Abstract
To test the hypothesis that ultraviolet B (UVB) can activate the hypothalamic-pituitary-adrenal (HPA) axis, the shaved back skin of C57BL/6 mice was exposed to 400 mJ/cm2 of UVB or was shame irradiated. After 12 and 24 h of exposure, plasma, skin, brain, and adrenals were collected and processed to measure corticotropin-releasing hormone (CRH), urocortin (Ucn), β-endorphin (β-END), ACTH and corticosterone (CORT) or brain was fixed for immunohistochemical detection of CRH. UVB stimulated plasma levels of CRH, Ucn, β-END, ACTH and CORT, and increased skin expression of Ucn, β-END and CORT at the gene and protein/peptide levels. UVB stimulated CRH gene and protein expression in the brain that was localized to the paraventricular nucleus of the hypothalamus. In adrenal glands it increased mRNAs of melanocortin receptor type 2, StAR and CYP11B1. Hypophysectomy abolished UVB stimulation of plasma but not of skin CORT levels, and had no effect on UVB stimulation of CRH and Ucn levels in the plasma, demonstrating the requirement of an intact pituitary for the systemic effect. In conclusion, we identify mechanism of the regulation of body homeostasis by UVB through activation of the HPA axis that originates in the skin and requires pituitary for the systemic effect.
Keywords: skin, pituitary, hypothalamus, CRH, POMC, corticosterone
Introduction
Skin with underlying subcutis is armed with neuroendocrine capabilities and represents the largest and one of the most complex organs in the human body (Slominski et al., 2012). Strategically located at the interface with external environment, skin detects, integrates, and responds to stressors including ultraviolet radiation (UVR) (Fritsche et al., 2007; Paus et al., 2006; Slominski and Wortsman, 2000; Slominski et al., 2012; Slominski et al., 2013b). UVB (290–320 nm) radiation has powerful biological actions not only on cutaneous biology but it also impacts many regulatory pathways involved in immune homeostasis that are both vitamin D-dependent and independent (Becklund et al., 2010; Campbell et al., 1993; Fritsche et al., 2007; Holick, 2003; Krutmann et al., 2012; Ndiaye et al., 2013; Pawelek et al., 1992; Schwarz et al., 2011). The mechanisms of UVB-induced immune suppression (Vink et al., 1997) are not completely understood. However, evidence is accumulating that DNA damage and other mechanisms such as the photoisomerization of urocanic acid, free-radical formation, and signal transduction-mediated activation of transcription factors and induction of neuroendocrine signaling may also contribute to the resulting pathological conditions as well (Fritsche et al., 2007; Ndiaye et al., 2013; Slominski and Pawelek, 1998; Slominski et al., 2012; Vink et al., 1997).
The main regulatory algorithm to maintain body homeostasis is the hypothalamic-pituitary-adrenal (HPA) axis, which requires activation of a complex range of responses involving the endocrine, nervous, and immune systems, collectively known as the stress responses (Chrousos, 2009; Selye, 1976; Smith and Vale, 2006; Vale et al., 1981). Neural signals encoded by the limbic system as stressors trigger neurons of the paraventricular nucleus (PVN) of hypothalamus to produce and release corticotropin releasing hormone (CRH) into the hypophyseal portal circulation (Smith and Vale, 2006; Vale et al., 1981). Next, CRH binds to CRH receptor type 1 (CRH-R1) on pituitary corticotropes, and induces the release of POMC-derived adrenocorticotropic hormone (ACTH) into the systemic circulation. The melanocortin receptor type 2 (MC2R), expressed in the adrenal cortex, stimulates glucocorticoid (GC) synthesis and secretion after binding of ACTH (Chrousos, 2009; Miller and Auchus, 2011; Smith and Vale, 2006). GC (i.e., cortisol (COR) in humans and corticosterone (CORT) in rodents) maintain metabolic and stress-responses, suppress immune activity and are self-regulated, with negative feedback to the hypothalamus and pituitary to mute the HPA axis (Chrousos, 2009; Miller and Auchus, 2011).
The skin has neuroendocrine capabilities that also encompasses all elements of the “cutaneous HPA axis” that follow the organization of the central HPA axis [reviewed in (Slominski et al., 2007)]. This concept was based on the evidence that vertebrate skin expresses CRH with functional CRH-R1 [reviewed in (Slominski et al., 2013b)] and related POMC macromolecule, which is further processed to ACTH (Ito et al., 2005; Schauer et al., 1994; Slominski et al., 1998; Slominski et al., 1992; Slominski et al., 2000c), which, after interaction with MC2R, induces steroidogenesis with the final production of highly adaptable COR or CORT (Cirillo and Prime, 2011; Ito et al., 2005; Skobowiat et al., 2011; Slominski et al., 2000a; Slominski et al., 2013a; Slominski et al., 2005a; Slominski et al., 2005b; Slominski et al., 2006a; Vukelic et al., 2011). Furthermore, Hiramoto et al (Hiramoto et al., 2003) have demonstrated that exposure of the eye to the UVB increases plasma α-MSH levels with systemic stimulation of epidermal melanocytes.
Based on the above we have decided to test the hypothesis that UVB acting on the skin can regulate body homeostasis through activation of central HPA. Using mouse model we evidence that UVB activates the HPA axis both on the local (skin) and systemic (brain, adrenal and plasma) levels, with a latter requiring an intact pituitary. The UVB induced increases in corticosterone production explain immunosuppressive effects of the UVB, while that of β-endorphin could explain a phenomenon of “UV addiction”.
Results
General design of UVB exposure
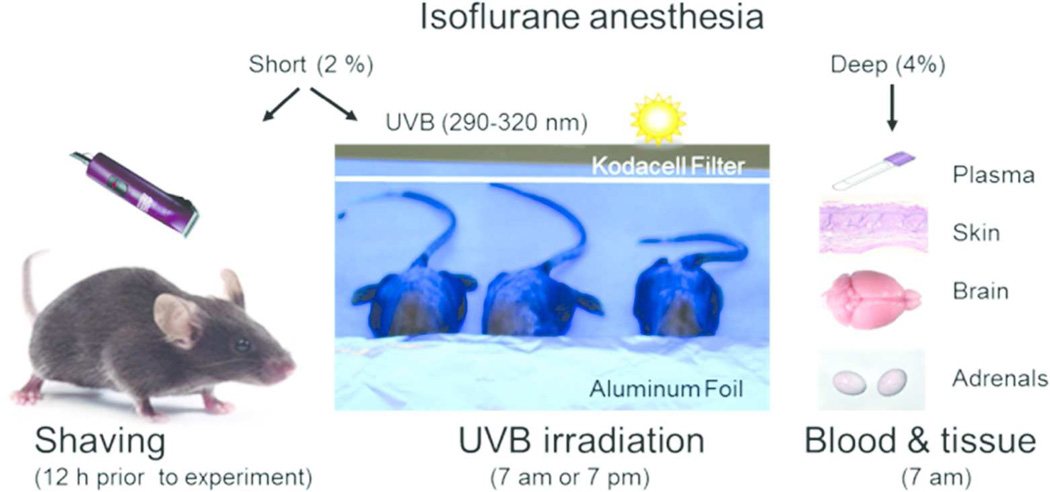
To prevent retinal or non-retinal eye signal transmission, the heads including eyes, were covered with aluminum foil, and the skin on the back was irradiated with UVB (Figure 1).
Figure 1.
Outline of the experimental design. Under short (max. 1 min) vapor isoflurane (2 %) anesthesia, the back skin was shaved with animal clippers 12 h before the experimental procedures. The next day, animals were repeatedly anesthetized, eyes covered with aluminum foil (to prevent retinal signal transmission), and the skin on the back was irradiated with UVB (400 mJ/cm2 for most experiments), either at 7 am (24 h group) or at 7 pm (12 h group). These optimal doses and times after exposure were based on initial testing of UVB at ranges of 100 – 400 mJ/cm2 vs control and time post-exposure of 3, 6, 12 and 24 hours.
Dose and Time Dependent Effects of UVB in the Skin
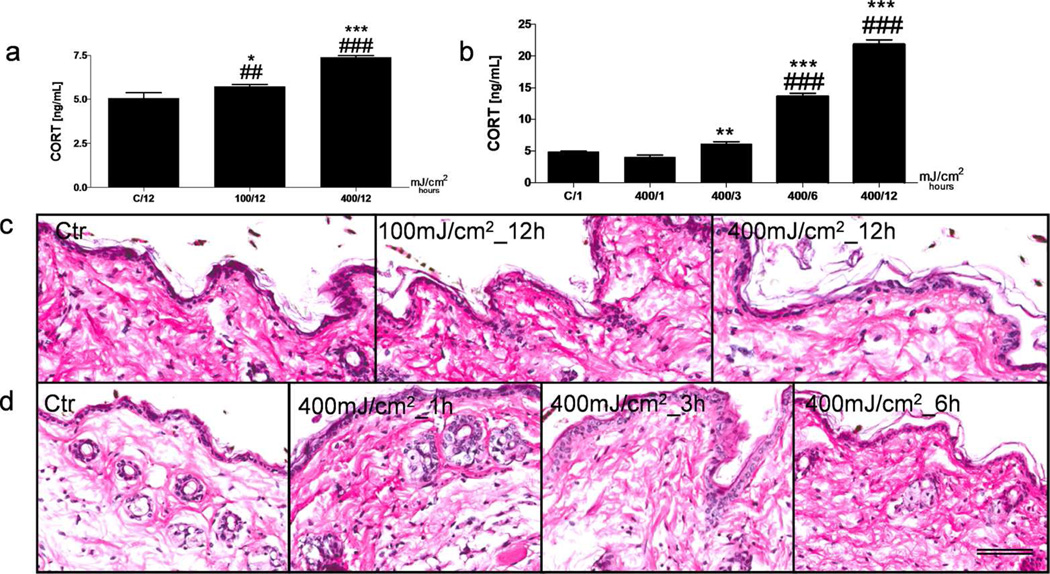
Previously, we have documented that UVB (290–320 nm), but not less energetic UVA (320–400 nm), is effective in stimulating HPA axis elements in human and mouse skin organ culture in vitro (Skobowiat et al., 2011; Skobowiat et al., 2013a; Skobowiat et al., 2013b). In current experiments we first measured the CORT in the skin in vivo using different doses and time after UVB exposure and found that the dose of 400 mJ/cm2 (2.1 minimal erythema doses (MED), Table S1) and 12 and 24 h after exposure were most optimal for enhancement of the CORT levels (Figure 2a,b). Lower dose (100 mJ/cm2) and shorter times of observation (3 and 6 h) showed significantly lower stimulatory effect. Similarly, markedly stronger stimulation of plasma CORT levels was observed at 400 in comparison to 100 mJ/cm2 and at 12 in comparison to 3 h after UVB exposure (Figure S1). The histopathological analysis demonstrated that UVB at 400 mJ/cm2 did not produce noticeable epidermal necrosis nor trigger marked/moderate inflammatory infiltrate (Figure 2c). However, small increase in infiltrating neutrophils and eosinophils was observed at 1–6 h after UVB exposure, which after 12 or 24 h returned to the control (Figure 2d). Therefore, based on the previous (Skobowiat et al., 2013a) and current in vivo experiments we have chosen the dose of 400 mJ/cm2 (2.1 MED) and a time of 12 and 24 h after UVB exposure for further experiments.
Figure 2.
Time and dose-dependent changes in CORT production and skin histological evaluation after UVB radiation.
Dose- (a) and time- (b) dependent increases in CORT production after UVB exposure in C57BL6 shaved mouse skin, evaluated with ELISA. A dose-dependent histological evaluation observed 12 h after UVB irradiation with a dose of 100 and 400 mJ/cm2 (c). Time-dependent histological changes evolved after 1, 3 and 6 h followed by UVB exposure of 400 mJ/cm2 (d). H&E staining on formalin fixed and paraffin embedded skin representative sections. Data were analyzed using Student’s t-test, * p<0.05, ** p<0.01, and *** p<0.001 or one-way ANOVA, ## p<0.01; ### p<0.001. Scale bar = 100 µm.
UVB effects on cutaneous expression of CRH and Ucn
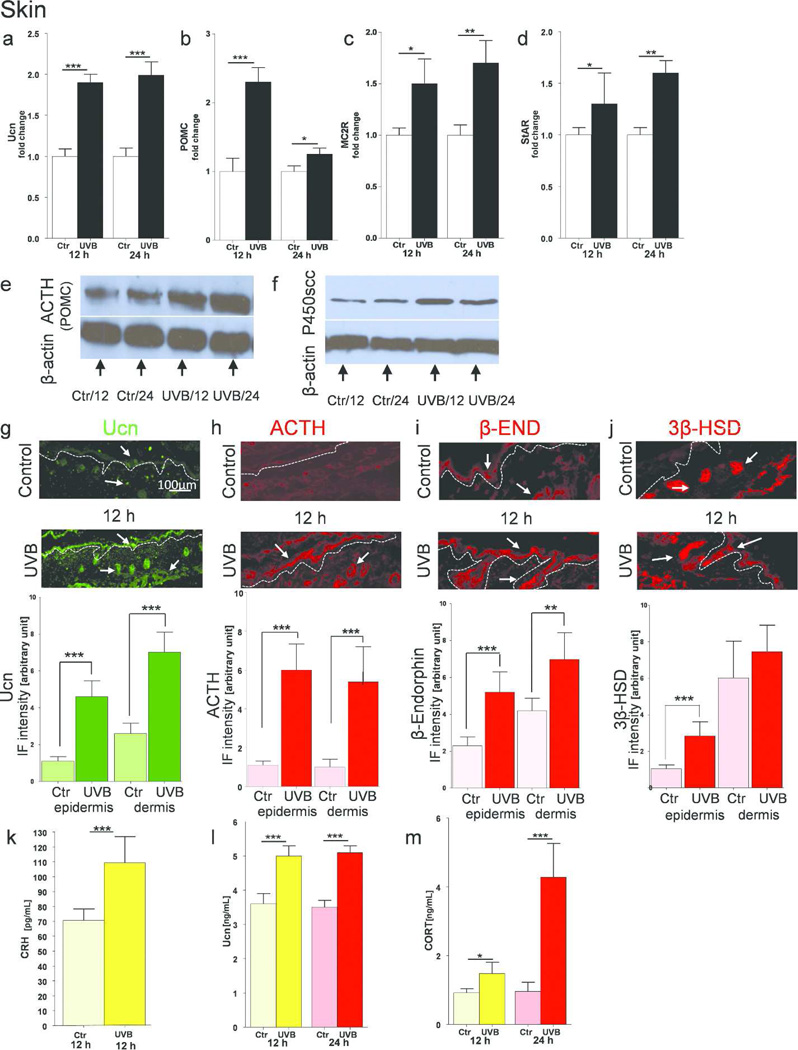
Since the CRH gene is not expressed in C57BL/6 skin (Slominski et al., 2001; Slominski et al., 2013b), and can be replaced by Ucn in activating HPA axis elements (Slominski et al., 2000b; Slominski et al., 2013b), we examined Ucn cutaneous expression as a potential triggering regulator. We also measured the concentration of CRH since this peptide can be released locally from cutaneous nerve fibers (Roloff et al., 1998; Slominski et al., 1996b). UVB radiation stimulated the Ucn expression at the gene (Figure 3a) and peptide levels (Figure 3l) with the similar patterns at 12 and 24 h post-radiation. In situ localization studies showed increased expression of Ucn in the main skin compartments including the epidermis, adnexal structures and stratum paniculosum (Figure 3g). There was also an increase in CRH peptide concentration in the skin after UVB exposure, as evaluated by ELISA (Figure 3k).
Figure 3.
Cutaneous equivalent of the HPA axis in C57BL6 mice is stimulated upon UVB radiation.
Expression of genes coding Ucn (a), POMC (b), MC2R (c) and StAR (d) after UVB exposure compared to control (shame-treated) animals. Data presented as fold change ± SD. Protein estimation with Western Blot for ACTH/POMC (e) and P450scc (f). In situ expression of Ucn (g), ACTH (h), β-END (i) and 3β-HSD (j) antigens measured by immunofluorescence with corresponding quantification of immunopositive signal intensity (inserts to the subpannels). Arrows indicate examples of positive signals. ELISA evaluation of peptide CRH (k), Ucn (l) and steroid CORT (m) concentrations. Data are presented in pg or ng/mL per 4 µg of total proteins extracted, and analyzed using Student’s t-test, * p<0.05, ** p<0.01, and *** p<0.001.
UVB effects on cutaneous expression of POMC, ACTH and β-END
Next, we checked cutaneous POMC expression, a “pituitary” element of the systemic HPA axis. UVB light stimulated a 2.5-fold increase in expression of POMC mRNA after 12 h, and which was still present after 24 h, although at lower levels (Figure 3b). Immunoblotting with antibodies directed against ACTH, which recognize the 33 kDA POMC precursor, confirmed increased expression of this molecule (Figure 3e). UVB-induced increased ACTH production was also confirmed with quantitative IHC (Figure 3h) as was expression of POMC-derived β-END stimulated by UVB (Figure 3i).
UVB effects on cutaneous expression of MC2R, CYP11A1, StAR, 3β-HSD and CORT
The expression of the next crucial element of the HPA axis, the MC2R (responsible for initiation of steroidogenesis upon ACTH activation), was upregulated 1.5 times after 12 h and almost two times after 24 h (Figure 3c). We also investigated StAR gene expression, which is required to transfer cholesterol from the outer to the inner mitochondrial membrane, and showed that its up-regulation occurred after 12 and 24 hrs post-UVB exposure (Figure 3d). Moreover, we evaluated the expression of the rate-limiting enzyme of steroidogenesis, the cytochrome P450scc (CYP11A1), which cleaves the cholesterol side chain to produce pregnenolone, a precursor of all steroids. Western blot analysis revealed high expression of P450scc at 12 h and 24 h post-UVB exposure, compared to appropriate controls (Figure 3f). Furthermore, immunohistochemistry for 3β-HSD, (the enzyme that transforms pregnenolone to progesterone), showed that this antigen is highly expressed in cutaneous adnexal structures and in the stratum paniculosum, but weakly expressed in the epidermis of untreated skin. UVB radiation enhanced 3β-HSD expression, especially in epidermal cells (Figure 3j). The final product of HPA axis activation, CORT was produced at significantly high level at 12 h and increased further at 24 h after UVB irradiation (Figure 3m).
UVB effects on CRH expression in the Hypothalamus
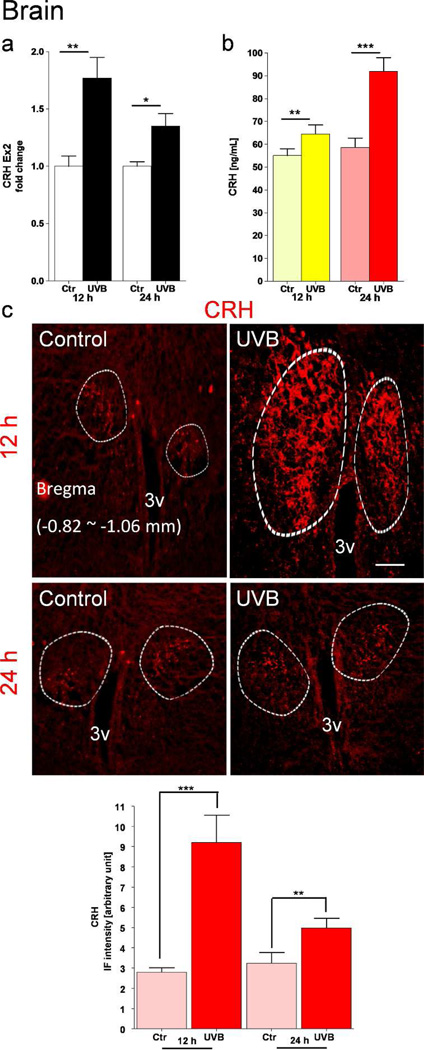
The area corresponding to the hypothalamus (Bregma ~ −0.34 to −2.70 mm) was dissected out and processed either for CRH mRNA expression or peptide measurements. There was a significant increase of CRH gene expression (Figure 4a) and CRH peptide production (Figure 4b) both 12 and 24 h after UVB exposure. Furthermore, immunohistochemistry showed an increased number of CRH-immunopositive neurons and nerve fibers surrounding the PVN area. The highest immunopositive signal was observed at 12 h after UVB exposure, and its relative values are calculated and presented as an insert to Figure 4c.
Figure 4.
Up-regulation of CRH expression in the murine hypothalamus after exposure of shaved back skin to UVB. CRH gene expression is shown as fold change ± SD (a). CRH peptide was measured by ELISA (b). Data are presented in pg/mL in relation to the same protein concentration (28 µg/µL). CRH immunoreactivity in situ in the PVN was evaluated by immunofluorescence (c). Circled areas show in situ localization of CRH antigen visualized in perikarya and nerve fibers. Calculation of positive signals intensity is presented on the graph in the lower panel c. Data are analyzed using t-test, * p<0.05, ** p<0.01, and *** p<0.001. Scale bar = 100 µm.
Changes in Adrenals after UVB exposure
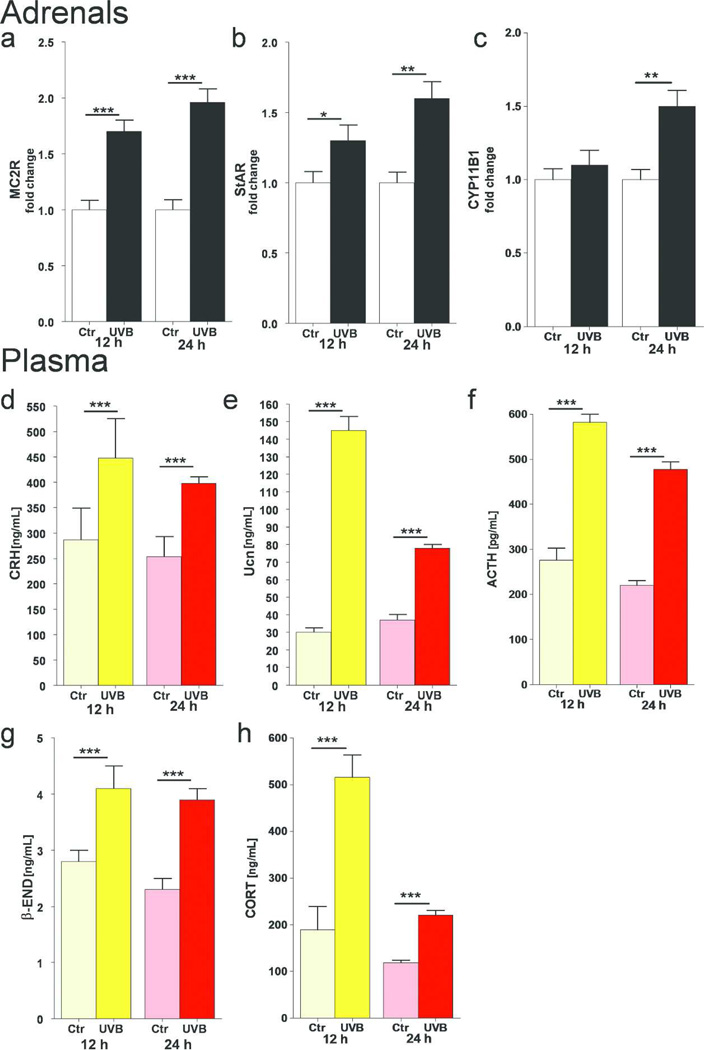
QPCR analyses showed that MC2R mRNA was up-regulated after 12 and 24 h of UVB radiation (Figure 5a). Similarly, StAR mRNA was increased at the same time points (Figure 5b). Expression of the CYP11B1 gene was also up-regulated but only after 24 h of UVB exposure (Figure 5c).
Figure 5.
UVB stimulates the systemic HPA axis in C57BL/6 mice.
UVB stimulated expression of gene coding MC2R (a), StAR (b) and CYP11B1 (c) in C57Bl/6 adrenals. Data are presented as fold changes ± SD. UVB enhanced plasma concentrations of CRH (d), Ucn (e), ACTH (f), β-END (g), and CORT (h). Data are presented as pg or ng/mL after appropriate dilutions were performed separately for each assay and analyzed using t-test, * p<0.05, ** p<0.01, and *** p<0.001.
Changes in Plasma after UVB exposure
CRH peptide content was highly increased at 12 and 24 h after UVB irradiation, compared to that in sham-irradiated animals (Figure 5d). A similar effect was observed for Ucn (Figure 5e). ACTH concentrations were markedly stimulated at 12 and 24 h after UVB exposure Figure 5f). Interestingly, UVB enhanced plasma concentrations of another peptide, β-END (Figure 5g), that results from POMC cleavage. The final component of the HPA stress-axis in rodents, CORT, was highly elevated after 12 and 24 h post-UVB exposure (Figure 5h).
UVB effects in Hypox Animals
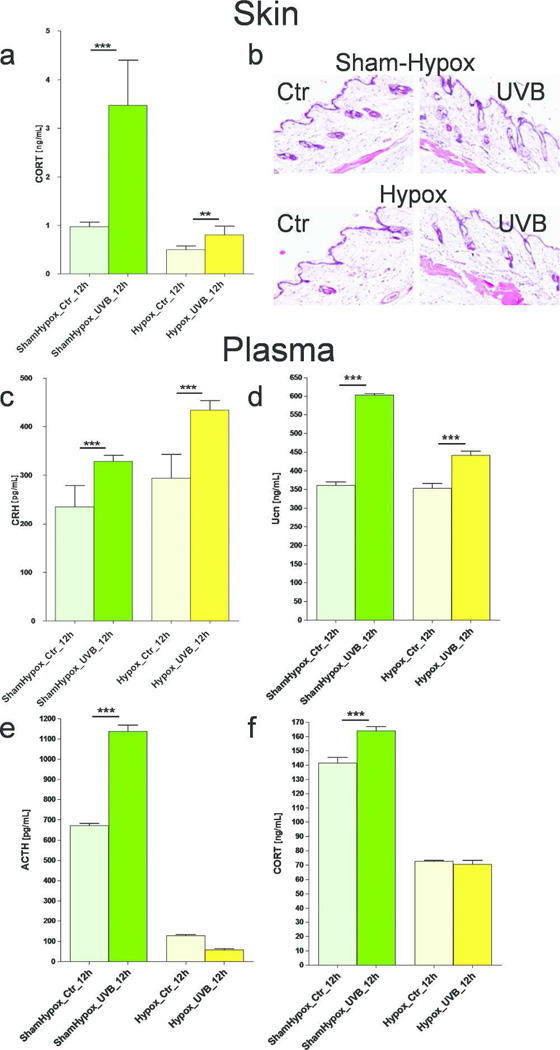
First, we tested the skin, and showed that both basal and UVB-stimulated cutaneous HPA activity was pronounced in mice with intact pituitary (sham-hypox controls) than in hypophysectomized (lacking the pituitary, hypox) mice (Figure 6a). Histological evaluation showed no significant change in the skin morphology between hypox and sham-hypox mice skin after UVB exposure (Figure 6b).
Figure 6.
Differential effects of UVB on the HPA axis in hypox (pituitary removed) and sham-hypox (pituitary intact) C57BL6 mice. CORT levels were evaluated with ELISA and presented as ng/mL after adjustment of total protein content to 4 µg/µL (a). Comparison of histological evaluation between hypox and sham-hypox mouse skin after UVB (400 mJ/cm2) exposure (b). Plasma levels of peptide CRH (c), Ucn (d), ACTH (e) and CORT (f) after exposure of shaved back skin to UVB. Data presented as pg or ng/mL after prior dilution performed separately for each assay, and analyzed using t-test, ** p<0.01, and *** p<0.001.
Second, changes evoked by UVB at the plasma level in mice with intact pituitary were markedly different from those of mice lacking the pituitary (hypox). Although, CRH and Ucn followed similar pattern of activation in both animal groups (Figure 6c,d), hypox mice had much lower plasma levels of ACTH and CORT (Figure 5e,f) and UVB failed to stimulate plasma levels of ACTH and CORT (Figure 6e,f).
Discussion
This manuscript shows, that the exposure of the skin to UVB can activate the systemic HPA axis culminating in increased plasma levels of corticosterone, which requires a functional pituitary. These studies parallel an important discovery by Hiramoto’s group that exposure of the eyes to UVB stimulates plasma levels of α-MSH and increases number of epidermal melanocytes (Hiramoto et al., 2003). Although the mechanism of this stimulation remains to be established (evidence for activation of any the hypothalamic nucleus awaits experimental demonstration), the requirement for an intact pituitary was demonstrated (Hiramoto et al., 2003). However, the final product of UVB induced in the eye axis is α-MSH, accompanied by an increased expression of pituitary prohormone convertase 2 (Hiramoto et al., 2013; Hiramoto et al., 2014), which contrasts the classical HPA axis. In the HPA axis, after pituitary stimulation by hypothalamic CRH, the main product of POMC process is ACTH that acts on the adrenal glands to stimulate glucocorticosteroidogenesis (Turnbull and Rivier, 1999). While Hiramato et al show a novel mechanism of UVB activity at the central level that is different from the HPA axis, our studies demonstrate activation of the classical HPA axis by skin derived factors acting at different entry points Figure S2, for detailed discussion see below).
We also show that UVB can induce in mouse skin in vivo expression of all the elements of the HPA axis including CRH, POMC and corticosteroidogenic pathway. Since UVB did not induce CRH gene expression in skin cells, CRH must be delivered from nerve endings supplying dermo-epidermal or follicular junctions. This is consistent with previous studies showing increases in skin CRH after trauma1-induced hair cycling in C57BL/6 mouse (O'Kane et al., 2006; Roloff et al., 1998; Slominski et al., 1996b; Slominski et al., 2004; Slominski et al., 2001; Slominski et al., 1999), and detection of CRH in skin nerve bundles (Roloff et al., 1998). CRH, together with Ucn (of which both gene and protein expression are up-regulated by UVB), can interact with CRH-R1 and CRH-R2 to promote local POMC activity (Slominski et al., 2013b) followed by the stimulation of local steroidogenesis (Slominski et al., 2013a; Slominski et al., 2014). Concomitantly, UVB enhanced ACTH and β-END production in the skin, with increased expression of crucial regulators of streoidogenesis such as MC2R (receptor for ACTH), StAR (transporter of cholesterol) and steroidogenic enzymes (CYP11A1/P450scc and 3β-HSD) culminating with the final production of CORT. Thus, UVB activates all elements of the HPA axis in mouse skin in vivo, which is in agreement with previous studies on UVB induction of cutaneous POMC (Chakraborty et al., 1999; Schiller et al., 2004) and of all or selected elements of the HPA axis in skin cells (Slominski et al., 1996a; Slominski et al., 2006b; Zbytek et al., 2006) or skin organ culture (Skobowiat et al., 2011; Skobowiat et al., 2013a).
The most striking and previously unreported observations were the stimulation of CRH in the brain localized to the PVN of the hypothalamus and plasma increases of CRH, Ucn, ACTH, β-END and CORT that were accompanied by up-regulation of adrenal MC2R, StAR and CYP11B1. Activation of all of these elements upon skin exposure to UVB clearly indicates that activation of the systemic stress response is centered in the HPA axis and is invoked by skin signals induced by UVB. The crucial role of skin factors in this activation is documented by our experimental design that shielded the head from UVB exposure, preventing retinal signal transmission. The possible mechanisms of this activation are outlined in Figure S1, which includes both the neural route and humoral signals sent from the skin to the central regulatory elements. UVB enhancement of the production of CRH mRNA and peptide in the hypothalamus with localization at the PVN supports the hypothesis that cutaneous signals are conveyed via DRG- spinal cord- dorsal column to the PVN, the center where the HPA axis begins (Slominski et al., 2013b; Smith and Vale, 2006). However, the detailed mapping of this routing is beyond the confines of this project and represents a future challenge.
The nature of humoral signaling from the skin to increase systemic CORT is more complex because in addition to production of ACTH, Ucn and CRH (see above), UVB stimulates cytokine production (IL1, IL6 and TNFα) and release into circulation (Kirnbauer et al., 1991; Muthusamy and Piva, 2010; Schwarz and Luger, 1989), all of which can activate the pituitary POMC (Chrousos, 2009; McEwen, 2007; Slominski et al., 2000c; Turnbull and Rivier, 1999). Although dissection of potential contributions of cytokines signals can be difficult or impossible to quantitate in this experimental setting, the most logical explanation would be the activation of CRH-R1 in anterior pituitary by the circulating natural ligands: CRH and Ucn, induced by UVB, to initiate the following cascade of HPA activity:
CRH + Ucn→CRH-R1→POMC →ACTH→MC2R→CORT
To substantiate this hypothesis, we employed hypophysectomized mice. After UVB exposure, there was significant enhancement of CRH and Ucn peptide levels in the skin and plasma of hypox and sham-hypox animals, with plasma increases of ACTH and CORT seen only in sham-hypox (control) mice. This documents that the pituitary is necessary for UVB activation of a final element of the HPA axis in rodents, CORT. Moreover, the elevated CRH, Ucn and ACTH in the skin after UVB exposure were insufficient for direct (omitting pituitary) humoral activation of adrenals. However, they were capable of stimulating cutaneous CORT but without the corresponding increases in the plasma. Thus, UVB is another trigger of the HPA axis that requires a functional pituitary to stimulate secretion of ACTH and plasma glucocorticoids, while activation of local (skin) HPA activity is restricted to this organ without systemic involvement, as previously predicted (Slominski et al., 2000c).
The net phenotypic consequences of these UVB-induced processes will include homeostatic and immunosuppressive effects resulting from the action of glucocorticoids and POMC-derived peptides, consistent with the established role and function of the HPA axis in the regulation of body homeostasis (Chrousos, 2009; Selye, 1976; Slominski et al., 2013b; Smith and Vale, 2006; Turnbull and Rivier, 1999). These activities would explain the powerful systemic UVB immunosuppressive effects (Kripke, 1994; Schwarz et al., 2011) as well as systemic beneficial effects in attenuation of autoimmune processes that are independent of vitamin D3 (Becklund et al., 2010; Juzeniene and Moan, 2012; Schwarz et al., 2012). Thus, it may be possible to attenuate severity of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease or scleroderma, by stimulation of endogenous glucocorticoids in an organized fashion through UVB induction of the HPA axis.
The stimulation of β-END levels in the skin and plasma offers a mechanistic explanation for the recently described phenomenon of “UV addiction” (Kourosh et al., 2010; Nolan et al., 2009), as a secondary effect of UVB-induced production of POMC-derived s-END (Slominski et al., 2012). The above data on β-END are supported by most recent report from Dr. Fisher group showing UVB mediated stimulation of β-END in the skin and serum (Fell et al., 2014).
In summary, we provide the evidence that UVB can activate the central HPA axis and, based on its organization and function, we propose that this mode of action represents unique mechanism regulating body homeostasis in response to UVB spectrum of solar light.
Materials and Methods
Animals
All procedures involving mouse experiments and tissue handling were approved by the IACUC at the UTHSC, Memphis TN. Eighty seven week-old females C57BL/6 mice (n=6) were purchased from the Taconic Farms (Hudson, NY). After arrival, animals were kept for 4 days to avoid transportation stress with free access to standard laboratory chow and water, maintained on a 12:12 light/dark cycle and room temperature (RT) ranged from 20 to 24 C. Eight week-old animals with all hairs at the telogen stage as judged by a lack of skin pigmentation (Slominski and Paus, 1993) were used for the experiments. This was further confirmed by histological examination of the hair cycle stage confirming that hairs were at the telogen (resting-quiescence) stage where the expression of neuroendocrine factors is expected to be the lowest (Slominski and Paus, 1993; Slominski et al., 2000c) and, therefore, the predicted UVB stimulation would be the highest. To test the role of the pituitary in UVB-induced HPA-activation, hypophysectomized and control (sham hypophysectomized) ninety eight (n=6) B6 females, 7 weeks old, were purchased from Charles River Laboratories (Wilmington, MA) and were kept on 5% sucrose-supplemented water according to the vendor’s guidelines.
General Experimental Design
Details of experimental design are in Figure 1 and its legend. Shortly, after irradiation, animals (3 animals per cage) were kept for 12 or 24 h, according to the group assignment. Finally, under deep anesthesia (isoflurane 4 %) blood was collected by retro-orbital phlebotomy into EDTA tubes, and animals were euthanized by cervical dislocation. Plasma was obtained by centrifugation (1,600 g, 5 min, 4 C), skin, brain and adrenals harvested and frozen immediately at −80 C or fixed with paraformaldehyde. We repeated experiments 3 times during the spring, summer and winter to avoid confounding effects caused by the seasonal fluctuation of the HPA axis activity (Cohen et al., 2012). To test the role of the pituitary, we employed the hypophysectomized (hypox) and sham operated controls (sham-hypox) mice. The animals were treated as above. Blood and tissues were collected in the morning (6 – 7 am, 12 and 24 h after irradiation).
Irradiation
UVB (290–320 nm) irradiation was performed with a Spectroline XX-15A lamp (Spectronics Corp., Westbury, NY) equipped with a UVB waveband bulb (USHIO G15T8E) from the distance of 2.5 inches (see Table S1. The UV dosimetry was described in (Skobowiat et al., 2013b). During irradiation, the bulb was covered with a cellulose triacetate sheet (Kodacel filter, Kodacel™, Eastman Kodak, Rochester, NY) which cuts-off wavelengths shorter than 290 nm, as described in (Skobowiat et al., 2013b). The time of UVB irradiation was calculated upon the formula Time (s) = Dose (J/cm2)/Intensity (W/cm2) and presented as a standard erythema dose (SED) and MED in Table S1. One SED is equivalent to an erythemal effective radiant exposures of 100 J/m2 (MKS) or 0.01 J/cm2 (CGS)(International Commission on Illumination (CIE), 1999).
Quantitative real time RT-PCR (QRT-PCR)
A detailed description of QRT-PCR is given in Supplemental materials and methods. The list of primers used for amplification with SYBR Green polymerase (Kapa Biosystems, Inc., Woburn, MA) is in Table S2. Results are presented as fold change based on the ΔΔ ct method ± SD.
ELISA/EIA
A detailed description is given in Supplemental materials and methods. Commercially available kits used in this study are listed in Table S3. Results are presented as mean ± SD in either pg/mL or ng/mL after recalculations by total protein concentration (brain, skin) or dilution (plasma).
Immunohistochemistry
Detailed protocols are in supplementary file. Briefly, the hypothalamus was isolated at the level of anterior Bregma +1 mm up to posterior Bregma −2.70 mm, by the use of the Brain Slicer Matrix (Zivic Instrument, Pittsburgh, PA). Region resembling the whole hypothalamus (Bregma ~ −0.34 to −2.70) were characterized under light microscope based on the The Allen Reference Atlas (http://mouse.brain-map.org/static/atlas) and 10 µm coronal sections were mounted onto silanized slides (Dako, Carpinteria, CA), and subjected to immunofluorescence protocols described in supplementary file.
Western Blot
A detailed description is in (Skobowiat et al., 2011). Briefly, equal amounts of protein from a combination of 3 skins for each sample was denatured with Laemli buffer, subjected to SDS/PAGE, and proteins were transferred to a PVDF membrane and incubated with antibodies listed in Table S4. Next, the membrane was incubated with secondary IgG-HRP, and detection of immunocomplexes was performed with chemiluminescence.
Statistics
Data are presented as means ± SD and are analyzed using Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted by * (Student’s t-test for two groups) or # (one-way ANOVA Tuckey test for more than two groups), where p<0.05 is considered as statistically significant.
Supplementary Material
Acknowledgments
The paper is dedicated to the memory of Dr Wylie Vale. We gratefully acknowledge careful reading of the manuscript and recommendations by Dr Jean Rivier (Salk Institute), Dr John Pawelek (Yale University) and Drs Arnold Postlethwaite and Robert Williams (UTHSC). We also thank Ms Deborah Doan (Salk Institute) for the editing of the manuscript.
This study was supported by grants from the National Science Foundation (# IOS-0918934) and the National Institutes of Health (#1R01AR056666-01A2 and R01AR052190) to AS.
Abbreviations
- ACTH
adrenocorticotropic hormone
- α-MSH
α-melanocyte stimulating hormone
- β-END
β-endorphin
- B6
C57BL/6 mice
- CIE
Commission Internationale de L'Éclairage (International Commission on Illumination)
- CORT
corticosterone
- COR
cortisol
- CRH
corticotropin releasing hormone
- CRH-R1
CRH receptor type 1
- CYP11B1
gene coding of Steroid 11β-hydroxylase
- P450scc
cytochrome P450 side-chain cleavage enzyme
- DRG
dorsal root ganglia
- MC2R
Melanocortin receptor 2
- POMC
proopiomelanocortin
- PVN
paraventricular nucleus
- SED
standard erythemal dose
- StAR
steroidogenic acute regulatory protein
- Ucn
Urocortin
- 3β-HSD
3-β-hydroxysteroid dehydrogenase
Footnotes
Conflict of interest: The authors state no conflict of interest.
References
- Becklund BR, Severson KS, Vang SV, et al. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci U S A. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, Quinn AG, Angus B, et al. Wavelength specific patterns of p53 induction in human skin following exposure to UV radiation. Cancer Res. 1993;53:2697–2699. [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, et al. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–116. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, et al. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157:1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto K, Yamate Y, Kobayashi H, et al. Ultraviolet B irradiation of the mouse eye induces pigmentation of the skin more strongly than does stress loading, by increasing the levels of prohormone convertase 2 and alpha-melanocyte-stimulating hormone. Clin Exp Dermatol. 2013;38:71–76. doi: 10.1111/j.1365-2230.2012.04439.x. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid suppresses ultraviolet B eye irradiation-induced melanocyte activation by decreasing the levels of prohormone convertase 2 and alpha-melanocyte-stimulating hormone. Photodermatology, photoimmunology & photomedicine. 2014 doi: 10.1111/phpp.12131. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Yanagihara N, Sato EF, et al. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. The Journal of investigative dermatology. 2003;120:123–127. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- International Commission on Illumination (CIE) International Standard ISO 17166:1999(E) - CIE 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. 1st edn. Geneva, Switzerland: International Organization for Standardization (ISO); 1999. [Google Scholar]
- Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Journal. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Juzeniene A, Moan J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol. 2012;4:109–117. doi: 10.4161/derm.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Kock A, Neuner P, et al. Regulation of epidermal cell interleukin-6 production by UV light and corticosteroids. J Invest Dermatol. 1991;96:484–489. doi: 10.1111/1523-1747.ep12470181. [DOI] [PubMed] [Google Scholar]
- Kourosh AS, Harrington CR, Adinoff B. Tanning as a behavioral addiction. The American journal of drug and alcohol abuse. 2010;36:284–290. doi: 10.3109/00952990.2010.491883. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- Krutmann J, Morita A, Chung JH. Sun exposure: what molecular photodermatology tells us about its good and bad sides. J Invest Dermatol. 2012;132:976–984. doi: 10.1038/jid.2011.394. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- Ndiaye MA, Nihal M, Wood GS, et al. Skin, Reactive Oxygen Species, and Circadian Clocks. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan BV, Taylor SL, Liguori A, et al. Tanning as an addictive behavior: a literature review. Photodermatology, photoimmunology & photomedicine. 2009;25:12–19. doi: 10.1111/j.1600-0781.2009.00392.x. [DOI] [PubMed] [Google Scholar]
- O'Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–153. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pawelek JM, Chakraborty AK, Osber MP, et al. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5:348–356. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Roloff B, Fechner K, Slominski A, et al. Hair cycle-dependent expression of corticotropin-releasing factor (CRF) and CRF receptors in murine skin. FASEB J. 1998;12:287–297. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Kock A, et al. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Brzoska T, Bohm M, et al. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and alpha-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122:468–476. doi: 10.1046/j.0022-202X.2004.22239.x. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Navid F, Sparwasser T, et al. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol. 2011;128:826–833. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Navid F, Sparwasser T, et al. 1,25-dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression. J Invest Dermatol. 2012;132:2762–2769. doi: 10.1038/jid.2012.238. [DOI] [PubMed] [Google Scholar]
- Schwarz T, Luger TA. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989;4:1–13. doi: 10.1016/1011-1344(89)80097-1. [DOI] [PubMed] [Google Scholar]
- Selye H. The Stress of Life, revised edn. New York: McGraw-Hill Book Company; 1976. p. 515. [Google Scholar]
- Skobowiat C, Dowdy JC, Sayre RM, et al. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Nejati R, Lu L, et al. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene. 2013a;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobowiat C, Sayre RM, Dowdy JC, et al. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013b;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Baker J, Ermak G, et al. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS letters. 1996a;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkareva NV, Botchkarev VA, et al. Hair cycle-dependent production of ACTH in mouse skin. Biochimica et biophysica acta. 1998;1448:147–152. doi: 10.1016/s0167-4889(98)00124-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Hwang J, et al. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996b;1289:247–251. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Gomez-Sanchez CE, Foecking MF, et al. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000a;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–54. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16:503–515. doi: 10.1016/s0738-081x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Tobin DJ, et al. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Roloff B, Curry J, et al. The skin produces urocortin. J Clin Endocrinol Metab. 2000b;85:815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000c;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tuckey RC, et al. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013a;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Semak I, et al. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005a;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005b;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, et al. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006a;126:1177–1178. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006b;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R. Is there a "skin stress response system?". Ann N Y Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Experimental dermatology. 2014;23:369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, et al. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, et al. Key Role of CRF in the Skin Stress Response System. Endocr Rev. 2013b;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vink AA, Moodycliffe AM, Shreedhar V, et al. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc Natl Acad Sci U S A. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Molecular endocrinology. 2006;20:2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.