Abstract
The present study aimed to compare presynaptic and postsynaptic actions of direct current polarization in the spinal cord, focusing on DC effects on primary afferents and motoneurons. To reduce the directly affected spinal cord region, a weak polarizing direct current (0.1–0.3 μA) was applied locally in deeply anaesthetized cats and rats; within the hindlimb motor nuclei in the caudal lumbar segments, or in the dorsal horn within the terminal projection area of low threshold skin afferents. Changes in the excitability of primary afferents activated by intraspinal stimuli (20–50 μA) were estimated using increases or decreases in compound action potentials recorded from the dorsal roots or peripheral nerves as their measure. Changes in the postsynaptic actions of the afferents were assessed from intracellularly recorded monosynaptic EPSPs in hindlimb motoneurons and monosynaptic extracellular field potentials (evoked by group Ia afferents in motor nuclei, or by low threshold cutaneous afferents in the dorsal horn). The excitability of motoneurons activated by intraspinal stimuli was assessed using intracellular records or motoneuronal discharges recorded from a ventral root or a muscle nerve. Cathodal polarization was found to affect motoneurons and afferents providing input to them to a different extent. The excitability of both was markedly increased during DC application, although post-polarization facilitation was found to involve presynaptic afferents and some of their postsynaptic actions, but only negligibly motoneurons themselves. Taken together, these results indicate that long-lasting post-polarization facilitation of spinal activity induced by locally applied cathodal current primarily reflects the facilitation of synaptic transmission.
Key points
Trans-spinal DC stimulation affects both postsynaptic neurons and the presynaptic axons providing input to these neurons. In the present study, we show that intraspinally applied cathodal current replicates the effects of trans-spinal direct current stimulation in deeply anaesthetized animals and affects spinal neurons both during the actual current application and during a post-polarization period.
Presynaptic effects of local cathodal polarization were expressed in an increase in the excitability of skin afferents (in the dorsal horn) and group Ia afferents (in motor nuclei), both during and at least 30 min after DC application. However, although the postsynaptic facilitation (i.e. more effective) activation of motoneurons by stimuli applied in a motor nucleus was very potent during local DC application, it was only negligible once DC was discontinued.
The results suggest that the prolonged effects of cathodal polarization are primarily associated with changes in synaptic transmission.
Introduction
The present study aimed to compare the long-lasting effects of DC applied within the spinal cord on postsynaptic neurons and on the presynaptic fibres providing input to them. We expected to clarify the mechanisms underlying spinal post-polarization facilitation and neuronal plasticity, which are both of great theoretical interest, as well as of potential therapeutic importance.
Long-lasting DC effects were first reported to be evoked in nerve fibres in peripheral nerves (Nodera & Kaji, 2006). In the central nervous system, long-lasting effects were found to be evoked by transcranial direct current stimulation (tDCS) in the cerebral cortex (Paulus, 2011; Stagg & Nitsche, 2011; Nitsche et al. 2012) and recently also subcortically (Bolzoni et al. 2013a; Bolzoni et al. 2013b; Bączyk & Jankowska, 2014). They were also evoked in the spinal cord by trans-spinal DC stimulation (tsDCS) (Cogiamanian et al. 2008; Winkler et al. 2010; Cogiamanian et al. 2011; Ahmed & Wieraszko, 2012; Ahmed, 2013a).
However, detailed analysis of the mechanisms underlying the effects of DC focused on events occurring during DC application, and less attention was paid to effects outlasting the polarization. For example, in the most advanced in vitro preparations used for this purpose, DC was applied for only 1 s (Rahman et al. 2013), or for not more than 5 min (Kabakov et al. 2012), specifically to avoid evoking lasting changes, and, in both studies, the effects evoked during (but not after) the polarization were examined. DC effects on the spinal cord were originally analysed when DC was applied in vivo for a few seconds (Eccles et al. 1962) and no after-effects were reported. Only a few cases of effects of longer-lasting polarization were described in these studies; for example, the after-effects of ∼12 s of tetanization, which were found to last for 1–2 min (Eccles et al. 1961).
We previously demonstrated that prolonged DC effects may be evoked not only when cathodal or anodal current is applied at a distance from the analysed neurons, as in the cases of tDCS and tsDCS, but also when DC is applied locally (Bączyk & Jankowska, 2014). Because the local polarization evokes much more spatially restricted effects, we aimed to use this to relate DC effects to selected spinal neurons or fibres at different sites along the affected neuronal pathways. In particular, we aimed to examine effects of local cathodal polarization on (i) the excitability of cutaneous afferents stimulated within the terminal projection region of these afferents in the dorsal horn, using changes in the compound action potentials recorded in a cutaneous nerve as its measure; (ii) the excitability of group Ia muscle afferents stimulated within a motor nucleus, as estimated from records from a dorsal root, or a muscle nerve when the corresponding ventral roots (VRs) were transected; (iii) directly evoked discharges of motoneurons stimulated within a motor nucleus, recorded in a VR or a muscle nerve when the corresponding dorsal roots were transected; (iv) monosynaptic EPSPs evoked by group Ia afferents in hindlimb motoneurons, based on intracellular records from motoneurons, or extracellular records of monosynaptic field potentials evoked by these afferents in motor nuclei; and (v) monosynaptically evoked discharges of motoneurons (monosynaptic reflexes) recorded from a VR.
For (i), (ii) and (iii), the polarization would primarily affect the initiation of reponses evoked by intraspinally applied electrical stimuli; in most general terms, it would reproduce the situation when tDCS effects are tested on responses evoked by magnetic or electric stimulation of the cortex, or by electrical stimuli. The situation will be different in (iv) and (v), in the case of DC effects on responses elicited by stimuli applied to peripheral nerves, far away from the site of the polarization, where neuronal networks required for the mediation but not for the initiation of the responses would depend on the DC. Together, they should help to clarify what happens when tDCS and tsDCS facilitate indirect activation of cortcal, subcortical or spinal neurons by affecting both their initiation and the excitability of the neurons that mediate them.
The experiments were undertaken expecting that local depolarization facilitates trans-synaptic activation of spinal neurons by primary afferents to the same extent as the activation of rubro-spinal neurons by interposito-rubral neurons, as previously demonstrated by (Bączyk, 2014). It was also hypothesized that the mechanisms for the facilitation of the activity of spinal neurons by DC polarization are generally the same as those for the facilitation of subcortical neurons by local DC or by tDCS, and may explain the basic effects of tDCS on cortical neurons.
The present study focused on mechanism of post-polarization effects of local cathodal polarization because previous observations indicated that they might be longer-lasting and/or more marked than the post-polarization effects of anodal polarization. This appeared to be the case both within the Red Nucleus (Bączyk & Jankowska, 2014) and in the spinal cord, as indicated by the preliminary experiments conducted for the present series. A further analysis of mechanisms underlying post-polarization effects will be extended to anodal as well as to cathodal current application in a subsequent study.
Methods
Ethical approval
All experiments were approved by the Regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and comply with NIH and EU guidelines for animal care and with the ethical policies and regulations of The Journal of Physiology (Drummond, 2009). The animals were housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on five deeply anaesthetised cats (2.9–4.2 kg) and 19 adult rats of both sexes (Wistar; 210–470 g). Cats were used for the most technically demanding experiments, involving intracellular recording from motoneurons, parallel records of field potentials and/or monosynaptic reflexes from 2 or 3 different muscle nerves, whereas simpler experiments were performed on rats.
In cats, anaesthesia was induced with sodium pentobarbital (Apoteksbolaget, Göteborg, Sweden; 40–44 mg kg−1 i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, Vitry sur Seine, France; 5 mg kg−1) administered every 1–3 h, up to 65 mg kg−1 i.v.). Additional doses of α-chloralose were given when motor reactions were evoked during dissection and when increases in the continuously monitored blood pressure or heart rate were evoked by any experimental procedures. Following the initial cephalic vein, femoral artery and tracheal cannulation, the spinal cord was exposed by laminectomy at the level of the L5–S1segments and several peripheral nerves were dissected free. These included: the posterior biceps (PB), semitendinosus (ST), medial gastrocnemius (MG), lateral gastrocnemius-soleus (LGS) and sural (SUR). During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sollentuna, Sweden; 0.3 mg kg i.v. supplemented with ∼0.2 mg kg−1 h−1) and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and end-tidal CO2 at 3.9–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). The body temperature was kept at ∼37.5°C by servo-controlled heating lamps. The experiments were terminated by a lethal dose of pentobarbital i.v.
In rats, anaesthesia was induced with isoflurane (Baxter Medical AB, Kista, Sweden) followed by α-chloralose (80 mg kg−1 i.p. supplemented by pentobarbital sodium (10–15 mg kg i.p.) and with 3 or 4 additional doses of α-chloralose up to 140–160 mg kg−1. The additional doses were administered when the animal started to respond with muscle twitches to any stimuli and/or when the continuously monitored heart rate increased above 500 min−1. At the deepest levels of anaesthesia at which no withdrawal reflexes were evoked, the heart rate was ∼400–450 min−1. When needed (e.g. at later stages of the experiments), respiration was assisted by a high frequency (60–70 min−1) and low volume (0.3–0.4 ml min−1) of artificial ventilation (using respiratory pump CWE, model SAR-830/P; CWE, Inc., Ardmore, PA, USA) to maintain the expired CO2 level at ∼3.5–4.2%. The neuromuscular transmission remained intact, as in the study of Bolzoni et al. (2013a), or was temporarily blocked by pancuronium bromide (0.3 mg kg−1). The core body temperature was maintained at ∼38 °C by servo-controlled heating lamps. To compensate for fluid loss, 10–15 ml of acetate buffer were injected s.c. at the beginning of the experiments. All other fluids were injected i.p. via an indwelling catheter. The experiments were terminated by a lethal dose of pentobarbital i.p., decapitation or heart excision. The preliminary dissection included tracheal intubation, insertion of an i.p. catheter, dissection of the left or both left and right Sur nerves, or the deep peroneal nerves, and exposing the L4–L5 spinal segments by laminectomy. Paraffin oil pools were constructed above the dissected tissues from skin flaps.
Recording and stimulation
Intracellular records from motoneurons (in cats) were obtained using glass micropipettes filled with 2 m solution of KCitr (tip ∼1.5 μm, impedance 3–5 MΩ) and a conventional high impedance amplifier. Extracellular field potentials (in cats and rats) were recorded with glass micropipettes filled with 3 m solution of NaCl (tip ∼2 μm, impedance 1.5–5 MΩ). Responses from the nerves (in cats and rats) were recorded using a pair of silver/silver chloride electrodes on which they were placed in a paraffin oil pool. Afferent volleys were recorded with a silver ball electrode in contact with the surface of the spinal cord at the L7 (cat) or the L2 (rat) spinal level against a large reference electrode in contact with a back muscle. Both original records and averages of records evoked by 10 or 20 stimuli applied once or twice per second were stored on line.
Stimulation of peripheral nerves was evoked via silver/silver chloride electrodes in a paraffin oil pool using constant voltage current pulses of 0.2 ms in duration, near-threshold to near maximal for the afferent volleys but not exceeding twice the threshold stimuli. Intraspinal stimuli, constant current pulses of 7–50 μA and 0.2 ms in duration, were applied via tungsten electrodes manufactured from 0.2 mm wires insulated up to the very tip (impedance 30–300 kΩ). An intraspinal recording glass micropipette and a stimulating tungsten electrode were mounted in pairs in two holders of a double-headed manipulator that were operated by separate step motors, allowing the placement of the two electrodes within a pre-selected distance from each other (Engberg et al. 1972). When both electrodes were above the spinal cord, their tips were aligned so that they almost touched each other. Subsequently, the tungsten electrode was withdrawn a known distance, the microelectrode was placed at a selected site and the tungsten electrode lowered to the original position. The microelectrodes were placed in a motor nucleus under guidance of recording antidromic field potentials evoked by stimulation of a VR or a muscle nerve. In the intermediate zone and the dorsal horn, they were placed at the site of the largest monosynaptic field potentials evoked by group I afferents and low threshold (1.3–1.6 threshold) skin afferents, respectively.
Local polarization
The polarization was applied using a purpose constructed battery driven constant current stimulator (by D. Magnusson at the University of Gothenburg). The manipulator supplied continuously monitored current within a range of intensities of 0–1 μA. As a rule, the current was passed via the same (for exceptions, see text) tungsten electrode as that used for intraspinal stimulation (0.2 mm wires insulated up to the very tip, impedance 50–300 kΩ) against another larger electrode in contact with contralateral back muscles. The polarizing current was applied five times, each time for 5 min, separated by 5 min intervals, in accordance with the same experimental protocol as for tDCS (Bolzoni et al. 2013a, 2013b; Baczyk et al. 2014). Because the polarization did not interfere with the recording, a series of records was taken at the beginning and at the end of each polarization and between-polarization periods and, subsequently, every five minutes during the post-polarization period.
Methodological problems related to local polarization
The effects of electric fields generated by tDCS and tsDCS depend on a number of factors. In most recent studies, they were related to the channelling effects of the skin, the skull and the cerebrospinal spinal fluid, with current vectors tending to be oriented towards the closest higher conducting region, and current density decreasing with an increasing distance from the electrodes (Wagner et al. 2014; Toshev et al. 2014). To avoid some of these factors, and in particular to overcome the difficulty in predicting the density of the current at a distance from the source of electric fields, we applied local rather than transcranial DC stimulation to examine its effects within subcortical structures (Bączyk, 2014). The results obtained showed that effects of the two means of polarization may be comparable once the proper current parameters are selected.
Local application of DC nevertheless posed some problems. The main problem was to select a current intensity sufficient for facilitatory actions but not too strong to evoke anodal surround (Katz & Miledi, 1965; Ranck, 1975), which might counteract the effects of cathodal polarization or result in a cathodal block (Skoglund, 1945; Bhadra & Kilgore, 2004). Because, in some experiments (Bączyk, 2014), the thresholds almost doubled when the polarization exceeded 1 μA, in keeping with effects of the anodal surround, the current intensities used in the present study were <1 μA. However, even at intensities of 0.4–1.0 μA, the responses were sometimes facilitated during the initial few minutes of cathodal polarization but subsequently decreased, or even disappeared, especially when the current was applied close to neurons activated at particularly low (<10 μA) intensities of intermittent current pulses. The reported effects of spinally applied polarization are therefore based on actions of 0.1–0.3 μA DC. Although these intensities may have been submaximal, they were well below current intensities reported to induce cathodal block in peripheral nerves (e.g. 6–125 μA for frog nerves; Bhadra & Kilgore, 2004) or even damage (100–500 μA for rabbit and cat nerves; Ravid et al. 2011).
A further problem was that of possible interactions between the direct current and the intermittent current pulses delivered through the same electrodes, even if both were obtained from constant current stimulators. For technical reasons, we were unable to use separate electrodes for stimulation and for polarization within the very small areas to be explored. However, we have previously confirmed that the polarization did not affect the parameters of constant current pulses concurrently or subsequently applied through the same electrode (Bączyk, 2014). Furthermore, changes in the electrode/tissue interface or electrode polarization (Merrill et al. 2005) were expected to be negligible in view of low intensities of both the intermittent (<50 μA, 0.2 ms) and constant (<1 μA) current stimuli and also because no significant changes in electrode impedance were found to be caused by 0.75 mA applied for some 10–20 min in the experiments reported by Ravid et al. (2011). Electrochemical reactions at the electrode–nerve interface and possibly resulting damage could not be excluded, although they would be rather unlikely to contribute to only transient increases in the excitability of the neurons or fibres.
Statistical analysis
The effects of the polarizing current were estimated from changes in the size and/or latencies of potentials evoked during control periods and during or after application of the polarization. They were estimated by comparing areas of the averaged potentials measured during time windows from the onset to approximately one-third of the declining phase of these potentials, as indicated in Fig. 1B. The areas were assessed using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg) and were normalized with respect to the mean value of the control areas (i.e. the areas of potentials evoked prior to the first polarization period) for each series of records. The normalized areas from all experiments were averaged for each period during, between or after the polarization and the comparisons between each period (15 levels) were performed with repeated measures ANOVA. When a significant effect was found (P < 0.05), Tukey's post hoc honestly significant difference (HSD) test was performed. A one-sample t test was also used to estimate statistically significant differences between the lowest values in a series (when different from others in the same plots) or all averaged values when the difference was found with ANOVA and the control values.
Figure 1. Comparison of excitability of cutaneous afferents before, during and after cathodal DC polarization in the rat.
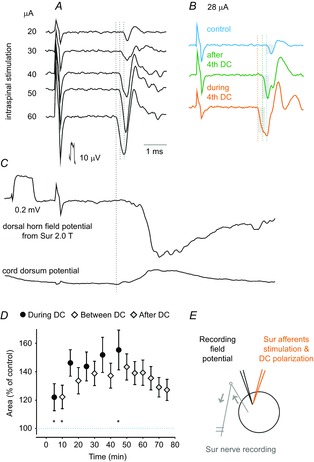
A, records of antidromic volleys evoked in Sur afferent fibres after intraspinal stimuli of increasing intensities (20–60 μA) before DC polarization. Averages of 20 records. B, as in A but before, during and after DC polarization, as indicated, using 28 μA to evoke the volleys. C, field potential evoked by Sur stimulation at the site where the intraspinal stimuli were applied and the corresponding record from the cord dorsum. Vertical horizontal lines in B indicate latencies of antidromic volleys evoked during, after and before DC polarization corresponding to latencies of antidromic volleys evoked by 20, 40 and 60 μA in the series of records in A, with the shortest ones corresponding to the latency of the afferent volley recorded from the cord dorsum. In this and subsequent figures, negativity is up for records from the peripheral nerves and cord dorsum and down in microelectrode records (extracellular records of field potentials or intracellular). D, mean areas of the antidromic volleys in 8 sural nerves during successive polarization, between polarization and post polarization periods. Values (mean ± sem) are expressed as a percentage of the mean control area (i.e. the area of the volley recorded prior to the first polarization). E, the recording, stimulation, and polarization sites. Arrows indicate the direction of the neural traffic. For the comparison in D, repeated measures ANOVA indicated a significant effect (P = 0.0073; F14,98 = 2.35); Tukey's post hoc HSD test revealed significant differences between the last period of polarization (45 min) and the first two periods (5 min, P = 0.014; 10 min, P = 0.0016). A one-sample t test revealed that the first point (5 min) is significantly larger than the control, P = 0.02. Because this point showed the lowest values, the conclusion applies to all data points.
Results
Long-lasting increase in the excitability of primary afferents by local cathodal polarization
Changes in the excitability of afferent fibres were tested by comparing compound action potentials induced in a dorsal root or in peripheral nerves by 10–30 μA intraspinal stimuli. Responses recorded from a dorsal root were attributed to group Ia afferents when intraspinal stimuli were applied in the motor nuclei because these are the only afferents (in addition to a small fraction of slower conducting group II afferents) synapsing with motoneurons and because the stimuli were sufficiently weak not to affect afferents terminating outside motor nuclei. Responses recorded from a muscle nerve were similarly attributed to group Ia afferents when they were evoked from motor nuclei in preparations in which motoneuron axons in the VRs were transected. Changes in the excitability of skin afferents were tested on responses recorded from the Sur nerve after intraspinal stimuli applied within their terminal projection area within the dorsal horn.
The size of peripherally recorded potentials was used as a measure of the number of fibres excited by intraspinal stimuli, which, in turn, was a measure of the fibre excitability, as in the original studies of Wall (1958) and Eccles et al. (1962). The reasons for using this measure are illustrated in Fig. 1. The series of records in Fig. 1A shows that increasing intensities of intraspinal stimuli evoked volleys of increasing sizes and decreasing latencies. The records in Fig. 1B show that, during DC polarization, the 28 μA stimuli evoked volleys (bottom trace) as large as those originally evoked by 50–60 μA stimuli (i.e. became more effective) and, in addition, these volleys were evoked at a 0.3 ms shorter latency. The volleys evoked during the subsequent post-polarization period (middle trace) were of intermediate sizes and latencies and were comparable to those originally evoked by 40 μA stimuli.
The effects of cathodal DC polarization on excitability of cutaneous afferents in 8 nerves were analysed in 6 rats. The afferents were stimulated at the sites at which monosynaptic field potentials from the Sur nerve were maximal and from which the antidromically evoked volleys appeared in this nerve at the same latency as that of afferent volleys recorded from the cord dorsum (Fig. 1A and C). The responses were increased during polarization, between-polarization and post-polarization periods (Table1). In Fig. 1D, the changes found during and after the successive 5 min polarization periods are plotted as means for all 8 Sur nerves, respectively.
Table 1.
Summary of effects of local cathodal polarization on various tests in the cat and rat spinal cord
| Area as a percentage of control (± sem) | ||||||
|---|---|---|---|---|---|---|
| Number of | Number of | During last | Twenty minutes after | Statistical | ||
| Tests | tests | animals | DC period | the last polarization | significance | |
| Rat | ||||||
| (a) | Responses of cutaneous afferents | 8 | 6 | 154.2 ± 12.1 | 135.5 ± 8.2 | See Fig. 1 |
| (b) | Field potentials from cutaneous afferents | 8 | 7 | 64.2 ± 7.5 | 78.1 ± 6.1 | See Fig. 7 |
| (c) | Directly evoked motoneuron responses | 7 | 6 | 166.3 ± 27.3 | 116.5 ± 9.7 | Not significant |
| Cat | ||||||
| (d) | Responses of group la afferences | 7 | 5 | 168.3 ± 23.2 | 150.5 ± 19.5 | See Fig. 2 |
| (e) | Field potentials from group la afferents | 8 | 3 | 128.9 ± 8.3 | 120.9 ± 10.1 | See Fig. 5 |
| (f) | Monosynaptic EPSPs in motoneuronst | 18 | 2 | 114.2 ± 7.0 | 119.7 ± 18.0 | |
| (g) | Monosynaptic reflex | 7 | 3 | 191.1 ± 16.6 | 161.9 ± 13.9 | See Fig. 6 |
| (h) | Presynaptic volleys | 9 | 3 | 98.0 ± 4.4 | 96.2 ± 3.4 | Not significant |
| (i) | Directly evoked motoneuron responses | 9 | 5 | 148.6 ± 8.1 | 117.5 ± 6.1 | Not significant |
(a) Stimuli and DC polarization were applied in the dorsal horn. Activity in cutaneous afferents was recorded from the Sur nerve. (b) Field potentials were evoked in the dorsal horn by stimulating the Sur nerve. DC was applied via recording electrodes in the dorsal horn. (c) Stimuli and DC polarization were applied in the DP motor nucleus. Motoneuronal discharges were recorded from the DP nerve after transection of the L2–L6 dorsal roots. (d) Stimuli and DC polarization were applied in the PBST and/or GS motor nuclei. Activity in Ia afferents was recorded from the dorsal roots or from the PBST or GS nerves when the DRs were transected. (e) Field potentials were evoked within motor nuclei by stimulating the PBST and/or GS nerves. DC was applied via recording electrodes in the motor nuclei. (f) Monosynaptic EPSPs were evoked in motoneurons by stimulating the PBST or GS nerves. DC was applied in the same motor nucleus. EPSPs were recorded during and after only one period of polarization. (g) Monosynaptic reflexes were evoked by stimulating the PBST and/or GS nerves. They were recorded from the S1 and/or L7 VR. DC was applied in the PBST and/or GS motor nuclei. (h) Presynaptic volleys from group Ia afferents preceding field potentials evoked in motor nuclei were recorded at the same time as the field potentials in series (e). (i) Stimuli and DC polarization were applied in the PBST and/or GS motor nuclei. Motoneuronal discharges were recorded from the PBST and/or GS nerves after transection of the L7 and S1 VRs or from the VRs. In the 2nd column, the ‘number of tests’ indicates the number of the series of records in which DC effects were evaluated. As a rule, except for those in (f) (see text), they were obtained either in separate experiments or from the left and right side in the same rat.
The effects of DC polarization on group Ia afferents were analysed in cats. The afferents were stimulated within the region of PBST and MG motor nuclei. The antidromically conducted responses were recorded from four dorsal roots and three muscle nerves (one or two nerves per cat). The effects are illustrated in Fig. 2A and summarized in Fig. 2B and C and Table1. DC polarization increased these volleys to a different extent but facilitation developed gradually during, as well as between and after, the polarization periods, and remained well above the control level for at least 30 min after the last polarization period had been discontinued.
Figure 2. Comparison of antidromic volleys in group Ia afferents in a muscle nerve evoked by intraspinal stimuli before, during and after DC polarization.
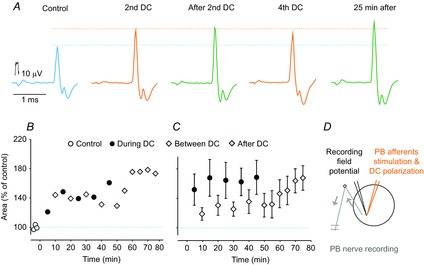
A, records from the PB nerve after constant current intraspinal stimuli (0.1 μA) applied in the PBST motor nucleus in a preparation in which both the L7 and S1 VRs were transected. The records were obtained before, during and after cathodal DC polarization (0.1 μA), as indicated. Averages of 20 records. Lower and upper dotted horizontal lines indicate maximal amplitudes of the volleys evoked before and during the DC application. The latency of the control responses was shortened by 0.13 ms. B, areas of the antidromic volleys illustrated in A at the end of the control, during the series of alternating polarization and between polarization periods and during the post polarization period. C, as in B but for mean areas of antidromic volleys in 7 group Ia afferents. Repeated measures ANOVA indicated a significant effect (P = 0.0022; F14,84 = 2.74); Tukey's post hoc HSD test revealed no significant differences between the points. D, stimulation, polarization and recording sites. Arrows indicate the direction of the neural traffic.
Effects of local cathodal DC polarization on monosynaptically evoked postsynaptic actions of group Ia and cutaneous afferents
Subsequent experiments aimed to examine whether the depolarization of primary afferents affected synaptic actions evoked by them in their target cells. The afferents were depolarized within their terminal branching region but action potentials induced in them were initiated by stimuli applied far away from this region (i.e. to peripheral muscle or cutaneous nerves). DC could thus not affect the initiation of the action potentials and could only affect synaptic transmission, including the invasion of the terminals, release of the transmitter and its actions on postsynaptic membrane receptors. This situation differed from that in the previously explored Red Nucleus in which the rubrospinal neurons were activated by stimuli applied within the depolarized region, where the depolarized presynaptic fibres could be more effectively activated.
Local cathodal polarization was applied either in a motor nucleus (within a region where large antidromic field potentials were evoked from a muscle nerve and monosynaptic field potentials were evoked from group Ia afferents in this nerve), or in the dorsal horn where monosynaptic field potentials were evoked from a skin nerve. Depolarized group Ia afferents evoked larger monosynaptic EPSPs in motoneurons, larger extracellular field potentials in motor nuclei and more effective activation of motoneurons, whether monitored intracellularly, or as monosynaptic reflexes. By contrast, depolarization of cutaneous afferents was invariably followed by a decrease in monosynaptic extracellular field potentials in the dorsal horn, with the time course of both these opposite effects being similar. Cathodal polarization was thus unexpectedly found to elicit opposite effects on synaptic actions of group Ia afferents on motoneurons and on synaptic actions of cutaneous afferents on neurons in the dorsal horn (Table1).
Facilitation of monosynaptic EPSPs in intracellularly recorded motoneurons
The sample of motoneurons on which effects of cathodal polarization were tested included 18 motoneurons (8 MG, 10 PB or ST) recorded in 2 cats. In these particular experiments, the tungsten electrode used for polarization was positioned at the border between the L7 and S1 spinal segments at a location where antidromic field potentials were evoked by stimulation of the S1 VR (VR) and monosynaptic field potentials were evoked from either the MG or PB nerves. The electrode remained stationary when the micropipette used for penetrating the motoneurons was moved with respect to it within a distance of 300 μA rostrally and 100 μA medially or laterally. In all of the tested motoneurons, the membrane potential decreased by less than 5 mv from the original 55–70 mV for at least 15 min, whereas it remained stable for 30 min, or more, in only five motoneurons. Accordingly, the effects of the first period of polarization were examined in the entire 18 motoneurons, whereas the effects of the subsequent periods of polarization were examined in only five motoneurons. On average, the area of EPSPs recorded during the first 5 min period of polarization increased to 114.2 ± 7% (mean ± sem) and, during the first 5 min of the post-polarization period, to 119.7 ± 18% (Table1). The difference between the areas of the EPSPs recorded before and during polarization was statistically significant (P = 0.009; t test for paired samples assuming equal variances), whereas no difference was found between areas of EPSPs recorded during DC application and during the immediately after between polarization and post-polarization periods. Only a weak facilitation was thus detected, although its extent is probably an underestimate because the facilitatory effects were found to develop during three to five periods of polarization. In addition, each of the three, four or five or even single periods of polarization might have residual effects so that control records from motoneurons penetrated even 1 h after polarization applied when recording from a previous motoneuron might have been obtained at a higher level of excitability of Ia afferents than before the very first polarization, and therefore be influenced to a smaller extent. Residual effects could also affect the extent of facilitation of EPSPs when the tungsten electrode was reinserted 1–2 mm more rostral or caudal in the same nucleus because DC current would spread over such distances. In addition, even though the polarizing current was applied within the new region, it was likely to reach partly the same afferents because a very high proportion, if not all afferents of a muscle, forms synaptic contacts with individual motoneurons innervating this muscle (Watt et al. 1976) and synapse along up to 1–2 mm long dendrites.
Considering that the effects of DC found on most of the EPSPs were underestimated, only the largest increases in the EPSPs might be of interest. One of these is illustrated in Fig. 3A–C. Only an occasional action potential was initiated in the motoneuron during the control period but several action potentials appeared at the end of the first polarization from the top of the increased EPSPs. The EPSPs continued to increase during the successive periods of polarization and post-polarization, or remained facilitated (Fig. 3D), although action potentials failed to be evoked when the membrane potential of the motoneuron started to decline (Fig. 3C).
Figure 3. Increase in monosynaptic EPSPs from group Ia afferents during and after the first period of cathodal polarization in the PBST motor nucleus in the cat.
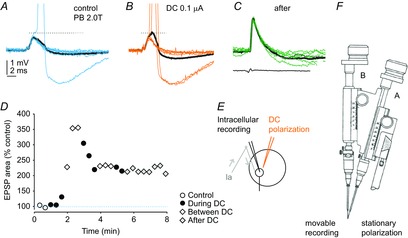
A, records of EPSPs and the only action potential evoked in a PB motoneuron by stimulation of the PB nerve at 2 times threshold for group I afferents during the last control series. Black trace, average of 20 records. Blue traces, a selection of single records illustrating a small range of amplitudes of EPSPs. B, EPSPs evoked at the end of the 1st period of cathodal polarization (0.1 μA, at 100 μm distance from the tip of the micropipette). Black trace, average of 20 EPSPs. Orange traces, EPSPs of the same amplitude as the control EPSPs but giving rise to action potentials at a shorter latency than in A. C, EPSPs evoked at the end of the first between-polarization period when action potential were no longer initiated; bottom trace, incoming afferent volleys recorded from the cord dorsum. Black trace, average of 20 EPSPs. Green traces, EPSPs illustrating a small range of their amplitudes. Action potentials in A and B are truncated. Dotted lines indicate the threshold for generation of action potentials. Voltage and time calibrations in A are for all records. D, areas of the average EPSPs (within 1 ms from their onset) during the control period and during the cathodal polarization, between polarization and post-polarization periods, as indicated. E, stimulation and recording sites. Arrows indicate the directions of the neural traffic. F, the double manipulator (Engberg et al. 1972). In the illustrated experiment, the tungsten electrode was held in the stationary drive (A) and the glass microelectrode was held in the movable drive (B).
Figure 4A–C illustrates a slower building-up of effects of the polarization in a motoneuron in which monosynaptic EPSPs were facilitated during the second but not the first period of cathodal polarization. In this particular case, the EPSPs did not increase during the first 5 min of the polarization, nor during the subsequent after-polarization period. The EPSPs only began to increase at the end of the second polarization period (Fig. 4D), although an early facilitation occurred in other motoneurons (Figs 3 and 5G and H). No action potentials were initiated by near threshold stimuli applied during the control period (Fig. 4A) or during the first polarization period but appeared when the EPSPs increased during the second polarization and post-polarization periods (Fig. 4B and C).
Figure 4. An example of delayed increases in monosynaptic EPSPs from group Ia afferents during cathodal polarization in the MG motor nucleus in the cat.
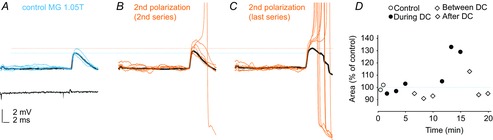
A, records of EPSPs evoked in an MG motoneuron by near-threshold stimulation of the MG nerve during the first control series. Black trace, average of 20 records. Blue traces, single records illustrating a range of amplitudes of EPSPs, all below threshold for inducing an action potential. B, EPSPs evoked at the beginning of the 2nd period of cathodal polarization (0.2 μA, within 300 μm distance from the tip of the micropipette). Black trace, average of 20 successive EPSPs. Orange traces, a selection of EPSPs illustrating the upper range of amplitudes of the EPSPs; one of which gave rise to an action potential. C, EPSPs evoked at the end of the 2nd period of polarization. Black trace, average of EPSPs evoked during a series of records when only 2 out of 20 stimuli evoked action potentials. Orange traces, EPSPs evoked during a subsequent series of records when 14 out of 20 stimuli were followed by action potentials. During the period of recording, the membrane potential dropped by only 2 mV from ∼60 mV just after the penetration. Calibration pulses 0.2 mV. Action potentials are truncated at the top. The two horizontal dotted lines indicate the original amplitude of the EPSPs and the average amplitude of EPSPs evoked during the last series of DC polarization. D, areas of the early parts of the average EPSPs (measured within 1.9 ms from their onset) during the control period, during 2 periods of cathodal polarization and during two subsequent post-polarization periods, as indicated.
Figure 5. Facilitation of monosynaptic field potentials from group Ia afferents in a motor nucleus in the cat.
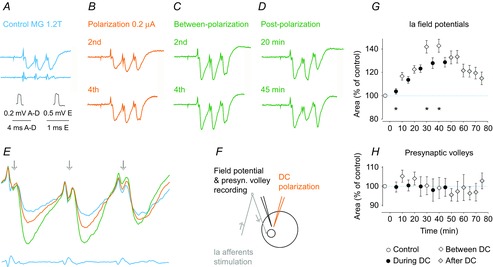
A–D, averaged records (n = 20) of field potentials evoked by submaximal stimulation of group I afferents in the MG nerve. They were evoked before, during and after cathodal polarization (0.2 μA), applied as indicated in F. In A, they are accompanied by records of afferent volleys from the cord dorsum (bottom trace). E, superimposed expanded records of control field potentials and of those evoked during and after the 4th period of polarization. Note that the field potentials are increased but the presynaptic volleys that preceded them (arrows) were not. F, stimulation and recording sites with arrows indicating the direction of the nervous traffic. G, time course of changes in the whole sample of 9 field potentials tested. Values (mean ± sem) are expressed as a percentage of control (i.e. the area of the potentials recorded prior to the first polarization). Repeated measures ANOVA indicated a statistically significant effect (P = 0.006; F14,98 = 3.05); Tukey's post hoc HSD test revealed significant differences between field potentials evoked 30 and 40 min from the beginning of the DC polarization (P = 0.021 and P = 0.0014, respectively) and during the first period of the polarization (5 min). H, mean changes in presynaptic volleys preceding 9 field potentials; all were evoked by the first stimulus in a train of stimuli. Repeated measures ANOVA did not reveal any statistically significant differences.
Facilitation of extracellular field potentials evoked by group I afferents in motor nuclei
In view of the great variability of effects of polarization on monosynaptic EPSPs evoked in individual motoneurons, the timing of DC effects upon synaptic actions of group Ia afferents was examined on extracellular field potentials evoked in a motor nucleus, which reflect EPSPs of group Ia origin in a populations of motoneurons. In total, eight field potentials were recorded at three locations in PBST and five locations in MG motor nuclei in two cats using a glass micropipette attached to the stationary drive of a double-headed manipulator (Fig. 3F) when the current was applied through a tungsten electrode positioned by the movable drive, as described in the Methods. When more than one region in a given motor nucleus was explored, both electrodes were moved 1–2 mm in a rostral or caudal direction. All eight field potentials were facilitated by cathodal DC applied within 10–100 μm from the tip of the recording micropipette (Table1). The extent of the facilitation of field potentials recorded at different locations or during different experiments varied, although the extent of facilitation also varied for field potentials recorded at the same location evoked by successive stimuli in a train. For example, in the case of field potentials illustrated in Fig. 5A–E, those evoked by the first stimulus were facilitated to the greatest extent, whereas increases in field potentials evoked by the second stimulus were more marked in other cases (Fig. 6E and F). However, irrespective of the extent of facilitation, the facilitation of all of the field potentials outlasted the period of DC application by at least 30 min (Fig. 5H).
Figure 6. Facilitation of monosynaptically evoked activation of motoneurons by local cathodal DC.
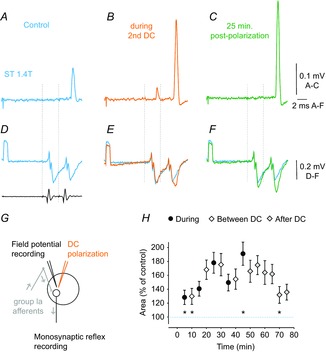
A–C, averaged records (n = 20) of monosynaptic reflexes evoked from the ST nerve in the S1 VR before, during and after 0.2 μA cathodal DC application in ST motor nucleus. Note in B that DC current not only increased the response to the 2nd stimulus, but also made a small response appear after the first stimulus. The size of the monosynaptic reflex evoked by the 2nd stimulus might thus have been reduced by the refractory period. D–F, field potentials simultaneously recorded within the PBST motor nucleus and incoming volleys recorded from the cord dorsum (in D). Note that increases in in the monosynaptic reflex coincided with increases in the field potentials. Dotted lines indicate stimulus artefacts. G, stimulation and recording sites. H, mean changes in the areas of monosynaptic reflexes evoked from 7 nerves in the cat during and after cathodal polarization applied in motor nuclei. Values (mean ± sem) are expressed as a percentage of the control (i.e. the area of the reflex recorded prior to the first polarization). Repeated measures ANOVA indicated a statistically significant effect (P = 0.0008; F14,84 = 3.016); Tukey's post hoc HSD test revealed significant differences between the last period of polarization (45 min) and the first two periods (5 min, P = 0.015; 10 min, P = 0.020) and the after-polarization period (70 min, P = 0.030). A one-sample t test revealed that the first point is significantly different from zero (i.e. larger than the control, P = 0.04). Because these points showed the lowest values, this conclusion applies to all the data points.
The records shown in Figs 5A–E and 6D–F, and especially the superimposed records in Fig. 5E, also show a marked difference between the effects of cathodal polarization on extracellular field potentials and on the presynaptic volleys preceding these potentials, as indicated by arrows. During cathodal polarization, the presynaptic volleys either remained unaltered or were reduced, whereas their synaptic actions were greatly facilitated. Similar differences were found between the presynaptic volleys and the field potentials recorded from Ia afferents at 9 locations in PBST and/or GS motor nuclei, as shown in Fig. 5H. None of the volleys were increased by cathodal polarization. Presynaptic volleys were thus changed in the opposite direction to changes in the field potentials, as well as to changes in the excitability of Ia afferents to stimuli applied in motor nuclei illustrated in Fig. 2. The excitability of afferents stimulated at the location from which the field potentials shown in Fig. 5A–E were recorded was increased by 200–250%. In view of these opposite effects, it is unlikely that a more effective invasion of terminal branches of Ia afferents synapsing with motoneurons contributes in any essential way to the facilitatory effects of cathodal polarization. Instead, polarization results in a more effective synaptic transmission between the afferents and the motoneurons.
Long-lasting facilitation of monosynaptic reflexes
Monosynaptic reflexes were evoked by stimulation of group I afferents in seven muscle nerves (3 MG and 4 PB or ST) at intensities submaximal for group I afferents. The resulting activation of motoneurons was recorded as discharges in the L7 or S1 VR and was evaluated by measuring the areas of the earliest components of these discharges. We found that the discharges were potently facilitated by local DC application (Fig. 6A–C) both during the polarization and during the post-polarization period of 30 min (Fig. 6H and Table1). Because, at the end of this period, the mean areas of the monosynaptic responses exceeded the areas of the control responses by more than 130% (Fig. 6H), the total period of their post-polarization facilitation would be longer.
The facilitation of the monosynaptic reflexes illustrated in Fig. 6 coincided with the facilitation of field potentials that were recorded concurrently in the depolarized motor nuclei (compare Fig. 6B and C and Fig. 6E and F). The overall effects of DC on monosynaptic reflexes in Fig. 6H also showed a tendency to develop in parallel with the facilitation of field potentials of Ia origin (Fig. 5G).
Depression of monosynaptic actions of cutaneous afferents
By contrast to the facilitatory actions of DC application described above, all 8 extracellular field potentials evoked by cutaneous afferents in the rat dorsal horn were consistently depressed (Table1). As in the experiments described above, the field potentials were recorded with a glass micropipette when cathodal current (0.1–0.2 μA) was passed through a tungsten electrode placed within 100 μm of its tip. The depression is shown in Fig. 7A–C with a decrease of a field potential evoked from Sur in a rat during the 5th period of DC application, as well as 15 min after the polarization had been discontinued. The extent of depression in the whole sample of field potentials is plotted in Fig. 7D. The plot shows a trend for the depression to be enhanced during successive periods of depolarization and the field potentials remained decreased for at least 30 min after the polarization was discontinued. The reasons for the opposite effects of cathodal polarization on synaptic actions of the cutaneous afferents in the dorsal horn and of group Ia afferents in the motor nuclei remain to be clarified. However, one possible reason for these differences, related to DC effects on cutaneous and group Ia afferents, may already be eliminated in view of the very similar changes in the excitability of these afferents evoked under the same experimental conditions (compare Fig. 1 and Fig. 2).
Figure 7. Effect of local cathodal polarizing current on field potentials evoked by cutaneous afferents in the rat.
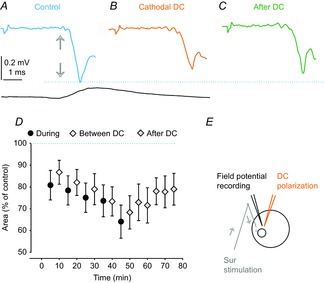
A, averaged record (n = 20) of field potentials evoked in the dorsal horn by stimulation of Sur at 1.7 threshold in the rat and a cord dorsum potential. Arrows indicate the arrival of the afferent volleys. B, as in A but during the 5th application of 0.1 μA cathodal DC current through a tungsten electrode positioned within 50 μm from the site of recording. C, as in A but 15 min after the last period of polarization. Arrows indicate the timing of arrival of the afferent volleys. D, plot of mean changes in the area of the earliest part of the field potentials evoked by local polarization in eight rats. E, stimulation and recording sites. In D, the values (mean ± sem) are expressed as a percentage of control (i.e. the area of the field potentials recorded before the first polarization). Repeated measures ANOVA did not indicate any time effect. However, a one-sample t test revealed a significant difference from zero (P = 0.012).
Effects of local cathodal DC polarization on motoneurons
The results reported so far link the facilitation of the activation of motoneurons with the facilitation of input to these neurons and/or more effective synaptic transmission. However, local cathodal polarization would also affect the motoneurons themselves. In subsequent experiments, we therefore examined the effects of cathodal polarization on the excitability of motoneurons both during the polarization and after the period of current application. Accordingly, the motoneurons were activated by current pulses delivered within a motor nucleus, as indicated in Fig. 8I, at a location at which maximal antidromic field potentials were evoked by stimulation of a muscle nerve and/or of a VR. The latency of antidromic field potentials after stimulation of a VR (Fig. 8D) defined the expected conduction time from the stimulated motor nucleus to the VR (0.3–0.4 ms). Accordingly, we could restrict VR discharges attributable to direct activation of motoneurons to discharges evoked at such latencies and exclude from them any later discharges (e.g. those evoked after additional 0.6 ms), which might have been induced trans-synaptically. By comparing the effects of intraspinal stimuli with increasing intensities, the submaximal intensity leaving a sufficient sub-threshold fringe for the expression of DC facilitation could also be selected. The results revealed marked differences in the effects of cathodal polarization during current application and during post-polarization periods. During 0.1–0.2 μA cathodal polarization, the VR discharges were almost doubled (Fig. 8A and B), with the motoneurons responding as they did to the application of 5–7 μA stronger stimuli. However, there were no indications for facilitatory effects within the post-polarization period, or they appeared to be negligible. Qualitatively, the same effects were found on VR responses evoked from all 9 GS or PBST motor nuclei stimulated at similar current parameters in the cats and from 7 deep peroneal (DP) motor nuclei in the rats (Fig. 8 and Table1).
Figure 8. Strong facilitation of direct activation of motoneurons by stimuli applied in a motor nucleus during but not after cathodal DC polarization.
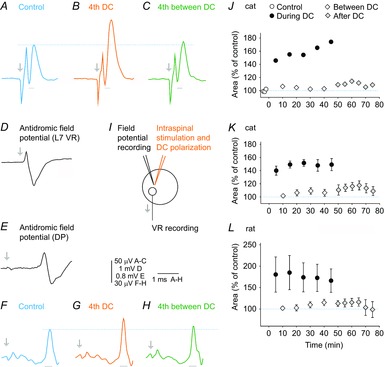
A, record of averaged discharges (n = 20) evoked in the L7 VR by stimuli (18 μA) applied in the MG motor nucleus in a cat. B, as in A but during the fourth application of 0.2 μA direct cathodal current. C, as in A but 5 min after DC application. D, antidromic field potential at a location at which the intraspinal stimuli were applied. F, G and H, as in A–C but with records from the DP nerve from an experiment in a rat (with the dorsal roots L2–L6 transected); the discharges were evoked by stimuli (20 μA) applied in the DP motor nucleus at the site of the antidromic field potential illustrated in E. In A–D and E–H, arrows indicate stimulus artefacts and dotted lines indicate peaks of control responses. I, stimulation and recording sites. J, changes in the area of the early VR discharges illustrated in A–C during successive periods of DC application, the in-between periods and the post-polarization periods, as indicated. The areas were measured during a time window of 0.26 ms from the onset of the discharges (indicated below traces in A–C). They corresponded to the earliest part of the antidromic field potential in D. K, as in J but for values (mean ± sem) for discharges evoked from 9 stimulation sites in 5 cats. Repeated measures ANOVA indicated a time effect (P = 0.0000; F14112 = 15.869); Tukey's post hoc HSD test revealed that all the values ‘during’ are significantly different from all the values ‘after’ (P always < 0.05). A one-sample t test revealed that the mean of the values ‘during’ is different from the control (P < 0.05), whereas the mean of the values ‘after’ does not differ from the control. The polarization acted thus only when applied, facilitating the activation of motoneurons, at the same time as leaving the responses evoked after its application practically unaltered. L, as for K but for discharges evoked in the DP nerve from 7 stimulation sites in 6 rats. Repeated measures ANOVA indicated in this case a significant effect (P = 0.0028; F14,84 = 2.66), whereas no significant differences were revealed by Tukey's post hoc HSD test.
The results thus lead to the conclusion that the facilitation of synaptically evoked activation of motoneurons during cathodal DC application is related to the effects exerted on both the presynaptic afferents and the motoneurons, whereas facilitation during the post-polarization period would be to a much greater extent related to effects on the afferents than on the motoneurons themselves, as well as to synaptic transmission between them.
Discussion
The results of the present study show that some effects of local cathodal polarization in the spinal cord are long-lasting (e.g. a higher excitability of primary afferents stimulated within the regions of their terminal branching and an increased number of fibres activated by the same intensity stimuli). By contrast, other effects, especially changes in the excitability of motoneurons, appear to be restricted to the period of polarization and do not persist after it has been terminated, as indicated by similar thresholds for direct activation of motoneurons by stimuli applied in motor nuclei before and after DC application. Our results also show that cathodal polarization within a motor nucleus facilitates synaptic actions evoked by peripherally stimulated afferents including intracellularly recorded EPSPs in individual motoneurons as well as extracellularly recorded population EPSPs (field potentials) and monosynaptically evoked activation of motoneurons. The facilitation of synaptic actions of group Ia afferents stimulated within a muscle nerve could not be attributed to an increase in the excitability of these fibres at the site of their stimulation because DC was applied within the motor nuclei. These results thus appear to be more compatible with long-lasting post-polarization facilitation of spinal synaptic transmission than with increases in the excitability evoked either presynaptically or postsynaptically. However, local cathodal depolarization was found to depress rather than enhance synaptic actions of cutaneous afferents on their target cells in the dorsal horn, affecting them in an opposite way compared to synaptic actions of Ia afferents on motoneurons, thereby indicating a certain differentiation of the actions of DC.
Pre- and postsynaptic sites of the effects of local cathodal DC polarization
Plasticity in the operation of spinal neuronal networks and in properties of various spinal neurons has been demonstrated repeatedly (Pearson, 2000; Edgerton et al. 2006; Rossignol et al. 2008; Wolpaw, 2010) and various forms of spinal cord stimulation were found to be facilitatory, whether epidural (Edgerton & Harkema, 2011), intra-spinal (Mushahwar et al. 2004) or trans-spinal (Cogiamanian et al. 2011; Ahmed & Wieraszko, 2012; Ahmed, 2013a). Some of these stimuli were found to have prolonged effects, although no attempts have yet been made to localize these prolonged effects.
In the present study, we found that, during local cathodal polarization, the excitability of both primary afferents and spinal motoneurons was potently increased. Much weaker intraspinal stimuli were required to excite the same number of afferents or motoneurons, and the same intensity stimuli excited their considerably increased numbers. However, after polarization had been terminated, the situation changed. The excitability of afferents remained increased during the post-polarization period but the excitability of motoneurons themselves usually returned to more or less control levels as soon as the current was switched off.
The reason for this difference remains an open question. It does not appear to be related to the generally higher thresholds of the activation of motoneurons under our experimental conditions (under anaesthesia) because direct activation of motoneurons was enhanced during DC application as potently as the activation of primary afferents. Furthermore, even if activation of motoneurons required relatively higher intensities of intraspinal stimuli than activation of afferents, the DC effects were generally most marked for effects of near-threshold stimuli and should therefore have been detectable on even the weakest activation of motoneurons. These observations are thus in keeping with the easier to detect post-polarization facilitation of indirectly rather than directly evoked activation of rubrospinal neurons reported by Bolzoni et al. (2013a) and Bączyk & Jankowska (2014). Relatively weak effects of cathodal polarization on motoneurons are also in keeping with observations on spinal lamina I neurons in vitro in which repeated depolarization by intracellularly applied direct current (under whole-cell patch-clamp conditions) often failed to induce any long-term potentiation (LTP), even when it was leading to a similar rise in [Ca2+]1 such as during the paired high frequency stimulation that induced LTP (Naka et al. 2013). LTP was nevertheless found in the cells in which combined pulsed stimulation was combined with sustained depolarization reminiscent of plateau potentials.
More potent DC effects on peripheral afferents than on spinal motoneurons are also consistent with the evidence for a stronger trans-synaptic activation of spinal circuitry by epidural electric stimulation than for direct effects on motoneurons and interneurons, both in modelling studies and in vivo without anaesthesia (Capogrosso et al. 2013) (for the possible effects of anaesthesia, see also the end of the Discussion). On the other hand, they may be at variance with the case reported by Di Lazzaro et al. (2013) who found that facilitation of direct activation of axons of corticospinal neurons (D-wave) by tDCS was longer-lasting than facilitation of indirect activation of these neurons (I-wave).
With respect to the effects of local polarization on primary afferents, it should be noted that we still lack information on the extent to which cathodal post-polarization facilitation is linked to the terminal branches of the afferents, as well as to their pre-terminal regions. Action potentials induced by intraspinal stimuli would have to be initiated within one of the nodes of Ranvier (i.e. at least at one intermodal distance from the terminals) and intraspinal stimuli of 10–20 μA might not only reach these nodes, but also initial parts of motor axons, some 100–200 μm away (Roberts & Smith, 1973; Gustafsson & Jankowska, 1976). In addition, the residual depolarization of presynaptic fibres might involve both the myelinated parts of the fibres and the non-myelinated terminal branches and there is only scarce information available on the long-lasting effects of cathodal polarization on myelinated axons. The longest reported after-effects of transcutaneous cathodal polarization on motor axons in a peripheral muscle nerve were only investigated for a period of 2 min after DC offset (Ardolino et al. 2005), with earlier studies on peripheral nerves using intervals in the order of milliseconds or seconds (Nodera & Kaji, 2006). Somewhat longer lasting (15 min) facilitatory effects of tDCS on axons or initial segments of corticospinal neurons (the D-wave) were reported in a patient by Di Lazzaro et al. (2013). However, in the few cases in the present study where motoneurons were affected by local depolarization applied within a motor nucleus during the post-polarization period, the facilitation was seen during 10–20 min (Fig. 8J–L).
How to reconcile facilitation of synaptic actions of presynaptic fibres with their depolarization?
Facilitation of synaptic actions of group Ia afferents depolarized close to their terminals would be at variance with the well-established effects of polarization of presynaptic terminals because, as a rule, facilitation of synaptic actions is evoked when the terminals are hyperpolarized by anodal DC, rather than when they are depolarized by cathodal DC (Hagiwara & Tasaki, 1958; Eccles et al. 1962; Hubbard & Willis, 1962a,b). With respect to effects of electric fields in slice preparations of Fritsch et al. (2010) or Rahman et al. (2013), it may be more difficult to determine to what extent the stronger synaptic actions found in the superficial cortical layers of fibres stimulated within the deeper layers of M1 region were a result of the relatively stronger depolarization or hyperpolarization of the fibres at the site of their intracortical stimulation. They were nevertheless concluded to be consistent with increases in synaptic efficacy by terminal hyperpolarization and somatic depolarization, with the relative influence of DC electric fields being determined by the orientation of postsynaptic neurons and fibres providing input to them with respect to these fields. Studies on hippocampal slices (Bikson et al. 2004, Kabakov et al. 2012) have led to similar conclusions.
In the present study we found three expressions of facilitation of synaptic actions on motoneurons evoked by stimulation of group Ia afferents in peripheral nerves, far away from the site of the cathodal polarization where the excitability of the afferents could not be affected by intraspinal cathodal polarization. These were larger intracellularly recorded EPSPs, larger monosynaptically evoked extracellular field potentials and more effective monosynaptic reflexes.
The depolarization of terminal branches of Ia afferents might nevertheless play a role, perhaps by affecting ephaptic activation of nearby axonal branches (Debanne et al. 2011) and thereby an increase in the overall transmitter release from a greater number of these branches. Alternatively, cathodal polarization might facilitate transmission at axonal branch points of Ia afferents and the invasion of their terminal axonal branches in a motor nucleus. However, there might be a higher probability of a block of transmission at a branch point by depolarization rather than facilitation (Debanne et al. 2011). In addition, our observations on the effects of local polarization on presynaptic volleys in Ia afferents oppose this possibility. As shown in Fig. 5, these presynaptic volleys remained unchanged or were reduced rather than increased by the local cathodal polarization, whereas concurrently evoked synaptic actions of Ia afferents were facilitated.
Opposite effects of the depolarization of skin and group Ia afferents on their synaptic actions represent another argument against a simple link between the extent of depolarization of presynaptic fibres and their synaptic actions.
Together with negligible effects on the activation of motoneurons, the results of the present study thus lead to the conclusion that neither presynaptic, nor postsynaptic actions of cathodal facilitation probably play any major role in the long-lasting facilitation of synaptically evoked activation of motoneurons by cathodal polarization. Consequently, DC effects on synaptic transmission itself may be much more decisive in this respect.
Parallels with other cases of facilitation of synaptic transmission
Several features of the after-effects of local cathodal polarization might suggest that similar mechanisms are involved in the prolonged effects of cathodal polarization as in other cases of plasticity of synaptic transmission in the spinal cord. Particularly relevant in this context might be changes in synaptic transmission between premotor interneurons and spinal phrenic motoneurons related to LTP of the activation of these motoneurons (Baker-Herman & Mitchell, 2002). Facilitation of the activation of phrenic motoneurons requires repeated (but not continuous) episodes of hypoxia of a few minutes in length. It begins only minutes after the termination of the last episode and develops over a period of at least 30–60 min. Mutatis mutandis, its time course would thus show a fair degree of similarity with the time course of the various effects of local cathodal polarization, as found both in the present study and previously reported by Bączyk & Jankowska (2014). Furthermore, long-term facilitation after hypoxia was found to depend both on serotonin release and on a new protein synthesis (as revealed by the effects of serotonin receptor antagonists and by protein synthesis inhibitors), which might be comparable to the effects of serotonin reuptake antagonists (Nitsche et al. 2009) and protein synthesis inhibitors (Gartside, 1968) on tDCS.
Long-lasting facilitation of spinal activity by tsDCSwas previously associated with several factors, in particular with an increased release of glutamate in the spinal cord (Ahmed & Wieraszko, 2012). However, the reported cases of tsDCS evoked facilitation involved neurons over considerable distances from the cathode and might have resulted from either depolarization or hyperpolarization of presynaptic fibres or neurons within the whole width of the spinal grey matter. In addition, no information has been provided on any long-lasting changes in the concentration of other transmitters or modulators, or concerning the K+ concentration (Kriz et al. 1974; Jimenez et al. 1983), which would have been evoked by the same stimuli, nor on changes in the electric fields induced by them (Faber & Korn, 1989). Functional consequences of increased release of glutamate in the spinal cord might nevertheless correspond to changes in synaptic transmission recently reported to occur in the rat cerebral cortex by Loebel et al. (2013). In the latter study, it was concluded that glutamate related changes in synaptic transmission over long time scales reflect slowly developing modifications in the number of presynaptic release sites matched with postsynaptic changes, which, together, determine the effectiveness of synaptic transmission. If prolonged and repeated cathodal polarization results in such matched presynaptic and postsynaptic modifications, this might explain why synaptic transmission is facilitated rather than depressed by local depolarization and why the facilitatory effects develop fairly slowly.
An even slower developing facilitation of monosynaptic field potentials was reported by Fritsch et al. (2010). These field potentials were recorded in the superficial cortical layers in a slice preparation, in response to deeper delivered intracortical stimuli, and were facilitated by anodal DC applied across the width of the motor cortex. The potentiation of the field potentials began several minutes after DC onset and outlasted the duration of the polarization by more than 2 h. DC was shown to enhance NMDA receptor dependent long-lasting LTP and required coupling of DC with repetitive low-frequency synaptic activation (Fritsch et al. 2010). DC was also reported to contribute to the induction of NMDA receptor-dependent long-term potentiation of synaptic activity at CA3-CA1 synapses in hippocampus slices in the rat and was increased by anodal and reduced by cathodal DC (Ranieri et al. 2012). It would therefore be of interest to determine whether similarly timed effects are associated with the prolonged actions of locally applied cathodal current independently of LTP.
Provided that changes in synaptic transmission similar to those reported on phrenic motoneurons (Baker-Herman & Mitchell, 2002) and/or increases in Ca2+ as observed in cortical neurons (Loebel et al. 2013) are induced in synapses between Ia afferents and motoneurons, it remains an open question as to why cathodal polarization does not have similar effects on synaptic transmission between cutaneous afferents and their target cells in the dorsal horn. It would be of particular interest to investigate whether these differences might be in keeping with the differences in the monoaminergic modulation of transmission between primary afferents and neurons in the dorsal horn and in more ventral laminae, as demonstrated previously (Jankowska et al. 2000; Hammar & Jankowska, 2003; Hammar et al. 2004).
On some opposite effects of cathodal polarization
The effects of local cathodal polarization found in the present study demonstrated considerable variability. Even when the activation of primary afferents, motoneurons or field potentials was facilitated, the overall effects reflected both weak and strong facilitation, with some being manifested immediately, whereas others only developed slowly. This is not unexpected in view of the unavoidable variability of distances between the stimulating/polarizing electrodes and their target neurons or fibres and consequent differences in the efficacy of the direct current. However, the effects of the cathodal block turned out to be a complicating factor. The cathodal block (Bhadra & Kilgore, 2004) appeared to be evoked most often when the DC intensity was probably too high for motoneurons or afferents closest to the electrode tip and/or activated at a lower than average threshold. The lack of appearance of facilitation in some of these tests may thus be explained by the cathodal block counteracting the facilitatory effects evoked in other neurons or afferents. Weak facilitation might therefore reflect not only a non-optimal placement of the stimulating electrode, or non-optimal choice of stimulus parameters, but also a mixture of cathodal block and of facilitation. Different phases of the facilitation, when it was sometimes stronger at the beginning, and then declined and increased again, might also be the result of such mixed effects. Several cases of different phases of the facilitation (e.g. stronger at the beginning, declining and increasing) might also be a result of the expression of such a mixture. For these reasons, we aimed not to identify a maximal cathodal facilitation but rather to optimize the conditions under which it could be reliably attributed to either presynaptic or postsynaptic actions of DC, as well as examine whether it would outlast the period of the cathodal polarization.
On the possible effects of anaesthesia
It is difficult to determine which of the different effects of the cathodal polarization in our animal preparations might be linked to effects of anaesthesia. If a mixture of chloralose and pentobarbital affected the excitability of presynaptic fibres less than the excitability of motoneurons, this would raise the question whether our failures to reveal post-polarization facilitation of direct activation of motoneurons were not secondary to anaesthesia. No definite answer could be given to this question, although a very potent facilitation of direct activation of motoneurons during DC application (see above) would oppose their low excitability related to anaesthesia. Similarly, a very potent facilitation of synaptic activation of motoneurons by group Ia afferents, both during DC application and during the post-polarization period, would be at variance with such a possibility. The probability of stronger effects of anaesthesia on motoneurons than on afferents in our preparations would also be weakened by a failure to detect the facilitation of monosynaptic reflexes in most of the experiments in humans, whereas tsDCS potently facilitated the operation of polysynaptic neuronal pathways (Priori et al. 2014). Furthermore, in animal in vivo experiments, it is a common experience that anaesthesia affects synaptically evoked activation of motoneurons, as potently facilitated during the post-polarization period. The reported differences in the effects of cathodal polarization might nevertheless depend not only on the current parameters, but also on the state of the animals related to the effects of anaesthesia.
Some functional applications
The potential of trans-spinal polarization was been recently discussed in the context of rehabilitation after various spinal injuries (Cogiamanian et al. 2008; Ahmed, 2013b, 2014; Toshev et al. 2014). By contrast, the clinical applicability of the highly invasive local intraspinal polarization would be much more restricted. However, the benefits of intraspinal stimulation were considered in some cases of severe spinal injuries (Mushahwar et al. 2004; Guevremont et al. 2007) and the effects of such stimulation and/or of epidural stimulation (Capogrosso et al. 2013) might be enhanced by combining it with the application of cathodal polarization, similar to that employed in the present study; especially because local cathodal polarization improves the operation of neuronal networks activated by peripheral stimuli and is particularly effective when the stimuli are applied within or near motor nuclei and affect input to spinal motoneurons. The prolonged facilitation of synaptic actions of peripheral afferents on motoneurons by DC polarization could thus improve the output of intraspinal stimulation, whenever applied, not only during its application, but also over a longer time scale.
Acknowledgments
We thank Drs Bengt Gustafsson and Ingela Hammar for their comments on a preliminary version of this paper, as well as Jytte Grännsjö for providing technical assistance during the experiments.
Glossary
Abbreviations
- DP
deep peroneal
- DR
dorsal root
- HSD
honestly significant difference
- L
lumbar
- LG
lateral gastrocnemius
- LTP
long-term potentiation
- MG
medial gastrocnemius
- PAD
primary afferent depolarization
- PB
posterior biceps
- ST
semitendinosus
- Sur
sural
- tDCS
transcranial direct current stimulation
- tsDCS
trans-spinal direct current stimulation
- VR
ventral root
Additional information
Competing interests
The authors declare that there are no competing interests.
Author contributions
The experiments were performed at the Department of Physiology University of Gothenburg. Both authors contributed to the design of the experiments, as well as to the collection, analysis and interpretation of the data, and also the drafting of the article. Both authors approved the final version of the manuscript submitted for publication.
Funding
The work was supported by the National Institutes of Health (R01 NS040863 to E.J.) and the University of Gothenburg.
References
- Ahmed Z. Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements. J Neurosci. 2013a;33:14949–14957. doi: 10.1523/JNEUROSCI.2793-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Electrophysiological characterization of spino-sciatic and cortico-sciatic associative plasticity: modulation by trans-spinal direct current and effects on recovery after spinal cord injury in mice. J Neurosci. 2013b;33:4935–4946. doi: 10.1523/JNEUROSCI.4930-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Trans-spinal direct current stimulation alters muscle tone in mice with and without spinal cord injury with spasticity. J Neurosci. 2014;34:1701–1709. doi: 10.1523/JNEUROSCI.4445-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Wieraszko A. Trans-spinal direct current enhances corticospinal output and stimulation-evoked release of glutamate analog, D-2,3-(3)H-aspartic acid. J Appl Physiol. 2012;112:1576–1592. doi: 10.1152/japplphysiol.00967.2011. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S. Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bączyk M, Pettersson LG. Jankowska E. Facilitation of ipsilateral actions of corticospinal tract neurons on feline motoneurons by transcranial direct current stimulation. Eur J Neurosci. 2014;40:2628–2640. doi: 10.1111/ejn.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bączyk M. Jankowska E. Presynaptic actions of transcranial and local direct current stimulation in the Red Nucleus. J Physiol. 2014;592:4313–4328. doi: 10.1113/jphysiol.2014.276691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL. Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N. Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004;12:313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H. Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiology. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Bączyk M. Jankowska E. Subcortical effects of transcranial direct current stimulation in the rat. J Physiology. 2013a;591:4027–4042. doi: 10.1113/jphysiol.2013.257063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson LG. Jankowska E. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiology. 2013b;591:3381–3399. doi: 10.1113/jphysiol.2012.244764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G. Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33:19326–19340. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S. Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2008;119:2636–2640. doi: 10.1016/j.clinph.2008.07.249. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S. Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain. 2011;152:370–375. doi: 10.1016/j.pain.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E. Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ranieri F, Profice P, Pilato F, Mazzone P, Capone F, Insola A. Oliviero A. Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stimul. 2013;6:641–643. doi: 10.1016/j.brs.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM. Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG. Schmidt RF. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J Physiol. 1962;162:138–150. doi: 10.1113/jphysiol.1962.sp006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR. Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother. 2011;11:1351–1353. doi: 10.1586/ern.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP. Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23:560–570. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- Engberg I, Kallstrom Y. Marshall KC. Double manipulator for independent impalements of one neurone with two electrodes. Acta Physiol Scand. 1972;184:4–5A. [Google Scholar]
- Faber DS. Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev. 1989;69:821–863. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG. Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 1968;220:383–384. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- Guevremont L, Norton JA. Mushahwar VK. Physiologically based controller for generating overground locomotion using functional electrical stimulation. J Neurophysiol. 2007;97:2499–2510. doi: 10.1152/jn.01177.2006. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Tasaki I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958;143:114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA. Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–1316. doi: 10.1111/j.l460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I. Jankowska E. Modulatory effects of alpha1-, alpha2-, and beta - receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci. 2003;23:332–338. doi: 10.1523/JNEUROSCI.23-01-00332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JI. Willis WD. Mobilization of transmitter by hyperpolarization. Nature. 1962a;193:174–175. doi: 10.1038/193174a0. [DOI] [PubMed] [Google Scholar]
- Hubbard JI. Willis WD. Reduction of transmitter output by depolarization. Nature. 1962b;193:1294–1295. doi: 10.1038/1931294a0. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B. Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jimenez I, Rudomin P, Solodkin M. Vyklicky L. Specific and potassium components in the depolarization of the la afferents in the spinal cord of the cat. Brain Res. 1983;272:179–184. doi: 10.1016/0006-8993(83)90378-5. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE. Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–1889. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Miledi R. Propagation of Electric Activity in Motor Nerve Terminals. Proc R Soc Lond B Biol Sci. 1965;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kriz N, Sykova E, Ujec E. Vyklicky L. Changes of extracellular potassium concentration induced by neuronal activity in the sinal cord of the cat. J Physiology. 1974;238:1–15. doi: 10.1113/jphysiol.1974.sp010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel A, Le Be JV, Richardson MJ, Markram H. Herz AV. Matched pre- and post-synaptic changes underlie synaptic plasticity over long time scales. J Neurosci. 2013;33:6257–6266. doi: 10.1523/JNEUROSCI.3740-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Bikson M. Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Aoyagi Y, Stein RB. Prochazka A. Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol. 2004;82:702–714. doi: 10.1139/y04-079. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo MF, Karrasch R, Wachter B, Liebetanz D. Paulus W. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biolog Psychiatry. 2009;66:503–508. doi: 10.1016/j.biopsych.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Muller-Dahlhaus F, Paulus W. Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590:4641–4662. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodera H. Kaji R. Nerve excitability testing and its clinical application to neuromuscular diseases. Clin Neurophysiol. 2006;117:1902–1916. doi: 10.1016/j.clinph.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Plasticity of neuronal networks in the spinal cord: modifications in response to altered sensory input. Progr Brain Res. 2000;128:61–70. doi: 10.1016/S0079-6123(00)28007-2. [DOI] [PubMed] [Google Scholar]
- Priori A, Ciocca M, Parazzini M, Vergari M. Ferrucci R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol. 2014;592:3345–3369. doi: 10.1113/jphysiol.2013.270280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC. Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591:2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V. Grassi C. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1868–1880. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- Ravid EN, Gan LS, Todd K. Prochazka A. Nerve lesioning with direct current. J Neural Eng. 2011;8:016005. doi: 10.1088/1741-2560/8/1/016005. [DOI] [PubMed] [Google Scholar]
- Roberts WJ. Smith DO. Analysis of threshold currents during microstimulation of fibres in the spinal cord. Acta Physiol Scand. 1973;89:384–394. doi: 10.1111/j.1748-1716.1973.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Barriere G, Frigon A, Barthelemy D, Bouyer L, Provencher J, Leblond H. Bernard G. Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev. 2008;57:228–240. doi: 10.1016/j.brainresrev.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Skoglund CR. Modification by electrotonus of the transmission in the artificial synapse formed by severed mammalian nerve. J Neurophysiol. 1945;8:377–386. doi: 10.1152/jn.1945.8.6.377. [DOI] [PubMed] [Google Scholar]
- Stagg CJ. Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Toshev PK, Guleyupoglu B. Bikson M. Informing dose design by modeling transcutaneous spinal direct current stimulation. Clin Neurophysiol. 2014;125:2147–2149. doi: 10.1016/j.clinph.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Wagner S, Rampersad SM, Aydin U, Vorwerk J, Oostendorp TF, Neuling T, Herrmann CS, Stegeman DF. Wolters CH. Investigation of tDCS volume conduction effects in a highly realistic head model. J Neural Eng. 2014;11:016002. doi: 10.1088/1741-2560/11/1/016002. [DOI] [PubMed] [Google Scholar]
- Wall PD. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958;142:i3–21. [PMC free article] [PubMed] [Google Scholar]
- Watt DG, Stauffer EK, Taylor A, Reinking RM. Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976;39:1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Winkler T, Hering P. Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol. 2010;121:957–961. doi: 10.1016/j.clinph.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory. Neuroscientist. 2010;16:532–549. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]


