Abstract
Dentinogenesis is a complex and multistep process, which is regulated by various growth factors, including members of the Fibroblast Growth Factor (FGF) family. Both positive and negative effects of FGFs on dentinogenesis have been reported but the underlying mechanisms of these conflicting results are still unclear. To gain better insight into the role of FGF2 in dentinogenesis, we used dental pulp cells from various transgenic mice, in which fluorescent protein expression identifies cells at different stages of odontoblast differentiation. Our results showed that continuous exposure of pulp cells to FGF2 inhibited mineralization and revealed both stimulatory and inhibitory effects of FGF2 on expression of markers of dentinogenesis and various transgenes. During the proliferation phase of in vitro growth FGF2 increased expression of markers of dentinogenesis and the percentages of DMP1-GFP+ functional odontoblasts and DSPP-Cerulean+ odontoblasts. Additional exposure to FGF2 during the differentiation/mineralization phase of in vitro growth decreased the extent of mineralization, expression of markers of dentinogenesis, and expression of DMP1-GFP and DSPP-Cerulean transgenes. Recovery experiments showed that the inhibitory effects of FGF2 on dentinogenesis were related to the blocking of differentiation of cells into mature odontoblasts. These observations together showed stage-specific effects of FGF2 on dentinogenesis by dental pulp cells and provide critical information for the development of improved treatments for vital pulp therapy and dentin regeneration.
Keywords: odontoblast differentiation, dental pulp, progenitors, FGF2, GFP
Introduction
Fibroblast growth factors (FGFs) are a family of signaling molecules shown to play essential roles in development, repair and regeneration of damaged skeletal tissues [Hatch, 2010; Miraoui and Marie, 2010; Marie, 2012; Marie et al., 2012; Kim et al., 2014]. Currently, the FGF family contains 22 members, which elicit their effects through interaction with four highly conserved transmembrane tyrosine kinase receptors (FGFR1, FGFR2, FGFR3 and FGFR4) in concert with heparin or heparan sulfate proteoglycans [Hatch, 2010; Miraoui and Marie, 2010]. Three major downstream signaling pathways, which mediate effects of FGF/FGFR signaling on cellular processes, include MAPK, PI3K/Akt and PLCγ [Miraoui and Marie, 2010; Marie, 2012; Marie et al., 2012].
FGF signaling plays essential roles in osteogenesis and dentinogenesis, and among FGFs FGF2 is widely expressed in the cells of odontoblast and osteoblast lineages and has been identified as a potent regulator of mineralization in vivo and in vitro [Roberts-Clark and Smith, 2000; Madan and Kramer, 2005; Cooper et al., 2010; Miraoui and Marie, 2010; Marie, 2012; Marie et al., 2012; Smith et al., 2012].
Earlier studies on osteogenic cells reported conflicting effects of FGF signaling on osteoblast differentiation and production of mineralized matrix [Miraoui and Marie, 2010; Marie, 2012; Marie et al., 2012]. Later studies showed that the effects of FGF signaling were depended on the stage of osteoblast maturation. In immature osteoblasts FGF signaling induced proliferation leading to increased osteogenesis in long term, whereas in mature osteoblasts FGF signaling inhibited differentiation and mineralization [Miraoui and Marie, 2010; Marie, 2012; Marie et al., 2012].
FGF signaling has been shown to play important roles in primary and reparative dentinogenesis [Smith et al., 2012; Li et al., 2014]. FGF2 affects the proliferation, homing and migration of healthy and inflamed dental pulp cells [Nakao et al., 2004; Morito et al., 2009; Shimabukuro et al., 2009; Xiao et al., 2009; Osathanon et al., 2011; Suzuki et al., 2011; Kim et al., 2014]. FGF2 also increase the expression of markers of mesenchymal stem cells Oct4, Nanog and Rex1 and the percentage of STRO-1+ cells in the dental pulp [Morito et al., 2009; Osathanon et al., 2011; Wu et al., 2012].
Although there appears to be a general agreement on the effects of FGF signaling on cell proliferation, there are conflicting results on its effects on dentinogenesis and mineralization. Several studies showed that FGF2 inhibited dentinogenesis and the expression of dentin sialophosphoprotein (Dspp) [Tsuboi et al., 2003; Shimabukuro et al., 2009; Xiao et al., 2009; Kim et al., 2010; Kim et al., 2014]. On the other hand, other studies showed that FGF2 stimulated the expression of Dspp in vitro and the formation of osteodentin in vivo [Kikuchi et al., 2007; Ishimatsu et al., 2009; Kim et al., 2010; Kim et al., 2014].
Thus, the precise effects of FGF signaling on differentiation of cells in odontoblast lineage are still not well understood and most likely involve multiple intra- and extracellular mediators and differential responses of various cell populations [Dailey et al., 2005]. This is partially due to the lack of availability of stage-specific markers for studying progression of cells in the odontoblast lineage.
To gain a better understanding of the progression of progenitor cells in the odontoblast lineage, we have used a series of GFP reporter transgenic mice that display stage-specific activation of transgenes during odontoblast differentiation in vivo and in vitro [Braut et al., 2003; Balic et al., 2010b; Balic and Mina, 2011]. These studies showed that 2.3-GFP and 3.6-GFP transgenes were activated at early stages of odontoblast differentiation (i.e., polarizing odontoblasts and prior to the expression of Dmp1 and Dspp), whereas DMP1-GFP was first activated in functional/secretory odontoblasts (cells expressing Dmp1 and low levels of Dspp) [Balic et al., 2010b; Balic and Mina, 2011]. All three transgenes (2.3-GFP, 3.6-GFP and DMP1-GFP) were also expressed at high levels in fully differentiated/mature odontoblasts, and their temporal and spatial patterns of expression mimicked those of endogenous transcripts and proteins [Balic et al., 2010b; Balic and Mina, 2011].
In addition, we have recently generated new transgenic mice using the bacterial artificial chromosome (BAC), which directs expression of DSPP-Cerulean transgene to functional and fully differentiated odontoblasts (unpublished data). Our in vivo and in vitro studies showed that expression of DSPP-Cerulean transgene was limited to odontoblasts and correlated closely with the expression of endogenous Dspp and can be used to identify fully differentiated odontoblasts in the heterogeneous pulp cultures (unpublished data).
Therefore, in the present study we have used dental pulp cells from various transgenic mice, which display stage-specific activation of transgenes during odontoblast differentiation, to gain further insight into effects of FGF2 on mineralization and dentinogenesis of dental pulp cells.
Materials and Methods
Primary dental pulp cultures
All experimental protocols involving animal tissues in the present study were approved by Institutional Animal Care and Use Committee of University of Connecticut Health Center. The coronal portions of the pulps from first and second molars were isolated from 5–7-day-old hemizygous pOBCol3.6GFP (referred to as 3.6-GFP), pOBCol2.3GFP (referred to as 2.3-GFP), DMP1-GFP, DSPP-Cerulean and non-transgenic pups as described previously [Balic et al., 2010a]. All mice were maintained in the CD1 background. After isolation, 8.75×104 cells/cm2 were grown first in Dulbecco's modified Eagle’s medium (DMEM), 20% fetal bovine serum (FBS), 2 mM L-glutamine, 40 U/ml penicillin, 40 µg/ml streptomycin and 0.1 µg/ml Fungizone (Invitrogen). Three days later, the medium was changed to DMEM containing 5% FBS. At day 7, mineralization was induced by addition of Minimum Essential Medium alpha (αMEM) medium, 5% FBS, with 50 µg/ml fresh ascorbic acid and 4 mM β-glycerophosphate. Medium was changed every other day.
Primary bone marrow stromal cell (BMSC) cultures
BMSCs were prepared from femurs and tibiae of 5–7-day-old pups as described before [Balic et al., 2010a]. Briefly, single cell suspension was prepared from flushed marrows, plated at a density of 6.5×105 cells/cm2 and grown in αMEM containing 10% FBS, 40 U/ml penicillin and 40 μg/ml streptomycin. Three days later, the medium was changed to αMEM and 5% FBS. At day 7, when the cells became confluent, the medium was switched to the mineralization-inducing medium containing αMEM, 5% FBS, with 50 µg/ml fresh ascorbic acid and 4 mM β-glycerophosphate. Medium was changed every other day.
FGF2 treatment of primary cultures
Cultures were exposed to low molecular weight (18 kDa) bovine FGF2 (R&D systems, Inc., Minneapolis, MN) or vehicle (VH, 0.1% BSA fraction V in PBS) every other day during the proliferation and differentiation/mineralization phases of in vitro growth (between days 3–21).
Detection and quantification of mineralization in cultures
Mineralization in live cultures was examined by Xylenol Orange (XO) staining as described previously [Balic et al., 2010b]. The mean fluorescence intensity of XO staining was measured using a multidetection monochromator microplate reader (Safire2, Tecan, Research Triangle Park, NC) as described previously [Kuhn et al., 2010] with minor modifications. Fluorometric measurements were performed at 570/610 nm wavelength (excitation/emission) and gain of 80. The entire area of each well was read at a scan density of 6×6 regions (high sensitivity flash mode). Background fluorescence for XO was measured using cultures from preodontoblastic Q705 cell line [Priam et al., 2005] that lacks a mineralization potential. The background fluorescence values were subtracted from respective XO measurements.
Mineralization in fixed cultures was examined using a modified von Kossa silver nitrate staining protocol as described previously [Balic et al., 2010a]. After staining, cultures were rinsed and images were acquired using a scanner. The area of mineralization (black precipitate) in each well was quantified using NIH ImageJ software and is represented as the percentage of total area analyzed as described before [Balic et al., 2010a].
Immunocytochemistry
Pulp cells derived from the DSPP-Cerulean transgenic mice were treated with VH or FGF2 and processed for immunocytochemistry as described previously [Mulrooney et al., 2001] with some modifications. Cells were fixed with 3.7% formaldehyde in PBS for 4 minutes at room temperature (RT), incubated with 0.5% Triton X in PBS for 10 minutes at RT, blocked with 3% milk for 1 hr at RT, and then incubated with 1:1000 dilution of anti-GFP Alexa Fluor 488 conjugated antibody (Molecular Probes, Invitrogen) in 0.3% Triton X in PBS overnight at +4°C. In these cultures, the anti-GFP antibody binds specifically to the Cerulean fluorescent protein to enhance its visualization. The nuclei were stained with 1.0 µg/ml Hoechst 33342 dye (Invitrogen) for 15 minutes at RT. After staining, coverslips were mounted using Dako Fluorescent Mounting Medium (Dako North America, Inc., Carpinteria, CA) and cultures were visualized under the microscope using filters for DAPI and GFPtpz for detection of Hoechst 33342 and GFP, respectively.
Percentage of DSPP-Cerulean+ odontoblasts in cultures was calculated as the ratio of cells stained with anti-GFP antibody (DSPP-Cerulean+ cells) to the total number of Hoechst+ cells. In each experiment, approximately 20,000–30,000 Hoechst+ cells were counted from 20–40 different areas of VH- and FGF2-treated cultures. Negative controls included primary BMSC cultures derived from the DSPP-Cerulean littermates and then stained with anti-GFP antibody, and primary dental pulp cultures derived from the DSPP-Cerulean littermates without addition of anti-GFP antibody.
Digital imaging and epifluorescence analysis of cell cultures
GFP expression in cell cultures at various time points was examined using Zeiss AxioObserver Z.1 microscope equipped with AxioCam MRc digital camera and appropriate filters. Exposure times were adjusted for optimum imaging and kept consistent for each time point of the culture. Panoramic images of larger areas of the cultures were obtained using a computer-controlled motorized imaging workstation and Zeiss AxioObserver Z.1 microscope.
Fluorescence intensity of GFP
The mean fluorescence intensity of GFP transgenes in each well was measured as described for XO staining. Fluorometric measurement was performed at 483/525 nm wavelength (excitation/emission) for 2.3-GFP transgene and at 500/540 nm wavelength for 3.6-GFP and DMP1-GFP transgenes (gain 80 for all three transgenes). Background fluorescence for GFP was measured using dental pulp cultures from non-transgenic littermates, and these values were subtracted from respective GFP measurements. Fluorometric measurements were also obtained in DSPP-Cerulean cultures stained with anti-GFP antibody (500/540 nm wavelength and gain 80) and Hoechst 33342 dye (343/483 nm wavelength and gain 70). Background fluorescence for GFP was measured using BMSC cultures from the DSPP-Cerulean littermates and stained with anti-GFP antibody, and these values were subtracted from respective GFP measurements.
RNA extraction and quantitative PCR (qPCR) analysis
Total RNA was isolated using TRIzol reagent (Invitrogen) followed by cDNA synthesis and TaqMan qPCR analysis. TaqMan primers for Bsp, Dmp1, Dspp, Gapdh, Osteocalcin and Type I collagen were purchased from Applied Biosystems (Suppl. Table 1). All qPCR reactions were run using 7900HT Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 50°C for 2 min, 95°C for 10 min, and 40 cycles with denaturation at 95°C for 15 sec and extension at 60°C for 1 min. Amplification efficiency was determined using internal standard curves derived from a purified amplicon diluted 2-fold (0.14 – 9.0 ng), and was close to 100% for all qPCR reactions. We defined the acceptable range of CT values representing gene expression to be between 10 and 35 cycles, according to manufacturer’s recommendations (Applied Biosystems).
WST-1 cell proliferation assay
Cell proliferation was examined by WST-1 rapid cell proliferation assay according to manufacturer’s instructions (EMD Millipore Corporation, Billerica, MA). The assay is based on the cleavage of the tetrazolium salt, WST-1, to formazan by cellular mitochondrial dehydrogenases. Increases in the number of viable cells result in increases in the amount of formazan dye formed. Pulp cells were cultured in a 96-well microtiter plate (17×103 cells/cm2), treated with VH or FGF2 (20 ng/ml) starting day 3 (0 hrs) and processed for WST-1 assay at 24, 48, 72 and 96 hrs after treatment. Cells were incubated with WST-1 reagent for 2 hrs at 37°C and the amount of the formazan dye produced was quantified by the optical density (OD) at 450 nm using a Synergy™ HT Multi-detection microplate reader and analyzed using Gen5™ 1.09 Data Analysis Software (BioTek Instruments, Winooski, VT). Background absorbance levels were measured using wells without cells (culture medium only), and these values were subtracted from respective VH and FGF2 values.
Flow cytometric (FACS) analysis and sorting
Cells from 3.6-GFP, 2.3-GFP and DMP1-GFP pups were processed for FACS analysis at day 7 by mild 0.05% trypsin/EDTA (Invitrogen, USA) digestion followed by centrifugation at 4°C. Cells were then resuspended in 300–400 µl of the staining medium (1× HBSS, 2% FBS, 10 mM HEPES, in distilled H2O, pH 7.2) containing 1.0 µg/ml propidium iodide (PI), and strained through a 70-µm strainer to obtain single-cell suspension. Approximately 50,000–100,000 cells/sample were collected by a BD™ LSR-II FACS cytometer (BD Biosciences, San Jose, CA) using a blue laser (excitation 488 nm at 20 mW; collected emission at 515–545 nm). Percentages of GFP+ and GFP– cells were determined using BD FACSDiva™ 6.2 software. Pulp cells from non-transgenic littermates served as a negative control for GFP expression in all experiments.
For FACS sorting, pulp cells from 2.3-GFP pups were grown under control culture conditions for 7 days. At day 7, cells were detached with 0.05% trypsin/EDTA (Invitrogen) followed by centrifugation at +4°C. Cells were then resuspended in 300–400 µl of the staining medium containing 1.0 µg/ml PI, and strained through a 70-µm strainer. FACS based on GFP expression was performed on 2.5×106 cells/ml by UCHC FACS facility using a BD FACSAria™ II cell sorter (130 µm nozzle at 12 PSI) (BD Biosciences, San Jose, CA). GFP was excited at 488 nm with an argon laser and a 550/30 emission filter was utilized. Upon separation, reanalysis confirmed that the purity of isolated 2.3-GFP+ and 2.3-GFP– populations was higher than 98%. Live GFP+ and GFP– cells were collected into DMEM with 20% FBS, recounted and replated at the same density as the primary cultures (8.75×104 cells/cm2). Cultures were treated with VH or FGF2 (20 ng/ml) between days 3–14 and processed for various analyses as described for unsorted cultures.
Statistical analysis of data
was performed by GraphPad Prism 6 software using one-way ANOVA analysis with the Bonferroni’s multiple comparison post-test or unpaired two-tailed Student t-test. Values in all experiments represented mean ± SEM of at least three independent experiments, and a *p-value ≤ 0.05 was considered statistically significant.
Results
Effects of FGF2 on mineralization and dentinogenesis in primary dental pulp cultures
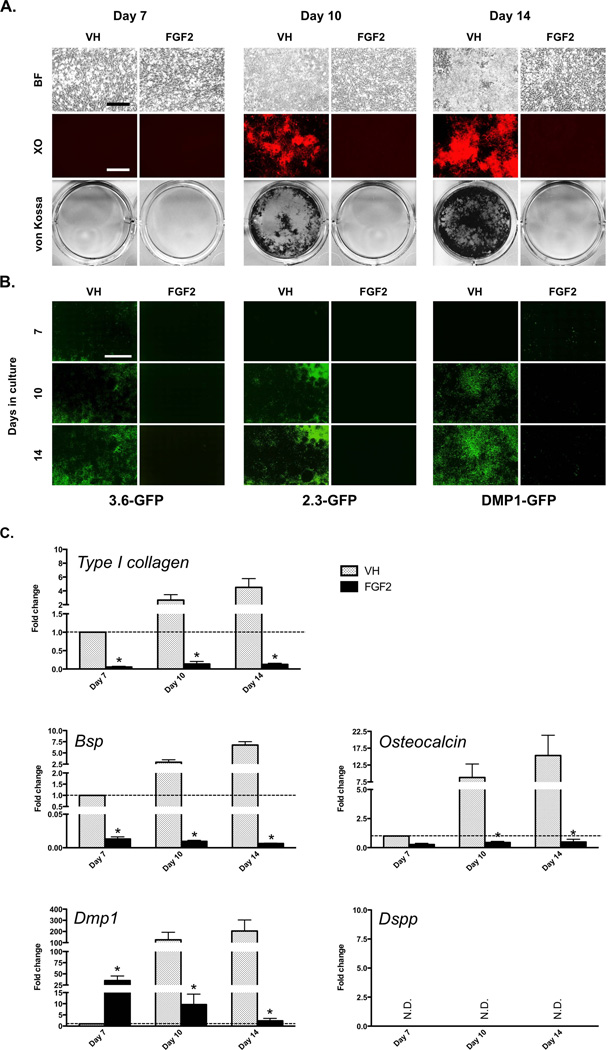
Previous studies in our laboratory showed that when placed in primary culture, progenitor/stem cells in the pulp from unerupted molars proliferated rapidly and reached confluence around day 7 (proliferation phase of in vitro growth). Following addition of the mineralization-inducing medium at day 7, these cells underwent differentiation and gave rise to an extensive amount of mineralized matrix (differentiation/mineralization phase of in vitro growth). The first sign of mineralization appeared around day 10 with significant increases in the extent of mineralization thereafter. At day 21 almost the entire culture dish was covered with a sheet of mineralized tissue [Balic et al., 2010a].
Using this well-characterized dental pulp culture system, we examined the effects of FGF2 on mineralization and dentinogenesis. In these experiments, primary pulp cultures were exposed to VH (control) or FGF2 between days 3–21 (during both proliferation and differentiation/mineralization phases of in vitro growth).
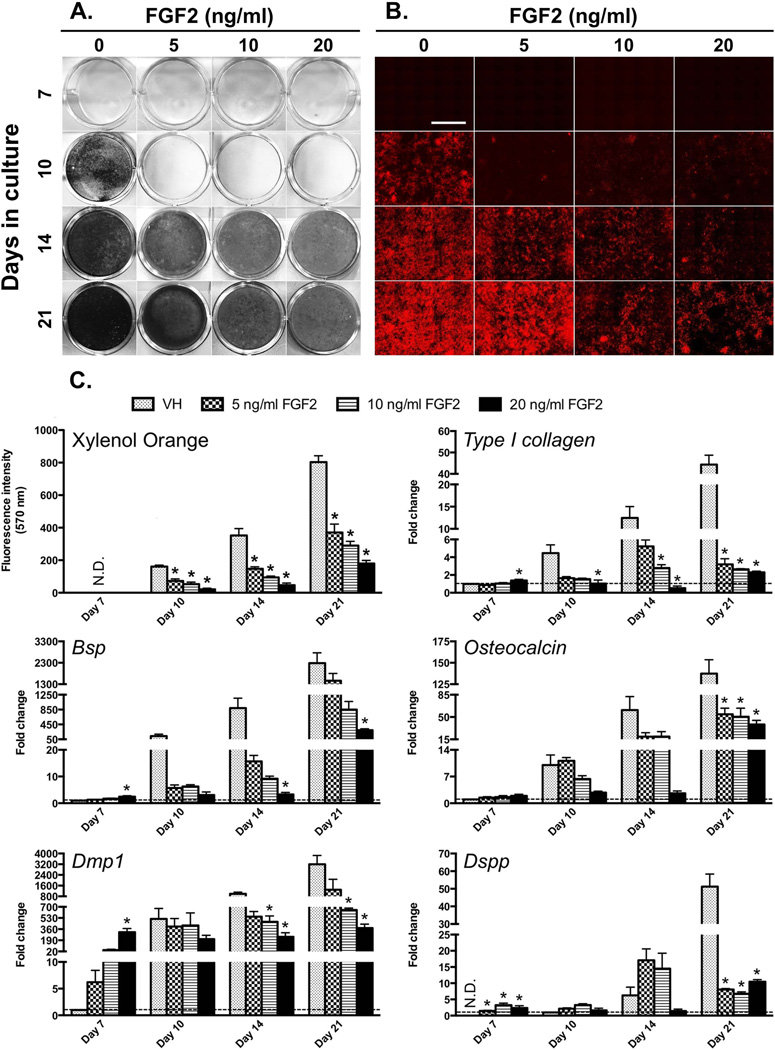
XO and von Kossa staining showed marked and concentration-dependent decreases in the extent of mineralization in FGF2-treated cultures as compared to control (Figures 1A–B). QPCR analysis also showed concentration-dependent changes in FGF2-treated cultures as compared to control (Figure 1C). FGF2-treated cultures showed increases in the levels of expression of all markers at day 7 followed by decreases between days 10–21 as compared to control (Figure 1C). The most marked increases at day 7 in FGF2-treated cultures were in the expression of Dmp1 (up to ~310-fold) followed by increases in the expression of Dspp, Bsp and Osteocalcin (~2-fold) (Figure 1C). These observations revealed both stimulatory and inhibitory effects of FGF2 on the expression of markers of mineralization and dentinogenesis.
Figure 1. Concentration-dependent effects of FGF2 on mineralization and the expression of markers of mineralization and dentinogenesis in primary dental pulp cultures.
(A) Representative images of von Kossa-stained dishes.
(B) Representative composite of 5× scanned images of XO-stained live cultures at various time points. The magnifications of all micrographs are identical. Scale bar = 2 mm.
(C) Histogram showing the changes in the intensity of XO fluorescence and in the levels of expression of various markers. The intensity of XO staining is expressed as absolute values and the expression levels of markers of mineralization and dentinogenesis are expressed as relative values. Expression of all mRNAs except Dspp is normalized to VH at day 7, which is arbitrarily set to 1 and indicated by the dashed line. The expression of Dspp is normalized to VH at day 10, which is arbitrarily set to 1 and indicated by the dashed line. Results in all histograms represent mean ± SEM of at least three independent experiments; *p ≤ 0.05 relative to VH at each time point. N.D. = not detected. Note the increases in the expression of markers of mineralization and dentinogenesis at day 7 followed by decreases in their expression at days 10–21 in FGF2-treated cultures as compared to control.
Effects of FGF2 on cell proliferation in primary dental pulp cultures
To examine if the increases in the expression of various markers of dentinogenesis in FGF2-treated cultures at day 7 were related to increases in the cell number, the effects of FGF2 on cell proliferation in the whole culture were examined by the WST-1 assay 24–96 hrs after exposure to FGF2 (days 4–7 of the culture). Cell proliferation in the control and FGF2-treated cultures peaked around 48–72 hrs and declined at 96 hrs. FGF2-treated cultures showed up to ~1.6-fold increase in proliferation at 24–96 hrs as compared to control (Table 1). These observations showed that FGF2 increased cell proliferation as compared to control.
Table 1. Effects of FGF2 on proliferation of dental pulp cells (WST-1 assay).
Cultures were established from 5–7-day-old non-transgenic mice and treated with VH or 20 ng/ml FGF2 starting day 3 (0 hrs). Cultures were processed for the WST-1 proliferation assay at 24, 48, 72 and 96 hrs after FGF2 treatment as described in the Materials and Methods. Results represent mean ± SEM of absorbance in at least three independent experiments;
| Hours after treatment |
VH | FGF2 | Fold change |
|---|---|---|---|
| 24 | 0.56 ± 0.01 | 0.62 ± 0.01* | ~1.11 |
| 48 | 0.82 ± 0.05 | 1.04 ± 0.07* | ~1.27 |
| 72 | 0.77 ± 0.03 | 1.18 ± 0.03* | ~1.53 |
| 96 | 0.61 ± 0.03 | 1.00 ± 0.05* | ~1.64 |
p ≤ 0.05 relative to control at each time point. Fold changes represent the FGF2 value divided by the control value for each time point.
Effects of FGF2 on expression of various GFP reporter transgenes in primary dental pulp cultures
Next we examined the effects of FGF2 on pulp cultures from various GFP reporter transgenic mice. In these studies expression of 2.3-GFP and 3.6-GFP transgenes before the onset of mineralization were used as markers for cells at early stages of odontoblast differentiation (polarizing odontoblasts that lack expression of Dmp1 and Dspp) [Balic et al., 2010b]. DMP1-GFP and DSPP-Cerulean transgenes were used as markers for cells at later stages of odontoblast differentiation (functional and fully differentiated odontoblasts) [Balic and Mina, 2011](unpublished data).
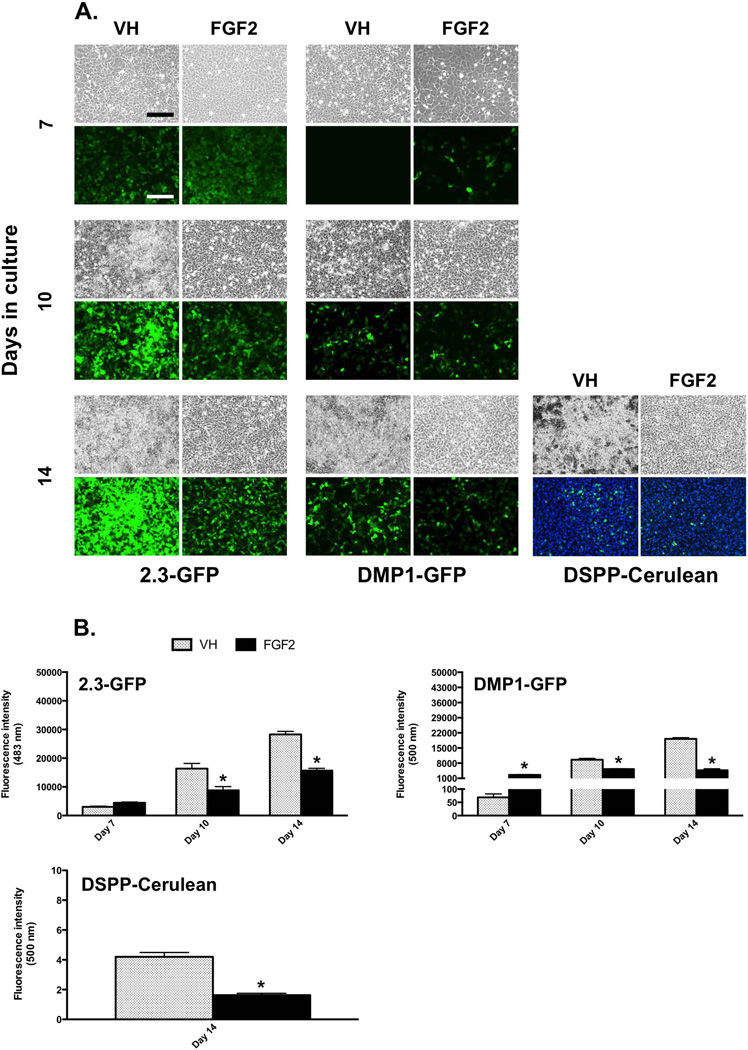
Epifluorescence analyses of live cultures at day 7 showed slight increases in the intensity of 2.3-GFP and 3.6-GFP transgenes and marked increases (~38-fold) in the intensity of DMP1-GFP transgene (Figures 2A and 2B, and data not shown) in FGF2-treated cultures as compared to the respective controls. These increases in FGF2-treated cultures were followed by marked decreases in the intensity of the expression of all transgenes as compared to the respective controls between days 10–14 (Figures 2A and 2B, and data not shown). The percentage of DSPP-Cerulean+ odontoblasts in FGF2-treated cultures at day 14 was less than half of that in control cultures (control: 6.50 ± 0.18%; FGF2-treated: 3.00 ± 0.15%; ~2.3-fold) (Figure 2A).
Figure 2. Effects of FGF2 on the expression of GFP transgenes in primary dental pulp cultures.
(A) Each panel represents images of the same areas in cultures from transgenic animals at different time points analyzed under brightfield and epifluorescent light using filters for GFPemd (for detection of 2.3-GFP transgene) or GFPtpz (for detection of DMP1-GFP transgene). The magnifications of all micrographs are identical. Scale bar = 200 µm.
(B) Histograms showing changes in the intensity of 2.3-GFP, DMP1-GFP and DSPP-Cerulean transgene expression in VH- and FGF2-treated cultures. For detection of DSPP-Cerulean, cells were processed for immunocytochemistry using anti-GFP antibody at day 14 and epifluorescent image represents Hoechst/GFP overlay. The magnifications of all micrographs are identical. Scale bar = 200 µm.
Results in all histograms are expressed as absolute values and represent mean ± SEM of at least three independent experiments; *p ≤ 0.05 relative to VH at each time point. Note the marked increase in the intensity of DMP1-GFP at day 7 in FGF2-treated cultures as compared to control. Also note decreases in the intensity of 2.3-GFP and DMP1-GFP at days 10–14 in FGF2-treated cultures as compared to control.
To determine if increases in the intensity of these transgenes in FGF2-treated cultures at day 7 were related to increases in the number of the GFP+ cells, FACS analysis was performed at day 7 (Table 2). FGF2-treated cultures displayed slight but not significant increases in the percentages of the 2.3-GFP+ and 3.6-GFP+ populations as compared to the respective controls (Table 2). Pulp cultures from DMP1-GFP transgenic animals showed marked increases in the percentage of DMP1-GFP+ cells as compared to control (Table 2). Immunocytochemical analysis of pulp cultures from DSPP-Cerulean mice at day 7 also showed marked increases in the percentage of DSPP-Cerulean+ odontoblasts in FGF2-treated cultures as compared to control (Table 2).
Table 2. Effects of FGF2 on the percentage of GFP+ cells in primary dental pulp cultures.
Cultures were treated with VH or 20 ng/ml FGF2 starting day 3. Cells were subjected for GFP-based FACS analysis and immunocytochemistry for DSPP-Cerulean at day 7. Results represent mean ± SEM of at least three independent experiments;
| Control | FGF2 | |||
|---|---|---|---|---|
| %GFP– cells | %GFP+ cells | %GFP– cells | %GFP+ cells | |
| 2.3-GFP | 15.10 ± 0.80 | 84.90 ± 0.77 | 12.57 ± 0.64 | 87.43 ± 0.66 |
| 3.6-GFP | 17.31 ± 0.18 | 82.69 ± 0.14 | 15.62 ± 0.35 | 84.38 ± 0.40 |
| DMP1-GFP | 94.60 ± 0.27 | 5.40 ± 0.27 | 64.60 ± 2.98* | 35.40 ± 2.98* |
| DSPP-Cerulean | ND | 0.11 ± 0.05 | ND | 1.91 ± 0.25* |
p ≤ 0.05 relative to control at each time point.
N.D. = not detected.
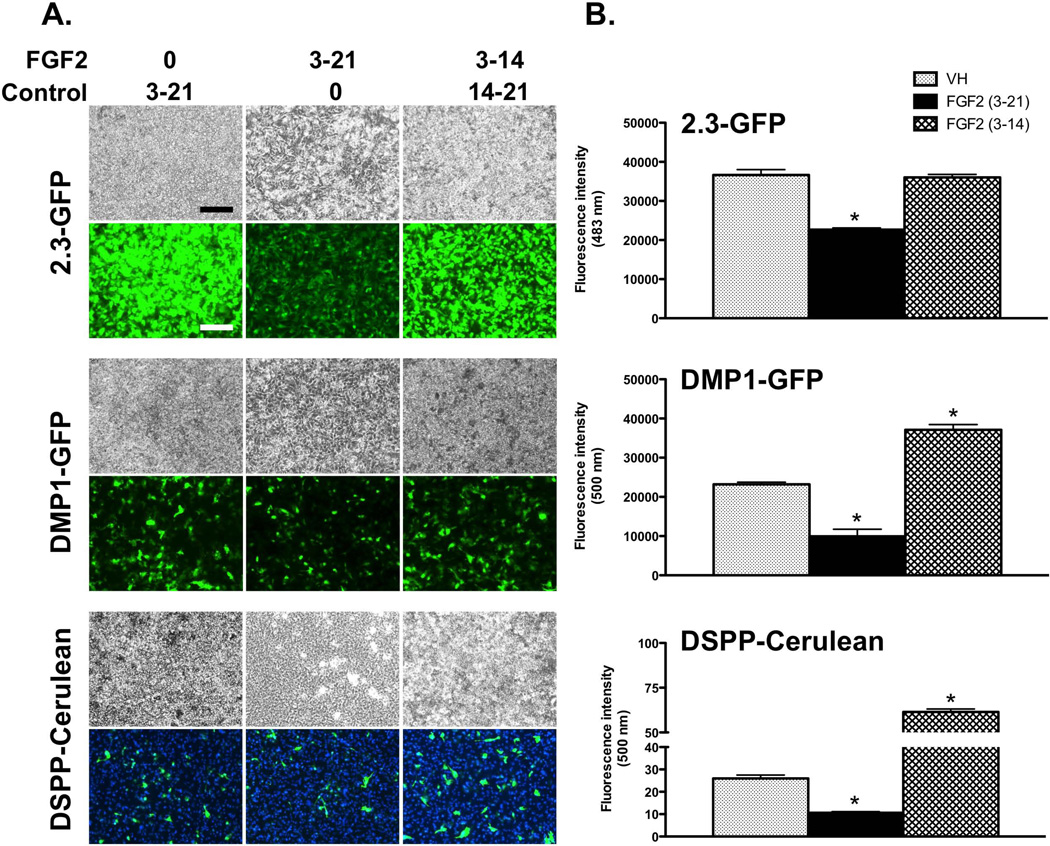
Effects of FGF2 on FACS-sorted 2.3-GFP+ and 2.3-GFP– populations
The presence of a mixture of GFP+ and GFP− populations made it difficult to study the effects of FGF2 on activation of these transgenes during proliferation and mineralization/dentinogenesis. Therefore, as the next step, we studied the effects of FGF2 on FACS-sorted populations. Our previous observations showed that FACS-sorted 2.3-GFP+ and 2.3-GFP– populations represented proliferative cells enriched in polarizing odontoblasts and undifferentiated progenitors respectively [Balic et al., 2010b]. Based on these observations, we examined the effects of FGF2 on relatively homogeneous populations of FACS-sorted 2.3-GFP+ and 2.3-GFP– cells (≥ 98% purity of isolated populations; Suppl. Figure 1).
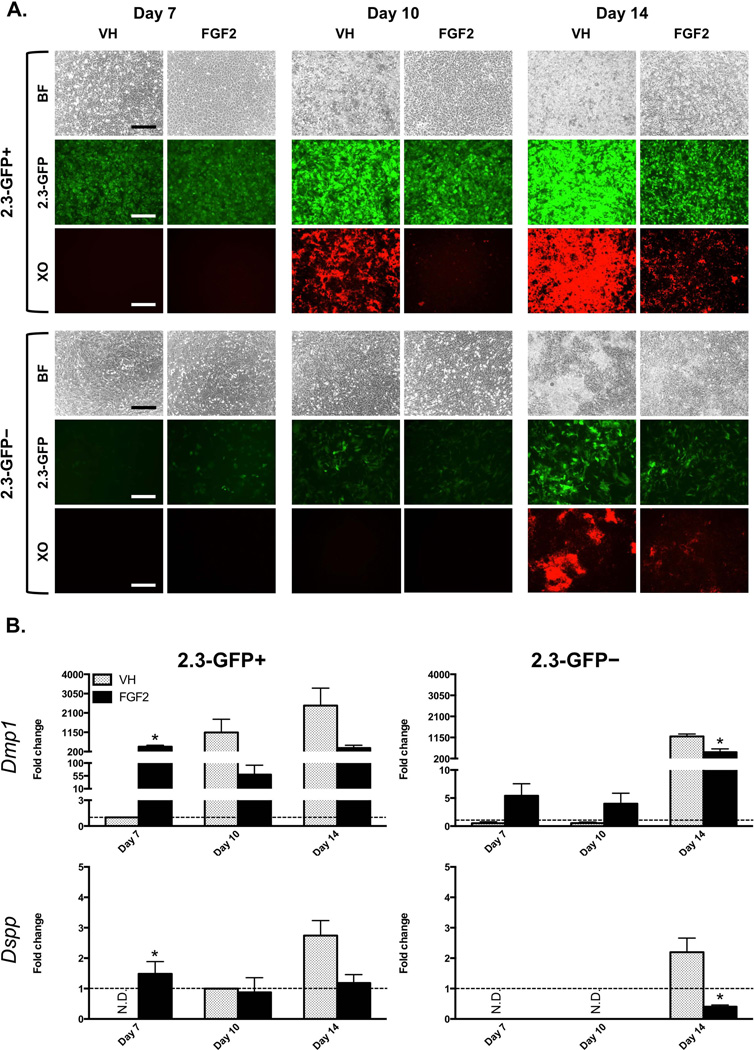
In cultures established from the 2.3-GFP+ population, GFP expression was detected initially and was maintained throughout the entire culture period (Figure 3A). The first sign of mineralization was around day 10 with significant increases thereafter (Figure 3A). In these cultures low levels of Dmp1 and Dspp were detected around days 7 and 10, respectively. Expression of markers of mineralization and dentinogenesis increased with more advanced stages of differentiation in vitro (Figure 3B).
Figure 3. Effects of FGF2 on mineralization and expression of the 2.3-GFP transgene in FACS-sorted 2.3-GFP+ and 2.3-GFP– populations.
Primary pulp cultures from the 2.3-GFP transgenic mice were grown under control culture conditions and processed for FACS to separate relatively homogeneous 2.3-GFP+ and 2.3-GFP– populations. FACS-sorted populations were plated (day 0) and exposed to VH or 20 ng/ml FGF2 between days 3–14.
(A) Each panel represents images of the same areas in cultures at different time points analyzed under brightfield and epifluorescent light using appropriate filters for detection of GFP and XO. The magnifications of all micrographs are identical. Scale bar = 200 µm. Note the decreases in mineralization in both populations in response to FGF2.
(B) Histograms showing the changes in the expression of Dmp1 and Dspp in both populations. Expression levels of Dmp1 in both populations are normalized to VH-treated 2.3-GFP+ population at day 7, which is arbitrarily set to 1 and indicated by the dashed line. Expression levels of Dspp in both populations are normalized to VH-treated 2.3-GFP+ population at day 10, which is arbitrarily set to 1 and indicated by the dashed line.
Results represent mean ± SEM of at least three independent experiments; *p ≤ 0.05 relative to VH at each time point. N.D. = not detected. In the 2.3-GFP+ population FGF2-treated cultures displayed increases in the levels of Dmp1 and Dspp at day 7 followed by decreases in the levels of their expression at days 10–14 as compared to control. In the 2.3-GFP– population, FGF2-treated cultures showed increases in the levels of Dmp1 at day 7 as compared to control. At later time points these cultures displayed decreases in expression of Dmp1 and Dspp as compared to control.
In cultures established from the 2.3-GFP– population, GFP was not detected initially, but appeared at day 7 in a few isolated cuboidal cells and increased thereafter (Figure 3A). In these cultures low levels of Dmp1 were detected at day 7, and mineralization and expression of Dspp were detected only at day 14 (Figures 3A and 3B). The delayed expression of GFP and the delayed appearance of XO-stained mineralized nodules together with the lack of expression of Dspp at days 7 and 10 in cultures from 2.3-GFP– population confirmed that as compared to the 2.3-GFP+ population, 2.3-GFP– population was enriched in cells at earlier stages of differentiation.
FGF2-treated cultures showed marked decreases in the intensity of GFP expression and the extent of mineralization as compared to the respective controls. However, in 2.3-GFP+ cultures FGF2 increased the expression of Dmp1 and Dspp at day 7 followed by decreases at days 10 and 14 as compared to control. In the 2.3-GFP– population, FGF2 increased the levels of Dmp1 at days 7 and 10 followed by decreases at day 14 as compared to control (Figure 3B). In the 2.3-GFP– population, expression of Dspp was detected only at day 14 and at lower levels in FGF2-treated cultures as compared to control (Figure 3B).
FGF2 inhibited progression of cells into the final stage of differentiation
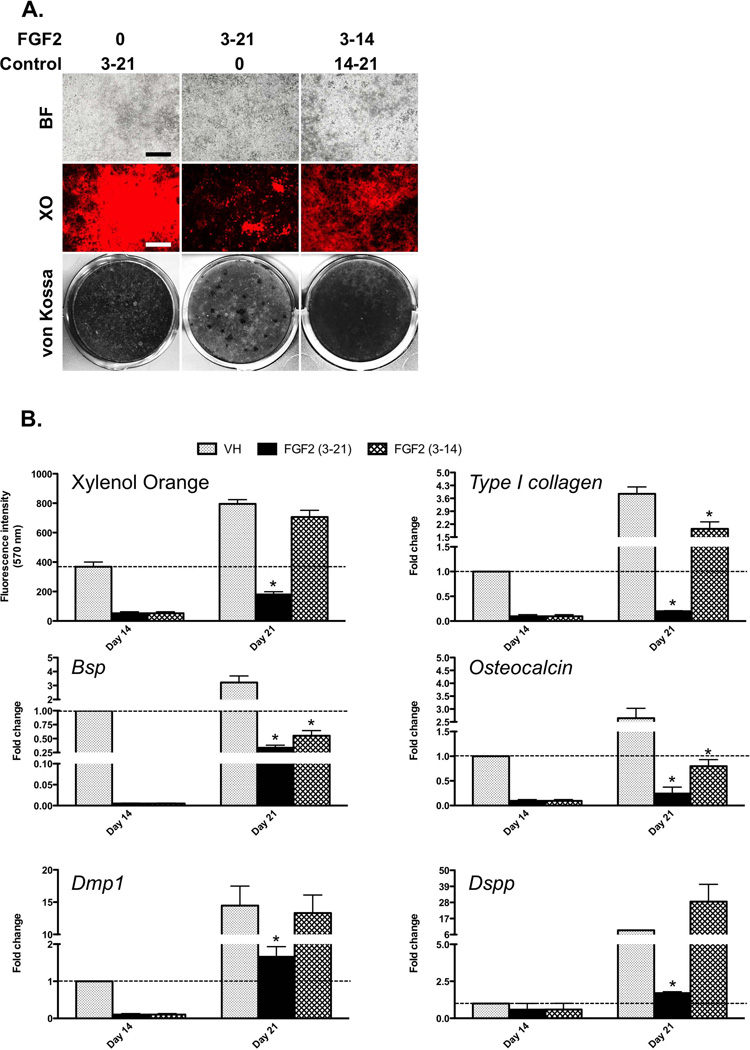
Despite decreases at days 10 and 14 as compared to the respective controls, the intensity of 2.3-GFP and DMP1-GFP in FGF2-treated cultures remained relatively unchanged (Figure 2). These observations suggested that FGF2 did not de-differentiate cells and maintained healthy number of 2.3-GFP+ and DMP1-GFP+ cells, in which further differentiation into mature odontoblasts was inhibited. To test this possibility, we examined the effects of withdrawal of FGF2 on differentiation of pulp cells. In these experiments dental pulp cells were exposed to FGF2 between days 3–14 and then grown in control medium (without FGF2) for additional 7 days. The effects of withdrawal of FGF2 on the extent of mineralization and dentinogenesis in these cultures were compared to control cultures (not exposed to FGF2) and cultures continuously exposed to FGF2 between days 3–21.
Withdrawal of FGF2 for 7 days allowed almost complete recovery of mineralization and the expression of markers of mineralization and dentinogenesis (Figures 4A and 4B). The intensity of XO staining at day 21 in these cultures was similar to that in control cultures (Figures 4B). Levels of expression of Type I collagen, Bsp and Osteocalcin in these cultures at day 21 were higher than those in cultures continuously exposed to FGF2, but did not reach those in control (Figure 4B). On the other hand, the levels of Dmp1 in these cultures were similar to those in control, and the levels of Dspp were higher (~3.2-fold) than those in control (Figure 4B).
Figure 4. Effects of withdrawal of FGF2 on mineralization and the expression of markers of mineralization and dentinogenesis in primary dental pulp cultures.
Cultures were treated with VH or 20 ng/ml FGF2 between days 3–14. At day 14, FGF2 was withdrawn and cells were grown for additional 7 days in the medium without FGF2.
(A) Images of the same areas in cultures at day 21 analyzed under brightfield (upper row) and epifluorescent light using TRITC Red filter for detection of XO staining (middle row). The lower row shows representative von Kossa-stained dishes. The magnifications of all micrographs are identical. Scale bar = 200 µm.
(B) Histograms showing the changes in the intensity of XO staining (relative values). Levels of expression of all mRNAs were normalized to those of VH at day 14, which is arbitrarily set to 1 and indicated by the dashed line.
Results in all histograms represent mean ± SEM of values from at least three independent experiments; *p ≤ 0.05 relative to VH at each time point. Note almost complete recovery in the extent of mineralization and in the levels of expression of Dmp1 and Dspp in cultures after withdrawal of FGF2 for 7 days.
Epifluorescence analyses of cultures from various transgenic animals showed that 7 days following the withdrawal of FGF2, the intensity of the expression of 2.3-GFP and 3.6-GFP transgenes reached that in the respective control cultures (Figures 5A and 5B, and data not shown). Interestingly, the intensity of DMP1-GFP and DSPP-Cerulean transgenes and the percentage of DSPP-Cerulean+ odontoblasts (control: 6.88 ± 0.28%. FGF2-treated: 8.39 ± 0.21%; 1.22-fold) were higher than those in the respective controls (Figures 5A and 5B).
Figure 5. Effects of withdrawal of FGF2 on the expression of GFP transgenes in primary dental pulp cultures.
(A) Representative images of the same areas in cultures analyzed under brightfield and epifluorescent light using appropriate filters at day 21. DSPP-Cerulean was detected with anti-GFP antibody by immunocytochemistry and examined using filter for GFPtpz. The epifluorescent image represents Hoechst/GFP overlay. The magnifications of all micrographs are identical. Scale bar = 200 μm.
(B) Histograms showing the effects of withdrawal of FGF2 on the expression of various transgenes at day 21. Results in all histograms are expressed in absolute values and represent mean ± SEM of at least three independent experiments; *p ≤ 0.05 relative to VH. Note that after withdrawal of FGF2 the intensity of the expression of 2.3-GFP reached that in control, and the intensity of DMP1-GFP and DSPP-Cerulean exceeded that in control.
Effects of FGF2 on primary BMSC cultures
Our previous studies showed that primary dental pulp cultures from unerupted molars contained progenitors capable of giving rise to both osteoblasts and odontoblasts [Balic et al., 2010a]. This makes it difficult to distinguish the effects of FGF2 on cells of osteogenic vs. dentinogenic lineages.
To distinguish between the effects of FGF2 on cells of these lineages, we examined the effects of FGF2 on BMSC cultures, as they do not contain odontoprogenitors and are used routinely to examine mineralization and osteoblast differentiation in vitro. Previous studies have also shown that in the osteoblast lineage 3.6-GFP is activated in pre-osteoblasts, 2.3-GFP in mature osteoblasts and DMP1-GFP in late osteoblasts and osteocytes [Kalajzic et al., 2002; Kalajzic et al., 2004].
Exposure of BMSCs to FGF2 between days 3–14 completely inhibited mineralization (Figure 6A) and led to marked decreases in the expression of markers of early and late stages of osteoblast differentiation at day 7 except for Dmp1 that was transiently increased (~35-fold) as compared to control (Figure 6C). At days 10 and 14 expression of all these markers was markedly reduced in FGF2-treated cultures as compared to control. Analysis of BMSC cultures from various transgenic animals showed that FGF2 completely inhibited the expression of 2.3-GFP and 3.6-GFP transgenes at all time points as compared to the respective controls. FGF2-treated BMSC cultures from DMP1-GFP animals showed a few DMP1-GFP+ cells at day 7 followed by complete inhibition of the expression of this transgene at days 10 and 14 as compared to control (Figure 6B). Expression of Dspp and DSPP-Cerulean transgene was not detected in control and FGF2-treated cultures at any time point (Figure 6C and data not shown).
Figure 6. Effects of FGF2 on mineralization, expression of transgenes and markers of mineralization in primary BMSC cultures.
Primary BMSC cultures were treated with VH or 20 ng/ml FGF2 between days 3–14.
(A) Representative images of the same areas in cultures at different time points were analyzed under brightfield (upper row) and epifluorescent light using TRITC Red filter for detection of XO staining (middle row). The magnifications of all micrographs are identical. Scale bar = 200 µm. The lower row shows representative images of von Kossa-stained cultures. Note the lack of mineralization in FGF2-treated cultures as compared to control.
(B) Representative composite of 5× scanned images of live cultures at various time points analyzed under epifluorescent light using filters for GFPtpz (for detection of 3.6-GFP and DMP1-GFP transgenes) and GFPemd (for detection of 2.3-GFP transgene). The magnifications of all micrographs are identical. Scale bar = 2 mm. Note the lack of expression of all transgenes in FGF2-treated cultures as compared to control.
(C) Histograms showing the changes in the levels of expression of various markers of mineralization. Expression of all mRNAs is normalized to VH at day 7, which is arbitrarily set to 1 and indicated by the dashed line. Results represent mean ± SEM of at least three independent experiments. *p ≤ 0.05 relative to VH at each time point. N.D. = not detected. Note the marked increases in the levels of Dmp1 in FGF2-treated cultures as compared to control at day 7. Also note the marked decreases in the expression of all markers of mineralization in FGF2-treated cultures at days 10 and 14 as compared to control.
Discussion
Members of the FGF family of growth factors including FGF2 play essential roles in various functions of dental pulp cells during reparative dentinogenesis, including proliferation, migration, differentiation and self-renewal of dental pulp stem and progenitor cells [Nakao et al., 2004; He et al., 2008; Morito et al., 2009; Shimabukuro et al., 2009; Osathanon et al., 2011; Suzuki et al., 2011; Wu et al., 2012]. However, the effects of FGF2 on mineralization and dentinogenesis have remained controversial, as both inhibitory and stimulatory roles of FGF2 have been reported.
It has been shown that continuous exposure of primary dental pulp cultures and tooth organ cultures to FGF2 decreased the extent of mineralization and the expression of various markers of mineralization and dentinogenesis, including Dmp1 and Dspp [Tsuboi et al., 2003; He et al., 2008; Osathanon et al., 2011; Wu et al., 2012]. Inhibition of FGF2 signaling in tooth organ cultures by specific antisense oligonucleotides increased Alp and Dspp expression [Tsuboi et al., 2003]. On the other hand, several studies have shown that FGF2 increased expression of Dmp1 and Dspp in primary pulp cultures [Nakao et al., 2004; Kim et al., 2010], immortalized human dental pulp cells [Kim et al., 2010] and E15 (cap stage) tooth organ cultures [Tsuboi et al., 2003].
Our study showed that the effects of FGF2 on differentiation of pulp cells were stage-specific and depended of the stage of maturity of cells. Our results provided a strong support that in the odontoblast lineage, FGF2 stimulated/promoted the differentiation of early progenitors into functional odontoblasts (Figure 7). Exposure of pulp cells to FGF2 during the proliferation phase of in vitro growth increased the levels of the expression of all markers of mineralization and dentinogenesis, including marked increases in the expression of Dmp1 and marked increases in intensity of DMP1-GFP transgene, shown to be activated in functional odontoblasts [Balic and Mina, 2011]. Furthermore, our studies on FACS-sorted populations showed that FGF2 stimulated the expression of Dmp1 at day 7 in both undifferentiated progenitors (2.3-GFP–) and cells at early stages of differentiation (polarizing odontoblasts, 2.3-GFP+). FGF2 stimulated Dspp expression only in the 2.3-GFP+ population.
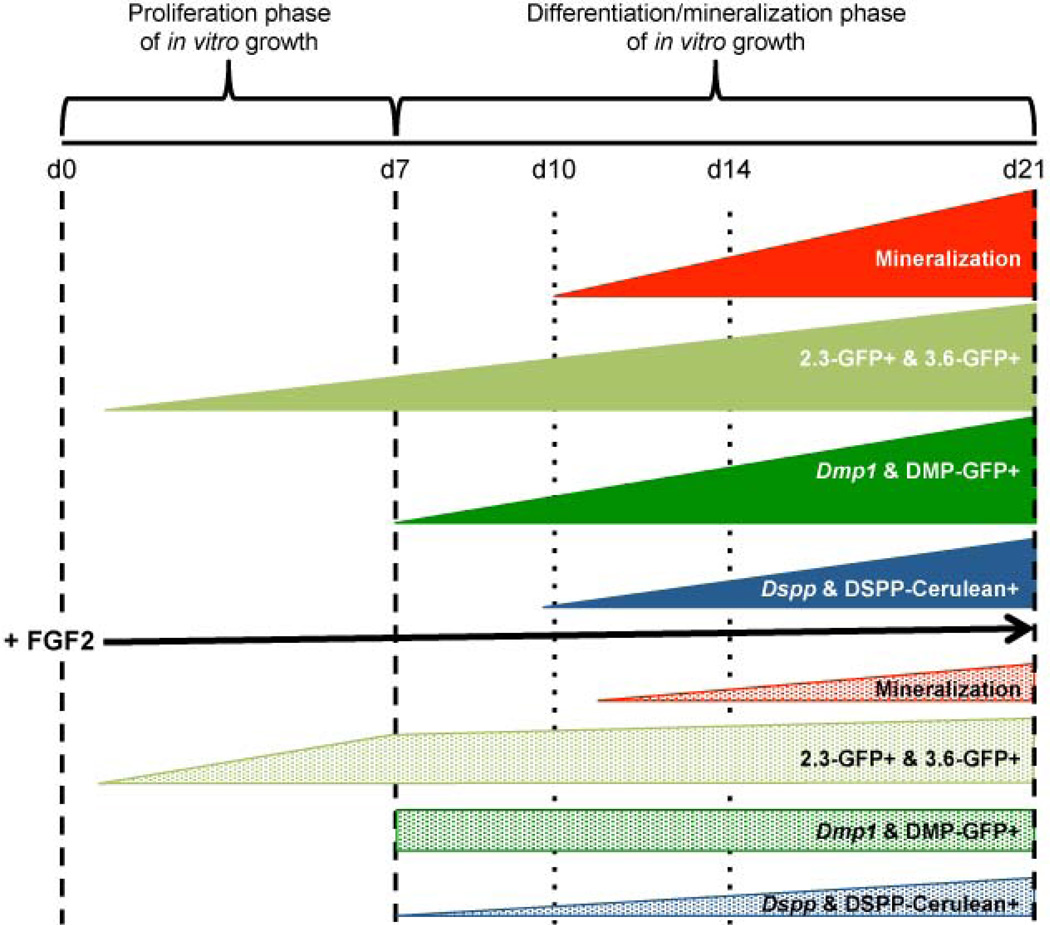
Figure 7. Summary of changes in pulp cultures grown in presence and absence of FGF2.
During the proliferative phase of in vitro growth (first 7 days), pulp cultures undergo proliferation and contain early progenitors. Following addition of the mineralization-inducing medium at day 7, these cells undergo differentiation and give rise to an extensive amount of mineralized matrix (differentiation/mineralization phase of in vitro growth). The first sign of mineralization is around day 10 with significant increases in the extent of mineralization thereafter. In these cultures Dmp1 and Dspp are expressed at low levels at day 7 and 10 respectively. DMP1-GFP+ and DSPP-Cerulean+ cells are detected at day 7 and 10 respectively, with increases thereafter.
Continuous exposure of pulp cultures to FGF2 resulted in decreases in the extent of mineralization. FGF2-treated cultures displayed increases in the levels of Dmp1, and percentage of DMP1-GFP+ cells at day 7 followed by decreases between days 10–21 as compared to control. However, despite decreases, the intensity of DMP1-GFP transgene and expression of Dmp1 in FGF2-treated cultures between days 7–21 remained relatively unchanged. FGF2-treated cultures also displayed increases in the levels of Dspp at day 7, followed by decreases in the levels of Dspp and percentage of DSPP-Cerulean+ cells between days 10–21 as compared to control.
Our results also showed that despite these early stimulatory effects, additional exposure of pulp cells to FGF2 reduced mineralization, expression of Dmp1 and Dspp, all transgenes and the number of DSPP-Cerulean+ odontoblasts as compared to control (Figure 7). These observations together with those on FACS-sorted populations suggested that in the odontoblast lineage, FGF2 inhibited the differentiation of functional odontoblasts into fully differentiated odontoblasts. The rapid and almost complete recovery of mineralization, expression of markers of dentinogenesis and various GFP transgenes 7 days after withdrawal of FGF2 suggested that the inhibitory effects of FGF2 on mineralization and dentinogenesis were primarily related to its negative effects on final stages of cell differentiation.
Taken together, these results show stage-specific effects of FGF2 on differentiation of cells of the odontoblast lineage and suggest positive roles of FGF2 in the formation of functional odontoblasts and negative roles in further differentiation of these cells. Additional experiments are in progress to examine the underlying mechanisms mediating the stimulatory and inhibitory effects of FGF2 on pulp cells. These observations provide insight into conflicting results for positive and negative effects of FGF2 on mineralization and dentinogenesis.
It is well documented that FGF signaling produces diverse biological responses in various cell types. The mechanisms of specific cellular responses to FGF signaling are dependent on many factors, including cell type, expression of specific ligands and receptors, the signal transduction pathways utilized, and the transcriptional regulation of tissue-specific genes [Dailey et al., 2005]. Moreover, studies on bone showed that the response to FGF signaling in a specific cell type was also stage-specific. FGF signaling stimulated the proliferation of immature osteoblasts but inhibited mineralization and increased apoptosis in more differentiated cells [Mansukhani et al., 2000; Fakhry et al., 2005; Eda et al., 2008; James et al., 2008; Xiao et al., 2013].
Effects of FGF2 on primary BMSC cultures reveal differences between the effects of FGF2 on osteoprogenitors and odontoprogenitors
The formation of both bone- and dentin-like tissues in primary pulp cultures [Balic et al., 2010a] raises the possibility that some of the effects of FGF2 on dental pulp cultures may be related to its effects on cells of the osteoblast lineage. However, the differences between the early and later effects of FGF2 on BMSC and pulp cultures in our study suggest that the effects of FGF2 in pulp cultures are primarily on cells of the odontoblast lineage.
Our results showed that continuous exposure of BMSCs to FGF2 completely inhibited mineralization and decreased the expression of markers of early and late stages of osteoblast differentiation, and is consistent with previously reported studies [Kalajzic et al., 2003; Marie, 2012; Marie et al., 2012; Yamachika et al., 2012]. The transient increase in Dmp1 in FGF2-treated cultures is also consistent with other studies that showed that exposure to FGF2 during the proliferation phase of in vitro growth resulted in rapid and marked increases in the expression of Dmp1 and other osteocyte-associated markers (E11, Cx43, Phex) in osteoblast- and osteocyte-like cells (ROS17/2.8 and MC-4, MLO-Y4) and BMSC [Kyono et al., 2012; Nakayama et al., 2012]. Although the underlying mechanisms of the stimulatory effects of FGF2 on Dmp1 are not fully understood, available evidence suggests the involvement of FGFR/MEK/Erk1/2 in this regulation [Kyono et al., 2012].
Furthermore, consistent with previous results [Kalajzic et al., 2003] our study showed that FGF2 completely inhibited the expression of 3.6-GFP and 2.3-GFP transgenes in BMSC cultures, indicating that the inhibition of osteogenesis by FGF2 was mediated by blocking the onset of preosteoblast differentiation.
Our results showed that in pulp cultures FGF2 induced transient increases in the expression of all markers of mineralization and dentinogenesis at day 7 and reduced (but did not eliminate) the expression of 3.6-GFP and 2.3-GFP transgenes between days 10–21.
In addition, rapid and almost complete recovery of mineralization in pulp cultures after withdrawal of FGF2 in our study is different from that in BMSC cultures. Upon withdrawal of FGF2 from BMSC cultures, full osteoblast differentiation and mineralization did not appear in vitro and was detected only after subcutaneous implantation of FGF2-treated cells in SCID/Beige mice in vivo [Kalajzic et al., 2003].
These observations suggest significant differences in the response of odontoprogenitors and osteoprogenitors to FGF2 and/or differences in osteoprogenitors residing in the dental pulp vs. bone marrow. The differences in the activation of 2.3-GFP and DMP1-GFP in cells of the osteogenic vs. dentinogenic lineage will allow us to gain a better understanding of these differences. Previous studies have showed that 2.3-GFP and DMP1-GFP are activated in mature and late osteoblasts and osteocytes, respectively, which are cell populations at relatively advanced stages of osteoblast differentiation [Kalajzic et al., 2002; Kalajzic et al., 2004]. Our studies indicated that 2.3-GFP and DMP1-GFP were activated in polarizing and functional odontoblasts, respectively, which are cell populations at early and intermediate stages of odontoblast differentiation [Balic et al., 2010b; Balic and Mina, 2011].
Supplementary Material
Acknowledgements
We would like to thank all individuals who provided reagents, valuable input and technical assistance in various aspects of this study, including Drs. David Rowe, Peter Maye and Anamaria Balic, Gloria Gronowicz, Mrs. Barbara Rodgers, members of Molecular Core and Flow Cytometry Facilities at UCHC. This work was supported by R01-DE016689 & T90-DE022526 grants from the National Institute of Health (NIDCR).
Abbreviations used in this paper
- FGF
Fibroblast Growth Factor
- FGFR
Fibroblast Growth Factor Receptor
- BMSC
Bone marrow stromal cell
- ADSC
Adipose-tissue-derived stromal cell
- Dspp
Dentin sialophosphoprotein
- BAC
Bacterial artificial chromosome
- FBS
Fetal bovine serum
- VH
Vehicle
- XO
Xylenol Orange
- qPCR
Quantitative PCR
- FACS
Flow cytometric sorting
- αMEM
Minimum Essential Medium alpha
- DMEM
Dulbecco's modified Eagle's medium
- RT
Room temperature
- OD
Optical Dentisty
- PI
Propidium Iodide
Footnotes
The authors declare no conflicts of interest.
References
- Balic A, Aguila HL, Caimano MJ, Francone VP, Mina M. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 2010a;46(6):1639–1651. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Aguila HL, Mina M. Identification of cells at early and late stages of polarization during odontoblast differentiation using pOBCol3.6GFP and pOBCo12.3GFP transgenic mice. Bone. 2010b;47(5):948–958. doi: 10.1016/j.bone.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Mina M. Identification of secretory odontoblasts using DMP1-GFP transgenic mice. Bone. 2011;48(4):927–937. doi: 10.1016/j.bone.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1-2.3-GFP transgene. Int J Dev Biol. 2003;47(4):281–292. [PubMed] [Google Scholar]
- Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. Inflammation-regeneration interplay in the dentine-pulp complex. Journal of dentistry. 2010;38(9):687–697. doi: 10.1016/j.jdent.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16(2):233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Eda H, Aoki K, Marumo K, Fujii K, Ohkawa K. FGF-2 signaling induces downregulation of TAZ protein in osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 2008;366(2):471–475. doi: 10.1016/j.bbrc.2007.11.140. [DOI] [PubMed] [Google Scholar]
- Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, Pacifici M, Kirschner RE, Nah HD. Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36(2):254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hatch NE. FGF signaling in craniofacial biological control and pathological craniofacial development. Crit Rev Eukaryot Gene Expr. 2010;20(4):295–311. doi: 10.1615/critreveukargeneexpr.v20.i4.20. [DOI] [PubMed] [Google Scholar]
- He H, Yu J, Liu Y, Lu S, Liu H, Shi J, Jin Y. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell biology international. 2008;32(7):827–834. doi: 10.1016/j.cellbi.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Ishimatsu H, Kitamura C, Morotomi T, Tahata Y, Nishihara T, Chen KK, Terashita M. Formation of Dentinal Bridge on Surface of Regenerated Dental Pulp in Dentin Defects by Controlled Release of Fibroblast Growth Factor-2 From Gelatin Hydrogels. J Endod. 2009;35(6):858–865. doi: 10.1016/j.joen.2009.03.049. [DOI] [PubMed] [Google Scholar]
- James AW, Xu Y, Wang R, Longaker MT. Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plastic and reconstructive surgery. 2008;122(1):53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35(1):74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Kalajzic Z, Hurley MM, Lichtler AC, Rowe DW. Stage specific inhibition of osteoblast lineage differentiation by FGF2 and noggin. J Cell Biochem. 2003;88(6):1168–1176. doi: 10.1002/jcb.10459. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(1):15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Kitamura C, Morotomi T, Inuyama Y, Ishimatsu H. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J Endod. 2007;33(10):1198–1202. doi: 10.1016/j.joen.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Kim J, Park JC, Kim SH, Im GI, Kim BS, Lee JB, Choi EY, Song JS, Cho KS, Kim CS. Treatment of FGF-2 on stem cells from inflamed dental pulp tissue from human deciduous teeth. Oral Dis. 2014;20(2):191–204. doi: 10.1111/odi.12089. [DOI] [PubMed] [Google Scholar]
- Kim YS, Min KS, Jeong DH, Jang JH, Kim HW, Kim EC. Effects of fibroblast growth factor-2 on the expression and regulation of chemokines in human dental pulp cells. J Endod. 2010;36(11):1824–1830. doi: 10.1016/j.joen.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Kuhn LT, Liu Y, Advincula M, Wang YH, Maye P, Goldberg AJ. A nondestructive method for evaluating in vitro osteoblast differentiation on biomaterials using osteoblast-specific fluorescence. Tissue Eng Part C Methods. 2010;16(6):1357–1366. doi: 10.1089/ten.TEC.2009.0701. [DOI] [PubMed] [Google Scholar]
- Kyono A, Avishai N, Ouyang Z, Landreth GE, Murakami S. FGF and ERK signaling coordinately regulate mineralization-related genes and play essential roles in osteocyte differentiation. J Bone Miner Metab. 2012;30(1):19–30. doi: 10.1007/s00774-011-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Prochazka J, Goodwin AF, Klein OD. Fibroblast growth factor signaling in mammalian tooth development. Odontology. 2014;102(1):1–13. doi: 10.1007/s10266-013-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan AK, Kramer B. Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth. Journal of molecular histology. 2005;36(3):171–178. doi: 10.1007/s10735-005-2684-1. [DOI] [PubMed] [Google Scholar]
- Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149(6):1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ. Fibroblast growth factor signaling controlling bone formation: an update. Gene. 2012;498(1):1–4. doi: 10.1016/j.gene.2012.01.086. [DOI] [PubMed] [Google Scholar]
- Marie PJ, Miraoui H, Severe N. FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors. 2012;30(2):117–123. doi: 10.3109/08977194.2012.656761. [DOI] [PubMed] [Google Scholar]
- Miraoui H, Marie PJ. Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Science signaling. 2010;3(146):re9. doi: 10.1126/scisignal.3146re9. [DOI] [PubMed] [Google Scholar]
- Morito A, Kida Y, Suzuki K, Inoue K, Kuroda N, Gomi K, Arai T, Sato T. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch Histol Cytol. 2009;72(1):51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- Mulrooney JP, Hong T, Grabel LB. Serine 785 phosphorylation of the beta1 cytoplasmic domain modulates beta1A-integrin-dependent functions. J Cell Sci. 2001;114(Pt 13):2525–2533. doi: 10.1242/jcs.114.13.2525. [DOI] [PubMed] [Google Scholar]
- Nakao K, Itoh M, Tomita Y, Tomooka Y, Tsuji T. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochem Biophys Res Commun. 2004;325(3):1052–1059. doi: 10.1016/j.bbrc.2004.10.136. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yang L, Takai H, Kaneko H, Abiko Y, Ogata Y. Fibroblast growth factor 2 and forskolin induce mineralization-associated genes in two kinds of osteoblast-like cells. J Oral Sci. 2012;54(3):251–259. doi: 10.2334/josnusd.54.251. [DOI] [PubMed] [Google Scholar]
- Osathanon T, Nowwarote N, Pavasant P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCgamma signaling pathway. J Cell Biochem. 2011;112(7):1807–1816. doi: 10.1002/jcb.23097. [DOI] [PubMed] [Google Scholar]
- Priam F, Ronco V, Locker M, Bourd K, Bonnefoix M, Duchene T, Bitard J, Wurtz T, Kellermann O, Goldberg M, Poliard A. New cellular models for tracking the odontoblast phenotype. Archives of oral biology. 2005;50(2):271–277. doi: 10.1016/j.archoralbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Archives of oral biology. 2000;45(11):1013–1016. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Ueda M, Ozasa M, Anzai J, Takedachi M, Yanagita M, Ito M, Hashikawa T, Yamada S, Murakami S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J Endod. 2009;35(11):1529–1535. doi: 10.1016/j.joen.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. Dentine as a bioactive extracellular matrix. Archives of oral biology. 2012;57(2):109–121. doi: 10.1016/j.archoralbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 2011;90(8):1013–1018. doi: 10.1177/0022034511408426. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Mizutani S, Nakano M, Hirukawa K, Togari A. Fgf-2 regulates enamel and dentine formation in mouse tooth germ. Calcif Tissue Int. 2003;73(5):496–501. doi: 10.1007/s00223-002-4070-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Huang GT, He W, Wang P, Tong Z, Jia Q, Dong L, Niu Z, Ni L. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J Endod. 2012;38(5):614–622. doi: 10.1016/j.joen.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Esliger A, Hurley MM. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(1):35–45. doi: 10.1002/jbmr.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Liu P, Li X, Doetschman T, Coffin JD, Drissi H, Hurley MM. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J Biol Chem. 2009;284(5):3170–3182. doi: 10.1074/jbc.M804900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamachika E, Tsujigiwa H, Matsubara M, Hirata Y, Kita K, Takabatake K, Mizukawa N, Kaneda Y, Nagatsuka H, Iida S. Basic fibroblast growth factor supports expansion of mouse compact bone-derived mesenchymal stem cells (MSCs) and regeneration of bone from MSC in vivo. Journal of molecular histology. 2012;43(2):223–233. doi: 10.1007/s10735-011-9385-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.