage (overlap distance between the external tibial plateau margin and the internal meniscus margin), quantitative measures of meniscus extrusion and size, and semi-quantitative scores of meniscus extrusion and damage were viewed as exploratory and/or explanatory. Two-tailed P-values <0.025 were considered statistically significant to account for two parallel confirmatory tests performed (i.e., ACdAB.Covp in JSN1 vs no-JSN and in JSN 2/3 vs no-JSN knees).
The maximum (semi-quantitative) MOAKS morphology score across the entire meniscus (anterior horn, posterior horn and meniscus body) was calculated and compared between mJSN1 vs CL no-JSN knees, and between mJSN2/3 vs CL no-JSN knees using a Wilcoxon signed rank test. The same statistical testing procedures as above were applied to MOAKS extrusion scores.
Results
Medial meniscus tibial plateau coverage
Knees with mJSN grade 1 had significantly less medial tibial plateau coverage (36.0 ± 8.8%) than CL no-JSN knees (45.1 ± 8.4%; Table I; Fig. 4). Knees with mJSN grade 2/3 also had significantly less medial tibial plateau coverage (31.3 ± 9.3%) than CL no-JSN knees (46.2 ± 6.1%; Table II; Fig. 4). The relative position of the internal margin of the meniscus compared with the external margin of the tibial plateau (mean overlap distance) was observed to have less negative values (less coverage) in mJSN1 and mJSN2/3 vs CL no-JSN knees (Table I & II). Similar relationships were observed for the maximum overlap distance, and for the overlap distance in the central five slices (Table I & II).
Table I.
Knees with mJSN grade 1 vs CL knees without JSN: tibial coverage, meniscus position and meniscus size
| mJSN1 (n = 43)
|
CL no-JSN
|
Diff*
|
P-value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean [95% CI] | ||
| Medial meniscus | ||||
| Tibial plateau coverage | ||||
| ACdAB.Covp [%] | 36.0 ± 8.75 | 45.1 ± 8.36 | −9.14 [−12.2–(−6.08)] | <0.001 |
| OvD.Me [mm] | −9.01 ± 2.13 | −11.3 ± 2.57 | 2.26 [1.56–2.96] | <0.001 |
| OvD.Max [mm] | −2.43 ± 1.69 | −3.88 ± 1.92 | 1.44 [0.85–2.03] | <0.001 |
| OvD.c5 [mm] | 4.08 ± 2.31 | 6.79 ± 3.20 | 2.71 [1.90–3.51] | <0.001 |
| Meniscus extrusion | ||||
| Ex.Me [mm] | 3.45 ± 1.46 | 2.11 ± 1.51 | 1.34 [0.92–1.76] | <0.001 |
| Ex.Max [mm] | 7.05 ± 1.84 | 6.60 ± 1.48 | 0.45 [−0.04–0.94] | 0.068 |
| Ex.c5 [mm] | 3.09 ± 1.81 | 1.84 ± 1.26 | 1.25 [0.76–1.74] | <0.001 |
| TA.uncovp [%] | 26.5 ± 11.4 | 16.3 ± 8.11 | 10.2 [6.84–13.6] | <0.001 |
| Meniscus size | ||||
| Wid.Me [mm] | 8.13 ± 1.50 | 9.24 ± 1.57 | −1.11 [−1.54–(−0.68)] | <0.001 |
| Wid.Max [mm] | 14.1 ± 2.68 | 16.3 ± 3.15 | −2.21 [−3.02–(−1.41)] | <0.001 |
| Wid.c5 [mm] | 7.39 ± 2.20 | 9.39 ± 2.94 | −1.76 [−2.58–(−0.95)] | <0.001 |
| Th.Me [mm] | 2.67 ± 0.502 | 2.72 ± 0.532 | −0.05 [−0.15–0.06] | 0.399 |
| Th.Mav [mm] | 6.63 ± 1.52 | 6.44 ± 1.32 | 0.18 [−0.18–0.54] | 0.318 |
| V [mm3] | 1930 ± 747 | 2112 ± 871 | −182[−330–(−34.1)] | 0.017 |
| TOTA [mm2] | 1470 ± 371 | 1553 ± 412 | −83.5 [−148–(−18.9)] | 0.013 |
| Lateral meniscus | ||||
| Tibial plateau coverage | ||||
| ACdAB.Covp [%] | 57.2 ± 5.61 | 57.8 ± 5.21 | −0.59 [−2.38–1.20] | 0.510 |
| OvD.Me [mm] | −15.9 ± 2.58 | −16.1 ± 2.16 | 0.12 [−0.28–0.52] | 0.559 |
| OvD.Max [mm] | −8.70 ± 2.28 | −8.60 ± 2.17 | −0.10 [−0.50–0.29] | 0.600 |
| OvD.c5 [mm] | −9.95 ± 2.42 | −9.89 ± 2.45 | −0.06 [−0.51–0.40] | 0.798 |
| Meniscus extrusion | ||||
| Ex.Me [mm] | −1.41 ± 1.99 | −1.31 ± 1.86 | −0.11 [−0.62–0.41] | 0.681 |
| Ex.Max [mm] | 7.24 ± 1.81 | 7.49 ± 2.01 | −0.25 [−0.86–0.36] | 0.409 |
| Ex.c5 [mm] | 0.50 ± 1.19 | −0.28 ± 1.13 | −0.21 [−0.53–0.11] | 0.183 |
| TA.uncovp [%] | 3.90 ± 4.20 | 4.45 ± 3.97 | −0.54 [−1.80–0.71] | 0.386 |
| Meniscus size | ||||
| Wid.Me [mm] | 8.85 ± 1.41 | 8.99 ± 1.26 | −0.14 [−0.42–0.15] | 0.348 |
| Wid.Max [mm] | 12.7 ± 1.96 | 12.7 ± 1.61 | −0.02 [−0.45–0.41] | 0.912 |
| Wid.c5 [mm] | 10.6 ± 2.21 | 10.7 ± 2.17 | −0.12 [−0.62–0.38] | 0.628 |
| Th.Me [mm] | 2.64 ± 0.445 | 2.62 ± 0.392 | 0.02 [−0.06–0.10] | 0.627 |
| Th.Mav [mm] | 6.60 ± 1.05 | 6.61 ± 1.05 | −0.01 [−0.22–0.20] | 0.929 |
| V [mm3] | 1964 ± 652 | 2001 ± 602 | −36.9 [−148.2–74.4] | 0.508 |
| TOTA [mm2] | 1509 ± 334 | 1536 ± 303 | −26.9 [−84.4–30.7] | 0.351 |
Mean of the pairwise differences (may deviation from difference between group means); ACdAB.Covp: area of cartilage surface covered with meniscus in percent; Ex.Me: mean external extrusion; Ex.Max: maximal external extrusion; OvD.Me: mean overlap distance; OvD.Max: maximal overlap distance. Note that a positive value for meniscal extrusion indicates an external position relative to the external border of the tibial plateau. Whereas a negative value indicates an internal position relative to the external border. A more negative value for the overlap distance indicates a more internal position of the inner margin of the meniscus; TA.uncovp: tibial area of the meniscus not covering the tibial plateau in percent; TOTA: sum of all three surface areas of the meniscus; V: volume of the meniscus; Th.Me: mean thickness of the meniscus; Th.Mav: average thickness of the meniscus; Wid.Me: mean width of the meniscus; Wid.max: maximal width of the meniscus; Ex.c5: mean extrusion in the central five slices; Wid.c5: mean width in the central five slices; OvD.c5: mean overlap distance in the central five slices.
Fig. 4.
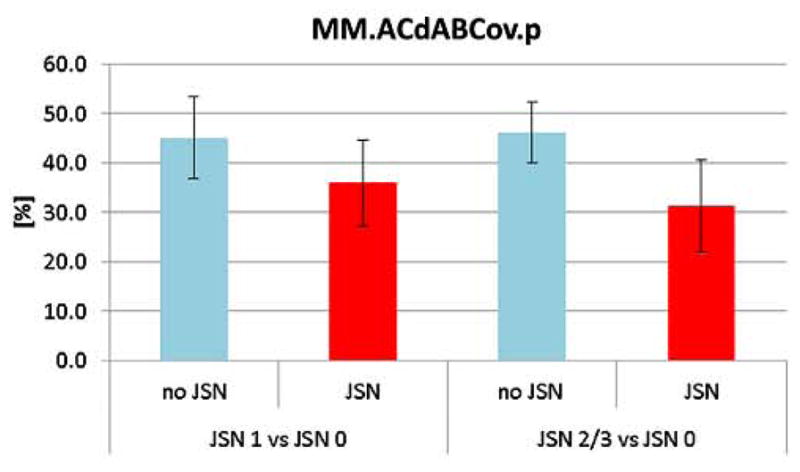
Bar graph showing the tibial plateau coverage by the medial meniscus in CL knees with and without JSN 1 and 2/3.
Table II.
Knees with mJSN grade 2/3 vs CL knees without JSN: Tibial coverage, meniscus position and meniscus size
| mJSN2/3(n = 17)
|
CL no-JSN
|
Diff*
|
P-value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean [95% CI] | ||
| Medial meniscus | ||||
| Tibial plateau coverage | ||||
| ACdAB.Covp [%] | 31.3 ± 9.29 | 46.2 ± 6.14 | −14.8 [−21.6–(−8.03)] | <0.001 |
| OvD.Me [mm] | −7.76 ± 2.40 | −10.8 ± 1.44 | 3.08 [1.54–4.62] | 0.001 |
| OvD.Max [mm] | −1.79 ± 1.54 | −3.71 ± 1.42 | 1.91 [0.80–3.03] | 0.002 |
| OvD.c5 [mm] | −3.46 ± 1.81 | −5.95 ± 2.43 | 2.50 [1.01–4.00] | 0.003 |
| Meniscus extrusion | ||||
| Ex.Me [mm] | 4.62 ± 1.23 | 2.50 ± 1.29 | 2.12 [1.06–3.18] | 0.001 |
| Ex.Max [mm] | 7.86 ± 1.61 | 7.25 ± 1.39 | 0.61 [−0.53–1.74] | 0.273 |
| Ex.c5 [mm] | 4.10 ± 1.85 | 1.79 ± 1.32 | 2.31 [1.01–3.62] | 0.002 |
| TA.uncovp [%] | 36.4 ± 15.6 | 16.2 ± 7.28 | 20.2 [10.1–30.3] | 0.001 |
| Meniscus size | ||||
| Wid.Me [mm] | 8.25 ± 1.22 | 8.96 ± 1.02 | −0.72 [−1.44–0.002] | 0.051 |
| Wid.Max [mm] | 14.1 ± 2.33 | 16.4 ± 2.74 | −2.31 [−4.07–(−0.54)] | 0.014 |
| Wid.c5 [mm] | 7.88 ± 2.08 | 8.46 ± 1.94 | −0.58 [−1.93–0.76] | 0.373 |
| Th.Me [mm] | 2.88 ± 0.397 | 2.68 ± 0.340 | 0.20 [−0.05–0.44] | 0.103 |
| Th.Mav [mm] | 7.09 ± 1.13 | 6.58 ± 0.712 | 0.51 [−0.16–1.18] | 0.128 |
| V [mm3] | 2037 ± 574 | 2031 ± 526 | 6.37 [−343–356] | 0.970 |
| TOTA [mm2] | 1507 ± 294 | 1547 ± 265 | −40.6 [−232–151] | 0.660 |
| Lateral meniscus | ||||
| Tibial plateau coverage | ||||
| ACdAB.Covp [%] | 62.2 ± 6.77 | 58.8 ± 5.99 | 3.43 [−1.79–8.66] | 0.183 |
| OvD.Me [mm] | −17.1 ± 1.93 | −16.2 ± 2.20 | −0.90 [− 2.51–0.71] | 0.253 |
| OvD.Max [mm] | −10.2 ± 2.45 | −9.48 ± 2.65 | −0.68 [−2.60–1.23] | 0.459 |
| OvD.c5 [mm] | −11.9 ± 2.44 | −10.7 ± 2.80 | −1.25 [−3.25–0.75] | 0.204 |
| Meniscus extrusion | ||||
| Ex.Me [mm] | −1.62 ± 1.44 | −1.38 ± 1.74 | −0.24 [−1.46–0.98] | 0.683 |
| Ex.Max [mm] | 7.36 ± 1.76 | 7.06 ± 1.08 | 0.30 [− 0.73–1.32] | 0.548 |
| Ex.c5 [mm] | −0.716 ± 0.90 | −0.476 ± 1.13 | −0.24 [− 1.08–0.60] | 0.554 |
| TA.uncovp [%] | 3.34 ± 2.79 | 3.89 ± 3.92 | −0.55 [−3.06–1.96] | 0.648 |
| Meniscus size | ||||
| Wid.Me [mm] | 9.60 ± 1.52 | 9.07 ± 1.20 | 0.54 [−0.46–1.53] | 0.269 |
| Wid.Max [mm] | 14.2 ± 2.42 | 13.4 ± 2.01 | 0.78 [−0.86–2.41] | 0.330 |
| Wid.c5 [mm] | 12.6 ± 2.41 | 11.4 ± 2.64 | 1.15 [−0.74–3.04] | 0.214 |
| Th.Me [mm] | 2.65 ± 0.279 | 2.59 ± 0.322 | 0.05 [−0.11–0.22] | 0.495 |
| Th.Mav [mm] | 6.45 ± 0.930 | 6.47 ± 0.969 | −0.02 [−0.44–0.41] | 0.934 |
| V [mm3] | 1953 ± 519 | 1877 ± 532 | 76.3 [−231–384] | 0.606 |
| TOTA [mm2] | 1503 ± 310 | 1462 ± 273 | 41.2 [−128–211] | 0.615 |
For abbreviations, see Table 1.
Medial meniscus extrusion
The mean extrusion of the entire medial meniscus was observed to be greater in mJSN vs no-JSN knees (mJSN1: 3.45 ± 1.46 vs 2.11 ± 1.51 mm: mJSN2/3: 4.62 ± 1.23 vs 2.50 ± 1.29 mm: Table I & II; Fig. 5) and so was the mean extrusion in the central five slices (mJSN1: 3.09 ± 1.81 vs 1.84 ± 1.26 mm; mJSN2/3: 4.10 ± 1.85 vs 1.79 ± 1.32 mm; Table I & II). Further the medial meniscus surface area extruding the tibial plateau was observed to be greater in mJSN than in no-JSN knees (mJSN1: 27 ± 11 vs 16 ± 8.1%; mJSN2/3: 36 ± 16 vs 16 ± 7.3%) and so was the maximum extrusion across the meniscus (Table I & II).
Fig. 5.
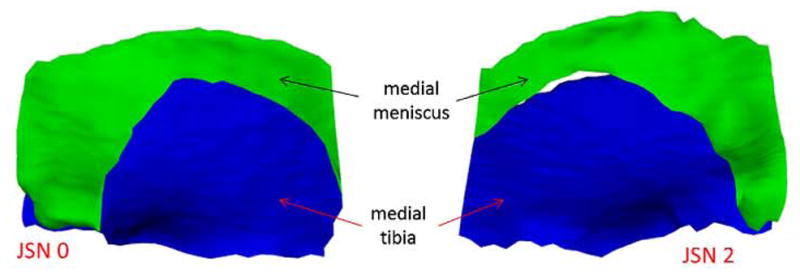
3D reconstruction of the medial meniscus (green) and the medial tibial plateau (blue) for no-JSN (left) vs JSN2 (right) knees, showing the greater amount of extrusion of the medial meniscus for JSN knees.
Other quantitative measures of the medial and lateral meniscus
Measures of meniscus size did not show obvious differences between mJSN vs CL no-JSN knees (Table I & II). The only exception was the meniscus width, which was observed to be smaller in mJSN than in the no-JSN knees (entire meniscus and central five slices; Table I & II). No obvious differences in any of the quantitative measures of lateral meniscus position or size were observed in mJSN vs CL no-JSN knees (Table I & II).
Semi-quantitative results
The average maximum lesions score in the medial meniscus was observed to be greater in mJSN one than in CL no-JSN knees (mean 3.3 vs 1.7; median 3 vs 1), and was also greater in mJSN2/3 than in no-JSN knees (mean 3.9 vs 2.0; median 5 vs 1). The mean average score in the lateral meniscus was similar between mJSN and CL no-JSN knees (mJSN1: 0.7 vs 0.8, P = 0.7; mJSN2/3: 1.1 vs 0.5, P = 0.31).
The presence of meniscus tears (MOAKS 2–5) and maceration (MOAKS 6–7) for the medial and lateral meniscus in different subgroups is shown in Table III. 65% of the mJSN1 knees and only 37% of the no-JSN knees had any medial meniscus damage (MOAKS 2–7); 65% of the mJSN2/3 knees had any damage vs 47% of the no-JSN knee, with mJSN2/3 knees displaying a high percentage (47%) of partial or complete maceration (Table III). The frequency of lateral meniscus tears was not obviously different between mJSN and no-JSN knees (mJSN1: 16 vs 21%, mJSN2/3 24 vs 12%). There was no maceration observed in any lateral meniscus.
Table III.
Semi-quantitative evaluation of the medial meniscus morphology and extrusion according to the MOAKS grading system
| mJSN1 n = 43 | CL no-JSN n = 43 | mJSN2/3 n = 17 | CL no-JSN n = 17 | |
|---|---|---|---|---|
| Morphology | ||||
| Grade 0/1 | 34.9% | 62.8% | 35.3% | 52.9% |
| Grade 2–5 | 32.6% | 30.2% | 17.6% | 35.3% |
| Grade 6–7 | 32.6% | 7.0% | 47.1% | 11.8% |
| Extrusion of the meniscus body | ||||
| Grade 0 | 30.2% | 37.2% | 5.6% | 52.9% |
| Grade 1 | 20.9% | 39.5% | 17.6% | 17.6% |
| Grade 2 | 34.9% | 20.9% | 35.3% | 23.5% |
| Grade 3 | 14.0% | 2.3% | 41.1% | 5.88% |
Meniscus morphology: 1 = signal change; 2 = radial tear; 3 = horizontal tear; 4 = vertical tear; 5 = complex tear; 6 = partial maceration; 7 = complete maceration; Extrusion grades: 0: <2 mm; 1: 2–2.9 mm; 2: 3–4.9 mm; 3: >5 mm33.
The mean average extrusion score in the medial meniscus was observed to be greater in mJSN than in no-JSN knees (mJSN1: 1.3 vs 0.9; mJSN2/3: 2.0 vs 0.0). The mean average score in the lateral meniscus was the same in mJSN1 as in no-JSN knees (0.3 vs 0.3), and did not show an obvious difference between mJSN2/3 and no-JSN knees (0.4 vs 0.2). Meniscal root tears were observed in three knees with mJSN2/3, in one with mJSN1, and in one knee with no-JSN.
Discussion
The current study is the first to report the association of radiographic disease stage with 3D quantitative measures (specifically tibial plateau coverage) and semi-quantitative measures (using MOAKS) of the medial and lateral meniscus, specifically in individuals with bilateral painful knees discordant on mJSN. We hypothesized that knees with mJSN have substantially less medial tibial coverage than CL knees without mJSN. The key finding is that medial tibial plateau coverage is significantly and substantially lower in mJSN1 than in (CL) no-JSN knees and in mJSN2/3 than in (CL) no-JSN knees. Observed medial meniscus extrusion and morphology lesion scores were greater in mJSN than in no-JSN knees, whereas no obvious differences in meniscus size (e.g., volume, thickness) were detected between CL knees. Further, no differences were observed in quantitative measures of the lateral meniscus.
A limitation of this study is its moderate sample size, particularly of knees with mJSN2/3, although knees were selected from a very large sample. This is because knee OA often is a symmetric bilateral disease and knees rarely are discordant by 2 or more JSN grades, when both being frequently painful. Further, in some knees (mostly with mJSN2/3) the meniscus could not be segmented due to complete maceration. Nevertheless, highly significant differences were identified between mJSN vs no-JSN knees in tibial plateau coverage and extrusion. The strength of the study is the choice of a between-knee, within-person comparison14,30–32, which eliminates between-person confounding, such as differences in sex, age, weight, height, BMI, occupation/physical activity levels, and others. For instance, differences in medial meniscus position and extrusion have been reported between men and women23,43. The between-knee, within-person approach also involves greater statistical efficiency, by allowing one to apply a paired test. Further studies need to show whether the findings made here are generalizable to between-subject differences at different radiographic disease stages.
Another limitation is that segmentation of the meniscus was done using only coronal (but not sagittal) MR imaging. Coronal images are ideal for evaluating the meniscal body and meniscus extrusion of the body in external direction, but preclude measurement of anterior extrusion23, because of the partial volume effects in this region with coronal slices. Yet, the coronal protocol was shown to display satisfactory intra-observer29,41,42 and inter-observer reproducibility40, and the primary outcome to be studied was tibial plateau coverage, which can be adequately measured using the coronal protocol. A strength, however, of the methodology used here is that tibial coverage by the meniscus as well as overlap and extrusion were measured for the entire medial and lateral tibial plateau and were not confined to one or several (central) slices. A 3T DESSwe sequence was used for segmentation which is not used to clinically evaluate the meniscus, but has been validated for the purpose of cartilage measurement38,39 and delineates the cartilage surface (the segmentation of which is required to measure coverage and extrusion) with high spatial resolution. Further, quantitative meniscus measurements obtained from the 3T DESSwe have shown satisfactory agreement with those from the IW-TSE. which is commonly used for the clinical evaluation of the meniscus40.
The prevalence of medial meniscus damage found in painful mJSN knees in our study (approx. 65%) agrees well with the prevalence rate observed in knees with frequent symptoms and radiographic evidence of knee osteoarthritis (Kellgren Lawrence grade 2 or higher) reported in a large population-based study7. Our measures of medial meniscus extrusion in mJSN knees (central five slices) also are in good agreement with similar measurements of Vanwanseele et al.21 in a cohort of subjects with predominantly (82%) medial knee OA (3.86 mm), and our extrusion results in the medial meniscus of no-JSN knees with those of Hwang et al. in subjects with end-stage lateral knee OA (2.5 mm in women, 1.7 mm in men). However, our measures of mean medial meniscus extrusion in the central five slices of mJSN knees are somewhat smaller than those reported by Jung et al.22 for the medial meniscus body in knees with varus OA (6.1 mm).
The observation that knees with mJSN show greater medial meniscus extrusion than those without confirm previous comparisons made using two-dimensional measurement in single MRI slices between-subject knees with and without mJSN18,44. However, we did not find consistent difference in meniscus size or signs of meniscus hypertrophy22 between mJSN and no mJSN knees.
The medial tibial plateau coverage by the medial meniscus in the no-JSN knees in our current study (approx. 45%) is somewhat smaller than that previously described in a healthy reference cohort of men and women (50%), whereas the lateral tibial plateau coverage in the current study is identical to the healthy reference subjects (58%)43. As the no-JSN knees in the current study displayed frequent pain and were CL to knees with advanced medial radiographic OA, they can be assumed to be at an early state of (medial) knee OA, which appears to be associated with an reduction by approximately 5% of medial tibial plateau coverage (from 50% to 45%). Knees with mJSN1, in contrast, displayed a much larger reduction of the medial tibial plateau coverage to 36%, and those with mJSN2/3 to only 31%. These between-knee differences are much larger than those previously observed between painful vs (CL) painless knees (41% vs 44% medial plateau coverage) with the same JSN status42. The dramatic reduction in medial tibial plateau coverage by the medial meniscus in knees with medial radiographic JSN very likely is associated with substantially reduced mechanical protection of the medial tibial plateau cartilage. Although this needs to be further explored in longitudinal studies, it is plausible that the greater mechanical stress acting on the cartilage in JSN knees with less medial tibial plateau coverage may explain why knees with (medial) radiographic JSN show much greater rates of (medial) femorotibial cartilage loss than osteoarthritis knees without JSN13–17. Further, the quantitative measurement methodology proposed here for the meniscus may be applied longitudinally, for instance in knees without JSN, trying to predict the incidence and/or progression of JSN. This also opens possibility of assessing the effectiveness of meniscal repairs or replacement, and potential disease modifying OA drugs that attempt to halt structural progression.
In conclusion we find that knees with mJSN1 and mJSN2/3 show substantially less tibial plateau coverage by the medial meniscus than CL no-JSN knees in the same person. We suggest that the substantially lesser degree of medial tibial plateau coverage and potentially less degree of mechanical protection by the meniscus in knees with mJSN may be a mechanical reason why previous studies found greater rates of medial femorotibial cartilage loss in knees with radiographic JSN than in those without.
Acknowledgments
We would like to thank the OAI participants, OAI investigators and OAI Clinical Center’s staff for generating this publicly available image data set. The study and image acquisition were supported by the Osteoarthritis Initiative (OAI). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The image analysis was supported by the Paracelsus Medical University (PMU) Forschungsfond (PMU FFF R-12702/036/BLO).
David Hunter is funded by an Australian Research Council Future Fellowship. Martin Englund is funded by Swedish Research Council, the Royal Physiographic Society in Lund, King Gustav 80-Year Birthday Fund and Koch Foundations.
Footnotes
Declaration of potentially competing interests
Katja Bloecker, David Hunter, Martin Englund, Herbert Resch and Kent Kwoh have no competing interests.
Ali Guermazi is President and co-owner of the Boston Core Imaging Lab (BICL), a company providing MRI reading services to academic researchers and to industry. He provides consulting services to Novartis, Genzyme, Stryker, MerckSerono and AstraZeneca.
Wolfgang Wirth has a part-time appointment with Chondrometrics GmbH, a company providing MR image analysis services, and is co-owner of Chondrometrics GmbH.
Olivier Benichou is employee of Eli Lilly & Co.
Felix Eckstein is CEO and co-owner of Chondrometrics GmbH. He provides consulting services to MerckSerono, Novartis, Sanofi Aventis, Perceptive, Bioclinica and Abbot.
Author’s contribution
All authors have made substantial contributions to (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
- the conception and design of the study: KB, AG, OB, DH, FE,
- acquisition of data: CK,
- analysis and interpretation of data: KB, AG, WW, OB, CK, DH, ME, HR, FE,
- Drafting the article: KB, FE,
- Revising the article critically for important intellectual content: KB, AG, WW, OB, CK, DH, ME, HR, FE,
- Final approval of the version submitted: KB, AG, WW, OB, CK, DH, ME, HR, FE,
- Statistical expertise: WW, FE,
- Obtaining of funding: KB,
- Collection and assembly of data: KB, WW.
KB takes responsibility for the integrity of the work as a whole, from inception to finished article. KB was involved in conception and design of the study, analysis and interpretation of the data, drafting of the article, critical revision of the article for important intellectual content, and final approval of the article.
References
- 1.Hunter DJ, Buck R, Vignon E, Eckstein F, Brandt K, Mazzuca SA, et al. Relation of regional articular cartilage morphometry and meniscal position by MRI to joint space width in knee radiographs. Osteoarthritis Cartilage. 2009;17:1170–6. doi: 10.1016/j.joca.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Krause WR, Pope MH, Johnson RJ, Wilder DG. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58:599–604. [PubMed] [Google Scholar]
- 3.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Chivers MD, Howitt SD. Anatomy and physical examination of the knee menisci: a narrative review of the orthopedic literature. J Can Chiropr Assoc. 2009;53:319–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;(149):283–90. [PubMed] [Google Scholar]
- 6.Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010;24:39–46. doi: 10.1016/j.berh.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennie WJ, Finlay DB. Meniscal extrusion in young athletes: associated knee joint abnormalities. AJR Am J Roentgenol. 2006;186:791–4. doi: 10.2214/AJR.04.1181. [DOI] [PubMed] [Google Scholar]
- 9.Choi CJ, Choi YJ, Lee JJ, Choi CH. Magnetic resonance imaging evidence of meniscal extrusion in medial meniscus posterior root tear. Arthroscopy. 2010;26:1602–6. doi: 10.1016/j.arthro.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, Lee BS, Kim JM, Yang KS, Cha EJ, Park JH, et al. Predictors of degenerative medial meniscus extrusion: radial component and knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:222–9. doi: 10.1007/s00167-010-1274-2. [DOI] [PubMed] [Google Scholar]
- 11.Crema MD, Roemer FW, Felson DT, Englund M, Wang K, Jarraya M, et al. Factors associated with meniscal extrusion in knees with or at risk for osteoarthritis: the multicenter osteoarthritis study. Radiology. 2012;264:494–503. doi: 10.1148/radiol.12110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34:645–87. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy conrols: a multicentre study using 3. 0 Tesla MRI and Lyon–Schuss radiography. Ann Rheum Dis. 2010;69:155–62. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–25. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckstein F, Le Graverand MP, Charles HC, Hunter DJ, Kraus VB, Sunyer T, et al. Clinical, radiographic, molecular and MRI-based predictors of cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2011;70:1223–30. doi: 10.1136/ard.2010.141382. [DOI] [PubMed] [Google Scholar]
- 16.Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography – data from the OA initiative. Osteoarthritis Cartilage. 2011;19:689–99. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: results from 831 participants from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010;63:311–9. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–32. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 21.Vanwanseele B, Eckstein F, Smith RM, Lange AK, Foroughi N, Baker MK, et al. The relationship between knee adduction moment and cartilage and meniscus morphology in women with osteoarthritis. Osteoarthritis Cartilage. 2010;18(7):894–901. doi: 10.1016/j.joca.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Jung KA, Lee SC, Hwang SH, Yang KH, Kim DH, Sohn JH, et al. High frequency of meniscal hypertrophy in persons with advanced varus knee osteoarthritis. Rheumatol Int. 2010;30:1325–33. doi: 10.1007/s00296-009-1153-7. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SH, Jung KA, Lee WJ, Yang KH, Lee DW, Carter A, et al. Morphological changes of the lateral meniscus in end-stage lateral compartment osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:110–6. doi: 10.1016/j.joca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Stone KR, Stoller DW, Irving SG, Elmquist C, Gildengorin G. 3D MRI volume sizing of knee meniscus cartilage. Arthroscopy. 1994;10:641–4. doi: 10.1016/s0749-8063(05)80062-3. [DOI] [PubMed] [Google Scholar]
- 25.Stone KR, Freyer A, Turek T, Walgenbach AW, Wadhwa 5, Crues J. Meniscal sizing based on gender, height and weight. Arthroscopy. 2007;23:503–8. doi: 10.1016/j.arthro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Bowers ME, Tung CA, Oksendahl HL, Hulstyn MJ, Fadale PD, Machan JT, et al. Quantitative magnetic resonance imaging detects changes in meniscal volume in vivo after partial meniscectomy. Am J Sports Med. 2010;38:1631–7. doi: 10.1177/0363546510364054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowers ME, Tung GA, Fleming BC, Crisco JJ, Rey J. Quantification of meniscal volume by segmentation of 3T magnetic resonance images. J Biomech. 2007;40:2811–5. doi: 10.1016/j.jbiomech.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson MS, Prescott JW, Best TM, Powell K, Jackson RD, Haq F, et al. Semi-automated segmentation to assess the lateral meniscus in normal and osteoarthritic knees. Osteoarthritis Cartilage. 2010;18:344–53. doi: 10.1016/j.joca.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirth W, Frobell RB, Souza RB, Li X, Wyman BT, Le Graverand MP, et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magn Reson Med. 2010;63:1162–71. doi: 10.1002/mrm.22380. [DOI] [PubMed] [Google Scholar]
- 30.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. http://dx.doi.org/10.1136/bmj.b2844.:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckstein F, Wirth W, Hunter DJ, Guermazi A, Kwoh CK, Nelson DR, et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis – data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18:760–8. doi: 10.1016/j.joca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benichou OD, Hunter DJ, Nelson DR, Guermazi A, Eckstein F, Kwoh K, et al. One-year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62:924–31. doi: 10.1002/acr.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 36.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols – comparative data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18:547–54. doi: 10.1016/j.joca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siorpaes K, Wenger A, Bloecker K, Wirth W, Hudelmaier M, Eckstein F. Interobserver reproducibility of quantitative meniscus analysis using coronal multiplanar DESS and IWTSE MR imaging. Magn Reson Med. 2012;67:1419–26. doi: 10.1002/mrm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloecker K, Wirth W, Hudelmaier M, Burgkart R, Frobell R, Eckstein F. Morphometric differences between the medial and lateral meniscus in healthy men - a three-dimensional analysis using magnetic resonance imaging. Cells Tissues Organs. 2012;195:353–64. doi: 10.1159/000327012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenger A, Englund M, Wirth W, Hudelmaier M, Kwoh K, Eckstein F. Relationship of 3D meniscal morphology and position with knee pain in subjects with knee osteoarthritis: a pilot study. Eur Radiol. 2012;22:211–20. doi: 10.1007/s00330-011-2234-z. [DOI] [PubMed] [Google Scholar]
- 43.Bloecker K, Englund M, Wirth W, Hudelmaier M, Burgkart R, Frobell RB, et al. Size and position of the healthy meniscus, and its correlation with sex, height, weight, and bone area -a cross-sectional study. BMC Musculoskelet Disord. 2011;12:248. doi: 10.1186/1471-2474-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams JG, McAlindon T, Dimasi M, Carey J, Eustace S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54:502–6. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]