Abstract
Most clinical strains of Pseudomonas aeruginosa, a leading agent of nosocomial infections, are multiresistant to antibiotherapy. Because of the paucity of new available antibiotics, the investigation of strategies aimed at limiting the action of its major virulence factors has gained much interest. The type 3 secretion system of P. aeruginosa and its effectors are known to be major determinants of toxicity and are required for bacterial dissemination in the host. Bacterial transmigration across the vascular wall is considered to be an important step in the infectious process. Using human endothelial primary cells, we demonstrate that forskolin (FSK), a drug inducing cyclic AMP (cAMP) elevation in eukaryotic cells, strikingly reduced the cell retraction provoked by two type 3 toxins, ExoS and ExoT, found in the majority of clinical strains. Conversely, cytotoxicity of a strain carrying the type 3 effector ExoU was unaffected by FSK. In addition, FSK altered the capacity of two ExoS/ExoT strains to transmigrate across cell monolayers. In agreement with these findings, other drugs and a cytokine inducing the increase of cAMP intracellular levels have also protected cells from retraction. cAMP is an activator of both protein kinase A and EPAC, a GTPase exchange factor of Rap1. Using activators or inhibitors of either pathway, we show that the beneficial effect of FSK is exerted by the activation of the EPAC/Rap1 axis, suggesting that its protective effect is mediated by reinforcing cell-cell and cell-substrate adhesion.
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen and a leading agent of nosocomial infections. The largest cohorts of P. aeruginosa-infected patients are found in three pathological settings: ventilator-associated pneumonia, bacteremia, and cystic fibrosis. In acute infections, P. aeruginosa disseminates from the primary infection site to the blood and other organs, leading to sepsis and multiple organ failure. From a clinical point of view, vascular barrier breakdown is thus considered to be a key step in the pathophysiology of infection (1).
Most P. aeruginosa clinical isolates are multidrug or even extremely drug resistant to antibiotics, which explains the high fatality rates of P. aeruginosa infections. The pathogen has been recently included in a family of so-called “ESKAPE” bacterial pathogens, a group which also includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Enterobacter species, that are able to efficiently “escape” the effects of available antibacterial drugs and for which there is an urgent need for developments of novel types of drugs (2, 3). In this context, the investigation of new strategies limiting the action of the virulence factors rather than bactericidal agents has gained much interest.
P. aeruginosa is extremely well equipped in virulence determinants, which are membrane-embedded protein machineries dedicated for effector/toxin export (4). The type 3 secretion system (T3SS) and its effectors are recognized as the most important virulence factor, based on clinical studies and animal models of infection (5–8). Notably, the T3SS effectors are required for bacterial dissemination in the body (8). The T3SS consists of an injectisome that is built up in the bacterial envelope by a dozen proteins encoded in the P. aeruginosa chromosome (9–11). This molecular syringe is devoted to secretion and translocation of exotoxins directly into the cytoplasm of target cells. Four exotoxins have been identified, ExoS, ExoT, ExoY, and ExoU, but most strains secrete a maximum of three type 3 toxins, ExoS and ExoU being mutually exclusive. A large number of studies investigated the cellular targets of these toxins. The most powerful toxin, ExoU, encoded by ca. 30% of the strains (12, 13), is a highly efficient phospholipase provoking rapid plasma membrane disruption (10, 13, 14). However, the most frequent isolates secrete ExoS and ExoT, two highly homologous bifunctional toxins. Both ExoS and ExoT possess a GTPase-activating domain that inhibits the activity of Rho, Rac, and Cdc42, three GTPases organizing the actin cytoskeleton. ExoS also harbors a potent ADP-ribosyltransferase activity targeting and inhibiting various GTPases, including Rac, Cdc42, some Ras and Rab family proteins, and the ezrin, radixin, and moesin family of proteins (reviewed in references 10 and 15). ExoT ADP-ribosyltransferase activity targets Crk1 and Crk2, two adaptors located in the focal contact complex. The main consequence of ExoS/ExoT action at the cell level is the dismantlement of the actin cytoskeleton and the focal contacts, leading to cell retraction (16). ExoY is a potent adenylate cyclase, which has no effect on cell retraction when injected alone and even induces a slight but significant spreading when cells are infected at a low multiplicity of infection (MOI) with a mutant strain secreting ExoY as the sole type 3 toxin (16, 17), while ExoY induces the disruption of the microtubules at a high MOI and longer infection times (18).
Investigations aimed at preventing the action of P. aeruginosa's virulence factors involve chemical agents altering the quorum sensing or the lectins, as well as monoclonal antibodies directed against the T3SS or lipopolysaccharides and proteins of the bacterial envelope (reviewed in reference 19). Although these approaches are promising and tested in clinical trials (ClinicalTrials.gov identifiers NCT01455675, NCT00851435, NCT00638365, NCT00876252, and NCT01563263), none are used in the clinic yet. Taking into account the tight interplay between bacterial effectors and host proteins for establishing infection, alternative antivirulence approaches may rely on targeting eukaryotic components required for effector function. Here, we investigated the action of drugs known to alter eukaryotic signaling pathways on T3SS-dependent P. aeruginosa toxicity, using human endothelial cell monolayers as a model of the vascular barrier. We found that forskolin (FSK), a drug elevating intracellular cyclic AMP (cAMP) levels in the host, significantly reduced ExoS/T-induced cell retraction in endothelial cells. FSK affects ExoS/T toxicity by using the EPAC/Rap1 signaling pathway, rather than protein kinase A (PKA) activation. Other synthetic or natural agents triggering cAMP synthesis similarly protected the cells from P. aeruginosa-dependent retraction.
MATERIALS AND METHODS
Reagents.
Antibodies to β-actin were from Sigma-Aldrich. The secondary antibodies coupled to Alexa 488 were purchased from Invitrogen, and those coupled to peroxidase were from Jackson ImmunoResearch. PopB and PcrV antibodies were previously described (20). FSK, H89, prostaglandin E2 (PGE2), salmeterol, and rolipram were purchased from Sigma-Aldrich, and N-benzoyl–cAMP (BNZ) and 8-(4-chlorophenylthio)-2′-O-methyl–cAMP (CPT) were purchased from Biolog.
Bacterial strains and culture.
The P. aeruginosa strains used in the present study are described in Table 1. P. aeruginosa was grown in liquid Luria broth (LB) medium at 37°C with agitation until the cultures reached an optical density at 600 nm of 1.0.
TABLE 1.
P. aeruginosa strains used in this study
| Strain | Description | Abbreviation | Reference(s) |
|---|---|---|---|
| PAO1F (RP1831) | Wild-type PAO1 (exoS exoT exoY) | Pa-WT | 43 |
| CHA | Wild-type CHA (exoS exoT exoY) | CHA | 44 |
| PP34 | Wild-type PP34 (exoU exoT exoY) | PP34 | 12 |
| PAO1FΔST (RP1947) | PAO1F ΔexoS ΔexoT | Pa-Y | 8, 45 |
| PAO1FΔSY (RP1924) | PAO1F ΔexoS ΔexoY | Pa-T | 8, 45 |
| PAO1FΔTY (RP1948) | PAO1F ΔexoT ΔexoY | Pa-S | 8, 45 |
| PAO1FΔpscD (RP1871) | PAO1F devoid of T3SS | PaΔpscD | 45 |
| PAO1FΔY (RP562) | PAO1F ΔexoY | Pa-ST | 8 |
| PAO1FΔ3Tox/exoSADPRT− | PAO1F only secreting ExoS mutated in the ADPRT domain | Pa-SADPRT- | 16 |
| PAO1ΔSTY/exoS-bla | PAO1F strain with deletions of exoS, exoT, and exoY genes and chromosomal insertion of the exoSADPRT-GAP-/β-lactamase fusion gene | PAO1ΔSTY/exoS-bla | 25 |
Cell culture and infection.
Human umbilical vein endothelial cells (HUVECs) were isolated according to previously described protocols (16). Recovered cells were cultured in endothelial-basal medium 2 (EBM-2; Lonza) supplemented as recommended by the manufacturer. Medium was replaced 30 min before infection with fresh nonsupplemented medium. Cells were infected with a bacterial MOI of 10 and infection was stopped at 4 h postinfection (hpi) by cell lysis or fixation. For microscopy experiments, cells were grown on glass coverslips previously coated with fibronectin.
Cell retraction assay.
The cell retraction assay method was described previously (16). HUVECs were seeded at 105 in 24-well plates containing a fibronectin-coated glass coverslip and left confluent for 48 h. The cell medium was changed 30 min before infection with fresh nonsupplemented EBM-2, including drugs in some experiments. Then, cells were infected with late-exponential-phase bacteria at an MOI of 10. At the indicated times, cells were fixed in methanol at −20°C for 10 min and labeled for actin. Images were captured with a low-magnification objective lens (×16) and examined using ImageJ software. Briefly, images of actin staining were binarized, and the total cell surface was calculated for each image.
Cytotoxicity assay.
Cell death was evaluated by measuring the lactate dehydrogenase (LDH) released in the supernatant, as previously described (21). Briefly, strains were grown to late exponential phase (A600 = 1) and used to infect cells at an MOI of 10. The LDH was measured at different time points using a cytotoxicity detection kit from Roche Applied Science, following the recommended protocol.
Bacterial transmigration across cell monolayers.
HUVECs were seeded onto tissue culture inserts containing porous membranes (Greiner Thincert, 3-μm pore size). After 2 days of confluence, the cells were infected as described above by the addition of bacteria in the upper compartment. Bacterial transmigration was measured by plating serial dilutions of samples withdrawn in the lower compartment on Pseudomonas isolation agar plates, followed by CFU counting. The results shown were calculated for the total volume of the lower compartment.
Rap GTPase activity assay.
Assays were performed in triplicate using a pull-down kit from Cytoskeleton. Briefly, the active form of Rap1 (Rap1-GTP) was pulled down using the Rap1-binding domain from one of its effectors, RalGDS, fused to the glutathione S-transferase and glutathione beads. Protein precipitates, as well as the total extracts, were electrophoresed in parallel to monitor active and total Rap1, respectively. Proteins were analyzed by Western blotting with Rap1 antibody, and image capture was performed with a Chemidoc system from Bio-Rad, allowing direct measurement of signal intensities. Only signals in the linear range of sensitivity were used.
Mass spectrum analysis of Rap1 modifications.
Thirty micrograms of HUVEC Triton X-100-extracted proteins was electrophoresed using SDS-PAGE. For trypsin digestion, 0.15 μg of modified trypsin (Promega, sequencing grade) in 25 mM NH4HCO3 was added to a hydrated piece of gel for an overnight incubation at 37°C. The peptides were then extracted from gel pieces in three 15-min sequential extraction steps with 30 μl of 50% acetonitrile, 30 μl of 5% formic acid, and finally 30 μl of 100% acetonitrile. The pooled supernatants were then dried under vacuum. These operations were performed automatically using a robot apparatus (EVO150; Tecan).
For nano-liquid chromatography-tandem mass spectrometry (nano-LC-MS/MS) analysis, the dried extracted peptides were resuspended in water containing 2.5% acetonitrile and 2.5% trifluoroacetic acid. Nano-LC-MS/MS analysis was then performed (Ultimate 3000, Dionex, and LTQ-Orbitrap XL; Thermo Fischer Scientific). The method consisted of a 40-min gradient at a flow rate of 300 nl/min using a gradient from solvent A (5% acetonitrile and 0.1% formic acid in water) and solvent B (80% acetonitrile and 0.08% formic acid in water). The system includes a 300-μm-by-5-mm PepMap C18 precolumn and a 75-μm-by-250-mm C18 PepMap. MS and MS/MS data were acquired using Xcalibur (Thermo Fischer Scientific) and processed automatically using Mascot Daemon software (Matrix Science).
Intranet version 2.4 of Mascot was used for the searches performed on a Uniprot_decoy database using “human” as the taxonomy. Peptide modifications allowed during the search were set as follows: fixed modifications, carbamidomethyl (C), and variable modifications, oxidation (M) and ADP-ribosylation (K and R). The other parameters were as follows: peptide tolerance, 10 ppm; MS/MS tolerance, 0.8 Da; three missed cleavage sites by trypsin allowed.
Statistical analysis.
For single comparison, a two-tailed Student t test was used. For multiple comparisons, a one-way analysis of variance (ANOVA) test was performed, followed by Dunnett's test for differences with the control or Tukey's test for pairwise comparisons. Statistics were calculated using SigmaPlot software.
RESULTS
FSK protects endothelial cells from P. aeruginosa-induced retraction.
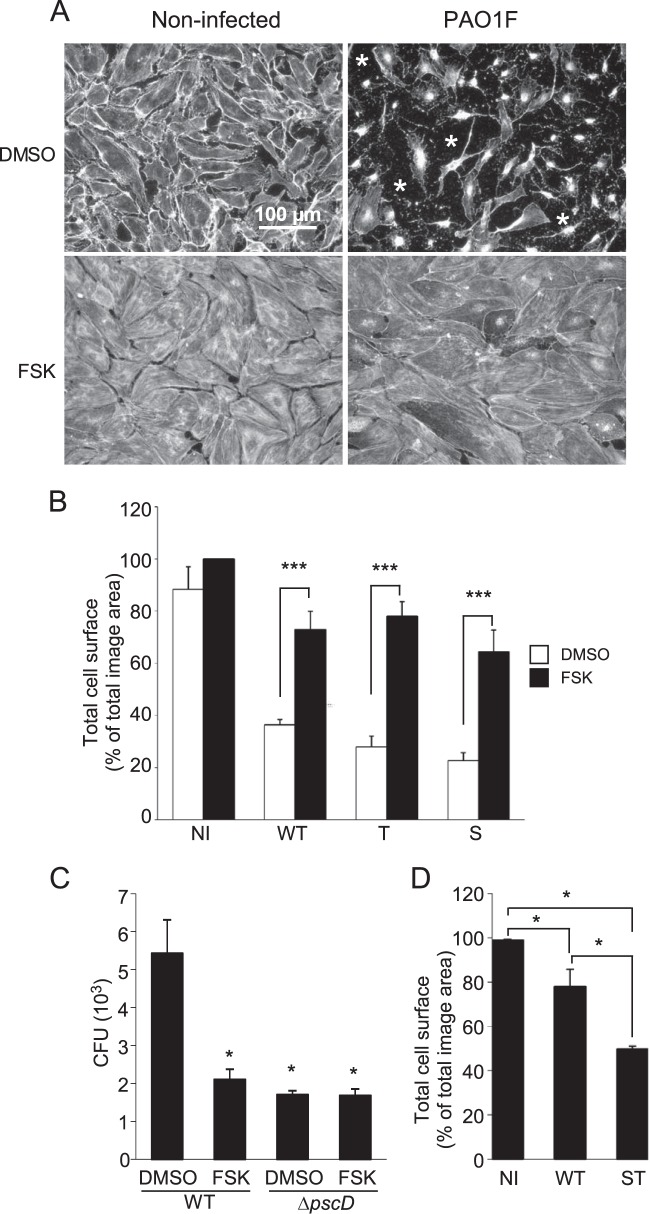
The major observable effect of ExoS/ExoT toxin injection in endothelial cells is a striking cell retraction, subsequent to the disruption of the actin cytoskeleton and the loss of the focal contacts (16). We reasoned that preventing the disassembly of these two cellular components could slow down the intoxication process and the breakdown of the endothelial barrier. Hence, we screened several pharmacological molecules known to act on the actin cytoskeleton and cell adhesion to examine whether one of them could inhibit P. aeruginosa-induced cell retraction. In this experiment, we used primary endothelial cells derived from human umbilical cords (HUVECs) infected by the P. aeruginosa strain PAO1F (Table 1). This strain is known to inject exotoxins S, T, and Y through its T3SS. At 3 hpi with PAO1F at an MOI of 10, the cells were fixed, labeled with anti-β-actin antibody to stain the entire cell body, and observed by fluorescence microscopy. As illustrated in Fig. 1A, the cells exhibited a massive retraction when experiments were performed in the presence of dimethyl sulfoxide (DMSO; the vehicle control), confirming previous studies. One of the tested molecules, forskolin (FSK), was able to inhibit P. aeruginosa-induced cell retraction (Fig. 1A). This experiment was reproduced many times with identical results. FSK is a potent stimulator of adenylate cyclase that raises cAMP intracellular levels (see Fig. S1A in the supplemental material), which in turn positively impacts the actin cytoskeleton, the cell-cell junctions and cell-matrix adhesion (22–24).
FIG 1.
Forskolin decreases P. aeruginosa-induced endothelial cell retraction and monolayer permeability. (A) Confluent HUVECs were infected for 4 h with PAO1F at an MOI of 10, in the presence of DMSO or FSK at 10 μM, as indicated. Cells were fixed and labeled with anti-actin antibody to label the entire cell body. Noninfected controls (NI) are shown on the left. *, Absence of cell coverage. (B) HUVECs were infected with Pa-WT (WT), Pa-T (T), or Pa-S (S) in the presence of DMSO or FSK. The total cell surface was calculated by measuring the total surface occupied by cells in actin immunofluorescence images. The data represent the mean percentages of the cell surface (plus the standard deviations [SD]) calculated for three images. As determined by using a Student t test, FSK induced a significant increase (***, P < 0.001) compared to DMSO treatment for each strain type. (C) Confluent HUVECs grown on a porous membrane (see Materials and Methods) were infected in the upper compartment with Pa-WT or PaΔpscD (ΔpscD) at an MOI of 10 in the presence of DMSO or FSK (10 μM). At 1 hpi, samples of the lower compartment were assessed for the presence of P. aeruginosa CFU. The data represent the mean number of CFU calculated for the total compartment (plus the SD) in three independent wells. Statistics were established by multiple-comparison one-way ANOVA (P < 0.001), followed by Dunnett's test for comparison with Pa-WT/DMSO (*, P < 0.05). (D) Cell retraction assay as in panel A, except that HUVECs were infected for 2 h with Pa-WT or Pa-ST (ST) or were uninfected. A multiple-comparison one-way ANOVA (P < 0.001) was performed, followed by Tukey's test for pairwise comparisons (*, P < 0.05). All data shown are representative of three independent experiments.
The effect of FSK on cell retraction was quantified by measuring the surface occupied by the cells, which were confluent before infection, in three microscopic fields at low magnification. As shown in Fig. 1B, FSK strongly decreased the cell retraction induced by PAO1F. Furthermore, FSK was also efficient when cells were infected with P. aeruginosa strains injecting only ExoS or ExoT (Pa-S or Pa-T; Fig. 1B). To further establish the protective effect of FSK, we measured the capacity of the bacteria to transmigrate through a monolayer of endothelial cells in a Boyden chamber assay (Fig. 1C). Bacteria were loaded in the upper compartment, and samples were withdrawn in the lower compartment at 2 hpi for CFU counting. Transmigration was significantly delayed when the cells were infected in the presence of FSK, hence confirming the capacity of FSK to preserve the endothelial barrier. A mutant of PAO1F devoid of T3SS, strain PaΔpscD, was also tested in this assay to evaluate the bacterial transmigration due to factors other than the T3SS (Fig. 1C). Strain PaΔpscD levels were significantly reduced compared to those of the wild-type in the absence of FSK (2.6-fold) but were similar to the wild-type in the presence of FSK, indicating that the T3SS-dependent bacterial transmigration was abrogated by FSK. Interestingly, FSK did not influence PaΔpscD transmigration (Fig. 1C). This feature suggests that FSK has no effect on transmigration besides its role on the type 3 toxin-dependent effects.
As previously mentioned, ExoY possesses an adenylate cyclase activity once injected into host cells that may have a similar effect as FSK and counteract ExoS/T action. To test this hypothesis, we infected HUVECs with a mutant devoid of ExoY but secreting ExoS and ExoT, i.e., Pa-ST, and compared its effect to that of PAO1F in a cell retraction assay (Fig. 1D). At 4 hpi, we did not measure a significant difference between Pa-ST and PAO1F retraction capacities (data not shown). However, at the beginning of the retraction process (2 hpi), retraction was significantly increased with Pa-ST (Fig. 1D), suggesting that ExoY attenuates the action of ExoS/T at early time points.
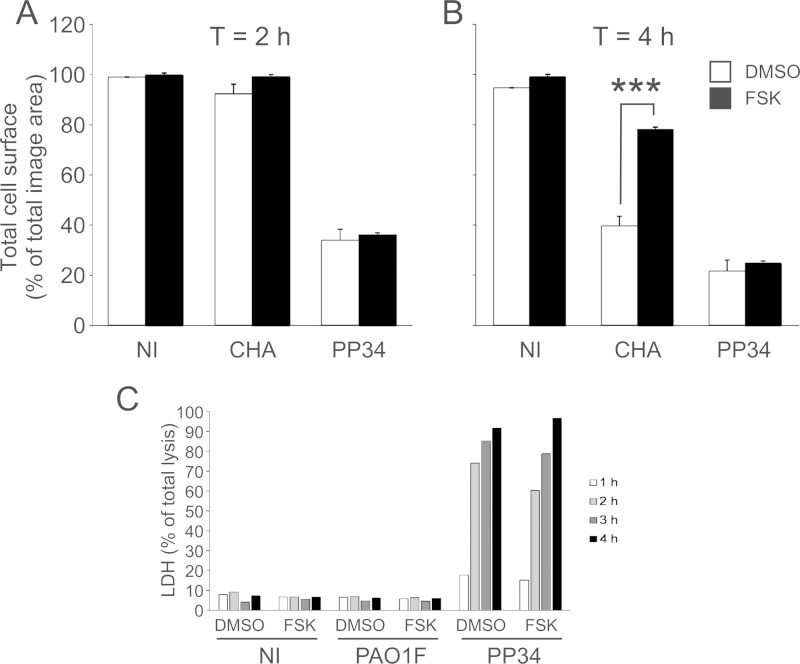
The ability of FSK to prevent P. aeruginosa-induced retraction was also tested on two other P. aeruginosa strains: CHA, which also injects ExoS, ExoT, and ExoY, and PP34, which injects ExoU, ExoT, and ExoY. As shown in Fig. 2A and B, the action of CHA was prevented by FSK, whereas this compound had no effects on PP34-induced cell retraction. Similarly, FSK did not protect cells from ExoU-dependent membrane permeabilization (Fig. 2C). As expected, PAO1F or CHA (Fig. 2C and data not shown) had no effect on plasma membrane permeability. Altogether, these data suggest that FSK may antagonize the action of ExoS and ExoT once injected into the target cells. FSK likely does not affect the injection or secretion capacities of the T3SS or limits bacterial growth.
FIG 2.
Forskolin action depends on the type of type 3 toxin. HUVECs were infected with CHA and PP34 strains at an MOI of 10, and the cells were fixed at 2 hpi (A) or 4 hpi (B). As established using a Student t test, FSK induced a significant increase (***, P < 0.001) when the cells were infected with CHA compared to DMSO, but not when the cells were infected with PP34. The data are representative of three independent experiments. (C) HUVECs were infected by PAO1 or PP34 at an MOI of 10 in the presence or absence of FSK at 10 μM. Cell death was examined by measuring the LDH released in the supernatant at different time points using a colorimetric assay. The data are presented as the percentages of total cell lysis, obtained after treatment with 1% Triton X-100. FSK did not protect cells from ExoU-dependent cell lysis.
To unravel these various possibilities, we performed a series of experiments aiming at characterizing the effect of FSK on P. aeruginosa. We first investigated the effect of various concentrations of FSK on the synthesis and secretion of two T3SS components, PopB and PcrV, when triggered by a low-calcium switch (see Fig. S2A in the supplemental material). Under all conditions, the secreted proteins were present in similar amounts in the secretome, thus showing that FSK did not influence the production of T3SS components. The effectiveness of T3SS injection into HUVECs in the presence of FSK was examined by using a mutant strain of PAO1F devoid of type 3 toxin genes that has been complemented with an ADPRT- and GAP-inactive form of ExoS coupled to β-lactamase used as a reporter enzyme (25). After infection, endothelial cells were loaded with a bifluorescent substrate of β-lactamase, CCF2, emitting a green fluorescence resonance energy transfer (FRET) signal. Once cleaved by the enzyme, the energy transfer between the two fluorophores is lost, and T3S-injected cells are revealed by a blue fluorescence. As shown in Fig. S2B in the supplemental material, the percentages of injected cells were similar whether HUVECs were infected in the presence or in the absence of FSK. Furthermore, bacterial growth was not affected by FSK (see Fig. S2C in the supplemental material). Altogether, these data demonstrate that FSK exerts its protective role by an action on the host rather than an alteration of P. aeruginosa virulence or growth. Furthermore, FSK did not alter the injection capacity of the T3S apparatus either on the bacterial side or on the host side.
Induction of EPAC/Rap1 pathway is crucial for the protective effect of forskolin.
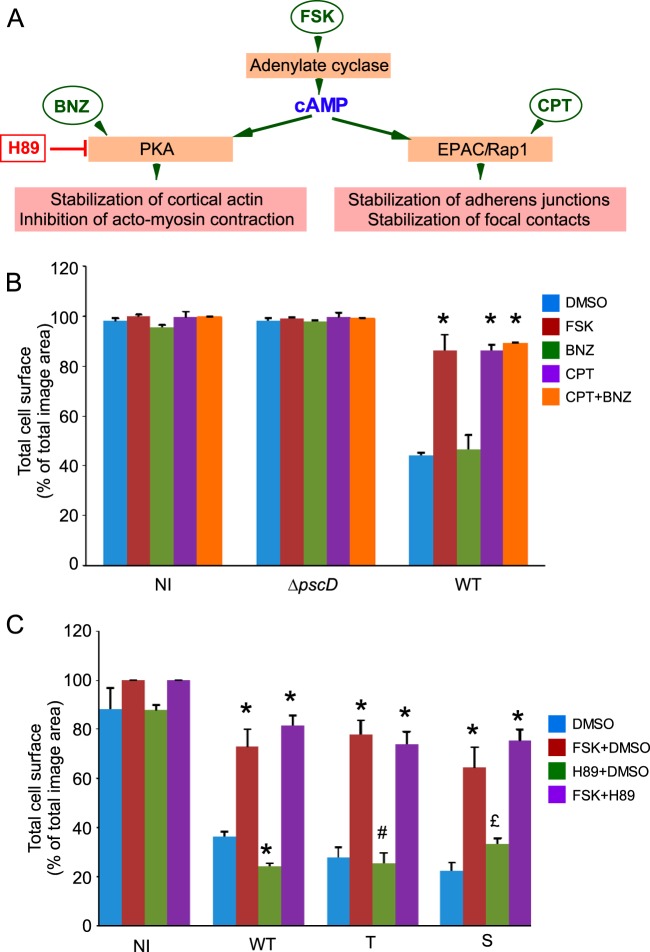
cAMP is a signal activator of two different pathways: (i) it stimulates protein kinase A (PKA), which in turn inhibits actomyosin contraction and stabilizes cortical actin, and (ii) it activates EPAC, a GTPase exchange factor (GEF) for the GTPase Rap1 that has emerged as a major regulator of cell-cell and cell-matrix adhesion (Fig. 3A and discussion below). To determine which pathway (PKA or EPAC) is involved in FSK's protective effect, we used drugs known to specifically activate or inhibit one pathway and not the other. We used cell-permeant cAMP analogs, BNZ and CPT, that activate either PKA or EPAC, respectively, and a PKA inhibitor, H89 (Fig. 3A). The drugs were first tested on AKT kinase phosphorylation at Ser473, which is differentially affected by PKA and EPAC pathways (26). As shown in Fig. S1B in the supplemental material, CPT (EPAC activator) increased AKT phosphorylation, while BNZ (PKA activator) decreased it, in agreement with previous data (26). FSK alone increased AKT phosphorylation, indicating that cAMP elevation has a preponderant effect on the EPAC pathway. The addition of both H89 and FSK increased AKT phosphorylation, a finding consistent with its inhibition of PKA. Next, we performed a cell retraction assay using these compounds. In these experiments, only CPT, the EPAC activator, had a striking protective effect on cell retraction comparable to that of FSK (Fig. 3B). In contrast, BNZ (PKA activator) had very little (although statistically significant for Pa-WT and Pa-S) or no effect in this assay (Pa-T). To further determine which pathway is important to reverse the P. aeruginosa cell retraction effect, we challenged FSK in infection assays with H89, the PKA inhibitor. H89 did not interfere with the protective effect of FSK, confirming that the EPAC/Rap1 pathway is solely required for the FSK effect (Fig. 3C). The addition of H89 alone did not modify P. aeruginosa-induced cell retraction, indicating that H89 has no effect on this process in itself (Fig. 3C). In this experiment, we also infected cells with Pa-T or Pa-S and the results were similar to those for infection with Pa-WT, indicating that FSK inhibition of cell retraction induced by either exotoxin targets the EPAC/Rap pathway.
FIG 3.
Specific activation of protein kinase A or EPAC/Rap1 differentially affects cell retraction. (A) Diagram representing the production of cAMP by adenylate cyclase and its downstream pathways. The effects of pharmacological activators (FSK, BNZ, and CPT) and inhibitor (H89) are shown. (B) HUVECs were infected by Pa-WT or PaΔpscD in the presence of FSK at 10 μM, BNZ at 200 μM (a specific PKA activator), CPT at 200 μM (a specific EPAC activator), or both CPT and BNZ (at 200 μM each). Noninfected cells (NI) are shown as a control. Drug effects were measured in a cell retraction assay. The data represent the mean surface percentage (plus the SD) of noninfected cells (NI) or cells infected with either PaΔpscD or Pa-WT (n = 4 to 8). Statistics were established by a multiple-comparison one-way ANOVA (P < 0.001), followed by Dunnett's t test for differences between drug- and control DMSO-treated samples in Pa-WT infections (*, P < 0.05). (C) HUVECs were infected by Pa-WT, Pa-T, or Pa-S in the presence of the PKA inhibitor H89 at 1 μM, in the presence of FSK, or in the presence of both. Cell surfaces were measured as described above (n = 3 in each experiment). Differences with control DMSO were established by statistical analysis, as described above, for each infection type, with an overall P value of <0.001 for each infection type: Pa-WT, Pa-T, and Pa-S. Significant differences with DMSO-treated samples are indicated (*, P < 0.05). In panels B and C, the data are representative of three independent experiments.
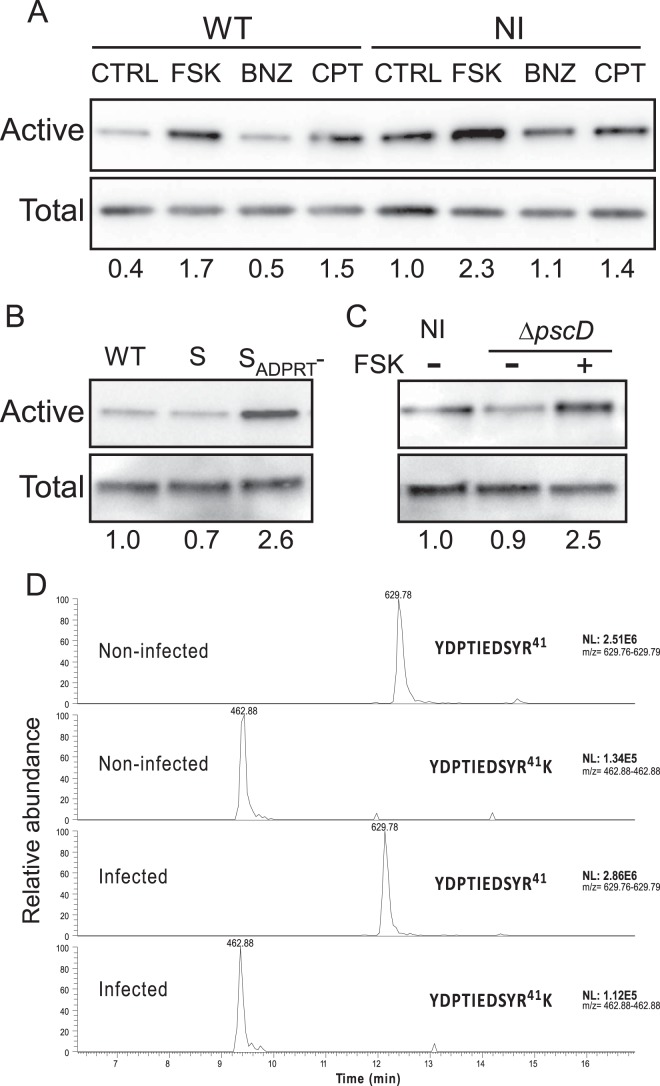
To confirm that Rap1 was indeed activated in infected endothelial cells in the presence of FSK, we examined the amount of activated Rap1 (Rap1 linked to GTP) in comparison to total Rap1 using a pulldown assay (Fig. 4A). In uninfected cells, FSK and CPT, but not BNZ, increased the GTP-Rap1/total Rap1 ratio, as expected. The infection of HUVECs dramatically decreased the Rap1 activity, while this activity was increased in infected cells challenged with FSK or CPT but not BNZ. Thus, the cAMP/EPAC/Rap1 pathway is downregulated in infected cells but reactivated by FSK or CPT. A bacterial strain secreting only ExoS (Pa-S) had a similar effect as PAO1F on Rap1 activity (Fig. 4B). Conversely, a strain secreting only an ExoS mutant in which the ADP-ribosyltransferase is inactivated (Pa-SADPRT-) did not downregulate Rap1, thus demonstrating that the ExoS ADP-ribosyl transferase domain is solely responsible for Rap1 downregulation. As previously shown (16), Pa-SADPRT- induced only a little retraction in HUVECs, indicating that the ExoS GAP domain has no major effect on the cytoskeleton either.
FIG 4.
Rap1 activation by FSK in infected HUVECs. (A) Levels of Rap1-active cells (Rap1-GTP) investigated by pull down, followed by Western blotting in PAO1F-infected (MOI = 10) or in noninfected (NI) cells at 4 h posttreatment, in the presence of DMSO (control [CTRL]), FSK at 10 μM, BNZ at 200 μM, or CPT at 200 μM. Cell lysates were analyzed in parallel to show the total amount of Rap in each sample. The Rap1-GTP/total Rap1 signal ratios are indicated below the lanes. (B) Levels of Rap1-active cells assayed as in panel A after infection with Pa-S or Pa-SADPRT-. (C) Levels of Rap1-active cells infected by PaΔpscD in the presence or absence of FSK. The data are representative of three independent experiments. (D) Analysis of Rap1 potential ADP-ribosylation on Arg41 by LC-MS/MS (see Materials and Methods). Chromatograms of the two tryptic peptides containing Arg41 in PAO1F-infected or uninfected HUVEC protein extracts. Signal intensities (NL, normalized levels) of both peptides are shown on the right.
To investigate the role of other bacterial factors apart from the T3SS on Rap1 regulation, we infected cells with a mutant strain defective in the T3SS (PaΔpscD). Infection with this strain had no impact on the proportion of active Rap1 compared to the uninfected condition, and FSK upregulated Rap1 activity, as when infected with PAO1F (Fig. 4C). Hence, the T3SS is the only component, among the other P. aeruginosa virulence factors, that controls Rap1 activity.
Rap1 was reported to be ADP-ribosylated by ExoS in epithelial cells (27–29), and the modification was shown to occur at Arg41 (30). Therefore, we wondered whether Rap1 could be a direct substrate of ExoS ADP-ribosyltransferase in endothelial cells. As shown in Fig. 4A to C, HUVEC infection did not induce an electrophoretic mobility shift of Rap1, as opposed to epithelial cells (28–30), suggesting that Rap1 is not ADP-ribosylated in endothelial cells.
To further evaluate Rap1 potential ADP-ribosylation in infected endothelial cells, we analyzed trypsin digests of infected cell extracts by mass spectrometry, using an LC-MS/MS technique (see Materials and Methods). Two tryptic peptides containing Arg41, YDPTIEDSYR41 and YDPTIEDSYR41K, were identified in both infected and uninfected conditions (Fig. 4D). Analysis of MS data using Mascot software (see Materials and Methods) or by scanning MS/MS spectra for characteristic fragmentation of the modification (m/z = 428 and 348) did not identify any ADP-ribosylated Rap1 peptide (data not shown). Furthermore, ADP-ribosylation of Arg41 would prevent trypsin digestion between Arg41 and Lys42; no modification in peptide signal intensities on the chromatograms was observed between the uninfected and the infected conditions (Fig. 4D), confirming that no ADP-ribosylation occurred on Arg41 in infected endothelial cells. Rap1 downregulation during infection is thus not caused by Rap1 ADP-ribosylation.
Physiological or pharmacological activators of adenylate cyclase hamper P. aeruginosa-induced cell retraction.
We next examined whether protection against P. aeruginosa-induced retraction could also be produced by physiological activators of adenylate cyclase. Prostaglandin E2 (PGE2) is a naturally occurring hormone that selectively binds and activates the PGE2 receptor. This receptor, also called EP2, activates the G-protein Gs, a potent activator of adenylate cyclase (31) that significantly increased cAMP levels in HUVECs (see Fig. S1A in the supplemental material). In the cell retraction assay, PGE2 significantly protected the cell surface decrease induced by P. aeruginosa (Fig. 5).
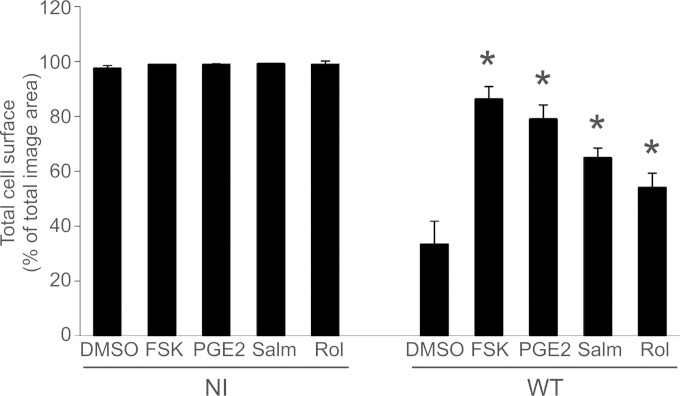
FIG 5.
Effect of physiological and pharmacological agents on P. aeruginosa-induced cell retraction. HUVECs were either noninfected (NI) or infected with PAO1F (WT) for 4 h and then challenged with FSK at 10 μM, PGE2 at 1 nM, salmeterol (Salm) at 100 μM, or rolipram (Rol) at 100 μM. The total cell surface was calculated as in Fig. 1B. In PAO1F-infected cells, the effects of agonist or drugs were compared to control DMSO-treated samples by one-way ANOVA (P < 0.001), followed by Dunnett's t test (*, P < 0.05). The data are representative of three independent experiments.
We also used two other pharmacological agents: (i) salmeterol, a Gs-coupled beta-receptor agonist known to activate adenylate cyclase (32), and (ii) rolipram, a selective phosphodiesterase (PDE) type IV inhibitor (33), which blocks the degradation of cAMP by this phosphodiesterase. These molecules were selected because beta-receptors are present on the surface of endothelial cells and because type IV PDE is the major phosphodiesterase isotype located in endothelial cells. Salmeterol and rolipram significantly increased cAMP in endothelial cells (see Fig. S1A in the supplemental material). Elevation of intracellular cAMP levels either by activation of adenylate cyclase (salmeterol) or by inhibition of its degradation (rolipram) significantly reduced P. aeruginosa-induced cell retraction (Fig. 5).
DISCUSSION
In this study, we show that one of the most striking and rapid effects of P. aeruginosa on endothelial cells—cell retraction—can be significantly attenuated by pharmacological drugs increasing the intracellular cAMP level (e.g., FSK, salmeterol, or rolipram) or by PGE2, a natural cytokine. The data of the monolayer transmigration assay further demonstrated that this effect on cell morphology has a real outcome on the capacity of the bacteria to cross the endothelial monolayer.
The FSK effect was observed for strains secreting ExoS/ExoT toxins, such as PAO1F and CHA. Furthermore, FSK attenuated both ExoS- and ExoT-induced retraction, as shown with the mutant PAO1F strains. Conversely, FSK had no effect on infection with an ExoU-positive strain, even at early time points. This was expected, since ExoU intoxicates cells using a completely different mode of action (10, 34). Fortunately, the highly toxic ExoU-secreting strains are less common in the clinic than the ExoS/ExoT-secreting strains (12, 13).
The only known biological activity of ExoY in host cells is an adenylate cyclase. Interestingly, the Pa-Y mutant, which expresses only ExoY among the T3SS exotoxins, induces cell spreading instead of cell retraction (16). The seemingly paradoxical ExoY activity in host cells is in fact in agreement with the action of cAMP-elevating agents shown in the present study. Confirming these findings, we show that the absence of ExoY accelerates cell retraction (Fig. 1D).
Altogether, our data are consistent with the necessity of developing targeted therapies depending on the type of P. aeruginosa strain, based on the nature of the secreted type 3 toxins. We demonstrate that the FSK effect was due to its action on host cells, not on bacteria (see Fig. S2 in the supplemental material). Along the same lines, PP34 (ExoU-positive strain) action was not altered by FSK (Fig. 2), confirming that the T3SS machinery is not affected by FSK. Thus, the action of this drug takes place once the toxins are injected, rather than inducing an alteration of the host cell permissivity to the T3SS.
cAMP is a potent second messenger in eukaryotic cells, activating both PKA and EPAC, a GEF specific to Rap1. Both pathways are critical for cell reaction to various stimuli. Our experiments, using specific activators of each pathway and with the PKA inhibitor H89, showed that activation of the EPAC/Rap1 axis has a major protective role in cell morphology. The biological activity of Rap1 has been extensively studied, and Rap1 upregulation by cAMP/EPAC is well known to stimulate integrin function and to increase endothelial cell-cell adhesion (22–24). Accordingly, Rap1 activation was shown to reduce vascular permeability and leukocyte transmigration (35–37). Rap1 has several effectors, including RapL, which stabilize integrin-mediated adhesion, extend focal adhesion, and promote the interaction of the integrin complex with actin filaments (reviewed in reference 38). In addition, Rap1 stabilizes endothelial adherens junctions by regulating the junctional localization of Krit1/CCM1, where it stabilizes junction integrity by anchoring junctional components to actin bundles (39, 40). The overall conclusion of these previous studies is that activation of the cAMP/EPAC/Rap1 axis enhances the barrier properties of endothelial cell monolayers. The data presented here extend the known effects of cAMP/EPAC/Rap1 signaling to the situation of infected cells. Importantly, Rap1 activates Cdc42 and Rac1, leading to profound modifications of the actin cytoskeleton: it releases tension of radial stress fibers and increases tension of junctional actin (41). The cAMP/EPAC/Rap1-dependent activation of Rac1 and Cdc42 may thus counteract the action of ExoS and ExoT on these two GTPases.
Rap1 was less active after infection with PAO1F (Fig. 4). Although there are some controversies in the literature, Rap1 was shown by three groups to be ADP-ribosylated by ExoS in epithelial cells (27–30). Rap1 ADP-ribosylation by ExoS occurs at Arg41 and prevents its activation by C3G, a Rap1 GEF (30). Here, we show, by various approaches, that Rap1 is not ADP-ribosylated by ExoS in endothelial cells. More generally, it is interesting to note that endothelial cells respond differently to P. aeruginosa infection than epithelial cells; for example, moesin phosphorylation is not altered in endothelial cells by T3SS effectors, as opposed to epithelial cells (16).
One remaining issue is to understand how ExoS and ExoT induce Rap1 downregulation. Rap1 activation is promoted by several GEF, including PDZ-GEF1, which is activated by endothelial cell-cell junctions and responsible for basal Rap1 activation (reviewed in reference 41). In connection with this, we previously showed that ExoS and ExoT both induce cell retraction and cell junction disruption (Fig. 1B) (16). Thus, ExoS/ExoT-induced loss of cell-cell junctions may inhibit Rap1 by downregulation of PDZ-GEF1.
Specific therapies aiming at the improvement of vascular barrier function in patients with acute infection are still lacking. Taken together, our results show that increasing intracellular cAMP levels may be beneficial to the host to maintain endothelial barriers in infected organs. However, a systemic increase of cAMP levels in the whole body may lead to undesired effects, such as vasodilation, increased heart rate, and activation of several ion channels. Therefore, elevation of cAMP at the organism level may require specific delivery in endothelial cells exposed to the pathogen and/or a specific action on Rap1, not PKA, in order to prevent harmful secondary effects. In this context, CPT is probably the best agent to specifically activate Rap1 in vivo; however, a drug delivery system for CPT is not yet available. Recently, the oxidized form of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine was shown to enhance endothelium barrier properties through Rap1 activation (42). This compound may represent an alternative to CPT in the context of P. aeruginosa infection.
In conclusion, we propose here a mechanism to counteract the action of ExoS and ExoT on the vascular barrier. Further studies are needed to evaluate this strategy in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Commissariat à l'Energie Atomique, the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and Grenoble Alp University. Part of this study was also supported by the Alliance pour les Sciences de la Vie et de la Santé Program in Infectiology.
We thank Christophe Masselon and Sylvie Kieffer from the EDyP platform for the mass spectrometry analysis and Arne Rietsch for Pseudomonas aeruginosa mutant strains.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00010-15.
REFERENCES
- 1.Lee WL, Slutsky AS. 2010. Sepsis and endothelial permeability. N Engl J Med 363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D, Infectious Diseases Society of America. 2013. 10×'20 progress: development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson LR. 2009. Bad bugs, no drugs: no ESCAPE revisited. Clin Infect Dis 49:992–993. doi: 10.1086/605539. [DOI] [PubMed] [Google Scholar]
- 4.Filloux A. 2011. Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front Microbiol 2:155. doi: 10.3389/fmicb.2011.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. 2012. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 40:1157–1163. doi: 10.1097/CCM.0b013e3182377906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Le Berre R, Nguyen S, Nowak E, Kipnis E, Pierre M, Quenee L, Ader F, Lancel S, Courcol R, Guery BP, Faure K, Pyopneumagen G. 2011. Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit Care Med 39:2113–2120. doi: 10.1097/CCM.0b013e31821e899f. [DOI] [PubMed] [Google Scholar]
- 8.Vance RE, Rietsch A, Mekalanos JJ. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galle M, Carpentier I, Beyaert R. 2012. Structure and function of the type III secretion system of Pseudomonas aeruginosa. Curr Protein Peptide Sci 13:831–842. doi: 10.2174/138920312804871210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izore T, Perdu C, Job V, Attree I, Faudry E, Dessen A. 2011. Structural characterization and membrane localization of ExsB from the type III secretion system (T3SS) of Pseudomonas aeruginosa. J Mol Biol 413:236–246. doi: 10.1016/j.jmb.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Berthelot P, Attree I, Plesiat P, Chabert J, de Bentzmann S, Pozzetto B, Grattard F, Groupe d'Etudes des Septicemies Pseudomonas. 2003. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: evidence for a possible association between O serotypes and exo genes. J Infect Dis 188:512–518. doi: 10.1086/377000. [DOI] [PubMed] [Google Scholar]
- 13.Frank DW. 2012. Research topic on Pseudomonas aeruginosa, biology, genetics, and host-pathogen interactions. Front Microbiol 3:20. doi: 10.3389/fmicb.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gendrin C, Contreras-Martel C, Bouillot S, Elsen S, Lemaire D, Skoufias DA, Huber P, Attree I, Dessen A. 2012. Structural basis of cytotoxicity mediated by the type III secretion toxin ExoU from Pseudomonas aeruginosa. PLoS Pathog 8:e1002637. doi: 10.1371/journal.ppat.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbieri JT, Sun J. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152:79–92. [DOI] [PubMed] [Google Scholar]
- 16.Huber P, Bouillot S, Elsen S, Attree I. 2014. Sequential inactivation of Rho GTPases and Lim kinase by Pseudomonas aeruginosa toxins ExoS and ExoT leads to endothelial monolayer breakdown. Cell Mol Life Sci 71:1927–1941. doi: 10.1007/s00018-013-1451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganter MT, Roux J, Su G, Lynch SV, Deutschman CS, Weiss YG, Christiaans SC, Myazawa B, Kipnis E, Wiener-Kronish JP, Howard M, Pittet JF. 2009. Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am J Respir Cell Mol Biol 40:108–118. doi: 10.1165/rcmb.2007-0454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. 2012. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem 287:25407–25418. doi: 10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley MN, Camara M, Smyth AR. 2012. Novel approaches to the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Eur Respir J 40:1014–1023. doi: 10.1183/09031936.00042012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goure J, Pastor A, Faudry E, Chabert J, Dessen A, Attree I. 2004. The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect Immun 72:4741–4750. doi: 10.1128/IAI.72.8.4741-4750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsen S, Huber P, Bouillot S, Coute Y, Fournier P, Dubois Y, Timsit JF, Maurin M, Attree I. 2014. A type III secretion negative clinical strain of Pseudomonas aeruginosa employs a two-partner secreted exolysin to induce hemorrhagic pneumonia. Cell Host Microbe 15:164–176. doi: 10.1016/j.chom.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, Ji Z, Tsalkova T, Mei F. 2008. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin 40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. 2008. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol 87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Retta SF, Balzac F, Avolio M. 2006. Rap1: a turnabout for the crosstalk between cadherins and integrins. Eur J Cell Biol 85:283–293. doi: 10.1016/j.ejcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Verove J, Bernarde C, Bohn YS, Boulay F, Rabiet MJ, Attree I, Cretin F. 2012. Injection of Pseudomonas aeruginosa Exo toxins into host cells can be modulated by host factors at the level of translocon assembly and/or activity. PLoS One 7:e30488. doi: 10.1371/journal.pone.0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. 2002. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 27.Fraylick JE, Rucks EA, Greene DM, Vincent TS, Olson JC. 2002. Eukaryotic cell determination of ExoS ADP-ribosyltransferase substrate specificity. Biochem Biophys Res Commun 291:91–100. doi: 10.1006/bbrc.2002.6402. [DOI] [PubMed] [Google Scholar]
- 28.Henriksson ML, Sundin C, Jansson AL, Forsberg A, Palmer RH, Hallberg B. 2002. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Biochem J 367:617–628. doi: 10.1042/BJ20020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuffie EM, Frank DW, Vincent TS, Olson JC. 1998. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun 66:2607–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riese MJ, Wittinghofer A, Barbieri JT. 2001. ADP-ribosylation of Arg41 of Rap by ExoS inhibits the ability of Rap to interact with its guanine nucleotide exchange factor, C3G. Biochemistry 40:3289–3294. doi: 10.1021/bi002729q. [DOI] [PubMed] [Google Scholar]
- 31.Norel X. 2007. Prostanoid receptors in the human vascular wall. Sci World J 7:1359–1374. doi: 10.1100/tsw.2007.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M. 1990. The pharmacology of salmeterol. Lung 168(Suppl):115–119. [DOI] [PubMed] [Google Scholar]
- 33.Weishaar RE, Cain MH, Bristol JA. 1985. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem 28:537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- 34.Sato H, Frank DW. 2004. ExoU is a potent intracellular phospholipase. Mol Microbiol 53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 35.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. 2005. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 36.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. 2005. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. 2005. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 38.Frische EW, Zwartkruis FJ. 2010. Rap1, a mercenary among the Ras-like GTPases. Dev Biol 340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 39.Glading A, Han J, Stockton RA, Ginsberg MH. 2007. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell-cell junctions. J Cell Biol 179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. 2010. Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through alpha- and beta-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Mol Biol Cell 21:584–596. doi: 10.1091/mbc.E09-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pannekoek WJ, Post A, Bos JL. 2014. Rap1 signaling in endothelial barrier control. Cell Adhesion Migration 8:100–107. doi: 10.4161/cam.27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. 2011. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol 226:2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleves S, Viarre V, Salacha R, Michel GP, Filloux A, Voulhoux R. 2010. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int J Med Microbiol 300:534–543. doi: 10.1016/j.ijmm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Toussaint B, Delic-Attree I, Vignais PM. 1993. Pseudomonas aeruginosa contains an IHF-like protein that binds to the algD promoter. Biochem Biophys Res Commun 196:416–421. doi: 10.1006/bbrc.1993.2265. [DOI] [PubMed] [Google Scholar]
- 45.Cisz M, Lee PC, Rietsch A. 2008. ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol 190:2726–2738. doi: 10.1128/JB.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.