Abstract
Thoracic aortic aneurysm (TAA) is progressive fatal aortic pathological dilation. However, the underlying molecular mechanisms are still largely unknown. Evidences suggest that endothelial cells and renin-angiotensin system may participate in the pathogenesis of TAA. This study aimed to investigate whether angiotensin II type 2 receptor (AT2) positive cells are involved in TAA formation. The mRNA level of AT2 is dramatically elevated in TAA compared with in controls. CD4+AT2+ cells increased in both aortic wall and circulation of TAA patients. The levels of IL-1β and IL-17B in CD4+AT2+ cells were lower than those in CD4+AT2- cells. When compared with endothelial cells (ECs) cultured alone, CD4+AT2+ cells showed an inhibitory effect on proliferation and MMP2 expression in ECs, but CD4+AT2- cells promoted proliferation and MMP2 expression in ECs. Both CD4+AT2+ and CD4+AT2- cells suppressed apoptosis of ECs. In conclusion, we have identified a novel population of CD4+AT2+ T lymphocytes that show protective effect in TAA through inhibition of growth, apoptosis, and MMP2 expression in ECs.
Keywords: Angiotensin II type 2 receptor, endothelial cell, thoracic aortic aneurysm, matrix metalloprotease 2, inflammation
Introduction
Thoracic aortic aneurysm (TAA) is one of leading causes of morbidity and mortality worldwide. The presentation of TAA is usually silent and detected during a routine physical examination or when tests for an unrelated medical evaluation. Accumulated evidence has revealed that TAA is a pathology with complex etiology that involves genetic and environmental risk factors, such as hypertension, atherosclerosis, inflammatory diseases, smoking, and inherited disease [1]. Although the exact mechanism of TAA is not entirely known, its most common cause is cystic degeneration in the medial layer of the aorta. Therefore, elucidating the underlying mechanisms of TAA not only facilitates earlier diagnosis but also optimizes the management of TAA.
Aortic aneurysm has some etiologies, but all forms present common features, including tissue remodeling [2] and presence of inflammatory cells [3]. Transmural inflammation consisting primarily of mononuclear phagocytes, lymphocytes, and blood plasma cells is one of the principal histological features of abdominal aneurysm [4]. These cells facilitate the pathological changes through the secretory of IL-1β, TNF-a, INF-r [5], which induce matrix metalloproteinases (MMPs) expression in endothelial cells (ECs) [6] and smooth muscle cells (SMCs) [7]. Increased levels of MMPs are observed in patients with aortic aneurysms, especially smoking patients [8-10]. MMP-mediated degradation of media matrix plays a foundational role in the pathogenesis of aortic aneurysm formation. Attenuation of inflammation can prevent aortic aneurysm formation and progression [9,11-15].
Chronic angiotensin II (AngII) infusion promotes vascular inflammation and formation of aortic aneurysms in hypercholesterolemic mice [16-19]. Ang II exerts its effects on aortic aneurysms by binding to AngII type 1 (AT1) receptor [19-21]. The AT1 receptor antagonist losartan has been shown to prevent aortic aneurysm in Marfan syndrome [22] and diminish AngII-induced aortic aneurysm [19,20]. AngII type 2 (AT2) receptor is characterized by effects different from and often opposing those of AT1 [23]. AT2 is rarely expressed in healthy adult tissues, but strongly upregulated following tissue injury [24], including vascular injury [25] and myocardial infarction [26]. AT2 exhibits tissue-protective properties through inhibition of inflammation, fibrosis and apoptosis [24]. However, the role of AT2 in TAA remains largely unknown. Here, we observed increased CD4+AT2+ T lymphocytes in the circulation of TAA patient and local aneurysm tissues, which downregulated MMP2 expression in ECs, and inhibited the apoptosis and proliferation of ECs.
Materials and methods
Human TAA tissues
Protocol was approved by the Ethical committee of Ren Ji Hospital, and written informed consent was obtained from all subjects. A total of 20 TAA tissues were collected from patients with primary TAA between January 2012 and June 2014. TAA secondary to known causes such as Marfan syndrome and aortic dissection were excluded from analysis. Furthermore, we also collected non-aneurysmal thoracic aortic specimens from patients undergoing coronary artery bypass surgery (n = 20) or aortic valve replacement with aorta trim (n = 3) who had no aortic aneurysms.
Immunofluorescence staining
Frozen tissues were cut into 5 um thick sections. After fixed with cold acetone for 10 minutes, sections were rinsed in PBS three times and blocked in 5% donkey serum for 1 hour at 37°C. After incubated with primary antibodies overnight at 4°C, sections were then washed in PBS and secondary antibodies were added for another 1 hour at room temperature. Finally, sections were counterstained with DAPI. Stained slides were examined under fluorescence microscope (Nikon, Tokyo, Japan). NIS-Elements D software (Nikon, Tokyo, Japan) was used to quantify the cell count and tissue area measurement. Three double-blind counts were performed.
For cells immunofluorescence experiments, cells were washed in PBS, and fixed in 4% paraformaldehyde, permeating using 0.1% Triton. The cells were then rinsed with PBS and blocked in 5% donkey serum for one hour at 37°C. The cells were then incubated with primary antibodies overnight at 4°C. After rinsing with PBS, the cells were incubated with secondary antibodies. Finally DAPI staining was performed to identify nuclei. Fluorescence was visualized on a fluorescence microscope (Nikon, Tokyo, Japan).
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted using with Microprep Kit (Zymo research, USA) according to manufacturer’s instructions. Primer sequences were following: β-actin, 3’-AGAAAATCTGGCACCACACC-5’ (forward) and 3’-AGAGGCGTACAGGGATAGCA-5’ (reverse); AT2, 3’-ACTGGCTCTTTGGACCTGTG-5’ (forward) and 3’-GCCATACACCAAACAAGGGG-5’ (reverse); IL-1β, 3’-ACGGACCCCAAAAGATGAAG-5’ (forward) and 3’-TTCTCCACA- GCCACAATGAG-5’ (reverse); IL-17B, 3’-GAGCCCCAAAAGCAAGAGGAA-5’ (forward) and 3’-TGCGGGCATACGGTTTCATC-5’ (reverse). The relative expression levels of AT2, IL-1β and IL-17B were quantified relative to the expression of β-actin using the 2-ΔΔct method.
Flow cytometry analysis
Mononuclear cells were extracted from peripheral blood specimens by gradient centrifugation using lymphocyte separation media (Biowest, Spanish) according to manufacturer’s instructions. The cells (1 × 106) were incubated PE-conjugated mouse anti-CD4 (BD, USA) antibody and rabbit anti-AT2 antibody at 4°C for 20 min. After washed with dilute with PBS, cells were incubated with Alexa Fluor® 647 donkey anti rabbit antibody (Life technologies, USA) at 4°C for 20 min. Finally, cells were resuspended with DPBS, and then analyzed using FACSAria (BD, New Jersy, USA). Data were analyzed using C6 software (BD, New Jersy, USA).
Apoptosis and proliferation
Human aortic endothelial cells were purchased from Promocell Corporation. After subculture, cells were planted in a 24 wells culture plate at a density of 0.5 × 105 per well. Since the highest activity of cells was achieved between the third and seventh day, after sorting on the third day, AT2+CD4+ and AT2-CD4+ cells were added into each wells at a density of 2 × 105 in 1:1 RPMI/ECGM (Promocell, German). ECs were separated from CD4+ cells by a transwell membrane. Cells were collected on the seventh day for further assay. For apoptosis analysis, cells were treated with either AnnexIV (Invitrogen, USA) or Proliferation Kit (Promega, USA), following the manufacturer’s instructions, and quantified by FACScan flow cytometer (BD Bioscience, CA, USA). Cell proliferation assays were performed using CellTiter 96® AQueous One Solution Reagent, according to the manufacturer’s instructions.
Statistical analyses
Data were expressed as mean ± SEM. Comparison between two groups was analyzed by two-tailed Student t test. Multiple comparisons were analyzed with one-way ANOVA followed by Bonferroni post hoc test. Differences were considered significant at P < 0.05.
Result
CD3+ T lymphocytes and CD31+MMP2+ cells in TAA
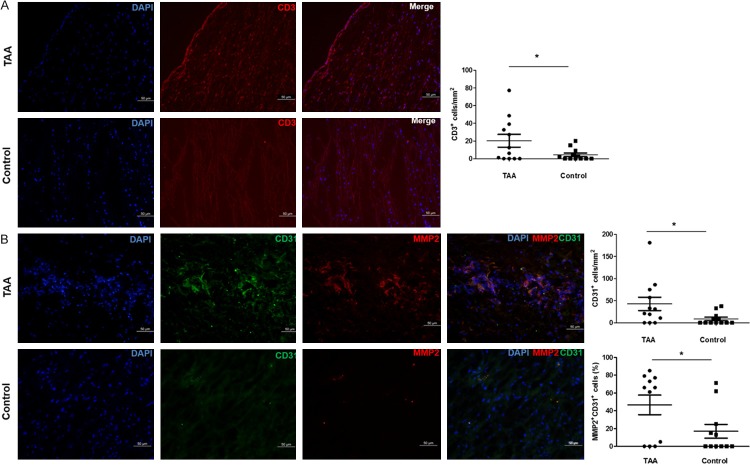
To investigate the inflammation response in TAA, immunofluorescence analyses were performed on arterial cross section. There was a significant difference in CD3+ T lymphocyte counts between aneurysmal and non-aneurysmal thoracic aortas (P = 0.045). CD3+ T lymphocyte accumulated in TAA (20.3 ± 7.2/mm2, n = 12), especially in the adventitia and media of TAA, but was relative rarely present in non-aneurysmal thoracic aortas (4.5 ± 1.9/mm2, n = 12; Figure 1A).
Figure 1.
Immunofluorescence staining of CD3, CD31 and MMP2 in TAA. A. CD3+ T lymphocytes were significantly more frequent in TAA compared with non-aneurysmal thoracic aortas. B. CD31+ and CD31+MMP2+ cells were significantly increased in TAA compared with non-aneurysmal thoracic aorta. *P < 0.05.
To evaluate the pathological changes of ECs function, CD31 and MMP2 staining were carried out simultaneously on serial cross sections. The density of CD31+ cells in TAA (42.8 ± 15/mm2, n = 12) was significantly increased when compared with that in non-aneurysmal thoracic aortas (8.7 ± 3.8/mm2, n = 12, P = 0.039; Figure 1B). Furthermore, CD31+MMP2+ cells were crowded in cluster in TAA, but rarely present in non-aneurysmal aortas. The percentage of CD31+MMP2+ cells in TAA was significantly higher than that in non-aneurysmal thoracic aortas (46.5 ± 11.1% vs 16.9 ± 7.8%, n = 11, P = 0.040; Figure 1B).
Increased CD4+AT2+ T lymphocytes in the aortic walls of TAA
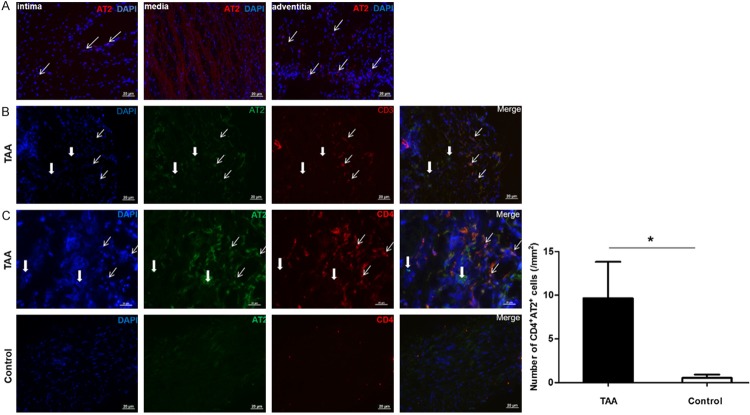
To investigate the clinical relevance of AT2 in TAA, AT2 expression was analyzed in TAA and non-aneurysmal thoracic aortas. RT-qPCR analysis indicated that the level of AT2 in TAA was significantly upregulated compared with those in non-aneurysmal aortas (P < 0.001, Figure 2). Anti-AT2 immunostaining showed that AT2+ cells were predominantly present in adventitia, but rarely observed in media (Figure 3). Further analyses revealed the existence of CD3+AT2+ and CD4+AT2+ cells together with CD3-AT2+, CD3+AT2-, CD4-AT2+ and CD4+AT2- cells. The density of CD4+AT2+ cells in TAA (0.52 ± 0.37 cells/mm2, n = 15) was higher than that in controls (9.64 ± 4.17 cells/mm2, n = 16, P = 0.044). Moreover, comparative analyses of sequential sections of aneurysm tissue showed that some AT2+ cells exhibited a CD3/CD4 double-positive phenotype (Figure S1).
Figure 2.
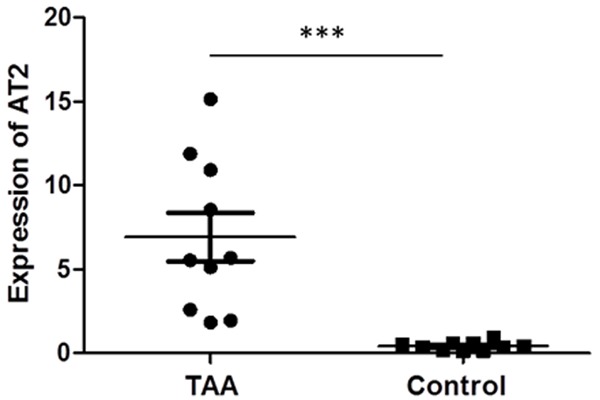
The mRNA level of AT2 expression is upregulated in TAA. ***P < 0.001.
Figure 3.
Immunofluorescence staining of AT2, CD3, CD4 in TAA. A. AT2+ cells were found primary in adventitia, but rarely observed in media and intima of TAA. B. The majority of AT2+ cells were AT2+CD3+ cells (spindly arrows), whereas thick arrows show CD3+AT2- and CD3-AT2+ cells in adventitia of TAA. C. CD4+AT2+ cells (spindly arrows) were significantly more frequent in adventitia of TAA compared with adventitia of non-aneurysmal thoracic aortas. Thick arrows show CD4-AT2+ and CD4+AT2- cells in adventitia. *P < 0.05.
Increased circulating CD4+AT2+ T lymphocytes in TAA patients
Lymphocytes in peripheral blood reflect the body immune mobilization to local pathological changes. CD4+AT2+ cells occupied 1.9% of all T cells in PBMCs from TAA patients, which was significant higher than 0.5% in controls (P = 0.003, Figure 4A and 4B). The CD4+AT2+ cells accounted for 92.5% of CD3+ cell population in PBMCs from TAA patients, which was consistent with the result in TAA (Figure 4C).
Figure 4.
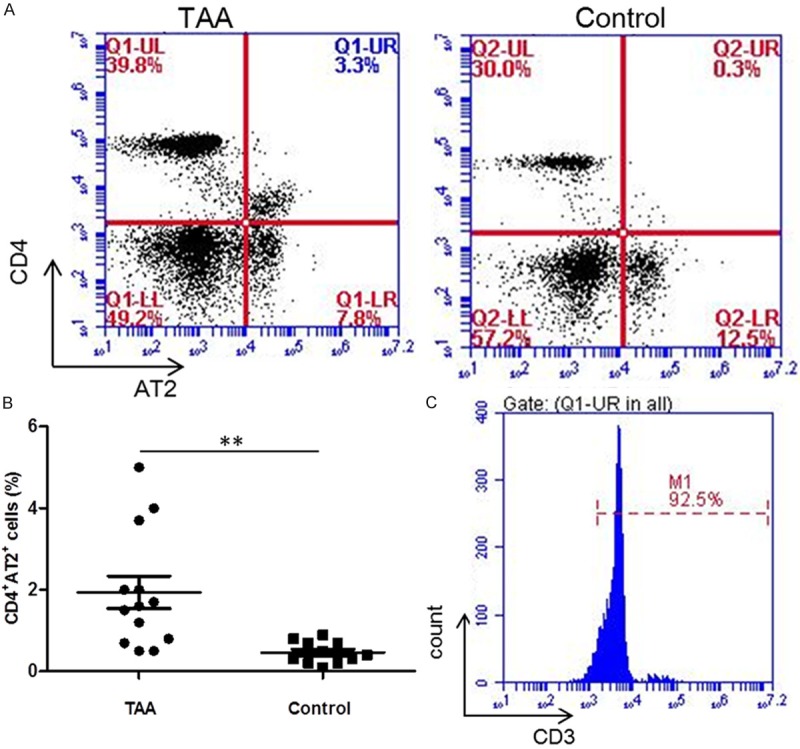
Flow cytometric analysis of CD4+AT2+ cells in circulation of TAA patients. The percentage of CD4+AT2+ cells was significantly increased in TAA compared with non-aneurysmal thoracic aortas (A and B). Further analysis showed that 92.5% CD4+AT2+ cells were CD3+ cells (C). **P < 0.01.
IL-1β and IL-17B were downregulated in circulating CD4+AT2+ T lymphocytes
To evaluate the cytokine secretion in the distinct cell subsets, IL-1β and IL-17B, two pro-inflammation cytokines, were analyzed by RT-qPCR. The levels of both IL-1β and IL-17B in CD4+AT2+ cells were significantly lower than those in CD4+AT2- cells (P < 0.05, Figure 5).
Figure 5.
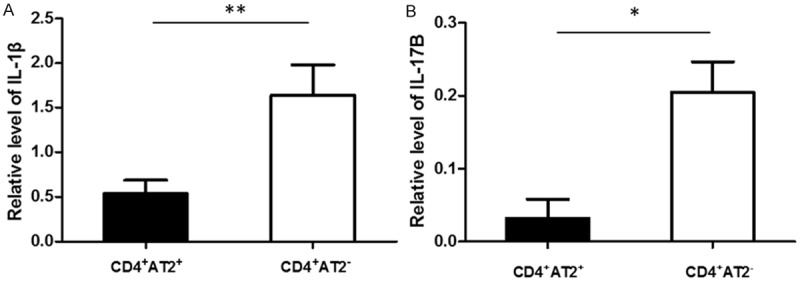
The levels of IL-1β and IL-17B in CD4+AT2+ cells were lower than in AT2-CD4+ cells. (A) IL-1β. (B) IL-17B. **P < 0.01.
Effects of CD4+AT2+ T lymphocytes on the proliferation and apoptosis of aortic ECs
Aortic ECs were cocultured with CD4+AT2+ and CD4+AT2- cells, respectively, in order to investigate their effects on the proliferation and apoptosis of aortic ECs. After 4 days of coculture, the proliferative index of ECs was measured. As shown in Figure 6, CD4+AT2+ cells suppressed ECs proliferation (0.71 ± 0.01), but CD4+AT2- cells promoted ECs growth (1.36 ± 0.13), when compared with ECs cultured alone (0.89 ± 0.05, P < 0.05). Both CD4+AT2+ and CD4+AT2- cells inhibited apoptosis of ECs, when compared with ECs cultured alone (P < 0.05). Furthermore, CD4+AT2- cells had stronger inhibitory effect on apoptosis of ECs than CD4+AT2+ cells (P = 0.012).
Figure 6.
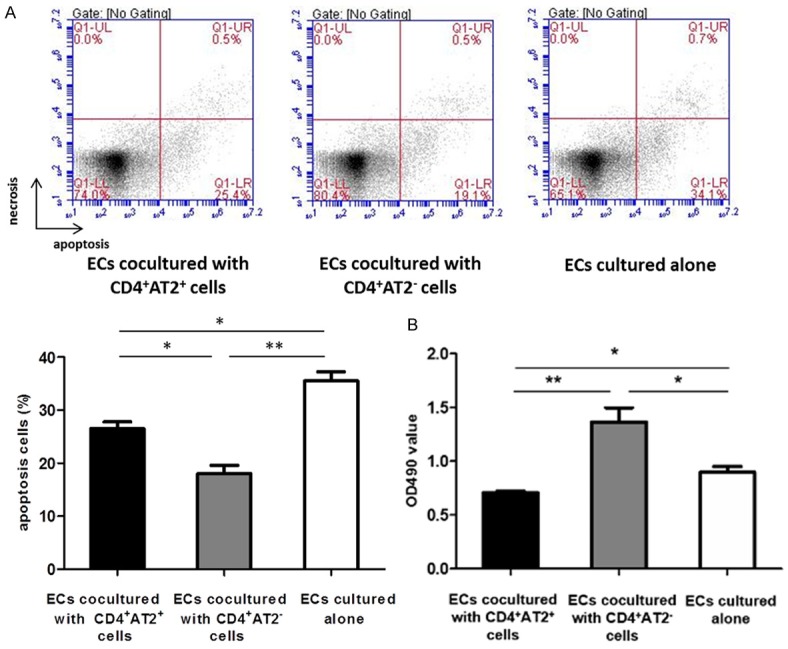
CD4+AT2+ cells inhibit apoptosis and proliferation of ECs. A. Both CD4+AT2+ and CD4+AT2- cells inhibited apoptosis of ECs. Furthermore, there was a significant difference in apoptosis rate of ECs between CD4+AT2+ and CD4+AT2- cell groups. B. CD4+AT2+ cells inhibited proliferation of ECs, whereas CD4+AT2- cells promoted proliferation of ECs. *P < 0.05, **P < 0.01.
Effects of CD4+AT2+ T lymphocytes on MMP2 expression of aortic ECs
We further investigated the effects of CD4+AT2+ and CD4+AT2- cells on MMP2 expression of ECs. After 4 days of coculture, the immunofluorescence staining was carried out. Positive immunostaining for MMP2 was detected in 51.8% of ECs cocultured with CD4+AT2+ cells, and 87.7% of ECs cocultured with CD4+AT2- cells, whereas 75.6% of ECs cultured alone stained positively for MMP2 (P < 0.05, Figure 7). Therefore, CD4+AT2+ cells showed an inhibitory effect on MMP2 expression in ECs, but CD4+AT2- cells promoted MMP2 expression in ECs.
Figure 7.
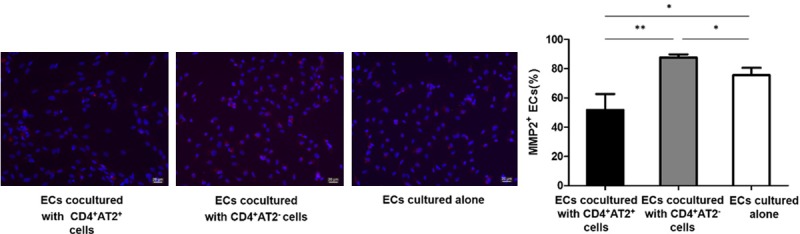
MMP2 expression in ECs is inhibited when cocultured with CD4+AT2+ cells. Compared with ECs culture alone, CD4+AT2+ cells inhibited MMP2 expression in ECs, whereas CD4+AT2- cells promoted MMP2 expression in ECs. *P < 0.05, **P < 0.01.
Discussion
Previous study showed a possible protective role of AT2 in aortic aneurysm [20,27], but underlying mechanism is poorly understood. In this study, we found an elevated level of AT2 in TAA, and identified a novel CD4+AT2+ lymphocyte subset, which was increased in both TAA and circulation of TAA patients. CD4+AT2+ cells inhibited both proliferation and apoptosis of ECs, and downregulated MMP2 expression of ECs. Furthermore, IL-1β and IL-17B are downregulated in CD4+AT2+ cells. These finding advanced our knowledge of the pathogenesis of TAA and characterized protective function of CD4+AT2+ cells in TAA.
It has been well known that AT2 has beneficial vascular effects through AT2-mediated anti-inflammatory effects [28]. Given that AT2 is upregulated in vascular injury [25], and not expressed in ECs and SMCs (data not shown), elevated AT2 in TAA is clearly expressed by inflammatory cells, which is an indispensable participant in the inflammatory process. In this study, TAA showed CD4+AT2+ cells aggregates, especially located in the adventitial, which could result in elevated level of AT2 in TAA tissues. Lymphocytes regulate tissue inflammation through cytokine secretion, whereas pro-inflammation cytokine such as IL-1β and IL-17B, in turn, lead to T lymphocytes recruitment and enhanced more inflammation response. Previous studies have demonstrated that IL-1β neutralization attenuates aortic aneurysm formation and progression [14,15]. Compared with CD4+AT2- cells, CD4+AT2+ cells had low levels of IL-1β and IL-17B, indicated that its anti-inflammation effect may be mediated through AT2 pathway.
Accumulating CD3+ cells predominantly in the adventitia and media of TAA is consistent with the outside-in mechanism of aortic aneurysm in which vascular inflammation is initiated in the adventitia during the course of disease and progresses inward toward the intima [29]. Since CD4+AT2+ cells accumulating in the adventitia, we further evaluated its effect on biological traits of ECs. CD4+AT2+ cells showed inhibitory effect on both proliferation and apoptosis of ECs. Both angiogenesis and lymphangiogenesis can lead to remodeled vascular structure, and therefore contribute to destructive processes within aortic aneurysm wall and play a vital role in aortic aneurysm development and rupture [30,31]. A recent study revealed neovessel formation in TAA media through deposition of blood-borne zymogens [31]. These findings indicate that CD4+AT2+ cells could inhibit angiogenesis in TAA wall. Endothelial damage is involved in aortic aneurysm formation, and is initiated by inflammation. Although the role of CD4+AT2+ cells in ECs apoptosis is not clear, inhibition of apoptosis of ECs may be beneficial to prevent intima thickness and thus slow down the progression of TAA.
MMP2 is one of the MMPs essential for aneurysm formation and is upregulated in aortic aneurysm [32,33]. Target deletion of MMP2 prevents aneurysm formation and matrix degradation [34], whereas decreased MMP2 expression caused by knockdown of tissue inhibitor of metalloproteinase-2 (TIMP-2) attenuates aneurysm development [35]. In this study, we found that CD4+AT2+ cells also inhibited MMP2 expression in ECs. Therefore, reduced MMP2 level results in less components of the extracellular matrix hydrolyzed. The underlying mechanism of decreased MMP2 expression in ECs may involve elevated TIMPs and inhibition of proliferation of ECs in TAA regulated by CD4+AT2+ cells.
In summary, we have identified and characterized a novel population of CD4+AT2+ T lymphocytes that were increased in TAA and circulation of TAA patients. The findings provide evidence that these cells have protective effect in TAA through inhibition of growth, apoptosis, and MMP2 expression in ECs. An improved mechanistic understanding of CD4+AT2+ T lymphocytes in protective effect in TAA may provide novel treatment options.
Acknowledgements
This article was supported by the project of Ren Ji Hospital in Shanghai China (No. RJZZ13-010)and projects of Shanghai Science and Technology Commission (No. 12DZ1940800 and No. 124119a2100).
Supporting Information
References
- 1.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 2.Annambhotla S, Bourgeois S, Wang X, Lin PH, Yao Q, Chen C. Recent advances in molecular mechanisms of abdominal aortic aneurysm formation. World J Surg. 2008;32:976–986. doi: 10.1007/s00268-007-9456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagadesham VP, Scott DJ, Carding SR. Abdominal aortic aneurysms: an autoimmune disease? Trends Mol Med. 2008;14:522–529. doi: 10.1016/j.molmed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto A, Kawashiri M, Ishibashi-Ueda H, Sugamoto Y, Yoshimuta T, Higashikata T, Ogino H, Tada H, Konno T, Hayashi K, Yamagishi M. Expression and Function of Ephrin-B1 and Its Cognate Receptor EphB2 in Human Abdominal Aortic Aneurysm. Int J Vasc Med. 2012;2012:127149. doi: 10.1155/2012/127149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagleton MJ. Inflammation in abdominal aortic aneurysms: cellular infiltrate and cytokine profiles. Vascular. 2012;20:278–283. doi: 10.1258/vasc.2011.201207. [DOI] [PubMed] [Google Scholar]
- 6.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993;296:803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newby AC. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol. 2012;56:232–244. doi: 10.1016/j.vph.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Dilme JF, Bellmunt S, Camacho M, Sola-Villa D, Romero JM, Escudero JR, Vila L. Influence of cardiovascular risk factors on levels of matrix metalloproteinases 2 and 9 in human abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;48:374–381. doi: 10.1016/j.ejvs.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe A, Ichiki T, Sankoda C, Takahara Y, Ikeda J, Inoue E, Tokunou T, Kitamoto S, Sunagawa K. Suppression of abdominal aortic aneurysm formation by inhibition of prolyl hydroxylase domain protein through attenuation of inflammation and extracellular matrix disruption. Clin Sci (Lond) 2014;126:671–678. doi: 10.1042/CS20130435. [DOI] [PubMed] [Google Scholar]
- 10.Rabkin SW. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm - comparison with and without bicuspid aortic valve: a meta-analysis. Vasa. 2014;43:433–442. doi: 10.1024/0301-1526/a000390. [DOI] [PubMed] [Google Scholar]
- 11.Hans CP, Koenig SN, Huang N, Cheng J, Beceiro S, Guggilam A, Kuivaniemi H, Partida-Sanchez S, Garg V. Inhibition of Notch1 signaling reduces abdominal aortic aneurysm in mice by attenuating macrophage-mediated inflammation. Arterioscler Thromb Vasc Biol. 2012;32:3012–3023. doi: 10.1161/ATVBAHA.112.254219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mieth A, Revermann M, Babelova A, Weigert A, Schermuly RT, Brandes RP. L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension. 2013;62:1098–1104. doi: 10.1161/HYPERTENSIONAHA.113.01986. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR Jr. and Ailawadi G. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR Jr, Ailawadi G. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130:S51–59. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann N Y Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE-/- mice. Clin Sci (Lond) 2010;118:681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 22.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namsolleck P, Recarti C, Foulquier S, Steckelings UM, Unger T. AT(2) receptor and tissue injury: therapeutic implications. Curr Hypertens Rep. 2014;16:416. doi: 10.1007/s11906-013-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin Angiotensin Aldosterone Syst. 2010;11:67–73. doi: 10.1177/1470320309347791. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci U S A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altarche-Xifro W, Curato C, Kaschina E, Grzesiak A, Slavic S, Dong J, Kappert K, Steckelings M, Imboden H, Unger T, Li J. Cardiac c-kit+AT2+ cell population is increased in response to ischemic injury and supports cardiomyocyte performance. Stem Cells. 2009;27:2488–2497. doi: 10.1002/stem.171. [DOI] [PubMed] [Google Scholar]
- 27.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 29.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swedenborg J, Mayranpaa MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–740. doi: 10.1161/ATVBAHA.110.213157. [DOI] [PubMed] [Google Scholar]
- 31.Kessler K, Borges LF, Ho-Tin-Noe B, Jondeau G, Michel JB, Vranckx R. Angiogenesis and remodelling in human thoracic aortic aneurysms. Cardiovasc Res. 2014;104:147–159. doi: 10.1093/cvr/cvu196. [DOI] [PubMed] [Google Scholar]
- 32.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 33.Goodall S, Crowther M, Hemingway DM, Bell PR, Thompson MM. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation. 2001;104:304–309. doi: 10.1161/01.cir.104.3.304. [DOI] [PubMed] [Google Scholar]
- 34.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong W, Knispel R, Mactaggart J, Baxter BT. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J Vasc Surg. 2006;44:1061–1066. doi: 10.1016/j.jvs.2006.06.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.