Abstract
Previous work using gambling tasks indicate that the feedback negativity (FN) reflects primary or salient stimulus attributes (often gain vs. loss), whereas the feedback-P300 appears sensitive to secondary stimulus information. A recent time-frequency approach has characterized separable theta (3–7 Hz) and delta (0–3 Hz) feedback processes, independently sensitive to primary feedback attributes, specifically loss and gain outcomes respectively (Bernat et al., 2011). The current study extends this time-frequency work to evaluate both primary and secondary (relative outcome and outcome magnitude) feedback attributes. Consistent with previous reports, theta indexed an initial, lower-level response sensitive to the primary (most salient) feedback attributes (specifically losses), while delta was sensitive to both primary attributes (specifically gains) and assessed secondary stimulus features.
Keywords: time-frequency analysis, theta, delta, feedback-negativity, FN, P300, gambling, event-related potential, ERP
Research using event related potentials (ERPs) demonstrates the presence of multiple cognitive processes that contribute to an individual’s evaluation of performance feedback. Two markers of these processes are the feedback negativity (FN), a negative going deflection maximal at frontocentral scalp sites around 250 ms post-feedback onset, and the P300, a positive-going deflection maximal more parietally around 300 ms. Although FN and P300 are widely acknowledged as important components of the feedback response (Gehring & Willoughby, 2002; Holroyd & Coles, 2002; Miltner, Braun, & Coles, 1997), isolation of these overlapping components using traditional time-domain measurement techniques is challenging and may be limiting progress toward understanding what each measure represents in the context of processing performance feedback.
Current Measures of Feedback Processing
Most paradigms evaluating the ERP response to performance feedback focus on the FN alone. For the most part this literature has told a fairly consistent story about FN as a relatively low-level response to negative outcomes. The initial tasks (e.g., gambling and feedback; Gehring & Willoughby, 2002; Miltner et al., 1997, respectively) used to measure the FN demonstrated that this component shows increased negativity following bad versus good performance feedback. More recently, task manipulations varying the experimental context have built a more nuanced account of FN. For example, when feedback stimuli provide multiple pieces of information, the FN responds to the most salient factor (usually loss vs. gain), often referred to as primary feedback attributes. The FN has been shown to be less sensitive to more complex secondary feedback attributes, such as relative outcome (i.e., given two options, this is the comparative value of the outcome the participant would have received had they selected the other option) or outcome magnitude (Gehring & Willoughby, 2002; Yeung & Sanfey, 2004). Consistent with the specific association of FN and salience, manipulations that alter the visual or functional salience of primary feedback attributes (e.g., making errors rather than losses more visually striking or more important for performing the task), have demonstrated that the FN becomes sensitive to the most salient attribute (i.e., error in this case) in the environment (Nieuwenhuis, Yeung, Holroyd, Schurger, & Cohen, 2004). Another aspect of outcome salience that has been assessed involves manipulating the value of bad and good outcomes relative to the task context. For example, in a block of trials offering no money vs. multiple gain magnitudes, the FN shows differentiation between non-reward versus all rewards (Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd, Hajcak, & Larsen, 2006). Thus, here, a failure to win acts as the bad outcome. Taken together, the literature supports the view that the FN represents a simple good vs. bad response that is dependent on the most salient attribute of the feedback stimulus given the current task context.
The P300, although frequently ignored in feedback-FN tasks, is another important component of feedback processing. Unlike the FN, the feedback-P300 appears to pick up on secondary feedback attributes such as the magnitude of reward and the valence of the alternative outcome (Gu, Wu, Jiang, & Luo, 2011; Pfabigan, Alexopoulos, Bauer, & Sailer, 2010; Sato et al., 2005; Yeung & Sanfey, 2004). With respect to primary feedback attributes (often loss vs. gain; we will use the term outcome valence to refer to this dimension), the majority of studies have found no relationship to P300 (Foti, Weinberg, Dien, & Hajcak, 2011; Hajcak, Holroyd, Moser, & Simons, 2005; Pfabigan, et al., 2010; Yeung & Sanfey, 2004), although some have found sensitivity of P300 to positive valence (Hajcak, Moser, Holroyd, & Simons, 2007; Zhou, Yu, & Zhou, 2010). Overall, these findings support the idea that, in contrast to FN, the feedback-P300 may be sensitive to more complex stimulus parameters requiring additional evaluation or comparison, consistent with the idea that P300s reflect elaborative, post-perceptual processing of stimuli that may be used to update working memory about the task context (Donchin, 1981; Polich, 2007).
While there appears to be a consistent distinction between what FN and feedback-P300 reflect (primary vs. secondary feedback attributes), within the literature on each time-domain component there remains some inconsistencies and ambiguity. For example, although the FN is thought to reflect a simple response to primary feedback attributes, some reports have found that FN and other early feedback-ERP components can be modulated by secondary feedback attributes (Goyer, Woldorff, & Huettel, 2008; Wu & Zhou, 2009). Similarly, it is somewhat surprising that the feedback-P300 would not reflect primary gain-loss differences in gambling tasks, given that it is theorized to reflect a response to motivationally salient outcomes (Nieuwenhuis, Aston-Jones, & Cohen, 2005), and is related to subsequent risk-taking behavior on subsequent trials based on the salient outcomes (Gehring & Willoughby, 2002; Nelson, Patrick, Collins, Lang, & Bernat, 2011).
A new potential explanation for these inconsistencies has emerged from recent work indicating the presence of a positive polarity signal, which increases specifically on reward trials, referred to as the reward positivity. One study identified this reward positivity through experimental manipulation using an oddball task. Holroyd and colleagues (Holroyd, Pakzad Vaezi, & Krigolson, 2008) found that the FN, rather than being dominated by the negative-going potential to bad outcomes, may actually be dominated by large positive-going potentials sensitive to good outcomes. Similarly, Foti and colleagues (2011), using temporospatial principal components analysis of the time-domain feedback-ERP, recently identified a large positive-going signal present exclusively on reward (gain) trials, the absence of which they hypothesized created the FN on loss trials. Together, these findings suggest that multiple processes are captured during the FN time window, and the simple association between FN and primary feedback characteristics may be overly simplistic. Moreover, given the inconsistent findings in the FN and P300 literature, it is possible that a measurement problem may be complicating findings in this area (Bernat, Nelson, Steele, Gehring, & Patrick, 2011; Nelson, et al., 2011). Certainly, FN and P300 are notoriously difficult to parse due to a large degree of temporal overlap (Foti, et al., 2011; Hajcak, et al., 2007; Holroyd, Pakzad Vaezi, & Krigolson, 2008; Miltner, et al., 1997), and researchers have devoted much effort considering how to best isolate processes during gambling feedback tasks.
Time-Frequency Measurement Approaches
One important measurement approach that has gained momentum in parsing processes during gambling feedback (as well as with ERP data more broadly) is time-frequency analysis. Substantial time-frequency work has indicated that activity associated with the FN and P300 occur primarily within distinct frequency bands, with FN occurring primarily in the theta (3–7 Hz) frequency band (Cavanagh, Zambrano Vazquez, & Allen, 2011; Cohen, Elger, & Ranganath, 2007; Gehring & Willoughby, 2004; van de Vijver, Ridderinkhof, & Cohen, 2011) and P300 in the delta (0–3 Hz) band (Başar-Eroglu, Başar, Demiralp, & Schürmann, 1992; Başar-Eroglu, Demiralp, Schurmann, & Başar, 2001; Bernat, Malone, Williams, Patrick, & Iacono, 2007; Demiralp, Ademoglu, Istefanopulos, Başar-Eroglu, & Başar, 2001; Gilmore, Malone, Bernat, & Iacono, 2010). As an approach to separating theta and delta time-frequency activity, we recently proposed applying time-frequency principal components analysis, which has proven effective for separating activity that overlaps in time but is distinct in frequency (Bernat, Williams, & Gehring, 2005). Indeed, this time-frequency principal components analysis approach has proven effective in partitioning overlapping ERP components in prior published studies of the response-ERN (Bernat, et al., 2005; Hall, Bernat, & Patrick, 2007), oddball target P300 (Bernat, et al., 2007) and, most importantly, FN and feedback-P300 in the gambling task used in the current study (Bernat, et al., 2011; Nelson, et al., 2011).
An important finding that emerged from these recent studies was an ordered description of how the phase (positive and negative polarity of the peaks) and amplitude for theta and delta combined to yield the traditional FN and P3 time-domain components. These findings suggested how these dynamics can complicate inferences from time-domain measures. More specifically, for the time-domain FN and P3, the faster oscillation of theta contributed increased amplitude to the negative polarity of the FN component, but positive amplitude at P3, due to the phase reversal of theta during these two components. By contrast, the slower changing phase of delta contributed positive amplitude to both the FN and P3 components. The dynamics of the phase (polarity) of theta and delta had a crucial relationship to gain–loss experimental effects when observed in the time domain: the increased delta activity to gain feedback corresponded to an enhanced positivity at both FN and P3, whereas enhanced theta activity for losses produced opposite effects at FN (increased negative amplitude) and P3 (increased positive amplitude). Together theta and delta combined additively at FN to create a large gain–loss difference but acted in a subtractive manner at P3 resulting in a non-significant gain–loss difference (Bernat, et al., 2011). These analyses indicated that time-domain FN and P300 measures can be considered mixtures of separable processes occurring in the theta and delta bands. Thus, from a measurement perspective, theta and delta measures offer substantial promise for better parsing functional processes occurring during gambling feedback.
The Current Study
The current study builds upon Bernat et al. (2011) by assessing the utility of the two-process time-frequency (theta and delta) model in accounting for multiple attributes of the feedback stimuli (i.e., the extent to which theta and delta are modulated by primary and secondary attributes). To accomplish this, feedback outcomes from the gambling task originally published by Gehring and Willoughby (2002) were subdivided to represent not only the primary outcome valence attribute (gain versus loss), but also a more complete array of common secondary feedback attributes depicted in the stimuli (relative outcome, outcome magnitude – detailed below).
Based on prior work indicating that theta and delta index more independent processes underlying FN and P300 measures, respectively, we expected that theta would be modulated mainly by loss (i.e., within the primary loss vs. gain, or outcome valence, feedback attribute), while delta would be modulated by the full array of primary and secondary feedback attributes (e.g., increases to gain outcomes in the primary outcome valence dimension, but also other secondary attributes). Further, based on the expectation that time-domain FN and P300 were confounded by overlapping influences of theta and delta activity, we also expected that the time-frequency measures would show purer relationships to the primary and secondary feedback attributes than their time-domain counterparts (e.g., to the extent that time-domain FN showed relationships to secondary feedback attributes, those relationships would be explainable by overlapping delta). Finally, we expected that differences in theta and delta phase (polarity) at the FN and P300, as described above, would account for any observed mixtures of theta and delta in the time-domain components. Broadly, identifying the extent to which time-frequency theta and delta are modulated by primary versus secondary feedback attributes could help clarify the nature of the FN and P300 responses during feedback processing, and could additionally reveal processes that have not been consistently identified in the time-domain measures (e.g., delta reward positivity, and additional secondary attributes of the feedback).
Method
Participants
Participants were 166 undergraduate students recruited from Introductory Psychology classes at the University of Minnesota who received either monetary compensation or course credit. To match the dataset used in the previous report (Bernat et al., 2011), the same eighteen subjects were excluded from analyses (eight because of incomplete questionnaire data in that study, three due to equipment problems during collection, four due to excessive artifacts, and two who discontinued prior to the completion of testing). Thus, the final study sample consisted of 149 participants (58 male, age M = 20.6, SD = 3.7).
Procedure
Testing was conducted in a dimly lit, sound-attenuated room. Experimental stimuli were presented centrally on a 21” Dell high-definition cathode ray tube color monitor, at a viewing distance of 100 cm, using E-Prime version 1.1 software (Psychology Software Tools, Inc.). Behavioral responses were made using the Psychology Software Tools’ Serial Response Box. The experimental task was a modified version of Gehring and Willoughby’s (2002) gambling task in which the participant chose between two monetary options on each trial and then received feedback indicating whether the choice resulted in winning or losing money on that trial. The modification was that feedback was presented 100 ms after the button press to have the feedback more immediately follow the choice. The target stimuli consisted of two adjacent squares, each enclosing a number (5 or 25) representing a monetary value (in cents). Participants chose one of the two squares (left or right), and a subsequent feedback stimulus displayed the outcome of their decision. That is, the chosen box turned either red or green to signify either a win or a loss (with red or green as the winning color counterbalanced across participants), and the unchosen box turned the other color (either green or red) to indicate what the outcome of the trial would have been had that box been chosen. All possible combinations of 5 and 25 (i.e., 5-5, 5–25, 25-5, and 25-25) were presented as targets, with each combination occurring an equal number of times in a randomized sequence. The target stimulus remained on the screen until a choice was made, after which a blank screen appeared for 100 ms. Next, a feedback stimulus appeared for 1000 ms, followed by a blank screen for 1500 ms, preceding the onset of the next trial. Participants completed 12 blocks of 32 trials. The target and feedback boxes were 5.5 cm high, viewed from 100 cm away, subtending 3.15° visual angle.
Electroencephalographic Recording
Participants in the study were tested in two waves. Participants in the first wave (N = 42) were tested using a 64-channel Neuroscan, Inc. Synamps amplifier, and those in the second wave (N = 125) were tested using a 64-channel Neuroscan Synamps2 amplifier. In each phase, EEG activity was recorded using 64-channel Quick-caps containing sintered Ag-AgCl electrodes positioned in accordance with the International 10–20 System (Jasper, 1958). Activity was recorded from a greater number of scalp sites in Wave 2, but only electrodes in common across the two waves were included in the analyses reported here. Additionally, problems with the FP1 and FP2 scalp sites in Wave 1 necessitated dropping these sites from both waves. Thus, 51 electrodes are included in the reported data, as follows: AF3, AF4, F7, F5, F3, F1, Fz, F2, F4, F6, F8, FT7, FC3, FC1, FCz, FC2, FC4, FT8, T7, C5, C3, C1, Cz, C2, C4, C6, T8, TP7, CP3, CP1, CPz, CP2, CP4, TP8, P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO5, PO3, POz, PO4, PO6, O1, Oz, O2. Ocular activity was monitored using electrodes positioned on the outer canthus of each eye (Horizontal EOG) as well as above and below the left eye (Vertical EOG). Impedances were kept below 10 kΩ. All EEG signals were referenced to CPz and digitized on-line at 1000 Hz. The signals were then epoched off-line from 1000 ms before to 2000 ms after feedback onset, and re-referenced to the average of activity at the left and right mastoids. Trial-level EEG data were corrected for ocular and movement artifacts using a regression-base algorithm (Semlitsch, Anderer, Schuster, & Presslich, 1986), as implemented in the Neuroscan Edit software, version 4.3. As a final step, the processed data were re-sampled off-line to 128 Hz.
Data Preprocessing
Trial-level ERPs were epoched (−1000 to 2000 ms) and baseline-corrected for the 150 ms preceding feedback stimulus presentation. A careful visual inspection of the data was undertaken to identify and exclude movement and other artifacts, in particular, to minimize their impact on the time-frequency principal components analysis decomposition (detailed below). Toward this end, several exclusionary criteria were applied. First, to exclude ocular artifacts remaining after ocular correction, trials on which activity at frontal electrode sites F1 or F2 exceeded 75 µV within a 1500 ms post-stimulus window (relative to median activity within a 750 ms window immediately preceding the stimulus) were excluded from further processing. Then, within each trial, individual electrode sites at which activity exceeded ± 75 µV in either the pre- (−750 to 0) or post-stimulus (0 to 1500) time regions (relative to one another) were also omitted from analysis. Applying these criteria, 9.9% of trials were excluded. Additionally, across all subjects and electrodes, 24 electrodes (out of 8517) became disconnected at some point during the procedure. Missing data for these leads were replaced with the average activity of their nearest-neighbors.
Data Reduction
Time-domain components: FN and P300
The time-domain FN component was defined as the maximum negative deflection in the ERP waveform occurring between 203 and 328 ms post stimulus onset relative to a −102 to −8 ms baseline; the P300 was defined as the maximum positive deflection occurring between 297 and 500 ms post stimulus onset, relative to the same baseline (with ms corresponding to bins of 128 Hz re-sampled signal). Electrode sites FCz and Cz were most proximal topographically to the center of FN and P300 Gain-Loss condition differences, respectively, and were thus employed in the time-domain statistical analyses reported below.
Time-frequency components: theta and delta
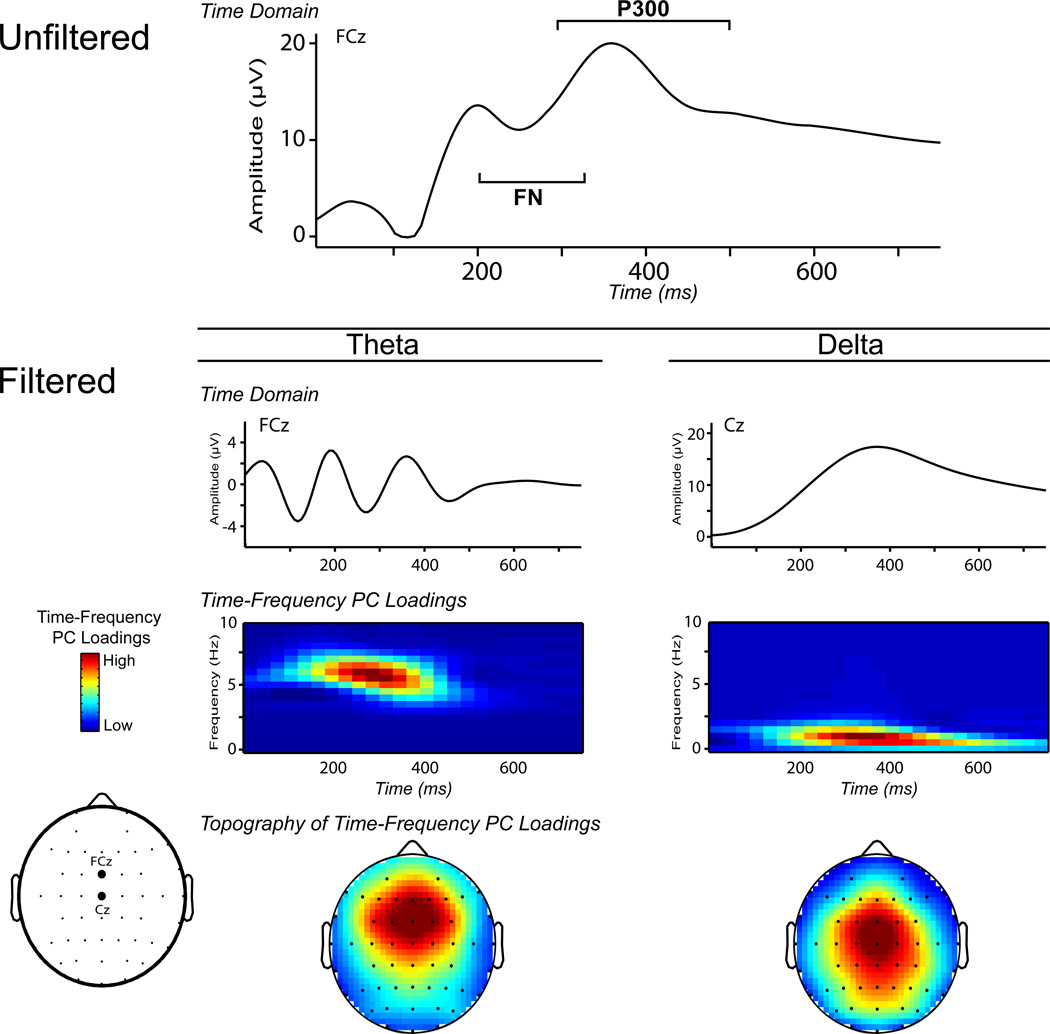
Figure 1 illustrates the time-frequency theta and delta measures used in this study. The method used to isolate theta-FN from delta-P300 was identical to that of Bernat et al. (2011) and Nelson et al. (2011). Specifically, the condition-averaged feedback-ERP signals were filtered in two alternative ways: 1) using a 3–9 Hz bandpass 3rd order Butterworth filter (high-pass at 3 Hz and low-pass at 9 Hz) to isolate activity in the theta frequency band and 2) using a 3 Hz lowpass 3rd order Butterworth filter (all filters implemented with the Matlab butter and filtfilt functions, Matlab version 7.4, Mathworks, Inc.) to isolate activity in the delta frequency band. Because higher-frequency activity (i.e., theta) is much smaller in amplitude than lower-frequency (delta) activity, pre-filtering the ERP allowed us to directly target the relatively weak theta component of the feedback-ERP that was known to best represent FN. Next, each filtered signal (theta and delta) was transformed into a time-frequency energy distribution (surface) with the binomial reduced interference distribution variant of Cohen’s class of time-frequency transforms (Bernat, et al., 2005). This was done using full epochs of −1 to 2 s, relative to feedback onset, in order to provide sufficient data to resolve frequencies at and around 1 Hz. For each of these time-frequency bands, principal components analysis was applied to an area corresponding to the 0 to 750 ms time range and 0 to 10 Hz frequency range; this yielded equivalent time windows for decomposition, but with filters having narrowed the frequency activity within the window to either theta or delta, as described above. Principal components analysis (PCA) was used to identify the primary activation component in each frequency band (delta, theta), corresponding to the largest principal component emerging from the principal components analysis (i.e., the component accounting for the greatest proportion of shared covariance across all time-frequency points). Details for this application of PCA to time-frequency surfaces have been previously published (Bernat, et al., 2005). Briefly, this involves first vectorizing the time-frequency surfaces (e.g., concatenating each frequency row end to end) such that the columns of the data matrix index data from different time-frequency points while the rows index the condition averages (separated by subject and electrode). The PCA decomposition is then conducted using this matrix, utilizing the covariance approach and varimax rotation. The vectorized components are then reassembled into time-frequency surface matrices for interpretation (as presented in Figure 1).
Figure 1.
Time-frequency decomposition of the feedback-ERP. Theta’s frontocentral topographical distribution of the principal components (PC) loadings closely matches that of the FN, and delta’s more centroparietal distribution of the principal components (PC) loadings matches the P300. Adapted with permission from the American Psychological Association.
The variance accounted for by the first principal component for each measure (theta: 40%; delta: 71%) substantially exceeded that accounted for by the next component (theta band: 10%; delta band: 9%) for each decomposition, indicating that retention of a single principal component was justifiable in each case. These time-frequency-based theta and delta principal components scores (depicted in Figure 1) served as the primary dependent measures in the analyses of brain reactivity to feedback stimuli reported below. Principal component scores were calculated as the mean across the principal component weighted time-frequency surface, in the same manner that principal component scores are calculated with questionnaire data. As with the time-domain FN and P300 measures, electrodes FCz and Cz were most proximal topographically to the maximum of the theta and delta gain-loss condition differences, respectively, so data from these electrode sites were employed in the statistical analyses of time-frequency component scores reported below.
Outcome Categories
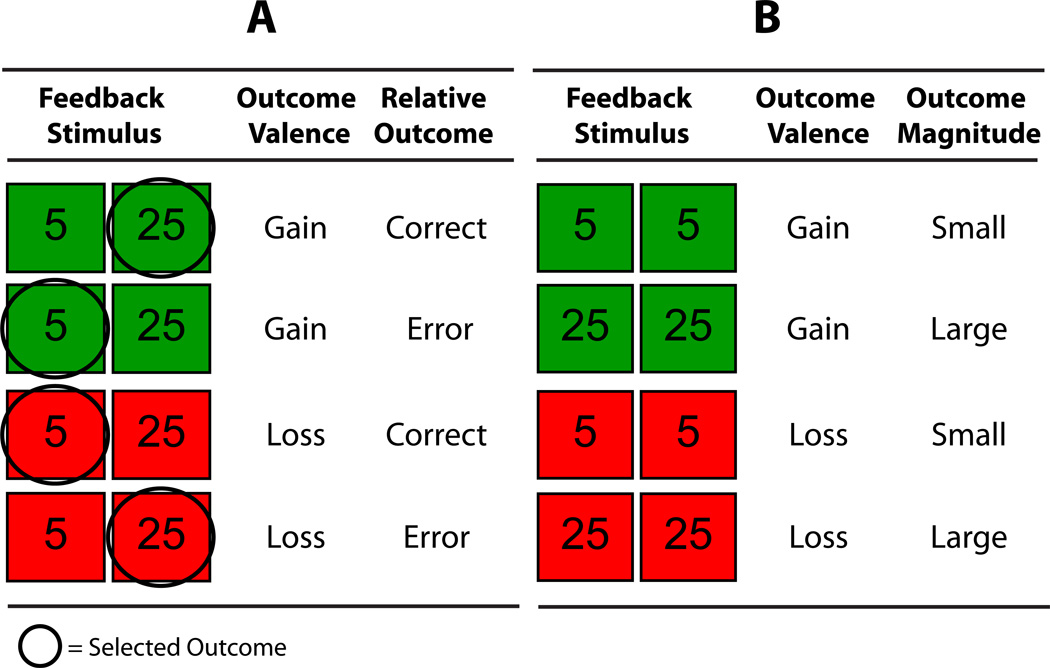
Based on the Gehring and Willoughby (2002) task, trials started with a two-alternative forced choice gamble, in two adjacent boxes, each containing either 5 or 25 cents (i.e., 5/5, 5/25, 25/5, or 25/25). The participant then chose either the left or right box as the gamble for the current trial. After the choice was made a feedback stimulus appeared displaying the two monetary choices from the previous screen, with colors filling in the background of both boxes to indicate the valence of the chosen and alternative outcome (red or green, assigned to (G)ain and (L)oss, counter balanced across subjects). Thus, the colors indicated both whether the chosen box produced a gain or loss and whether the participant would have won or lost had they chosen the other side (i.e., G/G, G/L, L/G, or L/L). With the 4 possible 5 and 25 cent gambling choices, and the 4 possible gain and loss combinations for the outcome, there were 16 possible feedback stimuli. In this array of feedback stimuli, whether the chosen box produced a gain or loss was the primary, or emphasized, dimension -- because the color change defining the feedback onset indicated winning or losing money, indicated as the goal of the study (cf. Nieuwenhuis et al., 2004). In this study, we refer to this primary gain/loss attribute as outcome valence. In addition to the primary outcome valence attribute, information about several secondary attributes was also present in the feedback. The current study assessed two such attributes which have been of interest in the literature: relative outcome and outcome magnitude. Relative outcome reflects the conditions referred to as error-correct information in the original report (Gehring & Willoughby, 2002), described as error-correct information. The outcome magnitude dimension (i.e., the amount of money) has also been assessed in the field (e.g., Yeung & Sanfi, 2004), but not with stimuli from the current task. These stimuli utilized for these analyses are detailed next.
The eight feedback stimuli assessed in this study are presented in Figure 2. For these stimuli (out of the 16 total feedback stimuli in the task), both of the boxes produced the same gain or loss outcome (both-gain or both-loss), rather than one side gain and the other side loss. Because the unchosen alternative would not have changed the outcome in terms of being a gain or a loss, this provided an opportunity for unconfounded assessments of the effects of the amount of money overall (outcome magnitude), and comparisons with the amount of money that would have been obtained had the other box been chosen (relative outcome). The relative outcome feedback stimuli are presented in Figure 2A. Here, for example, gain trials can be understood in terms of a secondary error or correct component when compared with the unchosen alternative. That is, a gain can be either small in comparison to the unchosen larger gain (error) or larger than the unchosen smaller gain (correct), and this error-correct comparison with the unchosen alternative holds for the two loss stimuli in Figure 2A (these four outcomes were similarly utilized by Gehring & Willoughby, 2002). Next, the reward magnitude stimuli are presented in Figure 2B. Because the two sides are equal both for the gain-loss dimension and also the amount of money, these stimuli provide the opportunity to assess the magnitude of the gain or loss outcomes in isolation, i.e., without competing alternative outcome attributes. The remaining 8 out of 16 feedback stimuli (which were left out of the analyses) had the same variation in the amount of money, but in each case the two boxes had opposing gain and loss outcomes (unlike the first 8 containing either both-gain or both-loss). Because these stimuli mixed gain-loss with the error-correct and magnitude dimensions, they did not represent unconfounded comparisons, and are not presented in this report.
Figure 2.
Gambling task feedback outcomes evaluated in the current study. Column A depicts the four conditions selected to evaluate outcome valence (loss vs. gain) × relative outcome (error vs. correct). Column B depicts the four conditions selected to evaluate valence × magnitude. Note that for the outcomes in B, actual and alternative outcomes are equivalent and thus there is no relative outcome effect possible.
Data Analysis
Data analyses took place in two main parts. The first part focused on assessing relationships between the time-domain and time-frequency measures across the dataset with two hypotheses: 1) that theta and delta time-frequency measures index more independent processes than FN and P300 time-domain measures, and 2) that FN and P300 are each better understood as a mixture of independent processes that can be indexed by theta and delta time-frequency activity. For hypothesis 1, the bivariate relationships within the time-domain and time-frequency measures were assessed using Pearson correlations (between FN and P300, and between theta and delta). Correlations were computed for the component grand averages across conditions and each condition difference. A Fisher z transformed Pearson-Filon statistic (ZPF) for comparing non-overlapping non-independent correlations (Raghunathan, Rosenthal, & Rubin, 1996) was computed between these correlations to assess whether the time-domain variables (FN and P300) were significantly more related to each other than the time-frequency variables (theta and delta). For hypothesis 2, regression analyses were conducted, in which each of the time-domain measures alternatively served as the dependent variable, and the theta and delta time-frequency measures served as simultaneously entered independent variables. Support for hypothesis 2 occurs when the time-frequency regression coefficients indicate significant and unique variance for each theta and delta in predicting either the FN or P300.
The second part of the data analysis assessed the hypotheses that theta would be more sensitive to the primary attributes, relative to the secondary attributes, while delta would be more similarly sensitive to primary and secondary attributes. To accomplish this, separate repeated-measures ANOVA (RM-ANOVA) analyses for theta and delta were conducted separately for feedback stimulus sets A and B, as defined in Figure 2. For each of these 4 RM-ANOVAs, Outcome Valence (Gain/Loss) was included as the primary stimulus attribute factor, with set A including also the Relative Outcome (Error/Correct) as the secondary attribute factor and set B including also Outcome Magnitude (Large/Small) as the secondary attribute factor. Follow-up comparisons between the results from the time-domain and time-frequency repeated-measures ANOVA were next conducted to statistically assess observed differences in the patterns of results relative to these hypotheses.
Results
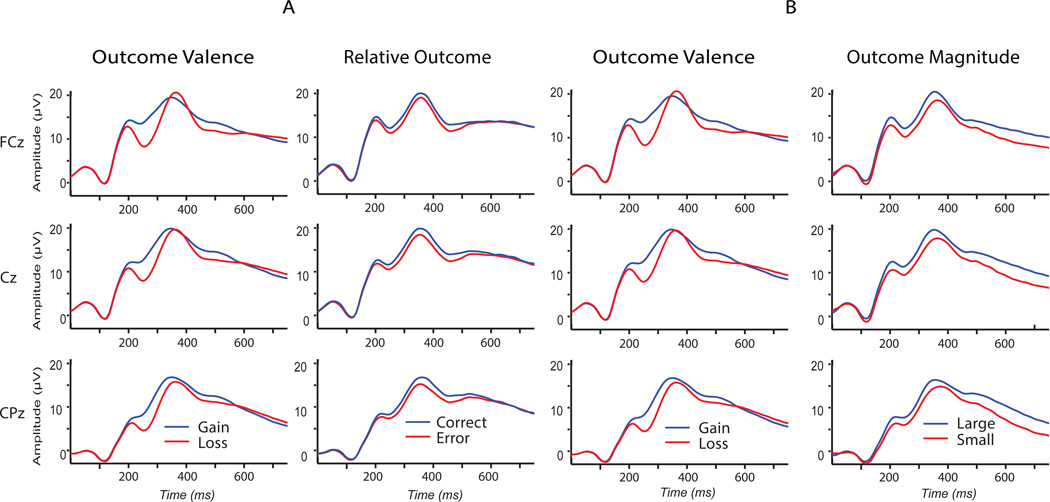
Figure 3 presents condition averages for unfiltered time-domain waveforms from midline electrodes FCz, Cz, and CPz for the primary Outcome Valence (Gain/Loss) stimulus attribute, as well as the secondary stimulus attributes Relative Outcome (Error/Correct) and Outcome Magnitude (Large/Small) from stimulus subsets A and B as defined in Figure 2. These waveforms are presented for comparison with traditional plots depicting activity across the midline sensors.
Figure 3.
Unfiltered time-domain waveforms for the midline electrodes (FCz, Cz, and CPz). Grand averages by condition are presented -- including the primary Gain/Loss comparisons, as well as the secondary Error/Correct and Large/Small comparisons. These waveforms are presented for comparison with traditional plots depicting activity across the midline sensors. For example, the expected larger Gain-Loss difference at FCz, as compared to CPz, is readily apparent.
Relationship Between Time and Time-Frequency Representations of FN and P300
Before evaluating for the statistical significance of primary and secondary stimulus attribute differences, the basic relationship between the time and time-frequency domain measures were assessed. As detailed in the data analysis section above, the aim of these analyses was to assess whether the conventional time-domain FN and P300 component measures can be understood as a mixture of more functionally separable theta and delta processes. Functional separation between theta and delta, and between FN and P300, were first assessed using correlations. Next, FN and P300 were each directly assessed for unique contributions from theta and delta activity using a regression analysis approach.
Correlations (Table 1)
Table 1.
Correlations for Time-Domain FN and P300 and Time-Frequency Theta and Delta
Because the present time-frequency decomposition was computed on a different set of ERP averages than in the Bernat et al. (2011) report, it was important to first replicate those analyses based on the overall average and Gain-Loss difference scores, using the new decomposition. Additionally, we computed Correct-Error difference scores (using the four conditions in column A of Figure 1) and Big-Small difference scores (using the four conditions in column B of Figure 1) in order to evaluate these relationships for the secondary stimulus attributes of interest in the present study. Correlations between theta and delta for each of these measures, as well as the correlations between FN and P300 for the same conditions for comparison, are detailed in Table 1.
Consistent with our previous report (Bernat et al., 2011), overall theta and delta component average measures were modestly correlated, suggesting that while theta and delta do share some variance, they are not simply yoked expressions of the same underlying process in the data. Indeed, the gain-loss difference scores for theta and delta were not significantly correlated, indicating that they index separable processes related to Gain and Loss feedback registration. Further, for each of the four measures presented in Table 1 time-frequency theta and delta were significantly less correlated than the time-domain FN and P300 counterparts (component averages, ZPF = 4.60, p < .001; gain-loss, ZPF = 2.63, p < .008; correct-error, ZPF = 4.61, p < .001; large-small, ZPF = 3.79, p < .001), in addition to the fact that theta/delta correlations for each of the three condition difference measures were non-significant while the FN/P300 correlations were significant. This supports the hypothesis that the time-frequency theta and delta measures indexed more independent activity than the time-domain FN and P300 measures.
Regressions
Table 2 shows four pairs of regression analyses, with one member of each pair using FN as the dependent variable and the other using P300. For each regression, theta and delta measures served as the two independent variables, entered simultaneously in one regression model. Each pair focused on one of the ERP measures (i.e., grand average amplitude, and gain-loss, correct-error, or large-small condition differences). These regression analyses were conducted to assess whether the FN and P300 components can be understood of mixtures of delta and theta activity for each of the assessed measures. This hypothesis was supported in each case, where theta and delta accounted for a significant and substantial portion of variance in each time-domain measure, and each contributed uniquely to the prediction of FN and P300.
Table 2.
Standardized Beta and t Parameters, and Model F and R2 Values, from Regression Models Predicting either FN or P300 with Theta and Delta
| Component Averages |
Gain-Loss Differences |
Correct-Error Differences |
Large-Small Differences |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Variable | Beta | t | Beta | t | Beta | t | Beta | t |
| FN | Theta | −.17 | −2.90** | −.62 | −11.22*** | −.16 | −2.21* | −.21 | −3.31** |
| Delta | .77 | 13.01*** | .34 | 6.16*** | .40 | 5.44*** | .65 | 10.36*** | |
| F | 86.12*** | 94.61*** | 18.47*** | 55.67*** | |||||
| Adj. R2 | .54 | .56 | .19 | .43 | |||||
| P300 | Theta | .20 | 7.54*** | .37 | 7.03*** | .22 | 4.29*** | .22 | 4.30*** |
| Delta | .87 | 32.69*** | .74 | 13.84*** | .78 | 15.10*** | .72 | 13.95*** | |
| F | 712.05*** | 108.13*** | 118.27*** | 116.00*** | |||||
| Adj. R2 | .91 | .59 | .61 | .61 | |||||
Analysis of Primary and Secondary Stimulus Attributes
The next set of analyses was conducted to evaluate the extent to which theta and delta measures were sensitive to the primary and secondary stimulus attributes as defined in figure 1A and 1B. This was accomplished using the RM-ANOVA approach defined in the data analysis section above.
Analysis A: Relative outcome (error/correct)
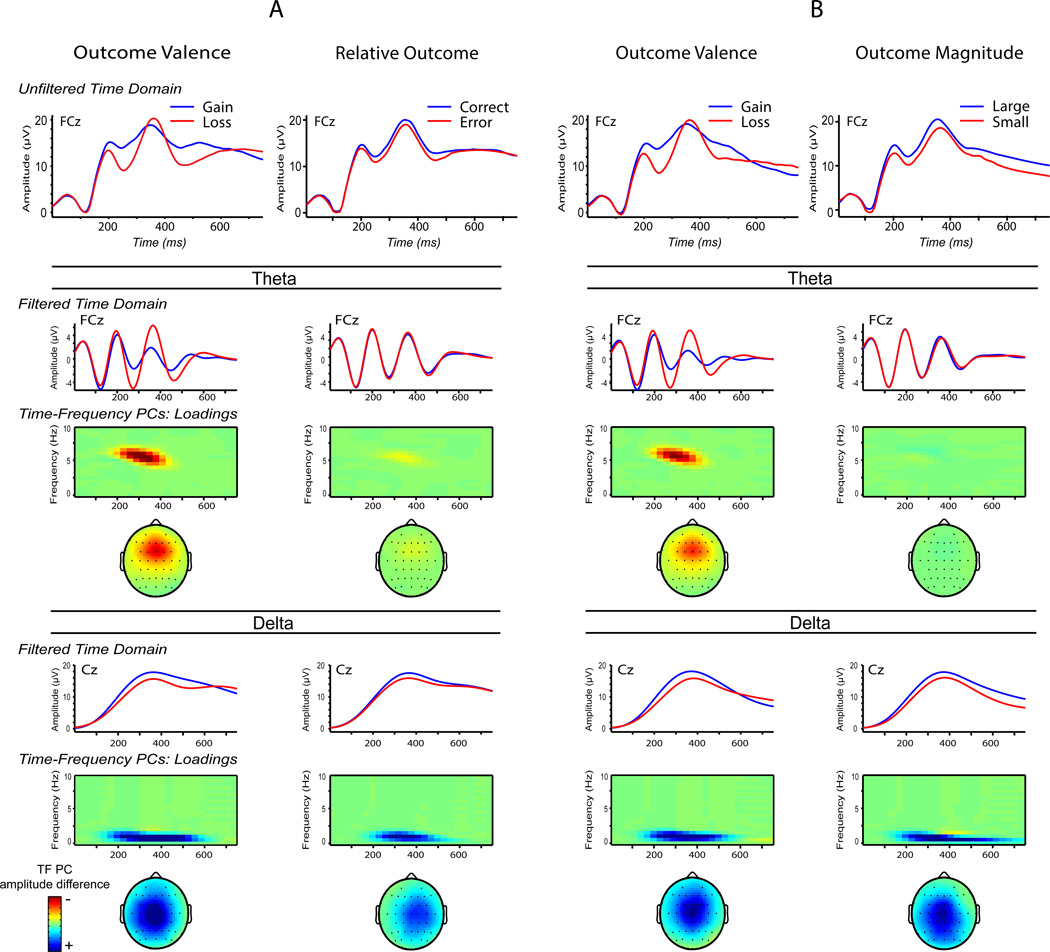
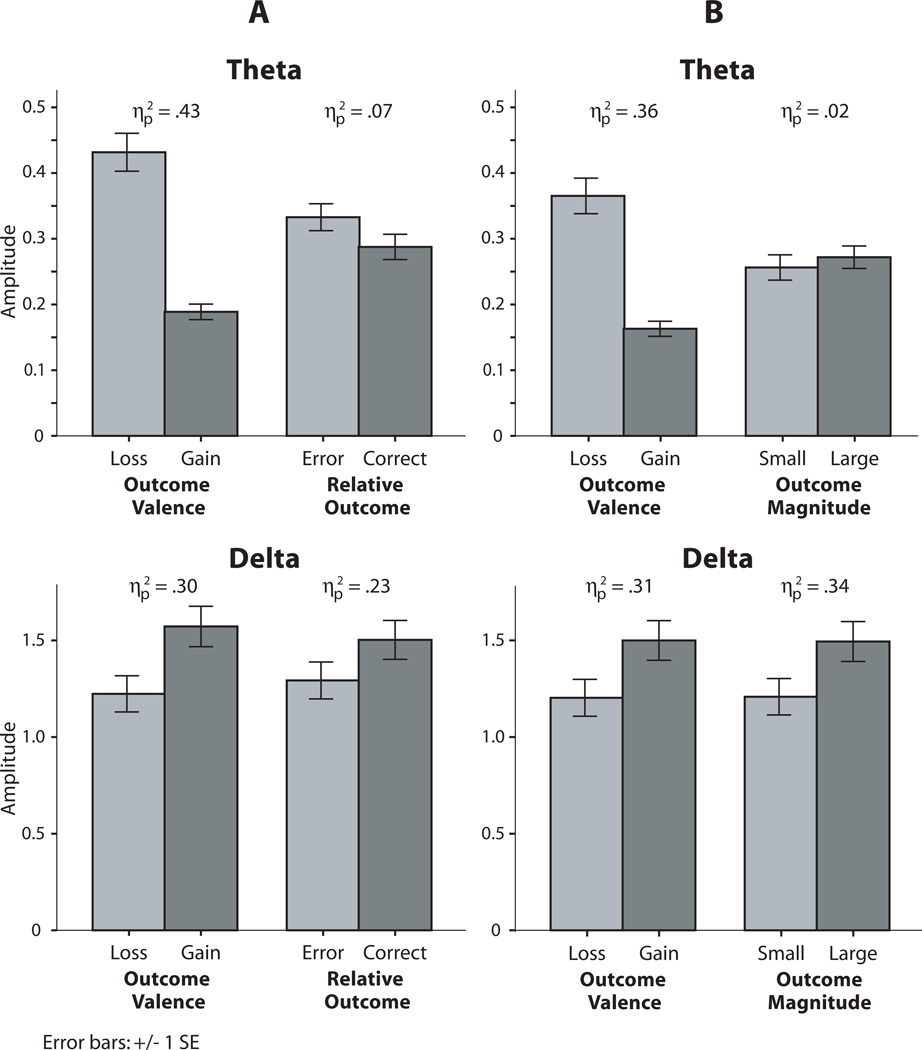
Theta and delta effects are presented in waveforms in Figure 4a, in bar charts in Figure 5a, and in statistical analyses in Table 3. First, both theta and delta evidenced significant main effects for both primary valence and secondary relative outcome stimulus attributes, in opposite directions. That is, theta increases were associated with primary loss valence and relative error outcomes, whereas delta increases were associated with primary gain valence and relative correct outcomes. This difference in direction is apparent in the waveforms and bar charts. Next the relative sensitivity of theta to the primary versus secondary stimulus attributes was evaluated. Consistent with hypotheses, the magnitude of the primary valence main effect (ηp2 = .43) was much larger than that of the secondary relative outcome difference (ηp2 = .07). To statistically test this a priori comparison, a t-test was conducted between these differences (i.e., bad-good versus error-correct for theta), which confirmed that that theta valence differences were significantly larger than theta relative outcome differences, t(148) = 7.62, p < .001. Next, these differences were compared between theta and delta, to evaluate the a priori hypothesis that these primary-secondary differences would be greater for theta than delta, i.e., theta ([bad-good] – [error-correct]) versus delta ([bad-good] – [error-correct]), which was supported, t(148) = 5.74, p < .001. Notably, beyond evidencing significantly smaller differences than theta between primary and secondary stimulus attributes, delta did not significantly differ in sensitivity between the primary (Valence) and secondary (Relative Outcome) stimulus attributes. Overall, there are two important results for theta and delta differences: 1) theta was increased for negative (loss and error) outcomes, while delta was increased for positive (gain and correct) outcomes, and 2) theta was significantly more sensitive to the primary (versus secondary) stimulus attributes than delta, whereas delta was similarly sensitive (not significantly different) to primary and secondary stimulus attributes.
Figure 4.
Time-domain waveforms and time-frequency surfaces are presented for the conditions. Portion A contains activity in response to the stimuli in Figure 2A, including Outcome Valence (loss vs. gain) and Relative Outcome (error vs. correct) stimulus attributes in the first and second labeled columns, respectively. Portion B contains activity in response to the stimuli in Figure 2B, including Outcome Valence and Outcome Magnitude (big vs. small, 25 vs. 5) stimulus attributes. For both A and B subsections, the upper row contains unfiltered time-domain waveforms for the conditions relevant to the corresponding column. The theta and delta sections just below the unfiltered waveforms contain filtered time-domain waveforms at the top, corresponding time-frequency condition difference surfaces in the middle, and topographical distribution of the condition differences in the lower part. Frontocentral theta activity corresponds most closely to time-domain FN and shows enhancement for loss outcomes relative to gains, in the Outcome Valence columns of analyses A and B, but qualitatively less for the secondary analyses (Relative Outcome in A and Outcome Magnitude in B). Centroparietal delta activity corresponds most to time-domain P300, but unlike theta, shows similar enhancements across all primary and secondary stimulus attributes – gain, correct, and large magnitude outcomes.
Figure 5.
Bar charts depicting condition difference main effects for theta and delta from each RM-ANOVA model. Subsection A contains mean activity in response to the stimuli in Figure 2A, including Outcome Valence (loss vs. gain) and Relative Outcome (error vs. correct) stimulus attributes in the first and second columns, respectively. Subsection B contains mean activity in response to the stimuli in Figure 2B, including Outcome Valence and Outcome Magnitude (big vs. small, i.e., 25 vs. 5) stimulus attributes. It can be seen here that theta was modulated by the primary (salient) stimulus parameter (enhanced for loss), but not by the secondary stimulus attributes (Relative Outcome and Outcome Magnitude). Delta was more equally modulated across the comparison types – by the primary stimulus parameters (Outcome Valence, enhanced for gain) and by the secondary feedback characteristics (relative outcome, enhanced for correct, and outcome magnitude, enhanced for large).
Table 3.
Outcome Valence (Loss vs. Gain) × Relative Outcome (Error vs. Correct) RM-ANOVA
| Theta | Delta | ||||
|---|---|---|---|---|---|
| df | F | ηp2 | F | ηp2 | |
| Valence | 1,147 | 108.9*** | .43 | 62.9*** | .30 |
| Relative Outcome | 1,147 | 11.3** | .07 | 43.0*** | .23 |
| Interaction | 1,147 | 1.3 | .01 | <1 | < .01 |
Note.
p < .05;
p < .01,
p < .001
Analysis B: Reward magnitude (large/small)
Theta and delta effects are presented in waveforms in Figure 4b, in bar charts in Figure 5b, and in statistical analyses in Table 4. First, theta and delta evidenced the same relationship to Valence as found in Analysis A: theta was increased to loss stimuli, while delta was increased to gain stimuli. For the critical reward magnitude effect, however, only significant for delta, where larger amplitude was associated with larger outcomes. Thus, for theta, only the primary Valence effect was observed, whereas for delta, both primary valence and secondary reward magnitude effects were observed (where delta effects on primary and secondary attributes were similar in magnitude, ηp2 = .31 versus .34).
Table 4.
Outcome Valence (Loss vs. Gain) × Reward Magnitude (Large vs. Small) RM-ANOVA
| Theta | Delta | ||||
|---|---|---|---|---|---|
| df | F | ηp2 | F | ηp2 | |
| Valence | 1,147 | 81.4*** | .36 | 66.2*** | .31 |
| Magnitude | 1,147 | 2.5 | .02 | 77.5*** | .34 |
| Interaction | 1,147 | 1.1 | .01 | 1.6 | .01 |
Note.
p < .05;
p < .01,
p < .001
Discussion
Previous research on feedback processing demonstrated that FN and P300 both tap cognitive processes responding to gambling outcomes, but a measurement problem (component overlap between FN and P300) limited progress to identify the range of attributes that modulate each measure individually. Our recent work suggested that time-frequency theta and delta index feedback processes thought to be associated with FN and P300 (respectively) but with greater specificity than their time-domain counterparts (Bernat et al., 2011). This prior work demonstrated the independent effects of primary (most salient) feedback attributes (gain vs. loss in this experimental task) on theta and delta, but no study using time-frequency has characterized the influence of secondary (less salient) feedback attributes (i.e., outcome magnitude and comparisons with alternative options not chosen here). Given that time-frequency approaches have been able to better parse responses related to primary dimensions (i.e., gain and loss) than time-domain approaches, the goal of the current study was to use time-frequency theta and delta to evaluate secondary attributes in hopes of further elucidating the functional significance of these measures.
Results supported the view that the theta, like time-domain FN, indexes an initial evaluation of the primary feedback attributes, while delta reflects more elaborative processing involving both primary and secondary feedback attributes. Further, while theta appears to be primarily sensitive to negative outcomes (loss, and to a lesser extent errors), delta is most sensitive to positive outcomes (i.e., both gain and correct). It is worth noting that the present results are consistent with the suggestion that the FN, as indexed by theta, will be most sensitive to whatever attribute of the stimuli was made primary (by virtue of the task emphasizing different attributes; cf. Nieuwenhuis et al., 2004).
These findings also provide additional support for initial indications by Bernat et al. (2011) that delta reflects a reward-sensitive component of feedback processing. This is consistent with other work in the field based on similar gambling feedback ERPs, identifying a positivity associated with reward occurring at the same time as the increased FN-negativity associated with loss (Baker & Holroyd, 2011; Foti, et al., 2011; Holroyd, et al., 2008), Further, the present results extend our previous work on the role of delta to show how, unlike the relatively simple theta-FN response, delta appears to reflect more elaborative processing of the feedback outcome (e.g., relative outcome and outcome magnitude) beyond the primary salient attribute of the feedback. Perhaps this is not surprising, given that delta made substantially larger contributions to P300 than theta in the assessed regression analyses, and P300/P3 (broadly defined) has been found to be associated with a vast array of stimulus attributes and condition differences during the long tradition of time-domain P300 ERP research.
Relationship Between Time-Domain and Time-Frequency Measures
The present results support the idea that time-frequency approaches can better parse components of feedback processing than traditional time-domain measures, particularly the secondary attributes. First, the time-domain FN/P300 correlations were significantly larger than the time-frequency theta/delta correlations – and theta and delta were not significantly related at all for the condition differences. This indicates that the time-domain measures index shared processes (presumably theta and delta contributions to each), which the separated time-frequency theta and delta measures do not. Additionally, in the regression analyses predicting the FN and P300, theta and delta accounted for unique variance in the component grand averages as well as each of the effects evaluated. This provides evidence that the time-domain measures in the current data can be viewed as mixtures of the time-frequency theta and delta measures. Together, these analyses support the view that time-frequency measures better index separable processes, which were confounded in the time-domain FN and P300 measures.
In addition to the conceptual nuances discussed with regard to reward processing, the present results advance the methodological understanding of the phase (polarity) of theta and delta as they relate to FN and P300. Recall that the phase (polarity) in theta and delta are related to FN and P300 in distinct ways. Theta is associated with the negative-going deflection of the FN and the positive-going deflection in P300. Conversely, delta is associated with a positive-going deflection at both the FN and P300. Replicating and extending findings from Bernat et al. (2011), theta phase appeared to be a primary mechanism underlying the observed relationship between time-frequency theta and delta and time-domain FN and P300 measures, but now for both the primary and secondary feedback attributes. Given the consistent patterns observed, we can infer two key factors that seem important for understanding the impact of the dynamics of theta and delta phase (polarity) on FN and P300 measures more broadly.
First, is the direction of condition effects. In the valence effects, for example, theta is increased to loss while delta is increased to gain, creating enhanced gain-loss differences at the FN and attenuated effects at P300 (Bernat et al., 2011). However, if theta and delta were instead both increased for losses, we would expect to see a muted difference at FN and an enhanced difference at P300. Indeed, we recently found exactly this effect when assessing theta and delta activity underlying N2 and P3 time-domain components in a go/no-go task (Harper, Malone, & Bernat, 2014). Specifically, while theta and delta activity were largely independent from each other as in the current report (although theta and delta go/no-go differences were modestly correlated, r = .27), both were robustly positively associated with the no-go condition relative to go (unlike the inverse relationships between theta/delta and gain/loss in the current report with gambling feedback ERPs). Critically, the associated time-domain results evidenced non-significant go/no-go differences for the N2 component and enhanced amplitude differences for the P3 component, as this hypothesis with regard to the dynamics of phase (polarity) would predict. Together, these findings provide increasing support for the idea that similar theta/delta dynamics of phase (polarity) are at work in common ERP components from other tasks, obscuring findings that could be better modeled in time-frequency theta and delta measures.
Second, is the relative weight of theta versus delta sensitivity to a given experimental manipulation. For example, if only one is modulated, or is much stronger than the other, then it will drive the observed FN and P300 amplitudes. This was the case with the secondary relative outcome and magnitude effects in the current study, which were associated only with delta. The idea that the relative weight of two subcomponents may underlie P300 has been detailed in work identifying two spatiotemporal subcomponents occurring during P300 (Spencer, Dien, & Donchin, 2001). One component is more anterior (Novelty-P3, maximal during novel stimuli), and the other is more posterior (P300, maximal during target stimuli), but both components occur in both conditions, with different weights. It would be of interest to assess whether the observed theta/delta dynamics in the current study map onto these spatiotemporal components, as suggested by work by Demiralp and colleagues indicating that theta contributes more to the P3a and delta more to the P3b (Demiralp, et al., 2001).
Limitations and Future Directions
Although not evaluated in the current study, another factor that would be interesting to evaluate with respect to theta and delta is the role of expectancies. Primary reinforcement learning conceptualizations of the FN involve expectancy violations (Holroyd & Coles, 2002; Holroyd & Yeung, 2012), and interesting new theories suggest expectancy may have a more broad role in the FN (Alexander & Brown, 2011). Similarly, there are a number of studies indicating that feedback-P300 is also more sensitive to unexpected outcomes (Bellebaum & Daum, 2008; Donchin, 1981).
Another more practical limitation of the current study is that only one theta and one delta component was extracted from each band. This was done to provide a parsimonious representation with strong explanatory power. However, extracting more than one principal component within each band could provide differentiation among processes in more complex experimental tasks.
Finally, in future studies, it may be of interest to directly manipulate parameters expected to selectively modulate theta or delta activity. For example, new experiments could be designed to manipulate different rewarding aspects of feedback stimuli, to assess how robust the present delta-gain effect is, and to make inferences about how delta is related to a more broad range of reward manipulations, and whether such relationships are also independent of theta.
Acknowledgments
This work was supported by grants MH080239, MH65137, and MH088143 from the National Institute of Mental Health and by funds from the Hathaway endowment at the University of Minnesota – Twin Cities.
References
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature neuroscience. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology. 2011;87(1):25–34. doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. International Journal of Psychophysiology. 1992;13(2):161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Demiralp T, Schurmann M, Başar E. Topological distribution of oddball 'P300' responses. International Journal of Psychophysiology. 2001;39(2–3):213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27(7):1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64(1):62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of abnormal psychology. 2011;120(2):352. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clinical Neurophysiology. 2005;116(6):1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano Vazquez L, Allen JJB. Theta lingua franca: A common mid frontal substrate for action monitoring processes. Psychophysiology. 2011;49(2):220–223. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroglu C, Başar E. Wavelet analysis of oddball P300. International Journal of Psychophysiology. 2001;39(2–3):221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!… surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. Errors, conflicts, and the brain. Current opinions on performance monitoring. 2004;14:20. [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event related potential, its associated time frequency components, and externalizing psychopathology. Psychophysiology. 2010;47(1):123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer JP, Woldorff MG, Huettel SA. Rapid electrophysiological brain responses are influenced by both valence and magnitude of monetary rewards. Journal of Cognitive Neuroscience. 2008;20(11):2058–2069. doi: 10.1162/jocn.2008.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Wu T, Jiang Y, Luo YJ. Woulda, coulda, shoulda: The evaluation and the impact of the alternative outcome. Psychophysiology. 2011 doi: 10.1111/j.1469-8986.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42(2):161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It's worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44(6):905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18(4):326. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP component activity in a go/no-go task. Clinical Neurophysiology. 2014;125:124–132. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research. 2006;1105(1):93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad Vaezi KL, Krigolson OE. The feedback correct related positivity: Sensitivity of the event related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences. 2012;16(2):122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10(2):371–375. [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011:1–10. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus--norepinephrine system. Psychological Bulletin. 2005;131(4):510. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cerebral Cortex. 2004;14(7):741. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Pfabigan DM, Alexopoulos J, Bauer H, Sailer U. Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event related brain potentials. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan T, Rosenthal R, Rubin DB. Comparing correlated but nonoverlapping correlations. Psychological Methods. 1996;1(2):178. [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, Kuboki T. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16(4):407. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38(2):343–358. [PubMed] [Google Scholar]
- van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal Oscillatory Dynamics Predict Feedback Learning and Action Adjustment. Journal of Cognitive Neuroscience. 2011;23(12):4106–4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain research. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. The Journal of Neuroscience. 2004;24(28):6258. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yu R, Zhou X. To do or not to do? Action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia. 2010;48(12):3606–3613. doi: 10.1016/j.neuropsychologia.2010.08.010. [DOI] [PubMed] [Google Scholar]