Abstract
Catechol-O-methyl transferase (COMT) plays a key role in the degradation of brain dopamine (DA). Specifically, low COMT activity results in higher DA levels in the prefrontal cortex (PFC), thereby reducing the vulnerability for attentional and cognitive deficits in both psychotic and healthy individuals. COMT activity is markedly reduced by a non-synonymous SNP that generates a valine-to-methionine substitution on the residue 108/158, by means of as-yet incompletely understood posttranslational mechanisms. One posttranslational modification is methionine sulfoxide, which can be reduced by the methionine sulfoxide reductase (Msr) A and B enzymes. We used recombinant COMT proteins (Val/Met108) and mice (wild-type (WT) and MsrA knockout) to determine the effect of methionine oxidation on COMT activity and COMT interaction with Msr, through a combination of enzymatic activity and Western blot assays. Recombinant COMT activity is positively regulated by MsrA, especially under oxidative conditions, while brains of MsrA knockout mice exhibited lower COMT activity (as compared with their WT counterparts). These results suggest that COMT activity may be reduced by methionine oxidation, and point to Msr as a key molecular determinant for the modulation of COMT activity in the brain. The role of Msr in modulating cognitive functions in healthy individuals and schizophrenia patients is yet to be determined.
Keywords: Methionine oxidation, Catechol-O-methyltransferase, prefrontal cortex, oxidative stress, postranslation modification
Introduction
The enzyme catechol-O-methyltransferase (COMT) catalyzes the O-methylation of catecholamine neurotransmitters, such as dopamine (DA) and norepinephrine (Axelrod & Tomchick ,1958), using S-adenosylmethionine (SAM) as a methyl donor (Männistö & Kaakkola,1999). The COMT protein occurs as two distinct isoforms with identical kinetic mechanisms, which result from differential transcriptions of the corresponding gene: a soluble form (S-COMT), found in the cell cytoplasm; and a membrane-bound form (MB-COMT), which features 50 additional residues in the N-terminal portion of the protein (Männistö & Kaakkola,1999; Huh & Friedhoff 1979).
Several lines of evidence have shown that COMT serves a primary role in DA degradation in the prefrontal cortex (PFC) (Karoum et al. 1994; Matsumoto et al. 2003). Given the well-documented implication of DAergic neurotransmission in the modulation of these tasks (Miller & Cohen, 2001; Cohen et al. 2002; Nieoullon, 2002), the functional role of COMT in the PFC has garnered substantial interest. In particular, numerous investigations have focused on rs4680, one of the best-characterized single-nucleotide polymorphisms (SNPs) of the COMT gene, resulting in the substitution of a valine (Val) for a methionine (Met) residue at position 108 of S-COMT and 158 of MB-COMT (Val108/158Met) (Lachman et al. 1996). The Met allele is associated with reduced COMT activity and lower DA metabolism; this functional characteristic has been shown to lead to a more efficient physiological response in the PFC across several functional domains, including cognitive flexibility, working memory, attentional control and emotional resilience (Malhotra et al. 2002; Goldberg et al. 2003; Blasi et al. 2005; Smolka et al. 2005).
The notion that low DAergic activity in the PFC may contribute to negative and cognitive symptoms of schizophrenia patients (Davis et al. 1991; Kahn & Davis, 1995), has also led several authors to investigate the potential influence of the rs4680 polymorphism of COMT on the severity of these deficits in psychotic disorders. Multiple studies have ascertained that the Met108/158 variant is associated with a slightly lower schizophrenia risk, as well as less severity of attentional, cognitive and information-processing deficits (Bray et al. 2003; Egan et al. 2001; Bilder et al. 2002; Gallinat et al. 2003; Tunbridge et al. 2006; Ehlis et al. 2007).
Previous research has shown that the Val108/158Met genotype affects protein expression, but not mRNA expression levels, indicating that this polymorphism may lead to post-translational changes in the protein (Matsumoto et al. 2003; Chen et al. 2004). Indeed, it has been shown that the methionine-to-valine substitution reduces the thermostability of the enzyme without affecting its structural and kinetic properties (Lachman et al. 1996; Lotta et al. 1995). Although the bases of this phenomenon are not fully elucidated, the lower catalytic activity of the Met108/158 variant has been associated with a markedly higher susceptibility to oxidation (Cotton et al. 2004). The most oxidation-sensitive amino acids are the sulfur-containing methionine and cysteine. Indeed, Cotton and co-workers (Cotton et al. 2004) identified a key role of cysteine residues in the vulnerability of the Met108/185 variant to oxidation; the specific role of methionine in this process, however, remains elusive. The oxidation of methionine residues leads to the formation of methionine sulfoxide (MetO); this well-known phenomenon is enhanced by oxidative stress and reversed by the methionine sulfoxide reductase (Msr) system, consisting of two enzymes (termed A and B) that reduce either S or R enantiomers of MetO, respectively (Moskovitz, 2005; Moskovitz et al. 2000, 2002).
In this study, we hypothesized that the lower catalytic activity of the Met108/158 variant may be also contributed by the oxidation of this and other methionine residues. To test for this possibility, we assessed whether COMT activity may be affected by Msr, using a number of complementary in vitro and ex vivo approaches.
Methods
Recombinant human COMT and MsrA proteins
Expression clones for recombinant His-tag soluble human COMT proteins (S-COMT, Val108 and Met108 forms) were kindly provided by Dr. Klinman (University of California, Berkeley). The expression and purification of the recombinant COMT proteins were performed as described by Zhang and Klinman (Zhang & Klinman, 2011). Recombinant His-tagged yeast MsrA protein was expressed and purified as previously described (Moskovitz et al. 1997). Oxidation of recombinant COMT proteins was performed by incubating the proteins with 200mM H2O2 for 24 h at room temperature. Then, the residual H2O2 was removed by dialysis against 25mM Tris-HCl (pH 7.4) at 4°C.
Animals
Wild type (WT) and MsrA knockout (KO) mice (n=10/group) were obtained as previously reported (Moskovitz et al. 2001). Animals were housed in group cages with ad libitum access to food and water. The room was maintained at 22°C, on a 12 h: 12 h light/dark cycle. Experimental procedures were in compliance with the accepted National Institute of Health guidelines (such as “Guiding principles in the care and use of animals” (DHEW Publications, NIH, 80-23) and approved by the Animal Use Committees of the University of Kansas.
Quantification of COMT levels in brains of WT and MsrA KO mice
Mice were euthanized at 6 and 12 months of age, by CO2 asphyxiation followed by cervical dislocation (n=5 per age group). The brains were dissected within 2 minutes of euthanasia and frozen on dry ice. The tissues were stored in −80oC until use. Tissues were homogenized using a Teflon homogenizer in the presence of 25mM Tris-HCl (pH 7.4) and protease inhibitors cocktail (Roche Applied Science, Branford, CT) at 4°C. Following centrifugation at 10,000 x g for 20 minutes, the supernatants were collected and their protein concentrations were assessed using a Bio-Rad (Hercules, CA) assay kit. Equal protein amounts were subjected to SDS-gel electrophoresis followed by western blot analyses using anti-COMT mouse antibodies (BD Biosciences, San Jose, CA) as the primary antibodies. HRP-conjugated goat anti-mouse antibodies (Santa Cruz Biotechnology, Dallas, TX) were used as the secondary antibodies. Anti-ß-actin mouse antibodies (Abcam, Cambridge, MA) were also used on the same blot as primary antibodies (after stripping the blot) for the assessment of protein loading control. Following exposure of the blot to an X-ray film, the resulting protein band images were quantified using the Image-J program (National Institute of Health).
COMT mRNA levels in brains of both mouse genotypes were determined by real-time RTPCR (using primers 1418701 and 144918, Affymetrix, Santa Clara, CA).
Statistical analyses
Normality and homoscedasticity of data distribution were verified by using Kolmogorov-Smirnov and Bartlett's tests. Non-parametric data were normalized by logarithmic transformation. Statistical analyses were performed using ANOVAs, followed by Newman-Keuls test for post-hoc comparisons. Significance threshold was set at 0.05. All statistical analyses were performed by STATISTICA 7 (Statsoft, Tulsa, OK).
Results
Effect of MetO reduction on recombinant COMT activity
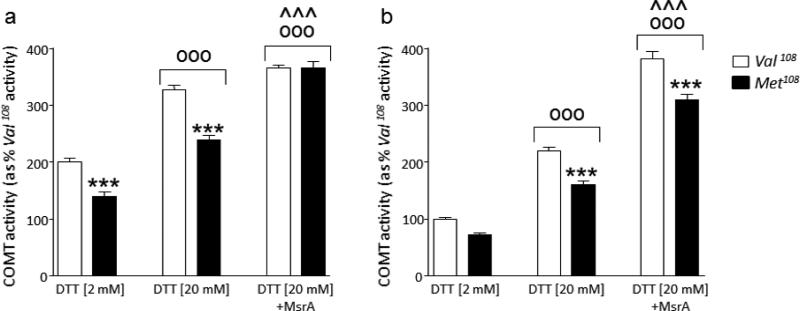
To determine the combined effect of the rs4680 polymorphism and methionine oxidation on S-COMT activity, we monitored the activity of purified His-tagged human recombinant COMT of Val108 (native) and Met108 forms. First, we analyzed the activity of the recombinant enzymes in the presence of 2mM DTT, a reducing agent that maintains cysteine residues in their reduced form (Fig. 1a). Under these conditions, the mean activity (± SEM) of the Met108 COMT variant was 69 ± 7.72% of the native form (P<0.001) (Fig. 1a). Increasing the DTT concentration to 20mM caused a significant increase in the activity of both COMT forms (P<0.001) (Fig. 1a), without affecting the activity gap between them (Fig.1a). The addition of recombinant MsrA in the presence of 20mM DTT further increased the activities of both forms of S-COMT (P<0.05), and ablated their difference in catalytic activity (Fig. 1a), suggesting that COMT activity is negatively affected by methionine oxidation in vitro, especially in the Met108 form. To further elucidate the possible negative effect of oxidizing COMT methionine residues (including Met108) on enzyme activity, both versions of the recombinant S-COMT were exposed to high concentrations of H2O2 prior to monitoring COMT activity. As shown in Fig. 1b, COMT activity was reduced in the Met108 variant in comparison to the Val108 variant, similar to the levels observed under non-oxidizing conditions, in the presence of DTT only. However, although the addition of recombinant MsrA caused significant increases in the activities of both COMT variants (P<0.001) (Fig. 1b), there was a significant gap between the two COMT variants. These data suggest that, under severe oxidative conditions in vitro, the difference in activity between Val108 and Met108 forms is not fully rescued.
Figure 1.
Effects of dithiothreitol (DTT) and MsrA on the activities of COMT Val108 and Met108 variants in vitro under (a) standard conditions and (b) high H2O2 concentrations. Values are displayed as means ± SEM. All analyses were run by 2-way ANOVAs, followed by Newman-Keuls test for post-hoc comparisons. ***, P<0.001 for comparisons vs corresponding Val108 (genotype x treatment interaction); °°°, P<0.001 for comparison vs 2mM DTT (Main treatment effect); ^^^, P<0.01 for comparison vs 20mM DTT (Main treatment effect). Main genotype effects are not shown.
Genetic ablation of MsrA leads to significant reduction of COMT activity in the mouse brain
To test the relevance in vivo of the relation between MsrA and COMT, we measured the activity of the latter enzyme in the brains of MsrA KO mice, as compared with WT counterparts. The primary sequences of murine and human COMT share sequence homology for 5 methionine residues. The mouse protein contains additional methionine residues at positions 93 and 244 (Table 1), and features a leucine residue (a hydrophobic amino acid like valine and methionine) in the position 151, homologue to 108/158 in human COMT (Table 2).
Table 1.
Protein sequence homology alignment between mouse and human catechol-O-methyl transferase (COMT). Met (M) residues are in bold and 158 Val of the human COMT is both in bold and underlined
| Mouse | 20 | RHLGWGLVAIGWFEFVQQPVHNLLMGGTKEQRILRHVQQHAKPGDPQSVLEAIDTYCSEK RH GWGL IGW EF+QP+HNLLMG TKEQRIL HV QHA+PG+QSVLEAIDTYC+K |
79 |
| Human | 27 | RHWGWGLCLIGWNEFILQPIHNLLMGDTKEQRILNHVLQHAEPGNAQSVLEAIDTYCEQK | 86 |
| Mouse | 80 | EWAMNVGDAKGQIMDAVIREYRPSLVLELGAYCGYSAVRMARLLPPGARLLTMEINPDYA EWAMNVGD KG+I+DAVI+E++PS++LELGAYCGYSAVRMARLL PGARL+T+EINPD A |
139 |
| Human | 87 | EWAMNVGDKKGKIVDAVIQEHQPSVLLELGAYCGYSAVRMARLLSPGARLITIEINPDCA | 146 |
| Mouse | 140 | AITQQMLDFAGLQDKVSILIGASQDLIPQLKKKYDVDTLDMVFLDHWKDRYLPDTLLLEE AITQ+M+DFAG++DKV++++GASQD+IPQLKKKYDVDTLDMVFLDHWKDRYLPDTLLLEE |
199 |
| Human | 147 | AITQRMVDFAG VKDKVTLVVGASQDIIPQLKKKYDVDTLDMVFLDHWKDRYLPDTLLLEE | 206 |
| Mouse | 200 | CGLLRKGTVLLADNVIVPGTPDFLAYVRGSSSFECTHYSSYLEYMKVVDGLEKAVYQGPG CGLLRKGTVLLADNVI PG PDFLA+VRGSS FECTHY S+LEY+VVDGLEKA+Y+GPG |
259 |
| Human | 207 | CGLLRKGTVLLADNVICPGAPDFLAHVRGSSCFECTHYQSFLEYREVVDGLEKAIYKGPG | 266 |
| Mouse | 260 | SS S |
261 |
| Human | 267 | SE | 268 |
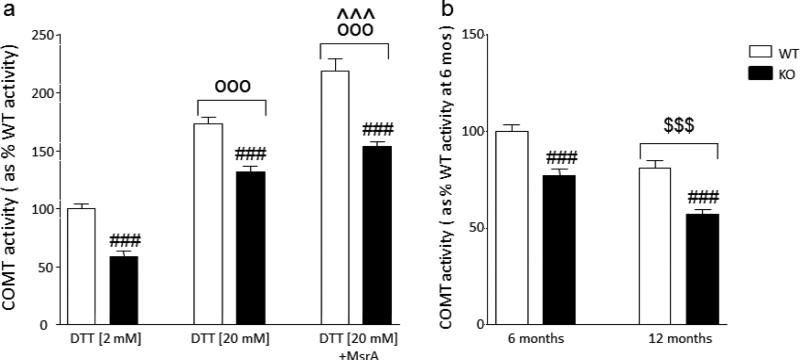
The brains of MsrA KO mice exhibited a marked reduction in COMT activity [Main genotype effect: F(1,24)=175.05, P<0.001] (Fig. 2a). The increase of DTT concentration to 20mM produced a significant enhancement in COMT activity in both WT and KO mice (173% and 132% in comparison to WT and MsrA KO treated with 2 mM DTT, respectively; Main treatment effect: F(1,24)=105.46, P<0.001 in comparison with 2 mM DTT). Finally, the addition of recombinant MsrA protein to the assay reaction (in the presence of 20mM DTT) further significantly increased COMT activity across both genotypes (Ps<0.001 compared with both 2 mM and 20 mM DTT (Fig. 2a). Notably, ANOVA failed to identify a significant genotype x treatment interaction, indicating that the treatment with DTT and MsrA did not abrogate the difference between genotypes.
Figure 2.
(a) Effects of DTT and MsrA on the brain activity of COMT in WT and MsrA KO mice, and (b) Effects of aging on COMT activity in WT and MsrA KO mice. Values are displayed as means ± SEM. All analyses were run by 2-way ANOVAs, followed by Newman-Keuls test for post-hoc comparisons. ###, P<0.001 for comparisons vs corresponding WT group (Main genotype effect); °°°, P<0.001 for comparison vs 2mM DTT (Main treatment effect); ^^^, P<0.01 for comparison vs 20mM DTT (Main treatment effect); $$$, P<0.001 for comparison vs 6 months (Main age effect).
We then analyzed whether the differences in COMT activity between WT and MsrA KO brains may be influenced by aging (Fig. 2b). While ANOVA found significant main effects for genotype [F(1,16)=47.40, P<0.001] and age [F(1,16)=32.64, P<0.001], no significant interaction between these two effects was found, indicating that the age-dependent decline in COMT activity was not dependent on MsrA genotype.
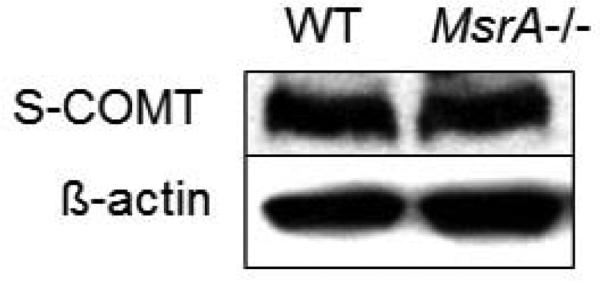
To confirm that the observed difference in COMT activity between the two mouse genotypes is not due to a difference in COMT mRNA/ protein expression levels, COMT protein and mRNA levels were determined in brains of both WT and MsrA KO mice. Accordingly, western blot analysis and real-time PCR determination of COMT's mRNA levels were performed. As shown in Fig. 3, the protein levels of COMT were equivalent across both mouse genotypes. Complementary to these data, there was no significant difference in the mRNA levels between the two mouse genotypes (data not shown).
Figure 3.
Representative blots from Western blot analyses (n=5) showing COMT expression in comparison with β-actin expression in brain samples from WT and MsrA KO mice.
Discussion
The findings of this study converge in support of a regulatory role of Msr on COMT activity in the brain, and, more specifically, the PFC. We documented that MsrA countered the reduction of COMT activity induced by oxidation; this effect was not only limited to the Met108 variant, but targeted also other methionine residues, as suggested by the ability of MsrA to enhance the catalytic activity of the Val108 variant (Fig. 1). In addition, we found that in the mouse brain, COMT activity was reduced by genetic MsrA deficiency and increased by addition of recombinant MsrA; nevertheless, the latter intervention did not fully rescue the deficits of COMT activity in MsrA KO mice. This phenomenon suggests that in these mutants, COMT may feature posttranslational modifications that may interfere with the reduction of R-MetO residues (Fig. 2). Supportive evidence for the involvement of posttranslational modifications to the observed reduced COMT activity in MsrA KO mice arises from the similar expression levels of COMT's mRNA (data not shown) and protein (Fig. 3) in both mouse strains.
These results suggest that COMT activity may be reduced by the oxidation of its methionine residues, and complements previous evidence documenting the implication of cysteine (the other sulfur-containing amino acid) in the higher susceptibility of the Met108/158 variant to oxidation (Cotton et al. 2004). Previous data have shown that methionine oxidation causes conformational changes (Berlett et al. 1996) and increases the hydrophobicity of proteins (Chao et al. 1997). Interestingly, the increase in hydrophobicity due to MetO formation has also been shown to enhance the proteolytic susceptibility of proteins (Levine et al. 1996), suggesting that the Met108/158 variant may be more vulnerable to protease-mediated degradation. Accordingly, the Met108/158 variant exhibits 20% of its activity at physiological temperature; this phenomenon may be due to the observed reduction in the protein expression level (possibly because of its enhanced degradation rate), but not mRNA expression level in brain tissue (Matsumoto et al. 2003; Chen et al. 2004; Lotta et al. 1995). The robust association between Msr and COMT is particularly noteworthy, in view of the primary role of COMT in the regulation of DA homeostasis in the PFC (Matsumoto et al. 2003; Gogos et al.1998). High COMT activity, such as that associated with the Val108/158 variant, leads to increased DA turnover, which may result in a higher predisposition for a number of information-processing, attentional, executive and cognitive deficits in patients with psychosis, as well as healthy individuals (Kahn & Davis, 1995; Weinberger et al. 2001, 2002; Barnett et al. 2007; Ira et al. 2013). Furthermore, it should be noted that alterations in oxidative stress in the PFC have been associated with multiple psychiatric disorders, including schizophrenia, bipolar disorder and major depression (Michel et al. 2007; Gawryluk et al. 2011; Andreazza et al. 2013), as well as cognitive and motivational deficits in mouse models (Johnson et al. 2013). These premises highlight the potential importance of Msr as a moderator of COMT function with respect to these functional domains regulated by the PFC. Future studies are needed to test this interesting hypothesis.
Previous studies have shown that MsrA KO mice exhibit high levels of DA in the brain (and, particularly, in the striatum), raising the possibility that the reduction in COMT activity may be partially responsible for such changes. Nevertheless, the contribution of COMT is unlikely to fully account for the enhancement of DA levels in MsrA KO mice; indeed, COMT KO mice only featured increases in DA in the PFC, but not in the striatum (Gogos et al.1998).
One of the main limitations of this study is that our observations were based on S-COMT, which is posited to play a relatively less important role in DA degradation in comparison with MB-COMT. Even with this limitation, the identification of a link between Msr and COMT activity points to the potential importance of methionine oxidation in the modulation of prefrontal activity and the pathophysiology of cognitive impairments of schizophrenia. Future clinical studies are warranted to test the implication of Msr in the function of COMT, particularly with respect to the regulation of dopamine neurotransmission and pathophysiology of schizophrenia.
Acknowledgements
This research was supported by grants from NICHD (R21HD070611, to MB) and the Hedwig Miller Fund for Aging Research (to JM). None of the institutions had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- COMT
Catechol-O-methyltransferase
- DA
dopamine
- WT
wild-type
- KO
knockout
- Msr
Methionine sulfoxide reductase
- PFC
prefrontal cortex
- SAM
S-adenosylmethionine
- MetO
Methionine sulfoxide
- DTT
dithiothreitol
Footnotes
Statement of Interest:
None.
References
- Andreazza AC, Wang JF, Salmasi F, Shao L, Young LT. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013 doi: 10.1111/jnc.12316. in press. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233(3):702–705. [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12(5):502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Friguet B, Yim MB, Chock PB, Stadtman ER. Peroxynitrite-mediated nitration of tyrosine residues in Escherichia coli glutamine synthetase mimics adenylylation: relevance to signal transduction. Proc Natl Acad Sci U S A. 1996;93(5):1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Kunz M, Chakos M, Cooper TB, Lieberman JA. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52(7):701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73(1):152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Ma YS, Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Natl Acad Sci U S A. 1997;94(7):2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cotton NJ, Stoddard B, Parson WW. Oxidative inhibition of human soluble catechol-O-methyltransferase. J Biol Chem. 2004;279(22):23710–23718. doi: 10.1074/jbc.M401086200. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. J Psychiatry. 1991;148(11):1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlis AC, Reif A, Herrmann MJ, Lesch KP, Fallgatter AJ. Impact of catechol-O-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders. Neuropsychopharmacology. 2007;32(1):162–170. doi: 10.1038/sj.npp.1301151. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Bajbouj M, Sander T, Schlattmann P, Xu K, Ferro EF, Goldman D, Winterer G. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry. 2003;54(1):40–48. doi: 10.1016/s0006-3223(02)01973-x. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14(1):123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger D. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60(9):889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Huh MM, Friedhoff AJ. Multiple molecular forms of catechol-O-methyltransferase. Evidence for two distinct forms, and their purification and physical characterization. J Biol Chem. 1979;254(2):299–308. [PubMed] [Google Scholar]
- Ira E, Zanoni M, Ruggeri M, Dazzan P, Tosato S. COMT, neuropsychological function and brain structure in schizophrenia: a systematic review and neurobiological interpretation. J Psychiatry Neurosci. 2013;38(3):120178. doi: 10.1503/jpn.120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013;110(30):12462–12467. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Davis KL. New developments in dopamine and schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1993–1204. [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93(26):15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28(8):1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Michel TM, Frangou S, Thiemeyer D, Camara S, Jecel J, Nara K, Brunklaus A, Zoechling R, Riederer P. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder--a postmortem study. Psychiatry Res. 2007;151(1-2):145–150. doi: 10.1016/j.psychres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703(2):213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci U S A. 1997;94(18):9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Poston JM, Berlett BS, Nosworthy NJ, Szczepanowski R, Stadtman ER. Identification and characterization of a putative active site for peptide methionine sulfoxide reductase (MsrA) and its substrate stereospecificity. J Biol Chem. 2000;275(19):14167–14172. doi: 10.1074/jbc.275.19.14167. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Singh VK, Requena J, Wilkinson BJ, Jayaswal RK, Stadtman ER. Purification and characterization of methionine sulfoxide reductases from mouse and Staphylococcus aureus and their substrate stereospecificity. Biochem Biophys Res Commun. 2002;290(1):62–65. doi: 10.1006/bbrc.2001.6171. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia, the prefrontal cortex, and a mechanism of genetic susceptibility. Eur Psychiatry Suppl. 2002;4:355s–362s. doi: 10.1016/s0924-9338(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Karayiorgou M. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Klinman JP. Enzymatic methyl transfer: role of an active site residue in generating active site compaction that correlates with catalytic efficiency. J Am Chem Soc. 2011;133(43):17134–17137. doi: 10.1021/ja207467d. [DOI] [PMC free article] [PubMed] [Google Scholar]