Abstract
Objective
Leptin and C-reactive protein (CRP) have each been linked to adverse cardiovascular events, and prior cross-sectional research suggests that increased levels of both biomarkers pose an even greater risk. The effect of increased levels of both leptin and CRP on mortality has not, however, been previously assessed.
Methods
We used data from the third National Health and Nutrition Examination Survey (NHANES III) to estimate the mortality effect of high leptin and high CRP levels. Outcomes were compared with the use of inverse-probability-weighting adjustment. Among 6,259 participants included in the analysis, 766 were in their sex-specific, population-weighted highest quartiles of both leptin and CRP. Median follow-up time was 14.3 years.
Results
There was no significant difference in adjusted all-cause mortality between the groups (risk ratio 1.22, 95% confidence interval [CI], 0.97 to 1.54). Similar results were noted with the use of several different analytic methods and in many subgroups, though high leptin and CRP levels may increase all-cause mortality in males (hazard ratio, 1.80, 95% CI, 1.32 to 2.46; P for interaction, 0.011). A significant difference in cardiovascular mortality was also noted (risk ratio, 1.54, 95% CI, 1.08 to 2.18), though that finding was not confirmed in all sensitivity analyses.
Conclusions
In this observational study, no significant difference in overall all-cause mortality rates in those with high leptin and high CRP levels was found, though high leptin and CRP levels appear associated with increased mortality in males. High leptin and CRP levels also likely increase risk for cardiovascular death.
Keywords: atherosclerosis, cardiovascular diseases, epidemiology, leptin, C-reactive protein
INTRODUCTION
Leptin is a polypeptide hormone involved in body weight regulation and insulin homeostasis.1–3 High leptin levels are associated with hypertension and diabetes mellitus4–6 as well as an increased risk for endothelial dysfunction.7–9 Leptin may adversely affect vascular wall lipid metabolism10 and may also increase the risk for adverse cardiovascular events.11–15 CRP is an acute-phase reactant shown to be a sensitive, non-specific marker of systemic inflammation16 and thought to be both maker and marker of the chronic arterial inflammation underlying atherosclerosis.17, 18 CRP is associated with traditional cardiovascular risk factors including obesity;19, 20 meta-analysis confirms it independently predicts adverse cardiovascular events.21
Leptin and CRP may interact in an important, yet undetermined, manner that adversely influences cardiovascular and all-cause mortality risk. Prior research suggests that leptin may induce expression of CRP22, and research supports a high correlation between the two biomarkers.23–25 A prior study suggested that increased levels of both leptin and CRP was significantly associated with increased rates of cardiovascular disease beyond that of CRP alone26, though its findings were limited by its cross-sectional study design.
It thus remains undetermined whether an association between increased levels in both leptin and CRP are of prognostic significance. To evaluate the potential prognostic value of high leptin and high CRP levels, we assessed prospectively collected data with long-term mortality follow-up using NHANES III.
METHODS
Study Population
From 1988 to 1994, the National Center for Health Statistics (NCHS) conducted NHANES III, a cross-sectional, stratified, multistage survey representative of the non-institutionalized U.S. population. Details of NHANES methodology have been previously described.27 In brief, the survey includes questionnaire, physical exam, and laboratory evaluation components. Individuals’ mortality status was subsequently determined through the National Death Index (NDI).28 The study complies with the Declaration of Helsinki. NHANES III protocols were approved by the NCHS and Centers for Disease Control and Prevention (CDC) Institutional Review Board. All participants gave written, informed consent.
There were 6,415 NHANES III adult subjects ≥20 years of age with available leptin data. Of these, individuals were excluded if they had missing CRP values (n=48), were pregnant (n=103), or had missing mortality data (n=5). The final sample consisted of 6,259 participants.
Baseline Measures
Fasting morning serum leptin concentrations were collected and stored at −70°C for an average of 8 years.29 Leptin levels were assessed by radioimmunoassay with a polyclonal antibody raised in rabbits against highly purified recombinant human leptin. The minimal detectable concentration of this assay was 0.5 mg/l and the limit of linearity was 100 mg/l. Intrassay and interassay coefficients of variation were both less than 5%.29 CRP levels in serum were measured within 2 months of sample collection, with a modified Behring latex-enhanced CRP assay.30
Mortality Follow-Up
Mortality status was based upon a NCHS search of the NDI through the year 2006. Details of the methodology are available elsewhere.28 In this analysis, we assessed deaths due to all causes and deaths due to cardiovascular disease (i.e., ICD-10 codes I00 to I99). Follow-up duration was calculated from the date of examination to either the date of death or to 31 December 2006.
Adjustment for Differences Between Groups
Based on individuals’ baseline characteristics, propensity scores were developed with the use of logistic regression to estimate the probability individuals were in the highest sex-specific, population-weighted quartiles of both leptin and CRP levels31, the same metric assessed in prior cross-sectional research.26 Continuous variables were modeled as a flexible polynomial with linear and quadratic components. Included in the propensity model were patients’ age, gender, race, body-mass index (BMI), waist circumference, smoking status, diabetes, hypertension, dyslipidemia, angina, chronic kidney disease (i.e., an glomerular filtration rate (GFR) <60 ml/min/1.73m2 as calculated by the Modification of Diet in Renal Disease equation32), and a family history of coronary artery disease, as well as a self-reported personal history of cerebrovascular disease, chronic lung disease (i.e., asthma, chronic bronchitis, or emphysema), intermittent claudication, heart failure, or myocardial infarction. Diabetes mellitus was defined as a self-reported history of diabetes, a fasting plasma glucose value ≥7.0 mmol/l (≥126 mg/dl), or a hemoglobin A1C ≥6.5.33 Intermittent claudication, following prior literature34, was defined as self-reported leg pain on walking quickly or uphill that was relieved by rest. Angina was defined using standard criteria from the Rose angina questionnaire35 and categorized as non-severe (Grade I) and severe (Grade II). Patients were classified as having hypertension if they reported having ever been diagnosed by a physician, whether they reported having ever taken blood pressure medication, or whether the average of up to three blood pressure measurements ≥140 mmHg for systolic blood pressure or ≥90 mmHg for diastolic blood pressure. Dyslipidemia was recorded if participants reported that a doctor had ever told them they had high cholesterol, they used cholesterol-lowering medications, had HDL cholesterol levels <40 mg/dl in men or <50 mg/dl in women, or had LDL cholesterol levels above ≥160 mg/dl.
Missing data were singly imputed using an expectation-maximization algorithm.36 Most variables had <1% missing data though 2.9% had missing waist circumference measurements and 2.7% had missing LDL data. Stabilized inverse probability weights (IPW) calculated from the propensity score were then used to adjust for differences between the two treatment groups.37 Performance of the propensity model was verified by comparing the distribution of covariates and propensity scores between treatment groups before and after IPW.
Statistical Analyses
Data were summarized as percentages in the case of categorical variables and as means with standard deviations in the case of continuous variables.χ2 and Wald tests were used to assess differences in baseline characteristics between groups for categorical and continuous variables, respectively.
Endpoints assessed all-cause mortality and cardiovascular mortality, as defined above, separately. The Kaplan-Meier method was used to estimate unadjusted survival curves.38 Adjusted survival curves employed IPW and represent the expected survival rate were all individuals to have had or not had both high leptin and CRP levels.39 Risk ratios at five-year intervals were calculated using estimated rates of survival using binomial log-linear regression40, with 95% confidence intervals calculated by bootstrapping. The comparison between those with and without both high leptin and high CRP was performed in the overall sample and in prospectively defined subgroups.
Sensitivity analyses were performed to assess the robustness of results. Survival curves were reestimated separately using Cox proportional-hazard models without propensity scores but using covariates identical to those used in the propensity model. In addition, we performed sensitivity analyses that combined IPW and model-based approaches in a “doubly robust” approach.41 The proportional hazards assumption in the Cox models was assessed with Schoenfeld residuals. All analyses were performed using Stata 11.2.
RESULTS
Characteristics of the study sample
After the exclusion criteria were applied, 6,259 participants were included in the analyses. Table 1 shows baseline participant characteristics. Before adjustment with IPW, patients with high leptin and CRP levels, as compared to those without such levels, were, on average, more likely to be older and obese. Participants with high leptin and high CRP levels were also more likely to have suffered from kidney, lung, and cardiovascular diseases including CHF, MI, CVA, claudication, and angina; and to have HTN, dyslipidemia, DM, or to have ever smoked.
Table 1. Baseline characteristics of the participants*.
Data are from NHANES III.
| Characteristic | Unadjusted Data | Data adjusted with use of inverse probability weighting | ||||
|---|---|---|---|---|---|---|
| CRP and leptin levels
|
P value | CRP and leptin levels
|
P value | |||
| Low (N=5493) | High (N=766) | Low (N=5493) | High (N=766) | |||
| Age (yr) | 47.1±18.9 | 53.1±17.6 | <0.001 | 47.9±18.5 | 50.4±21.6 | 0.16 |
| Male sex (%) | 46.9 | 43.6 | 0.082 | 46.1 | 39.4 | 0.067 |
| Race | <0.001 | 0.91 | ||||
| Non-Hispanic white | 42.6 | 41.0 | 42.4 | 41.2 | ||
| Non-Hispanic black | 26.1 | 34.5 | 27.5 | 29 | ||
| Mexican American | 26.9 | 21.8 | 26.0 | 24.8 | ||
| Other | 4.4 | 2.7 | 4.1 | 5.0 | ||
| Body-mass index† | 26.3±5.0 | 34.0±6.3 | <0.001 | 27.3±5.7 | 29.2±6.2 | <0.001 |
| Waist circumference (cm) | 90.9±13.0 | 109.8±12.9 | <0.001 | 93.4±14.3 | 98.2±14.8 | <0.001 |
| History of heart failure (%) | 2.4 | 5.1 | <0.001 | 2.9 | 5.4 | 0.11 |
| History of myocardial infarction (%) | 3.1 | 8.5 | <0.001 | 3.9 | 5.8 | 0.26 |
| Cerebrovascular disease (%) | 2.3 | 4.4 | <0.001 | 2.7 | 4.8 | 0.19 |
| Intermittent claudication (%) | 0.7 | 1.4 | 0.029 | 0.8 | 0.8 | 0.96 |
| Hypertension (%) | 31.8 | 54.4 | <0.001 | 34.9 | 42.0 | 0.052 |
| Dyslipidemia | 52.7 | 70.0 | <0.001 | 54.9 | 62.8 | 0.088 |
| Angina (%) | <0.001 | 0.080 | ||||
| None | 96.2 | 91.9 | 95.5 | 91.9 | ||
| Grade I | 2.3 | 4.3 | 2.6 | 5.4 | ||
| Grade II | 1.5 | 3.8 | 1.9 | 2.7 | ||
| Diabetes (%) | <0.001 | 0.16 | ||||
| None | 89.8 | 77.4 | 88.2 | 85.7 | ||
| Any | 9.9 | 21.3 | 11.4 | 13.9 | ||
| Requiring insulin | 0.4 | 1.3 | 0.4 | 0.4 | ||
| Chronic kidney disease (%) | 22.3 | 34.9 | <0.001 | 24.2 | 29.2 | 0.082 |
| Chronic lung disease (%) | 10.8 | 15.3 | <0.001 | 11.3 | 12.0 | 0.75 |
| Smoking status (%) | <0.001 | 0.53 | ||||
| Never smoker | 50.4 | 46.6 | 50.0 | 52.2 | ||
| Former smoker | 23.8 | 31.2 | 24.7 | 25.9 | ||
| Current smoker | 25.8 | 22.2 | 25.3 | 21.8 | ||
| Family history of myocardial infarction | 8.5 | 8.1 | 0.70 | 8.4 | 6.9 | 0.30 |
Plus-minus values are means ± SDs
The body-mass index is the weight in kilograms divided by the square of the height in meters
After adjustment with IPW, covariates showed improved balance (Table 1). As expected, patients without high leptin and CRP levels had a lower estimated probability of having high leptin and CRP levels (eFig. 1), with the median and interquartile range (IQR) of the propensity scores reflecting this difference (high leptin, high CRP group: median, 31.8%; IQR, 16.8 to 50.6; group without high leptin and high CRP: median, 3.4%; IQR, 0.9 to 11.2). These differences were notably less after IPW adjustment (high leptin, high CRP group: median, 9.4%; IQR, 3.8 to 23.0; group without high leptin and high CRP: median, 4.7%; IQR, 1.1 to 16.7).
Outcomes
The follow-up time ranged from ≤1 month to 18.1 years (average follow-up: overall, 13.4 years; high leptin, high CRP group, 12.5 years, and group without high leptin and high CRP, 13.5 years; median follow-up: overall, 14.3 years; high leptin, high CRP group, 13.7 years, and group without high leptin and high CRP, 14.3 years). Overall, there were 1,392 deaths observed (high leptin, high CRP group: 237; group without high leptin and high CRP: 1,155), of which 607 were due to cardiovascular causes (high leptin, high CRP group: 114; group without high leptin and high CRP group: 493).
Unadjusted survival curves for all-cause and cardiovascular mortality are shown in eFig. 2, and the survival curves adjusted with IPW are shown in Fig. 1. As displayed in eFig. 2, unadjusted mortality risk was consistently higher throughout the follow-up period for the group with high leptin and CRP levels. The unadjusted risk ratios demonstrated increased all-cause mortality (43.1% vs. 28.5% in the groups with and without high leptin and high CRP, respectively; risk ratio [RR], 1.47, 95% confidence interval [CI], 1.31 to 1.66) as well as increased cardiovascular mortality (19.1% vs. 12.3% in the groups with and without high leptin and high CRP, respectively; RR, 1.66, 95% CI, 1.37 to 2.00).
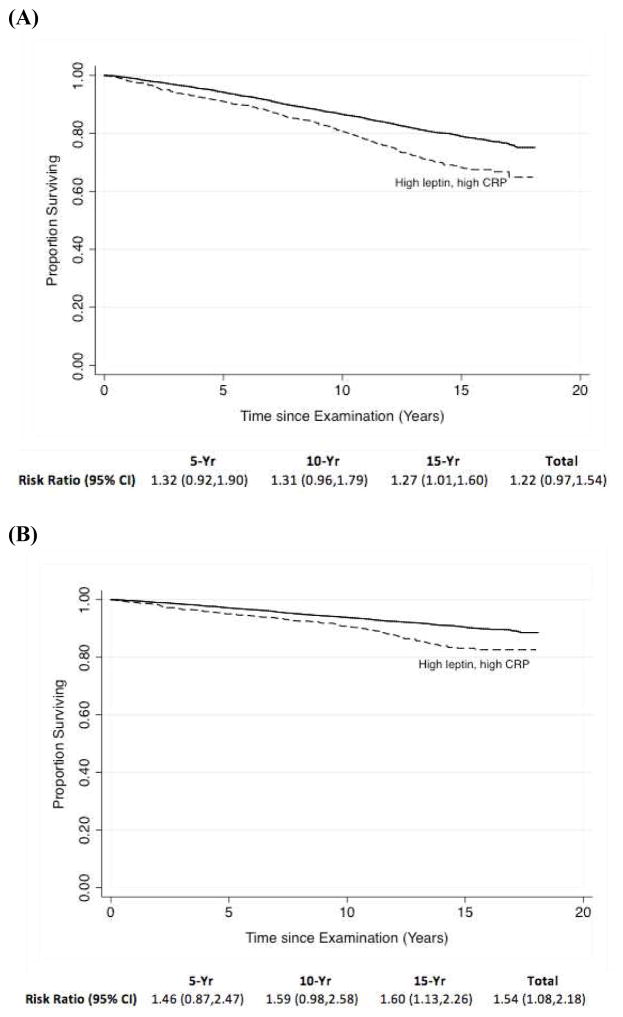
Figure 1. Kaplan-Meier curves of (A) all-cause and (B) cardiovascular mortality among NHANES participants with and without both high leptin and CRP levels, from an analysis adjusted with the use of inverse probability weighting.
Solid line, low leptin and CRP levels; dashed line, high leptin and CRP levels.
Adjustment for baseline covariates with IPW, however, demonstrated no significant effect of having both high leptin and high CRP on all-cause mortality (34.3% vs. 29.8% in the groups with and without high leptin and high CRP, respectively; RR 1.22, 95% CI, 0.97 to 1.54). These findings were largely confirmed when comparing relative all-cause mortality risks throughout the follow-up period. At 5 years, there was no significant difference in adjusted mortality between the groups (7.7% vs. 6.2% in the groups with and without high leptin and high CRP, respectively; RR, 1.32; 95% CI, 0.92 to 1.90). The adjusted 10-year mortality was 19.9% in the high leptin, high CRP group and 15.3% in the group without high leptin and high CRP (RR, 1.31; 95% CI, 0.96 to 1.79). At 15 years, mortality was 31.2% in the high leptin, high CRP group and 24.9% in the group without high leptin and high CRP (RR, 1.27; 95% CI, 1.01 to 1.60).
Adjustment with IPW did show a significant increase in overall cardiovascular mortality rates for those with high leptin and high CRP levels (16.6% vs. 12.8% in the groups with and without high leptin and high CRP, respectively; RR, 1.54, 95% CI, 1.08 to 2.18). While this finding was not observed at 5 (RR, 1.46, 95% CI 0.87 to 2.47) and 10 years (RR, 1.59, 95% CI 0.98 to 2.58) of follow-up, a significant difference in cardiovascular mortality rates was noted after 15 years (RR, 1.60, 95% CI, 1.13 to 2.26).
Sensitivity analyses performed with the use of a multivariate Cox model and a doubly robust approach employing multivariate analyses alongside IPW yielded results consistent with the primary analysis for all-cause mortality (Table 2). While the primary IPW analysis did find a relationship between high leptin and high CRP values with increased rates of cardiovascular mortality, as did the multivariate Cox model, the doubly robust approach found no such significant association. The hazard ratios showed similar results across most subgroups defined by age and race, as well as the presence or absence of diabetes, lung disease, hypertension, dyslipidemia, intermittent claudication, angina, chronic kidney disease, or obesity; an additional post hoc analysis also found no difference by the duration of follow-up. A significant interaction by sex was noted (P=0.011), however (Table 3). Among males, high leptin and CRP levels were associated with increased all-cause mortality rates (hazard ratio, 1.80, 95% CI, 1.32 to 2.46) while no such association was found among females (hazard ratio, 0.91, 95% CI, 0.60 to 1.39).
Table 2. Comparison of hazard ratios from sensitivity analyses.
Comparison of overall hazard ratio estimates from unadjusted analyses, as well as analyses adjusted by inverse probability weights, doubly robust methodology, and multivariate Cox proportional hazards regression.
| Method | Hazard ratio for all-cause mortality (95% CI) | Hazard ratio for cardiovascular mortality (95% CI) |
|---|---|---|
| Unadjusted | 1.59 (1.38,1.82) | 1.78 (1.44,2.19) |
| Adjusted with | ||
| IPW | 1.25 (0.94,1.67) | 1.53 (1.03,2.29) |
| IPW* | 1.18 (0.95,1.46) | 1.44 (1.02,2.02) |
| IPW† | 1.06 (0.86,1.30) | 1.25 (0.94,1.64) |
| Multivariate Cox regression | 1.15 (0.98, 1.34) | 1.28 (1.01,1.63) |
Additionally adjusts for body-mass index (BMI) and waist circumference.
Additionally adjusts for all covariates used to estimate the propensity score (i.e., the doubly robust methodology)
Table 3.
Hazard ratios for all-cause mortality by subgroup, from analyses adjusted with the use of inverse probability weighting.
| Category | Hazard Ratio (95% CI) | P for interaction |
|---|---|---|
| Sex | 0.011 | |
| Male | 1.80 (1.32,2.46) | |
| Female | 0.91 (0.60,1.39) | |
| Race | 0.84 | |
| White | 1.27 (1.30,1.88) | |
| Non-White | 1.23 (0.79,1.91) | |
| Age | 0.88 | |
| <65 | 1.09 (0.69,1.71) | |
| ≥65 | 1.13 (0.86,1.49) | |
| BMI | 0.55 | |
| <25 | 1.59 (0.56,4.46) | |
| ≥25 | 1.15 (0.94,1.41) | |
| Diabetes | 0.21 | |
| No | 1.30 (0.94,1.81) | |
| Yes | 0.96 (0.68,1.38) | |
| Lung disease | 0.28 | |
| No | 1.18 (0.87,1.60) | |
| Yes | 1.67 (0.96,2.90) | |
| Hypertension | 0.88 | |
| No | 1.12 (0.71,1.77) | |
| Yes | 1.16 (0.87,1.55) | |
| Dyslipidemia | 0.41 | |
| No | 1.04 (0.62,1.75) | |
| Yes | 1.34 (0.99,1.80) | |
| Intermittent claudication | 0.47 | |
| No | 1.24 (0.94,1.64) | |
| Yes | 1.81 (0.69,4.77) | |
| Angina | 0.37 | |
| No | 1.16 (0.89,1.52) | |
| Yes | 1.76 (0.74,4.16) | |
| Chronic kidney disease | 0.29 | |
| No | 0.99 (0.61,1.62) | |
| Yes | 1.34 (1.02,1.76) | |
| Overall | 1.25 (0.94,1.67) |
DISCUSSION
This study employed NHANES mortality follow-up data to assess whether increased levels of both leptin and CRP increased the risk of all-cause and cardiovascular mortality. No significant difference was noted in overall adjusted all-cause mortality between those with and without high levels of leptin and CRP. This finding was robust across multiple sensitivity analyses and across most subgroups. Notably, effect modification by sex was observed; high CRP and high leptin levels were significantly associated with increased all-cause mortality in males but not females. Results from the IPW analysis demonstrated that increased leptin and CRP levels do lead to increased overall risk of cardiovascular death, though results from certain sensitivity analyses suggest that these findings may not be robust.
These findings should be evaluated in light of prior research. Evidence suggests that a correlation between leptin and CRP exists, and a plausible biological pathway has been described.22–25, 42 One prior study, which also used NHANES III data, suggested that, in both males and females, increased levels of both leptin and CRP significantly improved estimates of cardiovascular disease.26 Importantly, that study was cross-sectional in nature while, by contrast, this study employs prospective data. In addition, IPW was used as a means by which to improve the causal interpretation of data. Results here suggest that high levels of leptin and CRP may be of limited prognostic significance. Adjusted results from this study suggest that high leptin and CRP levels are associated with a relatively small increased risk for cardiovascular mortality and that increased levels of both biomarkers have no effect on all-cause mortality. Taken together, having high levels of both leptin and CRP may reflect prior cardiovascular disease but does not appear to be a useful predictor of future overall cardiovascular mortality.
The difference by sex in the relationship between all-cause mortality and high levels of CRP and leptin merits comment. In the current study, a significant difference was noted between males and females in the mortality risk arising from high levels of these biomarkers. Males with high leptin and high CRP levels had significantly increased all-cause mortality; females did not. Though it did not assess CRP levels, a prior study examining the relationship between leptin, and cardiovascular disease and mortality in females with diabetes found no significant association.43 Differences in leptin levels by sex have been previously described, with onset of those differences at puberty.44, 45 Prior research suggests that the relationship between CRP and leptin is more robust in women than in men46, and that the observed sex differences may result not just as a consequence of differences in body fat mass but differences in sex hormones as well.47 This study’s design accounted for sex differences by examining levels of biomarkers in the highest sex-specific quartiles. Whether there remains an unappreciated effect of individuals’ sex hormone profiles on how leptin, and any interaction of leptin with CRP, alters cardiovascular risk remains undetermined. Nonetheless, that CRP and leptin are less correlated on average in males suggests that, at least as a proxy, high levels of both pose a comparatively greater risk in men than in women.
Leptin has been increasingly recognized as a pleiotropic hormone, and prior authors have suggested that the cardiovascular role of leptin is likely complex.47, 48 Leptin forms the core feedback mechanism regarding body fat availability yet obesity is associated with hyperleptinemia, which suggests the possibility of leptin resistance.48, 49 Nonetheless, the impact of leptin on mortality may be comparatively inconsequential if one accounts for obesity itself, as this study did, since increasing levels of BMI are associated with increased mortality.50 Moreover, subgroup analyses of individuals with known cardiovascular risk factors showed no difference from the overall sample. Leptin may have effects that are difficult to disentangle regarding their contribution to overall mortality risk, though. Leptin has vasodilatory properties, increases angiogenesis, and decreases lipid toxicity, all cardioprotective in nature, but it also has less desirable cardiovascular properties like enhancing platelet aggregation, being proinflammatory, and encouraging the proliferation of vascular smooth muscle cells.42 While estimates presented here adjust for known prior cardiovascular disease and risk factors, we were unable here to assess thoroughly the subclinical disease processes in which leptin may play a key mediating role.
The study is also not without analytical limitations. It is observational in nature, and the unadjusted profiles and propensity scores for having both high leptin and high CRP levels differed between the groups. After adjustment, differences in mean BMI and waist circumference remained, though they were much reduced compared to unadjusted comparisons. In addition, results from a sensitivity analysis also minimize the likelihood that imbalance between these covariates significantly biased the presented results; models combining IPW with additional adjustment for these factors did not differ materially from the overall findings. It remains theoretically tenable, however, that residual confounding remained, potentially biasing results in an unknown direction. Since the study employs a strategy to balance characteristics of both groups in order to assess the causal impact of high leptin and high CRP levels, it also cannot assess the differential impact of each among the balanced groups. The study’s design also assesses only baseline biomarker levels; how trajectories of these may influence cardiovascular risk remains undetermined.
Despite such limitations, this study has significant strengths when compared to prior research. It is the first study of which we are aware to examine whether elevated levels of both leptin and CRP increase all-cause and cardiovascular mortality using prospectively gathered data. It has a median follow-up time of 14.3 years. Data originate from a large, multiethnic sample, lending credence that results here may be generalizable. Though not without limitations, statistical techniques including IPW and the imputation of missing data through an algorithm previously shown to reduce potential biases have been used to improve causal inference. The robustness of these results were assessed by numerous sensitivity analyses.
In summary, this study used prospectively gathered NHANES data to evaluate whether increased levels of both leptin and CRP increased all-cause and cardiovascular mortality. We found the increased levels of both leptin and CRP did not increase all-cause mortality rates in the overall sample, but did increase all-cause mortality rates among males. Increased leptin and CRP levels also likely slightly increase risk of cardiovascular mortality.
Supplementary Material
Highlights.
Leptin and CRP have both individually been linked to adverse cardiovascular events
Increased levels of both leptin and CRP may pose an even greater risk to health
The effect on mortality of increased levels of both has not been assessed before
High levels of leptin and CRP increased cardiovascular mortality by 54%
High leptin and CRP levels nearly doubled overall mortality in males
Acknowledgments
We are grateful to Steven E. Lipschultz, MD for his comments on a draft of this manuscript and to Miguel Hernán, MD, DrPH for providing technical advise.
FUNDING SOURCES
None.
Abbreviations
- BMI
Body-mass index
- CDC
Centers for Disease Control and Prevention
- CRP
C-reactive protein
- ICD
International Classification of Disease
- IPW
Inverse probability weighting
- NCHS
National Center for Health Statistics
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Misra A, Garg A. Leptin, its receptor and obesity. J Investig Med. 1996;44(9):540–8. [PubMed] [Google Scholar]
- 2.Lönnqvist F. The obese (ob) gene and its product leptin—a new route toward obesity treatment in man? QJM. 1996;89(5):327–332. [Google Scholar]
- 3.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13(2):215–23. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sharma V, McNeill JH. The emerging roles of leptin and ghrelin in cardiovascular physiology and pathophysiology. Curr Vasc Pharmacol. 2005;3(2):169–80. doi: 10.2174/1570161053586868. [DOI] [PubMed] [Google Scholar]
- 6.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53(Suppl 1):S152–8. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 7.Porreca E, Di Febbo C, Fusco L, Moretta V, Di Nisio M, Cuccurullo F. Soluble thrombomodulin and vascular adhesion molecule-1 are associated to leptin plasma levels in obese women. Atherosclerosis. 2004;172(1):175–80. doi: 10.1016/j.atherosclerosis.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Malyszko J, Wolczynski S, Malyszko J, Mysliwiec M. Leptin correlates with some hemostatic parameters in CAPD patients. Nephron. 2002;92(3):721–4. doi: 10.1159/000064074. [DOI] [PubMed] [Google Scholar]
- 9.Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108(17):2074–81. doi: 10.1161/01.CIR.0000095272.67948.17. [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke L, Yeaman SJ, Shepherd PR. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes. 2001;50(5):955–61. doi: 10.2337/diabetes.50.5.955. [DOI] [PubMed] [Google Scholar]
- 11.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44(9):1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Soderberg S, Ahren B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246(4):409–18. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 13.Soderberg S, Stegmayr B, Stenlund H, Sjostrom LG, Agren A, Johansson L, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256(2):128–36. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 14.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104(25):3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 15.Sierra-Johnson J, Romero-Corral A, Lopez-Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, et al. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100(2):234–9. doi: 10.1016/j.amjcard.2007.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson J. CRP--marker or maker of cardiovascular disease? Arterioscler Thromb Vasc Biol. 2005;25(8):1527–8. doi: 10.1161/01.ATV.0000174796.81443.3f. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 19.Lemieux I, Pascot A, Prud’homme D, Almeras N, Bogaty P, Nadeau A, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21(6):961–7. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 20.Miller M, Zhan M, Havas S. High attributable risk of elevated C-reactive protein level to conventional coronary heart disease risk factors: the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2005;165(18):2063–8. doi: 10.1001/archinte.165.18.2063. [DOI] [PubMed] [Google Scholar]
- 21.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(9):e302–7. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12(4):425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 24.Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109(18):2181–5. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 25.Ble A, Windham BG, Bandinelli S, Taub DD, Volpato S, Bartali B, et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) Am J Cardiol. 2005;96(7):991–5. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, Thomas RJ, Singh P, Hoffmann M, et al. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5(7):418–25. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Health Statistics. Plan and operation of the Third National Heaith and Nutrition Examination Survey, 1988–94. Vital Health Stat. 1994;1(32) [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality Follow-up through 2006: Matching Methodology. Hyattsville, MD: National Center for Health Statistics, Office of Analysis and Epidemiology; 2009. [Google Scholar]
- 29.National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III Serum Leptin Data File (Series 11, No. 12A) Hyattsville, MD: Centers for Disease Control and Prevention; 2001. [Google Scholar]
- 30.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4(3):109–114. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Hsia SH, Pan D, Berookim P, Lee ML. A population-based, cross-sectional comparison of lipid-related indexes for symptoms of atherosclerotic disease. Am J Cardiol. 2006;98(8):1047–52. doi: 10.1016/j.amjcard.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 36.King G, Honaker J, Joseph A, Scheve K. Analyzing incomplete political science data: An alternative algorithm for multiple imputation. Amer Polit Sci Rev. 2001;95(1):49–70. [Google Scholar]
- 37.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–86. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statistical Assoc. 1958;53(282):457–481. [Google Scholar]
- 39.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160(4):301–305. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 41.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: An application to data on right heart catheterization. Health Services and Outcomes Research Methodology. 2001;2(3–4):259–278. [Google Scholar]
- 42.Wolk R, Somers VK. Leptin and vascular function: Friend or foe? Eur Heart J. 2006;27(19):2263–5. doi: 10.1093/eurheartj/ehl246. [DOI] [PubMed] [Google Scholar]
- 43.Brennan AM, Li TY, Kelesidis I, Gavrila A, Hu FB, Mantzoros CS. Circulating leptin levels are not associated with cardiovascular morbidity and mortality in women with diabetes: a prospective cohort study. Diabetologia. 2007;50(6):1178–85. doi: 10.1007/s00125-007-0635-y. [DOI] [PubMed] [Google Scholar]
- 44.Ruhl CE, Everhart JE. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr. 2001;74(3):295–301. doi: 10.1093/ajcn/74.3.295. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84(3):899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 46.Abdullah SM, Khera A, Leonard D, Das SR, Canham RM, Kamath SA, et al. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195(2):404–10. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol. 2013;33(1):54–65. doi: 10.1016/j.semnephrol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens. 2002;20(7):1245–50. doi: 10.1097/00004872-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.